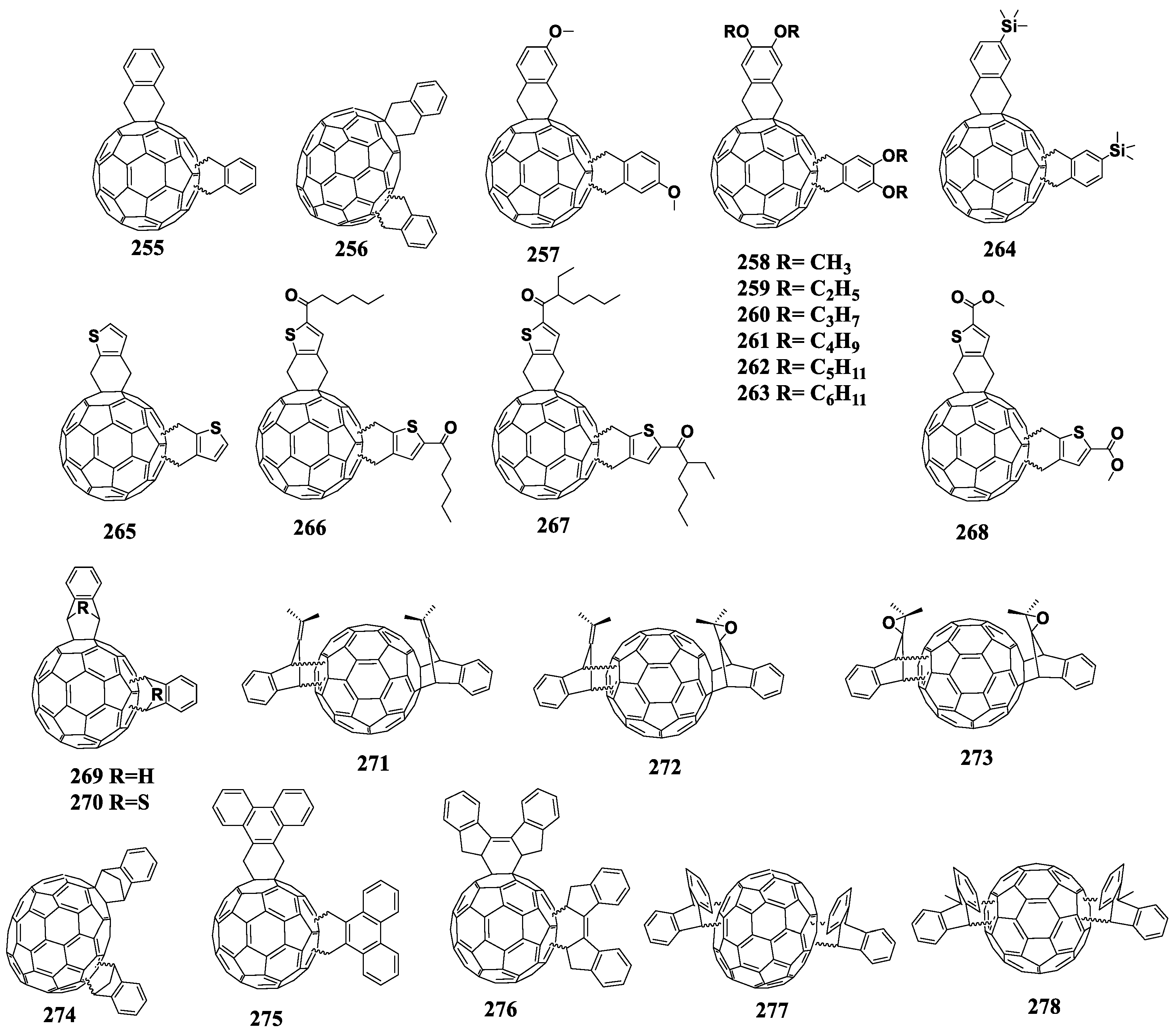Abstract
Organic solar cells (OSCs) represent a promising emerging photovoltaic technology offering such benefits as light weight, mechanical flexibility, semitransparency, environmental friendliness and aesthetic design of solar panels. Furthermore, organic solar cells can be produced using scalable and high-throughput solution-based printing and coating technologies, which are expected to lead to very low product costs. Fullerene derivatives have been used as acceptor materials in virtually all efficient organic solar cells for more than two decades, following the demonstration of the first proof-of-concept devices in the middle of 1990s. Still, the power conversion efficiencies of fullerene-based organic solar cells became stuck at around 12% due to the suboptimal optoelectronic properties of conventional fullerene acceptors. Therefore, the latest efficiency records (>18%) for organic solar cells were set using different types of non-fullerene acceptor (NFA) materials with tailorable properties. However, NFA materials appeared to be very sensitive to light, thus impairing the operational stability of OSCs. On the contrary, there is growing evidence that rationally designed fullerene-based acceptors enhance the photostability of conjugated polymers and also NFAs, when used in ternary blends. Hence, a renaissance of fullerene-based materials is currently expected in the context of their use in multicomponent organic solar cells (e.g., as stabilizers) and also lead halide perovskite solar cells, where they play an important role of electron transport materials. The success in both of these applications requires the tunability of optoelectronic characteristics of fullerene derivatives. In particular, electron affinity of the fullerene cage has to be reduced in many cases to match the energy levels of other absorber material(s). Herein, we present a systematic review of different strategies implemented to reduce the acceptor strength of the fullerene derivatives and the results of their performance evaluation in OSCs with model conjugated polymers. Particular attention is paid to correlations between the chemical structure of organic addends and their influence on the electronic properties of the fullerene core. We believe this review would be valuable to researchers working on the rational design of new fullerene-based materials with tailored properties for photovoltaic and other electronic applications.
1. Introduction
An annual increase in global energy consumption associated with the depletion of fossil fuels such as coal, natural gas, and oil, points to the need to develop new methods of electricity generation, transportation, and storage. Solar radiation is the most powerful alternative energy source. The energy that the Sun annually transmits to Earth is approximately seven thousand times higher than the current energy demand of the mankind. Solar cells represent the most direct way to utilize the sunlight energy by converting it to electricity. Currently, silicon-based solar cells represent the mainstream photovoltaic (PV) technology and account for >90% of the total solar panel market. Silicon solar cells deliver a relatively high power conversion efficiency (PCE) of nearly 27% [1] and have a long operational lifetime of more than 20 years [2,3]. However, the production of crystalline silicon solar cells is an extremely energy intensive process, while the resulting solar panels are fragile, heavy, and still quite expensive.
Organic solar cells represent a highly promising alternative photovoltaic technology, which could be complementary to silicon solar panels. Flexible, lightweight, aesthetically designed, and low-cost organic solar panels could be produced using efficient roll-to-roll printing and coating technologies [4,5,6,7]. It is remarkable that just a single industrial printing line can produce more than 1000 m2 of organic solar panels per minute. However, the power conversion efficiency (PCE) of organic solar cells is considerably lower than that of crystalline silicon PV devices, now reaching 18% in the best small-area laboratory samples [8,9,10]. The main limitation for improving the efficiency of OSCs is related to considerably higher open circuit voltage (VOC) losses of ca. 0.7–0.8 V as compared to the crystalline silicon (0.35 V), gallium arsenide (0.25 V), or thin-film chalcogenide (0.55 V for CdTe) photovoltaic cells [11]. This could be an intrinsic property of organic semiconductors related to the intra- and intermolecular vibrational disorder [12].
Nevertheless, theoretical modelling results suggest that PCE of >20% could be achieved in organic solar cells with a single p-n junction [13,14]. Since the theoretical limit is yet to be reached, many researchers are actively working to increase the light conversion efficiency in organic solar cells. One of the most promising approaches to solve this problem is the directed design of new photoactive n- and p-type semiconductor materials with desired properties. Significant progress has been made in the development of p-type electron donor materials and diverse non-fullerene acceptors (NFAs) have been emerging in the recent years.
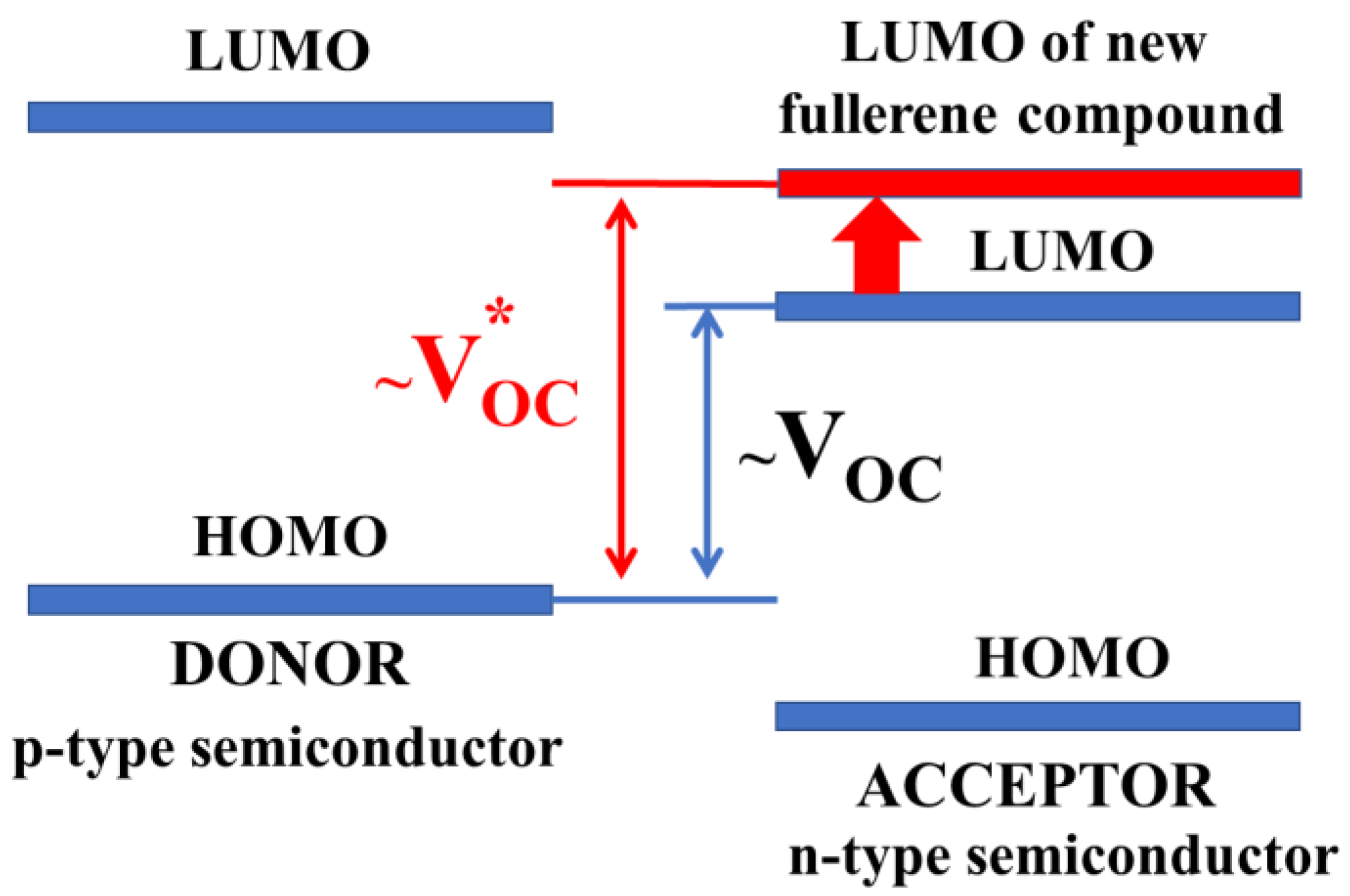
A somewhat decreased interest in the fullerene-based electron acceptor materials is associated, in particular, with the difficulty of tuning their optical and electronic characteristics. For example, fullerene derivatives [60]PCBM (phenyl-C61-butanoic acid methyl ester) and [70]PCBM (phenyl-C71-butanoic acid methyl ester) have been used for several decades as acceptor components for organic solar cells regardless of the structure and properties of the electron donor material. However, in most cases, acceptors with reduced electron affinity (relative to [60]PCBM and [70]PCBM) are required to achieve high open-circuit voltages and PCE (Figure 1).

Figure 1.
Scheme of the boundary orbitals of the p-type and n-type semiconductor materials in a bulk heterojunction photovoltaic cell. Increasing VOC with an increase in the LUMO energy of the fullerene derivative is shown.
Thus, the theory predicted an almost twofold increase in the efficiency of organic solar cells based on poly(3-hexylthiophene) (P3HT) when using fullerene derivatives with a significantly reduced electron affinity as electron acceptor materials [15]. A decrease in the electron affinity for the fullerene derivative is associated with an increase in the energy of the lowest unoccupied molecular orbital (LUMO). As can be seen from Figure 1, an increase in the LUMO energy of the fullerene derivative leads to a proportional increase in the maximum achievable VOC in the PV cell and, therefore, should lead to an increase in the efficiency.
In recent years, significant progress has been made in the synthesis of new fullerene-containing materials with reduced electron affinity. This review features only the fullerene-based materials whose low electron affinity (relative to [60]PCBM and/or [70]PCBM) has been confirmed by experiments, particularly the electrochemistry data. For convenience of analysis, all the described compounds are classified according to the type of organic addends attached to the fullerene framework.
2. Structure of Organic Solar Cells
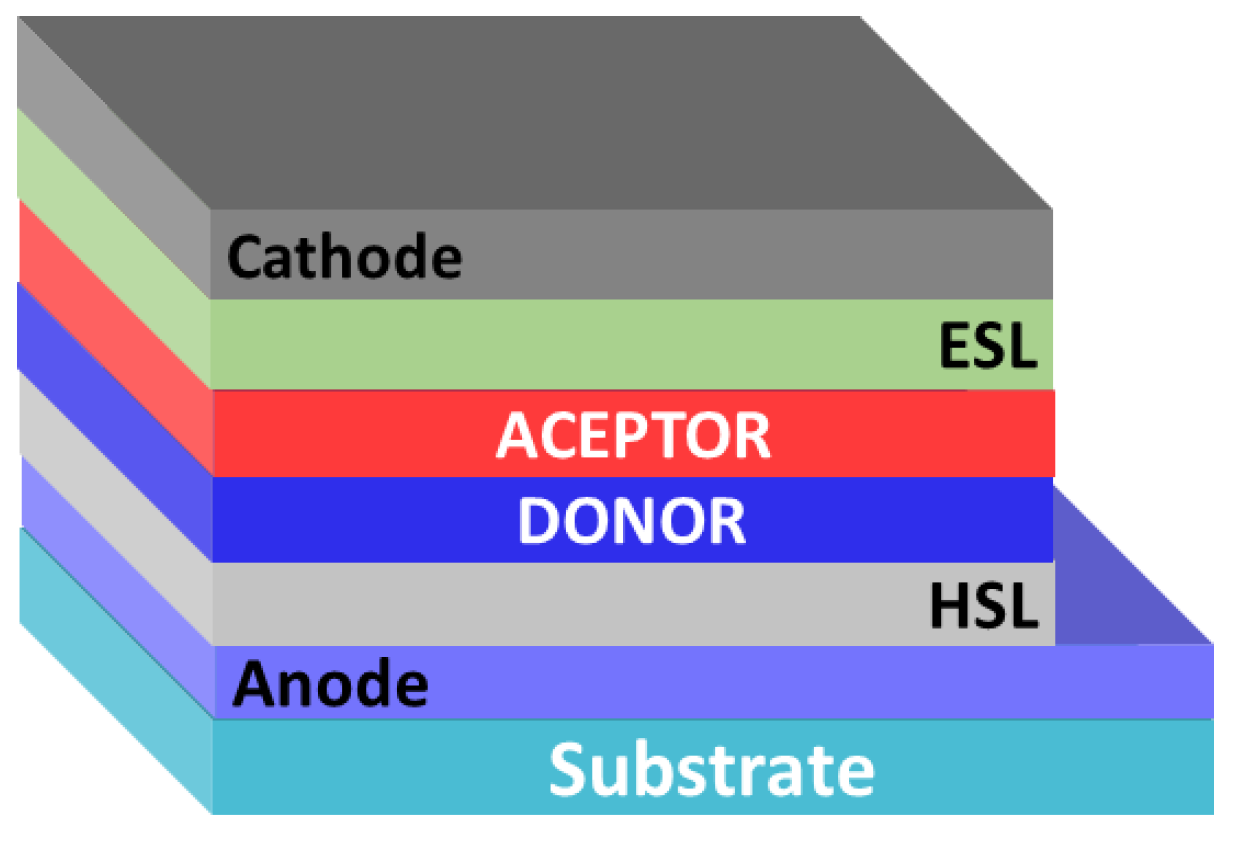
In 1986, C. Tang demonstrated the first bilayer organic solar cell [16]. As a result of the layer-by-layer deposition of electron donor (copper phthalocyanine) and electron acceptor (perylenediimide derivative) materials, the so-called lateral p-n heterojunction was formed. The presence of such a heterojunction made it possible to obtain the device with a record (for that time) PCE of about 1%. Note that, in general, the structures of all photovoltaic cells with a lateral heterojunction include an anode, a hole-selective layer (HSL), layers of electron donor and electron acceptor materials, an electron-selective layer (ESL), and a cathode (Figure 2).
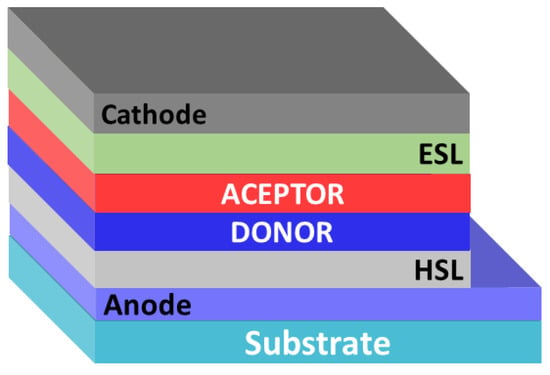
Figure 2.
Architecture of organic solar cells with a planar p-n heterojunction.
The presence of buffer layers makes it possible to selectively deliver charge carriers to the electrodes and to reduce their recombination [17]. The disadvantages of bilayer organic solar cells include the limited area of the heterojunction. Due to the short exciton diffusion lengths in organic semiconductor materials typically being within 5–13 nm only, a thin boundary layer contributes to photocurrent generation [18]. This means that the energy of the most part of the photons absorbed in the active layer of the solar cell dissipates in the form of heat, which negatively affects the PCE. Nevertheless, the bilayer structure of organic solar cells is a convenient model for studying the basic principles of their operation.
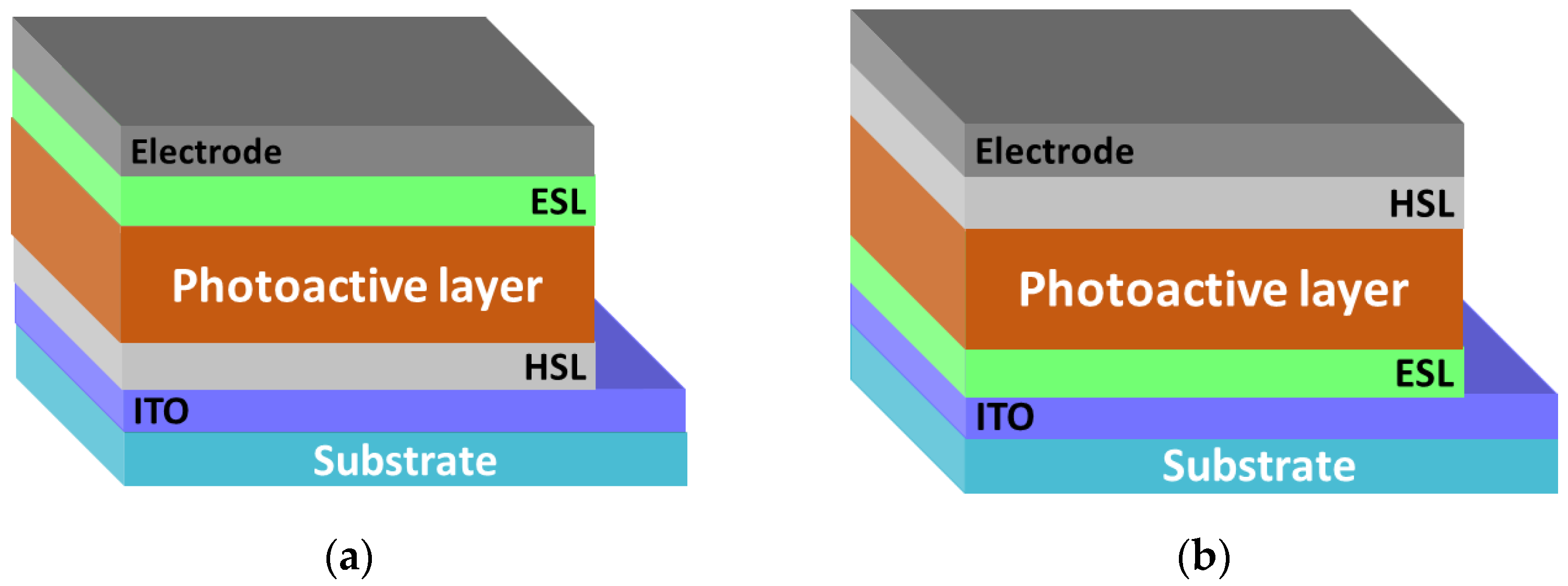
A significant increase in the efficiency of organic solar cells has been achieved by using a mixture of donor and acceptor materials as an active layer. In this case, the three-dimensional interpenetrating nanoscale networks of domains with hole and electron conductivity occurring in the active layer form a bulk heterojunction [19,20,21]. In the resulting nanoscale networks, the generation and transport of charge carriers to the electrodes is efficiently carried out. The bulk heterojunction makes it possible to increase the contact area between the phases of the donor and acceptor materials by several orders of magnitude in contrast to the lateral heterojunction, which ensures an almost 100% quantum efficiency of photoinduced charge separation [22]. Bulk heterojunction solar cells could be assembled in standard and inverted configurations. Due to good conductivity and optical characteristics of mixed indium-tin oxide (ITO), it is most commonly used as a transparent electrode. In the standard configuration (Figure 3a), ITO is coated with HSL, which is commonly represented by a PEDOT:PSS polymer or thin oxide films (for example, MoO3). A photoactive layer is formed on the top, which is a composite of donor and acceptor materials, and then ESL (for example, titanium oxide) is formed [23]. Finally, an electron-collecting electrode is deposited atop the ESL using vacuum deposition of metals with a low work function (calcium, barium, aluminum).
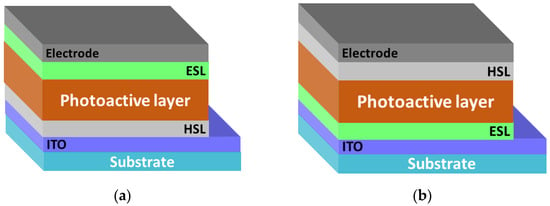
Figure 3.
(a) Bulk heterojunction organic solar cells: standard configuration with the top electron-collecting electrode and (b) inverted configuration with the top hole-collecting electrode.
In the inverted configuration, the bottom transparent electrode is the electron-collecting (Figure 3b). The use of a polymer buffer layer [24] or a transparent layer of metal oxide (for example, zinc oxide, titanium oxide) [25,26] as an ESL leads to the modification of the work function of the electrode material and the formation of the selective contact with the acceptor component in the photoactive layer. The HSL (PEDOT:PSS or a thin layer of, e.g., molybdenum oxide) is applied over the active layer, and then the electrode is formed from a high work function material (silver or gold) that is stable in air. High-efficiency organic solar cells are manufactured on the basis of both standard and inverted configurations [27,28]. Note that the inverted configuration of the solar cell has a number of advantages: the possibility of scalable manufacturing and enhanced stability with respect to environmental stress factors due to the absence of a low work function electrode.
3. Operation of Organic Solar Cells
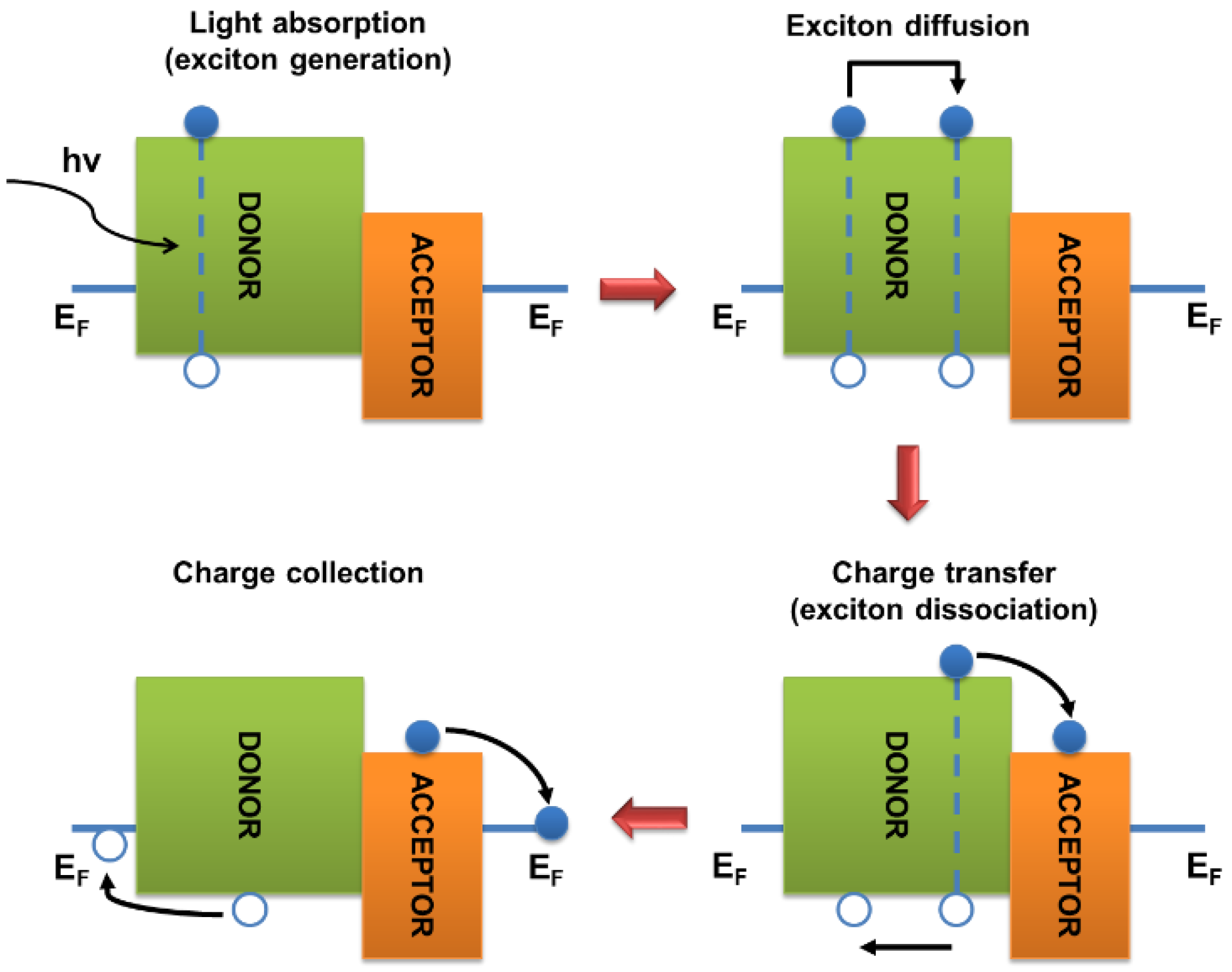
Figure 4 shows a simplified mechanism of solar energy conversion in organic photovoltaic cells using light absorption by a donor component as an example. As a result of the photon absorption by a material of the active layer (in this case, by the donor component), the molecule accomplishes a transition from the ground to excited state D*, i.e., the photon energy is used to transfer an electron from the HOMO to LUMO. Excitons are formed and diffuse in the semiconductor material. An important parameter is the exciton diffusion length, which defines the characteristic distance that an exciton can overcome before recombination with a probability of 62% [18]. When the phase boundary between the donor and acceptor components is reached, the exciton can dissociate into charge carriers if the difference between the LUMO energies of the active layer components is higher than the binding energy of the exciton. Many conflicting data can be found in the literature regarding the minimum difference between the energies of the LUMO of donor and acceptor materials, which is necessary for the efficient separation of excitons into free charge carriers. For the solar cells using the fullerene derivatives as n-type semiconductors, this difference should be about 0.1–0.3 eV [29,30]. During the dissociation of excitons formed in the donor material, an electron from D* is transferred to the LUMO of the acceptor molecule. A hole remains on the HOMO of the donor, so the molecule acquires a positive charge.
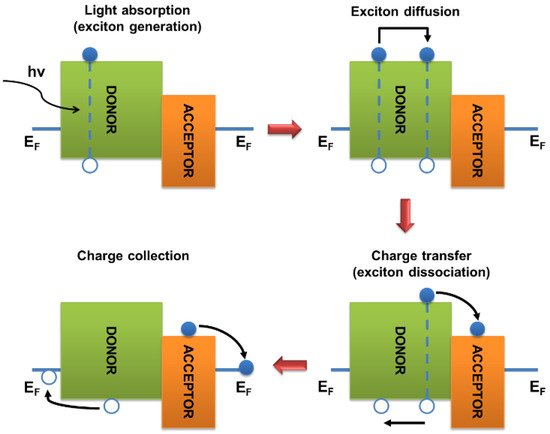
Figure 4.
Mechanism of light energy conversion in organic solar cells using light absorption by the donor component as an example.
In the case of the generation of A* excitons in an acceptor material, their dissociation results in the electron transition from the HOMO of the donor to the HOMO of the acceptor. At the HOMO level of the donor, a “hole” appears, i.e., the molecule acquires a positive charge, whereas the acceptor molecule gains an additional electron and negative neat charge. Thus, free charge carriers are formed. Subsequently, the charge carriers diffuse through appropriate materials (electrons in the acceptor phase and holes in the donor phase) to the opposite cell electrodes. It should be emphasized that the simplified scheme using the HOMO–LUMO boundary orbitals does not explain all the details of the charge generation process. In particular, it should be noted that the energy levels of the donor and acceptor components can change in the near-boundary regions due to the influence of charge-selective interlayers, electrode materials, and other factors [31]. The processes occurring during the operation of organic solar cells have been described in more detail in several reviews [32,33].
4. Main Parameters of Photovoltaic Cells
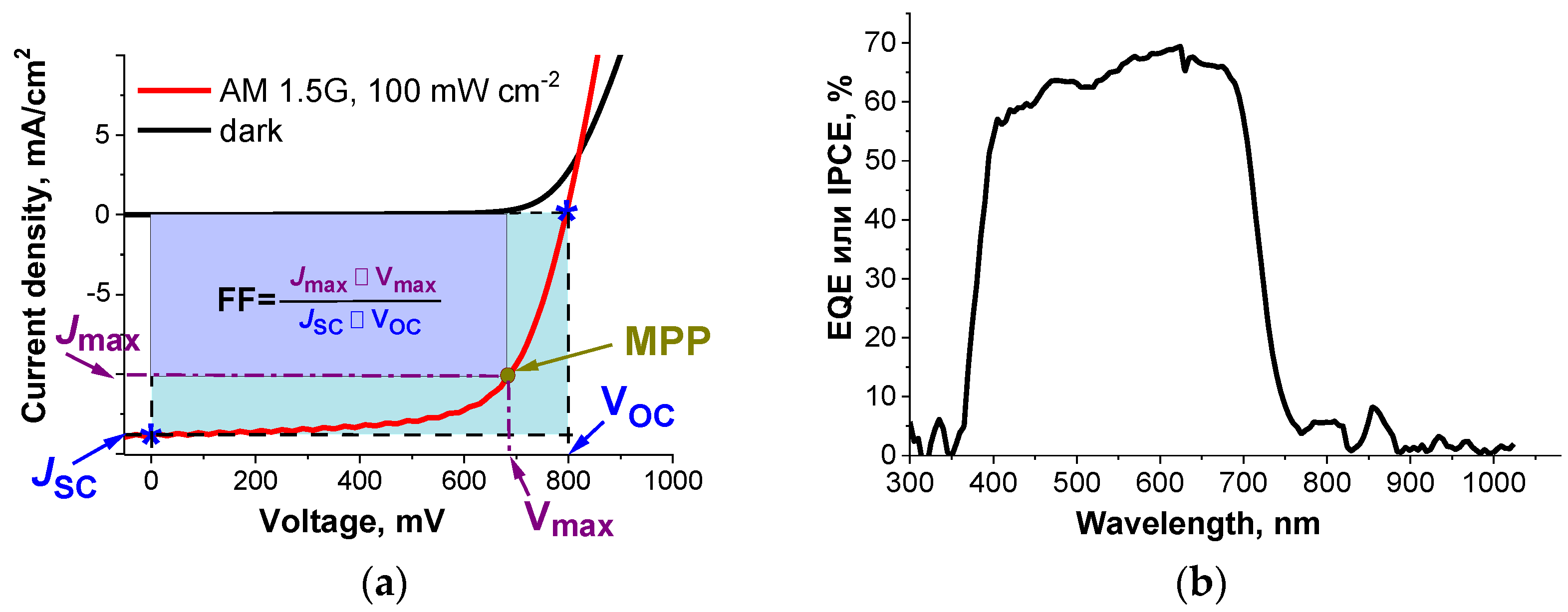
The parameters of solar cells are extracted from their current–voltage (J–V) curves obtained under standard conditions when irradiated with the light simulating the AM1.5G spectrum with an incident power of 100 mW cm−2 at a temperature of 25 °C (Figure 5a).

