Reactive Processes for H2S Removal
Abstract
:1. Introduction
Motivation
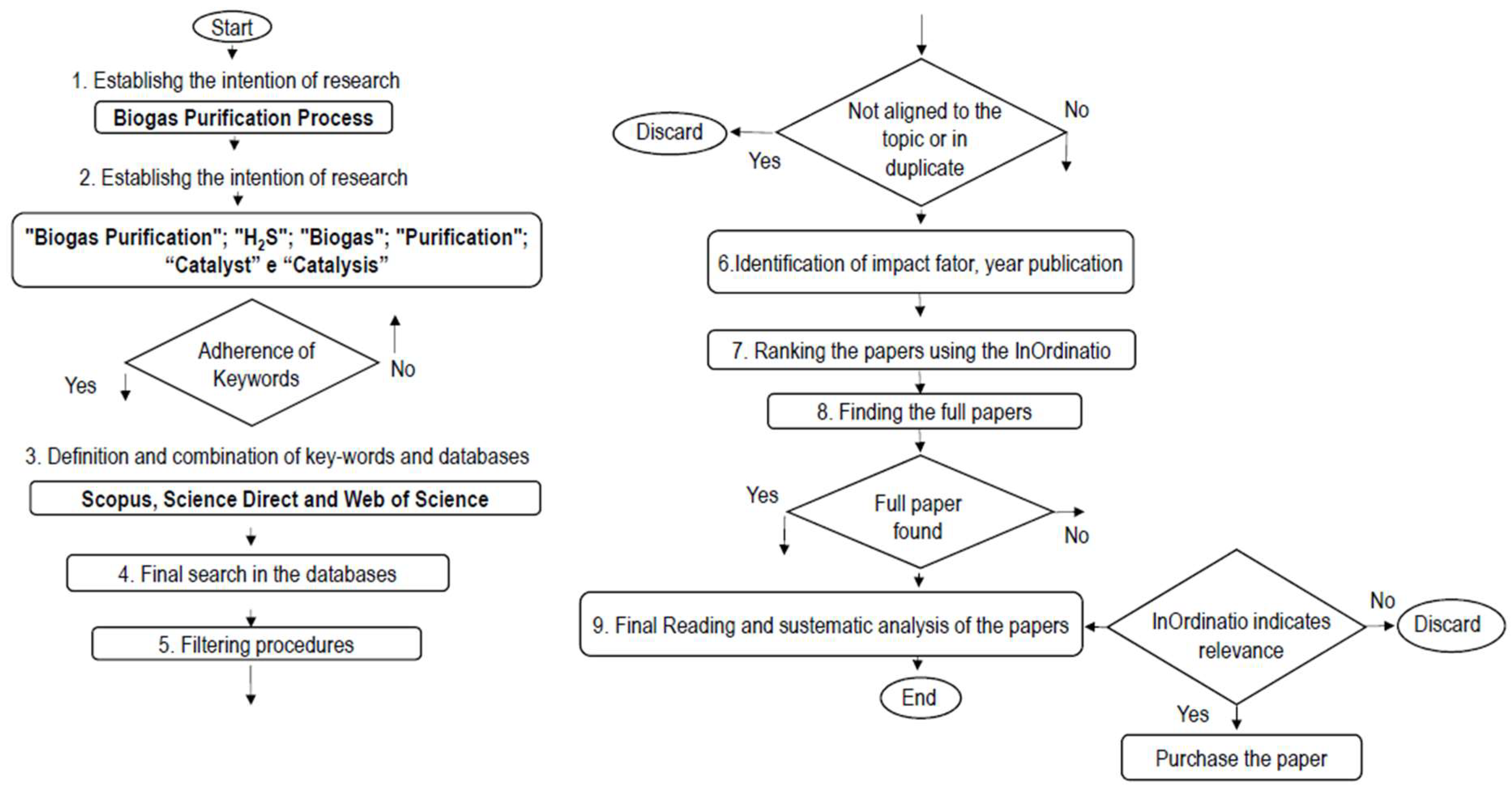
2. Materials and Methods
3. Results and Discussion
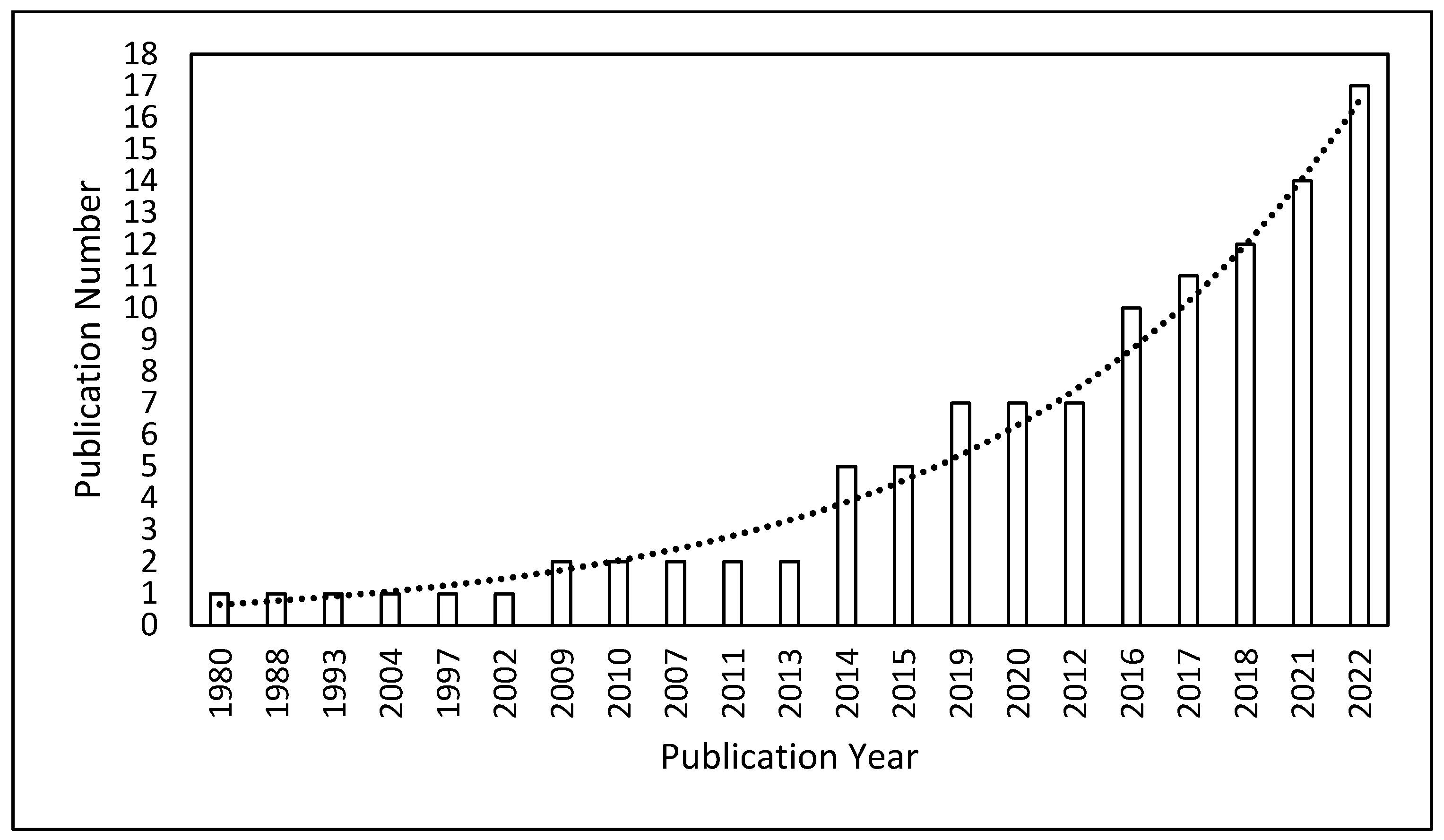
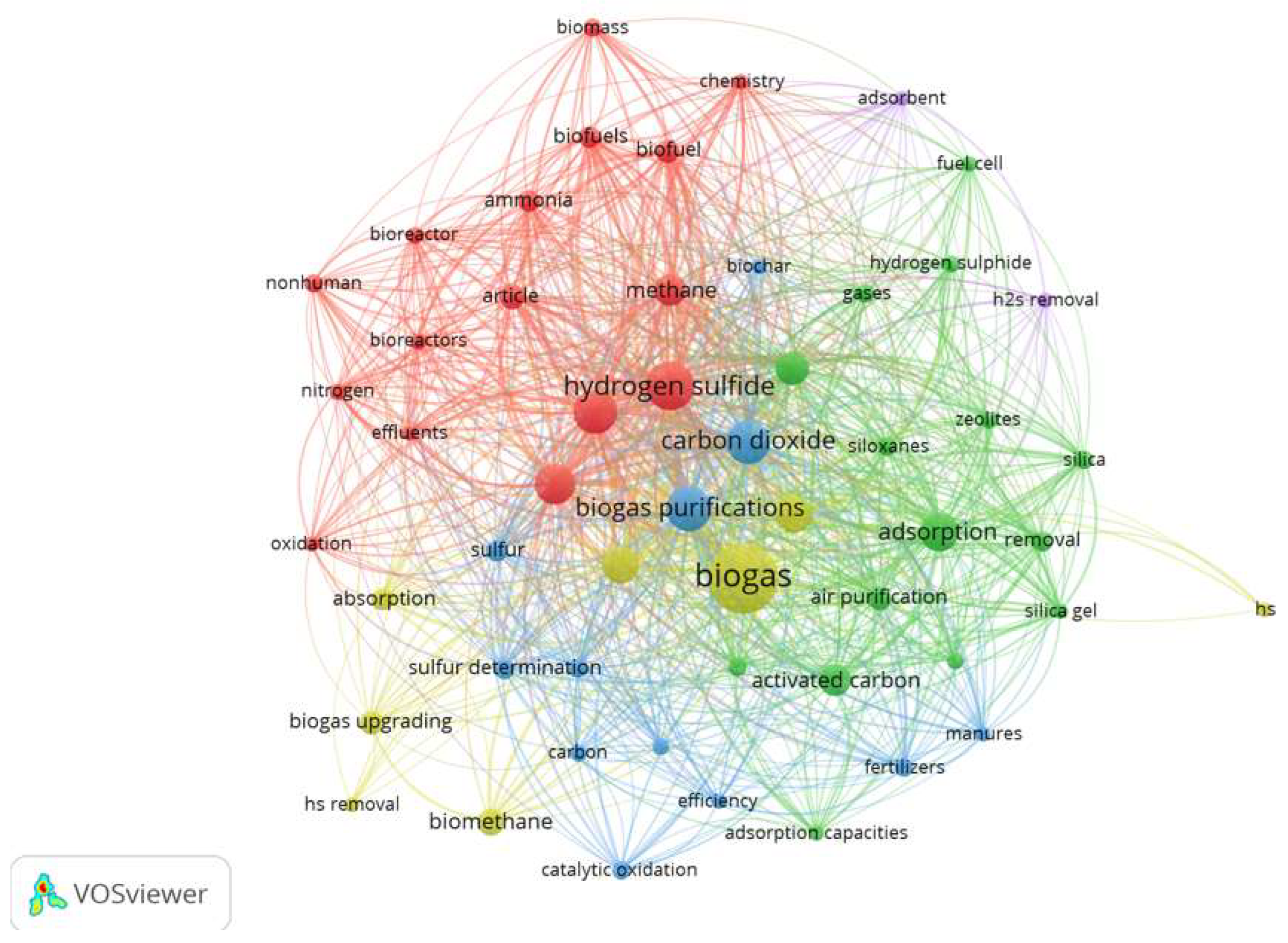
3.1. Bibliometric Analysis
3.2. Homogeneous Process
3.3. Chemisorption/Catalysis
3.4. Zeolites
3.5. H2S Removal Mechanisms
3.6. Limitations in the Materials Application
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank Publications: Washington, DC, USA, 2018. [Google Scholar]
- Wang, R.; Li, C.; Lv, N.; Pan, X.; Cai, G.; Ning, J.; Zhu, G. Deeper Insights into Effect of Activated Carbon and Nano-Zero-Valent Iron Addition on Acidogenesis and Whole Anaerobic Digestion. Bioresour. Technol. 2021, 324, 124671. [Google Scholar] [CrossRef]
- Kalaiselvan, N.; Glivin, G.; Bakthavatsalam, A.K.; Mariappan, V.; Premalatha, M.; Raveendran, P.S.; Jayaraj, S.; Sekhar, S.J. A Waste to Energy Technology for Enrichment of Biomethane Generation: A Review on Operating Parameters, Types of Biodigesters, Solar Assisted Heating Systems, Socio Economic Benefits and Challenges. Chemosphere 2022, 293, 133486. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, V.K.; Fdez-Güelfo, L.A.; Zhou, Y.; Álvarez-Gallego, C.J.; Garcia, L.I.R.; Ng, W.J. Anaerobic Co-Digestion of Organic Fraction of Municipal Solid Waste (OFMSW): Progress and Challenges. Renew. Sustain. Energy Rev. 2018, 93, 380–399. [Google Scholar] [CrossRef]
- Zamri, M.F.M.A.; Hasmady, S.; Akhiar, A.; Ideris, F.; Shamsuddin, A.H.; Mofijur, M.; Fattah, I.M.R.; Mahlia, T.M.I. A Comprehensive Review on Anaerobic Digestion of Organic Fraction of Municipal Solid Waste. Renew. Sustain. Energy Rev. 2021, 137, 110637. [Google Scholar] [CrossRef]
- Morone, P.; Imbert, E. Food Waste and Social Acceptance of a Circular Bioeconomy: The Role of Stakeholders. Curr. Opin. Green Sustain. Chem. 2020, 23, 55–60. [Google Scholar] [CrossRef]
