Recent Development of Fuel Cell Core Components and Key Materials: A Review
Abstract
1. Introduction
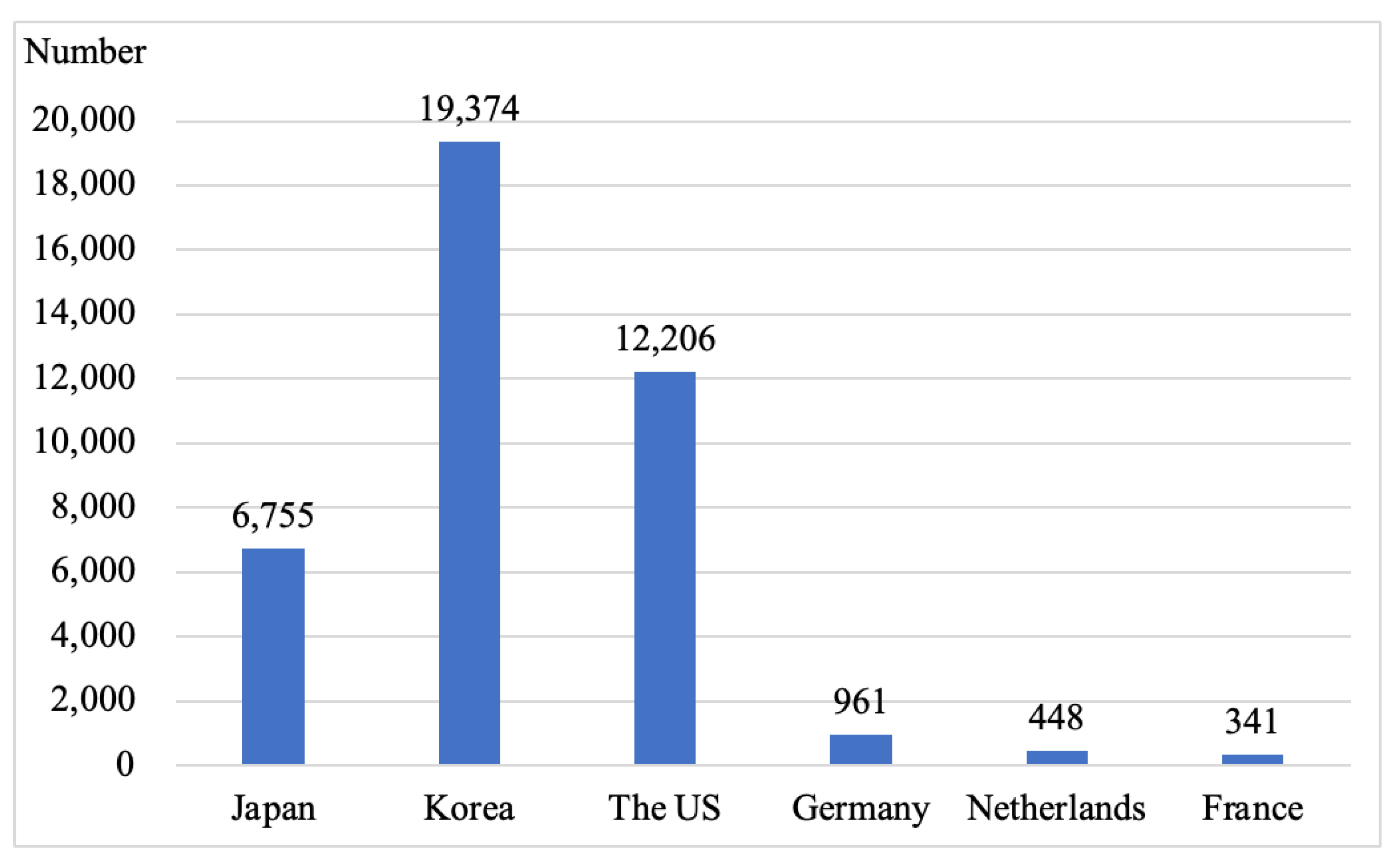
