Nanostructured Electrocatalysts for Advanced Applications in Fuel Cells
Abstract
1. Introduction
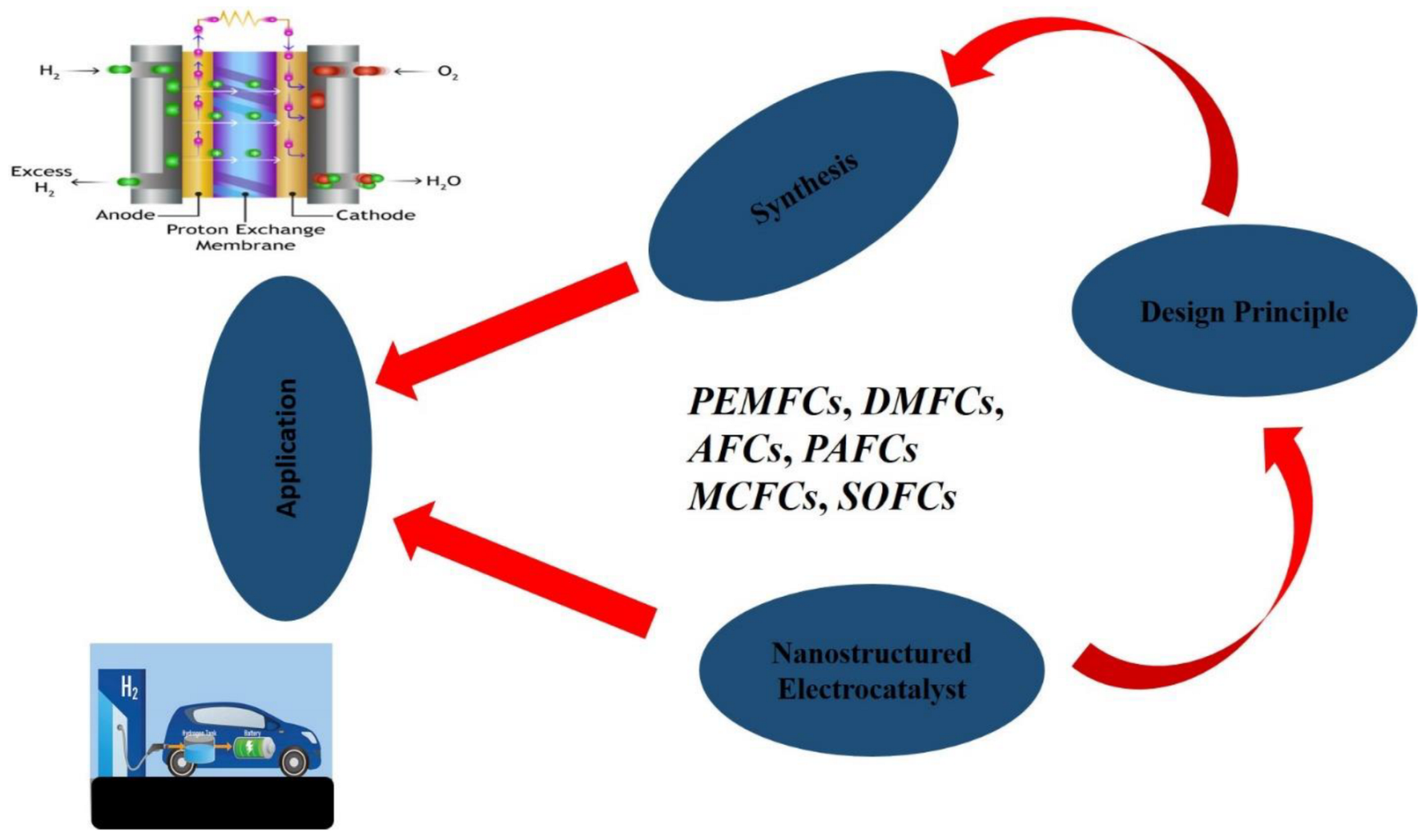
2. Design and Application of Nanostructured Electrocatalysts in Fuel Cell Devices
2.1. Porosity of Nanostructured Material
2.2. High Electronic Conducting Material Should Be Assembled
2.3. Increased Specific Surface Area
2.4. Percentage Increase of Active Facet Exposure
| Fuel Cells Type | Role of Nanostructured Electrocatalysts | Ref. |
|---|---|---|
| Polymer Electrolyte Membrane Fuel Cells (PEMFCs) | In PEM Fuel Cells, nanostructured electrocatalyst ensures high electronic-conduction pathway, homogenous dispersion of Pt-based catalyst particles, ionomer, and gas transport in porous media. The nanostructured electrocatalyst is very much helpful in Pt utilization, reduces Pt loading and helps remove carbon corrosion-induced oxidation on the cathode of Polymer Electrolyte Fuel Cells. The introduction of nanostructured electrocatalysts will enhance the sluggish kinetics of the oxygen reduction reaction (ORR), thereby bringing about fast and efficient catalytic activities in the electrodes. | [64] |
| Direct Methanol Fuel Cells (DMFCs) | The catalytic efficiency of Direct Methanol Fuel Cells can be improved by the addition of an optimized nanostructured electrocatalyst. Due to the excellent catalytic activity with respect to methanol oxidation of platinum at a low temperature in electrodes of DMFCs, nanostructured electrocatalyst has helped greatly in improving the overall efficiency of the system. They also reduce CO-poising effect and bring about high catalytic activity for methanol electrooxidation. | [57] |
| Alkaline Fuel Cells (AFCs) | Alkaline fuel cells are the most environmentally friendly of all the electrochemical energy sources. The application of nanostructured electrocatalyst results to improve kinetics at low potentials, reduce the possibility of crossover from its anodic components to the cathode side, minimize the corrosion risk for electrode material, and limit the risk of spectator-ions adsorption. The possibility of CO posing is minimal with the application of nanostructured-electrocatalyst material to the electrodes. | [38] |
| Phosphoric Acid Fuel Cells (PAFCs) | Phosphoric acid fuel cells have been successfully tested as energy-conversion technologies in stationary-energy- generation applications. The application of nanostructured- electrocatalyst material, especially the non-platinum group metals, has shown promising and encouraging immunity against surface poising by phosphate ions at room temperature. By using imaging microscopy, it was revealed that iron particles were isolated from the electrolyte of graphite layers, which ultimately protects the iron from phosphate-anion adsorption. | [65] |
| Molten Carbonate Fuel Cells (MCFCs) | MCFCs reduce high-temperature corrosion and breakdown of cell components, increase catalytic activities in the electrode, and ensure high power density. | [66] |
| Solid Oxide Fuel Cells (SOFCs) | The application of nanostructured electrocatalyst in Solid Oxide Fuel cells enhances the overall performance of the system. They increase the electrode-surface area and ensure a high oxygen- reduction-reaction rate at the electrode. | [19] |
3. Chemistry Base Synthesis Techniques
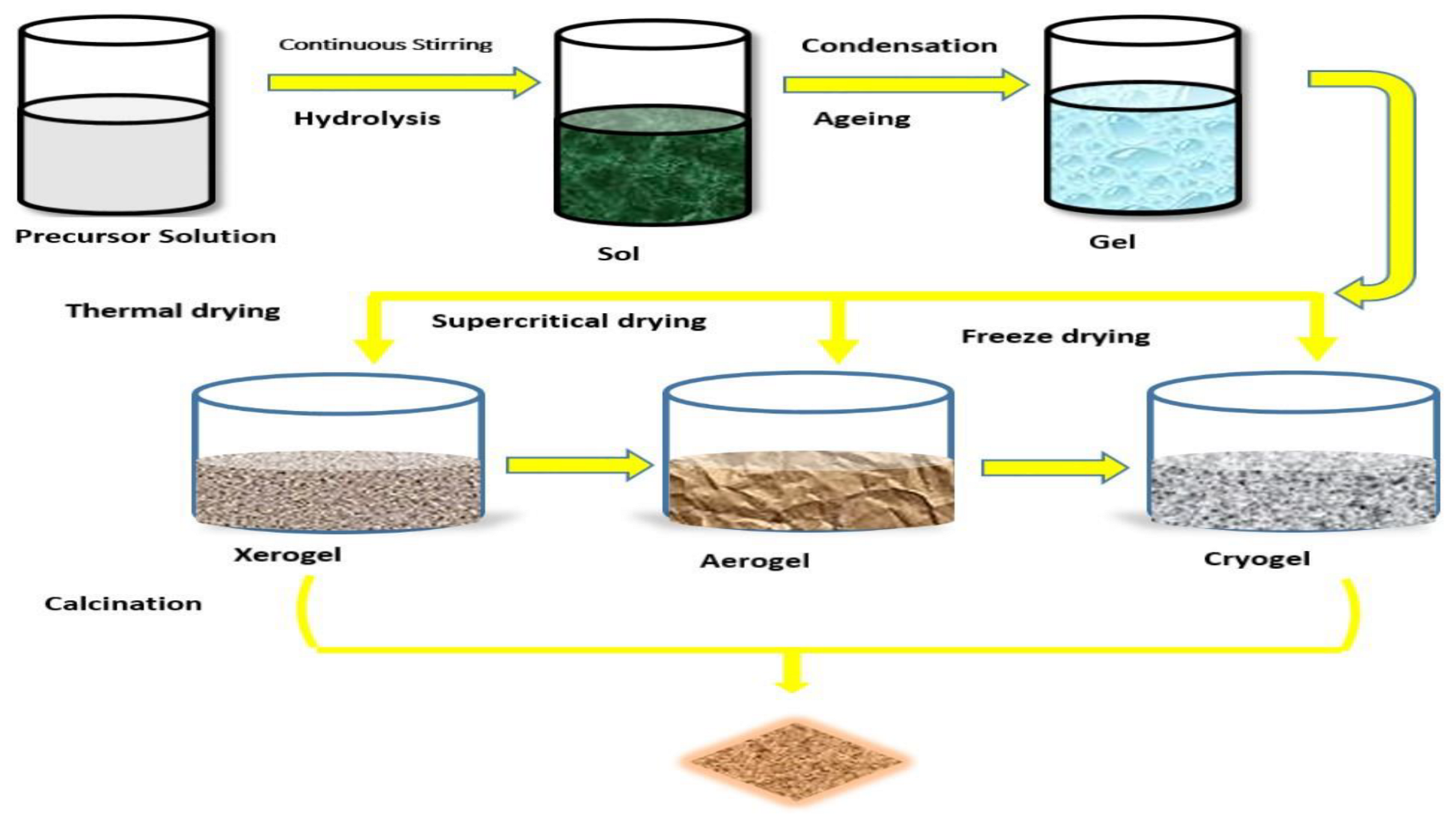
3.1. Sol-Gel Synthesis Method
- i.
- Making a homogeneous solution by dissolving organic or inorganic metal salts in water or a solvent.
- ii.
- Conversion of the formed solution into a sol
- iii.
- Gelation
- iv.
- Ageing
- v.
- Drying
- vi.
- Thermal heating
| Nanomaterials | Precursors | pH-Balance | Condition for Synthesis | Drying/Gel Formed | Size of Particles | Ref |
|---|---|---|---|---|---|---|
| MnFe2O4NPs | Mn(NO3)2·4 H2O, Fe (NO3)2, citric acid. | NaOH | Heated to temperatures ranging from 0 to 70 °C, evaporated to form a gel, dried to form flakes, and calcined for 2 h at 900 °C in a nitrogen atmosphere. | - | 45 nm | [81,82] |
| Cd2V2O7 NPs | Cd(NO3)2·4 H2O, NH4VO3, citric acid. | - | Stirred for 2 h at 100 °C, evaporation formed gel, dried in an oven at 80 °C, calcined for 2 h at various temperatures. | - | (10–20) nm | [83] |
| Al doped ZnO NPs | Zn(CH3COO)2·H2O, Al (NO3)3, methanol. | NAOH | Stirred 90 min, stirred 60 min after pH balancing, centrifuged 20 min at 10,000 rpm, washed, dried 2 h at 60 degrees Celsius, and calcined 2 h at 200 °C. | - | (20–50) nm | [84] |