Figure 5.
(a) Current–voltage curve and (b) the external quantum efficiency (EQE) spectrum of an organic solar cell.
Three basic parameters of a photovoltaic cell are determined from the J–V curves: short circuit current density (JSC), open circuit voltage (VOC), and fill factor (FF).The short circuit current density (JSC) is defined as a point on the J–V curves at zero voltage. To increase JSC, one should decrease the band gap of the photoactive layer materials, increase the light absorption coefficient, or increase the film thickness. In addition, to reduce recombination losses, it is necessary to ensure high mobility of charge carriers and optimal phase separation of the donor and acceptor components of the photoactive layer.
Open circuit voltage (VOC) is defined as a point on the J–V curves at zero current density. In an optimized solar cell, the electrodes form an ohmic contact with buffer charge-transport layers enabling a barrier-free transfer of charge carriers from the photoactive layer. In this case, VOC directly depends on the difference between the energies of the HOMO of the donor and the LUMO of the acceptor material minus the losses associated with the radiative and nonradiative recombination of holes and electrons in the device [34,35].
The fill factor (FF) defines the shape of the J–V curve and is calculated according to Equation (1)
where Jmax and Vmax are the current density and voltage at the maximum power point (MPP). The value of the fill factor is affected by the processes of charge transport in the photoactive layer and at the interlayer boundaries. Therefore, the fill factor can be increased by increasing the mobility of charge carriers in the photoactive layer and removing barriers for their transfer to the corresponding electrodes. It is also important to ensure balanced (i.e., as close as possible) mobilities of holes and electrons in the photoactive layer and the efficiency of their extraction onto the electrodes.
The power conversion efficiency or efficiency (ɳ) is calculated using Equation (2)
where Plight is the power of the incident light from an external source, and Pel is the electrical power provided by the photovoltaic cell.
Another important characteristic of photovoltaic cells is the external quantum efficiency spectrum (EQE or IPCE), which shows how efficiently the solar cell converts incident light to electrical energy at a given wavelength (Figure 5b). The principle of the EQE measurement is based on irradiating the sample with the monochromatic light and recording the electric current generated by the device. The ratio of the number of electrons reaching the electrodes of the solar cell to the total number of photons incident on the device gives the EQE value at a given wavelength. The integration of the EQE spectrum of a photovoltaic cell against the standard solar emission spectrum AM1.5G provides an accurate estimation of the short circuit current density of the device compared to the data obtained from J–V curves recorded using a solar simulator.
5. Evolution of Organic Semiconductor Materials for OSCs
One of the first reports on the photovoltaic cells using a conductive polymer and C60 fullerene appeared in 1993 [36]. Unfortunately, pristine fullerene has very low solubility in organic solvents, which makes processing fullerene-polymer composite films challenging. In this regard, the fullerene has to be modified with organic addends enabling good solubility of the resulting materials in organic solvents. Historically, the fullerene derivative [60]PCBM was first developed as a precursor for preparing fullerene derivatives with antiviral properties [37], then became the most widely used n-type semiconductor material in organic electronics. Similarly, a functional derivative of C70 fullerene known as [70]PCBM was synthesized and its improved optical properties (broader absorption spectrum) were demonstrated. Both [60]PCBM and [70]PCBM were the most important fullerene-based acceptor materials playing a crucial role in the development of OSCs for more than two decades.
With respect to the electron donor materials, initially polyphenylenevinylenes such as MEH-PPV and MDMO-PPV and later polythiophenes such as P3HT were the main running horses at the first stage of OSCs development. The efficiencies of 2–3% and 3–5% could be achieved, respectively, in photovoltaic cells based on the composites of MDMO-PPV and P3HT with [60]PCBM [38,39]. Historically, the next promising material was the conjugated copolymer known as PCDTBT (poly[N-9′-heptadecanyl-2,7-carbazole-alt-5,5-(4′,7′-di-2-thienyl-2′,1′,3′-benzothiadiazole)]) [40,41]. The PCE of photovoltaic cells based on composite [70]PCBM/PCDTBT reached 7% with an internal quantum efficiency approaching 100% [22,42,43,44].
Further evolution of electron donor components brought up hundreds or even thousands of promising materials [45,46]. Among them, a series of polymers comprised of benzodithiophene and thieno [3,4-b]thiophene blocks is worth noting [46]. Among them, PTB7 showed a decent efficiency of 9.5% [47] The devices based on conjugated polymers PffBT4T-2OD and PBTff4T-2OD in combination with the fullerene derivative [70]PCBM demonstrated PCE >10% [48,49].
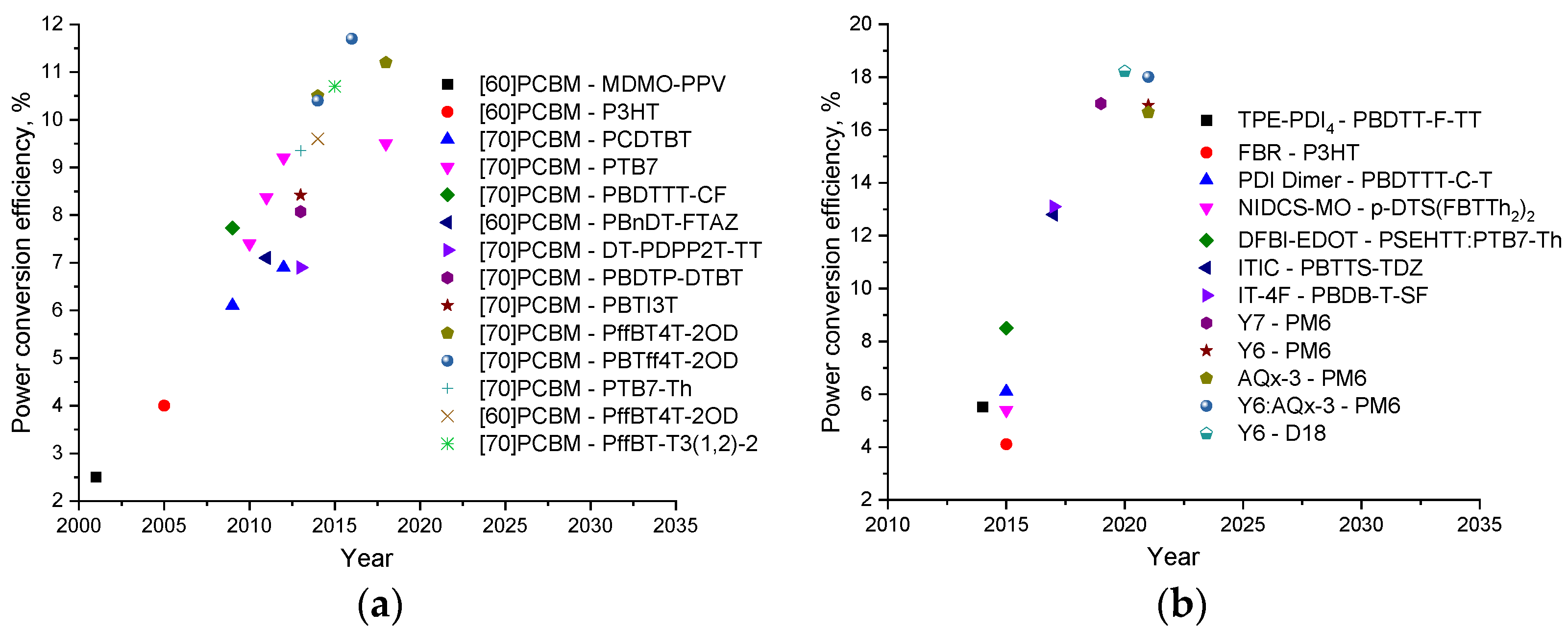
Thus, significant progress has been made in the design of new p-type semiconductor materials, especially conjugated polymers, delivering power conversion efficiencies of 10–12% in OSCs using fullerene-based acceptor materials (Figure 6 and Figure 7) [22,28,45,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62].
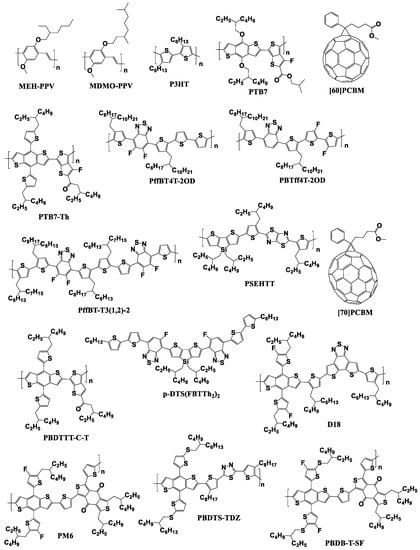
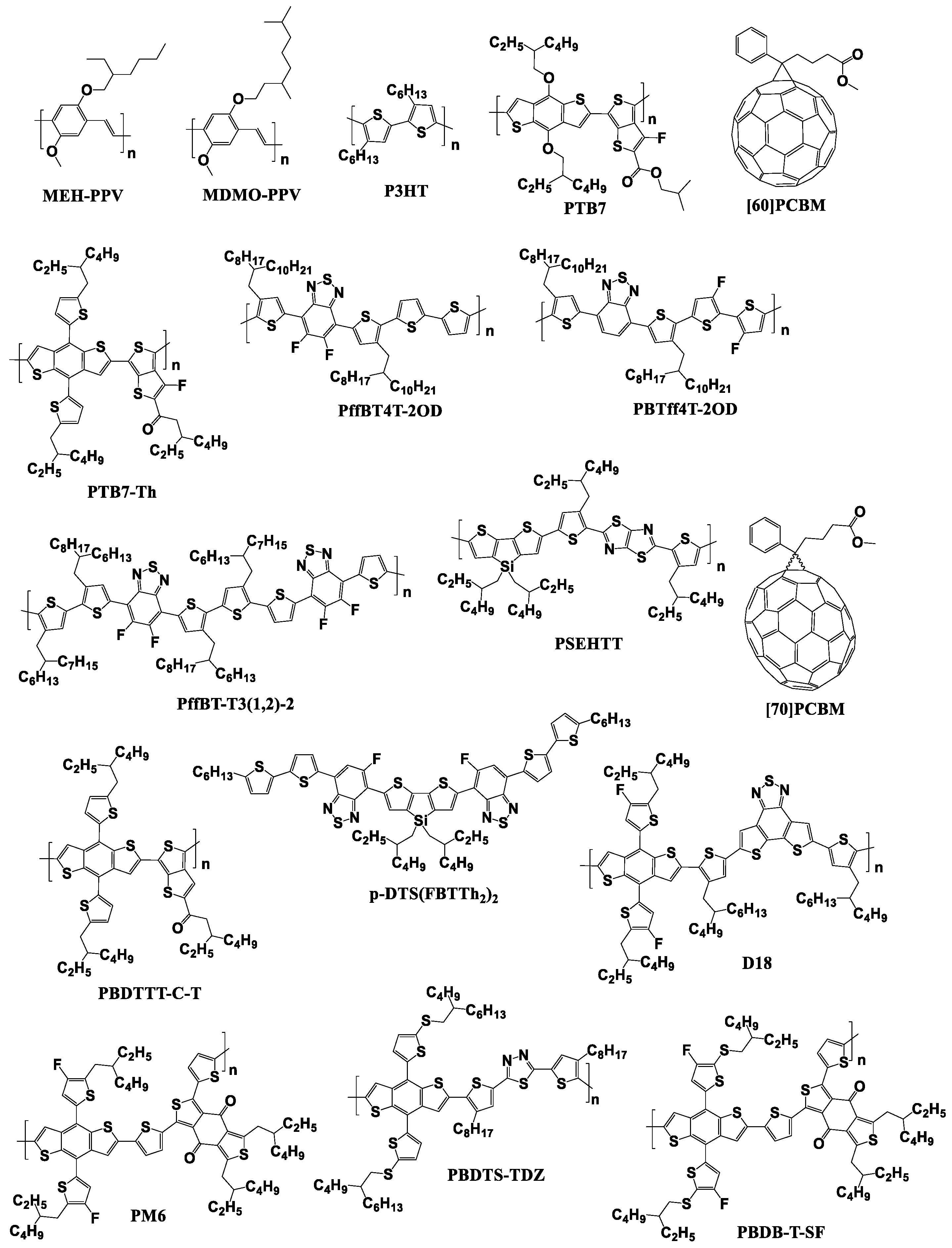
Figure 6.
Molecular structures of some of the fullerene-based acceptors and electron donor materials playing a historical role in the development of OSCs.
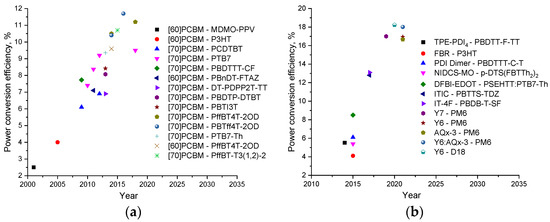
Figure 7.
Evolution of organic solar cells with (a) fullerene- and (b) non-fullerene acceptor materials.
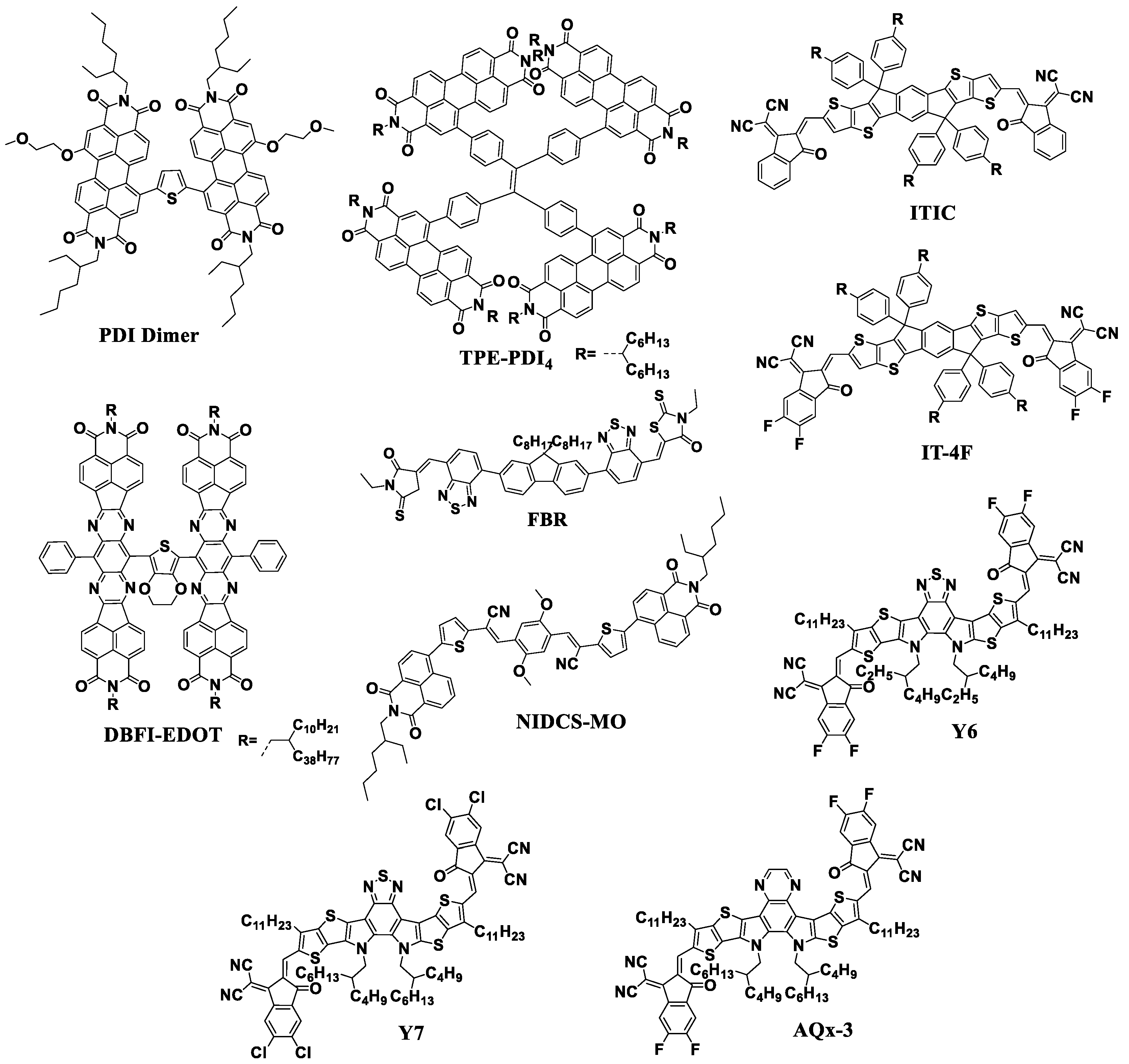
A further increase in the efficiency of OSCs up to the current ~18% has mainly been associated with the development of new non-fullerene acceptor (NFA) materials with tailored properties. To date, it has been possible to produce OSCs with PCE going beyond 18% using such NFAs as Y6 and AQx-3 [10,63]. Figure 8 shows the molecular structures of some NFAs that have proven to be promising n-type semiconductor materials for the high-efficiency OSCs [64,65,66,67,68,69,70,71].
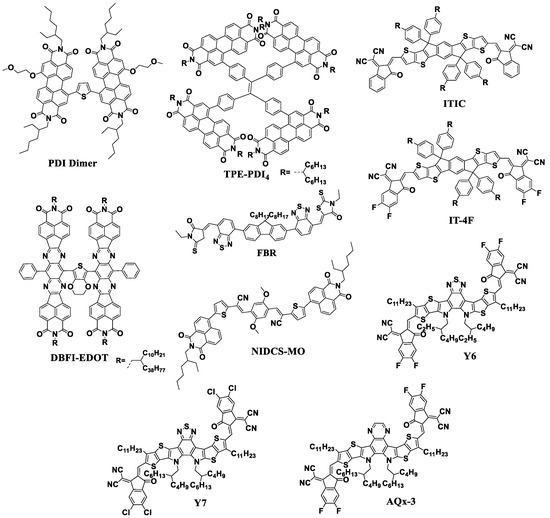
Figure 8.
Molecular structures of different types of NFAs: PDI Dimer, TPE-PDI4, FBR, NIDCS-MO, ITIC, IT-4F, Y6, Y7, and AQx-3.
The disadvantages of highly efficient NFAs include their complex and multistage synthesis resulting in a very high material cost. Furthermore, the presence of exocyclic double bonds adversely affects the photostability of these materials [72,73,74,75]. On the contrary, several reports have featured new fullerene derivatives, which were shown to be resistant to the light-induced photodimerization and could slow down the photodegradation of NFAs when used in ternary absorber blends [76,77,78]. In that context, fullerene-based acceptor materials could again become valuable cutting-edge components of highly efficient and stable OSCs.
6. Design of Fullerene Derivatives with Reduced Electron Affinity
To tailor the optoelectronic characteristics of the fullerene-based acceptors to match promising electron donor materials, some new fullerene derivatives with improved properties, in particular reduced electron affinity as compared to [60]PCBM, have been developed. The use of these fullerene derivatives as n-type semiconductors enables an increase in the VOC of the corresponding photovoltaic cells, but this does not ultimately lead to an increase in efficiency. An important criterion in the design of new fullerene derivatives is not only a reduced electron affinity but also the molecular structure and physicochemical properties, e.g., solubility of the resulting fullerene derivatives in organic solvents [79]. On the one hand, to obtain optimal characteristics of solar cells, the solubility of the fullerene derivative should be close to that of the donor component. On the other hand, when developing new fullerene derivatives with reduced electron affinity, the electron transport properties of the materials should also be taken into account. Unmodified fullerene C60 is one of the best n-type semiconductors, whereas the introduction of organic solubilizing addends has an adverse effect on the electron transport properties of the material. For example, the mobility of charge carriers in the [60]PCBM film is two to three orders of magnitude lower than the mobility of electrons in thin films of unmodified [60]fullerene [80,81]. Thus, the design of fullerene derivatives with reduced electron affinity for highly efficient organic solar cells is a non-trivial task and many parameters should be taken into account.
Information on the use of the fullerene derivatives with reduced electron affinity as electron acceptor materials for organic solar cells will be summarized and analyzed further in this review. Figure 9 shows the molecular structures of the p-type semiconductor materials that were used in photovoltaic cells in combination with the fullerene derivatives considered in this review.
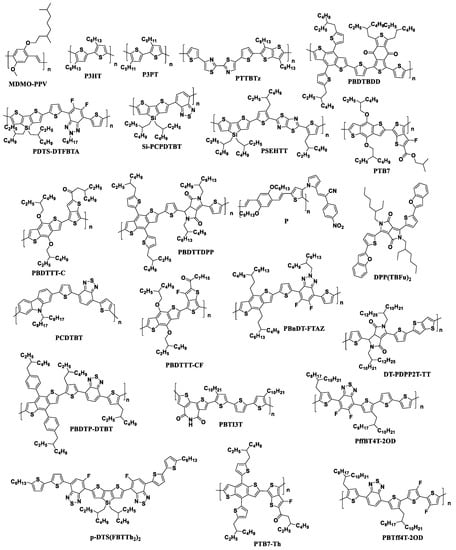
Figure 9.
Molecular structures of electron donor materials used in combination with the fullerene derivatives with reduced electron affinity.
6.1. Cyclopropane Fullerene Derivatives
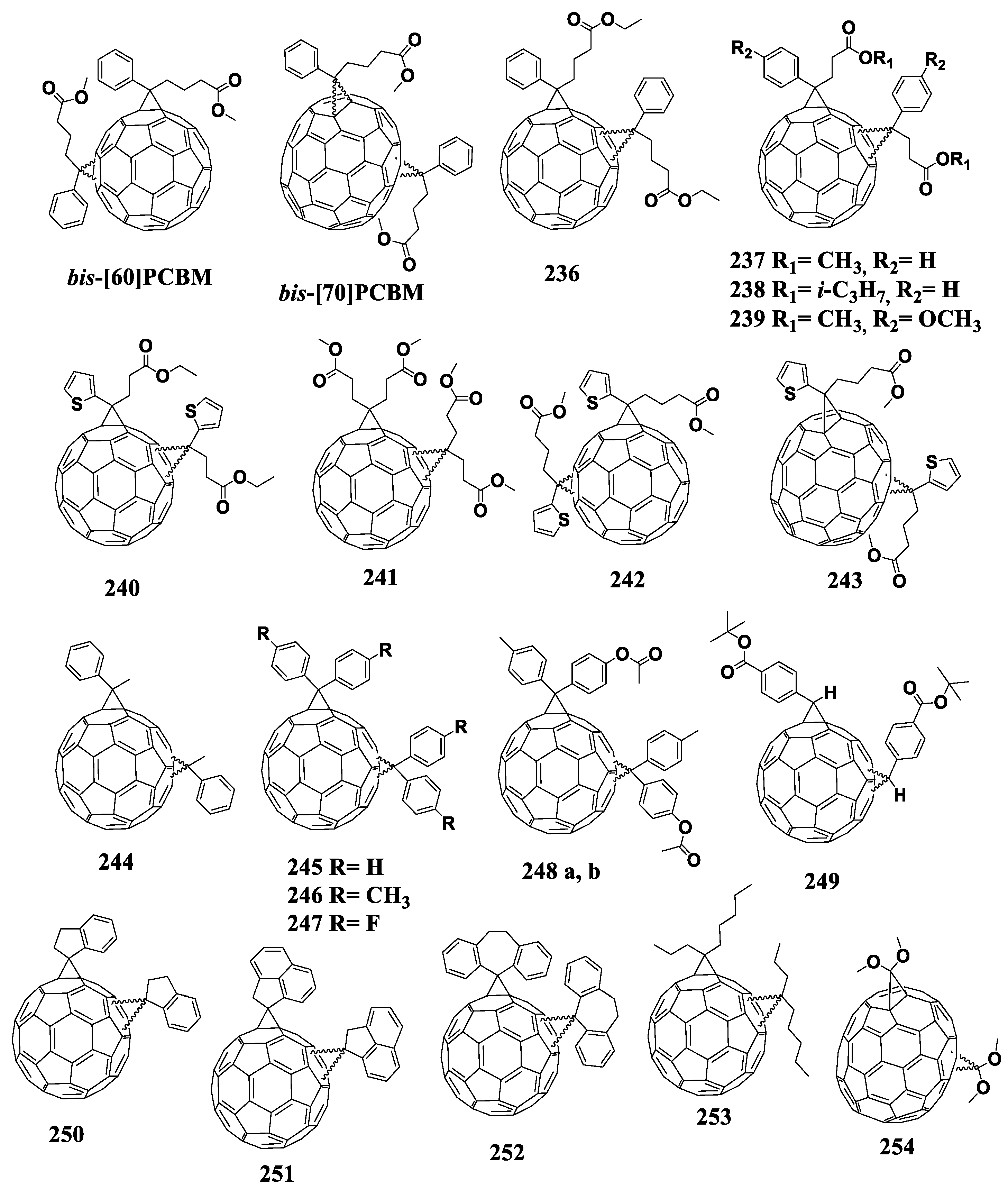
A common method for introducing a cyclopropane fragment at the fullerene cage is the Hummelen–Woodle method, which is the reaction of fullerenes with diazo compounds generated in situ from tosylhydrazones of aromatic aldehydes or ketones using sodium methoxide [37]. As a result of the reaction, fulleroid with an open 6-5 bond is first formed, which then isomerizes at a temperature of 180 °C to form a closed methanofullerene. The application of the Hummelen–Woodl method is limited by the formation of side products such as polyadducts, when the organic addend is attached to the fullerene framework several times, which must be carefully separated from the target fullerene derivative (monoadduct). Figure 10 shows the molecular structures of the fullerene derivatives in which the organic addend is attached to the fullerene framework through the cyclopropane fragment.
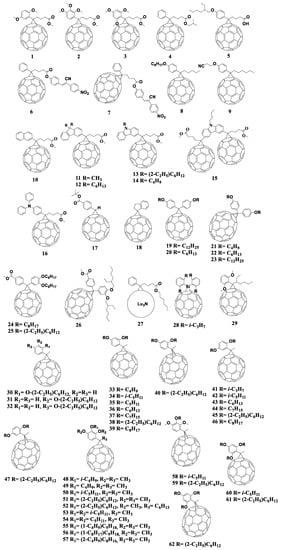
Figure 10.
Molecular structures of the cyclopropane derivatives of fullerenes with reduced electron affinity.
One of the methods to obtain new fullerene derivatives is the modification of the known molecule [60]PCBM with functional groups. Kooistra et al. introduced methoxy and thiomethyl electron donor substituents into the phenyl ring of [60]PCBM [82]. The introduction of thiomethyl groups had almost no effect on the first reduction potential of the resulting compounds. On the contrary, the corresponding modification with methoxy groups (compounds 1–3) noticeably affected the electronic properties of the formed fullerene derivatives since the first reduction potentials were cathodically shifted with respect to that of [60]PCBM. On the one hand, the authors noted that the ortho-position of the electron-donating group in the phenyl ring exerted a small effect on the electronic properties of the molecule despite the proximity of the oxygen or sulfur lone electron pair for the through-space interaction with the fullerene π-system. On the other hand, attention was drawn to a significant influence of the number of electron-donating substituents on the electronic properties of the molecule. When going from two to three methoxyl substituents, a significant decrease in the first reduction potential was observed. Unfortunately, the paper did not present all the characteristics of the solar cells based on the designed compounds, but the presented data indicated an increase in the VOC of the solar cells fabricated from the fullerene derivatives with a reduced electron affinity.
In the [60]PCBM molecule, the ester group was also modified. It was shown that the ester group did not affect the electronic properties of the fullerene derivatives but allowed one to control the solubility of the resulting compounds [79,83]. However, the photovoltaic cell based on compound 4 showed an increase in VOC by 40 mV against the reference device with [60]PCBM [84]. It is likely that this increase in VOC was related to the manufacturing process of the devices and is not due to the electronic effect of the alkyl substituent. Note that other characteristics of the solar cell were inferior as compared to the reference system. In the work of Yang et al., the alkoxy substituent was introduced into the [60]PCBM molecule in the para-position of the phenyl ring, and the ester group, on the contrary, was hydrolyzed to the carboxyl group [85]. Compound 5 had a lower electron affinity relative to [60]PCBM, which was explained by the authors as due to the inductive effect of the alkoxy substituent. The photovoltaic cells based on the fullerene derivative 5 showed VOC of ca. 40 mV higher than the reference cells with [60]PCBM, while other characteristics remained comparable.
An interesting example is the modification of the ester group by replacing the alkyl substituent with the 4-nitro-4’-hydroxy-α-cyanostilbene fragment (compounds 6 and 7) [86,87,88]. According to the authors, the presence of this fragment not only improved the optical properties and solubility of the fullerene derivative, but also shifted the reduction potential of the fullerene derivatives to the cathodic region as compared to that of [60]PCBM. The authors attributed this shift to the presence of two electron-withdrawing groups, nitro group and cyano group, which increased the electron acceptor strength of the molecule but, at the same time, paradoxically, they caused a cathodic shift of the reduction potentials and reduced the electron affinity of the fullerene cage. It should be noted that, unlike the authors of this article, most researchers, when developing new fullerene derivatives, try to reduce the electron affinity by introducing electron-donating substituents rather than electron-withdrawing ones. Therefore, this work does not inspire confidence, although the authors presumably managed not only to improve the electronic properties of the fullerene derivatives but also enhance the characteristics of solar cells based on them.
Another approach to modifying the [60]PCBM molecule is to replace the ester group with alkyl residue. An example is compound 8 in which the electron-donating alkoxy group in the para-position of the phenyl ring affects the electronic properties of the molecule [89].
However, the devices based on acceptor material 8 showed a comparable VOC and a deterioration in other parameters relative to the reference cells. Zhang et al. increased the dielectric constant of the fullerene derivative in comparison with that of [60]PCBM by introducing a polar substituent (fragment of 3-hydroxypropanenitrile (compound 9)) at the phenyl ring [90]. It was shown that the dielectric constant actually increased from 3.9 to 4.9. However, use of fullerene derivative 9 in solar cells in combination with PCDTBT polymer provided just a small increase in VOC (20 mV) and in PCE (~1%) in comparison to the reference system. Note that this is practically the only publication where the fullerene monocyclopropane adduct was studied in combination with the promising amorphous and stable conjugated polymer PCDTBT.
In a number of papers, the phenyl fragment in [60]PCBM was replaced by naphthyl, fluorenyl, or substituents based on carbazole or triphenylamine (compounds 10–16) [91,92,93,94,95]. In all cases, there was a slight change in the electronic properties of molecules (decreasing electron affinity), which was mainly attributed by the authors to the electron-donating effect of the introduced fragments. Note that the devices based on the 10/P3HT and 15/P3HT composites showed not only an increase in VOC, but also a slight increase in the PCE. Moreover, the devices based on acceptor materials 11 and 16 in combination with P3HT showed a high resistance to heat, which opens up new possibilities for the development of stable organic solar cells.
Usually, the electrochemical data for the fullerene derivatives are in good agreement with the VOC parameter of the corresponding solar cells [82]. An exception is compound 17 [96]. The cyclic voltammogram of fullerene derivative 17 showed anodic shift of all redox waves by 30 mV as compared to [60]PCBM. However, the photovoltaic cells based on this material showed VOC exceeding the values delivered by the reference system by 30–50 mV. The authors argued that the result obtained cannot be accidental, as confirmed by numerous experiments, but they gave no possible explanation for this phenomenon in the work.
Of great interest is compound 18 with a reduction potential lower by 60 mV as compared to that of [60]PCBM [97]. The reason for a decrease in the electron affinity is not entirely clear since there are no electron-donating substituents in the molecule. Furthermore, the authors did not investigate it in solar cells apparently due to poor solubility.