- Donner, M.; Gohier, R.; de Vries, H. A New Circular Business Model Typology for Creating Value from Agro-Waste. Sci. Total Environ. 2020, 716, 137065. [Google Scholar] [CrossRef]
- Horikawa, M.S.; Rossi, F.; Gimenes, M.L.; Costa, C.M.M.; da Silva, M.G.C. Chemical Absorption of H2S for Biogas Purification. Braz. J. Chem. Eng. 2004, 21, 415–422. [Google Scholar] [CrossRef]
- Tippayawong, N.; Thanompongchart, P. Biogas Quality Upgrade by Simultaneous Removal of CO2 and H2S in a Packed Column Reactor. Energy 2010, 35, 4531–4535. [Google Scholar] [CrossRef]
- Osorio, F.; Torres, J.C. Biogas Purification from Anaerobic Digestion in a Wastewater Treatment Plant for Biofuel Production. Renew. Energy 2009, 34, 2164–2171. [Google Scholar] [CrossRef]
- Dobslaw, D.; Engesser, K.-H.; Störk, H.; Gerl, T. Low-Cost Process for Emission Abatement of Biogas Internal Combustion Engines. J. Clean. Prod. 2019, 227, 1079–1092. [Google Scholar] [CrossRef]
- Sigot, L.; Ducom, G.; Germain, P. Adsorption of Hydrogen Sulfide (H2S) on Zeolite (Z): Retention Mechanism. Chem. Eng. J. 2016, 287, 47–53. [Google Scholar] [CrossRef]
- Moghadam, H.N.; Banaeia, A. Chemical Review and Letters Removal of Hydrogen Sulfide from Biogas by Using the Water Scrubbing Techniques. Chem. Rev. Lett. 2022, 5, 168–176. [Google Scholar]
- Lee, H.S.; Yang, J.H.; Lee, H.J.; Lee, H.; Jeon, S.C. Integrated Sol-Gel and Hydrothermal Synthesis of V2O5–TiO2 Nanocatalysts for Enhanced Catalytic Removal of H2S. J. Clean. Prod. 2021, 329, 129791. [Google Scholar] [CrossRef]
- Salehi, R.; Chaiprapat, S. Single-/Triple-Stage Biotrickling Filter Treating a H2S-Rich Biogas Stream: Statistical Analysis of the Effect of Empty Bed Retention Time and Liquid Recirculation Velocity. J. Air Waste Manag. Assoc. 2019, 69, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.H.; Meng, X.L.; Wang, Y.; Liu, L.D. Performance of Biotrickling Filters for Hydrogen Sulfide Removal under Starvation and Shock Loads Conditions. J. Zhejiang Univ. Sci. B 2009, 10, 595–601. [Google Scholar] [CrossRef]
