Pyrolysis of Waste Biomass: Technical and Process Achievements, and Future Development—A Review
Abstract
:1. Introduction
- -
- -
- Oxidative processes, where the main factor causing the fuel conversion is both free and chemically bound oxygen (e.g., in the form of CO2 or H2O);
- -
- Hydrogeneration processes, in which hydrogen is the main factor causing fuel conversion [6].
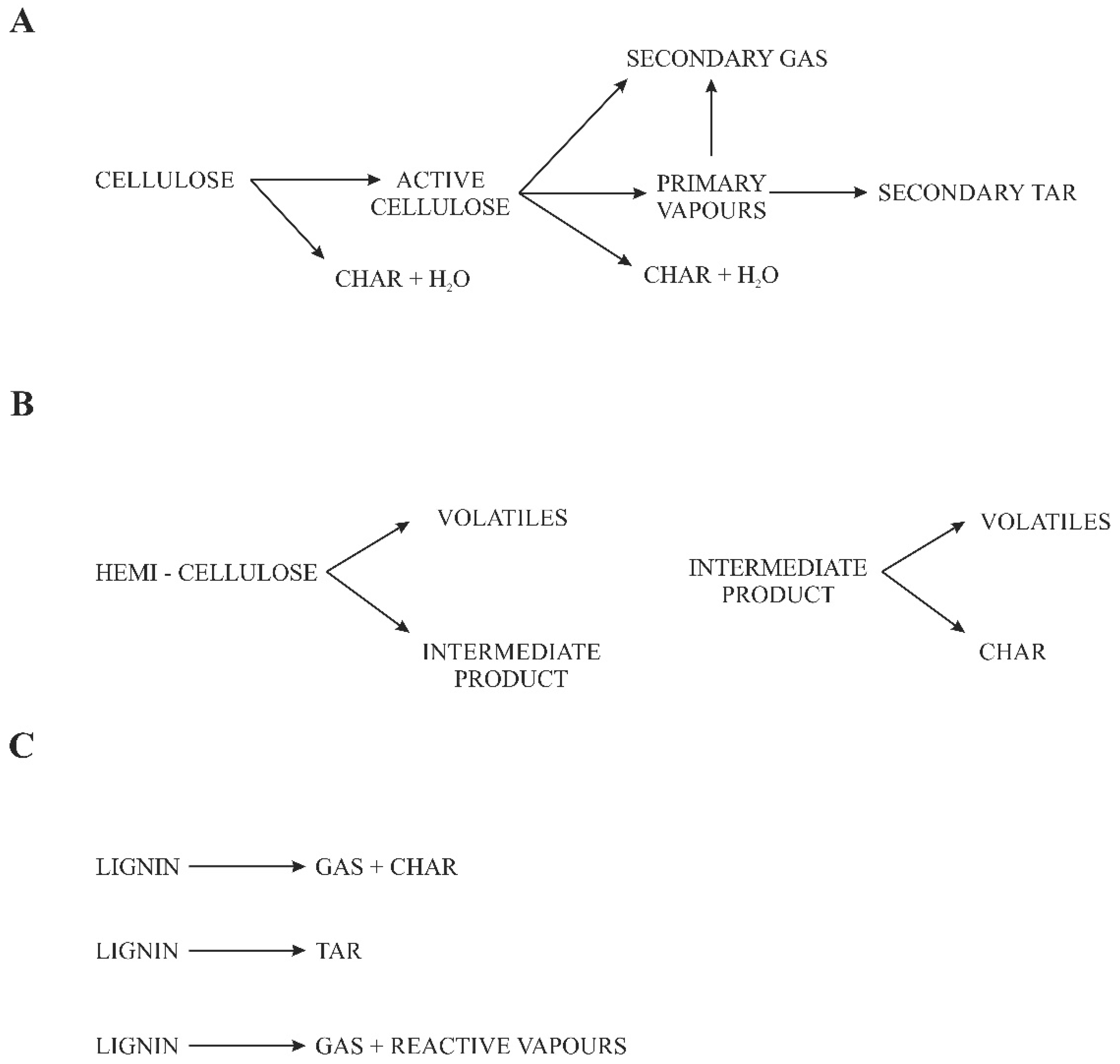
2. Pyrolysis Processing and Technology of Biomass
- -
- When the temperature of the wood rises from 105 °C to 120 °C, water is released which is not chemically bound to the organic matter of the wood (absorbed water), with a further increase in temperature from 245 °C to 265 °C;
- -
- Water chemically bound in the cellulose structure and the release of CO2, CO, and small amounts of condensing acetic acid and methanol vapors also begin to emit small amounts of wood tar;
- -
- Above 265 °C to 275 °C, the process becomes exothermic, with the strong release of methanol, acetic acid, acetone, lighter hydrocarbons, wood tar, and small amounts of hydrogen, and the amount of CO2 and CO released decreases;
- -
3. The Biofuel Production in the Pyrolysis Processes
3.1. Pyrolytic Gas
3.2. Pyrolytic Oil
3.3. Biochar
- -
- In the composting process as a structural material or ammonia abatement additive;
- -
- In bioenergy as a renewable fuel;
- -
- For carbon sequestration in soil;
- -
- For the production of organic fertilizers;
- -
- To remove pollutants from aqueous solutions, industrial and municipal wastewater, and process gases;
- -
- For the remediation of soils contaminated with organic and inorganic compounds;
- -
- To reduce ground and surface water pollution by retaining nutrients in the soil;
- -
- For improving the properties of agricultural soils [72].
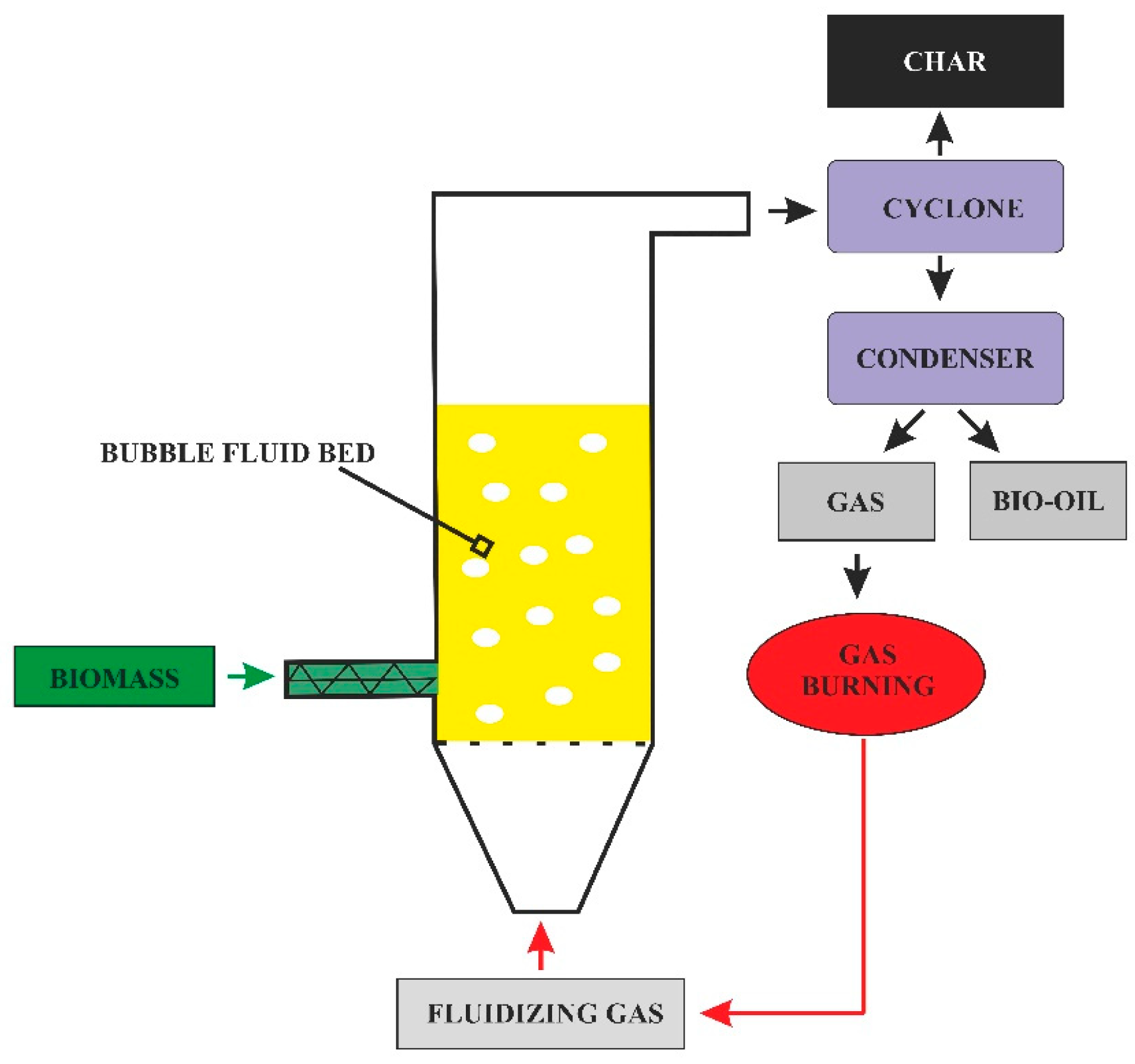
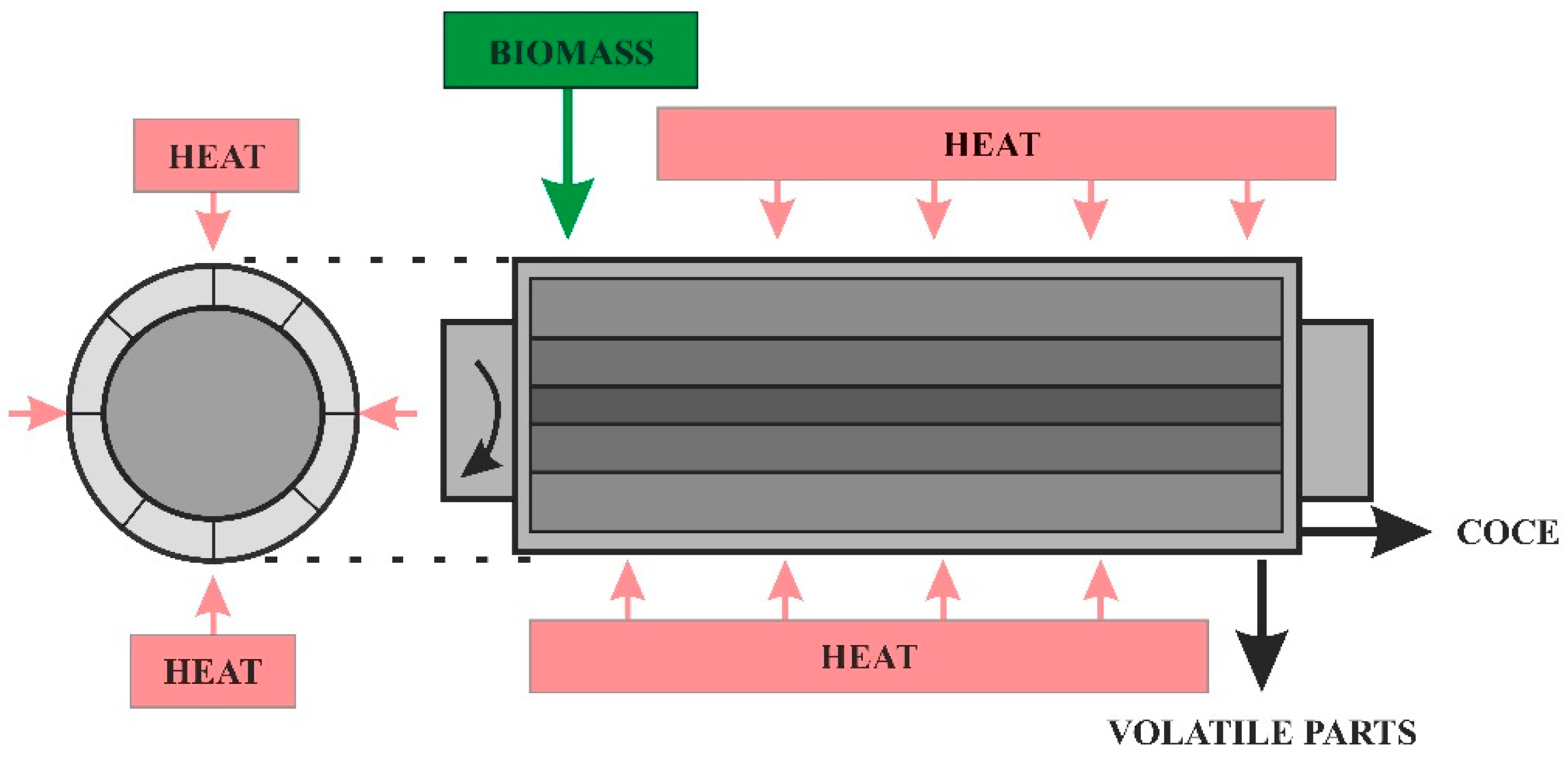
4. Pyrolytic Reactors—The Technical Aspects and Influence on the Process
- -
- Inert gas is not required;
- -
- Large particle sizes can be used;
- -
- Sufficient heat transfer;
- -
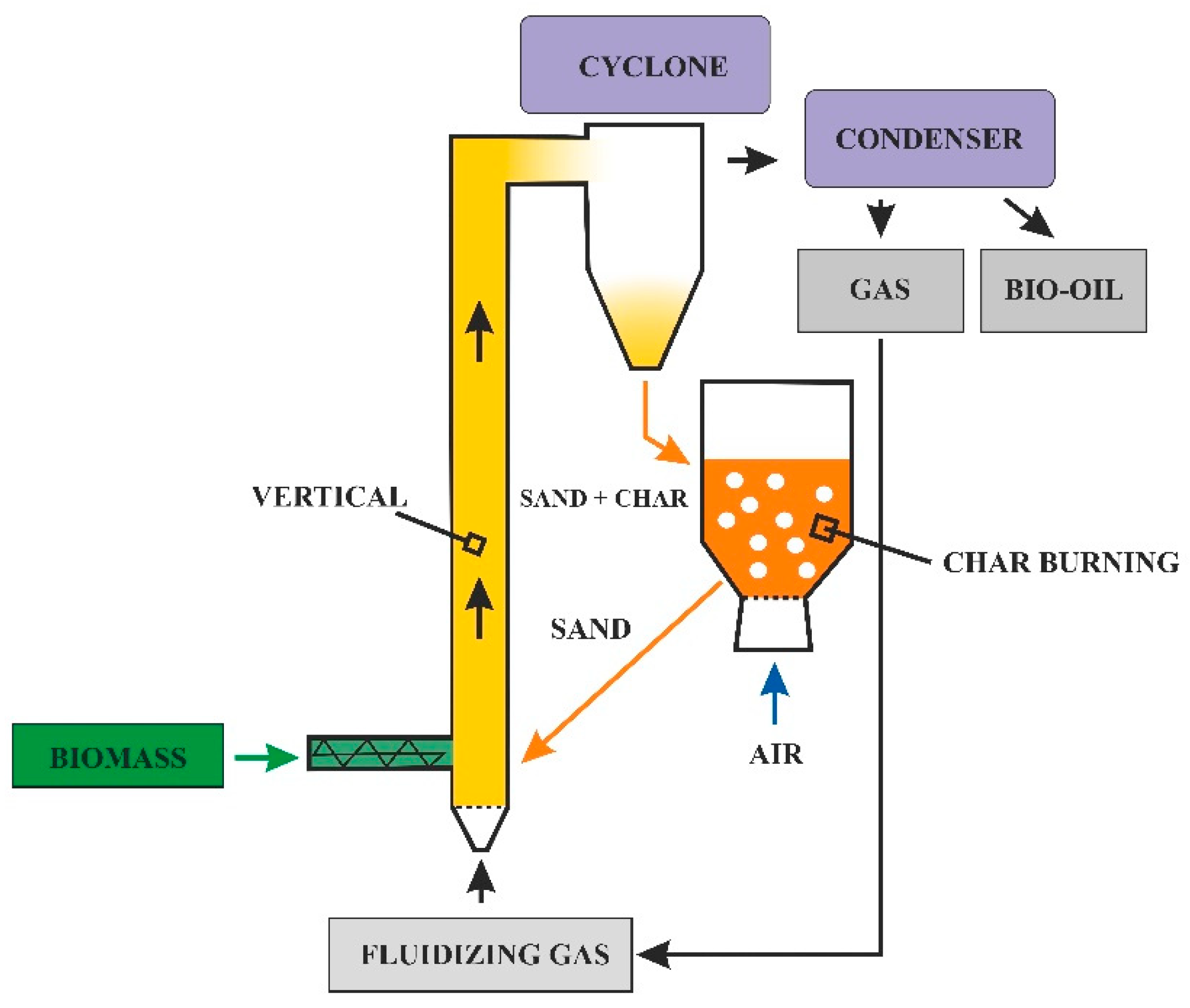
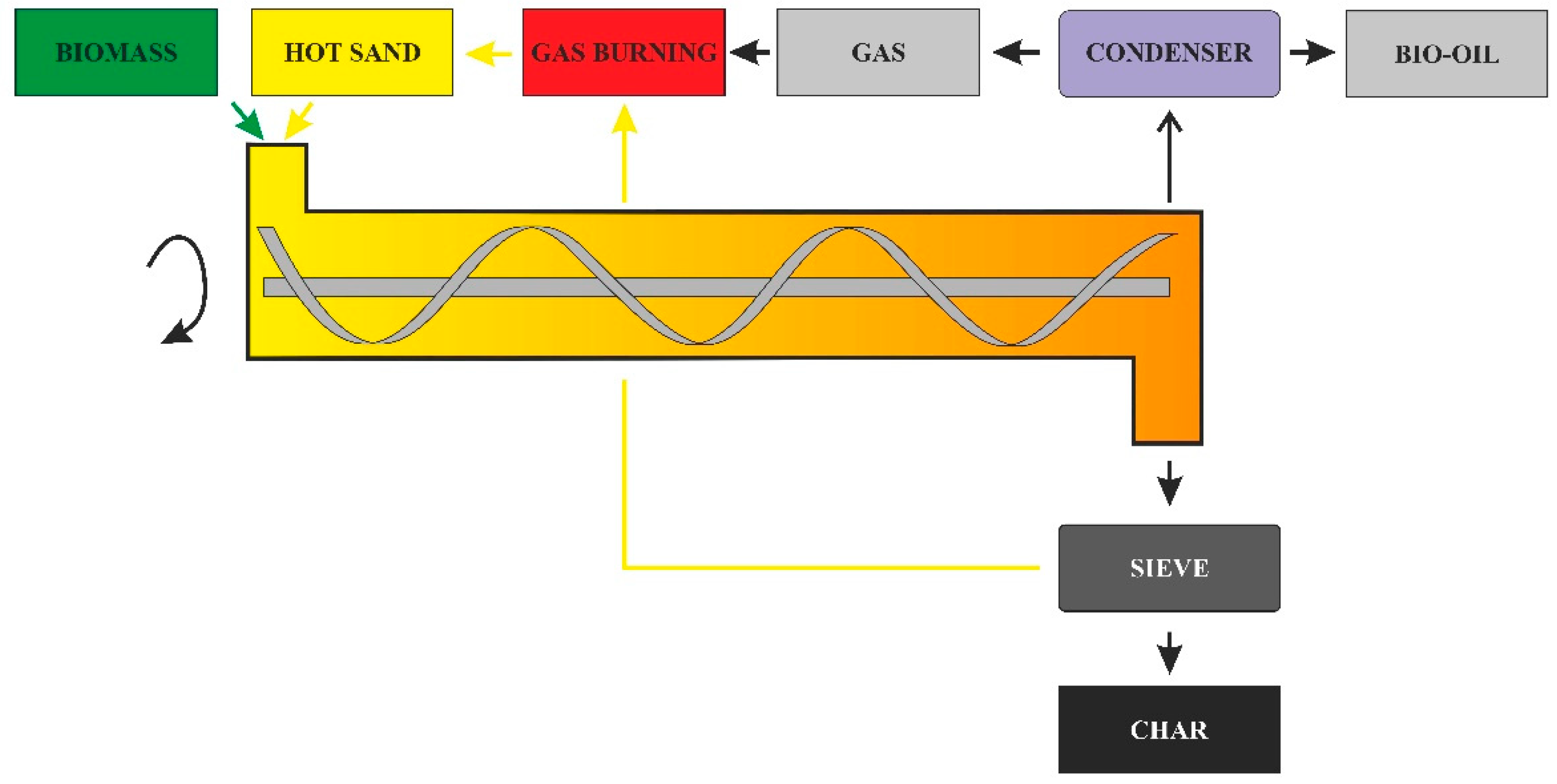
Reactor Heating Method
- -
- The heat is transferred to the reactor by the heat carrier;
- -
- From the carrier, the heat is transferred to the biomass to be pyrolyzed [47].
- -
- Heat exchange surfaces placed in appropriate places in the reactor;
- -
- Heating with fluidizing gas;
- -
- Removal and reheating of the bed (sand) in a separate reactor;
- -
- Adding some air, which can create hot spots and grow cracks, leading to tar formation.
5. Prospects and Developments in the Pyrolysis Technology
6. Energy Requirement of Pyrolysis Process
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Igliński, B.; Iglińska, A.; Kujawski, W.; Buczkowski, R.; Cichosz, M. Bioenergy in Poland. Renew. Sustain. Energy Rev. 2012, 15, 2999–3007. [Google Scholar] [CrossRef]
- Igliński, B.; Kiełkowska, U.; Piechota, G.; Skrzatek, M.; Cichosz, M.; Iwański, P. Can energy self-sufficiency be achieved? Case study of Warmińsko-Mazurskie Voivodeship (Poland). Clean Technol. Env. Policy 2021, 23, 2061–2081. [Google Scholar] [CrossRef]
- Baudry, G.; Macharis, C.; Vallée, T. Can microalgae biodiesel contribute to achieve the sustainability objectives in the transport sector in France by 2030? A comparison between first, second and third generation biofuels through a range-based Multi-Actor. Multi-Criteria Anal. Energy 2018, 155, 1032–1046. [Google Scholar] [CrossRef]
- Kaczor, Z.; Buliński, Z.; Werle, S. Modelling approaches to waste biomass pyrolysis: A review. Renew. Energy 2020, 159, 427–443. [Google Scholar] [CrossRef]
- Velmurugan, V. Review of research and development on pyrolysis process. Mater. Today Proc. 2022, 49, 3679–3686. [Google Scholar] [CrossRef]
- Liu, R.; Liu, G.; Yousaf, B.; Niu, Z.; Abbas, Q. Novel investigation of pyrolysis mechanism and kinetics for functional groups in biomass matrix. Renew. Sustain. Energy Rev. 2022, 153, 111761. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Y.; Liu, S.; Fan, L.; Zhou, N.; Peng, P.; Wang, Y.; Guo, F.; Cheng, Y.; Liu, Y.; et al. Fast microwave-assisted pyrolysis of wastes for biofuels production—A review. Bioresour. Technol. 2020, 297, 122480. [Google Scholar] [CrossRef]
- Du, Y.; Ju, T.; Meng, Y.; Lan, T.; Han, S.; Jiang, J. A review on municipal solid waste pyrolysis of different composition for gas production. Fuel Process. Technol. 2021, 224, 107026. [Google Scholar] [CrossRef]
- Haghighat, M.; Majidian, N.; Hallajisani, A.; Samipourgiri, M. Production of bio-oil from sewage sludge: A review on the thermal and catalytic conversion by pyrolysis. Sustain. Energy Technol. Assess. 2020, 42, 100870. [Google Scholar] [CrossRef]