| Bismuth ferrite NPs | Bi (NO)3·5 H2O, ethylene glycol, Fe (NO)3.9 H2O | - | Stirred for 2 h, heated to 60 degrees Celsius, and calcined for 4 h at 500 °C. | Evaporation/Xerogel | [85] | |
| CuO NPs | Cu(NO3)2·3 H2O, citric acid | - | Stirred at 90 °C until gel formed, then heated to 100 °C and annealed for 2 h at various temperatures of 200 °C, 300 °C, 400 °C, 500 °C, and 600 °C. | - | [86] | |
| ZnO NPs | ZnAc2, 2 H2O | NAOH | The precipitate was centrifuged, washed, and dried by lyophilization after being heated to temperatures ranging from 0 to 80 °C. | Freezing/Cry ogel | 37 nm | [87] |
| Co3O4 NPs | Cobalt acetate, polyvinylpyrrolidone (PVP), tri-ethanol | - | Stirred for 30 min, heated for 2 h at 300 °C, and annealed for 3 h at 450 °C. | - | 50 nm | [88] |
| Cu doped TiO2NPs | Titanium butoxide (C16H36O4Ti), copper acetate (Cu(CH3COO)2), HCl, methanol, ethanol | - | Stirred for 2 h at 50 °C, then annealed at 400 °C. | [89] | ||
| Fe doped TiO2 NPs | Iron (III) chloride 6-hydrate, C2H28O4Ti, ethanol, citrate acid, C5H8O2 | - | Stirred at 40 °C, refluxed at 120 °C for 6 h, gel obtained by heating 14 h at 80 °C, drying at 150 °C for 1 h, and annealing 1 h at 400 °C, 600 °C, and 800 °C. | Supercritical/Aerogel | (6–11) nm, (22–30) nm, (50–100) nm | [90] |
| Advantages | Disadvantages |
|---|---|
| They are cost-effective. | Organic chemicals present some health challenges. |
| They have a low processing temperature. | The reaction takes a longer time. |
| The technique is simple in making nanostructure and nanocomposites. | Purification of sample brings about post-treatment. |
| They have high purity. | |
| A modest amount of dopant is allowed into the sol, whose presence can be felt in the final product. |
3.2. Hydrothermal Synthesis Technique
| Nanomaterials | Stabilizing and Reducing Agent | Precursor | Autoclave of Hydrothermal | Condition of Synthesis | Size of Particle | Ref. |
|---|---|---|---|---|---|---|
| TiO2 NPs | 0.5 g BMI. Cl | 0.4 g TiO2 | Filled Teflon tube | Centrifuged and washed with Ethanol for 5 min before drying 80 °C, overnight | 35 nm | [99] |
| ZrO2 NPs | NH4OH | ZrOCl2·8 H2O | Filled Teflon-lined | Centrifuged, washed with acetone, and dried at 90 °C before being calcined at 450 °C for 60 min | 12 nm | [100] |
| NiO NPs | 50 mM urea | Ni(NO3)2·6 H2O | Filled Teflon-lined | Centrifuged, washed, and dried for 5 h at 50 °C, then annealed for 5 h at 40 °C. | (20–50) nm | [91] |
| Fe3O4 NPs | 50 mg polyvinyl | FeCl3·6 H2O | Filled 75 mL Teflon-lined | Filtered, washed, and dried for 3 h at 300 °C | ~65 nm | [101] |
| Ag NPs | Nanocellulose 20 ML | AgNO3 0.1 mL~0.5 ML | Filled 50 mL Teflon- lined | Filled with 50 mL Teflon-lined | 8 nm | [102] |
| Au NPs | “Hydrolyzed spider cobweb 33 ML” | HAuCl4 330 mg | Filled 50 mL Teflon vessel | Centrifuged for 5 min, washed with ethanol, and dried | 40 nm | [103] |
| CuO NPs | NaOH 10 mmol & 1 mL ethylene Diamine | Cu(NO3)2·3 H2O 10 mmol | Filled 60 mL Teflon-lined | Washed with ethanol and dried | ~27.7 nm | [104] |
| Advantages | Disadvantages |
|---|---|
| Control over the size is precise. | Autoclaves are expensive. |
| Low melting point, high vapour pressure, and pyrolysis are all guaranteed. | Crystal growth cannot be observed directly. |
| A high-crystallinity nanocrystal is obtained. | The control is difficult. |
4. Stability of the Basic Nanomaterials Arrays
5. Key Challenges, Limitations, and Future Considerations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations/Nomenclature
| Co3O4 | Cobalt (II, III) oxide |
| TiO2 | Titanium dioxide |
| ZrO2 | Zirconium dioxide |
| NiO | Nickel oxide |
| Fe3O4 | Iron (II, III) oxide |
| Ag | Silver |
| Au | Gold |
| PEMFCs | Polymer Electrolyte Membrane Fuel Cells |
| DMFCs | Direct Methanol Fuel Cells |
| AFCs | Alkaline Fuel Cells |
| PAFCs | Phosphoric Acid Fuel Cells |
| MCFCs | Molten Carbonate Fuel Cells |
| SOFCs | Solid Oxide Fuel Cells |
| MnFe2O4 | Manganese Ferrite |
| Cd2V2O7 | Cadmium pyrovanadate |
| ZnO | Zinc oxide |
| CuO | Copper (II) oxide or cupric oxide |
| NPs | Nanoparticles |
References
- Chinnappan, A.; Baskar, C.; Kim, H.; Ramakrishna, S. Carbon Nanotube Hybrid Nanostructures: Future Generation Conducting Materials. J. Mater. Chem. A 2016, 4, 9347–9361. [Google Scholar] [CrossRef]
