Development of a Dual-Chamber Pyrolizer for Biochar Production from Agricultural Waste in Sri Lanka
Abstract
:1. Introduction
Biochar System for Global Smallholder Agriculture
2. Materials and Methods
2.1. The Feedstocks
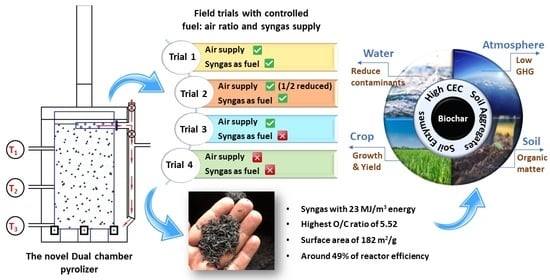
2.2. Dual-Chamber Pyrolizer
- Trial 1: all air supply doors were fully opened, and syngas was supplied as fuel from the beginning of syngas generation.
- Trial 2: air supply for the combustion was reduced by half by controlling the size of the air supply doors, and syngas was supplied as fuel from the beginning of syngas generation.
- Trial 3: all air supply doors were opened, and the produced syngas was taken out from the reactor.
- Trial 4: all air supply doors were closed, and the produced syngas was taken out from the reactor.

2.3. Performance Evaluation of Dual-Chamber Reactor
2.4. Quality of the Produced Biochar
2.5. Characterization
3. Results and Discussion
3.1. Characteristics of Feedstock
3.2. Mass Balance per Batch
3.3. Temperature Profiles
3.4. Syngas Composition and Energy Value
3.5. Heat Balance of the Reactor
3.6. Characteristics of Produced Biochar
3.6.1. Elemental Composition
3.6.2. Thermal Analysis (TGA and DSC) and Surface Area Results
3.6.3. Fourier Transform Infrared (FTIR) Spectroscopy
3.6.4. XRD
3.7. Challenges and Future Perspectives
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-Char Sequestration in Terrestrial Ecosystems—A Review. Mitig. Adapt. Strat. Glob. Chang. 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Wu, P.; Ata-Ul-Karim, S.T.; Singh, B.P.; Wang, H.; Wu, T.; Liu, C.; Fang, G.; Zhou, D.; Wang, Y.; Chen, W. A Scientometric Review of Biochar Research in the Past 20 Years (1998–2018). Biochar 2019, 1, 23–43. [Google Scholar] [CrossRef]
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The Concept and Future Prospects of Soil Health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, J.; Chen, R.; Yu, P.; Guo, S.; Wang, X. Adsorption and Reduction of Chromium(VI) from Aqueous Solution Using Polypyrrole/Calcium Rectorite Composite Adsorbent. Water Res. 2019, 160, 148–157. [Google Scholar] [CrossRef]
- Khataee, A.R.; Kasiri, M.B. Photocatalytic Degradation of Organic Dyes in the Presence of Nanostructured Titanium Dioxide: Influence of the Chemical Structure of Dyes. J. Mol. Catal. A Chem. 2010, 328, 8–26. [Google Scholar] [CrossRef]
- Haddad, M.Y.; Alharbi, H.F. Enhancement of Heavy Metal Ion Adsorption Using Electrospun Polyacrylonitrile Nanofibers Loaded with ZnO Nanoparticles. J. Appl. Polym. Sci. 2019, 136, 47209. [Google Scholar] [CrossRef]
- Haddad, M.; Oie, C.; Vo Duy, S.; Sauvé, S.; Barbeau, B. Adsorption of Micropollutants Present in Surface Waters onto Polymeric Resins: Impact of Resin Type and Water Matrix on Performance. Sci. Total Environ. 2019, 660, 1449–1458. [Google Scholar] [CrossRef]
- Mahvi, A.H. Application of Agricultural Fibers in Pollution Removal from Aqueous Solution. Int. J. Environ. Sci. Technol. 2008, 5, 275–285. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Mahvi, A.H.; Rastkari, N.; Saeedi, R.; Nazmara, S.; Iravani, E. Adsorption of Bisphenol A (BPA) from Aqueous Solutions by Carbon Nanotubes: Kinetic and Equilibrium Studies. Desalination Water Treat. 2015, 54, 84–92. [Google Scholar] [CrossRef]
