Water Wall Tubes’ High Temperature Corrosion Root Cause Investigation: A 300 MW Level Boiler Case
Abstract
:1. Introduction
2. Experimental Description
2.1. Boiler Basic Design Parameter
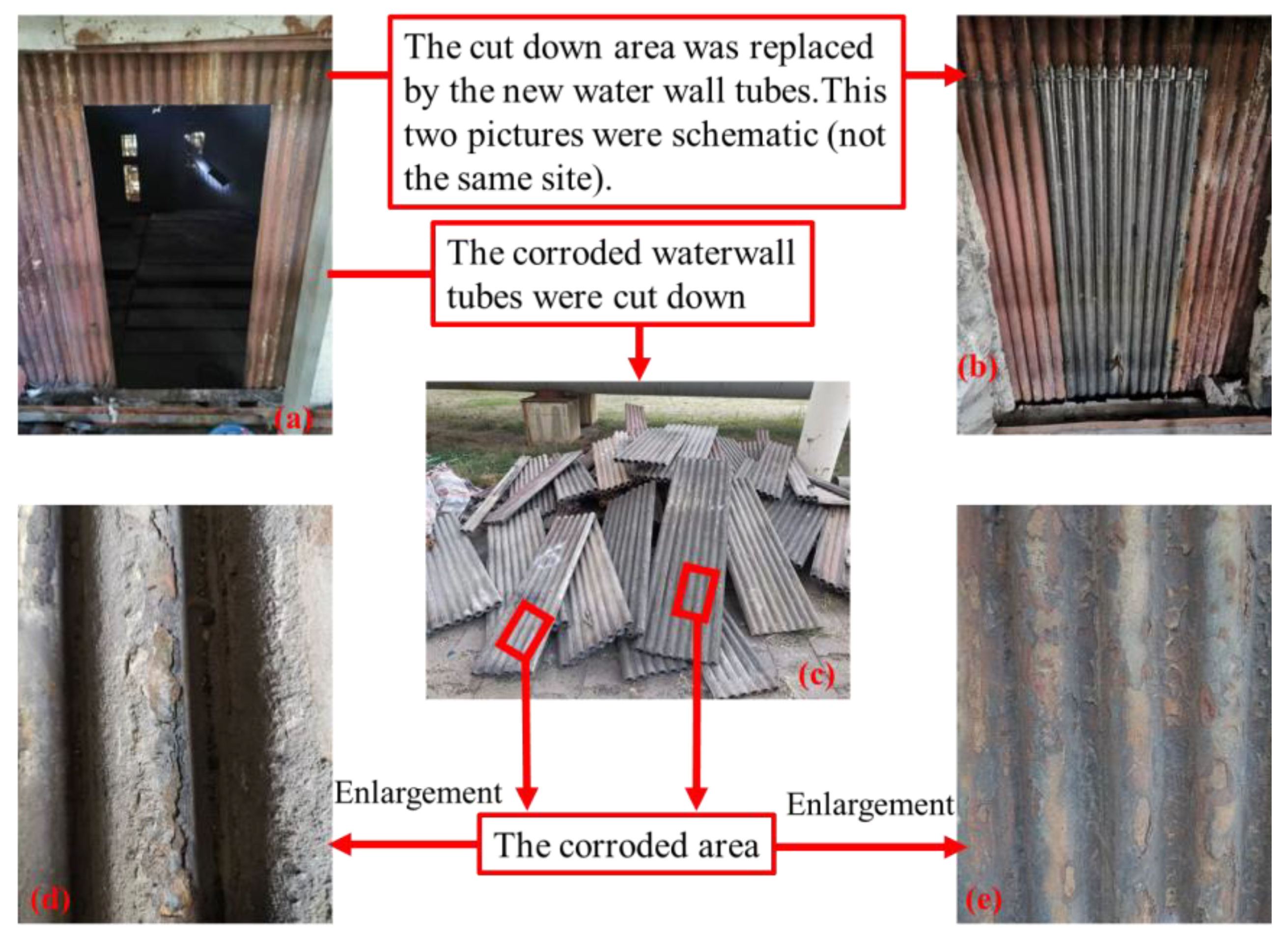
2.2. Corrosion Status
2.3. The Water Wall Tubes’ Ambience Gas Sampling Methods Description
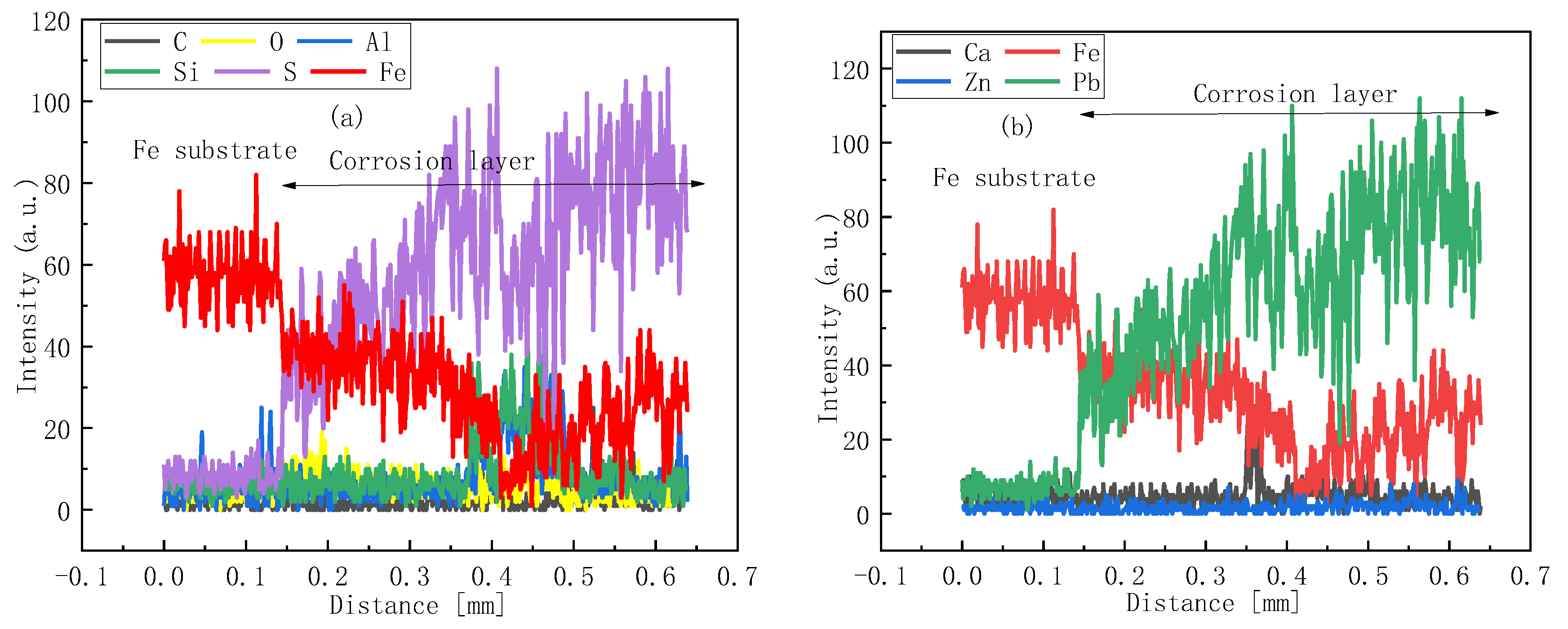
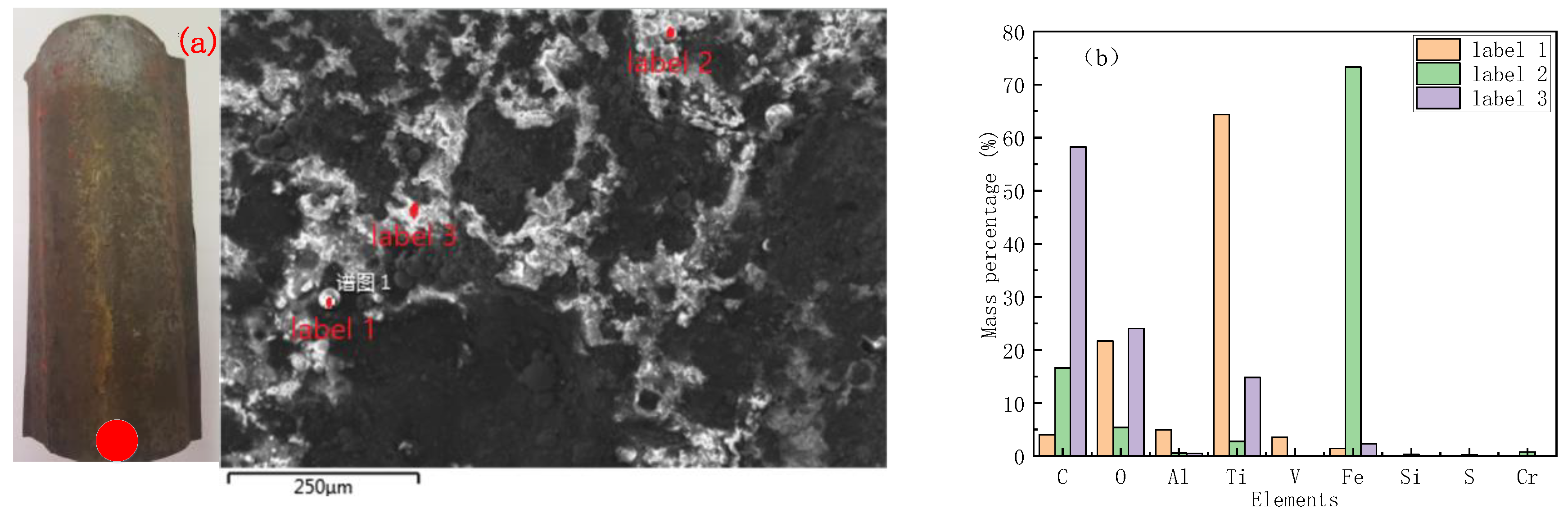
2.4. Corroded Tube Sampling and Characterization
3. Experimental Results
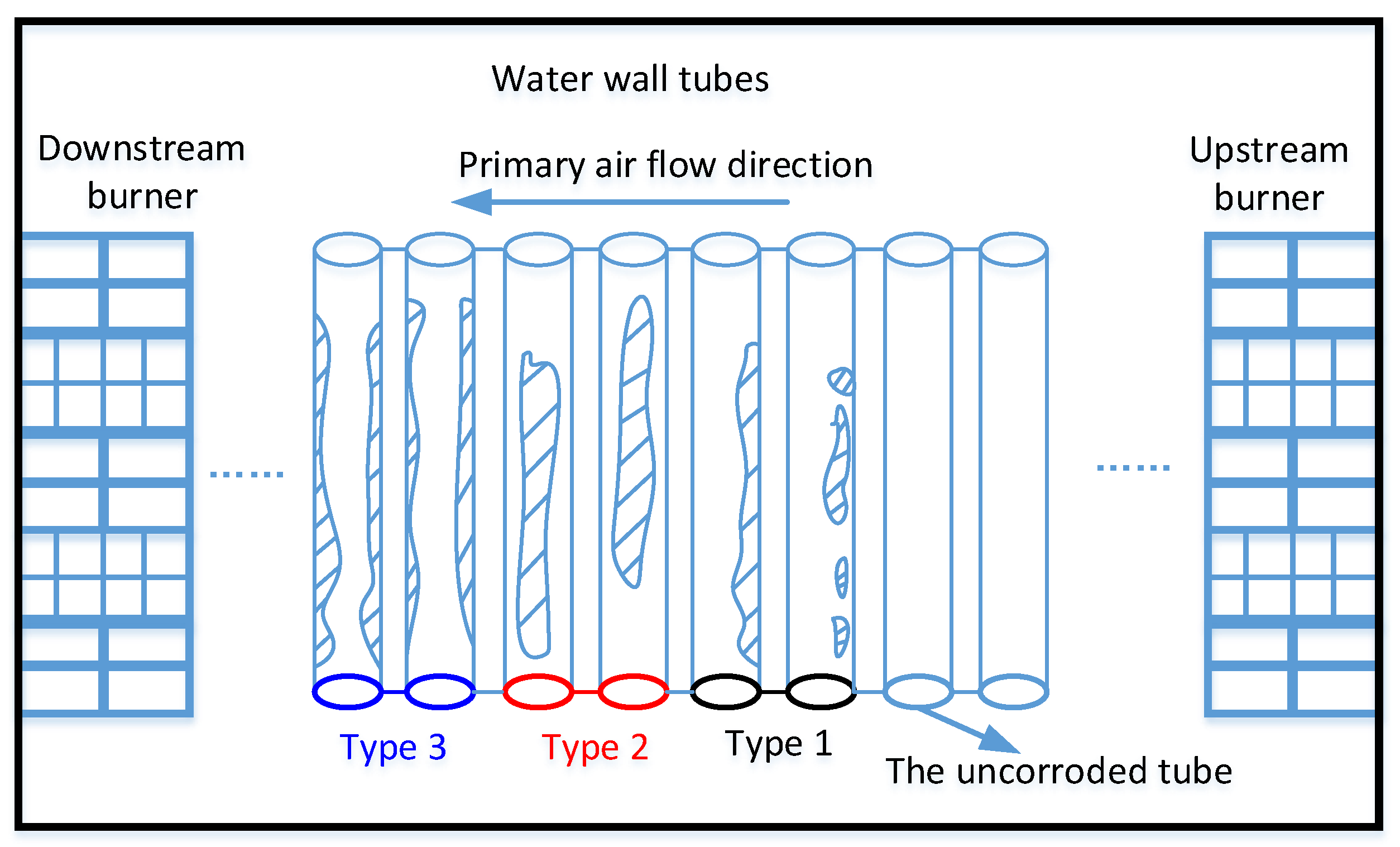
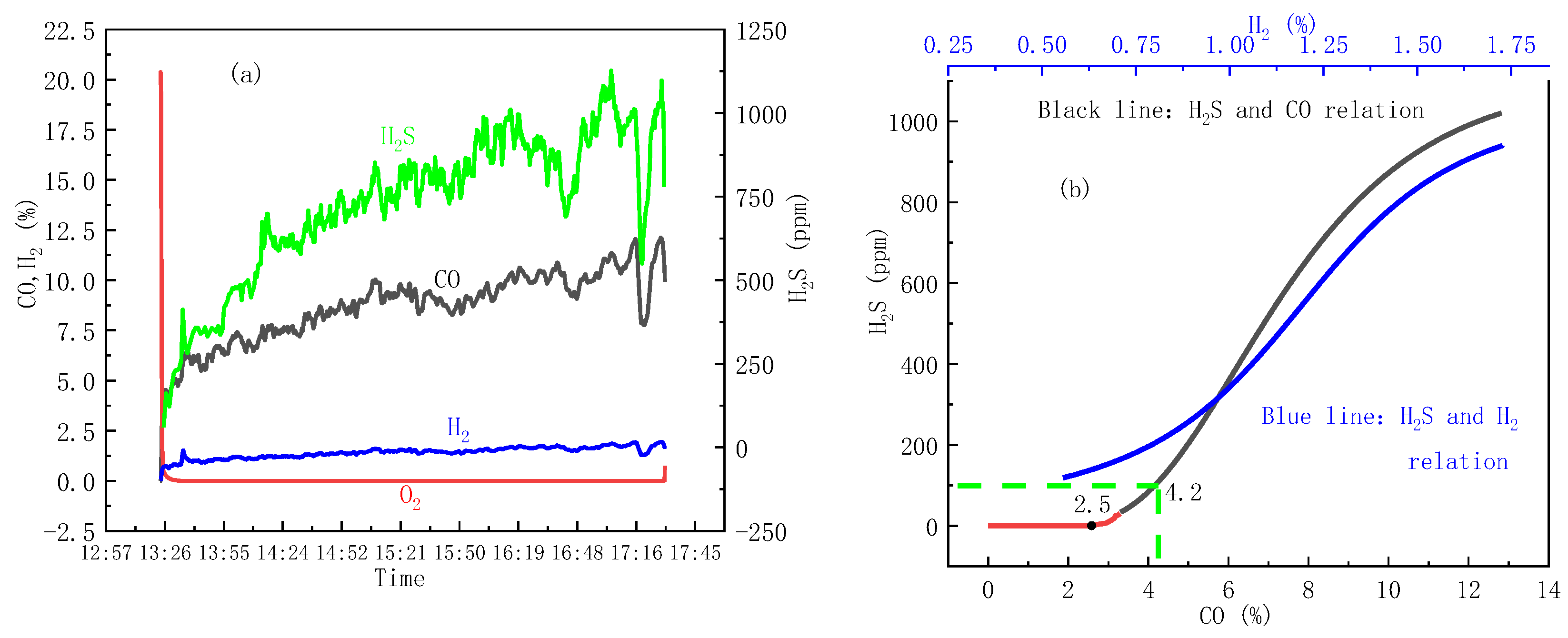
3.1. Water Wall Tube Corrosion Category Evaluation: H2S Corrosion
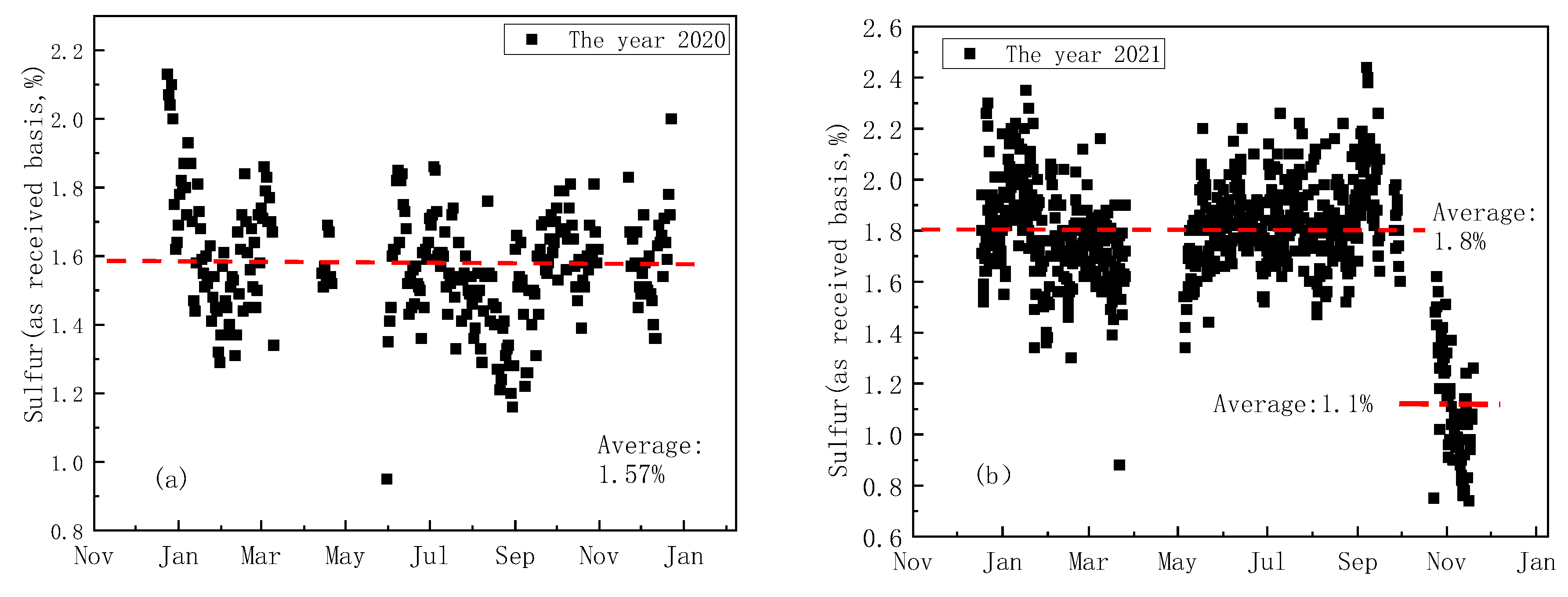
3.2. The Source of H2S and the Water Wall H2S Concentration
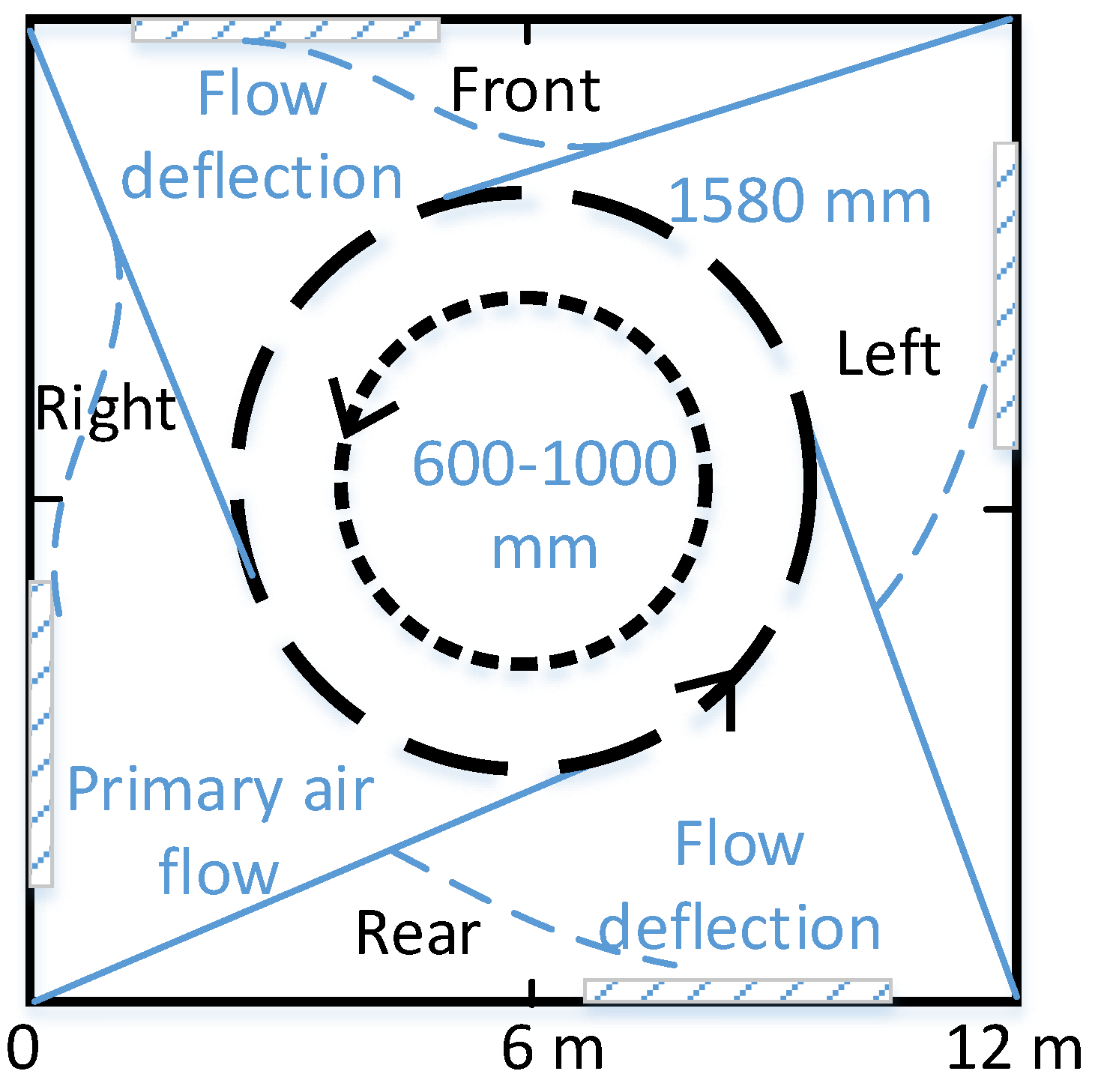
3.3. The Diameter of Imaginary Circle of Primary Air (DICPA) Analysis
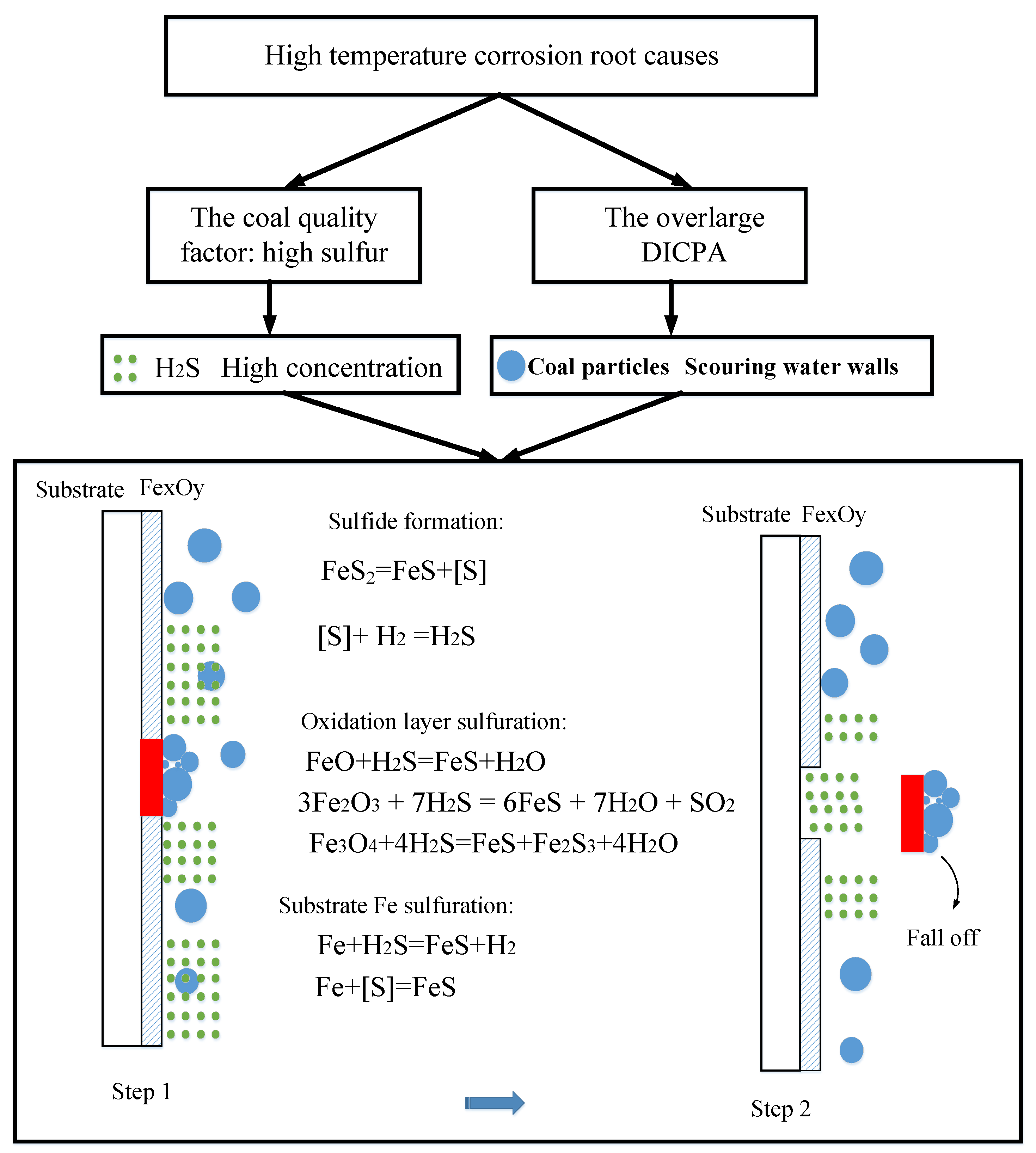
3.4. Water Wall High Temperature Corrosion Root Cause Analysis