- Sanli, D.; Bozbag, S.E.; Erkey, C. Synthesis of Nanostructured Materials Using Supercritical CO2: Part I. Physical Transformations. J. Mater. Sci. 2012, 47, 2995–3025. [Google Scholar] [CrossRef]
- Ghosh, A.; Ramaprabhu, S. An Efficient and Durable Novel Catalyst Support with Superior Electron-Donating Properties and Fuel Diffusivity for a Direct Methanol Fuel Cell. Catal. Sci. Technol. 2017, 7, 5079–5091. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen Production, Storage, Transportation and Key Challenges with Applications: A Review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Subramaniam, M.N.; Goh, P.S.; Lau, W.J.; Ismail, A.F. The Roles of Nanomaterials in Conventional and Emerging Technologies for Heavy Metal Removal: A State-of-the-Art Review. Nanomaterials 2019, 9, 625. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Amer-Hatem, F.; Azad, A.K.; Dedoussi, I.C.; de Joannon, M.; Fernandes, R.X.; Glarborg, P.; Hashemi, H.; He, X.; Mashruk, S.; et al. Review on Ammonia as a Potential Fuel: From Synthesis to Economics. Energy Fuels 2021, 35, 6964–7029. [Google Scholar] [CrossRef]
- Afif, A.; Radenahmad, N.; Cheok, Q.; Shams, S.; Kim, J.H.; Azad, A.K. Ammonia-Fed Fuel Cells: A Comprehensive Review. Renew. Sustain. Energy Rev. 2016, 60, 822–835. [Google Scholar] [CrossRef]
- Zhang, Q.; Uchaker, E.; Candelaria, S.L.; Cao, G. Nanomaterials for Energy Conversion and Storage. Chem. Soc. Rev. 2013, 42, 3127. [Google Scholar] [CrossRef]
- Radenahmad, N.; Azad, A.T.; Saghir, M.; Taweekun, J.; Bakar, M.S.A.; Reza, M.S.; Azad, A.K. A Review on Biomass Derived Syngas for SOFC Based Combined Heat and Power Application. Renew. Sustain. Energy Rev. 2020, 119, 109560. [Google Scholar] [CrossRef]
- Jiang, W.; Qu, Z.; Kumar, P.; Vecchio, D.; Wang, Y.; Ma, Y.; Bahng, J.H.; Bernardino, K.; Gomes, W.R.; Colombari, F.M.; et al. Emergence of Complexity in Hierarchically Organized Chiral Particles. Science 2020, 368, 642–648. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Herve Bang, Y.; Di Noto, V. Hybrid Inorganic-Organic Proton-Conducting Membranes Based on SPEEK Doped with WO3 Nanoparticles for Application in Vanadium Redox Flow Batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Ariga, K.; Leong, D.T.; Mori, T. Nanoarchitectonics for Hybrid and Related Materials for Bio-Oriented Applications. Adv. Funct. Mater. 2018, 28, 1702905. [Google Scholar] [CrossRef]
- Ariga, K.; Jia, X.; Song, J.; Hill, J.P.; Leong, D.T.; Jia, Y.; Li, J. Nanoarchitectonics beyond Self-Assembly: Challenges to Create Bio-Like Hierarchic Organization. Angew. Chem. Int. Ed. 2020, 59, 15424–15446. [Google Scholar] [CrossRef] [PubMed]
- Afif, A.; Rahman, S.M.; Tasfiah Azad, A.; Zaini, J.; Islan, M.A.; Azad, A.K. Advanced Materials and Technologies for Hybrid Supercapacitors for Energy Storage–A Review. J. Energy Storage 2019, 25, 100852. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, H. Review of the Development of First-Generation Redox Flow Batteries: Iron-Chromium System. ChemSusChem 2022, 15, e202101798. [Google Scholar] [CrossRef]
- Omeiza, L.A.; Azad, A.K.; Kozak, K.; Mafo, A.R.; Mamudu, U.; Daniel, A.O.D. COVID-19: Vaccine Hesitancy in Africa and the Way Forward. Probl. Ekorozwoju 2022, 17, 39–46. [Google Scholar] [CrossRef]
- Omeiza, L.A.; Azad, A.K.; Kozak, K.; Mamudu, U.; Daniel, A.O. Minimizing the Cost of Energy Consumption for Public Institutions in Nigeria. Present Environ. Sustain. Dev. 2022, 16, 123–138. [Google Scholar] [CrossRef]
- Azhar, A.; Li, Y.; Cai, Z.; Zakaria, M.B.; Masud, M.K.; Hossain, M.S.A.; Kim, J.; Zhang, W.; Na, J.; Yamauchi, Y.; et al. Nanoarchitectonics: A New Materials Horizon for Prussian Blue and Its Analogues. Bull. Chem. Soc. Jpn. 2019, 92, 875–904. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Azad, A.T.; Petra, P.M.I.; Begum, F.; Eriksson, S.G.; Azad, A.K. Nanomaterials for Solid Oxide Fuel Cells: A Review. Renew. Sustain. Energy Rev. 2018, 82, 353–368. [Google Scholar] [CrossRef]
- Liu, H.; Feng, Y.; Chen, D.; Li, C.; Cui, P.; Yang, J. Noble Metal-Based Composite Nanomaterials Fabricated via Solution-Based Approaches. J. Mater. Chem. A 2015, 3, 3182–3223. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Elnaghi, B.E.; Hossain, S.; Dawood, M.; Abdelrehim, O.; Azad, A.K. Nanotechnology Utilization in Energy Conversion, Storage and Efficiency: A Perspective Review. Adv. Energy Convers. Mater. 2020, 20, 30–54. [Google Scholar] [CrossRef]