- Dumont, E. H2S Removal from Biogas Using Bioreactors: A Review. Int. J. Energy Environ. 2015, 6, 479–498. [Google Scholar]
- Zain, M.M.; Mohamed, A.R. An Overview on Conversion Technologies to Produce Value Added Products from CH4 and CO2 as Major Biogas Constituents. Renew. Sustain. Energy Rev. 2018, 98, 56–63. [Google Scholar] [CrossRef]
- Okoro, O.V.; Sun, Z. Desulphurisation of Biogas: A Systematic Qualitative and Economic-Based Quantitative Review of Alternative Strategies. ChemEngineering 2019, 3, 76. [Google Scholar] [CrossRef]
- Gargiulo, N.; Peluso, A.; Aprea, P.; Marino, O.; Cioffi, R.; Jannelli, E.; Cimino, S.; Lisi, L.; Caputo, D. Chromium-Based MIL-101 Metal Organic Framework as a Fully Regenerable D4 Adsorbent for Biogas Purification. Renew. Energy 2019, 138, 230–235. [Google Scholar] [CrossRef]
- Schiavon Maia, D.C.; Niklevicz, R.R.; Arioli, R.; Frare, L.M.; Arroyo, P.A.; Gimenes, M.L.; Pereira, N.C. Removal of H2S and CO2 from Biogas in Bench Scale and the Pilot Scale Using a Regenerable Fe-EDTA Solution. Renew. Energy 2017, 109, 188–194. [Google Scholar] [CrossRef]
- De Campos, E.A.R.; Pagani, R.N.; Resende, L.M.; Pontes, J. Construction and Qualitative Assessment of a Bibliographic Portfolio Using the Methodology Methodi Ordinatio. Scientometrics 2018, 116, 815–842. [Google Scholar] [CrossRef]
- Pagani, R.N.; Kovaleski, J.L.; Resende, L.M. Methodi Ordinatio: A Proposed Methodology to Select and Rank Relevant Scientific Papers Encompassing the Impact Factor, Number of Citation, and Year of Publication. Scientometrics 2015, 105, 2109–2135. [Google Scholar] [CrossRef]
- Demmink, J.F.; Beenackers, A.A.C.M. Gas Desulfurization with Ferric Chelates of EDTA and HEDTA: New Model for the Oxidative Absorption of Hydrogen Sulfide. Ind. Eng. Chem. Res. 1998, 37, 1444–1453. [Google Scholar] [CrossRef]