The approach based on the introduction of two para-alkoxyphenyl substituents into the cyclopropane fragment annelated to the fullerene cage (fullerene derivatives 19–23) is worth noting [98,99,100,101]. According to the authors, the presence of two phenyl rings can favorably influence the ordering of molecules in solid films due to intermolecular π–π interactions. Compound 19 worked well as an acceptor material in combination with the P3HT polymer. Thus, the VOC of the devices based on the 19/P3HT composite increased by 100 mV as compared to the reference system. The authors attributed the increase in voltage to the improvement in the dark diode characteristic rather than to an increase in the acceptor LUMO energy. By reducing the length of para-alkoxy substituents from C12 to C6 (fullerene derivative 20), JSC was increased as compared to the solar cells based on compound 19. The VOC of the OSCs based on compound 20 increased by 100–130 mV as compared to the reference device, which was also observed for fullerene derivative 19. The first reduction potentials of the cyclopropane derivatives of [70]fullerene 21–23 turned out to be identical to that of the reference molecule [70]PCBM. Despite some increase in the VOC in comparison to the reference system, other characteristics of the solar cells based on compounds 21–23 in combination with the small electron donor molecule DPP(TBFu)2 (Figure 8) deteriorated.
Singh et al. synthesized compounds 24–26 containing the ester group in the para-position of one of the phenyl rings and two alkoxy groups in the meta- and para-positions of the others [102,103]. A decrease in the electron affinity of the designed materials and an increase in VOC and efficiency of the corresponding devices in comparison with the reference cells based on [60]PCBM and [70]PCBM were demonstrated.
An unusual method for lowering the electron affinity of the fullerene derivatives is the introduction of metal atoms into the fullerene framework. Ross et al. used endometallofullerene 27 as an acceptor material in combination with P3HT [104]. The electrochemical studies of the molecule showed a significant shift of the reduction potentials to the cathodic region by 280 mV against [60]PCBM. The VOC of the solar cells based on fullerene derivative 27 increased by 180 mV as compared to the reference device comprised of [60]PCBM/P3HT composite. Nevertheless, the use of this approach is severely limited because of low availability of endometallofullerenes.
Unusual methods for increasing the LUMO energy of the fullerene derivatives include replacing the carbon atom with silicon at the vertex of the cyclopropane fragment annelated to the fullerene framework (compound 28) [105]. The electrochemical studies have revealed a shift of the first reduction potential by 130 mV to the cathodic region as compared to unmodified fullerene. Unfortunately, no solar cells based on this material have been reported yet.
In 2016, Matsumoto et al. studied the effect of the polarity of fullerene derivatives on the efficiency of the corresponding OSCs using P3HT polymer [106]. It was shown that the photovoltaic cells based on fullerene derivative 29 demonstrated the highest efficiency. Note that the introduction of two methoxyl groups into the positions of 2 and 6 of the phenyl substituent, which are in close proximity to the fullerene core, increased the LUMO energy of the compound in comparison with that of [60]PCBM.
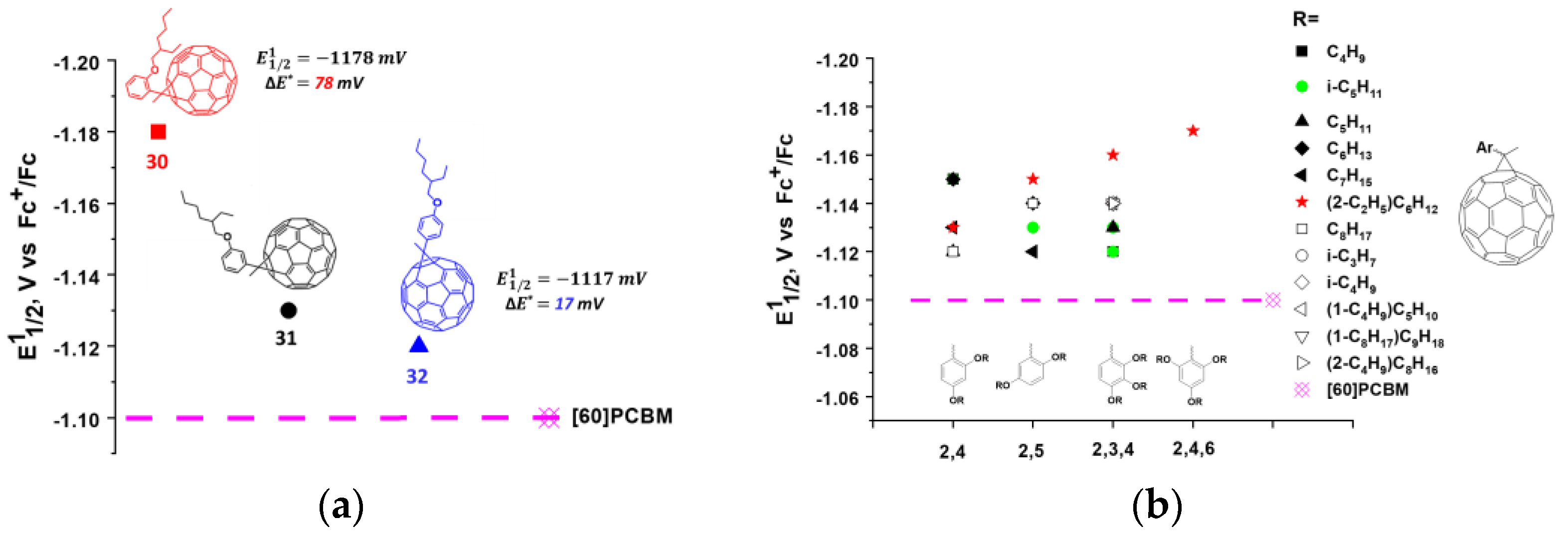
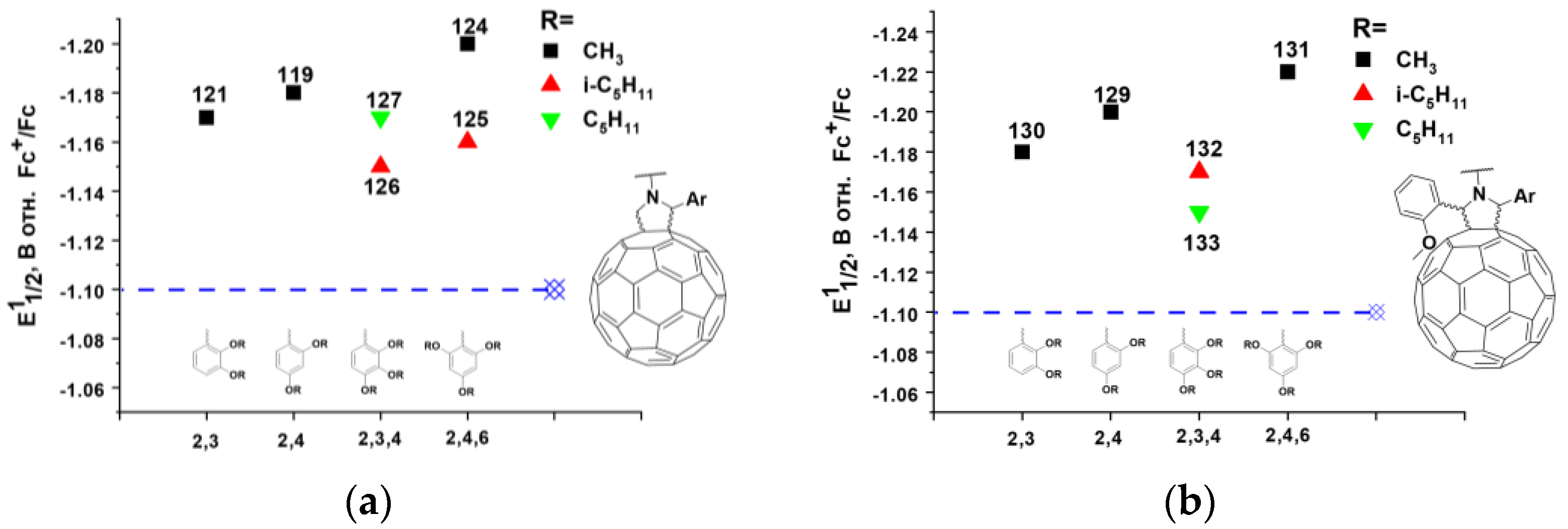
In our work, we synthesized an extensive series of fullerene monocyclopropane adducts (compounds 30–62) containing various electron-donor alkoxy groups (Figure 9). The effect of the position of the methoxyl group on the electronic properties of the molecule can clearly be seen from the obtained experimental data. Thus, the methoxy group in the para-position of the phenyl ring shifted the first reduction potential to the cathodic region by ~17 mV. Similar substitution in the meta-position led to a 32 mV shift, whereas the introduction of the same substituent in the ortho-position resulted in a 78 mV cathodic shift as compared to the potential of the [60]PCBM (Figure 11a). Thus, the methoxy group in the ortho-position of the phenyl substituent had the strongest effect on the electronic properties of the fullerene derivatives. We emphasized that the methoxy substituent in the ortho-position was in close proximity to the fullerene cage. An increase in the number of alkoxy groups in the phenyl fragment and their relative position did not result in a further substantial increase in the LUMO energy (Figure 11b). In most cases, the potential of the compounds increased slightly compared to that of fullerene derivative 30. This indicates that the number of alkoxy groups in the phenyl fragment and their relative positions do not significantly affect the electronic properties of the fullerene derivatives. The strongest effect on the electronic properties of the fullerene cage is caused by only one electron donor (alkoxy) group introduced into the ortho (2- or 6-) positions of phenyl substituent in the cyclopropane moiety of the fullerene derivatives.
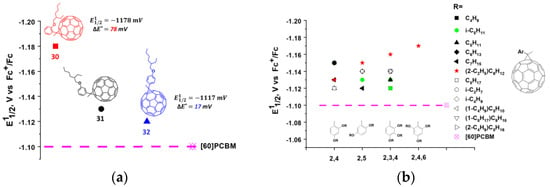
Figure 11.
(a) Dependence of the first reduction potentials (E11/2) obtained in solution on the position of the alkoxy substituent in the phenyl ring of methanofullerenes 30–32; (b) The effects of the positioning and number of alkoxy substituents in the phenyl fragment on reduction potentials of methanofullerenes.
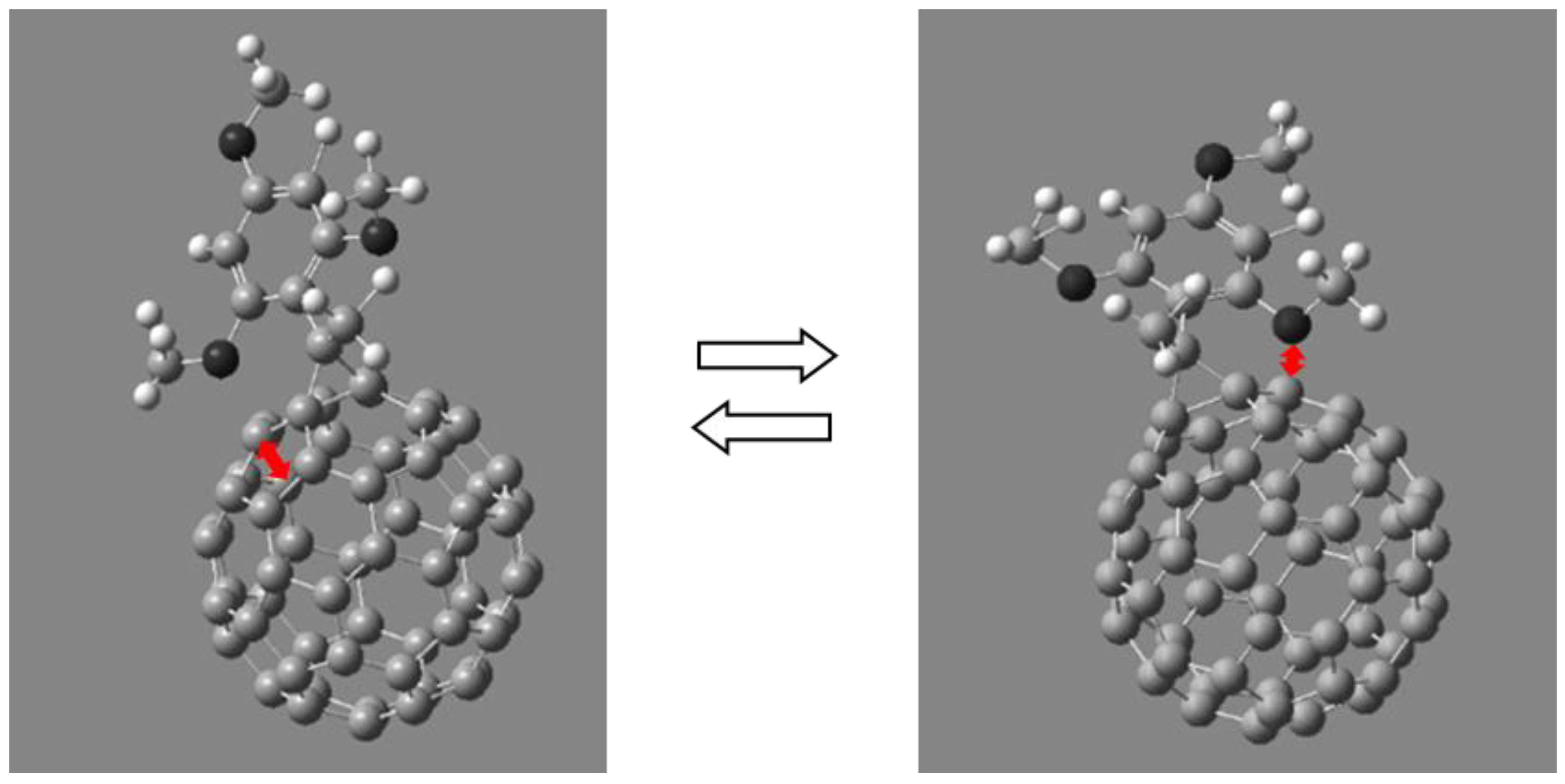
The results obtained confirm the assumption that electron-donor alkoxy groups interact with the fullerene π-system through the space rather than through a system of bonds. Moreover, such an interaction is possible only for alkoxy groups located in the positions of 2- and 6- of phenyl substituents in the cyclopropane fragment. Substituents located in other positions cannot efficiently interact with the fullerene framework due to larger distance between the lone electron pairs of the oxygen atom and the electronic system of the fullerene cage (Figure 12).
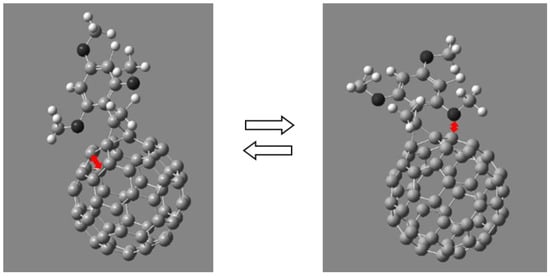
Figure 12.
Interaction of alkoxy groups at the positions of the 2- and 6-phenyl substituent in the cyclopropane fragment with the π-system of the fullerene framework.
An unexpected fact was the observation of a significant effect of the length and branching of alkyl chains on VOC and other characteristics of organic solar cells. Apparently, the improved photovoltaic properties of the fullerene derivatives with branched isoamyl and 2-ethylhexyl fragments are associated with their ability to form optimal morphology composites with conjugated polymers, which was confirmed by the atomic force and electron transmission microscopy data [107]. It was found that the composites based on fullerene derivatives 34, 50, 51, 58, and 60 and conjugated polymer P3HT showed a VOC of 80–90 mV higher than the reference system [60]PCBM/P3HT (Table 1). Unlike the vast majority of the aforementioned cyclopropane fullerene adducts, which were tested in OSCs mainly with P3HT, the fullerene derivatives 33, 34, 36–39, 42–46, 48, 50–53 were evaluated in combination with the promising conjugated polymer PCDTBT. Some of these fullerene derivatives showed a VOC of 100–180 mV higher as compared to the reference devices based on the [60]PCBM/PCDTBT composite (Table 1). The solar cells comprised of compounds 34, 42, 48, and 51 and PCDTBT outperformed analogous devices based on [60]PCBM not only with respect to VOC, but also in terms of efficiency, in particular for the 34/PCDTBT system. Note that the monocyclopropane adducts and other fullerene derivatives with reduced electron affinity typically do not show satisfactory characteristics in combination with PCDTBT and other electron donor copolymers. Apparently, the presented compounds are among the first fullerene-based materials with reduced electron affinity (relative to [60]PCBM), which provide high light conversion efficiencies in solar cells in combination with low band gap conjugated copolymers. In addition, the photovoltaic cells based on the fullerene derivative 51 and PTB7-Th polymer not only demonstrated enhanced open-circuit voltages, but also showed excellent thermal stability in contrast to the reference system [60]PCBM/PTB7-Th [108].

Table 1.
First reduction potentials and characteristics of the solar cells based on cyclopropane fullerene derivatives.
As can be seen from Table 1, modifying [60]PCBM or designing principally new electron-acceptor materials comprising a substituted cyclopropane fragment typically result in the cathodic shift of the reduction potential by 20–60 mV as compared to that of [60]PCBM. In some cases, the potential shifts by 200–380 mV (compounds 6 and 27). When using fullerene derivatives 6, 19, 20, and 27 as electron-acceptor materials, VOC of the fabricated solar cells was increased by 180, 100, 130, and 180 mV, respectively. In almost all cases, as the VOC of the photovoltaic cells increases, other characteristics deteriorate or remain unchanged as compared to the reference system. The approach of introducing metal atoms inside the fullerene sphere (compound 27) made it possible to increase both VOC and PCE of the corresponding devices. However, the use of such materials is challenging because of the low availability of the initial endometallofullerene.
Summarizing all of the aforementioned, we can conclude that methanofullerenes with reduced electron affinity represent promising n-type semiconductor materials for organic solar cells. The OSCs based on these materials demonstrate high VOC and PCE values in combination not only with the classical P3HT, but also in composites with the promising conjugated copolymer PCDTBT and PTB7-Th.
6.2. Pyrrolidinofullerenes
Pyrrolidinofullerenes are synthesized by [2+3]cycloaddition of azomethine ylides to the fullerene framework. The most common method (known as the Prato sarcosine method) is the reaction of N-substituted α-amino acids with carbonyl compounds and C60 [109]. The reaction by-products are polyadducts, i.e., compounds with several pyrrolidine addends on the fullerene cage.
Interestingly, the nitrogen atom of the pyrrolidine ring donates its electron density to the fullerene, which leads to an increase in the LUMO energy of pyrrolidinofullerenes [110]. In addition, there are indications in the literature that the substituents in the pyrrolidine ring can interact electronically with the fullerene skeleton [111]. Figure 13 shows the molecular structures of pyrrolidinofullerenes with reduced electron affinity.
Figure 13.
Molecular structures of pyrrolidinofullerenes with reduced electron affinity.
Compounds 63 and 64 contain the indole fragment, which was chosen by the authors because of its electron-donating properties [112,113,114]. The first reduction potentials of fullerene derivatives 63 and 64 are shifted to the cathodic region as compared to that of [60]PCBM by 80 and 110 mV, respectively. The VOC values of the photovoltaic cells are increased by 50 and 130 mV, respectively, whereas JSC is somewhat reduced, but, in general, the characteristics remain comparable to those of the reference cells. It is not entirely clear how the presence of an additional indole substituent attached to the nitrogen atom of the pyrrolidine ring in a molecule of the fullerene derivative affects the electronic properties of the compound. The distance between the indole fragment and fullerene cage is too large for the manifestation of the inductive effect or interaction through space. However, the devices based on similar acceptor material 65, prepared and studied by Wang et al., showed an increase in VOC by 70 mV compared to VOC of the reference devices [112].
A large series of pyrrolidinofullerenes 66–77 was prepared by Matsumoto et al. [115]. Solar cells based on the synthesized compounds showed an increase in VOC as compared to the reference cells with [60]PCBM. Unfortunately, the resulting pyrrolidinofullerenes were not studied by cyclic voltammetry. Nevertheless, the authors carried out a systematic analysis of the correlations between the molecular structure of the obtained compounds and the characteristics of the solar cells based on them. It was found that the position of the substituent in the phenyl ring, which is attached to the pyrrolidine fragment, significantly affects the characteristics of the solar cells. The devices based on compound 70 containing the ortho-methoxy group showed the best characteristics among all pyrrolidinofullerenes studied in this work and outperformed reference cells with [60]PCBM. An increase in VOC over the reference system with [60]PCBM was 77 mV, and the efficiency increased from 2.5% to 3.4%. Authors believe that the advantages of using compound 70 in solar cells are its stability under ambient conditions and easy preparation. Notably, in this work an increase in the efficiency of the solar cells was shown for the majority of the designed materials as compared to the efficiency of the reference devices, which, however, demonstrated an unusually low performance.
Compound 78 contains the thiophene fragment attached to the pyrrolidine ring [116]. The electrochemical study of the material showed a shift of the reduction potential to the cathodic region by 150 mV as compared to the reference [60]PCBM [117]. Despite encouraging results of the electrochemical study of fullerene derivative 78, the solar cells based on this derivative showed inferior characteristics as compared to the reference system based on the composite [60]PCBM and P3HT. The further modification of the compound by introducing the alkyl substituent at the β-position of thiophene (compound 79) led to an improved compatibility with the conjugated Si-PCPDTBT polymer [118]. Note that vast majority of the fullerene derivatives with reduced electron affinity are incompatible with donor-acceptor copolymers but work well in combination with the P3HT due to its regular structure and high crystallinity. In this case, the devices based on pyrrolidinofullerene 79 showed only a slight decrease in JSC, while VOC increased by 81 mV, and other characteristics were close to the parameters of the reference cells based on [60]PCBM. Thus, compound 79 is one of the first fullerene derivatives with reduced electron affinity compatible with conjugated copolymers other than P3HT.
Later, Yoshimura et al. synthesized compounds 80–84 assuming that the introduction of π-conjugated fragments into the pyrrolidine ring can affect the electronic properties of molecules [119]. The solar cells based on pyrrolidinofullerene 84 outperformed in terms of VOC the devices based on all other fullerene derivatives and reference [60]PCBM. Note also that the solar cells based on compounds 80–83 outperformed the reference [60]PCBM/P3HT cells in terms of efficiency. The introduction of the carbazole substituent (fullerene derivative 85) into the pyrrolidine ring increased both the LUMO energy of the molecule of the obtained compound and VOC of the corresponding solar cells [94].
Unfortunately, such a modification worsened the compatibility of the fullerene derivative with the conjugated polymer, so all other characteristics of the devices decreased in comparison with the reference system.
A very interesting study was carried out with compounds 86–87 [116]. The key idea was to attach bulky diarylfluorene addends to the pyrrolidinofullerene molecule, which would prevent strong intermolecular interactions of the fullerene derivative without a strong change in the electronic properties of the molecule. In addition, such a bulky substituent can be modified, for example, by introducing alkoxy groups. Unfortunately, despite a demonstrated reduced electron affinity, the solar cells showed unsatisfactory results.
Blanco et al. attached N-substituted phenothiazine to the pyrrolidine ring [120]. Although the photovoltaic devices based on compounds 88 and 89 showed an increase in VOC by 60–70 mV over the reference cells, the efficiency of the devices could not be improved. The PCEs of the solar cells based on N-phenyl-substituted pyrrolidinofullerenes 90 and 91 and P3HT are comparable to the parameters of the reference devices based on [60]PCBM [121]. In addition, pyrrolidinofullerenes 90 and 91 showed an acceptable performance in combination with the low band gap conjugated polymer PTB7: VOC values of the solar cells were 0.76 and 0.797 V, and the PCEs were 7.27 and 6.83%, respectively. The parameters of the control cell based on [60]PCBM were 0.74 V and 7.03%.
Karakawa et al. replaced the phenyl substituent with the benzyl fragment in pyrrolidinofullerenes 92–94 and studied the resulting fullerene derivatives in combination with the PTB7 polymer in photovoltaic devices [122]. VOC was increased for the solar cells based on these fullerenes, while the PCE was comparable to the characteristics of the reference [60]PCBM/PTB7 cells.
A large series of fullerene derivatives with N-methyl and N-phenyl moieties (95–102) was obtained by Liang et al. [123]. It was shown that the devices based on N-methyl-substituted pyrrolidinofullerenes (compounds 99–102) gave very low efficiency (below 1%), while the devices based on the N-phenyl-substituted fullerene derivatives (materials 95–98) showed a significantly improved efficiency ranging from 2.38 to 3.19%. An analysis of the presented data indicates a distinct positive effect of the phenyl substituent at the nitrogen atom on the photovoltaic characteristics of pyrrolidinofullerenes.
The modification of the phenyl fragment attached to the pyrrolidine ring with two trifluoromethyl groups (compound 103) made it possible to fabricate solar cells with record-breaking characteristics for pyrrolidinofullerenes [124]. In particular, the efficiencies of the devices based on the 103/PTB7 and 103/PTB7-Th composites were 6.8 and 8.6%, respectively. The control cells based on [60]PCBM showed efficiencies of 6.2 and 7.9%, respectively. Higher PCEs of the devices based on compound 103 were due to enhanced JSC and VOC, whereas the reasons for the observed increase in VOC when using the fullerene derivative with three strong electron-withdrawing CF3 substituents remain unclear.
In 2017, Karakawa et al. introduced fluorine atoms into the N-phenyl substituent (fullerene derivatives 104–108) [125]. The best photovoltaic cells based on pyrrolidinofullerene 107 in combination with the donor material PTB7 outperformed the control devices based on [60]PCBM/PTB7. Compound 109 with the anthracene substituent in the pyrrolidine ring showed an increase in VOC, but other parameters remained comparable to those of the [60]PCBM/P3HT system [126].
Yoshimura et al. studied 2,5-diarylpyrrolidinofullerenes (compounds 110–115) as an acceptor material [127]. The first reduction potentials of the compounds were slightly shifted to the cathodic region versus [60]PCBM. Among all the studied diarylpyrrolidinofullerenes the device based on the 113/P3HT composite showed the best parameters.
In our work, an extensive series of pyrrolidinofullerenes containing electron-donating alkoxy groups (compounds 116–133) was synthesized and investigated [128]. The electrochemical properties of the designed fullerene derivatives in solution were studied using cyclic voltammetry. It was found that the first reduction potentials of the compounds were shifted to the cathodic region as compared to that of [60]PCBM, and in some cases (compounds 124, 129, and 131) exceeded the reduction potentials of the monocyclopropane fullerene derivatives with attached fragments of alkoxy-substituted acetophenones 30–62 (Table 1). The photovoltaic characteristics of the pyrrolidinofullerenes with reduced electron affinity are summarized in Table 2.

Table 2.
First reduction potentials and characteristics of the solar cells based on pyrrolidinofullerenes.
Note that compounds 124, 125, and 129 contain in their structure two electron-donating alkoxy groups at the positions of the 2- and 6- of the phenyl rings, and compound 131 incorporates even three such substituents. The introduction of additional electron-donating substituents into the positions of the 3-, 4-, and 5-phenyl rings has no noticeable effect on the electronic properties of the fullerene derivatives (Figure 14a,b). Thus, electronic through-space interactions of electron-donating alkoxy groups and fullerene cage strongly influence electronic properties of pyrrolidinofulleres in a similar way as described above for substituted methanofullerenes.
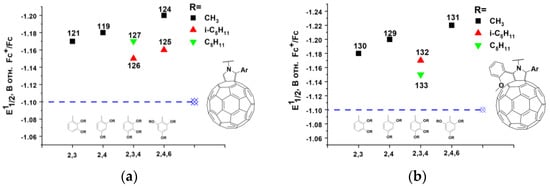
Figure 14.
Dependence of E11/2 in solution on the position of the alkoxy substituents in the phenyl rings of (a) 2-arylsubstituted and (b) 2,5-diarylsubstituted pyrrolidinofullerenes.
It should be noted that a fairly large group of pyrrolidinofullerenes with low electron affinity was prepared and studied. As can be seen from Table 2, in the most cases, an increase in VOC of the photovoltaic cells was 40–70 mV. The photovoltaic cells based on compounds 63, 66–77, 80–83, 109, 112, and 114 outperformed in terms of PCE reference cells based on [60]PCBM and P3HT. Compatibility with conjugated copolymers Si-PCPDTBT, PTB7, and PTB7-Th was demonstrated for pyrrolidinofullerenes 79 and 90–108. In particular, the characteristics of the devices based on fullerene derivatives 90, 103–105, and 107 exceeded those of the corresponding reference systems. The record enhancement in VOC values of the photovoltaic cells with pyrrolidinofullerenes was obtained for the devices based on the 131/P3HT composite. The VOC of the devices reached 772 mV, which is 173 mV higher than the values of the reference system with [60]PCBM and also 48 mV higher than the VOC of OSCs based on bis[60]PCBM (Table 2). The most important fundamental problem was solved for compound 131 used as an example: the electron affinity of the fullerene cage was significantly reduced (even more than in bis[60]PCBM with two organic addends on the fullerene cage) as a result of the addition of only one organic addend incorporating electron-donating substituents capable of electronic through-space interactions with the fullerene skeleton [129]. In addition, pyrrolidinofullerenes 119 and 121 were shown to be compatible with the stable conjugated polymer PCDTBT, and the solar cells based on the 121/PTB7-Th composite demonstrated outstanding thermal stability. Thus, great prospects appear for the use of such fullerene derivatives as n-type semiconductor components in organic solar cells. Summarizing the aforementioned, we can conclude that the further development of new pyrrolidinofullerenes with reduced electron affinity represents a promising research direction.
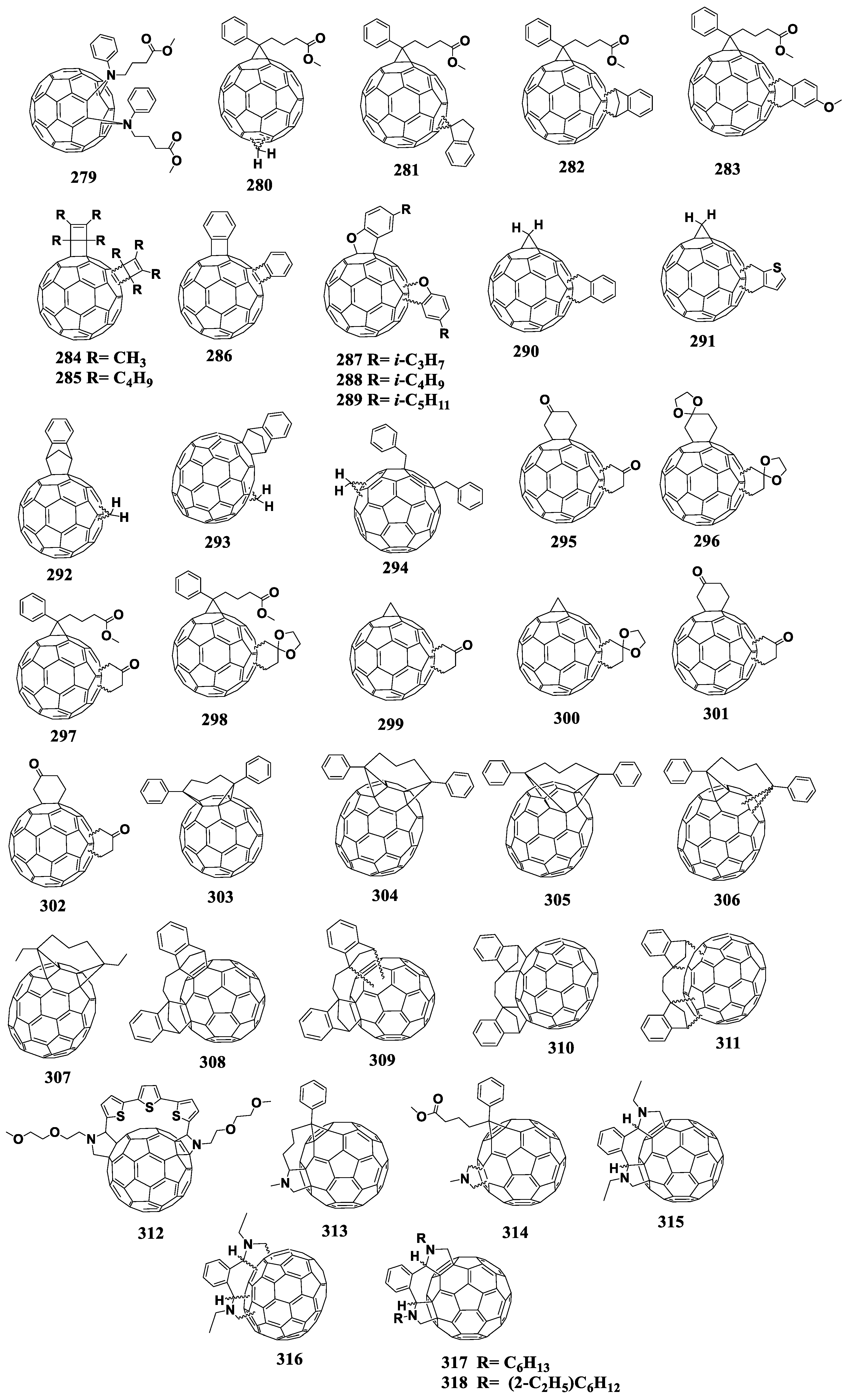
6.3. Cyclohexane Fullerene Derivatives
In 2013, the cyclohexane derivatives of fullerenes with reduced electron affinity were synthesized and studied in photovoltaic devices [130]. The target cyclohexane adducts were prepared in two stages. First, fullerene was introduced in the Diels–Alder reaction with a small excess of silicon-containing butadiene. Then the resulting product was decomposed with silica gel to form a fullerene derivative containing the attached cyclohexanone moiety. Finally, the resulting precursor reacted in the presence of TiCl4 with alcohols or thiols introduced into the reaction medium to form fullerene cyclohexane adducts 134–145 [131,132]. At the stage of [2+4]cycloaddition, the formation of polyadducts was possible. Figure 15 shows the molecular structures of the cyclohexane fullerene derivatives with reduced electron affinity. The resulting materials were characterized by thermal stability in an inert gas atmosphere up to 350 °C. It should be noted that the solar cells based on compound 137 showed comparable PCEs to the cells based on [60]PCBM/P3HT. Moreover, 137 was found to be compatible with the PTB7 conjugated copolymer.