- Roy, H.; Islam, M.S.; Arifin, M.T.; Firoz, S.H. Synthesis, Characterization and Sorption Properties of Biochar, Chitosan and ZnO-Based Binary Composites towards a Cationic Dye. Sustainability 2022, 14, 14571. [Google Scholar] [CrossRef]
- Yuan, Y.; Chesnutt, B.M.; Haggard, W.O.; Bumgardner, J.D. Deacetylation of Chitosan: Material Characterization and in Vitro Evaluation via Albumin Adsorption and Pre-Osteoblastic Cell Cultures. Materials 2011, 4, 1399–1416. [Google Scholar] [CrossRef]
- Regmi, P.; Garcia Moscoso, J.L.; Kumar, S.; Cao, X.; Mao, J.; Schafran, G. Removal of Copper and Cadmium from Aqueous Solution Using Switchgrass Biochar Produced via Hydrothermal Carbonization Process. J. Environ. Manag. 2012, 109, 61–69. [Google Scholar] [CrossRef]
- Beesley, L.; Marmiroli, M. The Immobilisation and Retention of Soluble Arsenic, Cadmium and Zinc by Biochar. Environ. Pollut. 2011, 159, 474–480. [Google Scholar] [CrossRef]
- Tan, K.B.; Vakili, M.; Horri, B.A.; Poh, P.E.; Abdullah, A.Z.; Salamatinia, B. Adsorption of Dyes by Nanomaterials: Recent Developments and Adsorption Mechanisms. Sep. Purif. Technol. 2015, 150, 229–242. [Google Scholar] [CrossRef]
- Maleki, A.; Mahvi, A.H.; Zazouli, M.A.; Izanloo, H.; Barati, A.H. Aqueous Cadmium Removal by Adsorption on Barley Hull and Barley Hull Ash. Asian J. Chem. 2011, 23, 1373–1376. [Google Scholar]
- Palansooriya, K.N.; Ok, Y.S.; Awad, Y.M.; Lee, S.S.; Sung, J.-K.; Koutsospyros, A.; Moon, D.H. Impacts of Biochar Application on Upland Agriculture: A Review. J. Environ. Manag. 2019, 234, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Wani, I.; Ramola, S.; Garg, A.; Kushvaha, V. Critical Review of Biochar Applications in Geoengineering Infrastructure: Moving beyond Agricultural and Environmental Perspectives. Biomass Convers. Biorefin. 2021, 1–29. [Google Scholar] [CrossRef]
- Xu, G.; Lv, Y.; Sun, J.; Shao, H.; Wei, L. Recent Advances in Biochar Applications in Agricultural Soils: Benefits and Environmental Implications. Clean 2012, 40, 1093–1098. [Google Scholar] [CrossRef]
- Roy, H.; Prantika, T.R.; Riyad, M.H.; Paul, S.; Islam, M.S. Synthesis, Characterizations, and RSM Analysis of Citrus Macroptera Peel Derived Biochar for Textile Dye Treatment. S. Afr. J. Chem. Eng. 2022, 41, 129–139. [Google Scholar] [CrossRef]
- Yrjälä, K.; Ramakrishnan, M.; Salo, E. Agricultural Waste Streams as Resource in Circular Economy for Biochar Production towards Carbon Neutrality. Curr. Opin. Environ. Sci. Health 2022, 26, 100339. [Google Scholar] [CrossRef]
- Nair, V.D.; Nair, P.K.R.; Dari, B.; Freitas, A.M.; Chatterjee, N.; Pinheiro, F.M. Biochar in the Agroecosystem–Climate-Change–Sustainability Nexus. Front. Plant Sci. 2017, 8, 02051. [Google Scholar] [CrossRef] [PubMed]
- Seeman, T.; Šrédl, K.; Prášilová, M.; Svoboda, R. The Price of Farmland as a Factor in the Sustainable Development of Czech Agriculture (A Case Study). Sustainability 2020, 12, 5622. [Google Scholar] [CrossRef]
- Lowder, S.K.; Skoet, J.; Raney, T. The Number, Size, and Distribution of Farms, Smallholder Farms, and Family Farms Worldwide. World Dev. 2016, 87, 16–29. [Google Scholar] [CrossRef]
- Small Family Farmers Produce a Third of the World’s Food. Available online: https://www.fao.org/newsroom/detail/Small-family-farmers-produce-a-third-of-the-world-s-food/en (accessed on 2 January 2023).