- Lin, W.C.; Chen, Y.P.; Tseng, C.P. Pilot-Scale Chemical-Biological System for Efficient H2S Removal from Biogas. Bioresour. Technol. 2013, 135, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Bak, C.U.; Lim, C.J.; Lee, J.G.; Kim, Y.D.; Kim, W.S. Removal of Sulfur Compounds and Siloxanes by Physical and Chemical Sorption. Sep. Purif. Technol. 2019, 209, 542–549. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, L.; Blasco, T.; Rodríguez-Castellón, E.; Nieto, J.M.L. Partial Oxidation of H2S to Sulfur on V-Cu-O Mixed Oxides Bronzes. Catal. Today 2019, 333, 237–244. [Google Scholar] [CrossRef]
- Kapłan, M.; Klimek, K.; Syrotyuk, S.; Konieczny, R.; Jura, B.; Smoliński, A.; Szymenderski, J.; Budnik, K.; Anders, D.; Dybek, B.; et al. Raw Biogas Desulphurization Using the Adsorption-Absorption Technique for a Pilot Production of Agricultural Biogas from Pig Slurry in Poland. Energies 2021, 14, 5929. [Google Scholar] [CrossRef]
- Sigot, L.; Ducom, G.; Benadda, B.; Labouré, C. Comparison of Adsorbents for H 2S and D4 Removal for Biogas Conversion in a Solid Oxide Fuel Cell. Environ. Technol. 2016, 37, 86–95. [Google Scholar] [CrossRef]
- Zhang, Y.; Kawasaki, Y.; Oshita, K.; Takaoka, M.; Minami, D.; Inoue, G.; Tanaka, T. Economic Assessment of Biogas Purification Systems for Removal of Both H2S and Siloxane from Biogas. Renew. Energy 2021, 168, 119–130. [Google Scholar] [CrossRef]
- Farghali, M.; Andriamanohiarisoamanana, F.J.; Ahmed, M.M.; Kotb, S.; Yamamoto, Y.; Iwasaki, M.; Yamashiro, T.; Umetsu, K. Prospects for Biogas Production and H2S Control from the Anaerobic Digestion of Cattle Manure: The Influence of Microscale Waste Iron Powder and Iron Oxide Nanoparticles. Waste Manag. 2020, 101, 141–149. [Google Scholar] [CrossRef]