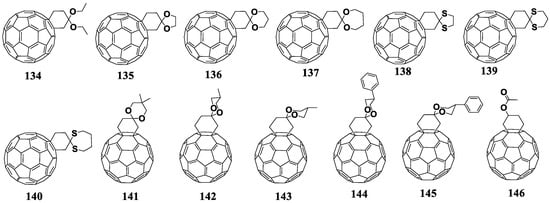
Figure 15.
Molecular structures of cyclohexane fullerene C60 adducts with reduced electron affinity.
At the next stage, the authors decided to increase the solubility of the compounds by introducing additional substituents (methyl or phenyl) into the structure of cyclohexane adduct 136 in the para-position with respect to the acetal group (methyl or phenyl). Thus, the authors obtained mixtures of endo and exo isomers of compounds 141–145, which were separated by column chromatography. It is interesting that the obtained isomers had the same electronic properties but operated in solar cells in combination with P3HT in a different manner. Thus, the devices with exo isomers 142 and 144 were superior in terms of photovoltaic characteristics as compared to the cells based on endo isomers 143 and 145.
Liu et al. prepared monoadduct 146 by the introduction of unmodified fullerene into the Diels–Alder reaction with 2-(trimethylsilyloxy)-1,3-butadiene [133]. The resulting product was then reduced and esterified. Cycloadduct 146 showed good solubility in organic solvents. The VOC of the devices based on the 146/P3HT composite was 20 mV higher than that of the control devices with [60]PCBM, whereas other characteristics were slightly reduced. The photovoltaic characteristics of the cyclohexane fullerene adducts with reduced electron affinity are summarized in Table 3.

Table 3.
The first reduction potentials and characteristics of the solar cells based on the cyclohexane fullerene derivatives.
It can be seen from the data presented in Table 3 that the cyclohexane adducts with reduced electron affinity have mainly been investigated only in combination with the highly crystalline polymer P3HT. On the average, their LUMO energy is just slightly higher than that of [60]PCBM (the reduction potential is shifted to the cathodic region by 20–60 mV only). At the same time, the synthesis of cyclohexane adducts of fullerenes is relatively complicated. Thus, this family of compounds could hardly be considered as promising acceptor materials for organic solar cells.
6.4. Cyclohexene Fullerenes Derivatives
Cyclohexene fullerenes derivatives are obtained as a result of the [2+4]cycloaddition reaction involving fullerene as a dienophile [134]. ortho-Quinodimethanes are often used as dienes, which are generated in situ. The disadvantages of the reaction include its reversibility and formation of polyadducts. Figure 16 shows the molecular structures of the cyclohexene fullerene adducts with reduced electron affinity.
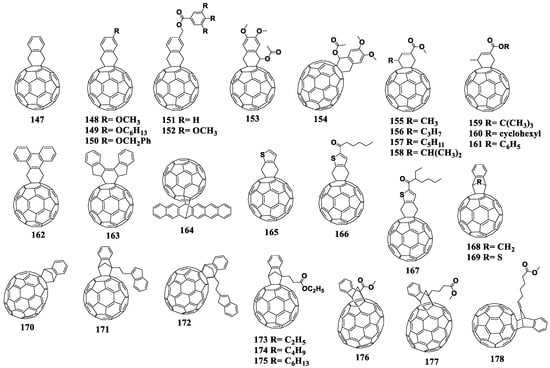
Figure 16.
Molecular structures of the fullerene derivatives with reduced electron affinity containing the cyclohexene fragment annelated to the fullerene cage.
The unsubstituted tetrahydronaphthalene fullerene derivative 147 was studied in several papers [135,136,137]. The reported first reduction potentials of compound 147 ranged from –1.02 to –1.3 V. The VOC value of the photovoltaic cells based on composite 147/P3HT exceeded that of the reference system by 40–70 mV (Table 4). In a number of cases, the PCE value also slightly increased.
At the next stage, electron donor alkoxy groups were introduced into the phenyl ring of compound 147 [138]. However, this modification did not lead to an increase in the LUMO energy of fullerene derivatives 148–150. As expected from the electrochemical data, the VOC of the solar cells based on such materials remain comparable to the characteristics of the devices based on unsubstituted tetrahydronaphthalene fullerene derivative 147 and P3HT.
The work describing fullerene-containing materials 151 and 152 is worth noting [139]. The electrochemical studies showed that the electronic properties of compounds 151 and [60]PCBM were very close. However, VOC of the photovoltaic cells based on the 151/P3HT composite exceeded that of the reference system [60]PCBM/P3HT by 44 mV. The introduction of three additional methoxyl substituents led to a shift in the reduction potential by 50 mV to the cathodic region, while the VOC of cells based on compound 152 decreased by 50 mV in comparison with the reference system. The authors explained this unusual result by the suboptimal morphology of the composite films based on compound 152 with P3HT. At the same time, the nature of the cathodic shift of the reduction potential of compound 152 with three methoxy groups remains unclear, since the distance between the electron-donor groups and the fullerene framework is too long for an inductive or spatial interactions.
The introduction of the ester group and two methoxy groups (compounds 153 and 154) into the tetrahydronaphthalene fragment attached to C60 and C70 cages allowed the authors to increase the LUMO energy by 50–60 meV versus that of [60]PCBM [140]. The compounds were investigated not only in combination with P3HT and also with the PBDTTT-C copolymer. The VOC values of the photovoltaic cells based on compounds 153 and 154 slightly increased in comparison with the reference devices, but JSC decreased.
Yamane et al. reported the preparation and study of a series of new fullerene derivatives with the cyclohexene fragment annelated to the fullerene cage (compounds 155–161) [141]. The authors varied both the alkyl substituent attached to the cyclohexene fragment (compounds 155–158) and the substituent in the ester group (fullerene derivatives 159–161). The obtained fullerene derivatives were studied in photovoltaic cells and an interesting effect was found: the efficiency of the devices based on the compounds containing an odd number of carbon atoms in the alkyl substituent was considerably higher than the efficiencies of devices based on similar compounds bearing side chains with even numbers of carbon atoms. It was shown by cyclic voltammetry that the studied compounds had a lower electron affinity as compared to that of [60]PCBM. In addition, the presence of the phenyl substituent in the ester group (compound 161) enabled improved compatibility of the material with P3HT and increased efficiency over the control devices (3.2 and 2.59%, respectively).
The introduction of polyaromatic substituents into the cyclohexene fragment resulted in compounds 162–164 [142,143,144]. The first reduction potential of fullerene derivative 162 containing the phenanthrene substituent shifted to the cathodic region by 50 mV. However, the authors emphasized that the low solubility of this fullerene derivative did not allow the fabrication of working photovoltaic cells. Fullerene adduct 163 containing the bis(indene) fragment is also interesting. Its reduction potential also shifted to the cathodic region by 50 mV versus [60]PCBM. At the same time, VOC of the solar cells based on compound 163 was 100 mV higher than that for the devices based on [60]PCBM. At the same time, JSC decreased almost twice in comparison to the reference system, which led to a relatively low PCE of ca. 2.2%. According to the authors, a significant decrease in JSC is associated with a poor morphology or deterioration of the electron transport properties, since the bis(indene) fragment is rather bulky.
The fullerene derivative obtained by addition of hexacene (compound 164) was also studied as an electron acceptor material. Despite a slight decrease in the electron affinity of compound 164 and an increase in VOC of the corresponding solar cells (by 70 mV), the overall photovoltaic performance turned out to be extremely low.
The search for fullerene derivatives with reduced electron affinity has led investigators to prepare compounds 165–167 [145]. They contain the thiophene fragment in their structure, which is attached to the fullerene cage through methylene groups. Note that unsubstituted compound 165 has the first reduction potential shifted to the cathodic region by 60 mV versus that of [60]PCBM. As can be seen from Table 4, VOC of the cells comprised of composite 165/P3HT is only 10 mV higher (630 mV) and JSC is twice lower (4.1 mA cm−2) than the corresponding characteristics of the reference devices using [60]PCBM (620 mV, 8.2 mA cm−2). Further modification of the compound with solubilizing fragments (materials 166 and 167) shifted the first reduction potential to the cathodic region by 10 and 40 mV, respectively. Compounds 166 and 167 showed only a slight increase in VOC (by 10 and 20 mV), whereas JSC also slightly increased (by 0.5 and 0.1 mA cm−2). Thus, the solar cells based on the 166/P3HT composite outperformed the reference cells with [60]PCBM by 0.3%.
The well-known fullerene derivative that has a reduced electron affinity is compound 168, which has been investigated by several research groups [117,135,146,147]. The shift of the first reduction potential of compound 168 to the cathodic region was ~ 40 mV, and the VOC increase in the corresponding OCSs reached ~50 mV in comparison to the control cells. Other characteristics remained comparable with the corresponding parameters of the reference system based on [60]PCBM and P3HT.
Compound 169 contains the bridging sulfur atom in the structure of an indene analogue attached to the fullerene cage [148]. The cyclic voltammogram showed a shift of the first reduction potential to the cathodic region by 40 mV versus [60]PCBM. Unfortunately, the authors studied this acceptor material only in planar-type solar cells. However, from the electrochemical data one should expect a slight increase in VOC (~40 mV) in bulk heterojunction devices.
Adduct 170 based on fullerene C70 is an analogue of compound 168 and showed an increase in VOC of the corresponding devices by 80 mV, while the cell efficiencies remained comparable to those of the reference devices based on [60]PCBM/P3HT [135,148].
R. Tao et al. developed the fullerene derivatives containing two indene fragments, one of which is directly linked to the fullerene skeleton (compounds 171 and 172) [149,150]. The first reduction potentials of adducts 171 and 172 shifted to the cathodic region versus that of [60]PCBM by 40 and 50 mV, respectively. The solar cells based on compound 171 showed no increase in VOC but demonstrated a significant deterioration in other performance parameters compared to the control system. The VOC values of OSCs based on cycloadduct 172 exceeded the voltage of the reference [70]PCBM/P3HT devices, but other parameters deteriorated.
The next step was the modification of the monoindene molecule with ester groups, which delivered compounds 173–177 [151,152,153]. In particular, the alkyl substituent in the ester group was varied in adducts 173–175. The best results were obtained for the devices based on the composite 174/P3HT. An increase in almost all parameters of solar cells was demonstrated, and the efficiency of the cells increased from 4.27 to 4.76%. Compounds 176 and 177 were developed on the basis of a molecule of compound 170. They also contained the ester group in the structure [152,153]. Fullerene cyclohexene adduct 176 was studied in combination with the highly crystalline P3HT polymer and low band gap polymers PBDTTT-C and PBDTTDPP. Compound 177 was tested with both polymer P3HT and copolymer PTB7. The characteristics of the photovoltaic devices based on compound 176 were superior to those of the reference system [70]PCBM/P3HT. The use of fullerene derivative 176 in combination with the PBDTTT-C and PBDTTDPP polymers provided an increase in VOC by 30 and 40 mV along with minor changes in JSC and fill factors with respect to the performance of the reference system. The solar OSCs based on 177/P3HT and 177/PTB-7 generally outperformed the control cells based on [70]PCBM/P3HT and [70]PCBM/PTB-7. The devices based on the 178/PTB-7 composite showed a 30 mV increase in VOC [154].
The photovoltaic performance of the solar cells using the cyclohexene fullerene derivatives as acceptor materials with reduced electron affinity are summarized in Table 4.

Table 4.
First reduction potentials and characteristics of the solar cells based on the cyclohexene fullerene derivatives.
Table 4.
First reduction potentials and characteristics of the solar cells based on the cyclohexene fullerene derivatives.
| Compound | E1 red, V | Polymer | VOC, V | JSC, mA cm−2 | FF, % | ɳ, % | Ref. |
|---|---|---|---|---|---|---|---|
| 147 | −1.02 (Ag/Ag+) | P3HT | 0.65 | 9.5 | 68 | 4.2 | [135] |
| ~−1.3 (Fc/Fc+) | 0.64 | 9.5 | 67 | 4.1 | [136] | ||
| −1.12 (Fc/Fc+) | 0.64 | 8.93 | 57 | 3.22 | [137] | ||
| [60]PCBM | −1.0 (Ag/Ag+) | 0.61 | 9.1 | 72 | 4.0 | [135] | |
| ~−1.1 (Fc/Fc+) | 0.57 | 9.6 | 70 | 3.8 | [136] | ||
| −1.14 (Fc/Fc+) | 0.59 | 9.47 | 66 | 3.68 | [137] | ||
| 148 | −0.93 (Ag/Ag+) | P3HT | 0.63 | 9.04 | 56.7 | 3.23 | [138] |
| 149 | −0.93 (Ag/Ag+) | 0.64 | 6.59 | 53.8 | 2.27 | ||
| 150 | −0.93 (Ag/Ag+) | 0.62 | 9.03 | 53.1 | 2.97 | ||
| [60]PCBM | −0.89 (Ag/Ag+) | 0.59 | 9.63 | 57.2 | 3.25 | ||
| 151 | −4.11 eV onset (Fc/Fc+) | P3HT | 0.654 | - | - | 4.5 | [139] |
| 152 | −4.16 eV onset (Fc/Fc+) | 0.562 | - | - | - | ||
| [60]PCBM | −4.11 eV onset (Fc/Fc+) | 0.61 | - | - | 4.4 | ||
| 153 | −0.65 (Ag/Ag+) | PBDTTT-C | 0.77 | 8.51 | 45.8 | 3.00 | [140] |
| P3HT | 0.62 | 8.10 | 71.3 | 3.63 | |||
| 154 | −0.66 (Ag/Ag+) | PBDTTT-C | 0.73 | 13.37 | 51.9 | 5.07 | |
| P3HT | 0.64 | 8.09 | 64.6 | 3.35 | |||
| [60]PCBM | −0.60 (Ag/Ag+) | PBDTTT-C | 0.71 | 10.83 | 65.0 | 4.99 | |
| P3HT | 0.59 | 9.03 | 68.6 | 3.64 | |||
| [70]PCBM | - | PBDTTT-C | 0.72 | 14.16 | 64.0 | 6.49 | |
| P3HT | 0.58 | 10.45 | 66.4 | 4.00 | |||
| 155 | −1.15 (Fc/Fc+) | P3HT | 0.619 | 6.96 | 62.9 | 2.71 | [141] |
| 156 | −1.16 (Fc/Fc+) | 0.627 | 6.46 | 63.4 | 2.57 | ||
| 157 | −1.16 (Fc/Fc+) | 0.625 | 6.16 | 5.87 | 2.26 | ||
| 158 | −1.16 (Fc/Fc+) | 0.625 | 7.71 | 58.6 | 2.83 | ||
| 159 | −1.11 (Fc/Fc+) | 0.652 | 5.26 | 41.2 | 1.41 | ||
| 160 | −1.11 (Fc/Fc+) | 0.672 | 5.24 | 53.6 | 1.89 | ||
| 161 | −1.14 (Fc/Fc+) | 0.642 | 8.13 | 61.4 | 3.2 | ||
| [60]PCBM | −1.09 (Fc/Fc+) | 0.601 | 6.91 | 62.5 | 2.59 | ||
| 162 | −1.05 (Fc/Fc+) | P3HT | - | - | - | - | [142] |
| [60]PCBM | −1.01 (Fc/Fc+) | 0.61 | 9.6 | 56.14 | 3.29 | ||
| 163 | −0.93 (Ag/Ag+) | P3HT | 0.68 | 5.41 | 60 | 2.21 | [143] |
| [60]PCBM | −0.88 (Ag/Ag+) | 0.58 | 10.8 | 62 | 3.88 | ||
| 164 | −1.27 (Fc/Fc+) | P3HT | 0.72 | 0.71 | 31 | 0.16 | [144] |
| [60]PCBM | −1.25 (Fc/Fc+) | 0.65 | 9.95 | 52 | 3.27 | ||
| 165 | −1.17 (Fc/Fc+) | P3HT | 0.63 | 4.1 | 57 | 1.7 | [145] |
| 166 | −1.12 (Fc/Fc+) | 0.63 | 8.7 | 61 | 3.9 | ||
| 167 | −1.15 (Fc/Fc+) | 0.64 | 8.3 | 55 | 3.4 | ||
| [60]PCBM | −1.11 (Fc/Fc+) | 0.62 | 8.2 | 61 | 3.6 | ||
| 168 | −1.04 (Fc/Fc+) | P3HT | 0.66 | 7.9 | 69 | 3.6 | [135] |
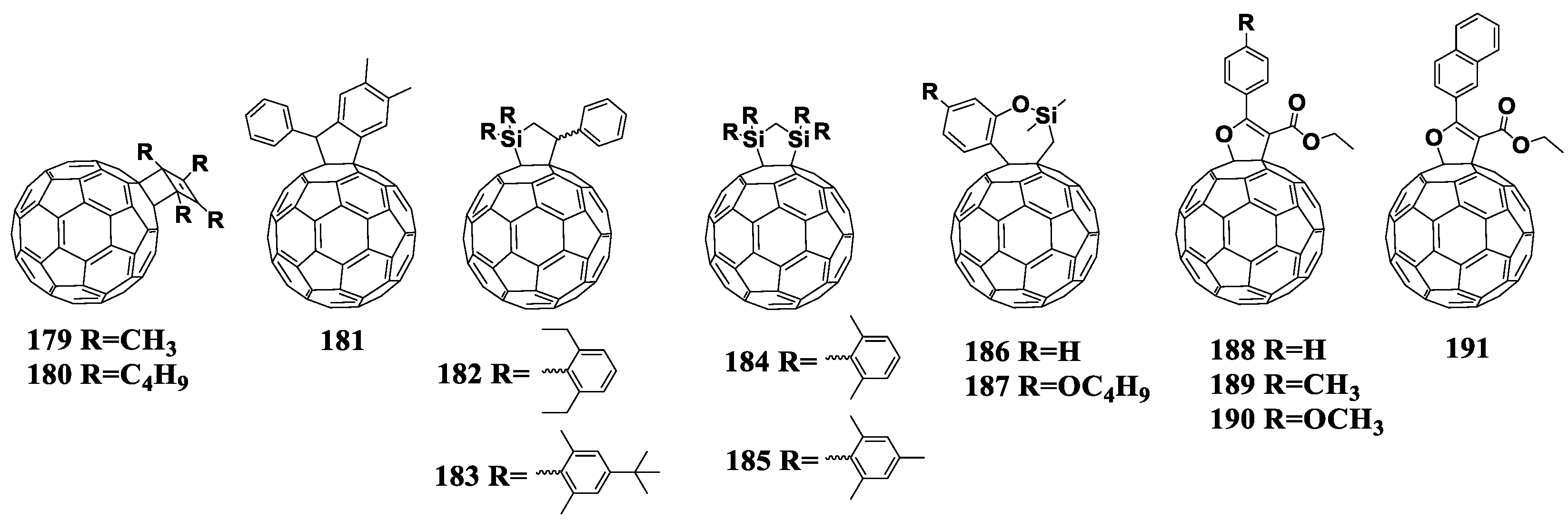
| −0.93 (Ag/Ag+) | 0.63 | 9.66 | 64 | 3.89 | [117] | ||
| −1.15 (Fc/Fc+) | 0.65 | 9.66 | 59 | 3.65 | [147] | ||
| [60]PCBM | −1.0 (Fc/Fc+) | 0.61 | 9.1 | 72 | 4.0 | [135] | |
| −0.88 (Ag/Ag+) | 0.58 | 10.8 | 62 | 3.88 | [117] | ||
| −1.11 (Fc/Fc+) | 0.62 | 9.71 | 62 | 3.74 | [146] | ||
| 169 | −1.01 (Fc/Fc+) | - | - | - | - | - | [148] |
| [60]PCBM | −0.97 (Fc/Fc+) | - | - | - | - | ||
| 170 | −1.04 (Fc/Fc+) | P3HT | 0.7 | 8.6 | 70 | 4.2 | [135] |
| −0.93 (Ag/Ag+) | - | - | - | - | [147] | ||
| [60]PCBM | −1.0 (Fc/Fc+) | 0.61 | 9.1 | 72 | 4.0 | [135] | |
| −0.88 (Ag/Ag+) | 0.59 | 9.26 | 65 | 3.55 | [147] | ||
| 171 | −0.94 (Ag/Ag+) | P3HT | 0.61 | 5.15 | 51 | 1.6 | [149] |
| [60]PCBM | −0.90 (Ag/Ag+) | 0.61 | 7.5 | 57 | 2.6 | ||
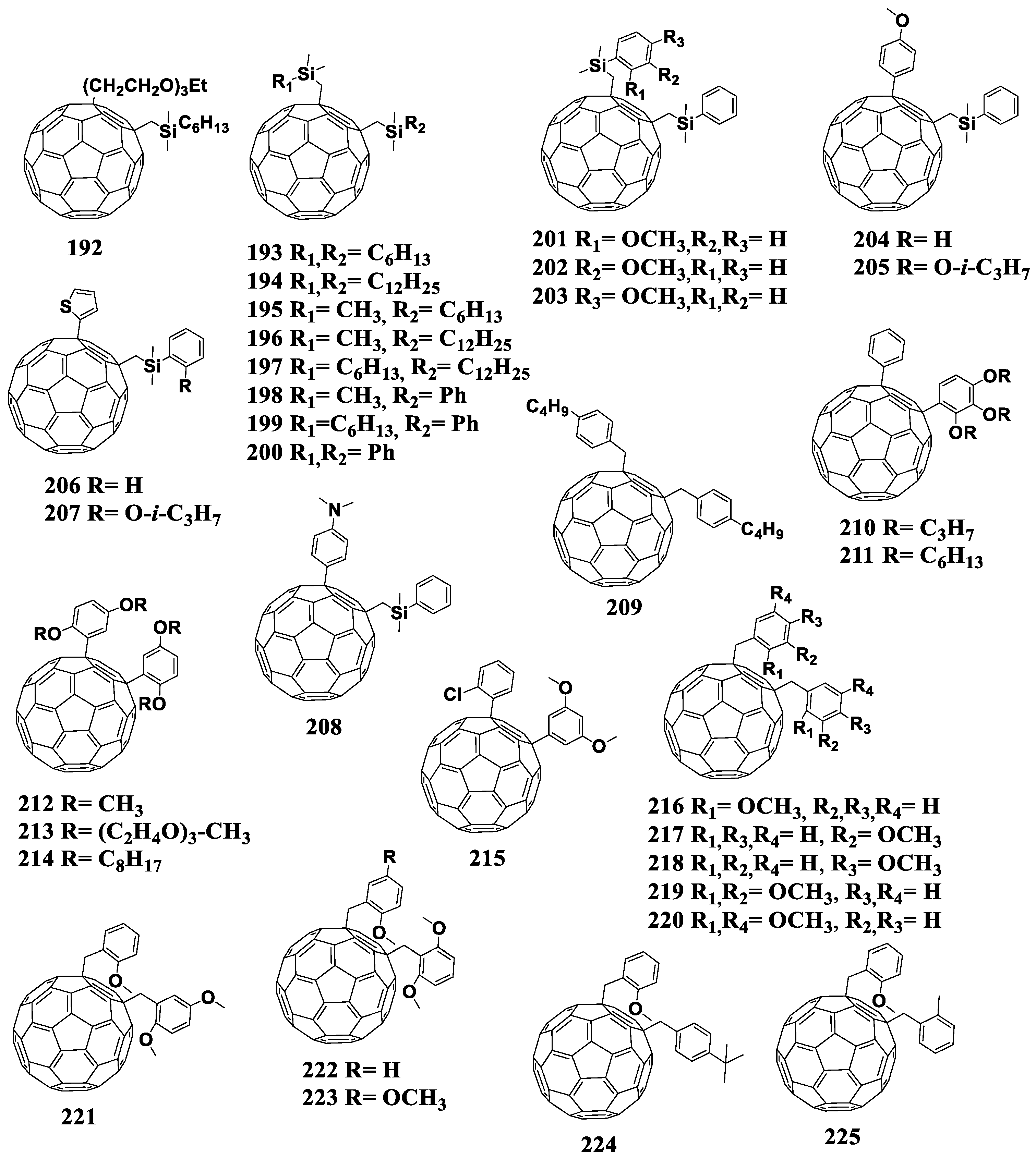
| 172 | −0.96 (Ag/Ag+) | P3HT | 0.64 | 5.9 | 57 | 2.2 | [150] |
| [70]PCBM | −0.91 (Ag/Ag+) | 0.6 | 8.6 | 73 | 3.8 | ||
| 173 | −1.11 (Fc/Fc+) | P3HT | 0.605 | 9.59 | 61 | 3.52 | [151] |
| 174 | - | 0.647 | 10.66 | 69 | 4.76 | ||
| 175 | - | 0.647 | 10.56 | 68 | 4.62 | ||
| [60]PCBM | −1.08 (Fc/Fc+) | 0.595 | 10.67 | 67 | 4.27 | ||
| 176 | −0.63 (Ag−Ag wire) | P3HT | 0.64 | 10.14 | 65 | 4.22 | [152] |
| PBDTTT-C | 0.75 | 14.18 | 57.6 | 6.00 | |||
| PBDTTDPP | 0.78 | 13.42 | 60 | 6.20 | |||
| [70]PCBM | −0.6 (Ag−Ag wire) | P3HT | 0.59 | 9.79 | 67 | 3.89 | |
| PBDTTT-C | 0.72 | 14.19 | 62 | 6.3 | |||
| PBDTTDPP | 0.74 | 13.60 | 65 | 6.5 | |||
| 177 | −0.52 (Fc/Fc+) | P3HT | 0.68 | 11.6 | 63.5 | 5.00 | [153] |
| PTB7 | 0.79 | 15.4 | 55.0 | 6.67 | |||
| [70]PCBM | −0.46 (Fc/Fc+) | P3HT | 0.62 | 11.2 | 58.0 | 4.04 | |
| PTB7 | 0.74 | 14.2 | 60.0 | 6.30 | |||
| 178 | −1.1 (Fc/Fc+) | PTB7 | 0.79 | 12.9 | 50 | 5.1 | [154] |
| [60]PCBM | −1.09 (Fc/Fc+) | 0.76 | 14.6 | 62 | 6.8 |
It can be seen from the data presented in Table 4 that the use of the fullerene derivatives with reduced electron affinity containing the cyclohexene fragment attached to the fullerene framework enabled an increase in VOC of the photovoltaic cells by 40–50 mV. However, in the most cases, other device characteristics either deteriorated or turned out to be comparable with the characteristics of the reference system. It is worth noting that fullerene derivatives 153, 154, 176, and 177 showed a compatibility not only with the classical donor material P3HT but also with promising donor–acceptor copolymers.
6.5. Other Cycloadducts
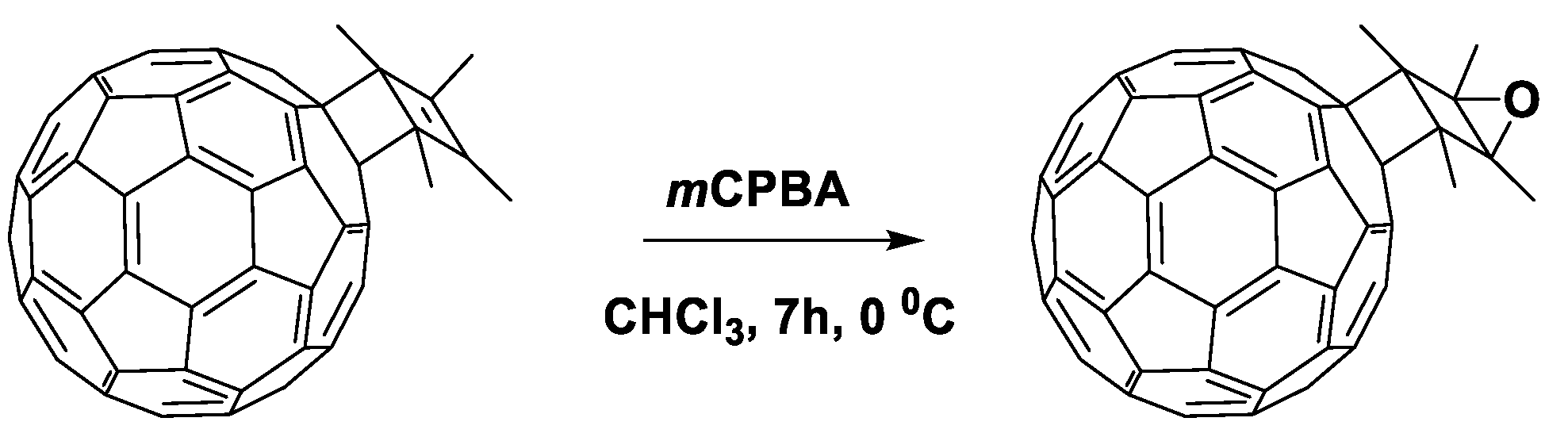
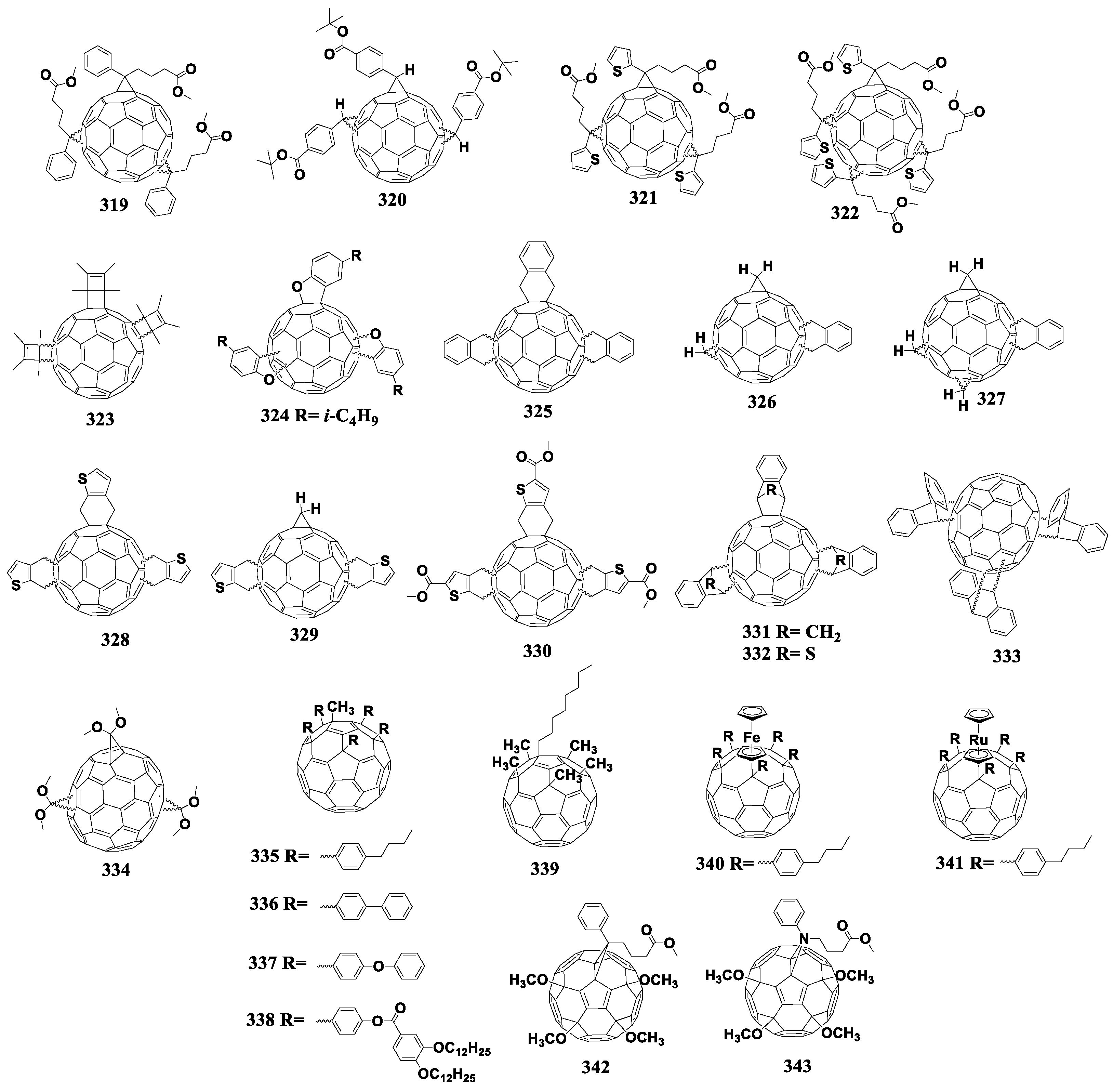
Currently, two fullerene monocyclobutane adducts are described in the literature, which have been studied electrochemically or used as acceptor materials in photovoltaic cells in combination with P3HT [155]. Using [2+2]cycloaddition, the authors of this work obtained fullerene derivatives 179 and 180 containing the bicyclo[2.2.0]hexene fragment (180a) annelated to the fullerene framework (Figure 17). The disadvantage of the [2+2]cycloaddition reaction to the fullerene framework is also the formation of polyadducts.