3.5. Practical Significance: High Temperature Corrosion Prevention
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, Y.M.; Chen, K.; Kang, J.N.; Chen, W.; Wang, X.Y.; Zhang, X. Policy and management of carbon peaking and carbon neutrality: A literature review. Engineering 2022, 14, 52–63. [Google Scholar] [CrossRef]
- Wei, H.; Lu, Y.; Yang, Y.; Zhang, C.; He, C.; Wu, Y.; Li, W.; Zhao, D. Research on influence of steam extraction parameters and operation load on operational flexibility of coal-fired power plant. Appl. Therm. Eng. 2021, 195, 117226. [Google Scholar] [CrossRef]
- Action Plan for Energy Conservation and Emission Reduction Upgrading and Transformation of Coal Fired Plants 2014–2020. National Development and Reform Commission, Ministry of Ecological Environment, National Energy Administration, China. EB/OL. Available online: http://www.gov.cn/gongbao/content/2015/content_2818468.htm (accessed on 30 December 2022).
- Sun, X.; Ning, Y.; Yang, J.; Zhao, Y.; Yang, Z.; Zhou, X. Study on high temperature corrosion mechanism of water wall tubes of 350 MW supercritical unit. Eng. Fail. Anal. 2021, 121, 105131. [Google Scholar] [CrossRef]
- Xu, L.; Huang, Y.; Zou, L.; Yue, J.; Wang, J.; Liu, C.; Liu, L.; Dong, L. Experimental research of mitigation strategy for high-temperature corrosion of waterwall fireside in a 630MWe tangentially fired utility boiler based on combustion adjustments. Fuel Process. Technol. 2019, 188, 1–15. [Google Scholar] [CrossRef]
- Cao, L.; Peng, R.; Deng, Z. Optimization study on high-temperature corrosion prevention of the water wall of a 1000 MW dual circle tangential boiler during operation. Energy Rep. 2021, 7, 915–925. [Google Scholar] [CrossRef]
- Zhao, Q.X.; Zhang, Z.X.; Cheng, D.N.; Wang, Y.; Deng, X. High temperature corrosion of water wall materials T23 and T24 in simulated furnace atmospheres. Chin. J. Chem. Eng. 2012, 20, 814–822. [Google Scholar] [CrossRef]