- Yu, H.-D.; Regulacio, M.D.; Ye, E.; Han, M.-Y. Chemical Routes to Top-down Nanofabrication. Chem. Soc. Rev. 2013, 42, 6006. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.S.; Yun, C.S.; Afroze, S.; Radenahmad, N.; Bakar, M.S.A.; Saidur, R.; Taweekun, J.; Azad, A.K. Preparation of Activated Carbon from Biomass and Its’ Applications in Water and Gas Purification, a Review. Arab, J. Basic Appl. Sci. 2020, 27, 208–238. [Google Scholar] [CrossRef]
- Ghosh, S.; Maiyalagan, T.; Basu, R.N. Recent Advances in Nanostructured Electrocatalysts for Low-Temperature Direct Alcohol Fuel Cells. In Electrocatalysts for Low Temperature Fuel Cells; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 347–371. [Google Scholar] [CrossRef]
- Dong, Z.; Hu, W.; Ma, Z.; Li, C.; Liu, Y. The Synthesis of Composite Powder Precursors via Chemical Processes for the Sintering of Oxide Dispersion-Strengthened Alloys. Mater. Chem. Front. 2019, 3, 1952–1972. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, H.; Qiao, J.; Feng, J.; Xu, C.; Wang, Z.; Sun, W.; Sun, K. Hierarchical Hollow Nanofiber Networks for High-Performance Hybrid Direct Carbon Fuel Cells. J. Mater. Chem. A 2017, 5, 17216–17220. [Google Scholar] [CrossRef]
- Mashola, T.A.; Matthews, T.; Msomi, P.F.; Maxakato, N.W. Novel Nanostructured Electrocatalysts for Fuel Cell Technology: Design, Solution Chemistry-Based Preparation Approaches and Application. Nano-Struct. Nano-Objects 2022, 29, 100831. [Google Scholar] [CrossRef]
- Huerta-Mata, C.A.; Chowdari, R.K.; Soto-Arteaga, C.E.; Infantes-Molina, A.; Alonso-Núñez, G.; Fuentes-Moyado, S.; Huirache-Acuña, R.; Díaz de León, J.N. Hydrothermal Synthesis of Bulk Ni Impregnated WO3 2D Layered Structures as Catalysts for the Desulfurization of 3-Methyl Thiophene. Chem. Eng. J. Adv. 2022, 11, 100312. [Google Scholar] [CrossRef]
- Sinha, T.K. Morphology-Dependent Visible Light Photocatalysis. In Nanostructured Materials for Visible Light Photocatalysis; Elsevier: Amsterdam, The Netherlands, 2022; pp. 375–412. [Google Scholar] [CrossRef]
- Wang, C.-I.; Wu, W.-C.; Periasamy, A.P.; Chang, H.-T. Electrochemical Synthesis of Photoluminescent Carbon Nanodots from Glycine for Highly Sensitive Detection of Hemoglobin. Green Chem. 2014, 16, 2509. [Google Scholar] [CrossRef]
- Nikam, A.V.; Prasad, B.L.V.; Kulkarni, A.A. Wet Chemical Synthesis of Metal Oxide Nanoparticles: A Review. CrystEngComm 2018, 20, 5091–5107. [Google Scholar] [CrossRef]
- Guo, T.; Yao, M.-S.; Lin, Y.-H.; Nan, C.-W. A Comprehensive Review on Synthesis Methods for Transition-Metal Oxide Nanostructures. CrystEngComm 2015, 17, 3551–3585. [Google Scholar] [CrossRef]
- Stachurski, C.D.; Click, S.M.; Wolfe, K.D.; Dervishogullari, D.; Rosenthal, S.J.; Jennings, G.K.; Cliffel, D.E. Optical and Electrochemical Tuning of Hydrothermally Synthesized Nitrogen-Doped Carbon Dots. Nanoscale Adv. 2020, 2, 3375–3383. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Ejsmont, A.; Freund, R.; Goscianska, J.; Schmidt, B.V.K.J.; Wuttke, S. Controlling the Morphology of Metal–Organic Frameworks and Porous Carbon Materials: Metal Oxides as Primary Architecture-Directing Agents. Chem. Soc. Rev. 2020, 49, 3348–3422. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-D. From Formation Mechanisms to Synthetic Methods toward Shape-Controlled Oxide Nanoparticles. Nanoscale 2013, 5, 9455. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Jiang, H.-L. Metal-Organic-Framework-Based Single-Atom Catalysts for Energy Applications. Chem 2019, 5, 786–804. [Google Scholar] [CrossRef]
- Ramli, Z.A.C.; Kamarudin, S.K. Platinum-Based Catalysts on Various Carbon Supports and Conducting Polymers for Direct Methanol Fuel Cell Applications: A Review. Nanoscale Res. Lett. 2018, 13, 410. [Google Scholar] [CrossRef]
- Sikeyi, L.L.; Matthews, T.; Adekunle, A.S.; Maxakato, N.W. Electro-oxidation of Ethanol and Methanol on Pd/C, Pd/CNFs and Pd−Ru/CNFs Nanocatalysts in Alkaline Direct Alcohol Fuel Cell. Electroanalysis 2020, 32, 2681–2692. [Google Scholar] [CrossRef]
- Shi, Y.; Peng, L.; Ding, Y.; Zhao, Y.; Yu, G. Nanostructured Conductive Polymers for Advanced Energy Storage. Chem. Soc. Rev. 2015, 44, 6684–6696. [Google Scholar] [CrossRef]
- Hossain, S.; Abdalla, A.M.; Suhaili, S.B.H.; Kamal, I.; Shaikh, S.P.S.; Dawood, M.K.; Azad, A.K. Nanostructured Graphene Materials Utilization in Fuel Cells and Batteries: A Review. J. Energy Storage 2020, 29, 101386. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for Fuel-Cell Technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef]