- Sitthikhankaew, R.; Chadwick, D.; Assabumrungrat, S.; Laosiripojana, N. Effects of Humidity, O2, and CO2 on H2S Adsorption onto Upgraded and KOH Impregnated Activated Carbons. Fuel Process. Technol. 2014, 124, 249–257. [Google Scholar] [CrossRef]
- Micoli, L.; Bagnasco, G.; Turco, M. H2S Removal from Biogas for Fuelling MCFCs: New Adsorbing Materials. Int. J. Hydrog. Energy 2014, 39, 1783–1787. [Google Scholar] [CrossRef]
- Juárez, M.F.-D.; Mostbauer, P.; Knapp, A.; Müller, W.; Tertsch, S.; Bockreis, A.; Insam, H. Biogas Purification with Biomass Ash. Waste Manag. 2018, 71, 224–232. [Google Scholar] [CrossRef]
- Zulkefli, N.N.; Masdar, M.S.; Isahak, W.N.R.W.; Bakar, S.N.H.A.; Hasan, H.A.; Sofian, N.M. Application of Response Surface Methodology for Preparation of Znac2/Cac Adsorbents for Hydrogen Sulfide (H2S) Capture. Catalysts 2021, 11, 545. [Google Scholar] [CrossRef]
- Quan, W.; Jiang, X.; Wang, X.; Song, C. Hydrogen Sulfide Removal from Biogas on ZIF-Derived Nitrogen-Doped Carbons. Catal. Today 2021, 371, 221–230. [Google Scholar] [CrossRef]
- Cepollaro, E.M.; Caputo, D.; Gargiulo, N.; Deorsola, F.A.; Cimino, S.; Lisi, L. H2S Catalytic Removal at Low Temperature over Cu- and Mg- Activated Carbon Honeycombs. Catal. Today 2022, 390–391, 221–229. [Google Scholar] [CrossRef]
- Yan, M.; Fang, W.; Feng, H.; Zhang, Y.; Wibowo, H.; Kanchanatip, E.; Rahim, D.A. Performance Evaluation of Zn-Fe Adsorbent for H2S Adsorption during Biogas Purification. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Zulkefli, N.N.; Mathuray Veeran, L.S.; Noor Azam, A.M.I.; Masdar, M.S.; Wan Isahak, W.N.R. Effect of Bimetallic-Activated Carbon Impregnation on Adsorption–Desorption Performance for Hydrogen Sulfide (H2S) Capture. Materials 2022, 15, 5409. [Google Scholar] [CrossRef]
- Cimino, S.; Lisi, L.; Erto, A.; Deorsola, F.A.; de Falco, G.; Montagnaro, F.; Balsamo, M. Role of H2O and O2 during the Reactive Adsorption of H2S on CuO-ZnO/Activated Carbon at Low Temperature. Microporous Mesoporous Mater. 2020, 295, 109949. [Google Scholar] [CrossRef]