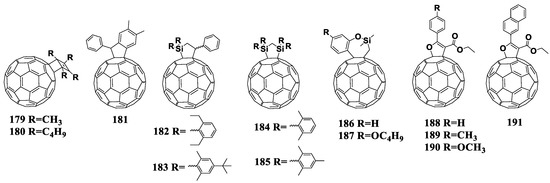
Figure 17.
Molecular structures of the fullerene cycloadducts with reduced electron affinity.
It can be seen from the data presented in Table 5 that the first reduction potential of compounds 179 and 180 shifted to the cathodic region by 60 mV. From the point of view of the authors, such a shift was due to a strong interaction between the double bond of the cyclobutene fragment and fullerene π-system. As a confirmation of this interaction, the fullerene derivative was obtained with the double bond epoxidized with 3-chloroperoxybenzoic acid (Scheme 1). The cyclic voltammogram of the new compound showed a shift of the first reduction potential by 50 mV to the anodic region.

Table 5.
First reduction potentials and characteristics of the solar cells based on the fullerene cycloadducts.
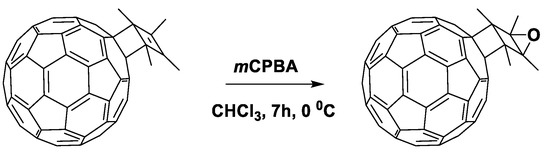
Scheme 1.
Epoxidation of the double bond in cyclobutene fragment of 179.
In fact, the resulting epoxide showed similar electrochemical properties to [60]PCBM. The obtained results confirmed the hypothesis about the interaction between the double bond of the organic addend and π-system of the fullerene cage. However, the presence of non-conjugated double bond can adversely affect the operational stability of OSCs. The solar cells with fullerene derivative 179 showed comparable characteristics with the reference devices based on [60]PCBM, but a slight increase in VOC (30 mV) and a decrease in Jsc were observed.
A representative of the cyclopentane derivatives of fullerenes is compound 181 (Figure 17). This compound was obtained by the solvent-free reaction under the high-frequency milling conditions in the presence of iron(III) chloride. As the authors noted, the reaction under these conditions enabled the synthesis of fullerene-based materials, which are challenging or even impossible to obtain by liquid-phase reactions [156]. The first reduction potential of compound 181 shifted to the cathodic region by 22 mV versus that of [60]PCBM. However, the evaluation of 181 in solar cells was not possible because of its insufficient solubility in organic solvents.
An alternative approach to increase the LUMO energy of a fullerene derivative is to replace one or more carbon atoms of the cyclopentane moiety with silicon atom(s). For example, only one carbon atom is substituted in compounds 182 and 183 [157]. The first reduction potential of fullerene derivatives 182 and 183 shifted to the cathodic region as compared to the unmodified fullerene by 150 and 160 mV, respectively. The next step was to replace two carbon atoms with the silicon atoms (cycloadducts 184 and 185) [105]. It turned out that the introduction of an additional silicon atom did not significantly affect the energy levels of the molecule.
Thus, the annelation of one cyclopentane fragment to the fullerene framework results in the shift the first reduction potential of the obtained compounds to the cathodic region by only 22 mV against [60]PCBM (compound 181) and by 150–160 mV against C60 (compounds 182–185). These materials were not studied in photovoltaic cells most probably because of insufficient solubility in organic solvents.
Matsuo et al. developed cycloadducts 186 and 187, which were obtained in two steps [158]. At the first stage, fullerene was transformed into the fullerene dimer (C60R)2 in which the carbon frameworks are linked by the single C–C bond, and the R substituents are (2-methoxyphenyl)dimethylsilylmethyl or (2-methoxy-4-butyloxyphenyl)dimethylsilylmethyl. The subsequent treatment of (C60R)2 with oxidizing agents (I2, CuCl2, CuBr2) led to the formation of the cation (RC60)+, which underwent cyclization with the formation of adducts 186 and 187. Electrochemical studies showed a shift of the first reduction potentials of compounds 186 and 187 by 20 mV to the cathodic region as compared to that of [60]PCBM. The solar cells based on the obtained acceptor materials showed decently high efficiency and VOC. Unfortunately, the paper presented no characteristics of the reference [60]PCBM/P3HT cells, which challenges the interpretation of the results obtained.
The work of Yan et al. presented a series of furan-containing fullerene derivatives 188–191 synthesized using the palladium-catalyzed one-step reaction [159]. Unfortunately, only the electrochemical properties of the compounds were studied: the first reduction potentials of cycloadducts 188–191 shifted to the cathodic region versus [60]PCBM, and the largest shift of 120 mV was shown by compounds 190–191 with the para-methoxyphenyl and 2-naphthyl substituents, respectively. A significant decrease in the electron affinity of compounds 190 and 191 makes them promising materials for photovoltaic devices. Thus, this area of research requires further development.
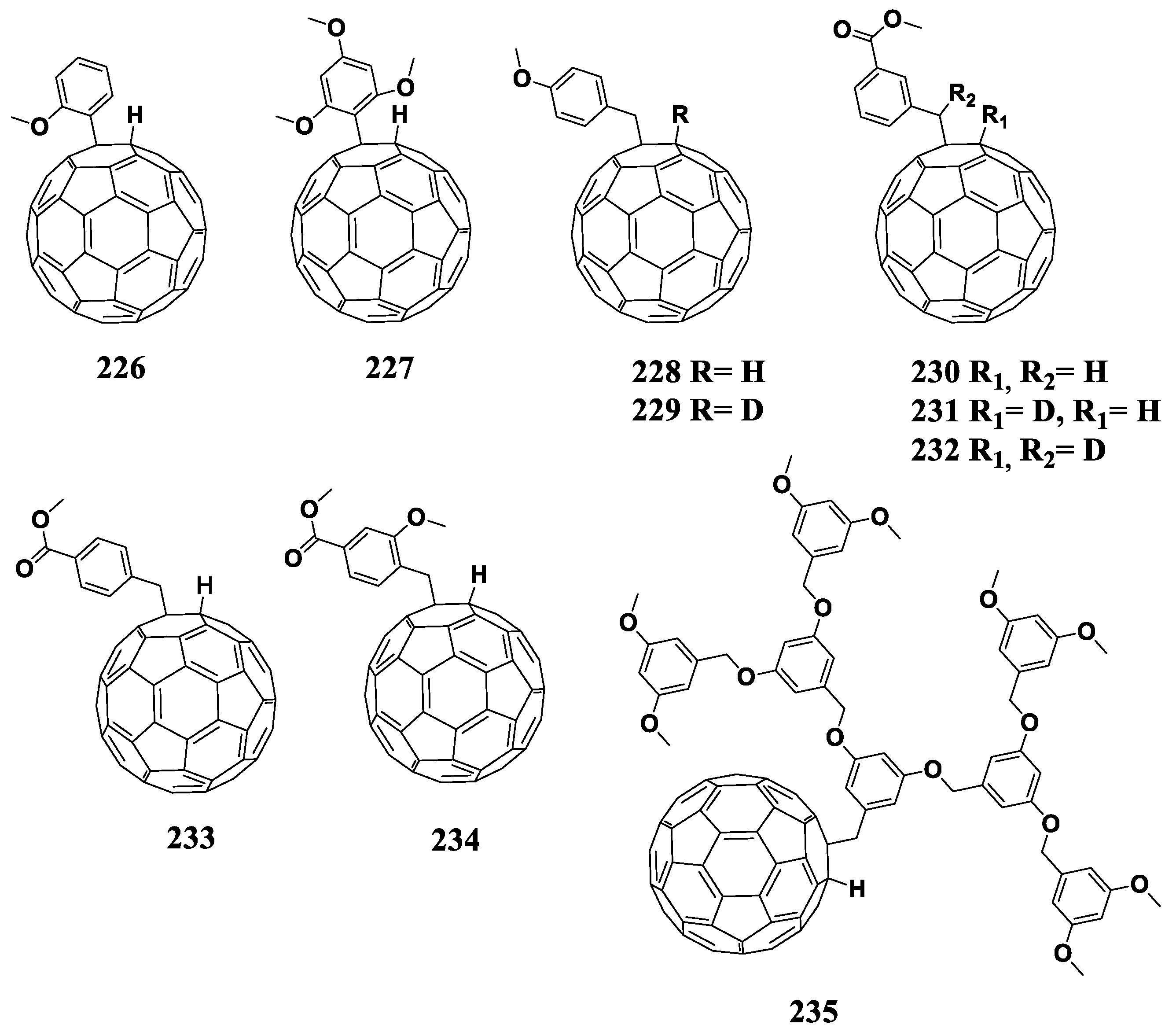
The experimental data obtained for the C60 cycloadducts with reduced electron affinity are summarized in Table 5.
6.6. 1,4-Fullerene Adducts
1,4-Adducts of C60 fullerene (Figure 18) have great potential as fullerene-based materials with reduced electron affinity owing to wide possibilities of the structural modification of attached organic addends.
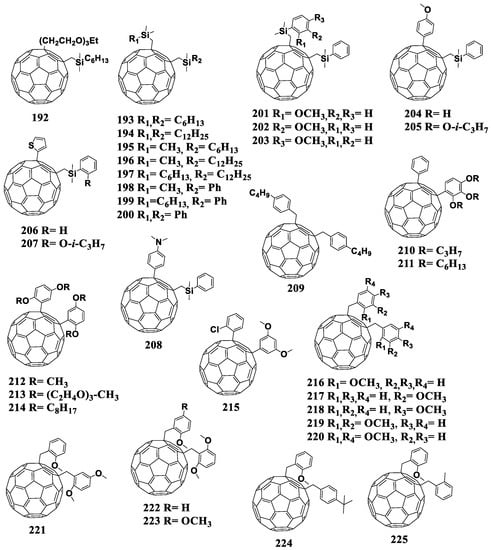
Figure 18.
Molecular structures of 1,4-adducts of C60 fullerene with reduced electron affinity.
The synthesis of 1,4-adducts of fullerene is carried out in two steps and leads to the formation of target compounds in high yields [160]. At the first step, fullerene is treated with the Grignard reagent in the presence of N,N-dimethylformamide (DMF), which leads to the formation of C60R1H hydrofullerene. At the next step, the [C60R]− anion obtained by the deprotonation of hydrofullerene is treated with electrophiles (alkyl halides, benzyl halides, etc.) leading to the formation of 1,4-adducts of fullerene C60R1R2 in a high yield. It should be emphasized that this synthetic approach does not lead to the formation of polyaddition products of organic addends to the fullerene cage (polyadducts), which represents its significant advantage.
A considerable work in this direction was completed by Matsuo et al., who studied the effect of organic addends attached to the fullerene framework on the electronic properties of the fullerene derivatives (compounds 192–200) [160,161]. It follows from the data presented in Table 6 that the first reduction potential of the fullerene derivatives containing only alkyl substituents shifted to the cathodic region by 30–40 mV versus [60]PCBM. The introduction of phenyl substituents instead of alkyl substituents resulted in additional shift of the first reduction potential to the cathodic region by 20 mV. Unfortunately, the designed compounds, except for fullerene derivative 200, have not been studied in bulk heterojunction solar cells. However, the authors showed that the film of compound 200 had an electron mobility of 1 × 10−4 cm2 V−1 s−1, while the electron mobility in thin films of [60]PCBM measured by the same method was 5 × 10−5 cm2 V−1 s−1. The VOC value of the photovoltaic cells based on the 200/P3HT composite exceeded the voltage of the reference cells by 100 mV, resulting in an efficiency increase of 0.4%. Jeon et al. studied compound 200 in combination with the p-type small molecule DPP(TBFu)2 [162]. The authors succeeded in obtaining higher VOC and PCE compared to the reference systems based on [60]PCBM/DPP(TBFu)2 (VOC = 1.03 V, ɳ = 4.57% for 200 vs. VOC = 0.92 V, ɳ = 4.38% for [60]PCBM).

Table 6.
First reduction potentials and characteristics of the solar cells based on the 1,4-fullerene adducts.
The methoxy group in compounds 201–203 was introduced at various positions of one of the phenyl rings to analyze its effect on the electronic properties of the molecule and the solar cells characteristics [162,163]. The data presented in Table 6 for compounds 201–203 show that a consistent shift of the first reduction potential to the cathodic region by 20 mV occurs when the methoxy group in the phenyl ring is moved from meta to ortho position and then further to para position. Thus, the methoxy group located at the para-position of the phenyl ring has the greatest influence on the electronic properties of the fullerene derivative. The reasons for this dependence are not clear and can hardly be explained by the classical notions of the inductive or mesomeric electronic effects of the substituent.
Compounds 204–208 incorporating various organic addends attached to the fullerene cage are described in the literature [161]. The first reduction potential of these fullerene derivatives shifted to the cathodic region by 80–90 mV against [60]PCBM. Using the adducts 205 and 208 in bulk heterojunction cells enabled a VOC increase by 60 and 70 mV over the reference cells, while all other characteristics deteriorated. The photovoltaic cells based on compound 209 also showed unsatisfactory characteristics [164]. The reason for this is probably related to incompatibility of the fullerene derivatives with the P3HT donor material and, as a result, the non-optimal morphology of the formed composite photoactive layer.
Attention should be given to compounds 210 and 211, which also belong to the class of fullerene 1,4-adducts, but their synthesis differs from that described earlier [165]. The precursor for their preparation is fullerenol, which was treated with trialkoxybenzenes in the presence of para-toluenesulfonic acid. Note that the yield of the target product in the reaction was only 19%. The authors of the article intentionally introduced the electron-donating aryl fragment into the molecule to increase the LUMO energy of the fullerene derivative. It can be seen from the presented data that the first reduction potential of compound 210 shifted by 100 mV to the cathodic region against [60]PCBM. The solar cells based on the 210/P3HT and 211/P3HT composites showed a higher VOC (by 110 and 70 mV) than the reference cells, while other device characteristics deteriorated. In the work of Cristofani et al., fullerene derivatives 212–215 were obtained [166]. Despite the detected shift of the first reduction potentials of all compounds to the cathodic region as compared to [60]PCBM, only compound 214 was studied in photovoltaic cells. An increase in VOC of the 214/P3HT devices by 70 mV was counterbalanced by the deterioration of other device characteristics.
Huang et al. obtained a big series of fullerene 1,4-adducts with an increased LUMO energy level as compared to [60]PCBM (compounds 216–225) [167]. It has been found that the introduction of methoxy groups at the phenyl rings significantly reduces the electron affinity of the obtained adducts. The devices based on 1,4-adducts 220 and 223 showed a 20% increase in the PCE compared to the cells based on the [60]PCBM/P3HT composite. Moreover, compound 220 has an acceptable compatibility with the PTB-7 copolymer.
Table 6 summarizes the experimental data obtained in the studies of 1,4-adducts of fullerene with reduced electron affinity, including those in photovoltaic cells.
The data presented in Table 6 indicate that the 1,4-fullerene adducts indeed have a high potential in the design of acceptor components of bulk heterojunction organic solar cells. Further development of this area of research is required, in particular, in order to ensure the compatibility of fullerene derivatives of this family with various conjugated polymers. There are also prospects for using 1,4-fullerene adducts in planar heterojunction OSCs [168].
6.7. 1,2-Fullerene Adducts
A very interesting family of fullerene derivatives is represented by 1,2-adducts (Figure 19). Note that arylated fullerene derivatives 226 and 227 were obtained by the reactions of substituted phenylboronic acids with fullerene in an inert atmosphere in the presence of the rhodium-based catalyst [169].
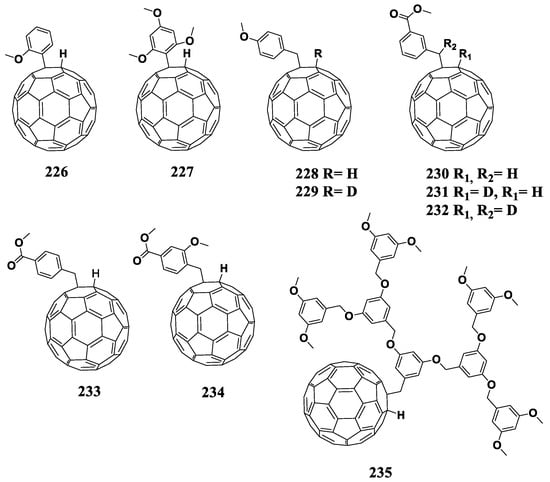
Figure 19.
Molecular structures of 1,2-adducts of fullerene C60 with reduced electron affinity.
Unfortunately, arylated fullerene derivatives 226 and 227 were studied only by cyclic voltammetry but were not evaluated as acceptor materials in photovoltaic cells. Apparently, the solubility of new compounds is insufficient for manufacturing solar cells. The first reduction potentials of the compounds shifted to the cathodic region by 135 and 180 mV, respectively, as compared to the pristine fullerene. The work is interesting, because the authors tried to understand the reason for this shift and came to the conclusion that the through-space interactions of the electron-donating methoxy groups in the 1,2-adducts, which are in close proximity to the fullerene framework, have a greater influence on the LUMO energy of the resulting compounds as compared to similar substituents in methanofullerenes and other types of fullerene derivatives.
1,2-Adducts 228–232 were obtained by reacting substituted benzyl bromides with fullerene in the presence of water or deuterated water and cobalt-containing catalyst [170,171]. In the approach of Lu et al., deuterated water was used to synthesize 1,2-adducts with deuterium atoms (adducts 229, 231, and 232) [171]. Introduction of deuterium was used in organic light-emitting diodes and led to an increase in the external quantum efficiency of the devices as compared to the reference diodes with proton-containing molecules [172]. Electrochemical measurements showed a shift of the first reduction potential of fullerene derivatives to the cathodic region by 20–30 mV against [60]PCBM. The devices based on 1,2-adducts of fullerene with deuterium atoms (229, 231, and 232) showed an increase in JSC and fill factors as compared to the devices with protic compounds 228 and 230.
The PCE of solar cells approached the efficiency of the reference system based on the [60]PCBM/P3HT composite. The organic solar cells based on 1,2-adduct 234 and P3HT showed a slight increase in VOC, whereas PCE remained comparable to that of the control cells. Table 7 summarizes the experimental data obtained for the 1,2-fullerene adducts.

Table 7.
The first reduction potentials and characteristics of solar cells based on the 1,2-fullerene adducts.
Thus, the use of the 1,2-adducts to increase the LUMO energy of the fullerene derivatives is a promising but still very underdeveloped area of research.
6.8. Bis(cyclopropane) Adducts
The most commonly used way to increase the LUMO energy of a fullerene derivative is to open two double bonds within the fullerene skeleton. To obtain bis(cyclopropane) adducts, the Hummelen–Woodl reaction (tosylhydrazone method) is typically used; the reaction conditions and the amount of reagents are adjusted in a way to obtain bis(cycloadduct) as a major product. The disadvantage of almost all bis(cycloadducts) is the formation of a large number of isomers. Figure 20 shows the molecular structures of the fullerene derivatives with reduced electron affinity containing two identical cyclopropane fragments annelated to the fullerene framework. A well-known example of the bis(cyclopropane) fullerene adduct is bis[60]PCBM containing two phenylbutyric acid methyl ester moieties, first reported by Lenes et al. [173]. Bis[60]PCBM shows increased LUMO energy by ~100 meV as compared to [60]PCBM. The photovoltaic devices based on bis[60]PCBM delivered inferior JSC values as compared to the reference cells with [60]PCBM but showed a significant increase in VOC by 144 mV, which resulted in an efficiency of about 4.5%, which was considerably higher than that of the reference cells with PCE of 3.8%. The authors emphasized that the resulting bis(cycloadduct) material consisted of many isomers (at least 17).
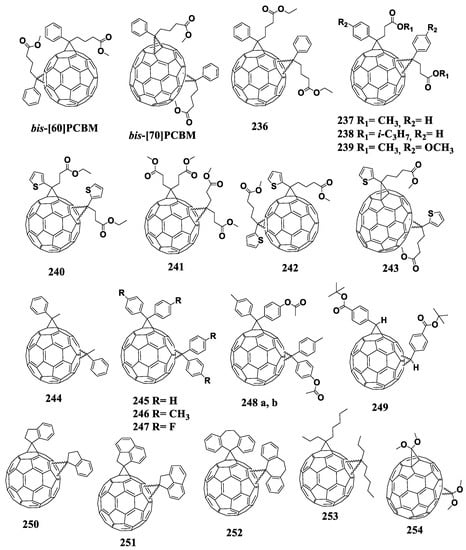
Figure 20.
Molecular structures of the bis(cyclopropane) adducts of fullerenes with reduced electron affinity.
Table 8 presents a number of studies in which bis[60]PCBM was studied as an acceptor component in organic solar cells [174,175,176,177]. Of particular interest is the study on using bis[60]PCBM in solar cells with the conjugated polymer PBDTBDD [175]. The authors were able to increase the VOC of the manufactured devices by 144 mV compared to the reference system with [60]PCBM and reach a value of 1 V. This was the first publication demonstrating the compatibility of the fullerene bis(cycloadduct) with the noncrystalline conjugated polymer. Lan et al. investigated bis[60]PCBM in combination with a small molecule of p-DTS(FBTTh2)2 [177]. The devices based on the bis[60]PCBM also showed an acceptable performance compared to the reference system based on the [70]PCBM/p-DTS(FBTTh2)2 composite. The use of bis[70]PCBM instead of bis[60]PCBM did not lead to any improvement in device performance.

Table 8.
First reduction potentials and the characteristics of solar cells based on the bis(cyclopropane) fullerene adducts.
Over the past few years, a considerable effort has been invested in the investigation of bis(cyclopropane) adducts of fullerenes. It has been shown that as in the case of fullerene monocycloadducts [79], the solubility of bis(cyclopropane) derivatives of fullerenes significantly affects the characteristics of solar cells [176]. This is well illustrated by the data presented in Table 8. Thus, despite the cathodic shift of the first reduction potentials of compounds 236–241 against [60]PCBM, the VOC of the photovoltaic cells does not always increase in comparison with the reference system and, in some cases, even deteriorates significantly (compounds 240 and 241). Therefore, when designing bis(cyclopropane) fullerene adducts for organic solar cells, it is important to take into account the solubility of the acceptor materials.
The replacement of the phenyl ring by the thienyl moiety in bis[60]PCBM and bis[70]PCBM (compounds 242 and 243) did not lead to significant changes in the characteristics of the corresponding devices [174,178]. As expected, VOC of the cells based on fullerene derivatives 242 and 243 exceeds that of the devices with [60]PCBM by 100–110mV. At the same time, the cell efficiencies remained comparable due to the decrease in JSC and fill factor values.
A systematic approach was implemented in the preparation of compounds 244–247 [179]. Despite the variation of the substituents in the para-position of the phenyl ring, the LUMO level of the bis(adducts) increased by only 100 meV. The authors attributed this to the fact that the arrangement of the phenyl group parallel to the fullerene surface leads to a weak orbital overlap and an insignificant electronic effect. This geometry of the molecule prevents the intermolecular aggregation and promotes an increase in the solubility of the compound. Despite the comparable electrochemical properties of fullerene derivatives 244–247, VOC of the corresponding devices differs significantly. Thus, the cells based on bis(adduct) 246 showed the VOC exceeding the voltage of the reference devices by 270 mV, while VOC should be increased by 100 mV only according to the electrochemical data. The solar cells based on compound 247 with fluorine-containing substituents showed an increase in VOC by 80 mV as compared to the reference system. The PCE of the devices was improved in comparison to the reference cells only when using bis(cyclopropane) 246.
Liao et al. showed that the steric effect of regioisomers in C60 bis(adduct) 248 had a greater influence on the photovoltaic characteristics of the devices than the effect of electron traps formed due to different energies of the resulting isomers [180]. However, the electrochemical data for two isomers of compound 248 showed approximately the same shift of the first reduction potential to the cathodic region by 110 and 130 mV, respectively.
The first reduction potential of fullerene derivative 249 studied by Liu et al. shifted to the cathodic region by 60 mV against [60]PCBM. However, the photovoltaic characteristics of 249 turned out to be unsatisfactory: VOC decreased by 40 mV, which was inconsistent with the electrochemistry data, and the low values of JSC were attributed by the authors to a large number of isomers of the fullerene derivative.
Compounds 250 and 251 developed by Tian et al. showed a cathodic shift of the first reduction potential as compared to that of [60]PCBM by 100 and 70 mV, respectively [181]. The VOC values of the cells based on bis(cycloadducts) 250 and 251 exceeded the characteristics of the reference devices by 70 and 80 mV, while other parameters deteriorated. A further increase in the volume of the substituent (compound 252) also did not lead to any improvements in the characteristics of OSCs, except for VOC, which increased by 70 mV [182]. The bis(cycloadduct) containing only alkyl substituents (compound 253) was investigated in combination with P3HT [183]. It was shown that an increase in the LUMO level of compound 253 led to an increase in the VOC values and the efficiency of the corresponding devices up to 0.73 V and 2.8% in the composites with P3HT. A derivative of C70 fullerene 254 containing two methoxy groups in each cyclopropane fragment showed only a slight decrease in the electron affinity versus [60]PCBM [184]. This example confirms that the introduction of electron-donating substituents, which are not capable of through-space interactions with the fullerene framework, does not allow one to significantly affect the electronic properties of the molecule.
Thus, a large number of bis(cyclopropane) derivatives of fullerenes with reduced electron affinity were synthesized and studied. In almost all cases, an increase in VOC of the corresponding photovoltaic cells was accompanied by a deterioration in other characteristics. However, we would like to draw attention to the work of Ye et al. in which bis[60]PCBM showed an acceptable compatibility with the amorphous conjugated polymer PBDTBDD, while other bis(cycloadducts) were generally compatible only with the highly crystalline polymer P3HT [166]. Compound 237 containing the fragment of benzoylpropionic acid methyl ester (structural analogue of [60]PCBM) worked well as an acceptor material for the devices with P3HT. A significant increase in VOC as compared to the reference [60]PCBM/P3HT system was shown by the cells with fullerene derivative 246 with the substituted diphenylmethane fragment annelated to the fullerene cage.
6.9. Bis(cyclohexene) Adducts
Bis(cyclohexene) adducts, such as cyclohexene adducts, are obtained as a result of the [2+4]cycloaddition involving fullerene as a dienophile. As noted above, the most popular dienes for the functionalization of C60 are ortho-quinodimethanes. The disadvantages of this way of obtaining bis(cycloadducts) include the reversibility of the reaction, the formation of several isomers of bis(cyclohexene) adducts, and the formation of polyadducts. Figure 21 shows the molecular structures of the bis(cyclohexene) fullerene adducts with reduced electron affinity. Many papers have reported the investigation of the unsubstituted tetrahydronaphthalene derivative of fullerene (255) in combination with P3HT in OSCs [135,136,137,185,186]. This material showed improved electrochemical properties with respect to [60]PCBM: the first reduction potential shifted to the cathodic region by 170–200 mV. The VOC values of the solar cells based on compound 255 increased by 220–240 mV compared to the reference system, while there was practically no deterioration in JSC and fill factors, which led to an improvement in PCE, which reached 4.7–5.3%. Apparently, the compact size of the substituents favorably affected the electron transport properties of the material and compatibility with the P3HT polymer. In some works, the authors succeeded in obtaining several pure isomers of adduct 255 and in studying their electronic properties and photovoltaic characteristics [187]. The use of an analogue of this compound based on C70 fullerene derivative 256 allowed Deng et al. to achieve an efficiency of 5.95%, while the reference [70]PCBM/P3HT system showed an efficiency of 4.32% [186].
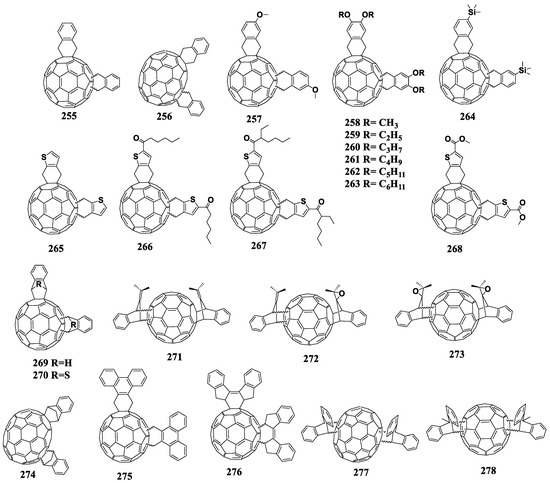
Figure 21.
Molecular structures of the bis(cyclohexene) fullerene adducts with reduced electron affinity.
The modification of the tetrahydronaphthalene fragment with one methoxyl substituent in the phenyl ring (compound 257) had practically no effect on the electrochemical properties of the fullerene derivative and characteristics of the corresponding solar cells. The devices showed an increase in VOC by 240 mV and enhanced PCE by 1.33% as compared to the reference device [138]. Meng et al. introduced two alkoxy groups (compounds 258–263) at the phenyl ring of the tetrahydronaphthalene fragment and varied the length of the solubilizing substituent [188]. It turned out that the fullerene derivative 258 containing methoxy groups demonstrated the worst performance in solar cells: VOC increased by only 100 mV, while other parameters decreased as compared to the reference [60]PCBM/P3HT system. Apparently, the introduction of methoxy substituents decreased the solubility of the compound and worsened its compatibility with P3HT. The best photovoltaic characteristics were obtained for bis(cycloadduct) 260 with propoxy groups: VOC increased by 190 mV, and the efficiency increased by 0.7% (810 mV and 4.1%, respectively).
In a number of studies, the authors managed to isolate individual isomers of compounds 261 and 263 and investigate their electronic properties and photovoltaic characteristics [189,190]. The introduction of the trimethylsilyl group (compound 264) into the phenyl fragment did not improve the efficiency of OSCs as compared to the [60]PCBM/P3HT system [191].
Subsequently, Zhang et al. obtained thiophene-containing C60 fullerene derivatives 265–267 [145]. The devices based on bis(cycloadduct) 265 containing no solubilizing substituents showed the best efficiency (5.1%) and the highest VOC (860 mV), which exceeded the characteristics of the reference devices by 1.5% and 240 mV. The introduction of additional substituents adversely affected JSC and fill factors. Apparently, bulky substituents prevented the formation of the optimal morphology of the composite layer, which led to a deterioration in the charge transport properties of the films. The introduction of the ester group into thiophene residues (fullerene derivative 268) also did not improve the performance of the devices with respect to the reference system [192].
The bis(indene) derivative of C60 (compound 269) is widely used [117,135,136,146,193,194,195,196,197,198,199,200]. The best devices based on the 269/P3HT composite showed PCE values of 6.5 and 7.5% [197,198]. Such a high efficiency was achieved due to the careful optimization of the solar cell fabrication process and the use of a special buffer layer. Note that compound 269 is one of the few bis(cycloadducts) studied in photovoltaic cells in combination with polymers other than P3HT. Good results have been obtained in the composites with the conjugated polymers PDTS-DTFBTA, PSEHTT, PTTBTz, and PTB7-Th.
Cao et al. isolated and studied individual isomers of 269 [193]. Zhen et al. obtained fullerene derivative 270 that differs from the acceptor molecule 269a by the bridging sulfur atom [201]. As shown by the electrochemistry studies, the first reduction potential of the compound 269a shifted to the cathodic region by 180 mV versus [60]PCBM. The paper presented the characteristics of OSCs, where tetrabenzoporphyrin and titanyl phthalocyanine were used as donor materials. The VOC value of the fabricated devices increased by 160 mV, and the efficiency increased by 0.5% as compared to the reference cells. When designing the compound, the authors expected that intermolecular S–S interactions would help to improve the charge transport properties of bis(cycloadduct) 270.