- Radenahmad, N.; Afif, A.; Abdalla, A.M.; Saqib, M.; Park, J.-Y.; Zaini, J.; Irvine, J.; Kim, J.H.; Azad, A.K. A New High-Performance Proton-Conducting Electrolyte for Next-Generation Solid Oxide Fuel Cells. Energy Technol. 2020, 8, 2000486. [Google Scholar] [CrossRef]
- Radenahmad, N.; Afif, A.; Petra, P.I.; Rahman, S.M.H.; Eriksson, S.-G.; Azad, A.K. Proton-Conducting Electrolytes for Direct Methanol and Direct Urea Fuel Cells–A State-of-the-Art Review. Renew. Sustain. Energy Rev. 2016, 57, 1347–1358. [Google Scholar] [CrossRef]
- Vignarooban, K.; Lin, J.; Arvay, A.; Kolli, S.; Kruusenberg, I.; Tammeveski, K.; Munukutla, L.; Kannan, A.M. Nano-Electrocatalyst Materials for Low Temperature Fuel Cells: A Review. Chin. J. Catal. 2015, 36, 458–472. [Google Scholar] [CrossRef]
- Hou, R.; Li, M.; Feng, S.; Liu, Y.; Wu, L.; Bi, Z.; Xu, X.; Ma, W.; Bo, Z. Fused Pentacyclic Electron Acceptors with Four Cis -Arranged Alkyl Side Chains for Efficient Polymer Solar Cells. J. Mater. Chem. A 2018, 6, 3724–3729. [Google Scholar] [CrossRef]
- Afroze, S.; Karim, A.; Cheok, Q.; Eriksson, S.; Azad, A.K. Latest Development of Double Perovskite Electrode Materials for Solid Oxide Fuel Cells: A Review. Front. Energy 2019, 13, 770–797. [Google Scholar] [CrossRef]
- Atkinson, A.; Barnett, S.; Gorte, R.J.; Irvine, J.T.S.; McEvoy, A.J.; Mogensen, M.; Singhal, S.C.; Vohs, J. Advanced Anodes for High-Temperature Fuel Cells. Nat. Mater. 2004, 3, 17–27. [Google Scholar] [CrossRef]
- Lee, T.-H.; Park, K.-Y.; Kim, N.-I.; Song, S.-J.; Hong, K.-H.; Ahn, D.; Azad, A.K.; Hwang, J.; Bhattacharjee, S.; Lee, S.-C.; et al. Robust NdBa0.5Sr0.5Co1.5Fe0.5O5+δ Cathode Material and Its Degradation Prevention Operating Logic for Intermediate Temperature-Solid Oxide Fuel Cells. J. Power Sources 2016, 331, 495–506. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Petra, P.M.; Ghasemi, M.; Azad, A.K. Achievements and Trends of Solid Oxide Fuel Cells in Clean Energy Field: A Perspective Review. Front. Energy 2020, 14, 359–382. [Google Scholar] [CrossRef]
- Jana, R.; Dey, A.; Das, M.; Datta, J.; Das, P.; Ray, P.P. Improving Performance of Device Made up of CuO Nanoparticles Synthesized by Hydrothermal over the Reflux Method. Appl. Surf. Sci. 2018, 452, 155–164. [Google Scholar] [CrossRef]
- Takacs, L. Self-Sustaining Reactions Induced by Ball Milling. Prog. Mater. Sci. 2002, 47, 355–414. [Google Scholar] [CrossRef]
- Li, C.; Tan, H.; Lin, J.; Luo, X.; Wang, S.; You, J.; Kang, Y.-M.; Bando, Y.; Yamauchi, Y.; Kim, J. Emerging Pt-Based Electrocatalysts with Highly Open Nanoarchitectures for Boosting Oxygen Reduction Reaction. Nano Today 2018, 21, 91–105. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, X.-X.; Huang, X.-Y.; Wang, A.-J.; Zhang, Q.-L.; Huang, H.; Feng, J.-J. Trimetallic PtRhCo Petal-Assembled Alloyed Nanoflowers as Efficient and Stable Bifunctional Electrocatalyst for Ethylene Glycol Oxidation and Hydrogen Evolution Reactions. J. Colloid Interface Sci. 2020, 559, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ge, X.; Lv, C.; Hu, C.; Guan, H.; Wu, J.; Wang, Z.; Yang, X.; Shi, Y.; Song, J.; et al. Oxygen Vacancy-Rich Amorphous Porous NiFe(OH) x Derived from Ni(OH) x /Prussian Blue as Highly Efficient Oxygen Evolution Electrocatalysts. Nanoscale 2020, 12, 9557–9568. [Google Scholar] [CrossRef] [PubMed]
- Sulciute, A.; Nishimura, K.; Gilshtein, E.; Cesano, F.; Viscardi, G.; Nasibulin, A.G.; Ohno, Y.; Rackauskas, S. ZnO Nanostructures Application in Electrochemistry: Influence of Morphology. J. Phys. Chem. C 2021, 125, 1472–1482. [Google Scholar] [CrossRef]
- Raseruthe, K.E.; Matthews, T.; Gwebu, S.S.; Pillay, K.; Maxakato, N.W. Investigating the Effect of Carbon Support on Palladium-Based Catalyst towards Electro-Oxidation of Ethylene Glycol. Mater. Res. Express 2021, 8, 015017. [Google Scholar] [CrossRef]
- Huang, H.; Wang, X. Recent Progress on Carbon-Based Support Materials for Electrocatalysts of Direct Methanol Fuel Cells. J. Mater. Chem. A 2014, 2, 6266–6291. [Google Scholar] [CrossRef]
- Azad, A.K.; Kim, J.H.; Irvine, J.T.S. Structure–Property Relationship in Layered Perovskite Cathode LnBa0.5Sr0.5Co2O5+δ (Ln=Pr, Nd) for Solid Oxide Fuel Cells. J. Power Sources 2011, 196, 7333–7337. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, Y.; Zhu, Y.; Wang, W.; Wang, C.; Yu, A.; Pu, X.; Zhai, J. A Flower-like CoS2/MoS2 Heteronanosheet Array as an Active and Stable Electrocatalyst toward the Hydrogen Evolution Reaction in Alkaline Media. RSC Adv. 2020, 10, 8973–8981. [Google Scholar] [CrossRef]