- Cristiano, D.M.; Mohedano, R.A.; Nadaleti, W.C.; de Castilhos Junior, A.B.; Lourenço, V.A.; Gonçalves, D.F.H.; Filho, P.B. H2S Adsorption on Nanostructured Iron Oxide at Room Temperature for Biogas Purification: Application of Renewable Energy. Renew. Energy 2020, 154, 151–160. [Google Scholar] [CrossRef]
- Bahraminia, S.; Anbia, M.; Koohsaryan, E. Hydrogen Sulfide Removal from Biogas Using Ion-Exchanged Nanostructured NaA Zeolite for Fueling Solid Oxide Fuel Cells. Int. J. Hydrog. Energy 2020, 45, 31027–31040. [Google Scholar] [CrossRef]
- Hao, X.; Hou, G.; Zheng, P.; Liu, R.; Liu, C. H2S In-Situ Removal from Biogas Using a Tubular Zeolite/TiO2 Photocatalytic Reactor and the Improvement on Methane Production. Chem. Eng. J. 2016, 294, 105–110. [Google Scholar] [CrossRef]
- Peluso, A.; Gargiulo, N.; Aprea, P.; Pepe, F.; Caputo, D. Nanoporous Materials as H2S Adsorbents for Biogas Purification: A Review. Sep. Purif. Rev. 2019, 48, 78–89. [Google Scholar] [CrossRef]
- Bareschino, P.; Mancusi, E.; Forgione, A.; Pepe, F. Biogas Purification on Na-X Zeolite: Experimental and Numerical Results. Chem. Eng. Sci. 2020, 223, 115744. [Google Scholar] [CrossRef]
- Dong, Y.N.; Chen, W.C.; Zhang, L.L.; Sun, B.C.; Chu, G.W.; Chen, J.F. Sulfur Recycle in Biogas Production: Novel Higee Desulfurization Process Using Natural Amino Acid Salts. Chemosphere 2022, 297, 134215. [Google Scholar] [CrossRef] [PubMed]

| CH4 | 60–70% |
| CO2 | 30–40% |
| N2 | <1% |
| H2S | 10–2000 ppm |
| Ref. | Adsorbent/Catalyst | Operational Condition | H2S Removal Capacity |
|---|---|---|---|
| [32] | Activated carbon (AC) | 3000 ppmv H2S, 100 mL/min, 30 °C, 1 g of activated carbon, fixed-bed reactor | 1.1 L/100 g |
| Steam activated AC | 2.6 L/100 g | ||
| AC impregnated with KOH | 4.3 L/100 g | ||