- Nimmervoll, M.; Mori, G.; Hönig, S.; Haubner, R. High-temperature corrosion of austenitic alloys in HCl and H2S containing atmospheres under reducing conditions. Corrosion 2022, 200, 110214. [Google Scholar] [CrossRef]
- Bai, L.; Peng, W.; Zhu, J.; Wu, X.; Shi, X.; Xiang, J.; Deng, X.; Wang, Y.; Sun, Z.; Yu, S. High temperature chloride corrosion behavior of AlFe2.5NiMoNbCr high-entropy alloy. Corros. Sci. 2022, 198, 110139. [Google Scholar] [CrossRef]
- Wei, C.; Wang, Z.; Chen, J. Sulfuration corrosion failure analysis of Inconel 600 alloy heater sleeve in high-temperature flue gas. Eng. Fail. Anal. 2022, 135, 106111. [Google Scholar] [CrossRef]
- Paz, M.D.; Phother-Simon, J.; Andersson, S.; Jonsson, T. High temperature corrosion memory in a waste fired boiler—Influence of sulfur. Waste Manag. 2021, 130, 30–37. [Google Scholar] [CrossRef]
- Yu, X.; Gong, B.; Gao, Q.; Zhao, Y.; Tian, C.; Zhang, J. Investigation of fireside corrosion at water-cooled wall from a coal-fired power plant in China. Appl. Therm. Eng. 2017, 127, 1164–1171. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Wang, M.; Tan, H. Formation of Sulfide Deposits and High-Temperature Corrosion Behavior at Fireside in a Coal-Fired Boiler. Energy Fuels 2020, 34, 13849–13861. [Google Scholar] [CrossRef]
- Xiong, X.; Liu, X.; Tan, H.; Deng, S. Investigation on high temperature corrosion of water-cooled wall tubes at a 300 MW boiler. J. Energy Inst. 2020, 93, 377–386. [Google Scholar] [CrossRef]
- Bukowski, P.; Hardy, T.; Kordylewski, W. Evaluation of corrosion hazard in PF boilers applying the oxygen content in flue gases. Arch. Combust. 2009, 29, 11–18. [Google Scholar]
- Hardy, T.; Modlinski, N. Development of Prognostic Tool for High-temperature Corrosion Risk Assessment in Pulverised Coal Boilers Based on the Waterwalls Boundary Layer Monitoring System and CFD Simulations. Energy Procedia 2017, 105, 1833–1838. [Google Scholar] [CrossRef]