- Fakayode, O.A.; Aboagarib, E.A.A.; Zhou, C.; Ma, H. Co-pyrolysis of lignocellulostic and macroalgae biomasses for the production of biochar—A review. Bioresour. Technol. 2020, 297, 122408. [Google Scholar] [CrossRef]
- Hu, X.; Gholizaed, M. Biomass pyrolysis: A review of the process development and challenges from initial researches up to the commercialisation stage. J. Energy Chem. 2019, 39, 109–143. [Google Scholar] [CrossRef]
- Wang, G.; Dai, Y.; Yang, H.; Xiong, Q.; Wang, K.; Zhou, J.; Li, Y.; Wang, S. A review of recent advances in biomass pyrolysis. Energy Fuels 2020, 34, 15557–15578. [Google Scholar] [CrossRef]
- Stelmach, S. Waste Pyrolysis as an Element of the Circular Economy; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2019. [Google Scholar]
- Gonnella, G.; Ischia, G.; Fambri, L.; Fiori, L. Thermal analysis and kinetic modeling of pyrolysis and oxidation of hydrochars. Energies 2022, 15, 950. [Google Scholar] [CrossRef]
- Hu, M.; Ye, Q.; Xue, Q.; Li, Z.; Wang, J.; Pan, Z. Towards understanding the chemical reactions between KOH and oxygen-containing groups during KOH-catalyzed pyrolysis of biomass. Energy 2022, 245, 123286. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.; Guo, J.; Xu, Z. Impact of the operating conditions on the derived products and the reaction mechanism in vacuum pyrolysis treatment of the organic material in waste integrated circuits. J. Clean. Prod. 2018, 197, 1488–1497. [Google Scholar] [CrossRef]
- Siddiqul, M.N.; Redhwi, H.H.; Antonakou, E.V.; Achilias, D.S. Pyrolysis mechanism and thermal degradation kinetics of poly(bisphenol A carbonate)-based polymers originating in waste electric and electronic equipment. J. Anal. Appl. Pyrolysis 2018, 132, 123–133. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.; He, J.; Kumar, R.; Lu, Q. Catalytic pyrolysis of lignocellulosic biomass: A review of variations in process factors and system structure. Renew. Sustain. Energy Rev. 2020, 134, 110305. [Google Scholar] [CrossRef]
- Stančić, H.; Manić, N.; Stojiljiković, D.; Vujanović, M.; Wang, X.; Duić, N. Thermogravimetric and kinetic analysis of biomass and polyurethane foam mixtures co-pyrolysis. Energy 2021, 237, 121592. [Google Scholar] [CrossRef]
- Lv, P.; Bai, Y.; Wang, J.; Song, X.; Su, W.; Yu, G.; Ma, Y. Investigation into interaction of biomass waste with industrial solid waste during co-pyrolysis and the synergetic effect of its char gasification. Biomass Bioenergy 2022, 159, 106414. [Google Scholar] [CrossRef]
- Zou, J.; Hu, H.; Xue, Y.; Li, C.; Li, Y.; Yellezuome, D.; He, F.; Zhang, X.; Rahman, M.M.; Cai, J. Exploring kinetic mechanisms of biomass pyrolysis using a generalized logistic mixture model. Energy Convers. Manag. 2022, 258, 115522. [Google Scholar] [CrossRef]
- Phuakpunk, K.; Chalermsininsuwan, B.; Assabumrungrat, S. Pyrolysis kinetic parameters investigation of single and tri-component biomass: Models fitting via comparative model-free methods. Renew. Energy 2022, 182, 494–507. [Google Scholar] [CrossRef]
- Gouws, S.M.; Carrier, M.; Bunt, J.R.; Neomagus, H.W.J.P. Lumped chemical kinetic modeling of raw torrefied biomass under pressurized pyrolysis. Energy Convers. Manag. 2022, 253, 115199. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Progess Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, Y.; Cai, D.; Sun, J.; Zhang, Y.; Li, L.; Zhang, Q.; Zou, G.; Song, Z.; Bai, Y. Enhanced pyrolysis of woody biomass under the interaction of microwave and needle-shaped metal and its production properties. Energy 2022, 249, 123667. [Google Scholar] [CrossRef]
- Elkhalifa, S.; Mariyam, S.; Mackey, H.R.; Al-Ansari, T.; McKay, G.; Parthasarathy, P. Pyrolysis valorization of vegetable wastes: Thermal, kinetic, thermodynamics, and pyrogas analyses. Energies 2022, 15, 6277. [Google Scholar] [CrossRef]
- Ansari, B.; Kamal, B.; Beg, S.; Khan, M.A.W.; Khan, M.S.; Al Mesfer, M.K.; Danish, M. Recent development in investigating reaction chemistry and transport effects in biomass fast pyrolysis: A review. Renew. Sustain. Energy Rev. 2021, 150, 111454. [Google Scholar] [CrossRef]
- Murakami, M.; Murakami, M. Cleavage of Carbon-Carbon Single Bonds by Transition Metals; Wiley-VCH GmbH & Co., KGaA: Weinheim, Germay, 2015. [Google Scholar] [CrossRef]
- Lutz, M.D.R.; Morandi, B. Metal-catalysed carbon-carbon bond cleavage of unstrained alcohols. Chemical Reviews 2021, 1, 300–326. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, P.; Chang, C.; Wang, X.; Song, J.; Fang, S.; Pang, S. Influence of metal chloride modified biochar on products characteristics from catalytic pyrolysis. Energy 2022, 250, 123776. [Google Scholar] [CrossRef]