- Nandan, R.; Pandey, P.; Gautam, A.; Bisen, O.Y.; Chattopadhyay, K.; Titirici, M.-M.; Nanda, K.K. Atomic Arrangement Modulation in CoFe Nanoparticles Encapsulated in N-Doped Carbon Nanostructures for Efficient Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2021, 13, 3771–3781. [Google Scholar] [CrossRef]
- Afif, A.; Zaini, J.; Rahman, S.M.H.; Eriksson, S.; Islam, M.A.; Azad, A.K. Scheelite Type Sr1−xBaxWO4 (x = 0.1, 0.2, 0.3) for Possible Application in Solid Oxide Fuel Cell Electrolytes. Sci. Rep. 2019, 9, 9173. [Google Scholar] [CrossRef]
- Gwebu, S.S.; Nomngongo, P.N.; Maxakato, N.W. Pt-Sn Nanoparticles Supported on Carbon Nanodots as Anode Catalysts for Alcohol Electro-Oxidation in Acidic Conditions. Electroanalysis 2018, 30, 1125–1132. [Google Scholar] [CrossRef]
- Azad, A.K.; Abdalla, A.M.; Afif, A.; Azad, A.; Afroze, S.; Idris, A.C.; Park, J.-Y.; Saqib, M.; Radenahmad, N.; Hossain, S.; et al. Improved Mechanical Strength, Proton Conductivity and Power Density in an ‘All-Protonic’ Ceramic Fuel Cell at Intermediate Temperature. Sci. Rep. 2021, 11, 19382. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Takasaki, F.; Noda, Z.; Hayashi, S.; Shiratori, Y.; Ito, K. Alternative Electrocatalyst Support Materials for Polymer Electrolyte Fuel Cells. ECS Trans. 2010, 33, 473–482. [Google Scholar] [CrossRef]
- Strickland, K.; Pavlicek, R.; Miner, E.; Jia, Q.; Zoller, I.; Ghoshal, S.; Liang, W.; Mukerjee, S. Anion Resistant Oxygen Reduction Electrocatalyst in Phosphoric Acid Fuel Cell. ACS Catal. 2018, 8, 3833–3843. [Google Scholar] [CrossRef]
- Cassir, M.; Ringuedé, A.; Lair, V. Molten Carbonates from Fuel Cells to New Energy Devices. In Molten Salts Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; pp. 355–371. [Google Scholar] [CrossRef]
- Zhu, X.; Hu, B.; Wang, C.; An, X.; He, J.; Wang, X.; Zhao, Y. Self-Assembly Induced Metal Ionic-Polymer Derived Fe-Nx/C Nanowire as Oxygen Reduction Reaction Electrocatalyst. J. Catal. 2020, 391, 1–10. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Green Synthesis, Characterization and Uses of Palladium/Platinum Nanoparticles. Nanoscale Res. Lett. 2016, 11, 482. [Google Scholar] [CrossRef]
- Issaoui, H.; Benali, A.; Bejar, M.; Dhahri, E.; Costa, B.F.O.; Graca, M.P.F.; Valente, M.A. Effect of Bi-Substitution into the A-Site of Multiferroic La0.8Ca0.2FeO3 on Structural, Electrical and Dielectric Properties. RSC Adv. 2020, 10, 16132–16146. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, H.; Zia, M.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, Characterization, Applications, and Challenges of Iron Oxide Nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef]
- Hasnidawani, J.N.; Azlina, H.N.; Norita, H.; Bonnia, N.N.; Ratim, S.; Ali, E.S. Synthesis of ZnO Nanostructures Using Sol-Gel Method. Procedia Chem. 2016, 19, 211–216. [Google Scholar] [CrossRef]
- Kayani, Z.N.; Butt, M.Z.; Riaz, S.; Naseem, S. Synthesis of NiO Nanoparticles by Sol-Gel Technique. Mater. Sci. 2018, 36, 547–552. [Google Scholar] [CrossRef]
- Xu, X.; Xie, Y.; Ni, S.; Azad, A.K.; Cao, T. Photocatalytic H2 Production from Spinels ZnGa2−CrO4 (0≤x≤2) Solid Solutions. J. Solid State Chem. 2015, 230, 95–101. [Google Scholar] [CrossRef]
- McCaugherty, S.; Grosvenor, A.P. Low-Temperature Synthesis of CaZrTi2O7 Zirconolite-Type Materials Using Ceramic, Coprecipitation, and Sol–Gel Methods. J. Mater. Chem. C 2019, 7, 177–187. [Google Scholar] [CrossRef]
- Vinogradov, A.V.; Vinogradov, V.V. Low-Temperature Sol–Gel Synthesis of Crystalline Materials. RSC Adv. 2014, 4, 45903–45919. [Google Scholar] [CrossRef]
- Hasany, F.S.; Ahmed, I.J.R.; Rehman, A. Systematic Review of the Preparation Techniques of Iron Oxide Magnetic Nanoparticles. Nanosci. Nanotechnol. 2013, 2, 148–158. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, G.; Li, X. Synthesis of Li(Ni0.6Co0.2Mn0.2)O2 by a Modified Sol-Gel Method for Lithium-Ion Batteries. Synth. Met. 2021, 281, 116905. [Google Scholar] [CrossRef]
- Rosset, A.; Djessas, K.; Goetz, V.; Grillo, S.; Plantard, G. Sol–Gel Synthesis and Solar Photocatalytic Activity of Ca-Alloyed ZnO Nanoparticles Elaborated Using Different Precursors. RSC Adv. 2020, 10, 25456–25466. [Google Scholar] [CrossRef]