- Shirai, H.; Ikeda, M.; Aramaki, H. Characteristics of hydrogen sulfide formation in pulverized coal combustion. Fuel 2013, 114, 114–119. [Google Scholar] [CrossRef]
- Tsuji, H.; Tanno, K.; Nakajima, A.; Yamamoto, A.; Shirai, H. Hydrogen sulfide formation characteristics of pulverized coal combustion -Evaluation of blended combustion of two bituminous coals. Fuel 2015, 158, 523–529. [Google Scholar] [CrossRef]
- Che, D. Boilers: Theory, Design and Operation; Xi’an Jiaotong University Press: Xi’an, China, 2008. [Google Scholar]
- Ma, H.; Zhou, L.; Ma, S.; Bai, Y. Progress in mechanism of H2S formation during pulverized coal combustion. Therm. Power Gener. 2019, 48, 5. [Google Scholar]
- Lv, S.; Wang, B.; Zhao, J.; Ma, H. A Study on detailed mechanism of H2S formation during pulverized coal combustion. Acad. J. Xi’an Jiaotong Univ. 2020, 54, 68–75. [Google Scholar]
- Xu, T. Boiler Combustion Equipment; Xi’an Jiaotong University Press: Xi’an, China, 1990. [Google Scholar]
- Xu, W.; Tan, H.; Liu, Y.; Wei, B.; Hui, S. Research on determination of high temperature corrosion tendency of water walls and limiting concentration range of H2S near walls. Electr. Power 2018, 51, 113–119. [Google Scholar]
- Barrau, O.; Boher, C.; Vergne, C.; Rezai-Aria, F.; Gras, R. Investigations of friction and wear mechanisms of hot forging tool steels. Karlstad Univ. 2002, 1, 81–94. [Google Scholar]
- Ma, H.; Zhou, L.; Ma, S.; Yang, S.; Zhao, Y.; Zhang, W.; Chew, J.W. Impact of multi-hole-wall air coupling with air-staged technology on H2S evolution during pulverized coal combustion. Fuel Process. Technol. 2018, 179, 277–284. [Google Scholar] [CrossRef]



| Material | C % | Si % | Mn % | P % | S % |
|---|---|---|---|---|---|
| 20G | 0.20 | 0.24 | 0.45 | 0.04 | 0.04 |
| Total Moisture, Mt % | Ash, Aad % | Volatile, Vdaf % | Fixed Carbon, Cd % | Total Sulfur, St,ar % | Lower Calorific Value, Qnet,ar, MJ/kg |
|---|---|---|---|---|---|
| 6.55 | 32.38 | 15.97 | 55.91 | 1.76 | 20.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, X.; Chen, F.; Li, L.; Tan, H. Water Wall Tubes’ High Temperature Corrosion Root Cause Investigation: A 300 MW Level Boiler Case. Energies 2023, 16, 1767. https://doi.org/10.3390/en16041767
Xiong X, Chen F, Li L, Tan H. Water Wall Tubes’ High Temperature Corrosion Root Cause Investigation: A 300 MW Level Boiler Case. Energies. 2023; 16(4):1767. https://doi.org/10.3390/en16041767
Chicago/Turabian StyleXiong, Xiaohe, Falin Chen, Liangyu Li, and Houzhang Tan. 2023. "Water Wall Tubes’ High Temperature Corrosion Root Cause Investigation: A 300 MW Level Boiler Case" Energies 16, no. 4: 1767. https://doi.org/10.3390/en16041767
APA StyleXiong, X., Chen, F., Li, L., & Tan, H. (2023). Water Wall Tubes’ High Temperature Corrosion Root Cause Investigation: A 300 MW Level Boiler Case. Energies, 16(4), 1767. https://doi.org/10.3390/en16041767