- Igliński, B.; Buczkowski, R.; Cichosz, M. Bioenergetics Technologies; Nicolaus Copernicus University: Toruń, Polan, 2009. [Google Scholar]
- Roy, P.; Dias, G. Prospects for pyrolysis technologies in the bioenergy sector: A review. Renew. Sustain. Energy Rev. 2017, 77, 59–69. [Google Scholar] [CrossRef]
- Homagain, K.; Shahi, C.; Luckai, N.; Sharma, M. Biochar-based bioenergy and its environmental impact in Northwestern Ontario Canada: A review. J. For. Res. 2014, 25, 737–748. [Google Scholar] [CrossRef]
- Hornung, A. Transformation of Biomass: Theory to Practice; John Wiley&Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohdan, D. Biochar as a sorbent for contamined management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Chintala, V. Production, up-gradation and utilization of solar-assisted pyrolysis fuels from biomass—A technical review. Renew. Sustain. Energy Rev. 2018, 90, 120–130. [Google Scholar] [CrossRef]
- Zhao, B.; Schmidt, S.; Qin, W.; Li, J.; Li, G.; Zhang, W. Towards the circular economy—A global meta-analysis of composting technologies reveals much potential for mitigating nitrogen losses. Sci. Total Environ. 2020, 704, 135401. [Google Scholar] [CrossRef]
- Retajczyk, M.; Wróblewska, A. Pyrolysis of biomass as a source of energy. Wiad. Chem. 2018, 72, 127–146. [Google Scholar]
- Zaman, C.Z.; Pal, K.; Yehye, W.A.; Sagadevan, S.; Shah, S.T.; Adebisi, G.A.; Mariana, E.; Rafique, R.F.; Johan, R.B. Pyrolysis: A sustainable way to generate energy from waste. In Pyrolysis; IntechOpen: London, UK, 2017; ISBN 978-953-51-3312-4. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, R.; Wang, D.; He, G.; Shao, S.; Zhang, J.; Zhong, Z. Biomass fast pyrolysis in a fluidized bed reactor under N2, CO2, CH4 and H2 atmospheres. Bioresour. Technol. 2021, 102, 4258–4264. [Google Scholar] [CrossRef]
- Bieniek, A.; Jerzak, W.; Sieradzka, M.; Mika, Ł.; Sztekler, K.; Magdziarz, A. Intermediate pyrolysis of brewer’s spent grain: Impact of gas atmosphere. Energies 2022, 15, 2491. [Google Scholar] [CrossRef]
- Niesler, M.; Stecko, J.; Stelmach, S. The use of softwood char as a substitute fuel in the iron ore sintering process. J. Met. Mater. 2020, 2, 2–14. [Google Scholar]
- Li, A.; Han, H.; Hu, S.; Zhu, M.; Ren, Q.; Wang, Y.; Xu, J.; Jiang, L.; Su, A.; Xiang, J. A novel sludge pyrolysis and biomass gasification integrated method to enhance hydrogen-rich gas generation. Energy Convers. Manag. 2022, 254, 115205. [Google Scholar] [CrossRef]
- Mariyam, S.; Shahbaz, M.; Al-Ansari, T.; Mackey, H.R. A critical review on co-gasification and co-pyrolysis for gas production. Renew. Sustain. Energy Rev. 2022, 161, 112349. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Hu, X.; Gholizadeh, M. Progress in the application of the pyrolytic lignin from pyrolysis of biomass. Chem. Eng. J. 2021, 419, 129560. [Google Scholar] [CrossRef]
- Terry, L.M.; Li, C.; Chew, J.J.; Aqsha, A.; How, B.S.; Loy, A.C.M.; Chin, B.L.F.; Khaerudini, D.S.; Hameed, N.; Guan, G.; et al. Bio-oil production from pyrolysis of oil palm biomass and the upgrading technologies: A review. Carbon Resour. Convers. 2021, 4, 239–250. [Google Scholar] [CrossRef]
- Wang, C.; Wang, R.; Chen, T.; Zhu, X. Visual experimental study on the effect of heat exchange area on the evaluation of biomass pyrolysis vapors in a vertical indirect condensing field. Bioresour. Technol. 2022, 348, 126686. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, J.; Liu, Y.; Li, S.; Chu, H. Research progress in the preparation of high-quality liquid fuels and chemicals by catalytic pyrolysis of biomass: A review. Energy Convers. Manag. 2022, 261, 115647. [Google Scholar] [CrossRef]
- Zhou, S.; Xue, Y.; Cai, J.; Cui, C.; Ni, Z.; Zhou, Z. An understanding for improved biomass pyrolysis: Towards a systematic comparison of different acid pretreatments. Chem. Eng. J. 2021, 411, 128513. [Google Scholar] [CrossRef]
- Nisar, J.; Ahmad, A.; Ali, G.; Rehman, N.U.; Shah, A.; Shah, I. Enhanced bio-oil yield from thermal decomposition of peanut shells using termite hill as the catalyst. Energies 2022, 15, 1891. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, N.; Zhang, S. Catalytic pyrolysis of biomass with potassium compounds for co-production of high-quality biofuels and porous carbons. Energy 2020, 190, 116431. [Google Scholar] [CrossRef]
- Fan, Y.; Zhao, W.; Shao, S.; Cai, Y.; Chen, Y.; Jin, L. Promotion of the vapors from biomass vacuum pyrolysis for biofuels under Non-thermal Plasma Synergistic Catyalysis (NPSC) system. Energy 2018, 142, 462–472. [Google Scholar] [CrossRef]