- Illankoon, W.A.M.A.N.; Milanese, C.; Girella, A.; Rathnasiri, P.G.; Sudesh, K.H.M.; Llamas, M.M.; Collivignarelli, M.C.; Sorlini, S. Agricultural Biomass-Based Power Generation Potential in Sri Lanka: A Techno-Economic Analysis. Energies 2022, 15, 8984. [Google Scholar] [CrossRef]
- Müller, S.; Backhaus, N.; Nagabovanalli, P.; Abiven, S. A Social-Ecological System Evaluation to Implement Sustainably a Biochar System in South India. Agron. Sustain. Dev. 2019, 39, 43. [Google Scholar] [CrossRef]
- Clare, A.; Shackley, S.; Joseph, S.; Hammond, J.; Pan, G.; Bloom, A. Competing Uses for China’s Straw: The Economic and Carbon Abatement Potential of Biochar. Bioenergy 2015, 7, 1272–1282. [Google Scholar] [CrossRef]
- Leach, M.; Fairhead, J.; Fraser, J. Green Grabs and Biochar: Revaluing African Soils and Farming in the New Carbon Economy. J. Peasant. Stud. 2012, 39, 285–307. [Google Scholar] [CrossRef]
- Schure, J.; Pinta, F.; Cerutti, P.O.; Kasereka-Muvatsi, L. Efficiency of Charcoal Production in Sub-Saharan Africa: Solutions beyond the Kiln. Bois For. Des. Trop. 2019, 340, 57–70. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Eletta, O.A.A.; Adeniyi, A.G. Biomass Carbonisation in Retort Kilns: Process Techniques, Product Quality and Future Perspectives. Bioresour. Technol. Rep. 2022, 17, 100934. [Google Scholar] [CrossRef]
- Mašek, O.; Buss, W.; Roy-Poirier, A.; Lowe, W.; Peters, C.; Brownsort, P.; Mignard, D.; Pritchard, C.; Sohi, S. Consistency of Biochar Properties over Time and Production Scales: A Characterisation of Standard Materials. J. Anal. Appl. Pyrolysis 2018, 132, 200–210. [Google Scholar] [CrossRef]
- Kirch, T.; Medwell, P.R.; Birzer, C.H.; van Eyk, P.J. Small-Scale Autothermal Thermochemical Conversion of Multiple Solid Biomass Feedstock. Renew Energy 2020, 149, 1261–1270. [Google Scholar] [CrossRef]
- Rodrigo, A.S.; Perera, S. Potential and Viability of Rice Husk Based Power Generation in Sri Lanka. Eng. J. Inst. Eng. Sri Lanka 2013, 46, 9. [Google Scholar] [CrossRef]
- Alahakoon, A.M.Y.W.; Karunarathna, A.K.; Dharmakeerthi, R.S.; Silva, F.H.C.A. Design and Development of a Double-Chamber Down Draft (DcDD) Pyrolyzer for Biochar Production from Rice Husk. J. Biosyst. Eng. 2022, 47, 458–467. [Google Scholar] [CrossRef]
- Qian, X.; Xue, J.; Yang, Y.; Lee, S.W. Thermal Properties and Combustion-Related Problems Prediction of Agricultural Crop Residues. Energies 2021, 14, 4619. [Google Scholar] [CrossRef]
- Schoeller, D.A. The Energy Balance Equation: Looking Back and Looking Forward Are Two Very Different Views. Nutr. Rev. 2009, 67, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.M.; Bhattacharya, S.C. Fuel Characteristics of Gasified Coconut Shell in a Fluidized and a Spouted Bed Reactor. Energy 2001, 26, 101–110. [Google Scholar] [CrossRef]