- Dai, Y.; Li, H.; Wang, Y.; Zhong, K.; Zhang, H.; Yu, J.; Huang, Z.; Yan, J.; Huang, L.; Liu, X.; et al. Zn-Doped CaFeO3 Perovskite-Derived High Performed Catalyst on Oxygen Reduction Reaction in Microbial Fuel Cells. J. Power Sources 2021, 489, 229498. [Google Scholar] [CrossRef]
- Qiang, M.; Zhang, X.; Song, H.; Pi, C.; Wang, X.; Gao, B.; Zheng, Y.; Peng, X.; Chu, P.K.; Huo, K. General Synthesis of Nanostructured Mo2C Electrocatalysts Using a Carbon Template for Electrocatalytic Applications. Carbon N. Y. 2022, 197, 238–245. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Zhou, X.-W.; Zeng, Y.; Amirkhiz, B.S.; Wang, M.-N.; Zhang, L.-Z.; Hua, B.; Li, J.; Li, J.-H.; Luo, J.-L. An Ingenious Ni/Ce Co-Doped Titanate Based Perovskite as a Coking-Tolerant Anode Material for Direct Hydrocarbon Solid Oxide Fuel Cells. J. Mater. Chem. A 2015, 3, 22830–22838. [Google Scholar] [CrossRef]
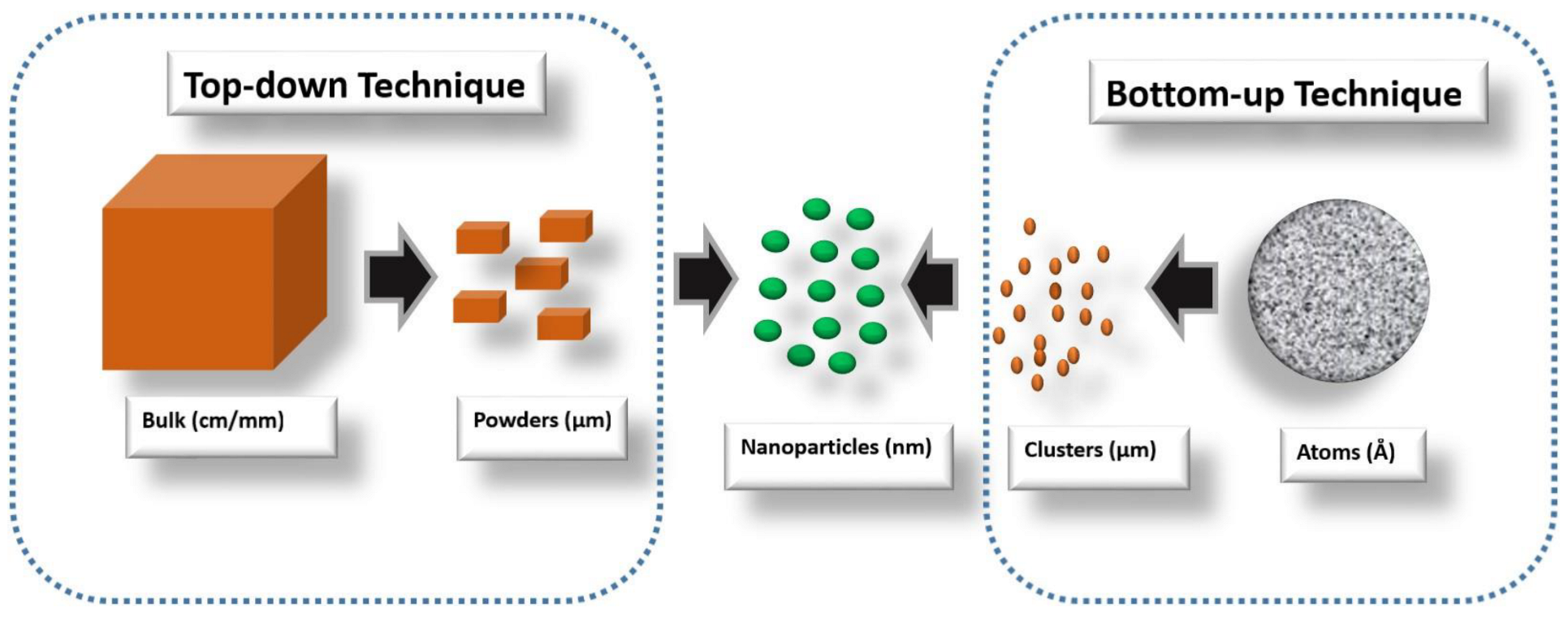
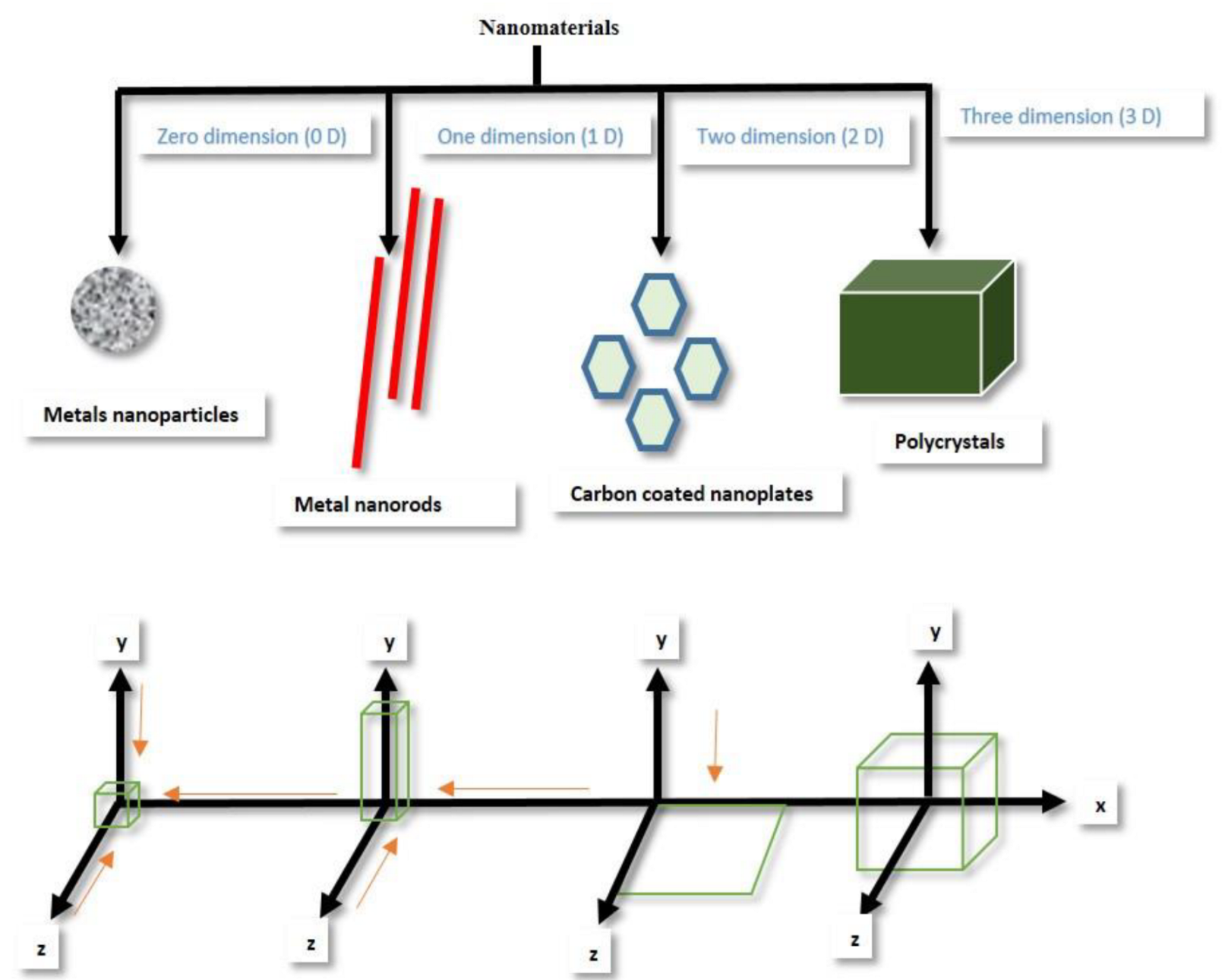
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of Nanomaterials Using Various Top-down and Bottom-up Approaches, Influencing Factors, Advantages, and Disadvantages: A Review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef]
- Kafshgari, L.A.; Ghorbani, M.; Azizi, A. Synthesis and Characterization of Manganese Ferrite Nanostructure by Co-Precipitation, Sol-Gel, and Hydrothermal Methods. Part. Sci. Technol. 2019, 37, 904–910. [Google Scholar] [CrossRef]
- Mazloom, F.; Ghiyasiyan-Arani, M.; Monsef, R.; Salavati-Niasari, M. Photocatalytic Degradation of Diverse Organic Dyes by Sol–Gel Synthesized Cd2V2O7 Nanostructures. J. Mater. Sci. Mater. Electron. 2018, 29, 18120–18127. [Google Scholar] [CrossRef]
- Mahdavi, R.; Talesh, S.S.A. Sol-Gel Synthesis, Structural and Enhanced Photocatalytic Performance of Al Doped ZnO Nanoparticles. Adv. Powder Technol. 2017, 28, 1418–1425. [Google Scholar] [CrossRef]
- Siddique, M.; Khan, N.M.; Saeed, M. Photocatalytic Activity of Bismuth Ferrite Nanoparticles Synthesized via Sol-Gel Route. Zeitschrift für Phys. Chemie 2019, 233, 595–607. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Folorunso, A.S.; Folorunso, F.A.; Oyebamiji, A.K. Green Synthesis of Copper Oxide Nanoparticles for Biomedical Application and Environmental Remediation. Heliyon 2020, 6, e04508. [Google Scholar] [CrossRef] [PubMed]
- Bekkari, R.; Laânab, L.; Boyer, D.; Mahiou, R.; Jaber, B. Influence of the Sol Gel Synthesis Parameters on the Photoluminescence Properties of ZnO Nanoparticles. Mater. Sci. Semicond. Process. 2017, 71, 181–187. [Google Scholar] [CrossRef]
- Vennela, A.B. Structural and Optical Properties of Co3O4 Nanoparticles Prepared by Sol-Gel Technique for Photocatalytic Application. Int. J. Electrochem. Sci. 2019, 14, 3535–3552. [Google Scholar] [CrossRef]
- Ikram, M.; Umar, E.; Raza, A.; Haider, A.; Naz, S.; Ul-Hamid, A.; Haider, J.; Shahzadi, I.; Hassan, J.; Ali, S. Dye Degradation Performance, Bactericidal Behavior and Molecular Docking Analysis of Cu-Doped TiO2 Nanoparticles. RSC Adv. 2020, 10, 24215–24233. [Google Scholar] [CrossRef]
- Genç, A.; Lütfi Öveçoğlu, M. Characterization Investigations during Mechanical Alloying and Sintering of Ni–W Solid Solution Alloys Dispersed with WC and Y2O3 Particles. J. Alloys Compd. 2010, 508, 162–171. [Google Scholar] [CrossRef]
- Mandal, G.; Srinivas, V.; Rao, V.V. Observation of Enhanced Positive Magnetoresistance at Low Temperatures in Ni0.8Fe0.2/C Granular Composites. J. Alloys Compd. 2010, 504, 110–114. [Google Scholar] [CrossRef]
- Yeganeh, M.; Shahtahmasebi, N.; Kompany, A.; Karimipour, M.; Razavi, F.; Nasralla, N.H.S.; Šiller, L. The Magnetic Characterization of Fe Doped TiO2 Semiconducting Oxide Nanoparticles Synthesized by Sol–Gel Method. Phys. B Condens. Matter. 2017, 511, 89–98. [Google Scholar] [CrossRef]
- Huot, J.; Ravnsbæk, D.B.; Zhang, J.; Cuevas, F.; Latroche, M.; Jensen, T.R. Mechanochemical Synthesis of Hydrogen Storage Materials. Prog. Mater. Sci. 2013, 58, 30–75. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal Nanoparticles Synthesis: An Overview on Methods of Preparation, Advantages and Disadvantages, and Applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Ghasaban, S.; Atai, M.; Imani, M. Simple Mass Production of Zinc Oxide Nanostructures via Low-Temperature Hydrothermal Synthesis. Mater. Res. Express 2017, 4, 035010. [Google Scholar] [CrossRef]