- Kumar, R.S.; Sivakumar, S.; Joshuva, A.; Deenadayalan, G.; Vishnuvardhan, R. Bio-fuel production from Martynia annua L. seeds using slow pyrolysis reactor and its effects on diesel engine performance, combustion and emission characteristics. Energy 2021, 217, 119327. [Google Scholar] [CrossRef]
- Armer, M.W.; Alhesan, J.S.A.; Ibrahim, S.; Qussay, G.; Marshall, M.; Al-Aye, O.S. Potential use of corn leaf waste for biofuel production in Jordan (physio-chemical study). Energy 2021, 214, 118863. [Google Scholar] [CrossRef]
- Zanzi, R.; Sjöström, E. Rapid pyrolisis of agricultural residues at high temperature. Biomass Bioenergy 2022, 23, 357–366. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Influence of bulking agents of physical, chemical, and microbiological properties during the two-stage composting of green waste. Waste Manag. 2016, 48, 115–126. [Google Scholar] [CrossRef]
- Kim, T.; Oh, S.; Kim, J.; Choi, I.; Choi, J.W. Study on the hydrodeoxygenative upgrading of crude bio-oil produced from woody biomass by fast pyrolysis. Energy 2014, 68, 437–443. [Google Scholar] [CrossRef]
- Wildschut, J.; Mahfud, F.H.; Venderbosch, R.H.; Heeres, H.J. Hydrotreatment of fast pyrolysis oil using heterogeneous noble-metal catalysts. Ind. Eng. Chem. Res. 2009, 48, 10324–10334. [Google Scholar] [CrossRef]
- Yin, W.; Venderbosch, R.H.; He, S.; Bykova, M.V.; Khromova, S.A.; Yakovlev, V.A.; Heeres, H.J. Mono-, bi-, and tri-metallic Ni-based catalysts for the catalytic hydrotreatment of pyrolysis liquids. Biomass Convers. Biorafin. 2017, 7, 361–376. [Google Scholar] [CrossRef]
- Boscagli, C.; Raffelt, K.; Grunwaldt, J. Reactivity of platform molecules in pyrolysis oil and in water during hydrotreatment over nickel and ruthenium catalysts. Biomass Bioenergy 2017, 106, 63–73. [Google Scholar] [CrossRef]
- Capunitan, J.A.; Capareda, S.C. Hydrotreatment of corn stover bio-oil using noble metal catalysts. Fuel Process. Technol. 2014, 125, 190–199. [Google Scholar] [CrossRef]
- Zheng, J.; Zhu, X.; Guo, Q.; Zhu, Q. Thermal conversion of rice husks and sawdust to liquid fuel. Waste Manag. 2006, 26, 1430–1435. [Google Scholar] [CrossRef]
- Weerachanchai, P.; Tangsathitkulchai, C.; Tangsathitkulchai, M. Effect of reaction conditions on the catalytic esterification of bio-oil. Korean J. Chem. Eng. 2012, 29, 182–189. [Google Scholar] [CrossRef]
- Cheng, D.; Wang, L.; Shahbazi, A.; Xiu, S.; Zhang, B. Catalytic cracking of crude bio-oil from glycerol-assisted liquefaction of swine manure. Energy Convers. Manag. 2014, 87, 378–384. [Google Scholar] [CrossRef]
- Tian, Y.; Zuo, W.; Ren, Z.; Chen, D. Estimation of a novel method to produce bio-oil from sewage sludge by microwave pyrolysis with the consideration of efficiency and safety. Bioresour. Technol. 2011, 102, 2053–2061. [Google Scholar] [CrossRef]
- Mushtaq, F.; Channa, A.S.; Mat, R.; Ani, F.N. Microwave assisted pyrolysis of waste biomass resources for bio-oil production. Appl. Mech. Mater. 2014, 554, 307–311. [Google Scholar] [CrossRef]
- Kuan, W.H.; Huang, Y.F.; Chang, C.C.; Lo, S.L. Catalytic pyrolysis of sugarcane bagasse by using microwave heating. Bioresour. Technol. 2013, 146, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, F.; Abdullah, T.A.T.; Mat, R.; Ani, F.N. Optimization and characterization of bio-oil produced by microwave assisted pyrolysis of oil palm shell waste biomass with microwave absorber. Bioresour. Technol. 2015, 190, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ma, X.; Li, L.; Hu, Z.; Guo, P.; Jiang, Y. The catalytic pyrolysis of food waste by microwave heating. Bioresour. Technol. 2014, 166, 45–50. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, Z.; Tian, X.; Dai, L.; Jiang, L.; Zhang, S.; Wu, Q.; Wen, P.; Fu, G.; Liu, Y.; et al. Production of bio-oil from agricultural waste by using a continuous fast microwave pyrolysis system. Bioresour. Technol. 2018, 269, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Al-Rumaihi, A.; Shahbaz, M.; Mckay, G.; Mackey, H.; Al-Ansari, T. A review of pyrolysis technologies and feedstock: A blending approach for plastic and biomass towards optimum biochar yield. Renew. Sustain. Energy Rev. 2022, 167, 112715. [Google Scholar] [CrossRef]
- Li, Y.; Xing, B.; Ding, Y.; Han, X.; Wang, S. A critical review of the production and advanced utilization of biochar via selective pyrolysis of lignocelluslosic biomass. Bioresour. Technol. 2020, 312, 1231614. [Google Scholar] [CrossRef]
- Shao, S.; Liu, C.; Xiang, X.; Liu, X.; Zhang, H.; Xiao, R.; Ca, Y. In situ catalytic fast pyrolysis over CeO2 catalyst: Impact of biomass source, pyrolysis temperature and metal ion. Renew. Energy 2021, 177, 1372–1381. [Google Scholar] [CrossRef]
- Składeczek, F.; Głodek-Bucyk, E. Research of using low-temperature pyrolysis for processing of waste biomass to biochar. Sci. Work. Inst. Ceram. Build. Mater. 2017, 28, 50–61. [Google Scholar]