- Jaya Prithika, A.; Sekar, S.K. Mechanical and Fracture Characteristics of Eco-Friendly Concrete Produced Using Coconut Shell, Ground Granulated Blast Furnace Slag and Manufactured Sand. Constr. Build. Mater. 2016, 103, 1–7. [Google Scholar] [CrossRef]
- Li, W.; Yang, K.; Peng, J.; Zhang, L.; Guo, S.; Xia, H. Effects of Carbonization Temperatures on Characteristics of Porosity in Coconut Shell Chars and Activated Carbons Derived from Carbonized Coconut Shell Chars. Ind. Crops Prod. 2008, 28, 190–198. [Google Scholar] [CrossRef]
- Nuriana, W.; Anisa, N. Martana Synthesis Preliminary Studies Durian Peel Bio Briquettes as an Alternative Fuels. Energy Procedia 2014, 47, 295–302. [Google Scholar] [CrossRef]
- Oyelere, A.T.; Oluwadare, A.O. Studies on Physical, Thermal and Chemical Properties of Wood Gliricidia Sepium for Potential Bioenergy Production. Int. J. Biomass Renew. 2019, 8, 28–38. [Google Scholar]
- Mainoo, A.A.; Ulzen-Appiah, F. Growth, Wood Yield and Energy Characteristics of Leucaena Leucocephala, Gliricidia Sepium and Senna Siamea at Age Four Years. Ghana J. For. 1996, 3, 69–79. [Google Scholar]
- Rahayu, S.; Hilyana, S.; Suryani, E.; Nazmi; Sari, H.; Ali, M. Analysis of Wood Pellet Quality from Calliandra Callothyrsus, Gliricida Sepium, and Sawdust as New and Renewable Energy. In Proceedings of the International Conference on Science and Technology (ICST), Mataram, Indonesia, 16–17 October 2021; Institute for Research and Research and Community Service: Kota Surakarta, Indonesia, 2021; pp. 110–115. [Google Scholar]
- Suryaningsih, S.; Nurhilal, O.; Yuliah, Y.; Mulyana, C. Combustion Quality Analysis of Briquettes from Variety of Agricultural Waste as Source of Alternative Fuels. IOP Conf. Ser. Earth Environ. Sci. 2017, 65, 012012. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, X.; Liu, Y.; Fang, Z.; Zhang, M.; Zhang, R.; You, L.; Li, T.; Liu, R.H. A Full Utilization of Rice Husk to Evaluate Phytochemical Bioactivities and Prepare Cellulose Nanocrystals. Sci. Rep. 2018, 8, 10482. [Google Scholar] [CrossRef]
- Olanipekun, E.A.; Olusola, K.O.; Ata, O. A Comparative Study of Concrete Properties Using Coconut Shell and Palm Kernel Shell as Coarse Aggregates. Build Environ. 2006, 41, 297–301. [Google Scholar] [CrossRef]
- Ranaraja, C.D.; Devasurendra, J.W.; Maduwantha, M.I.P.; Madhuwantha, G.A.L.; Hansa, R.Y.D. Optimization of an Industrial Boiler Operation. Ournal Res. Technol. Eng. 2020, 1, 126–134. [Google Scholar]
- Zhongqing Ma; Jiewang Ye; Chao Zhao; Qisheng Zhang Gasification of Rice Husk in a Downdraft Gasifier: The Effect of Equivalence Ratio on the Gasification Performance, Properties, and Utilization Analysis of Byproducts of Char and Tar. Bioresources 2015, 10, 2888–2902.