There are also reports on the synthesis of the fullerene adducts with isobenzofulvene (compounds 271–273) [202]. Photovoltaic cells based on these compounds showed an increase in VOC with deterioration of other characteristics. The authors noted that the introduction of the epoxy group significantly increased the value of JSC.
An analogue of compound 269 based on fullerene C70 (compound 274) was also obtained [127,150,196,203]. The characteristics of devices with acceptor 274, as a rule, exceeded the parameters of the reference cells. Mi et al. designed fullerene C60 derivative 275 containing phenanthrene substituents [142]. The first reduction half-wave potential of the compound shifted to the cathodic region by 180 mV against [60]PCBM. Solar cells based on 275 showed an increase in VOC by 70 mV and a significant deterioration of other characteristics with respect to the control cells. Apparently, the fullerene derivative had a poor compatibility with the P3HT polymer due to insufficient solubility, which adversely affected the morphology of the active layer.
He et al. synthesized and studied fullerene-based material 276 containing the bisindene fragment [143]. The first reduction half-wave potential of compound 276 shifted to the cathodic region by 170 mV versus [60]PCBM. Solar cells based on the 276/P3HT composite showed an increase in VOC by 240 mV and a significant deterioration in other characteristics as compared to the reference system. Ruff et al. annelated two fragments of anthracene (compound 277) to fullerene C70 [184]. An electrochemistry study of the fullerene derivative showed a shift of the first reduction potential to the cathodic region by 140 mV with respect to [60]PCBM. The studies of fullerene derivative 278 containing two 9-methylanthracene fragments annelated to the fullerene C60 skeleton in combination with the amorphous polymer PCDTBT showed an increase in VOC of the devices. However, other parameters deteriorated significantly as compared to the reference system [204].
The data on the first reduction potentials of the bis(cyclohexene) fullerene adducts and the results of their study in organic solar cells are presented in Table 9.

Table 9.
First reduction potentials and the characteristics of solar cells based on the bis(cyclohexene) fullerenes adducts.
Thus, a large group of the bis(cyclohexene) fullerene adducts has been obtained and studied. In most cases, the shift of the first reduction potential of the compounds to the cathodic region was 150–190 mV against [60]PCBM, which enabled an increase in the VOC of the corresponding photovoltaic devices by 200–250 mV. However, an increase in VOC was most frequently counterbalanced by the deterioration of other device characteristics. Fullerene derivatives 255, 269, and 274 deserve a special mention. Their use in combination with P3HT in solar cells enabled particularly high device efficiencies. However, their wide application as acceptor materials for OSCs is limited by poor compatibility with promising conjugated copolymers with a small band gap.
6.10. Other Bis(adducts)
The previously described bis(cycloadducts) had two absolutely identical organic addends annelated to the fullerene cage. In this section, we will consider fullerene-containing materials with two addends differing in structure, as well as other types of bis(adducts), the structure of which does not allow them to be attributed to the aforementioned families of compounds. The section also includes the fullerene derivatives with two organic addends, which are linked to each other by a spacer (Figure 22).
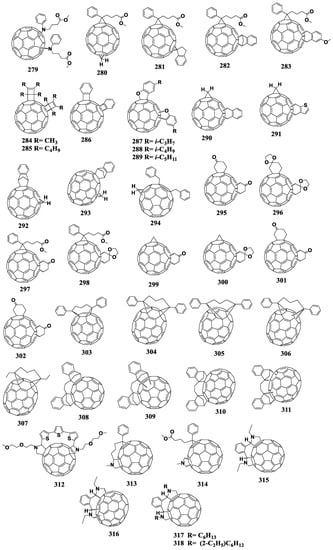
Figure 22.
Molecular structures of fullerene bis(adducts) 279–318 with reduced electron affinity.
In the work of Kim et al., bis(azafulleroid) 279 was obtained as a result of the [2+3]cycloaddition reaction of fullerene with the corresponding azide [205]. Note that, in contrast to classical bis(adducts) characterized by a chaotic distribution of substituents over the fullerene skeleton, in this case, only one compound containing organic addends located in close proximity to each other was formed. The first half-wave potential of compound 279 shifted by 30 mV to the cathodic region against [60]PCBM. The VOC of solar cells based on compound 279 increased by 110 mV as compared to the reference system, whereas other characteristics appeared to be quite low.
From the previously described examples of bis(adducts), it unequivocally follows that the presence of bulky substituents in the molecular structures of compounds result in severe deterioration of charge transport properties. On the contrary, a decrease in the volume of the addend leads to an improvement in the transport characteristics of the fullerene-containing material but worsens its solubility in organic solvents.
The understanding of these dependences inspired a realization of the approach based on the addition of only a small cyclic addend to the classical [60]PCBM molecule, which demonstrated good performance in the devices. Experimentally, this pathway was implemented by annelating the methylene fragment to the fullerene cage in compound 280, which enabled a considerable improvement in the characteristics of the solar cells with respect to the reference [60]PCBM/P3HT system [135,206]. The first reduction potential of compound 280 shifted to the cathodic region by 120 mV versus [60]PCBM, and the observed VOC values were 170 mV higher than those for the control devices. Wang et al. modified [60]PCBM by introducing indene to obtain fullerene derivative 281 [97]. The solar cells based on bis(adduct) 281 outperformed the reference cells in terms of FF and efficiency but, at the same time, JSC decreased slightly.
The opening of one more double bond within the fullerene cage of [60]PCBM upon the annelation of an additional indene-based cyclic fragment (compound 282) also led to a decrease in the electron affinity, an increase in VOC, and an increase in the efficiency of the OSCs as compared to the reference cells [198,207]. In the work of Liao et al., the photovoltaic cells based on composite 282 and P3HT demonstrated a VOC of about 0.86 V and PCE of 6.63% [198]. The devices based on bis(adduct) 283 showed a slight increase in VOC (0.58 V) and efficiency (3.67%) as compared to the [60]PCBM/P3HT system (0.54 V and 3.09%, respectively) [208].
The attachment of two identical tetraalkylcyclobutadiene fragments to the fullerene framework (compounds 284 and 285) resulted in the decreased electron affinity of the obtained compounds and improved VOC values of the corresponding devices [155]. However, other parameters of the photovoltaic cells deteriorated as compared to the control cells. Apparently, the unsatisfactory characteristics are associated with the low solubility of the resulting fullerene derivatives and the suboptimal morphology of the active layer. In contrast, the devices based on bis(benzocyclobutene) adduct 286 showed comparable characteristics with the bis[60]PCBM/P3HT reference system [209]. Bis(cycloadducts) 287–289 containing the benzofuran moiety also showed an increase in the LUMO energy with respect to [60]PCBM [210]. The characteristics of the best photovoltaic cells based on bis(adduct) 288 turned out to be comparable with that of the reference cells: VOC was 0.69 V and the efficiency was 3.4%.
The use of the methylene fragment as one of the addends attached to the fullerene framework allowed one to improve the electronic properties of the obtained compounds and enhance the characteristics of the corresponding devices. This approach was further used in the development of new fullerene derivatives 290–294 with reduced electron affinity [135,211,212,213].
In almost all cases, it enabled not only the reduction in the electron affinity, thereby increasing VOC, but also considerably improved the performance of organic solar cells. In particular, the photovoltaic cells based on the fullerene derivative 290 containing tetrahydronaphthalene and methylene fragments in the work of He et al. demonstrate a VOC of 0.84 V and a PCE of 5.86% [211]. Noteworthy is the compound 292 showing good compatibility with the donor small molecule DPP(TBFu)2. The solar cells based on the 292/DPP(TBFu)2 composite demonstrated an increase in VOC and efficiency as compared to the reference cells based on [60]PCBM/DPP(TBFu)2. Matsuo et al. obtained a rather high efficiency (6.4%) using an analogue of bis(adduct) 292 based on fullerene C70 (compound 293) in photovoltaic devices [135]. Mikie et al. systematically studied a series of bis(cycloadducts) 295–302 containing two identical and also different organic addends [200]. All compounds demonstrate an increase in the LUMO energy with respect to [60]PCBM and also have good solubility. The best results were shown by the photovoltaic cells based on adduct 302: VOC was 0.8 V and the efficiency was 4.43%, which exceeds the parameters of the reference device with the [60]PCBM/P3HT composite.
As noted above, the main disadvantage of the fullerene bis(adducts) is the formation of a large number of regio- and stereoisomers with different electronic and charge transport properties, which often leads to deterioration of OSCs characteristics [214,215]. The use of bifunctional reagents with a rigidly fixed geometry or linked by a spacer helps to reduce the number of isomers formed. Thus, the Hummelen–Woodl reaction with fullerenes C60 and C70 and bis(tosylhydrazone) of 1,3-dibenzoylpropane yielded compounds 303–305 [216]. The electrochemistry studies have shown that the first reduction potentials of the synthesized materials shifted to the cathodic region as compared to the pristine fullerenes by 200 and 300 mV. Unfortunately, the photovoltaic properties of the resulting bis(methanofullerenes) were not studied in this work.
Umeyama et al. obtained and studied a series of bis(cyclopropane) C70 adducts in combination with PCDTBT as the donor material [217]. The solar cells based on compounds 306 and 307 showed an increase in VOC as compared to the reference system, but other parameters deteriorated. Note that compound 306 is a mixture of two isomers, while compound 307 is the individual cis-2 isomer.
Tao et al. obtained a pure cis-2 isomer of bis(adduct) 308 as a result of the cycloaddition of two indene molecules linked by the ethylene unit to C60 [149]. The photovoltaic cells based on compound 308 showed a high VOC of 0.8 V and a PCE of 2.8%, which exceeded the parameters of the devices based on a mixture of the isomers (material 309) and [60]PCBM in combination with P3HT. The cis-2 isomer of bis(adduct) 310 based on fullerene C70 was also obtained and studied; it demonstrated an increase in VOC and PCE of the devices as compared to the cells assembled using a similar mixture of the isomers (compound 311) and [70]PCBM [150].
The bis(adduct) in which pyrolidine fragments annelated to the fullerene core are linked using terthiophene (compound 312) is particularly interesting [119]. However, the characteristics of the solar cells based on compound 312, except for VOC, turned out to be significantly worse as compared to the [60]PCBM/P3HT reference system. In other work, [60]PCBM was modified to form the cis-1 isomer of bis(adduct) 313 containing both cyclopropane and pyrrolidine rings linked to each other [218]. The solar cells based on compound 313 showed a superior efficiency and VOC in comparison with the cells based on [60]PCBM and a mixture of isomers 314.
Izquierdo et al. used phthalaldehyde and the corresponding N-alkylamino acid as precursors for the synthesis of fullerene derivatives 315 and 316 [219,220]. The authors revealed that only three isomers were formed as a result of the reaction of such a reagent having a rigid configuration with fullerene C60, and four isomers are formed in the case of fullerene C70. A small number of isomers should have a positive effect on the use of such fullerene derivatives as acceptor materials in solar cells. Unfortunately, the authors did not investigate these compounds in photovoltaic cells probably because of their low solubility. Nevertheless, the electrochemical data presented in this work indicate that this class of fullerene derivatives is highly promising. The first half-wave reduction potential of compound 315 is shifted to the cathodic region by 300 mV and that of compound 316 is shifted by 400 mV with respect to that of pristine fullerenes C60 and C70, respectively.
In our work, to increase the solubility of material 316, bulkier solubilizing alkyl groups were attached to the pyrrolidine nitrogen atoms [223. The resulting compounds 317 and 318 were studied in photovoltaic cells in combination with P3HT and PCDTBT polymers. The cyclic voltammograms of the materials showed a significant shift of the first reduction potentials to the cathodic region by 210 and 180 mV versus [60]PCBM, respectively. The bulk heterojunction organic solar cells based on 318/P3HT and 318/PCDTBT composites demonstrated an increased VOC compared to the reference devices based on the [60]PCBM/P3HT and [60]PCBM/PCDTBT composites. Moreover, fullerene derivatives 317 and 318 appeared to be resistant to photochemical dimerization, which is one of the parasitic reactions impacting the operational stability of organic solar cells using C60-based acceptors. It was also found that the designed compound 318 remarkably suppressed the photooxidation of conjugated polymers, thus being more efficient than other antioxidant additives described to date [221,222]. The results obtained convincingly show that bis(pyrrolidinofullerenes) are promising acceptor materials for highly efficient and stable organic solar cells.
The data on the first reduction potentials of the fullerene bis(adducts) and the results of their study in organic solar cells are presented in Table 10.

Table 10.
First reduction potentials and characteristics of OSCs based on different types of bis(adducts) with reduced electron affinity.
Thus, a broad variety of fullerene bis(adducts) bearing organic addends differing in structure and even chemical nature has been obtained. In particular, the use of methylene addends for the functionalization of PCBM and related compounds (290, 292, and 293), as well as a similar use of the indene fragment (compound 282), delivered the best results in terms of the efficiency of OSCs. An approach based on the use of bifunctional reagents with a rigid configuration seems to be especially promising, since it allows one to reduce significantly the amount of bis(cycloadduct) isomers formed. Note that the fullerene derivative 318 not only showed an acceptable compatibility with the PCDTBT copolymer but also exhibited efficient antioxidant properties.
6.11. Polyadducts
In this section, we will focus on the fullerene derivatives which have more than two organic addends attached to the fullerene framework (Figure 23). Based on general considerations, we can already assume that the tris(cycloadducts) should have further reduced electron affinity of the fullerene framework. However, the number of formed isomers with different electronic and charge transport properties increases, which can adversely affect the characteristics of the photovoltaic devices based on these materials.
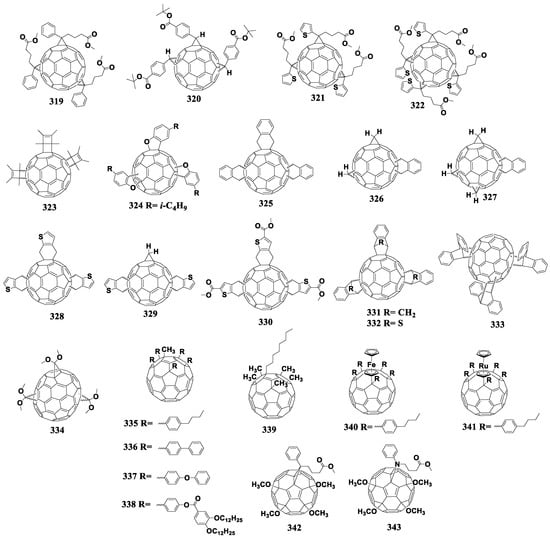
Figure 23.
Molecular structures of the polyadducts with reduced electron affinity.
Lenes et al. showed that the photovoltaic cell based on the [60]PCBM tris(adduct) (compound 319) with the P3HT polymer showed unsatisfactory performance [174]. Despite an increase in VOC by 200 mV (comparable to the gain when using bis(cycloadducts)), other device parameters decreased significantly, which is attributed to the inferior electron transport properties of the material. The use of tris(cyclopropane) adduct 320 as an alternative to [60]PCBM in photovoltaic devices also turned out to be unpromising: VOC increased by only 100 mV, while JSC and PCE decreased almost twice to 4.4 mA cm−2 and 1.68%, respectively [96]. Choi et al. obtained and studied the fullerene derivatives containing three (321) and four (322) thienyl fragments [178]. Despite the large shifts of the first reduction potentials to the cathodic region relative to [60]PCBM, the VOC values of the devices remained almost unchanged, while other parameters deteriorated significantly as compared to the reference system. The fullerene derivatives with three cyclobutane (compound 323) or three benzofuran (compound 324) moieties also proved to be suboptimal n-type semiconductor materials for organic solar cells [155,210]. The devices based on compounds 323 and 324 showed an increase in VOC of 70 mV and 130 mV, respectively, along with a significant deterioration of other parameters as compared to the reference system.
Kim et al. developed and studied the tris(tetrahydronaphthalene) fullerene derivative 325 [137]. The electrochemical data presented in this work show that the first reduction potential of 325 shifted by 330 mV to the cathodic region versus [60]PCBM. The VOC of the photovoltaic cells based on compound 325 and P3HT almost reached 1 V, which is 390 mV higher than that of the reference devices based on [60]PCBM/P3HT. However, there was a strong deterioration of JSC and fill factors, so the resulting efficiency was only 2.63%. Subsequently, using compound 326, which differs from 325 by replacement of two cyclohexene fragments by methylene units, allowed He et al. to significantly improve the characteristics of OSCs based on P3HT: VOC was 950 mV, and the efficiency was 6.43% [211]. Further introduction of the fourth methylene fragment (compound 327) increased VOC to 1 V, whereas the PCE of the cells deteriorated significantly.
Chen et al. isolated an adduct 328 containing three thienyl moieties attached to the fullerene cage [224]. The VOC of the devices based on 328 turned out to be high, but other parameters deteriorated. The replacement of one thienyl addend by the methylene unit yielded 329, which provided increased JSC, fill factors, and PCE as compared to the 328/P3HT reference system. The use of substituted thienyl moieties in trisadduct 330 worsened the device performance [192].
Kang et al. modified fullerene by three solubilizing indene moieties to form compound 331 [146]. Using the obtained trisadduct in photovoltaic devices, it was possible to increase the VOC values by 300 mV as compared to the P3HT/[60]PCBM reference cells, but other parameters deteriorated. Fullerene derivative 332, representing an analogue of compound 331 with sulfur atom in the indene bridge, has only been studied electrochemically [148]. The first reduction potential of compound 332 shifted to the cathodic region by 310 mV versus [60]PCBM. In the work of Ruffa et al., the electrochemical properties of compounds 333 and 334 were studied [176]. Tris(adduct) 333 showed the greatest decrease in the electron affinity, which follows from the cathodic shift of the first reduction potential by 270 mV against [60]PCBM.
The synthesis and investigation of a series of the fullerene derivatives with cyclopentadienyl type moiety 335–341 were reported by Niinomi et al. [225]. The fullerene derivatives were obtained by the reaction of C60 with organomagnesium reagents in the presence of the copper(I) salt as a catalyst. The authors were able to reduce the electron affinity of the fullerene framework, but they failed to achieve acceptable characteristics of the devices based on the designed compounds. The best efficiency of the solar cells based on the 337/P3HT composite was 1.1%. Apparently, the unsatisfactory characteristics were associated with the non-optimal morphology of the composite films of the studied compounds with P3HT. Indeed, in some cases, the formation of homogeneous solid solutions was observed, which leads to the deterioration of all cell parameters, since the transport of photogenerated charge carriers requires some degree of phase segregation.
The fullerene derivatives 342 and 343 in addition to the cyclopropane fragment contain also electron-donating methoxy groups [226]. The electrochemical studies have shown a shift of the first reduction potentials of compounds 342 and 343 to the cathodic region by 200 and 300 mV versus [60]PCBM, respectively. The photovoltaic devices based on compounds 342 and 343 demonstrated just a slight increase in VOC by 50 and 140 mV accompanied by the deterioration of all other parameters as compared to the reference cells based on [60]PCBM and P3HT. The data on the electrochemical and photovoltaic characteristics of the polyadducts are presented in Table 11.

Table 11.
First reduction potentials and characteristics of the solar cells based on the polyadducts with reduced electron affinity.
As can be seen from Table 11, polyadducts with a low electron affinity represent quite interesting materials for OSCs. However, cycloadducts have such disadvantages as a large number of isomers with different electronic and charge transport properties, whereas other types of multiadducts have also demonstrated poor morphological compatibility with conjugated polymers. The most successful examples are polyadducts 326 and 329 with compact methylene addends, which delivered high VOC and PCE values in OSCs.
7. Conclusions
A broad variety of the fullerene derivatives with reduced electron affinity has been obtained until now. About 50 fullerene derivatives with reduced electron affinity demonstrated acceptable characteristics in OSCs in combination with highly crystalline P3HT and actually do not work in composites with promising low band gap conjugated copolymers. Of the entire set of the presented fullerene derivatives with an increased LUMO energy, only a small fraction (<10 compounds) appeared to be compatible with such conjugated copolymers as PCDTBT, PTB7, PTB7-Th, etc. Notably, among this set of materials there are seven compounds containing only one organic addend. Thus, new strategies should be developed to manipulate the optoelectronic characteristics of the fullerene core, in particular, to decrease its electron affinity by adding only one organic addend to the carbon cage.
There is growing evidence that the electronic properties of the fullerene framework, in particular, its electron affinity, can be controlled via the through-space electronic interactions of its π-system with electron-donor groups introduced with the attached organic addend. These interactions enabled a shift in the first reduction potentials of the fullerene derivatives to the cathodic region by 50–100 mV, which is equivalent in magnitude of the observed effect to the opening of one more double bond in the fullerene cage (resulting in the formation of bis-adducts). The fullerene monoadducts with the lowest electron affinity revealed a substantial increase in VOC of the photovoltaic cells by 100–170 mV in comparison to that of the reference devices with [60]PCBM.
A particular important discovery was the ability of some of the fullerene derivatives such as 318 to suppress the photooxidation reactions of conjugated polymers more efficiently than specially designed antioxidant additives described to date. Thus, is seems reasonable to believe that the fullerene derivatives with a reduced electron affinity will continue to be demanded for the fabrication of efficient and stable organic solar cells. Furthermore, the importance of the development of new fullerene derivatives with a reduced electron affinity is emphasized by their successful application as electron transport materials in perovskite solar cells, which is currently attracting considerable attention [227,228,229].
Author Contributions
Writing—original draft preparation, A.V.M.; writing—review and editing, supervision, P.A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of Russian Federation (project 0089-2019-0010/AAAA-A19-119071190044-3).
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge the support of E. Khakina with the preparation of the first version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- NREL—Tranforming Energy. Best Research-Cell Efficiency Chart 2022. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 29 March 2022).
- Dunlop, E.D.; Halton, D. The Performance of Crystalline Silicon Photovoltaic Solar Modules after 22 Years of Continuous Outdoor Exposure. Prog. Photovolt. Res. Appl. 2006, 14, 53–64. [Google Scholar] [CrossRef]
- Skoczek, A.; Sample, T.; Dunlop, E.D. The Results of Performance Measurements of Field-Aged Crystalline Silicon Photovoltaic Modules: Field-Aged Crystalline Silicon Photovoltaic Modules. Prog. Photovolt. Res. Appl. 2009, 17, 227–240. [Google Scholar] [CrossRef]
- Norrman, K.; Ghanbari-Siahkali, A.; Larsen, N.B. 6 Studies of Spin-Coated Polymer Films. Annu. Rep. Prog. Chem. Sect. C 2005, 101, 174. [Google Scholar] [CrossRef]
- Hoth, C.N.; Schilinsky, P.; Choulis, S.A.; Balasubramanian, S.; Brabec, C.J. Solution-Processed Organic Photovoltaics. In Applications of Organic and Printed Electronics; Cantatore, E., Ed.; Springer: Boston, MA, USA, 2013; pp. 27–56. ISBN 9781461431596. [Google Scholar]
- DeJarnette, D. Roll-to-Roll Processing System for Transparent Organic Solar Cells Designed and Verified. Scilight 2018, 2018, 310002. [Google Scholar] [CrossRef]
- Qu, B.; Forrest, S.R. Continuous Roll-to-Roll Fabrication of Organic Photovoltaic Cells via Interconnected High-Vacuum and Low-Pressure Organic Vapor Phase Deposition Systems. Appl. Phys. Lett. 2018, 113, 053302. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.-L.; Lau, T.-K.; Lu, X.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core. Joule 2019, 3, 1140–1151. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, Y.; Wan, X.; Li, C.; Zhang, X.; Wang, Y.; Ke, X.; Xiao, Z.; Ding, L.; Xia, R.; et al. Organic and Solution-Processed Tandem Solar Cells with 17.3% Efficiency. Science 2018, 361, 1094–1098. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, L.; Liu, W.; Zhou, Z.; Yue, Q.; Zheng, W.; Sun, R.; Liu, W.; Xu, S.; Fan, H.; et al. Organic Solar Cells with 18% Efficiency Enabled by an Alloy Acceptor: A Two-in-One Strategy. Adv. Mater. 2021, 33, 2100830. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yamada, H.; Katsumata, Y.; Lee, K.-H.; Araki, K.; Kojima, N. Efficiency Potential and Recent Activities of High-Efficiency Solar Cells. J. Mater. Res. 2017, 32, 3445–3457. [Google Scholar] [CrossRef]
- Benduhn, J.; Tvingstedt, K.; Piersimoni, F.; Ullbrich, S.; Fan, Y.; Tropiano, M.; McGarry, K.A.; Zeika, O.; Riede, M.K.; Douglas, C.J.; et al. Intrinsic Non-Radiative Voltage Losses in Fullerene-Based Organic Solar Cells. Nat. Energy 2017, 2, 17053. [Google Scholar] [CrossRef]
- Kirchartz, T.; Taretto, K.; Rau, U. Efficiency Limits of Organic Bulk Heterojunction Solar Cells. J. Phys. Chem. C 2009, 113, 17958–17966. [Google Scholar] [CrossRef]
- Janssen, R.A.J.; Nelson, J. Factors Limiting Device Efficiency in Organic Photovoltaics. Adv. Mater. 2013, 25, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Koster, L.J.A.; Mihailetchi, V.D.; Blom, P.W.M. Ultimate Efficiency of Polymer/Fullerene Bulk Heterojunction Solar Cells. Appl. Phys. Lett. 2006, 88, 093511. [Google Scholar] [CrossRef]
- Tang, C.W. Two-layer Organic Photovoltaic Cell. Appl. Phys. Lett. 1986, 48, 183–185. [Google Scholar] [CrossRef]