- Sieradzka, M.; Kirczuk, C.; Kalemba-Rec, I.; Mlonka-Mędrala, A.; Magdziarz, A. Pyrolysis of biomass wastes into carbon materials. Energies 2022, 15, 1941. [Google Scholar] [CrossRef]
- Duan, D.; Chen, D.; Huang, L.; Zhang, Y.; Zhang, Y.; Wang, Q.; Xiao, G.; Zhang, W.; Lei, H.; Ruan, R. Activated carbon from lignocellulosic biomass as a catalyst: A review of the application in fast pyrolysis process. J. Anal. Appl. Pyrolysis 2021, 158, 105246. [Google Scholar] [CrossRef]
- Pallaréz, J.; González-Cencerrado, A.; Arazuro, I. Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam. Biomass Bioenergy 2017, 115, 54–73. [Google Scholar] [CrossRef]
- Köseoğlu, C.; Akmil-Başar, C. Preparation, structural evaluation and adsorptive properties of activated carbon from agricultural waste biomass. Adv. Powder Technol. 2015, 26, 811–818. [Google Scholar] [CrossRef]
- Tay, T.; Ucar, S.; Karagöz, S. Preparation and characterization of activated carbon from waste biomass. J. Hazard Mater. 2009, 165, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Sayğili, H.; Güzel, F. High surface area mesoporous activated carbon from tomato processing solid waste by zinc chloride activation: Process optimization, characterization and dyes adsorption. J. Clean. Prod. 2016, 113, 995–1004. [Google Scholar] [CrossRef]
- Duan, D.; Feng, Z.; Dong, X.; Chen, X.; Zhang, Y.; Wan, K.; Wang, Y.; Wang, Q.; Xiao, G.; Liu, H. Improving bio-oil quality from low-density polyethylene pyrolysis: Effects of varying activation and pyrolysis parameters. Energy 2021, 232, 121090. [Google Scholar] [CrossRef]
- Pan, J.; Jiang, J.; Xu, R. Adsorption of Cr(III) from acidic solutions by crop straw derived biochars. J. Environ. Sci. 2013, 25, 1957–1965. [Google Scholar] [CrossRef]
- Agrafioti, E.; Kalderis, D.; Diamadopoulos, E. Arsenic and chromium removal from water using biochars derived from rice husk, organic solid wastes and sewage sludge. J. Environ. Manag. 2014, 133, 309–314. [Google Scholar] [CrossRef]
- Wnetrzak, R.; Leahy, J.J.; Chojnacka, K.W.; Saeid, A.; Novotny, E.; Jensen, L.S.; Kwapinski, W. Influence of pig manure biochar mineral content on Cr(III) sorption capacity. J. Chem. Technol. Biotechnol. 2014, 89, 569–578. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Piechota, G.; Igliński, B. Biomethane in Poland: Current status, potential, perspective and development. Energies 2021, 14, 1517. [Google Scholar] [CrossRef]
- Mierzawa-Hersztek, M.; Gondek, K.; Jewarz, M.; Dziedzic, K. Assessment of energy parameters of biomass and biochars, leachability of heavy metals and phytotoxicity of their ashes. J. Mater. Cycles Waste Manag. 2019, 21, 786–800. [Google Scholar] [CrossRef]
- Karhu, K.; Mattila, T.; Bergstrom, I.; Regina, K. Biochar addition to agricultural soil increased CH4 uptake and water holding capacity—Results from a short-term pilot field study. Agric. Ecosyst. Environ. 2011, 140, 309–313. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, H.; Yu, L.; Sun, T. Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived biochars: Impact of structural properties of biochars. J. Hazard. Mater. 2013, 244–245, 217–224. [Google Scholar] [CrossRef]
- Qiu, M.; Ling, Q.; Cai, Y.; Yu, S.; Wang, S.; Fu, D.; Hu, B.; Wang, X. Biochar for the removal of contaminants from soil and water: A review. Biochar 2022, 4, 19. [Google Scholar] [CrossRef]
- Luo, J.; Sun, S.; Chen, X.; Lin, J.; Ma, R.; Zhang, R.; Fang, L. In-depth exploration of the Energy utilization and pyrolysis mechanism of advanced continuous microwave pyrolysis. Appl. Energy 2021, 292, 116941. [Google Scholar] [CrossRef]
- Ren, X.; Ghazani, M.S.; Zhu, H.; Ao, W.; Zhang, H.; Moreside, E.; Zhu, J.; Yang, P.; Zhong, N.; Bi, X. Challenges and opportunities in microwave-assisted catalytic pyrolysis of biomass: A review. Appl. Energy 2022, 315, 118970. [Google Scholar] [CrossRef]
- Sait, H.H.; Hussain, A.; Bassyouni, M.; Ali, I.; Kanthasamy, R.; Ayodele, B.V.; Elhenawy, Y. Hydrogen-rich syngas and biochar production by the non-catalytic valorization of date palm seeds. Energies 2022, 15, 2727. [Google Scholar] [CrossRef]
- Li, P.; Shi, X.; Wang, X.; Song, J.; Fang, S.; Bai, J.; Zhang, G.; Chang, C.; Pang, S. Bio-oil from biomass fast pyrolysis: Yields, related properties and energy consumption analysis of pyrolysis system. J. Clean. Prod. 2021, 328, 129613. [Google Scholar] [CrossRef]
- Ringer, M.; Putsche, V.; Scahill, J. Large-Scale Pyrolysis Oil Production: A Technology Assessment and Economic Analysis; Technical report; National Renewable Energy Laboratory: Golden, CO, USA, 2006. Available online: www.nrel.gov/docs/fy07osti/37779.pdf (accessed on 22 January 2023).