- Bhatia, B.E.A. Overview of Refractory Materials; Meadow Estates Drive Fairfax: Virginia, GA, USA, 2020. [Google Scholar]
- Gong, K.; Cao, Y.; Feng, Y.; Liu, S.; Qin, J. Influence of Secondary Reactions on Heat Transfer Process during Pyrolysis of Hydrocarbon Fuel under Supercritical Conditions. Appl. Therm. Eng. 2019, 159, 113912. [Google Scholar] [CrossRef]
- Strezov, V.; Evans, T.J.; Hayman, C. Thermal Conversion of Elephant Grass (Pennisetum Purpureum Schum) to Bio-Gas, Bio-Oil and Charcoal. Bioresour. Technol. 2008, 99, 8394–8399. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.-Y.; Lee, K.-L.; Lam, K.-L.; Chan, T.-Y.; Lee, C.-W.; Hui, C.-W. Operation Strategy for Multi-Stage Pyrolysis. J. Anal. Appl. Pyrolysis 2011, 91, 165–182. [Google Scholar] [CrossRef]
- Said, M.M.; Mhilu, C.F.; John, G.R. Thermal Characteristics And Kinetics Of Rice Husk For Pyrolysis Process. Int. J. Renew. Energy Res. 2014, 4, 275–278. [Google Scholar]
- Fonts, I.; Azuara, M.; Gea, G.; Murillo, M.B. Study of the Pyrolysis Liquids Obtained from Different Sewage Sludge. J. Anal. Appl. Pyrolysis 2009, 85, 184–191. [Google Scholar] [CrossRef]
- Domínguez, A.; Menéndez, J.A.; Inguanzo, M.; Pís, J.J. Production of Bio-Fuels by High Temperature Pyrolysis of Sewage Sludge Using Conventional and Microwave Heating. Bioresour. Technol. 2006, 97, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- ben Hassen Trabelsi, A.; Ghrib, A.; Zaafouri, K.; Friaa, A.; Ouerghi, A.; Naoui, S.; Belayouni, H. Hydrogen-Rich Syngas Production from Gasification and Pyrolysis of Solar Dried Sewage Sludge: Experimental and Modeling Investigations. Biomed Res. Int. 2017, 2017, 7831470. [Google Scholar] [CrossRef]
- Fan, H.; Zhou, H.; Wang, J. Pyrolysis of Municipal Sewage Sludges in a Slowly Heating and Gas Sweeping Fixed-Bed Reactor. Energy Convers. Manag. 2014, 88, 1151–1158. [Google Scholar] [CrossRef]
- Biomass Gasification—Biomass (Solid Waste/Sludge) Gasification. Available online: https://blog.nus.edu.sg/chbewch/gasification-technology/biomass-gasification/ (accessed on 23 December 2022).
- Wang, W. A Thermal Conversion Efficiency Study on Biomass Gasification of Arundo Donax and Woodchips. Master’s Thesis, Eastern Illinois University, Charleston, IL, USA, 2013. [Google Scholar]
- Biomass Gasification Research—HHV of Syn-Gas. Available online: https://www.eiu.edu/energy/hhv_of_syn_gas.pdf (accessed on 23 December 2022).
- Rahmat, N.F.H.; Rasid, R.A. Gasification of Empty Fruit Bunch with Carbon Dioxide in an Entrained Flow Gasifier for Syngas Production. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 012013. [Google Scholar] [CrossRef]
- Sultan, S.H.; Palamanit, A.; Techato, K.-A.; Amin, M.; Baloch, K.A. Physiochemical Characterization and Potential of Synthesis Gas Production from Rubber Wood Biomass by Using Downdraft Gasifier. Mehran Univ. Res. J. Eng. Technol. 2021, 40, 1–15. [Google Scholar] [CrossRef]
- Inguanzo, M.; Domínguez, A.; Menéndez, J.A.; Blanco, C.G.; Pis, J.J. On the Pyrolysis of Sewage Sludge: The Influence of Pyrolysis Conditions on Solid, Liquid and Gas Fractions. J. Anal. Appl. Pyrolysis 2002, 63, 209–222. [Google Scholar] [CrossRef]