| [33] | Cu-modified 13 zeolite (ion exchange) | 8 ppm of H2S in He, 0.1 L/min, 40 °C, 20 mg of sample, fixed-bed reactor | 1.17 mmol/g |
| Na2O3-AC | 2.46 mmol/g | ||
| [29] | Coconut-based activated carbon | 80 ppmv of H2S, 4 NL/min, 10–50 g of adsorbent | 1.3 mg H2S/g adsorbent |
| 13X zeolite | 46 mg H2S/g adsorbent | ||
| [34] | Biomass (wood) ash | 100–600 ppm, 2.6–5.2 m3/h, 10.5–22.4 °C | 0.56–1.25 kg/ton ash |
| [26] | Iron Oxide (48% wt FeOOH) | 1900 ppm, 300 mL/min, 25 °C, packed reactor, 40 mm | 132 mg/g |
| Iron Oxide Hydroxide (48% wt FeOOH) | 69 mg/g | ||
| [30] | Iron-oxide based adsorbent | 600–1160 ppmv of H2S, 1200 NMm3/h | 250–290 mg H2S/g Fe2O3 |
| [35] | Commercial activated carbon impregnated with ZnAc2 | 700–800 ppmv, 15 L/min–53 L/min | 2.37 mg/g adsorbent |
| [36] | Nitrogen-doped carbon | 500–1800 ppm, 15–40 mL/min, flow-bed reactor, 0.6 g of sample | 48.2 mg/g |
| [37] | 5%(wt) Cu-Activated carbon | 100 ppmv, 20 SL/h 30 °C | 222 mg/g |
| 5%(wt) Mg-Activated carbon | 242 mg/g | ||
| [38] | Zn-Fe/Al2O3 | 100–250 ppm, 25 °C, 2.5 g of adsorbent | 3.23 mg/g adsorbent |
| [39] | Coconut activated carbon samples impregnated with ZnAc2 and TiO2 | 5000 ppm, 5.5 L/min, 1 bar, 30 °C | 1.92 mg/g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Secco, C.; Fuziki, M.E.K.; Tusset, A.M.; Lenzi, G.G. Reactive Processes for H2S Removal. Energies 2023, 16, 1759. https://doi.org/10.3390/en16041759
Secco C, Fuziki MEK, Tusset AM, Lenzi GG. Reactive Processes for H2S Removal. Energies. 2023; 16(4):1759. https://doi.org/10.3390/en16041759
Chicago/Turabian StyleSecco, Carolinne, Maria Eduarda Kounaris Fuziki, Angelo Marcelo Tusset, and Giane Gonçalves Lenzi. 2023. "Reactive Processes for H2S Removal" Energies 16, no. 4: 1759. https://doi.org/10.3390/en16041759
APA StyleSecco, C., Fuziki, M. E. K., Tusset, A. M., & Lenzi, G. G. (2023). Reactive Processes for H2S Removal. Energies, 16(4), 1759. https://doi.org/10.3390/en16041759