- Jaworski, T.J. Waste and biomass pyrolysis reactors. Piece Kotły 2017, 1, 1–7. [Google Scholar]
- Li, X.; Peng, B.; Liu, Q.; Zhang, H. Microwave pyrolysis coupled with conventional pre-pyrolysis of the stalk for syngas and biochar. Bioresour. Technol. 2022, 348, 126745. [Google Scholar] [CrossRef] [PubMed]
- Jahiril, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels production through biomass pyrolysis—A technical review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Ronsse, F.; Dickinson, D.; Nachenius, R.; Prins, W. Biomass Pyrolysis and Biochar Characterization. Available online: https://www.oeaw.ac.at/forebiom/WS1lectures/SessionII_Ronsse.pdf (accessed on 24 January 2023).
- Li, X.T.; Grace, R.; Lim, C.J.; Watkinson, A.P.; Chen, H.P.; Kim, J.R. Biomass gasification in a circulating fluidized bed. Biomass Bioenergy 2004, 26, 171–193. [Google Scholar] [CrossRef]
- Marshall, A.J. Commercial Application of Pyrolysis Technology in Agriculture. 2013. Available online: https://ofa.on.ca/wp-content/uploads/2017/11/Pyrolysis-Report-Final.pdf (accessed on 26 January 2023).
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Gao, A.; Wang, Y.; Lin, G.; Liu, B.; Hu, X.; Huang, Y.; Zhang, S.; Zhang, H. Volatile-char interactions during biomass pyrolysis: Reactor design toward product control. Renew. Energy 2022, 185, 1–7. [Google Scholar] [CrossRef]
- Alves, V.R.D. Advances in the pyrolysis process and the generation of bioenergy. In Recent Perspectives in Pyrolysis Research; IntechOpen: London, UK, 2021; ISBN 978-1-83969-915-3. [Google Scholar]
- Hasan, M.M.; Rasul, M.G.; Khan, M.M.K.; Ashwath, N.; Jahirul, M.I. Energy recovery from municipal solid waste using pyrolysis technology: A review on current status and developments. Renew. Sustain. Energy Rev. 2021, 145, 111073. [Google Scholar] [CrossRef]
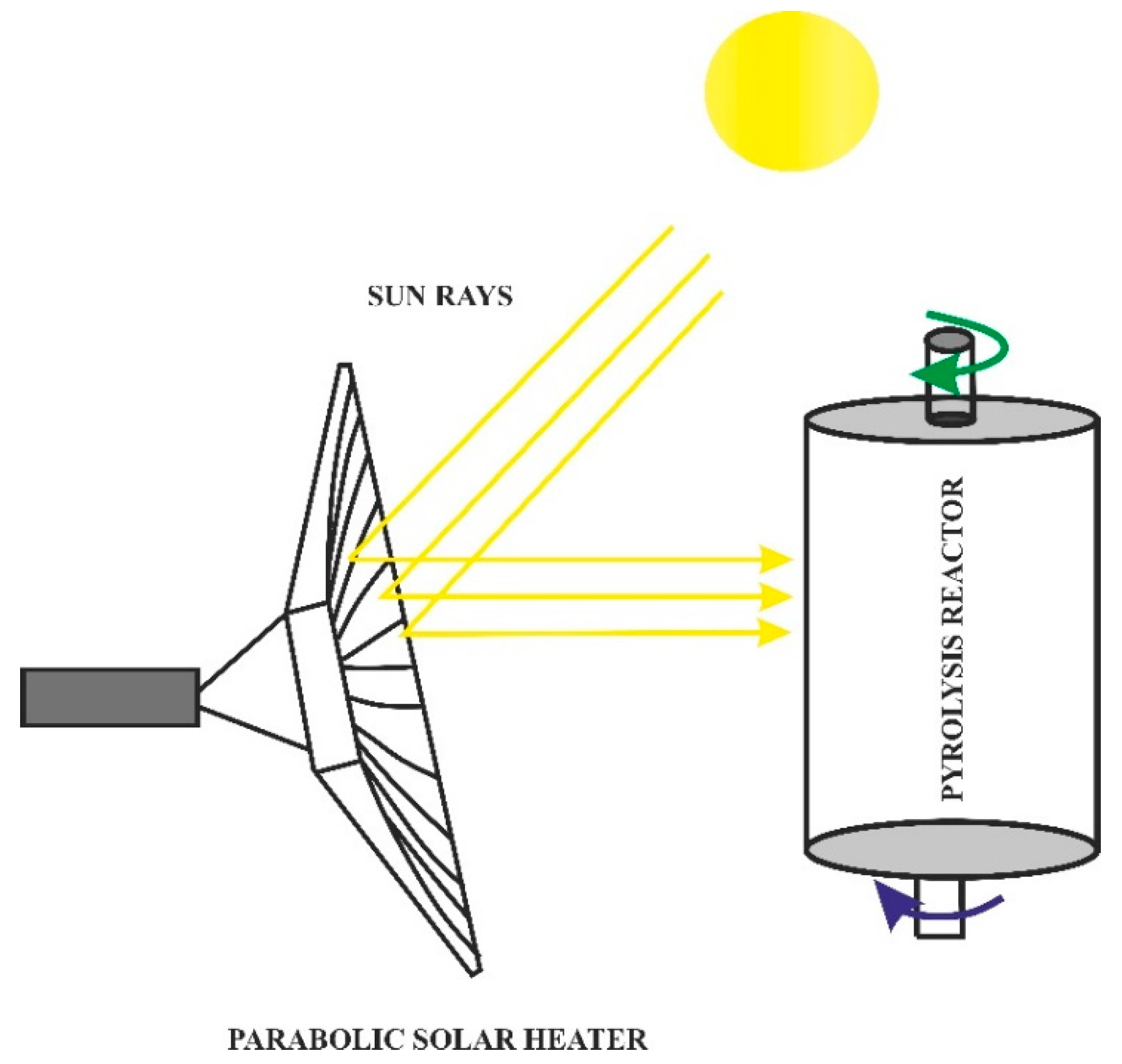
- Rahman, M.A.; Parvej, A.M.; Aziz, M. Concentrating technologies with reactor integration and effect of process variables on solar assisted pyrolysis: A critical review. Therm. Sci. Eng. Prog. 2021, 25, 100957. [Google Scholar] [CrossRef]
- Garcia-Nunez, J.A.; Pelaez-Samaniego, M.R.; Garcia-Perez, M.E.; Fonts, I.; Abrego, J.; Westerhof, J.M.; Garcia-Perez, M. Historical developments of pyrolysis reactors: A review. Energy Fuels 2017, 31, 5751–5775. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Al-Ansari, T.; Mackey, H.R.; Narayanan, K.S.; McKay, G. A review on prominent animal and municipal wastes as potential feedstocks for solar pyrolysis for biochar production. Fuel 2022, 316, 123378. [Google Scholar] [CrossRef]
- Ndukwu, M.C.; Horsfall, I.T.; Ubouh, E.A.; Orji, F.N.; Ekop, I.E.; Ezejiofor, N.R. Review of solar-biomass pyrolysis systems: Focus on the configuration of thermal-solar systems and reactor orientation. J. King Saud Univ.–Eng. Sci. 2021, 33, 413–423. [Google Scholar] [CrossRef]
- Sobek, S.; Werle, S. Solar pyrolysis of waste biomass: Part 2 kinetic modeling and methodology of the determination of the kinetic parameters for solar pyrolysis of sewage sludge. Renew. Energy 2020, 153, 962–974. [Google Scholar] [CrossRef]
- Boutin, O.; Lede, J.; Olalde, G.; Ferriere, A. Solar flash pyrolysis of biomass direct measurement of the optical properties of biomass components. J. Phys. Arch. 1999, 9, Pr3-367–Pr3-372. [Google Scholar] [CrossRef]
- Rony, A.H.; Daniel, M.; Zhao, S.; Qin, D.; Yuan, Z.; John, H.B.; Fan, M. A novel solar powered biomass pyrolysis reactor for producing fuels and chemicals. J. Anal. Appl. Pyrolysis 2018, 132, 19–32. [Google Scholar] [CrossRef]
- Sobek, S.; Werle, S. Solar pyrolysis of waste biomass: Part 1 reactor design. Renew. Energy 2019, 143, 1939–1948. [Google Scholar] [CrossRef]
- Su, G.; Zulkifli, N.W.M.; Ong, H.C.; Ibrahim, S.; Bu, Q.; Zhou, R. Pyrolysis of oil palm wastes for bioenergy in Malaysia: A review. Renew. Sustain. Energy Rev. 2022, 164, 112554. [Google Scholar] [CrossRef]
- Du, Z.; Li, Y.; Wang, X.; Wan, Y.; Chen, Q.; Wang, C.; Lin, X.; Liu, Y.; Chen, P.; Ruan, R. Microwave-assisted pyrolysis of microalgae for biofuel production. Bioresour. Technol. 2011, 102, 4890–4896. [Google Scholar] [CrossRef] [PubMed]
- Sekar, M.; Mathimani, T.; Alagumalai, A.; Chi, N.T.L.; Duc, P.A.; Bhatia, S.K.; Brindhadevi, K.; Pugazhendhi, A. A review on the pyrolysis of algal biomass for biochar and bio-oil—Bottlenecks and scope. Fuel 2021, 283, 119190. [Google Scholar] [CrossRef]
- Michalak, I.; Baśladyńska, S.; Mokrzycki, J.; Rutkowski, P. Biochar from a freshwater macroalga as a potential biosorbent for wastewater treatment. Water 2019, 11, 1390. [Google Scholar] [CrossRef]
- Wang, H.E.; Wang, H.; Zhao, H.; Yan, Q. Adsorption and Fenton-like removal of chelated nickel from Zn-Ni alloy electroplating wastewater using activated biochar composite derived from Taihu blue algae. Chem. Eng. J. 2020, 379, 122372. [Google Scholar] [CrossRef]
- Cheng, B.-H.; Zeng, R.J.; Jiang, H. Recent developments of post-modification of biochar for electrochemical energy storage. Bioresour. Technol. 2017, 246, 224–233. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H.; Cai, L.; Guo, J.; Wang, Y.; Ji, L.; Song, W. Preparation and characterization of macroalgae biochar nanomaterials with highly efficient adsorption and photodegradation ability. Materials 2018, 11, 1709. [Google Scholar] [CrossRef] [PubMed]
- Pourhosseini, S.E.M.; Norouzi, O.; Naderi, H.R. Study of micro/macro ordered porous carbon with olive-shaped structure derived from Cladophora glomerata macroalgae as efficient working electrodes of supercapacitors. Biomass Bioenergy 2017, 107, 287–298. [Google Scholar] [CrossRef]
- Pourhosseini, S.E.M.; Norouzi, O.; Salimi, P.; Naderi, H.R. Synthesis of a novel interconnected 3D pore network algal biochar constituting iron nanoparticles derived from a harmful marine biomass as high-performance asymmetric supercapacitor electrodes. ACS Sustain. Chem. Eng. 2018, 6, 4746–4758. [Google Scholar] [CrossRef]