- Jindo, K.; Mizumoto, H.; Sawada, Y.; Sanchez-Monedero, M.A.; Sonoki, T. Physical and Chemical Characterization of Biochars Derived from Different Agricultural Residues. Biogeosciences 2014, 11, 6613–6621. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Lehmann, J. Black Carbon Decomposition under Varying Water Regimes. Org. Geochem. 2009, 40, 846–853. [Google Scholar] [CrossRef]
- Harvey, O.R.; Herbert, B.E.; Kuo, L.-J.; Louchouarn, P. Generalized Two-Dimensional Perturbation Correlation Infrared Spectroscopy Reveals Mechanisms for the Development of Surface Charge and Recalcitrance in Plant-Derived Biochars. Environ. Sci. Technol. 2012, 46, 10641–10650. [Google Scholar] [CrossRef]
- Harvey, O.R.; Kuo, L.-J.; Zimmerman, A.R.; Louchouarn, P.; Amonette, J.E.; Herbert, B.E. An Index-Based Approach to Assessing Recalcitrance and Soil Carbon Sequestration Potential of Engineered Black Carbons (Biochars). Environ. Sci. Technol. 2012, 46, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, D.W.; Wershaw, R.L.; Rostad, C.E.; Kelly, C.N. Effect of Formation Conditions on Biochars: Compositional and Structural Properties of Cellulose, Lignin, and Pine Biochars. Biomass Bioenergy 2012, 46, 693–701. [Google Scholar] [CrossRef]
- Spokas, K.A. Review of the Stability of Biochar in Soils: Predictability of O:C Molar Ratios. Carbon Manag. 2010, 1, 289–303. [Google Scholar] [CrossRef]
- Wu, W.; Yang, M.; Feng, Q.; McGrouther, K.; Wang, H.; Lu, H.; Chen, Y. Chemical Characterization of Rice Straw-Derived Biochar for Soil Amendment. Biomass Bioenergy 2012, 47, 268–276. [Google Scholar] [CrossRef]
- Dong, X.; Ma, L.Q.; Zhu, Y.; Li, Y.; Gu, B. Mechanistic Investigation of Mercury Sorption by Brazilian Pepper Biochars of Different Pyrolytic Temperatures Based on X-Ray Photoelectron Spectroscopy and Flow Calorimetry. Environ. Sci. Technol. 2013, 47, 12156–12164. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Khodadad, C.L.M.; Zimmerman, A.R.; Green, S.J.; Uthandi, S.; Foster, J.S. Taxa-Specific Changes in Soil Microbial Community Composition Induced by Pyrogenic Carbon Amendments. Soil. Biol. Biochem. 2011, 43, 385–392. [Google Scholar] [CrossRef]
- Guo, J.; Chen, B. Insights on the Molecular Mechanism for the Recalcitrance of Biochars: Interactive Effects of Carbon and Silicon Components. Environ. Sci. Technol. 2014, 48, 9103–9112. [Google Scholar] [CrossRef] [PubMed]
- Mimmo, T.; Panzacchi, P.; Baratieri, M.; Davies, C.A.; Tonon, G. Effect of Pyrolysis Temperature on Miscanthus (Miscanthus × Giganteus) Biochar Physical, Chemical and Functional Properties. Biomass Bioenergy 2014, 62, 149–157. [Google Scholar] [CrossRef]
- Kalderis, D.; Kotti, M.S.; Méndez, A.; Gascó, G. Characterization of Hydrochars Produced by Hydrothermal Carbonization of Rice Husk. Solid Earth 2014, 5, 477–483. [Google Scholar] [CrossRef]
- Illankoon, W.A.M.A.N.; Milanese, C.; Girella, A.; Medina-Llamas, M.; Magnani, G.; Pontiroli, D.; Ricco, M.; Collivignarelli, M.C. Sabrina Sorlini Biochar derived from the rice industry by-products as sustainable energy storage material. In Proceedings of the 30th European Biomass Conference and Exhibition (EUBCE), Online, 9–12 May 2022; Chevet, P.-F., Scarlat, N., Grassi, A., Eds.; ETA-Florence Renewable Energies: Florence, Italy, 2022. [Google Scholar]