- Machmudah, S.; Widiyastuti, W.; Prastuti, O.P.; Nurtono, T.; Winardi, S.; Wahyudiono; Kanda, H.; Goto, M. Synthesis of ZrO2 Nanoparticles by Hydrothermal Treatment; American Institute of Physics: College Park, MD, USA, 2014; pp. 166–172. [Google Scholar] [CrossRef]
- Ravnsbæk, D.B.; Nickels, E.A.; Černý, R.; Olesen, C.H.; David, W.I.F.; Edwards, P.P.; Filinchuk, Y.; Jensen, T.R. Novel Alkali Earth Borohydride Sr(BH4)2 and Borohydride-Chloride Sr(BH4)Cl. Inorg. Chem. 2013, 52, 10877–10885. [Google Scholar] [CrossRef] [PubMed]
- Ley, M.B.; Ravnsbæk, D.B.; Filinchuk, Y.; Lee, Y.-S.; Janot, R.; Cho, Y.W.; Skibsted, J.; Jensen, T.R. LiCe(BH4)3Cl, a New Lithium-Ion Conductor and Hydrogen Storage Material with Isolated Tetranuclear Anionic Clusters. Chem. Mater. 2012, 24, 1654–1663. [Google Scholar] [CrossRef]
- Dorey, R. Routes to Thick Films, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Manjunath, K.; Reddy Yadav, L.S.; Jayalakshmi, T.; Reddy, V.; Rajanaika, H.; Nagaraju, G. Ionic Liquid Assisted Hydrothermal Synthesis of TiO2 Nanoparticles: Photocatalytic and Antibacterial Activity. J. Mater. Res. Technol. 2018, 7, 7–13. [Google Scholar] [CrossRef]
- Reddy, C.V.; Babu, B.; Reddy, I.N.; Shim, J. Synthesis and Characterization of Pure Tetragonal ZrO2 Nanoparticles with Enhanced Photocatalytic Activity. Ceram. Int. 2018, 44, 6940–6948. [Google Scholar] [CrossRef]
- Mitchell, E.; De Souza, F.; Gupta, R.K.; Kahol, P.K.; Kumar, D.; Dong, L.; Gupta, B.K. Probing on the Hydrothermally Synthesized Iron Oxide Nanoparticles for Ultra-Capacitor Applications. Powder Technol. 2015, 272, 295–299. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, H.; Tan, S.; Gao, J.; Fu, Y.; Liu, Z. Hydrothermal Synthesis of Ag Nanoparticles on the Nanocellulose and Their Antibacterial Study. Inorg. Chem. Commun. 2019, 100, 44–50. [Google Scholar] [CrossRef]
- Phan, H.T.; Haes, A.J. What Does Nanoparticle Stability Mean? J. Phys. Chem. C 2019, 123, 16495–16507. [Google Scholar] [CrossRef]
- Thanh, T.D.; Chuong, N.D.; Van Hien, H.; Kim, N.H.; Lee, J.H. CuAg@Ag Core–Shell Nanostructure Encapsulated by N-Doped Graphene as a High-Performance Catalyst for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2018, 10, 4672–4681. [Google Scholar] [CrossRef] [PubMed]
| Stability Parameter | Role |
| Size | The preservation of nanostructured-material dimension during the synthesis is critical to the overall performance of electrocatalyst in fuel-cell application. Their size contributes greatly to catalytic performance in the electrodes. |
| Aggregation | There is a need to preserve primary nanoparticles upon collisions to ensure the production of a stable material and avoid aggregation. The actual surface potential, chemical identity, structure, and functionality of surface chemistry are vital for nanostructured electrocatalysts. A large surface area to volume ratio ensures high catalytic activity. |
| Surface Chemistry | |
| Metal/Metal Oxide composition | The core’s unchanged chemical identity and crystallinity during an experiment or relevant time period influence the nature of nanostructure-electrocatalyst materials. |
| Shape | The preservation of local structure and the radius of curvature at an atomic and nanoscales level are essential for the stability of the nanostructured material obtained. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omeiza, L.A.; Abdalla, A.M.; Wei, B.; Dhanasekaran, A.; Subramanian, Y.; Afroze, S.; Reza, M.S.; Bakar, S.A.; Azad, A.K. Nanostructured Electrocatalysts for Advanced Applications in Fuel Cells. Energies 2023, 16, 1876. https://doi.org/10.3390/en16041876
Omeiza LA, Abdalla AM, Wei B, Dhanasekaran A, Subramanian Y, Afroze S, Reza MS, Bakar SA, Azad AK. Nanostructured Electrocatalysts for Advanced Applications in Fuel Cells. Energies. 2023; 16(4):1876. https://doi.org/10.3390/en16041876
Chicago/Turabian StyleOmeiza, Lukman Ahmed, Abdalla M. Abdalla, Bo Wei, Anitha Dhanasekaran, Yathavan Subramanian, Shammya Afroze, Md Sumon Reza, Saifullah Abu Bakar, and Abul Kalam Azad. 2023. "Nanostructured Electrocatalysts for Advanced Applications in Fuel Cells" Energies 16, no. 4: 1876. https://doi.org/10.3390/en16041876
APA StyleOmeiza, L. A., Abdalla, A. M., Wei, B., Dhanasekaran, A., Subramanian, Y., Afroze, S., Reza, M. S., Bakar, S. A., & Azad, A. K. (2023). Nanostructured Electrocatalysts for Advanced Applications in Fuel Cells. Energies, 16(4), 1876. https://doi.org/10.3390/en16041876