- Po, R.; Carbonera, C.; Bernardi, A.; Camaioni, N. The Role of Buffer Layers in Polymer Solar Cells. Energy Environ. Sci. 2011, 4, 285–310. [Google Scholar] [CrossRef]
- Lin, J.D.A.; Mikhnenko, O.V.; Chen, J.; Masri, Z.; Ruseckas, A.; Mikhailovsky, A.; Raab, R.P.; Liu, J.; Blom, P.W.M.; Loi, M.A.; et al. Systematic Study of Exciton Diffusion Length in Organic Semiconductors by Six Experimental Methods. Mater. Horiz. 2014, 1, 280–285. [Google Scholar] [CrossRef]
- Sariciftci, N.S.; Heeger, A.J. Conjugated Polymer-Acceptor Heterojunctions; Diodes, Photodiodes, and Photovoltaic Cells. U.S. Patent 5454880, 3 October 1995. [Google Scholar]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer Photovoltaic Cells: Enhanced Efficiencies via a Network of Internal Donor-Acceptor Heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef]
- Halls, J.J.M.; Walsh, C.A.; Greenham, N.C.; Marseglia, E.A.; Friend, R.H.; Moratti, S.C.; Holmes, A.B. Efficient Photodiodes from Interpenetrating Polymer Networks. Nature 1995, 376, 498–500. [Google Scholar] [CrossRef]
- Park, S.H.; Roy, A.; Beaupré, S.; Cho, S.; Coates, N.; Moon, J.S.; Moses, D.; Leclerc, M.; Lee, K.; Heeger, A.J. Bulk Heterojunction Solar Cells with Internal Quantum Efficiency Approaching 100%. Nat. Photonics 2009, 3, 297–302. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, S.H.; Lee, H.-H.; Lee, K.; Ma, W.; Gong, X.; Heeger, A.J. New Architecture for High-Efficiency Polymer Photovoltaic Cells Using Solution-Based Titanium Oxide as an Optical Spacer. Adv. Mater. 2006, 18, 572–576. [Google Scholar] [CrossRef]
- Zhou, Y.; Fuentes-Hernandez, C.; Shim, J.; Meyer, J.; Giordano, A.J.; Li, H.; Winget, P.; Papadopoulos, T.; Cheun, H.; Kim, J.; et al. A Universal Method to Produce Low-Work Function Electrodes for Organic Electronics. Science 2012, 336, 327–332. [Google Scholar] [CrossRef] [PubMed]
- White, M.S.; Olson, D.C.; Shaheen, S.E.; Kopidakis, N.; Ginley, D.S. Inverted Bulk-Heterojunction Organic Photovoltaic Device Using a Solution-Derived ZnO Underlayer. Appl. Phys. Lett. 2006, 89, 143517. [Google Scholar] [CrossRef]
- Waldauf, C.; Morana, M.; Denk, P.; Schilinsky, P.; Coakley, K.; Choulis, S.A.; Brabec, C.J. Highly Efficient Inverted Organic Photovoltaics Using Solution Based Titanium Oxide as Electron Selective Contact. Appl. Phys. Lett. 2006, 89, 233517. [Google Scholar] [CrossRef]
- Small, C.E.; Chen, S.; Subbiah, J.; Amb, C.M.; Tsang, S.-W.; Lai, T.-H.; Reynolds, J.R.; So, F. High-Efficiency Inverted Dithienogermole–Thienopyrrolodione-Based Polymer Solar Cells. Nat. Photonics 2012, 6, 115–120. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Hou, J.; Zhang, S.; Liang, Y.; Yang, G.; Yang, Y.; Yu, L.; Wu, Y.; Li, G. Polymer Solar Cells with Enhanced Open-Circuit Voltage and Efficiency. Nat. Photonics 2009, 3, 649–653. [Google Scholar] [CrossRef]
- Albrecht, S.; Vandewal, K.; Tumbleston, J.R.; Fischer, F.S.U.; Douglas, J.D.; Fréchet, J.M.J.; Ludwigs, S.; Ade, H.; Salleo, A.; Neher, D. On the Efficiency of Charge Transfer State Splitting in Polymer:Fullerene Solar Cells. Adv. Mater. 2014, 26, 2533–2539. [Google Scholar] [CrossRef] [PubMed]
- Jakowetz, A.C.; Böhm, M.L.; Zhang, J.; Sadhanala, A.; Huettner, S.; Bakulin, A.A.; Rao, A.; Friend, R.H. What Controls the Rate of Ultrafast Charge Transfer and Charge Separation Efficiency in Organic Photovoltaic Blends. J. Am. Chem. Soc. 2016, 138, 11672–11679. [Google Scholar] [CrossRef]
- Brédas, J.-L.; Norton, J.E.; Cornil, J.; Coropceanu, V. Molecular Understanding of Organic Solar Cells: The Challenges. Acc. Chem. Res. 2009, 42, 1691–1699. [Google Scholar] [CrossRef]
- Clarke, T.M.; Durrant, J.R. Charge Photogeneration in Organic Solar Cells. Chem. Rev. 2010, 110, 6736–6767. [Google Scholar] [CrossRef]
- Hains, A.W.; Liang, Z.; Woodhouse, M.A.; Gregg, B.A. Molecular Semiconductors in Organic Photovoltaic Cells. Chem. Rev. 2010, 110, 6689–6735. [Google Scholar] [CrossRef]
- Elumalai, N.K.; Uddin, A. Open Circuit Voltage of Organic Solar Cells: An in-Depth Review. Energy Environ. Sci. 2016, 9, 391–410. [Google Scholar] [CrossRef]
- Vollbrecht, J.; Brus, V.V. Effects of Recombination Order on Open-Circuit Voltage Decay Measurements of Organic and Perovskite Solar Cells. Energies 2021, 14, 4800. [Google Scholar] [CrossRef]
- Sariciftci, N.S.; Smilowitz, L.; Heeger, A.J.; Wudl, F. Semiconducting Polymers (as Donors) and Buckminsterfullerene (as Acceptor): Photoinduced Electron Transfer and Heterojunction Devices. Synth. Met. 1993, 59, 333–352. [Google Scholar] [CrossRef]
- Hummelen, J.C.; Knight, B.W.; LePeq, F.; Wudl, F.; Yao, J.; Wilkins, C.L. Preparation and Characterization of Fulleroid and Methanofullerene Derivatives. J. Org. Chem. 1995, 60, 532–538. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Yang, S.-H.; Hsu, C.-S. Synthesis of Conjugated Polymers for Organic Solar Cell Applications. Chem. Rev. 2009, 109, 5868–5923. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cao, Y. Development of Novel Conjugated Donor Polymers for High-Efficiency Bulk-Heterojunction Photovoltaic Devices. Acc. Chem. Res. 2009, 42, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Blouin, N.; Michaud, A.; Leclerc, M. A Low-Bandgap Poly(2,7-Carbazole) Derivative for Use in High-Performance Solar Cells. Adv. Mater. 2007, 19, 2295–2300. [Google Scholar] [CrossRef]
- Chan, K.K.H.; Tsang, S.W.; Lee, H.K.H.; So, F.; So, S.K. Charge Transport Study of Semiconducting Polymers and Their Bulk Heterojunction Blends by Capacitance Measurements. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 649–658. [Google Scholar] [CrossRef]
- Facchetti, A. π-Conjugated Polymers for Organic Electronics and Photovoltaic Cell Applications †. Chem. Mater. 2011, 23, 733–758. [Google Scholar] [CrossRef]
- Sun, Y.; Takacs, C.J.; Cowan, S.R.; Seo, J.H.; Gong, X.; Roy, A.; Heeger, A.J. Efficient, Air-Stable Bulk Heterojunction Polymer Solar Cells Using MoOx as the Anode Interfacial Layer. Adv. Mater. 2011, 23, 2226–2230. [Google Scholar] [CrossRef]
- Peters, C.H.; Sachs-Quintana, I.T.; Kastrop, J.P.; Beaupré, S.; Leclerc, M.; McGehee, M.D. High Efficiency Polymer Solar Cells with Long Operating Lifetimes. Adv. Energy Mater. 2011, 1, 491–494. [Google Scholar] [CrossRef]
- He, Z.; Zhong, C.; Huang, X.; Wong, W.-Y.; Wu, H.; Chen, L.; Su, S.; Cao, Y. Simultaneous Enhancement of Open-Circuit Voltage, Short-Circuit Current Density, and Fill Factor in Polymer Solar Cells. Adv. Mater. 2011, 23, 4636–4643. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, Y.; Feng, D.; Tsai, S.-T.; Son, H.-J.; Li, G.; Yu, L. Development of New Semiconducting Polymers for High Performance Solar Cells. J. Am. Chem. Soc. 2009, 131, 56–57. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, G.; Huang, D.; Kong, J.; Goh, T.; Huang, W.; Yu, J.; Taylor, A.D. Binary Solvent Additives Treatment Boosts the Efficiency of PTB7:PCBM Polymer Solar Cells to Over 9.5%. Sol. RRL 2018, 2, 1700144. [Google Scholar] [CrossRef]
- Li, W.; Cai, J.; Cai, F.; Yan, Y.; Yi, H.; Gurney, R.S.; Liu, D.; Iraqi, A.; Wang, T. Achieving over 11% Power Conversion Efficiency in PffBT4T-2OD-Based Ternary Polymer Solar Cells with Enhanced Open-Circuit-Voltage and Suppressed Charge Recombination. Nano Energy 2018, 44, 155–163. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Li, Z.; Mu, C.; Ma, W.; Hu, H.; Jiang, K.; Lin, H.; Ade, H.; Yan, H. Aggregation and Morphology Control Enables Multiple Cases of High-Efficiency Polymer Solar Cells. Nat. Commun. 2014, 5, 5293. [Google Scholar] [CrossRef]
- Shaheen, S.E.; Brabec, C.J.; Sariciftci, N.S.; Padinger, F.; Fromherz, T.; Hummelen, J.C. 2.5% Efficient Organic Plastic Solar Cells. Appl. Phys. Lett. 2001, 78, 841–843. [Google Scholar] [CrossRef]
- Li, G.; Shrotriya, V.; Yao, Y.; Yang, Y. Investigation of Annealing Effects and Film Thickness Dependence of Polymer Solar Cells Based on Poly(3-Hexylthiophene). J. Appl. Phys. 2005, 98, 043704. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, Z.; Xia, J.; Tsai, S.-T.; Wu, Y.; Li, G.; Ray, C.; Yu, L. For the Bright Future-Bulk Heterojunction Polymer Solar Cells with Power Conversion Efficiency of 7.4%. Adv. Mater. 2010, 22, E135–E138. [Google Scholar] [CrossRef]
- Price, S.C.; Stuart, A.C.; Yang, L.; Zhou, H.; You, W. Fluorine Substituted Conjugated Polymer of Medium Band Gap Yields 7% Efficiency in Polymer−Fullerene Solar Cells. J. Am. Chem. Soc. 2011, 133, 4625–4631. [Google Scholar] [CrossRef]
- He, Z.; Zhong, C.; Su, S.; Xu, M.; Wu, H.; Cao, Y. Enhanced Power-Conversion Efficiency in Polymer Solar Cells Using an Inverted Device Structure. Nat. Photonics 2012, 6, 591–595. [Google Scholar] [CrossRef]
- Moon, J.S.; Jo, J.; Heeger, A.J. Nanomorphology of PCDTBT:PC70BM Bulk Heterojunction Solar Cells. Adv. Energy Mater. 2012, 2, 304–308. [Google Scholar] [CrossRef]
- Li, W.; Hendriks, K.H.; Roelofs, W.S.C.; Kim, Y.; Wienk, M.M.; Janssen, R.A.J. Efficient Small Bandgap Polymer Solar Cells with High Fill Factors for 300 Nm Thick Films. Adv. Mater. 2013, 25, 3182–3186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gu, Y.; Guo, X.; Liu, F.; Zhang, S.; Huo, L.; Russell, T.P.; Hou, J. Efficient Polymer Solar Cells Based on Benzothiadiazole and Alkylphenyl Substituted Benzodithiophene with a Power Conversion Efficiency over 8%. Adv. Mater. 2013, 25, 4944–4949. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.-H.; Jhuo, H.-J.; Cheng, Y.-S.; Chen, S.-A. Fullerene Derivative-Doped Zinc Oxide Nanofilm as the Cathode of Inverted Polymer Solar Cells with Low-Bandgap Polymer (PTB7-Th) for High Performance. Adv. Mater. 2013, 25, 4766–4771. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, N.; Lou, S.J.; Smith, J.; Tice, D.B.; Hennek, J.W.; Ortiz, R.P.; Navarrete, J.T.L.; Li, S.; Strzalka, J.; et al. Polymer Solar Cells with Enhanced Fill Factors. Nat. Photonics 2013, 7, 825–833. [Google Scholar] [CrossRef]
- Hu, H.; Jiang, K.; Yang, G.; Liu, J.; Li, Z.; Lin, H.; Liu, Y.; Zhao, J.; Zhang, J.; Huang, F.; et al. Terthiophene-Based D–A Polymer with an Asymmetric Arrangement of Alkyl Chains That Enables Efficient Polymer Solar Cells. J. Am. Chem. Soc. 2015, 137, 14149–14157. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Yang, G.; Jiang, K.; Lin, H.; Ade, H.; Ma, W.; Yan, H. Efficient Organic Solar Cells Processed from Hydrocarbon Solvents. Nat. Energy 2016, 1, 15027. [Google Scholar] [CrossRef]
- Wang, D.H.; Park, K.H.; Seo, J.H.; Seifter, J.; Jeon, J.H.; Kim, J.K.; Park, J.H.; Park, O.O.; Heeger, A.J. Enhanced Power Conversion Efficiency in PCDTBT/PC70BM Bulk Heterojunction Photovoltaic Devices with Embedded Silver Nanoparticle Clusters. Adv. Energy Mater. 2011, 1, 766–770. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, Y.; Jin, K.; Qin, J.; Xu, J.; Li, W.; Xiong, J.; Liu, J.; Xiao, Z.; Sun, K.; et al. 18% Efficiency Organic Solar Cells. Sci. Bull. 2020, 65, 272–275. [Google Scholar] [CrossRef]
- Zhang, X.; Zhan, C.; Yao, J. Non-Fullerene Organic Solar Cells with 6.1% Efficiency through Fine-Tuning Parameters of the Film-Forming Process. Chem. Mater. 2015, 27, 166–173. [Google Scholar] [CrossRef]
- Holliday, S.; Ashraf, R.S.; Nielsen, C.B.; Kirkus, M.; Röhr, J.A.; Tan, C.-H.; Collado-Fregoso, E.; Knall, A.-C.; Durrant, J.R.; Nelson, J.; et al. A Rhodanine Flanked Nonfullerene Acceptor for Solution-Processed Organic Photovoltaics. J. Am. Chem. Soc. 2015, 137, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mu, C.; Jiang, K.; Zhao, J.; Li, Y.; Zhang, L.; Li, Z.; Lai, J.Y.L.; Hu, H.; Ma, T.; et al. A Tetraphenylethylene Core-Based 3D Structure Small Molecular Acceptor Enabling Efficient Non-Fullerene Organic Solar Cells. Adv. Mater. 2015, 27, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.K.; Park, J.-H.; Kim, D.W.; Park, S.K.; Park, S.Y. An All-Small-Molecule Organic Solar Cell with High Efficiency Nonfullerene Acceptor. Adv. Mater. 2015, 27, 1951–1956. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-J.; Li, H.; Courtright, B.A.E.; Subramaniyan, S.; Jenekhe, S.A. Nonfullerene Polymer Solar Cells with 8.5% Efficiency Enabled by a New Highly Twisted Electron Acceptor Dimer. Adv. Mater. 2016, 28, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yu, T.; Bi, Z.; Ma, W.; Li, Y.; Peng, Q. Realizing Over 13% Efficiency in Green-Solvent-Processed Nonfullerene Organic Solar Cells Enabled by 1,3,4-Thiadiazole-Based Wide-Bandgap Copolymers. Adv. Mater. 2018, 30, 1703973. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, S.; Yao, H.; Zhang, S.; Zhang, Y.; Yang, B.; Hou, J. Molecular Optimization Enables over 13% Efficiency in Organic Solar Cells. J. Am. Chem. Soc. 2017, 139, 7148–7151. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yao, H.; Hong, L.; Zhang, T.; Tang, Y.; Lin, B.; Xian, K.; Gao, B.; An, C.; Bi, P.; et al. Organic Photovoltaic Cell with 17% Efficiency and Superior Processability. Natl. Sci. Rev. 2020, 7, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.; Speller, E.M.; Wadsworth, A.; Wyatt, M.F.; Dimitrov, S.; Lee, H.K.H.; Li, Z.; Tsoi, W.C.; McCulloch, I.; Bagnis, D.; et al. Twist and Degrade—Impact of Molecular Structure on the Photostability of Nonfullerene Acceptors and Their Photovoltaic Blends. Adv. Energy Mater. 2019, 9, 1803755. [Google Scholar] [CrossRef]
- Speller, E.M.; Clarke, A.J.; Luke, J.; Lee, H.K.H.; Durrant, J.R.; Li, N.; Wang, T.; Wong, H.C.; Kim, J.-S.; Tsoi, W.C.; et al. From Fullerene Acceptors to Non-Fullerene Acceptors: Prospects and Challenges in the Stability of Organic Solar Cells. J. Mater. Chem. A 2019, 7, 23361–23377. [Google Scholar] [CrossRef]
- Park, S.; Son, H.J. Intrinsic Photo-Degradation and Mechanism of Polymer Solar Cells: The Crucial Role of Non-Fullerene Acceptors. J. Mater. Chem. A 2019, 7, 25830–25837. [Google Scholar] [CrossRef]
- Du, X.; Heumueller, T.; Gruber, W.; Almora, O.; Classen, A.; Qu, J.; He, F.; Unruh, T.; Li, N.; Brabec, C.J. Unraveling the Microstructure-Related Device Stability for Polymer Solar Cells Based on Nonfullerene Small-Molecular Acceptors. Adv. Mater. 2020, 32, 1908305. [Google Scholar] [CrossRef] [PubMed]
- Bartesaghi, D.; Ye, G.; Chiechi, R.C.; Koster, L.J.A. Compatibility of PTB7 and [70]PCBM as a Key Factor for the Stability of PTB7:[70]PCBM Solar Cells. Adv. Energy Mater. 2016, 6, 1502338. [Google Scholar] [CrossRef]
- Duan, L.; Meng, X.; Zhang, Y.; Yi, H.; Jin, K.; Haque, F.; Xu, C.; Xiao, Z.; Ding, L.; Uddin, A. Comparative Analysis of Burn-in Photo-Degradation in Non-Fullerene COi8DFIC Acceptor Based High-Efficiency Ternary Organic Solar Cells. Mater. Chem. Front. 2019, 3, 1085–1096. [Google Scholar] [CrossRef]
- Günther, M.; Blätte, D.; Oechsle, A.L.; Rivas, S.S.; Yousefi Amin, A.A.; Müller-Buschbaum, P.; Bein, T.; Ameri, T. Increasing Photostability of Inverted Nonfullerene Organic Solar Cells by Using Fullerene Derivative Additives. ACS Appl. Mater. Interfaces 2021, 13, 19072–19084. [Google Scholar] [CrossRef] [PubMed]
- Troshin, P.A.; Hoppe, H.; Renz, J.; Egginger, M.; Mayorova, J.Y.; Goryachev, A.E.; Peregudov, A.S.; Lyubovskaya, R.N.; Gobsch, G.; Sariciftci, N.S.; et al. Material Solubility-Photovoltaic Performance Relationship in the Design of Novel Fullerene Derivatives for Bulk Heterojunction Solar Cells. Adv. Funct. Mater. 2009, 19, 779–788. [Google Scholar] [CrossRef]
- Kobayashi, S.; Takenobu, T.; Mori, S.; Fujiwara, A.; Iwasa, Y. Fabrication and Characterization of C60 Thin-Film Transistors with High Field-Effect Mobility. Appl. Phys. Lett. 2003, 82, 4581–4583. [Google Scholar] [CrossRef]
- Waldauf, C.; Schilinsky, P.; Perisutti, M.; Hauch, J.; Brabec, C.J. Solution-Processed Organic n-Type Thin-Film Transistors. Adv. Mater. 2003, 15, 2084–2088. [Google Scholar] [CrossRef]
- Kooistra, F.B.; Knol, J.; Kastenberg, F.; Popescu, L.M.; Verhees, W.J.H.; Kroon, J.M.; Hummelen, J.C. Increasing the Open Circuit Voltage of Bulk-Heterojunction Solar Cells by Raising the LUMO Level of the Acceptor. Org. Lett. 2007, 9, 551–554. [Google Scholar] [CrossRef]
- Zheng, L.; Zhou, Q.; Deng, X.; Yuan, M.; Yu, G.; Cao, Y. Methanofullerenes Used as Electron Acceptors in Polymer Photovoltaic Devices. J. Phys. Chem. B 2004, 108, 11921–11926. [Google Scholar] [CrossRef]
- Yang, C.-H.; Chang, J.-Y.; Yeh, P.-H.; Guo, T.-F. Preparation and Characterization of Methanofullerenes for Polymer–Fullerene Bulk Heterojunction Solar Cells. Carbon 2007, 45, 2951–2956. [Google Scholar] [CrossRef]
- Yang, C.; Kim, J.Y.; Cho, S.; Lee, J.K.; Heeger, A.J.; Wudl, F. Functionalized Methanofullerenes Used as N-Type Materials in Bulk-Heterojunction Polymer Solar Cells and in Field-Effect Transistors. J. Am. Chem. Soc. 2008, 130, 6444–6450. [Google Scholar] [CrossRef] [PubMed]
- Mikroyannidis, J.A.; Kabanakis, A.N.; Sharma, S.S.; Sharma, G.D. A Simple and Effective Modification of PCBM for Use as an Electron Acceptor in Efficient Bulk Heterojunction Solar Cells. Adv. Funct. Mater. 2011, 21, 746–755. [Google Scholar] [CrossRef]
- Mikroyannidis, J.A.; Kabanakis, A.N.; Suresh, P.; Sharma, G.D. Efficient Bulk Heterojunction Solar Cells Based on a Broadly Absorbing Phenylenevinylene Copolymer Containing Thiophene and Pyrrole Rings. J. Phys. Chem. C 2011, 115, 7056–7066. [Google Scholar] [CrossRef]
- Singh, S.P.; Kumar, C.H.P.; Sharma, G.D.; Kurchania, R.; Roy, M.S. Synthesis of a Modified PC 70 BM and Its Application as an Electron Acceptor with Poly(3-Hexylthiophene) as an Electron Donor for Efficient Bulk Heterojunction Solar Cells. Adv. Funct. Mater. 2012, 22, 4087–4095. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Zhang, W.; Ai, X.-C.; Zhang, J.-P.; Wang, X.-F.; Kido, J. Influence of Fullerene Multiadducts on the Morphology and Charge Photogeneration of Their Photovoltaic Blends with Poly(3-Hexylthiophene). J. Phys. Chem. C 2013, 117, 25898–25907. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Z.; Liu, J.; Wang, L. Fullerene Adducts Bearing Cyano Moiety for Both High Dielectric Constant and Good Active Layer Morphology of Organic Photovoltaics. Adv. Funct. Mater. 2016, 26, 6107–6113. [Google Scholar] [CrossRef]
- Kim, H.U.; Kim, J.-H.; Kang, H.; Grimsdale, A.C.; Kim, B.J.; Yoon, S.C.; Hwang, D.-H. Naphthalene-, Anthracene-, and Pyrene-Substituted Fullerene Derivatives as Electron Acceptors in Polymer-Based Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 20776–20785. [Google Scholar] [CrossRef]
- Zhang, Y.; Yip, H.-L.; Acton, O.; Hau, S.K.; Huang, F.; Jen, A.K.-Y. A Simple and Effective Way of Achieving Highly Efficient and Thermally Stable Bulk-Heterojunction Polymer Solar Cells Using Amorphous Fullerene Derivatives as Electron Acceptor. Chem. Mater. 2009, 21, 2598–2600. [Google Scholar] [CrossRef]
- Kim, S.-O.; Sung Chung, D.; Cha, H.; Wan Jang, J.; Kim, Y.-H.; Kang, J.-W.; Jeong, Y.-S.; Park, C.E.; Kwon, S.-K. Thermally Stable Organic Bulk Heterojunction Photovoltaic Cells Incorporating an Amorphous Fullerene Derivative as an Electron Acceptor. Sol. Energy Mater. Sol. Cells 2011, 95, 432–439. [Google Scholar] [CrossRef]
- Kim, H.U.; Mi, D.; Kim, J.-H.; Park, J.B.; Yoon, S.C.; Yoon, U.C.; Hwang, D.-H. Carbazole-Containing Fullerene Derivatives for P3HT-Based Bulk-Heterojunction Solar Cells. Sol. Energy Mater. Sol. Cells 2012, 105, 6–14. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Yu, T.; Zhang, H.; Wei, C.; Liu, X. Synthesis and Photovoltaic Properties of Carbazole-Substituted Fullerene Derivatives. New J. Chem. 2017, 41, 4702–4706. [Google Scholar] [CrossRef]
- Liu, C.; Xu, L.; Chi, D.; Li, Y.; Liu, H.; Wang, J. Synthesis of Novel Acceptor Molecules of Mono- and Multiadduct Fullerene Derivatives for Improving Photovoltaic Performance. ACS Appl. Mater. Interfaces 2013, 5, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liao, X.; Wang, J.; Wang, C.; Chan, M.-Y.; Yam, V.W.-W. Indan-C60: From a Crystalline Molecule to Photovoltaic Application. Chem. Commun. 2013, 49, 9923. [Google Scholar] [CrossRef]
- Riedel, I.; Von Hauff, E.; Parisi, J.; Martín, N.; Giacalone, F.; Dyakonov, V. Diphenylmethanofullerenes: New and Efficient Acceptors in Bulk-Heterojunction Solar Cells. Adv. Funct. Mater. 2005, 15, 1979–1987. [Google Scholar] [CrossRef]
- Bolink, H.J.; Coronado, E.; Forment-Aliaga, A.; Lenes, M.; La Rosa, A.; Filippone, S.; Martín, N. Polymer Solar Cells Based on Diphenylmethanofullerenes with Reduced Sidechain Length. J. Mater. Chem. 2011, 21, 1382–1386. [Google Scholar] [CrossRef]
- Garcia-Belmonte, G.; Boix, P.P.; Bisquert, J.; Lenes, M.; Bolink, H.J.; La Rosa, A.; Filippone, S.; Martín, N. Influence of the Intermediate Density-of-States Occupancy on Open-Circuit Voltage of Bulk Heterojunction Solar Cells with Different Fullerene Acceptors. J. Phys. Chem. Lett. 2010, 1, 2566–2571. [Google Scholar] [CrossRef]
- Fernández, D.; Viterisi, A.; Ryan, J.W.; Gispert-Guirado, F.; Vidal, S.; Filippone, S.; Martín, N.; Palomares, E. Small Molecule BHJ Solar Cells Based on DPP(TBFu)2 and Diphenylmethanofullerenes (DPM): Linking Morphology, Transport, Recombination and Crystallinity. Nanoscale 2014, 6, 5871–5878. [Google Scholar] [CrossRef]
- Singh, S.P.; Kumar, C.P.; Nagarjuna, P.; Sharma, G.D.; Biswas, S.; Mikroyannidis, J.A. Diarylmethanofullerene: Efficient Polymer Solar Cells with Low-Band-Gap Copolymer. J. Phys. Chem. C 2013, 117, 13350–13356. [Google Scholar] [CrossRef]
- Singh, S.P.; Kumar, C.P.; Nagarjuna, P.; Kandhadi, J.; Giribabu, L.; Chandrasekharam, M.; Biswas, S.; Sharma, G.D. Efficient Solution Processable Polymer Solar Cells Using Newly Designed and Synthesized Fullerene Derivatives. J. Phys. Chem. C 2016, 120, 19493–19503. [Google Scholar] [CrossRef]
- Ross, R.B.; Cardona, C.M.; Guldi, D.M.; Sankaranarayanan, S.G.; Reese, M.O.; Kopidakis, N.; Peet, J.; Walker, B.; Bazan, G.C.; Van Keuren, E.; et al. Endohedral Fullerenes for Organic Photovoltaic Devices. Nat. Mater. 2009, 8, 208–212. [Google Scholar] [CrossRef]
- Suzuki, T.; Maruyama, Y.; Akasaka, T.; Ando, W.; Kobayashi, K.; Nagase, S. Redox Properties of Organofullerenes. J. Am. Chem. Soc. 1994, 116, 1359–1363. [Google Scholar] [CrossRef]
- Matsumoto, F.; Iwai, T.; Moriwaki, K.; Takao, Y.; Ito, T.; Mizuno, T.; Ohno, T. Controlling the Polarity of Fullerene Derivatives to Optimize Nanomorphology in Blend Films. ACS Appl. Mater. Interfaces 2016, 8, 4803–4810. [Google Scholar] [CrossRef]
- Mumyatov, A.V.; Goryachev, A.E.; Prudnov, F.A.; Mukhacheva, O.A.; Sagdullina, D.K.; Chernyak, A.V.; Troyanov, S.I.; Troshin, P.A. Monocyclopropanated Fullerene Derivatives with Decreased Electron Affinity as Promising Electron Acceptor Materials for Organic Solar Cells. Synth. Met. 2020, 270, 116565. [Google Scholar] [CrossRef]
- Zhang, C.; Mumyatov, A.; Langner, S.; Perea, J.D.; Kassar, T.; Min, J.; Ke, L.; Chen, H.; Gerasimov, K.L.; Anokhin, D.V.; et al. Overcoming the Thermal Instability of Efficient Polymer Solar Cells by Employing Novel Fullerene-Based Acceptors. Adv. Energy Mater. 2017, 7, 1601204. [Google Scholar] [CrossRef]
- Maggini, M.; Scorrano, G.; Prato, M. Addition of Azomethine Ylides to C60: Synthesis, Characterization, and Functionalization of Fullerene Pyrrolidines. J. Am. Chem. Soc. 1993, 115, 9798–9799. [Google Scholar] [CrossRef]
- Bagno, A.; Claeson, S.; Maggini, M.; Martini, M.L.; Prato, M.; Scorrano, G. [60]Fullerene as a Substituent. Chem. A Eur. J. 2002, 8, 1015. [Google Scholar] [CrossRef]
- Troshin, P.A.; Peregudov, A.S.; Mühlbacher, D.; Lyubovskaya, R.N. An Efficient [2+3] Cycloaddition Approach to the Synthesis of Pyridyl-Appended Fullerene Ligands. Eur. J. Org. Chem. 2005, 2005, 3064–3074. [Google Scholar] [CrossRef]
- Wang, N.; Bao, X.; Yang, C.; Wang, J.; Woo, H.Y.; Lan, Z.; Chen, W.; Yang, R. Design and Synthesis of Indole-Substituted Fullerene Derivatives with Different Side Groups for Organic Photovoltaic Devices. Org. Electron. 2013, 14, 682–692. [Google Scholar] [CrossRef]
- Valitov, M.I.; Romanova, I.P.; Gromchenko, A.A.; Shaikhutdinova, G.R.; Yakhvarov, D.G.; Bruevich, V.V.; Dyakov, V.A.; Sinyashin, O.G.; Paraschuk, D.Y. Indolinone-Substituted Methanofullerene—A New Acceptor for Organic Solar Cells. Sol. Energy Mater. Sol. Cells 2012, 103, 48–52. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, L.; Zheng, W.; Bao, X.; Wang, N.; Wang, T.; Yang, R. The Preparation and Properties of Bulk-Heterojunction Organic Solar Cells with Indole-Containing Fulleropyrrolidine Derivatives as Acceptors. Tetrahedron 2013, 69, 9544–9550. [Google Scholar] [CrossRef]
- Matsumoto, K.; Hashimoto, K.; Kamo, M.; Uetani, Y.; Hayase, S.; Kawatsura, M.; Itoh, T. Design of Fulleropyrrolidine Derivatives as an Acceptor Molecule in a Thin Layer Organic Solar Cell. J. Mater. Chem. 2010, 20, 9226. [Google Scholar] [CrossRef]
- Ren, B.-Y.; Ou, C.-J.; Zhang, C.; Chang, Y.-Z.; Yi, M.-D.; Liu, J.-Q.; Xie, L.-H.; Zhang, G.-W.; Deng, X.-Y.; Li, S.-B.; et al. Diarylfluorene-Modified Fulleropyrrolidine Acceptors to Tune Aggregate Morphology for Solution-Processable Polymer/Fullerene Bulk-Heterojunction Solar Cells. J. Phys. Chem. C 2012, 116, 8881–8887. [Google Scholar] [CrossRef]
- He, Y.; Chen, H.-Y.; Hou, J.; Li, Y. Indene−C60 Bisadduct: A New Acceptor for High-Performance Polymer Solar Cells. J. Am. Chem. Soc. 2010, 132, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Seo, J.H.; Park, E.Y.; Kim, T.-D.; Lee, K.; Lee, K.-S.; Cho, S.; Heeger, A.J. Increased Open-Circuit Voltage in Bulk-Heterojunction Solar Cells Using a C60 Derivative. Appl. Phys. Lett. 2010, 97, 193309. [Google Scholar] [CrossRef]
- Yoshimura, K.; Matsumoto, K.; Uetani, Y.; Sakumichi, S.; Hayase, S.; Kawatsura, M.; Itoh, T. Thiophene-Substituted Fulleropyrrolidine Derivatives as Acceptor Molecules in a Thin Film Organic Solar Cell. Tetrahedron 2012, 68, 3605–3610. [Google Scholar] [CrossRef]
- Blanco, G.D.; Hiltunen, A.J.; Lim, G.N.; Kc, C.B.; Kaunisto, K.M.; Vuorinen, T.K.; Nesterov, V.N.; Lemmetyinen, H.J.; D’Souza, F. Syntheses, Charge Separation, and Inverted Bulk Heterojunction Solar Cell Application of Phenothiazine–Fullerene Dyads. ACS Appl. Mater. Interfaces 2016, 8, 8481–8490. [Google Scholar] [CrossRef]
- Karakawa, M.; Nagai, T.; Adachi, K.; Ie, Y.; Aso, Y. N-Phenyl[60]Fulleropyrrolidines: Alternative Acceptor Materials to PC 61 BM for High Performance Organic Photovoltaic Cells. J. Mater. Chem. A 2014, 2, 20889–20895. [Google Scholar] [CrossRef]
- Karakawa, M.; Nagai, T. Interfacial Reaction of Fulleropyrrolidines Affecting Organic Photovoltaic Performance. ACS Appl. Mater. Interfaces 2017, 9, 21338–21345. [Google Scholar] [CrossRef]
- Liang, Y.; Hao, Y.; Liu, X.; Feng, L.; Chen, M.; Tang, Q.; Chen, N.; Tang, M.; Sun, B.; Zhou, Y.; et al. Efficiency Enhancement from [60]Fulleropyrrolidine-Based Polymer Solar Cells through N-Substitution Manipulation. Carbon 2015, 92, 185–192. [Google Scholar] [CrossRef]
- Nagarjuna, P.; Bagui, A.; Garg, A.; Gupta, V.; Singh, S.P. One-Step Synthesis of New Electron Acceptor for High Efficiency Solution Processable Organic Solar Cells. J. Phys. Chem. C 2017, 121, 26615–26621. [Google Scholar] [CrossRef]
- Karakawa, M.; Nagai, T.; Adachi, K.; Ie, Y.; Aso, Y. Precise Control over Reduction Potential of Fulleropyrrolidines for Organic Photovoltaic Materials. RSC Adv. 2017, 7, 7122–7129. [Google Scholar] [CrossRef]
- Kawajiri, K.; Kawanoue, T.; Yamato, M.; Terai, K.; Yamashita, M.; Furukawa, M.; Aratani, N.; Suzuki, M.; Nakayama, K.; Yamada, H. Fullerene-Based n-Type Materials That Can Be Processed by a Photoprecursor Approach for Photovoltaic Applications. ECS J. Solid State Sci. Technol. 2017, 6, M3068–M3074. [Google Scholar] [CrossRef]