- Ren, M.; Jia, Z.; Tian, Z.; Lopez, D.; Cai, J.; Titirici, M.-M.; Jorge, A.B. High performance n-doped carbon electrodes obtained via hydrothermal carbonization of macroalgae for supercapacitor applications. ChemElectroChem 2018, 5, 2686–2693. [Google Scholar] [CrossRef]
- Zeng, J.; Wei, L.U.; Guo, X. Bio-inspired high-performance solid-state supercapacitors with the electrolyte, separator, binder and electrodes entirely from kelp. J. Mater. Chem. A 2017, 48, 25282–25292. [Google Scholar] [CrossRef]
- Zhou, M.; Catanach, J.; Gomez, J.; Richins, S.; Deng, S. Effects of Nanoporous Carbon Derived from Microalgae and Its CoO Composite on Capacitance. ACS Appl. Mater. Interfaces 2017, 9, 4362–4373. [Google Scholar] [CrossRef]
- Chaudhary, J.P.; Gupta, R.; Mahto, A.; Vadodariya, N.; Dharmalingm, K.; Sanna Kotrappanavar, N.; Meena, R. Self-doped interwoven carbon network derived from Ulva fasciata for all-solid supercapacitor devices: Solvent-free approach to a scalable synthetic route. ACS Appl. Mater. Interfaces 2019, 7, 174–186. [Google Scholar] [CrossRef]
- Kang, D.; Liu, Q.; Gu, J.; Su, Y.; Zhang, W.; Zhang, D.I. “Egg-Box”—Assisted fabrication of porous carbon with small mesopores for high-rate electric double layer Capacitors. ACS Nano 2015, 9, 11225–11233. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, B.; Wu, K.; Zhao, B.; Yu, J.; Wang, S.; Tao, Y. Ex-situ catalytic pyrolysis of lignin using lignin-carbon (LG) catalyst combined with HZSM-5 to improve the yield of high-quality liquid fuels. Fuel 2022, 318, 123635. [Google Scholar] [CrossRef]
- Sun, J.; Luo, J.; Lin, J.; Ma, R.; Sun, S.; Fang, L.; Li, H. Study of co-pyrolysis endpoint and product conversion of plastic and biomass using microwave thermogravimetric technology. Energy 2022, 247, 123547. [Google Scholar] [CrossRef]
- Ayala-Cortés, A.; Lobato-Peralta, R.; Arreola-Ramos, C.E.; Martínez-Casillas, C.; Pacheno-Catalán, D.E.; Curntas-Gallegos, A.K.; Arancibia-Bulnes, C.A.; Villafán-Vidales, H.I. Exploring the influence of solar pyrolysis operation parameters on characteristics of carbon materials. J. Anal. Appl. Pyrolysis 2019, 140, 290–298. [Google Scholar] [CrossRef]
- Wang, Z.; Burra, K.G.; Lei, T.; Gupta, A.K. Co-pyrolysis of waste plastic and solid biomass for synergistic production of biofuels and chemicals—A review. Prog. Energy Combust. Sci. 2021, 84, 100899. [Google Scholar] [CrossRef]
- Haeldermans, T.; Campion, L.; Kuppens, T.; Vanreppelen, K.; Cuypers, A.; Schreurs, S. A comparative techno-economic assessment of biochar production from different residue streams using conventional and microwave pyrolysis. Bioresour. Technol. 2020, 318, 124083. [Google Scholar] [CrossRef] [PubMed]
- Ly, H.V.; Lee, B.; Sim, J.W.; Tran, Q.K.; Kim, S.-S.; Kim, J.; Brigljević, B.; Hwang, H.T.; Lim, H. Catalytic pyrolysis of spent coffee waste for upgrading sustainable bio-oil in bubbing fluidized-bed reactor: Experimental and techno-economic analysis. Chem. Eng. J. 2022, 427, 130956. [Google Scholar] [CrossRef]
- Wang, L.; Lei, H.; Ruan, R. Techno-economic analysis of microwave-assisted pyrolysis for production of biofuels. In Production of Biofuels and Chemicals with Microwave; Fang, Z., Smith, J.R.L., Qi, X., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 251–263. [Google Scholar] [CrossRef]
- Li, H.; Xu, J.; Nyambura, S.M.; Wang, J.; Li, C.; Zhu, X.; Feng, X.; Wang, Y. Food waste pyrolysis by traditional heating and microwave heating: A review. Fuel 2022, 324, 124574. [Google Scholar] [CrossRef]
- Malińska, K.; Dach, J. Potential applications of biochar for composting. Inż. Ekol. 2014, 36, 28–39. [Google Scholar]
- Bridgwater, A.V.; Toft, A.J.; Brammer, J.G. Techno-economic comparison of power production by biomass fast pyrolysis, with gasification and combustion. Renew. Sustain. Energy Rev. 2002, 6, 181–248. [Google Scholar] [CrossRef]
- Hess, J.R.; Kenney, K.; Laney, P.; Muth, D.; Pryfogle, P.; Radtke, C. Feasibility of a Producer Owned Ground-Straw Feedstock Supply System for Bioethanol and Other Products; Report INL/EXT-06-11815; Idaho National Laboratory: Falls, Idaho, 2006. [Google Scholar]
- Rogers, J.G.; Brammer, J.G. Estimation of the production cost of fast pyrolysis bio-oil. Biomass Bioenergy 2012, 36, 208–217. [Google Scholar] [CrossRef]
- Yahya, S.A.; Iqbal, T.; Omar, M.M.; Ahmad, M. Techno-economic analysis of fast pyrolysis of date palm waste for adoption in Saudi Arabia. Energies 2021, 14, 6048. [Google Scholar] [CrossRef]

| Type of Pyrolysis | Duration | Heating Speed | Temperature (°C) | Products | Ref. |
|---|---|---|---|---|---|
| Slow charring | 3–4 days | very slow | 400 | charcoal | [31] |
| Slow | hours/days | slow | 400 | charcoal, gas | [31] |
| Slow conventional | 5–30 min | slow | 600 | charcoal, oils, gas | [33] |
| Intermediate Fast | 10 min. 0.5–5 s | intermediate very fast | 300–450 300–1000 | charcoal oils, gas charcoal, oils gas | [34] [35] |
| Flash liquid | <1 s | fast | 650 | oils | [31] |
| Flash gas | <1 s | fast | 650 | chemicals, gas | [31] |
| Ultra-flash | <0.5 s | very fast | 1000 | chemicals, gas | [31] |
| Vacuum pyrolysis | 2–30 s | medium | 400 | oils | [31] |
| Hydro-pyrolysis | <10 s | medium | 500 | oils | [31] |
| Methane pyrolysis | <10 s | medium | 700 | chemicals | [31] |
| Component | Content in Bio-Oil (%) |
|---|---|
| Hydroxyacetic aldehyde Acetic acid Formic acid Acetaldehyde Hydroxyacetone Isoeugenol Furfuryl Alcohol 2,6-dimethyloxyphenol Etanedial Phenol Formic aldehyde Acetone Eugenol Ethylene glycol 1,4-dihydroxybenzene Cellobiose 1,6-anhydroglycofuran Fructose Levoglucosan Glucose | 0.9–13.0 0.5–12.0 0.3–9.1 0.1–8.5 0.7–7.4 0.1–7.2 0.1–5.2 0.7–4.8 0.9–4.6 0.1–3.8 0.1–3.3. 2.8 0.1–2.3 0.7–2.0 0.1–1.9 0.6–3.2 3.1 0.7–2.9 0.4–1.4 0.4–1.3 |
| Feedstock | Reaction Condition | Catalyst | Oil Yield (%) | References |
|---|---|---|---|---|
| Peanut shells | 3–30 °C/min, 30– | termite hill | [50] | |
| 800 °C | max. 57 | |||
| Martynia annua | 650 °C, 3 h | - | [53] | |
| seed | 30.77% of BTE | |||
| Corn leaf waste | 300–450 °C, | 57–73% of diesel | [54] | |