- Weber, K.; Quicker, P. Properties of Biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Lee, J.W.; Kidder, M.; Evans, B.R.; Paik, S.; Buchanan, A.C., III; Garten, C.T.; Brown, R.C. Characterization of Biochars Produced from Cornstovers for Soil Amendment. Environ. Sci. Technol. 2010, 44, 7970–7974. [Google Scholar] [CrossRef]
- Yuan, J.-H.; Xu, R.-K.; Zhang, H. The Forms of Alkalis in the Biochar Produced from Crop Residues at Different Temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
- Parr, J.F. Effect of Fire on Phytolith Coloration. Geoarchaeology 2006, 21, 171–185. [Google Scholar] [CrossRef]
- Wilding, L.P.; Brown, R.E.; Holowaychuk, N. Accessibility and Properties of occluded carbon in biogenetic opal. Soil Sci. 1967, 103, 56–61. [Google Scholar] [CrossRef]
- Gong, J.; Li, J.; Xu, J.; Xiang, Z.; Mo, L. Research on Cellulose Nanocrystals Produced from Cellulose Sources with Various Polymorphs. RSC Adv. 2017, 7, 33486–33493. [Google Scholar] [CrossRef] [Green Version]










| Production Type | Reactor Type | Advantages | Disadvantages |
|---|---|---|---|
| Small-scale batch-type processes | Earth pits and mounds, brick, concrete and double metal kilns, retorts | Portable, low-cost, and simple technological solution. | Inefficient, low yield, no heat recovery, hence, much of the feedstock is burned up. Pyrolysis gas and vapors are released into the air, polluting the environment. No control of the pyrolysis temperature, homogeneity of the final product. Some methods are unsafe and require high volumes of water to cool down biochar. |
| Industrial-scale continuous processes | Retort, multiple hearth reactors, screw type pyrolizers | Higher yields; feedstock flexibility; heat integration; possible co-generation of char and energy; technology that is easy to use and has been available for a long time; co-generation of char and energy; the unit that can be moved or be stationary (depending on size). | Systems that are more sophisticated and have higher costs than batch operations. There are no useful byproducts. |
| Paddle drum type reactors | Feedstock flexibility; proven technology; integrated char and energy generation; available as a portable or fixed system (depending on size); greater yields, heat integration, and potential co-production of char and energy. | Complex system; higher cost than batch processes. |
| System Component | Description | Dimensions (Diameter × Height) |
|---|---|---|
| Outer chamber | Material—Used oil barrel. Modifications were made in the oil barrel to achieve the design requirements. | 0.56 m × 0.88 m |
| Inner chamber | Material—Gauge 14 (0.016 m) thick steel metal sheet. A 0.01 m high stand was fabricated at the bottom of the cylinder to facilitate a proper heat supply from the bottom of the reactor. | 0.39 m × 0.76 m |
| Lid of the outer chamber | Material—Gauge 14 (0.016 m) thick steel metal sheet. The lid comprised an exhaust gas opening to connect the chimney. | 0.56 m × 0.08 m |
| Lid of the inner chamber | Material—Gauge 14 (0.016 m) thick steel metal sheet. Tightly fixed to minimize oxygen supply. | 0.39 m × 0.08 m |
| Chimney | Material—Gauge 14 (0.016 m) thick steel metal sheet. Fixed to the outer chamber lid to facilitate the updraft of the exhaust gas. | 0.10 m × 1.0 m |