- Yoshimura, K.; Sugawara, K.; Sakumichi, S.; Matsumoto, K.; Uetani, Y.; Hayase, S.; Nokami, T.; Itoh, T. Photovoltaic Properties of OPV Devices Using Cis—And Trans -2,5-Diarylfulleropyrrolidines as Acceptor Partners with P3HT on an ITO Electrode with or without PEDOT:PSS. Chem. Lett. 2013, 42, 1209–1211. [Google Scholar] [CrossRef]
- Mumyatov, A.V.; Prudnov, F.A.; Inasaridze, L.N.; Mukhacheva, O.A.; Troshin, P.A. High LUMO Energy Pyrrolidinofullerenes as Promising Electron-Acceptor Materials for Organic Solar Cells. J. Mater. Chem. C 2015, 3, 11612–11617. [Google Scholar] [CrossRef]
- Bouwer, R.K.M.; Wetzelaer, G.-J.A.H.; Blom, P.W.M.; Hummelen, J.C. Influence of the Isomeric Composition of the Acceptor on the Performance of Organic Bulk Heterojunction P3HT:Bis-PCBM Solar Cells. J. Mater. Chem. 2012, 22, 15412. [Google Scholar] [CrossRef]
- Kokubo, K.; Masuda, H.; Ikuma, N.; Mikie, T.; Oshima, T. Synthesis and Characterization of New Acetalized [60]Fullerenes. Tetrahedron Lett. 2013, 54, 3510–3513. [Google Scholar] [CrossRef]
- Mikie, T.; Saeki, A.; Masuda, H.; Ikuma, N.; Kokubo, K.; Seki, S. New Efficient (Thio)Acetalized Fullerene Monoadducts for Organic Solar Cells: Characterization Based on Solubility, Mobility Balance, and Dark Current. J. Mater. Chem. A 2015, 3, 1152–1157. [Google Scholar] [CrossRef]
- Mikie, T.; Saeki, A.; Yamazaki, Y.; Ikuma, N.; Kokubo, K.; Seki, S. Stereochemistry of Spiro-Acetalized [60]Fullerenes: How the Exo and Endo Stereoisomers Influence Organic Solar Cell Performance. ACS Appl. Mater. Interfaces 2015, 7, 8915–8922. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, W.; Li, W.; Hong, L.; Lei, T.; Mi, D.; Peng, R.; Ouyang, X.; Ge, Z. Reducible Fabrication Cost for P3HT-Based Organic Solar Cells by Using One-Step Synthesized Novel Fullerene Derivative. Sol. Energy Mater. Sol. Cells 2017, 159, 172–178. [Google Scholar] [CrossRef]
- Belik, P.; Gügel, A.; Spickermann, J.; Müllen, K. Reaction of Buckminsterfullerene with Ortho -Quinodimethane: A New Access to Stable C60 Derivatives. Angew. Chem. Int. Ed. Engl. 1993, 32, 78–80. [Google Scholar] [CrossRef]
- Matsuo, Y.; Kawai, J.; Inada, H.; Nakagawa, T.; Ota, H.; Otsubo, S.; Nakamura, E. Addition of Dihydromethano Group to Fullerenes to Improve the Performance of Bulk Heterojunction Organic Solar Cells. Adv. Mater. 2013, 25, 6266–6269. [Google Scholar] [CrossRef]
- Voroshazi, E.; Vasseur, K.; Aernouts, T.; Heremans, P.; Baumann, A.; Deibel, C.; Xue, X.; Herring, A.J.; Athans, A.J.; Lada, T.A.; et al. Novel Bis-C60 Derivative Compared to Other Fullerene Bis-Adducts in High Efficiency Polymer Photovoltaic Cells. J. Mater. Chem. 2011, 21, 17345. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kang, H.; Nam, S.Y.; Jung, J.; Kim, P.S.; Cho, C.-H.; Lee, C.; Yoon, S.C.; Kim, B.J. Facile Synthesis of o -Xylenyl Fullerene Multiadducts for High Open Circuit Voltage and Efficient Polymer Solar Cells. Chem. Mater. 2011, 23, 5090–5095. [Google Scholar] [CrossRef]
- Deng, L.-L.; Feng, J.; Sun, L.-C.; Wang, S.; Xie, S.-L.; Xie, S.-Y.; Huang, R.-B.; Zheng, L.-S. Functionalized Dihydronaphthyl-C60 Derivatives as Acceptors for Efficient Polymer Solar Cells with Tunable Photovoltaic Properties. Sol. Energy Mater. Sol. Cells 2012, 104, 113–120. [Google Scholar] [CrossRef]
- Backer, S.A.; Sivula, K.; Kavulak, D.F.; Fréchet, J.M.J. High Efficiency Organic Photovoltaics Incorporating a New Family of Soluble Fullerene Derivatives. Chem. Mater. 2007, 19, 2927–2929. [Google Scholar] [CrossRef]
- He, Y.; Chen, C.; Richard, E.; Dou, L.; Wu, Y.; Li, G.; Yang, Y. Novel Fullerene Acceptors: Synthesis and Application in Low Band Gap Polymer Solar Cells. J. Mater. Chem. 2012, 22, 13391. [Google Scholar] [CrossRef]
- Yamane, Y.; Sugawara, K.; Nakamura, N.; Hayase, S.; Nokami, T.; Itoh, T. Development of N-Type Semiconductor Based on Cyclopentene- or Cyclohexene-Fused [C60]-Fullerene Derivatives. J. Org. Chem. 2015, 80, 4638–4649. [Google Scholar] [CrossRef] [PubMed]
- Mi, D.; Park, J.B.; Xu, F.; Kim, H.U.; Kim, J.-H.; Hwang, D.-H. Synthesis and Characterization of Phenanthrene-Substituted Fullerene Derivatives as Electron Acceptors for P3HT-Based Polymer Solar Cells. Bull. Korean Chem. Soc. 2014, 35, 1647–1653. [Google Scholar] [CrossRef]
- He, Y.; Chen, H.-Y.; Zhao, G.; Hou, J.; Li, Y. Biindene-C60 Adducts for the Application as Acceptor in Polymer Solar Cells with Higher Open-Circuit-Voltage. Sol. Energy Mater. Sol. Cells 2011, 95, 899–903. [Google Scholar] [CrossRef]
- Su, W.-T.; Watanabe, M.; Chang, Y.J.; Chou, P.-T.; Ghosh, A.; Chow, T.J. Cycloaddition of Hexacene and Fullerene[60]. Tetrahedron Lett. 2015, 56, 1092–1095. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, S.; Xiao, Z.; Zuo, Q.; Ding, L. Synthesis of Mono- and Bisadducts of Thieno- o -Quinodimethane with C60 for Efficient Polymer Solar Cells. Org. Lett. 2012, 14, 1508–1511. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Cho, C.-H.; Cho, H.-H.; Kang, T.E.; Kim, H.J.; Kim, K.-H.; Yoon, S.C.; Kim, B.J. Controlling Number of Indene Solubilizing Groups in Multiadduct Fullerenes for Tuning Optoelectronic Properties and Open-Circuit Voltage in Organic Solar Cells. ACS Appl. Mater. Interfaces 2012, 4, 110–116. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhao, G.; Peng, B.; Li, Y. High-Yield Synthesis and Electrochemical and Photovoltaic Properties of Indene-C70 Bisadduct. Adv. Funct. Mater. 2010, 20, 3383–3389. [Google Scholar] [CrossRef]
- Zhen, Y.; Obata, N.; Matsuo, Y.; Nakamura, E. Benzo[c]Thiophene-C60 Diadduct: An Electron Acceptor for p-n Junction Organic Solar Cells Harvesting Visible to Near-IR Light. Chem. Asian J. 2012, 7, 2644–2649. [Google Scholar] [CrossRef]
- Tao, R.; Umeyama, T.; Higashino, T.; Koganezawa, T.; Imahori, H. A Single Cis-2 Regioisomer of Ethylene-Tethered Indene Dimer–Fullerene Adduct as an Electron-Acceptor in Polymer Solar Cells. Chem. Commun. 2015, 51, 8233–8236. [Google Scholar] [CrossRef]
- Tao, R.; Umeyama, T.; Higashino, T.; Koganezawa, T.; Imahori, H. Synthesis and Isolation of Cis -2 Regiospecific Ethylene-Tethered Indene Dimer–[70]Fullerene Adduct for Polymer Solar Cell Applications. ACS Appl. Mater. Interfaces 2015, 7, 16676–16685. [Google Scholar] [CrossRef]
- Sieval, A.B.; Treat, N.D.; Rozema, D.; Hummelen, J.C.; Stingelin, N. Diels–Alders Adducts of C 60 and Esters of 3-(1-Indenyl)-Propionic Acid: Alternatives for [60]PCBM in Polymer:Fullerene Solar Cells. Chem. Commun. 2015, 51, 8126–8129. [Google Scholar] [CrossRef]
- He, Y.; You, J.; Dou, L.; Chen, C.-C.; Richard, E.; Cha, K.C.; Wu, Y.; Li, G.; Yang, Y. High Performance Low Band Gap Polymer Solar Cells with a Non-Conventional Acceptor. Chem. Commun. 2012, 48, 7616. [Google Scholar] [CrossRef]
- He, Y.; Shao, M.; Xiao, K.; Smith, S.C.; Hong, K. High-Performance Polymer Photovoltaics Based on Rationally Designed Fullerene Acceptors. Sol. Energy Mater. Sol. Cells 2013, 118, 171–178. [Google Scholar] [CrossRef]
- Tseng, N.-W.; Yu, Y.; Li, Y.; Zhao, J.; So, S.K.; Yan, H.; Ng, K.M. Isobenzofulvene-Fullerene Mono-Adducts for Organic Photovoltaic Applications. J. Mater. Chem. C 2015, 3, 977–980. [Google Scholar] [CrossRef]
- Han, G.D.; Collins, W.R.; Andrew, T.L.; Bulović, V.; Swager, T.M. Cyclobutadiene-C 60 Adducts: N-Type Materials for Organic Photovoltaic Cells with High VOC. Adv. Funct. Mater. 2013, 23, 3061–3069. [Google Scholar] [CrossRef]
- Su, Y.-T.; Wang, G.-W. FeCl 3 -Mediated Cyclization of [60]Fullerene with N -Benzhydryl Sulfonamides under High-Speed Vibration Milling Conditions. Org. Lett. 2013, 15, 3408–3411. [Google Scholar] [CrossRef] [PubMed]
- Kako, M.; Iida, R.; Maeda, Y.; Yamada, M.; Hasegawa, T. Thermal Reactions of C 60 with Siliranes: Carbosilylation and Silylene Addition of Fullerenes. Fuller. Nanotub. Carbon Nanostruct. 2014, 22, 155–165. [Google Scholar] [CrossRef]
- Matsuo, Y.; Ogumi, K.; Zhang, Y.; Okada, H.; Nakagawa, T.; Ueno, H.; Gocho, A.; Nakamura, E. Fullerene Cation-Mediated Demethylation/Cyclization to Give 5- and 7-Membered Cyclo[60]Fullerene Derivatives. J. Mater. Chem. A 2017, 5, 2774–2783. [Google Scholar] [CrossRef]
- Yan, Y.-T.; Gao, W.; Jin, B.; Shan, D.-S.; Peng, R.-F.; Chu, S.-J. Palladium-Catalyzed Reaction of [60]Fullerene with Aroyl Compounds via Enolate-Mediated Sp2 C–H Bond Activation and Hydroxylation. J. Org. Chem. 2018, 83, 672–683. [Google Scholar] [CrossRef]
- Matsuo, Y.; Iwashita, A.; Abe, Y.; Li, C.-Z.; Matsuo, K.; Hashiguchi, M.; Nakamura, E. Regioselective Synthesis of 1,4-Di(Organo)[60]Fullerenes through DMF-Assisted Monoaddition of Silylmethyl Grignard Reagents and Subsequent Alkylation Reaction. J. Am. Chem. Soc. 2008, 130, 15429–15436. [Google Scholar] [CrossRef]
- Matsuo, Y.; Oyama, H.; Soga, I.; Okamoto, T.; Tanaka, H.; Saeki, A.; Seki, S.; Nakamura, E. 1-Aryl-4-Silylmethyl[60]Fullerenes: Synthesis, Properties, and Photovoltaic Performance. Chem. Asian J. 2013, 8, 121–128. [Google Scholar] [CrossRef]
- Jeon, I.; Delacou, C.; Nakagawa, T.; Matsuo, Y. Enhancement of Open-Circuit Voltage by Using the 58-π Silylmethyl Fullerenes in Small-Molecule Organic Solar Cells. Chem. Asian J. 2016, 11, 1268–1272. [Google Scholar] [CrossRef]
- Tanaka, H.; Abe, Y.; Matsuo, Y.; Kawai, J.; Soga, I.; Sato, Y.; Nakamura, E. An Amorphous Mesophase Generated by Thermal Annealing for High-Performance Organic Photovoltaic Devices. Adv. Mater. 2012, 24, 3521–3525. [Google Scholar] [CrossRef]
- Zhang, Y.; Matsuo, Y.; Li, C.-Z.; Tanaka, H.; Nakamura, E. A Scalable Synthesis of Methano[60]Fullerene and Congeners by the Oxidative Cyclopropanation Reaction of Silylmethylfullerene. J. Am. Chem. Soc. 2011, 133, 8086–8089. [Google Scholar] [CrossRef]
- Varotto, A.; Treat, N.D.; Jo, J.; Shuttle, C.G.; Batara, N.A.; Brunetti, F.G.; Seo, J.H.; Chabinyc, M.L.; Hawker, C.J.; Heeger, A.J.; et al. 1,4-Fullerene Derivatives: Tuning the Properties of the Electron Transporting Layer in Bulk-Heterojunction Solar Cells. Angew. Chem. Int. Ed. 2011, 50, 5166–5169. [Google Scholar] [CrossRef]
- Cristofani, M.; Menna, E.; Seri, M.; Muccini, M.; Prosa, M.; Antonello, S.; Mba, M.; Franco, L.; Maggini, M. Tuning the Electron-Acceptor Properties of [60]Fullerene by Tailored Functionalization for Application in Bulk Heterojunction Solar Cells. Asian J. Org. Chem. 2016, 5, 676–684. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, G.; Knutson, N.S.; Fontana, M.T.; Huber, R.C.; Ferreira, A.S.; Tolbert, S.H.; Schwartz, B.J.; Rubin, Y. Beyond PCBM: Methoxylated 1,4-Bisbenzyl[60]Fullerene Adducts for Efficient Organic Solar Cells. J. Mater. Chem. A 2016, 4, 416–424. [Google Scholar] [CrossRef]
- Matsuo, Y.; Sato, Y.; Niinomi, T.; Soga, I.; Tanaka, H.; Nakamura, E. Columnar Structure in Bulk Heterojunction in Solution-Processable Three-Layered p-i-n Organic Photovoltaic Devices Using Tetrabenzoporphyrin Precursor and Silylmethyl[60]Fullerene. J. Am. Chem. Soc. 2009, 131, 16048–16050. [Google Scholar] [CrossRef]
- Matsumoto, F.; Iwai, T.; Moriwaki, K.; Takao, Y.; Ito, T.; Mizuno, T.; Ohno, T. Design of Fullerene Derivatives for Stabilizing LUMO Energy Using Donor Groups Placed in Spatial Proximity to the C60 Cage. J. Org. Chem. 2012, 77, 9038–9043. [Google Scholar] [CrossRef]
- Lu, S.; Jin, T.; Yasuda, T.; Si, W.; Oniwa, K.; Alamry, K.A.; Kosa, S.A.; Asiri, A.M.; Han, L.; Yamamoto, Y. Deuterium Isotope Effect on Bulk Heterojunction Solar Cells. Enhancement of Organic Photovoltaic Performances Using Monobenzyl Substituted Deuteriofullerene Acceptors. Org. Lett. 2013, 15, 5674–5677. [Google Scholar] [CrossRef]
- Lu, S.; Jin, T.; Yasuda, T.; Islam, A.; Akhtaruzzaman, M.d.; Han, L.; Alamry, K.A.; Kosa, S.A.; Asiri, A.M.; Yamamoto, Y. Functional 2-Benzyl-1,2-Dihydro[60]Fullerenes as Acceptors for Organic Photovoltaics: Facile Synthesis and High Photovoltaic Performances. Tetrahedron 2013, 69, 1302–1306. [Google Scholar] [CrossRef]
- Tong, C.C.; Hwang, K.C. Enhancement of OLED Efficiencies and High-Voltage Stabilities of Light-Emitting Materials by Deuteration. J. Phys. Chem. C 2007, 111, 3490–3494. [Google Scholar] [CrossRef]
- Lenes, M.; Wetzelaer, G.-J.A.H.; Kooistra, F.B.; Veenstra, S.C.; Hummelen, J.C.; Blom, P.W.M. Fullerene Bisadducts for Enhanced Open-Circuit Voltages and Efficiencies in Polymer Solar Cells. Adv. Mater. 2008, 20, 2116–2119. [Google Scholar] [CrossRef]
- Lenes, M.; Shelton, S.W.; Sieval, A.B.; Kronholm, D.F.; Hummelen, J.C.K.; Blom, P.W.M. Electron Trapping in Higher Adduct Fullerene-Based Solar Cells. Adv. Funct. Mater. 2009, 19, 3002–3007. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, S.; Qian, D.; Wang, Q.; Hou, J. Application of Bis-PCBM in Polymer Solar Cells with Improved Voltage. J. Phys. Chem. C 2013, 117, 25360–25366. [Google Scholar] [CrossRef]
- Susarova, D.K.; Goryachev, A.E.; Novikov, D.V.; Dremova, N.N.; Peregudova, S.M.; Razumov, V.F.; Troshin, P.A. Material Solubility Effects in Bulk Heterojunction Solar Cells Based on the Bis-Cyclopropane Fullerene Adducts and P3HT. Sol. Energy Mater. Sol. Cells 2014, 120, 30–36. [Google Scholar] [CrossRef]
- Lan, S.; Yang, H.; Zhang, G.; Wu, X.; Ning, W.; Wang, S.; Chen, H.; Guo, T. Impact of Fullerene Structure on Nanoscale Morphology and Miscibility and Correlation of Performance on Small Molecules: Fullerene Solar Cell. J. Phys. Chem. C 2016, 120, 21317–21324. [Google Scholar] [CrossRef]
- Choi, J.H.; Son, K.-I.; Kim, T.; Kim, K.; Ohkubo, K.; Fukuzumi, S. Thienyl-Substituted Methanofullerene Derivatives for Organic Photovoltaic Cells. J. Mater. Chem. 2010, 20, 475–482. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Liao, M.-H.; Chang, C.-Y.; Kao, W.-S.; Wu, C.-E.; Hsu, C.-S. Di(4-Methylphenyl)Methano-C60 Bis-Adduct for Efficient and Stable Organic Photovoltaics with Enhanced Open-Circuit Voltage. Chem. Mater. 2011, 23, 4056–4062. [Google Scholar] [CrossRef]
- Liao, M.-H.; Lai, Y.-Y.; Lai, Y.-Y.; Chen, Y.-T.; Tsai, C.-E.; Liang, W.-W.; Cheng, Y.-J. Reducing Regioisomers of Fullerene-Bisadducts by Tether-Directed Remote Functionalization: Investigation of Electronically and Sterically Isomeric Effects on Bulk-Heterojunction Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 996–1004. [Google Scholar] [CrossRef]
- Tian, C.-B.; Deng, L.-L.; Zhang, Z.-Q.; Dai, S.-M.; Gao, C.-L.; Xie, S.-Y.; Huang, R.-B.; Zheng, L.-S. Bis-Adducts of Benzocyclopentane- and Acenaphthene-C60 Superior to Mono-Adducts as Electron Acceptors in Polymer Solar Cells. Sol. Energy Mater. Sol. Cells 2014, 125, 198–205. [Google Scholar] [CrossRef]
- Yang, T.; Jiang, Z.; Huang, X.; Wei, H.; Yuan, J.; Yue, W.; Li, Y.; Ma, W. Design and Synthesis of Soluble Dibenzosuberane-Substituted Fullerene Derivatives for Bulk-Heterojunction Polymer Solar Cells. Org. Electron. 2013, 14, 2184–2191. [Google Scholar] [CrossRef]
- Baran, D.; Erten-Ela, S.; Kratzer, A.; Ameri, T.; Brabec, C.J.; Hirsch, A. Facile Synthesis and Photovoltaic Applications of a New Alkylated Bismethano Fullerene as Electron Acceptor for High Open Circuit Voltage Solar Cells. RSC Adv. 2015, 5, 64724–64730. [Google Scholar] [CrossRef]
- Ruff, A.; Qian, X.; Porfyrakis, K.; Ludwigs, S. Effect of the Type and Number of Organic Addends on Fullerene Acceptors for N-Type Electronic Devices: Redox Properties and Energy Levels. ChemistrySelect 2018, 3, 5778–5785. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, W.; Tan, Z.; Du, C.; Li, C.; Bo, Z.; Li, Y.; Yang, X.; Zhen, M.; Jiang, F.; et al. Dihydronaphthyl-Based [60]Fullerene Bisadducts for Efficient and Stable Polymer Solar Cells. Chem. Commun. 2012, 48, 425–427. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, W.; Tan, Z.; Li, Y.; Ma, Y.; Wang, T.; Jiang, L.; Shu, C.; Wang, C. Highly Efficient and Thermally Stable Polymer Solar Cells with Dihydronaphthyl-Based [70]Fullerene Bisadduct Derivative as the Acceptor. Adv. Funct. Mater. 2012, 22, 2187–2193. [Google Scholar] [CrossRef]
- Li, Z.-J.; Wang, S.; Li, S.-H.; Sun, T.; Yang, W.-W.; Shoyama, K.; Nakagawa, T.; Jeon, I.; Yang, X.; Matsuo, Y.; et al. Regiocontrolled Electrosynthesis of [60]Fullerene Bisadducts: Photovoltaic Performance and Crystal Structures of C60 o-Quinodimethane Bisadducts. J. Org. Chem. 2017, 82, 8676–8685. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Xu, Q.; Zhang, W.; Tan, Z.; Li, Y.; Zhang, Z.; Jiang, L.; Shu, C.; Wang, C. Effects of Alkoxy Chain Length in Alkoxy-Substituted Dihydronaphthyl-Based [60]Fullerene Bisadduct Acceptors on Their Photovoltaic Properties. ACS Appl. Mater. Interfaces 2012, 4, 5966–5973. [Google Scholar] [CrossRef]
- Kitaura, S.; Kurotobi, K.; Sato, M.; Takano, Y.; Umeyama, T.; Imahori, H. Effects of Dihydronaphthyl-Based [60]Fullerene Bisadduct Regioisomers on Polymer Solar Cell Performance. Chem. Commun. 2012, 48, 8550. [Google Scholar] [CrossRef]
- Tao, R.; Umeyama, T.; Kurotobi, K.; Imahori, H. Effects of Alkyl Chain Length and Substituent Pattern of Fullerene Bis-Adducts on Film Structures and Photovoltaic Properties of Bulk Heterojunction Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 17313–17322. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.U.; Park, O.Y.; Park, J.B.; Hwang, D.-H. Trimethylsilyl o-Xylenyl-Substituted Fullerene Bis-Adduct as Electron Acceptor for Solution-Processed Polymer Solar Cells. J. Nanosci. Nanotechnol. 2016, 16, 10465–10469. [Google Scholar] [CrossRef]
- Sakthivel, P.; Ban, T.W.; Kim, S.; Kim, S.; Gal, Y.-S.; Chae, E.A.; Shin, W.S.; Moon, S.-J.; Lee, J.-C.; Jin, S.-H. Synthesis and Studies of Methyl Ester Substituted Thieno-o-Quinodimethane Fullerene Multiadducts for Polymer Solar Cells. Sol. Energy Mater. Sol. Cells 2013, 113, 13–19. [Google Scholar] [CrossRef]
- Min, J.; Zhang, Z.-G.; Zhang, M.; Li, Y. Synthesis and Photovoltaic Properties of a D–A Copolymer of Dithienosilole and Fluorinated-Benzotriazole. Polym. Chem. 2013, 4, 1467–1473. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, M.; Huo, L.; Cui, C.; Wu, Y.; Hou, J.; Li, Y. Poly(Thieno[3,2-b]Thiophene-Alt-Bithiazole): A D–A Copolymer Donor Showing Improved Photovoltaic Performance with Indene-C60 Bisadduct Acceptor. Macromolecules 2012, 45, 6930–6937. [Google Scholar] [CrossRef]
- Xin, H.; Subramaniyan, S.; Kwon, T.-W.; Shoaee, S.; Durrant, J.R.; Jenekhe, S.A. Enhanced Open Circuit Voltage and Efficiency of Donor–Acceptor Copolymer Solar Cells by Using Indene-C60 Bisadduct. Chem. Mater. 2012, 24, 1995–2001. [Google Scholar] [CrossRef]
- Sun, Y.; Cui, C.; Wang, H.; Li, Y. High-Efficiency Polymer Solar Cells Based on Poly(3-Pentylthiophene) with Indene-C70 Bisadduct as an Acceptor. Adv. Energy Mater. 2012, 2, 966–969. [Google Scholar] [CrossRef]
- Zhao, G.; He, Y.; Li, Y. 6.5% Efficiency of Polymer Solar Cells Based on Poly(3-Hexylthiophene) and Indene-C60 Bisadduct by Device Optimization. Adv. Mater. 2010, 22, 4355–4358. [Google Scholar] [CrossRef]
- Liao, S.-H.; Li, Y.-L.; Jen, T.-H.; Cheng, Y.-S.; Chen, S.-A. Multiple Functionalities of Polyfluorene Grafted with Metal Ion-Intercalated Crown Ether as an Electron Transport Layer for Bulk-Heterojunction Polymer Solar Cells: Optical Interference, Hole Blocking, Interfacial Dipole, and Electron Conduction. J. Am. Chem. Soc. 2012, 134, 14271–14274. [Google Scholar] [CrossRef]
- Huang, W.; Gann, E.; Chandrasekaran, N.; Prasad, S.K.K.; Chang, S.-Y.; Thomsen, L.; Kabra, D.; Hodgkiss, J.M.; Cheng, Y.-B.; Yang, Y.; et al. Influence of Fullerene Acceptor on the Performance, Microstructure, and Photophysics of Low Bandgap Polymer Solar Cells. Adv. Energy Mater. 2017, 7, 1602197. [Google Scholar] [CrossRef]
- Mikie, T.; Saeki, A.; Ikuma, N.; Kokubo, K.; Seki, S. Hetero Bis-Addition of Spiro-Acetalized or Cyclohexanone Ring to 58π Fullerene Impacts Solubility and Mobility Balance in Polymer Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 12894–12902. [Google Scholar] [CrossRef]
- Cao, T.; Chen, N.; Liu, G.; Wan, Y.; Perea, J.D.; Xia, Y.; Wang, Z.; Song, B.; Li, N.; Li, X.; et al. Towards a Full Understanding of Regioisomer Effects of Indene-C60 Bisadduct Acceptors in Bulk Heterojunction Polymer Solar Cells. J. Mater. Chem. A 2017, 5, 10206–10219. [Google Scholar] [CrossRef]
- Han, G.D.; Maurano, A.; Weis, J.G.; Bulović, V.; Swager, T.M. VOC Enhancement in Polymer Solar Cells with Isobenzofulvene–C60 Adducts. Org. Electron. 2016, 31, 48–55. [Google Scholar] [CrossRef]
- Fan, X.; Cui, C.; Fang, G.; Wang, J.; Li, S.; Cheng, F.; Long, H.; Li, Y. Efficient Polymer Solar Cells Based on Poly(3-Hexylthiophene):Indene-C70 Bisadduct with a MoO3 Buffer Layer. Adv. Funct. Mater. 2012, 22, 585–590. [Google Scholar] [CrossRef]
- Umeyama, T.; Shibata, S.; Imahori, H. Blend Films of an Amorphous Conjugated Polymer and a Thermal Precursor Fullerene: Effects of Annealing Temperatures on Film Structures and Photovoltaic Properties. RSC Adv. 2016, 6, 83758–83766. [Google Scholar] [CrossRef]
- Kim, B.; Lee, J.; Seo, J.H.; Wudl, F.; Park, S.H.; Yang, C. Regioselective 1,2,3-Bisazfulleroid: Doubly N-Bridged Bisimino-PCBMs for Polymer Solar Cells. J. Mater. Chem. 2012, 22, 22958. [Google Scholar] [CrossRef]
- Li, C.-Z.; Chien, S.-C.; Yip, H.-L.; Chueh, C.-C.; Chen, F.-C.; Matsuo, Y.; Nakamura, E.; Jen, A.K.-Y. Facile Synthesis of a 56π-Electron 1,2-Dihydromethano-[60]PCBM and Its Application for Thermally Stable Polymer Solar Cells. Chem. Commun. 2011, 47, 10082. [Google Scholar] [CrossRef]
- He, Y.; Peng, B.; Zhao, G.; Zou, Y.; Li, Y. Indene Addition of [6,6]-Phenyl-C61-Butyric Acid Methyl Ester for High-Performance Acceptor in Polymer Solar Cells. J. Phys. Chem. C 2011, 115, 4340–4344. [Google Scholar] [CrossRef]
- Jin, B.; Yu, Y.; Peng, R.; Fan, L.; Cai, L.; Fan, B.; Gou, X.; Chu, S. Functional Group Addition of [6,6]-Phenyl-C61-Butyric Acid Methyl Ester as Electron Acceptor in Polymer Solar Cells with High Performance. Synth. Met. 2016, 220, 141–146. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, C.-H.; Kang, H.; Kim, K.-H.; Park, S.; Kang, T.E.; Park, K.; Kim, B.J. Benzocyclobutene-Fullerene Bisadducts as Novel Electron Acceptors for Enhancing Open-Circuit Voltage in Polymer Solar Cells. Sol. Energy Mater. Sol. Cells 2015, 141, 87–92. [Google Scholar] [CrossRef]
- Liu, G.; Cao, T.; Xia, Y.; Song, B.; Zhou, Y.; Chen, N.; Li, Y. Dihydrobenzofuran-C60 Bisadducts as Electron Acceptors in Polymer Solar Cells: Effect of Alkyl Substituents. Synth. Met. 2016, 215, 176–183. [Google Scholar] [CrossRef]
- He, D.; Du, X.; Xiao, Z.; Ding, L. Methanofullerenes, C 60 (CH 2 ) n ( n = 1, 2, 3), as Building Blocks for High-Performance Acceptors Used in Organic Solar Cells. Org. Lett. 2014, 16, 612–615. [Google Scholar] [CrossRef]
- Ye, G.; Chen, S.; Xiao, Z.; Zuo, Q.; Wei, Q.; Ding, L. O-Quinodimethane-Methano[60]Fullerene and Thieno-o-Quinodimethane-Methano[60]Fullerene as Efficient Acceptor Materials for Polymer Solar Cells. J. Mater. Chem. 2012, 22, 22374. [Google Scholar] [CrossRef]
- Ryan, J.W.; Matsuo, Y. Increased Efficiency in Small Molecule Organic Solar Cells Through the Use of a 56-π Electron Acceptor—Methano Indene Fullerene. Sci. Rep. 2015, 5, 8319. [Google Scholar] [CrossRef]
- Faist, M.A.; Shoaee, S.; Tuladhar, S.; Dibb, G.F.A.; Foster, S.; Gong, W.; Kirchartz, T.; Bradley, D.D.C.; Durrant, J.R.; Nelson, J. Understanding the Reduced Efficiencies of Organic Solar Cells Employing Fullerene Multiadducts as Acceptors. Adv. Energy Mater. 2013, 3, 744–752. [Google Scholar] [CrossRef]
- Chen, H.; Peet, J.; Hsiao, Y.-C.; Hu, B.; Dadmun, M. The Impact of Fullerene Structure on Its Miscibility with P3HT and Its Correlation of Performance in Organic Photovoltaics. Chem. Mater. 2014, 26, 3993–4003. [Google Scholar] [CrossRef]
- Cerón, M.R.; Izquierdo, M.; Aghabali, A.; Valdez, J.A.; Ghiassi, K.B.; Olmstead, M.M.; Balch, A.L.; Wudl, F.; Echegoyen, L. Tethered Bisadducts of C60 and C70 with Addends on a Common Hexagonal Face and a 12-Membered Hole in the Fullerene Cage. J. Am. Chem. Soc. 2015, 137, 7502–7508. [Google Scholar] [CrossRef] [PubMed]
- Umeyama, T.; Takahara, S.; Shibata, S.; Igarashi, K.; Higashino, T.; Mishima, K.; Yamashita, K.; Imahori, H. Cis -1 Isomers of Tethered Bismethano[70]Fullerene as Electron Acceptors in Organic Photovoltaics. RSC Adv. 2018, 8, 18316–18326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Subbiah, J.; Lai, Y.-Y.; White, J.M.; Jones, D.J.; Wong, W.W.H. One-Pot Selective Synthesis of a Fullerene Bisadduct for Organic Solar Cell Applications. Chem. Commun. 2015, 51, 9837–9840. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.; Cerón, M.R.; Alegret, N.; Metta-Magaña, A.J.; Rodríguez-Fortea, A.; Poblet, J.M.; Echegoyen, L. Unexpected Isomerism in Cis-2 Bis(Pyrrolidino)[60]Fullerene Diastereomers. Angew. Chem. 2013, 125, 13166–13169. [Google Scholar] [CrossRef]
- Cerón, M.R.; Izquierdo, M.; Aghabali, A.; Vogel, S.P.; Olmstead, M.M.; Balch, A.L.; Echegoyen, L. Tethered Bis-Pyrrolidine Additions to C70: Some Unexpected and New Regioisomers. Carbon 2016, 105, 394–400. [Google Scholar] [CrossRef]
- Turkovic, V.; Prete, M.; Bregnhøj, M.; Inasaridze, L.; Volyniuk, D.; Obrezkov, F.A.; Grazulevicius, J.V.; Engmann, S.; Rubahn, H.-G.; Troshin, P.A.; et al. Biomimetic Approach to Inhibition of Photooxidation in Organic Solar Cells Using Beta-Carotene as an Additive. ACS Appl. Mater. Interfaces 2019, 11, 41570–41579. [Google Scholar] [CrossRef]
- Salvador, M.; Gasparini, N.; Perea, J.D.; Paleti, S.H.; Distler, A.; Inasaridze, L.N.; Troshin, P.A.; Lüer, L.; Egelhaaf, H.-J.; Brabec, C. Suppressing Photooxidation of Conjugated Polymers and Their Blends with Fullerenes through Nickel Chelates. Energy Environ. Sci. 2017, 10, 2005–2016. [Google Scholar] [CrossRef]
- Mumyatov, A.V.; Prudnov, F.A.; Sagdullina, D.K.; Martynov, I.V.; Inasaridze, L.N.; Chernyak, A.V.; Maskaev, A.V.; Kuznetsov, I.E.; Akkuratov, A.V.; Troshin, P.A. Bis(Pyrrolidino)[60]Fullerenes: Promising Photostable Fullerene-Based Acceptors Suppressing Light-Induced Absorber Degradation Pathways. Synth. Met. 2021, 271, 116632. [Google Scholar] [CrossRef]
- Chen, S.; Ye, G.; Xiao, Z.; Ding, L. Efficient and Thermally Stable Polymer Solar Cells Based on a 54π-Electron Fullerene Acceptor. J. Mater. Chem. A 2013, 1, 5562. [Google Scholar] [CrossRef]
- Niinomi, T.; Matsuo, Y.; Hashiguchi, M.; Sato, Y.; Nakamura, E. Penta(Organo)[60]Fullerenes as Acceptors for Organic Photovoltaic Cells. J. Mater. Chem. 2009, 19, 5804. [Google Scholar] [CrossRef]
- Deng, L.-L.; Xie, S.-L.; Yuan, C.; Liu, R.-F.; Feng, J.; Sun, L.-C.; Lu, X.; Xie, S.-Y.; Huang, R.-B.; Zheng, L.-S. High LUMO Energy Level C60(OCH3)4 Derivatives: Electronic Acceptors for Photovoltaic Cells with Higher Open-Circuit Voltage. Sol. Energy Mater. Sol. Cells 2013, 111, 193–199. [Google Scholar] [CrossRef]
- Kumari, M.A.; Swetha, T.; Singh, S.P. Fullerene Derivatives: A Review on Perovskite Solar Cells. Mat Express 2018, 8, 389–406. [Google Scholar] [CrossRef]
- Jia, L.; Chen, M.; Yang, S. Functionalization of Fullerene Materials toward Applications in Perovskite Solar Cells. Mater. Chem. Front. 2020, 4, 2256–2282. [Google Scholar] [CrossRef]
- Zahran, R.; Hawash, Z. Fullerene-Based Inverted Perovskite Solar Cell: A Key to Achieve Promising, Stable, and Efficient Photovoltaics. Adv. Mater. Inter. 2022, 9, 2201438. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).