| constant flow | - | fraction | ||
| rate of nitrogen | 23.6 | |||
| Poplar wood | 350 °C, 90 min | Pd/C | [57] | |
| Beech wood | 450 °C, 4 h, 35 MPa H2 | Ru/C | 60 | [58] |
| Pine wood | 350 °C, 4 h, 14 MPa H2 | NiMo/SiO2-Al2O3 | 42.4 | [59] |
| Wheat straw | 340 °C, 1.6 h, 8 MPa H2 | NiW/AC | 18.2 | [60] |
| Wheat straw | 340 °C, 1.6 h, 8 MPa H2 | Ni/TiO2 | 76.8 | [61] |
| Corn stover | 300 °C, 4 h, 12.5 MPa H2 | Ru/C | 54.4 | [62] |
| Rice husks | 465 °C, 30 min | SO42−/ZrO2 | 56 | [63] |
| Palm shell | 700 °C, 20 min | Amberlyst15 | 86.87 | [64] |
| Swine manure | 400 °C, 30 min, 0.69 MPa N2 | Modified zeolite | 45 | [65] |
| Sewage sludge | 330–1200 °C | Graphite | 7.16–49.79 | [66] |
| Bagasse | 300 W | Activated carbon (35%, 55%, 75%), | 13.95–18.95 | [67] |
| Sugarcane bagasse | 493–532 °C | NiO, CuO, CaO, MgO | 18.4–35 | [68] |
| Oil palm shell | 180–720 W | Activated carbon (18.8–91.2%), | 16.43–36.75 | [69] |
| Food waste | 300–600 W | CaO, MgO, CuO, Fe2O3, MnO2, CuCl2 | 10–36 | [70] |
| Agricultural waste | 400–600 °C | SiC | 14.56–31.86 | [71] |
| Raw Biomass | Agent | Temperature (°C) | Time (min) | Surface (m2/g) | Micropore Volume (cm3/g) | Ref. |
|---|---|---|---|---|---|---|
| Barley straw | Steam | 700 | 60 | 552 | 0.2304 | [78] |
| Barley straw | Steam | 800 | 60 | 534 | 0.2186 | [79] |
| Barley straw | CO2 | 700 | 60 | 211 | 0.0830 | [80] |
| Orange peels | K2CO3 | 700 | 60 | 477 | 0.21 | [81] |
| Orange peels | K2CO3 | 950 | 60 | 1352 | 0.22 | [81] |
| Orange peels | ZnCl2 | 700 | 60 | 822 | 0.09 | [81] |
| Soybean oil cake | K2CO3 | 600 | 60 | 643 | 0.272 | [82] |
| Soybean oil cake | KOH | 600 | 60 | 600 | 0.213 | [82] |
| Soybean oil cake | KOH | 800 | 60 | 619 | 0.143 | [82] |
| Tomato | ZnCl2 | 400 | 60 | 648 | 0.086 | [83] |
| Tomato | ZnCl2 | 600 | 60 | 1093 | 0.129 | [83] |
| Tomato | ZnCl2 | 800 | 60 | 492 | 0.058 | [83] |
| Chestnut shell | H3PO4 | 750 | 20 | 1138 | 0.424 | [84] |
| Chestnut shell | H3PO4 | 850 | 20 | 1413 | 0.562 | [84] |
| Raw Material | Pyrolysis Temperature (°C) | Initial Concentration Cr(III) | Sorption Capacity | References |
|---|---|---|---|---|
| The husk of rice | 300 | 185 (μg/dm3) | 15.1 (μg/dm3) | [39] |
| Soybean stalks | 400 | 260 (mg/dm3) | 14.6 (mg/dm3) | [50] |
| Slurry | 600 | 300 (mg/dm3) | 40.0 (mg/dm3) | [17] |
| Wood of conifers | 700 | 650 (mg/dm3) | 32.0 (mg/dm3) | [75] |
| Biomass | Reactor | Concentrator | Light Source | References |
|---|---|---|---|---|
| Wood | Quartz tube | Direct concentration | 5 kWarc Xenon bulb | [108] |
| Waste biomass | Indirect (conduction) | Elliptical reflector | 1.6 kW Xenon arc lamp | [109] |
| Mixed biomass components | Integrated sphere | Converging lenses | A xenon lamp | [110] |
| Pine sawdust | Cylindrical quartz reactor | Deep-dish parabolic concentrator | 5 kW Xenon arc lamps | [111] |
| Chicken litter | Copper, indirect (conduction) | Elliptical reflector | 0.6 kW Xenon arc lamp | [112] |
| Algae | Specific Surface Area (m2/g) | Energy Density (Wh/kg) | Cycle Stability | Cycle Stability Percent (%) | References |
|---|---|---|---|---|---|
| Chlorella | 1337.9 | 20 | 10,000 | 92 | [120] |
| Cladophora glomerata | 354 | 42.4 | 5000 | 99.2 | [121] |
| Cladophora glometa | 957 | 41.5 | 10,000 | 93.1 | [122] |
| Enteromorpha prolifera | 2000 | 7 | 10,000 | 96 | [123] |
| Kelp | 4425 | 8 | 20,000 | 92 | [124] |
| Nannochloropsis salina | 1784 | 26.1 | 5000 | 83 | [125] |
| Ulva fasciata | 376.82 | 46.1 | 5000 | 97.5 | [126] |
| Undaria pinnatifida | 3270 | 42 | 10,000 | 94 | [127,128] |
| Fraction Yield (%) | Heating Power (W) | |||
|---|---|---|---|---|
| 500 | 750 | 1000 | 1250 | |
| Oil fraction | 26 | 28.5 | 25 | 17 |
| Water fraction | 21 | 20 | 20 | 22 |
| Carbonizate | 28 | 24 | 25 | 25 |
| Gaseous fraction | 24 | 26.5 | 29 | 35 |
| Costs | Traditional Heating Pyrolysis | Microwave Pyrolysis |
|---|---|---|
| Capital costs | +++ | +++ Electrical panels, magnetron, reactor design |
| Production costs | ++ High throughput | ++ Low pyrolysis temperature and time |
| Revenue | + | ++ High-quality products |
| Steps in Pyrolysis Process | Energy Consumed (MJ/kg) | Energy Consumed (kWh/ton) | |
|---|---|---|---|
| A. Electrical Energy | |||
| Electricity consumption by fluidized bed | 200 | ||
| (i) | pyrolysis unit | - | |
| Electricity consumed for biomass | |||
| handling and | 40 | ||
| (ii) | pre-processing (chopping and | ||
| grinding) | - | ||
| Total electric energy consumed per ton | 240 | ||
| B. Thermal energy | |||
| (i) | 0.3 | 6.66 | |
| Drying (removal of residual moisture | |||
| from 8% to 0%), about 80 kg/ton | |||
| Heat required to raise the biomass to | |||
| (ii) | 500 °C and | 1.5 | 383.3 |
| Heat required to raise fluidizing gas | |||
| up to 500 °C from 50 °C quenching | |||
| (iii) | temperature (2.75 kg of | 0.60 | 458.33 |
| fluidizing gas per kg of biomass | |||
| Radiation, convection, and | |||
| conduction losses (3% of heat input | |||
| to pyrolysis reactor) | |||
| (iv) | Total thermal energy required per | ||
| ton of date | - | 25.45 | |
| palm waste, kWh | |||
| 872.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Igliński, B.; Kujawski, W.; Kiełkowska, U. Pyrolysis of Waste Biomass: Technical and Process Achievements, and Future Development—A Review. Energies 2023, 16, 1829. https://doi.org/10.3390/en16041829
Igliński B, Kujawski W, Kiełkowska U. Pyrolysis of Waste Biomass: Technical and Process Achievements, and Future Development—A Review. Energies. 2023; 16(4):1829. https://doi.org/10.3390/en16041829
Chicago/Turabian StyleIgliński, Bartłomiej, Wojciech Kujawski, and Urszula Kiełkowska. 2023. "Pyrolysis of Waste Biomass: Technical and Process Achievements, and Future Development—A Review" Energies 16, no. 4: 1829. https://doi.org/10.3390/en16041829
APA StyleIgliński, B., Kujawski, W., & Kiełkowska, U. (2023). Pyrolysis of Waste Biomass: Technical and Process Achievements, and Future Development—A Review. Energies, 16(4), 1829. https://doi.org/10.3390/en16041829