| Syngas circulation pipe | Material—1 mm thick galvanized square tube. Fabricated in two parts for easy dissembling during cleaning, 1 cm holes were drilled in the tube section inside the pyrolizer. | 0.032 m × 0.032 m (tube cross section) |
| Parameters | Feedstocks | ||
|---|---|---|---|
| Rice Husk | Gliricidia Wood | Coconut Shells | |
| Moisture content (%) | 7.7 a | 62.3 b [42] | 8.14–10.53 b [37,39] |
| Specific gravity (kg/m3) | 140 a | 670 b [42] | 1738 b [47] |
| Calorific value (MJ/kg) | 12. 85–14.02 b [44,49] | 19.0–20.5 b [42,43,48] | 34.1 b [40] |
| Fuelwood value index | - | 1255 b [42] | - |
| Lignin content (%) | 20–25 b [45,46] | 26.26–26.8 b [41,42] | 27 b [38] |
| Volatile matter (%) | 67.7 a | 73.1–82.9 b [41,43] | 67.7–78.3 b [37,39] |
| Fixed carbon (%) | 17.0 a | 10.3 b [43] | 17.6–20.96 b [37,39] |
| Ash (%) | 15.2 a | 1.11–1.28 b [41,43] | 0.74–0.73 b [39] |
| Trial | Wood | Rice Husk | |||
|---|---|---|---|---|---|
| Weight of Wood (kg) | Weight of Ash Residue (kg) | Weight of Rice Husk (kg) | Weight of Biochar (kg) | Biochar Yield (%) | |
| 1 | 28.6 | 0.5 | 10.8 | 4.7 | 43.52 |
| 2 | 28.1 | 1.3 | 11 | 4.96 | 45.05 |
| 3 | 28.2 | 1.13 | 11 | 4.66 | 42.37 |
| 4 | 28 | 1.58 | 11 | 5.42 | 49.28 |
| Trial | Rice Husk | Gliricidia Wood | Rice Husk Biochar | Rice Husk Biochar | Exhaust Gas (m3) * | |
|---|---|---|---|---|---|---|
| Mass (kg) | 1 | 10.8 | 28.6 | 4.7 | 30.12 | 4.88 |
| 2 | 11.0 | 28.1 | 4.96 | 26.8 | 4.83 | |
| 3 | 11.0 | 28.2 | 4.66 | 26.97 | 5.07 | |
| 4 | 11.0 | 28 | 5.42 | 26.42 | 5.07 | |
| Calorific value (MJ/kg) | 1 | 12.85 | 20.5 | 12.35 | 0.27 | 19.82 |
| 2 | 12.85 | 20.5 | 12.35 | 0.27 | 18.53 | |
| 3 | 12.85 | 20.5 | 12.35 | 0.27 | 22.47 | |
| 4 | 12.85 | 20.5 | 12.35 | 0.27 | 21.49 | |
| Energy content (MJ/batch) | 1 | 138.78 | 586.3 | 58.05 | 8.13 | 96.72 |
| 2 | 141.35 | 576.05 | 61.26 | 7.24 | 89.5 | |
| 3 | 141.35 | 578.1 | 57.55 | 7.28 | 113.92 | |
| 4 | 141.35 | 574 | 66.94 | 7.13 | 108.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Illankoon, W.A.M.A.N.; Milanese, C.; Karunarathna, A.K.; Alahakoon, A.M.Y.W.; Rathnasiri, P.G.; Medina-Llamas, M.; Collivignarelli, M.C.; Sorlini, S. Development of a Dual-Chamber Pyrolizer for Biochar Production from Agricultural Waste in Sri Lanka. Energies 2023, 16, 1819. https://doi.org/10.3390/en16041819
Illankoon WAMAN, Milanese C, Karunarathna AK, Alahakoon AMYW, Rathnasiri PG, Medina-Llamas M, Collivignarelli MC, Sorlini S. Development of a Dual-Chamber Pyrolizer for Biochar Production from Agricultural Waste in Sri Lanka. Energies. 2023; 16(4):1819. https://doi.org/10.3390/en16041819
Chicago/Turabian StyleIllankoon, W. A. M. A. N., Chiara Milanese, Anurudda Karunarathna Karunarathna, A. M. Y. W. Alahakoon, Puhulwella G. Rathnasiri, Maria Medina-Llamas, Maria Cristina Collivignarelli, and Sabrina Sorlini. 2023. "Development of a Dual-Chamber Pyrolizer for Biochar Production from Agricultural Waste in Sri Lanka" Energies 16, no. 4: 1819. https://doi.org/10.3390/en16041819
APA StyleIllankoon, W. A. M. A. N., Milanese, C., Karunarathna, A. K., Alahakoon, A. M. Y. W., Rathnasiri, P. G., Medina-Llamas, M., Collivignarelli, M. C., & Sorlini, S. (2023). Development of a Dual-Chamber Pyrolizer for Biochar Production from Agricultural Waste in Sri Lanka. Energies, 16(4), 1819. https://doi.org/10.3390/en16041819