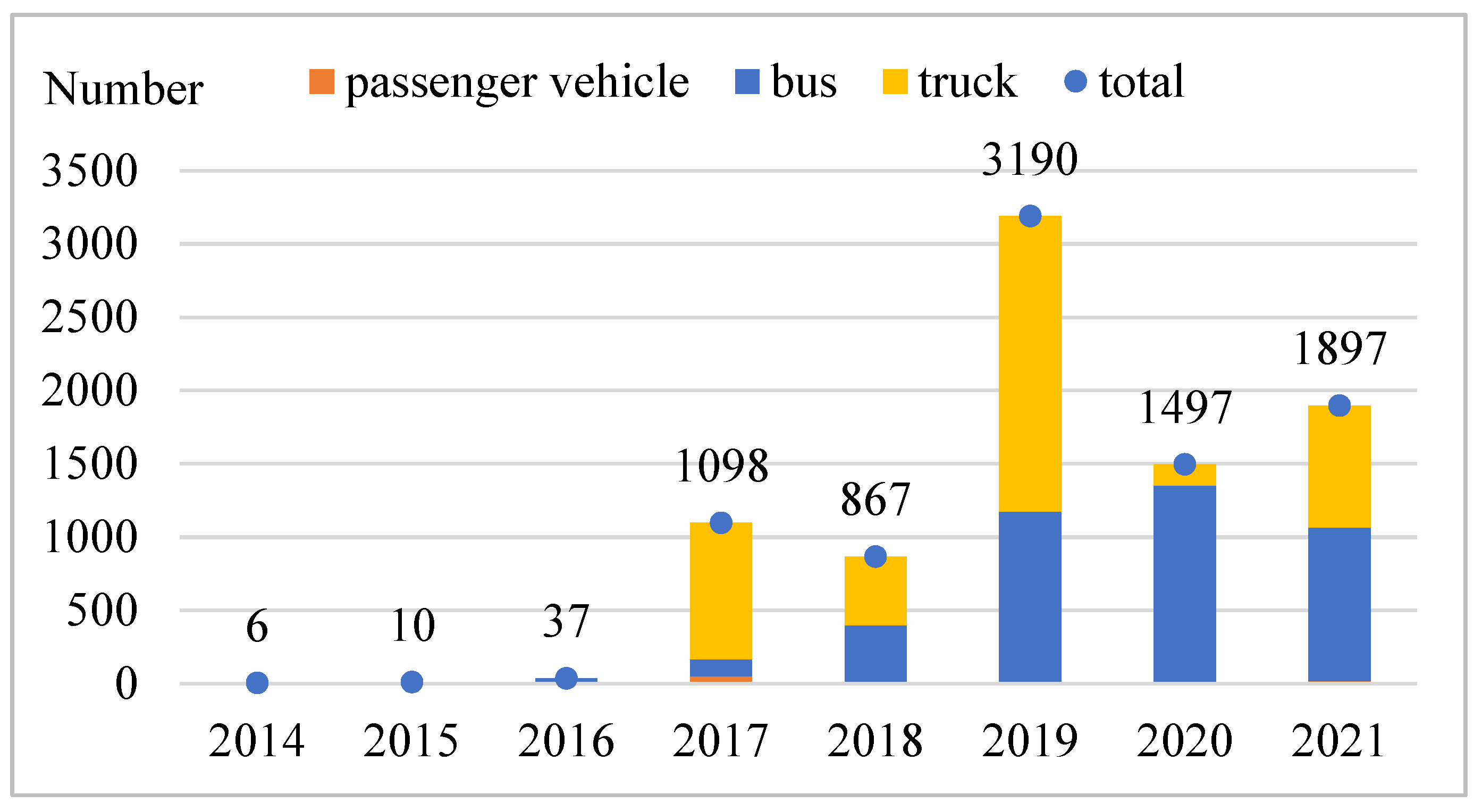
2. An Overview of the Technology and Industry Development of Key Parts of Fuel Cell Vehicles
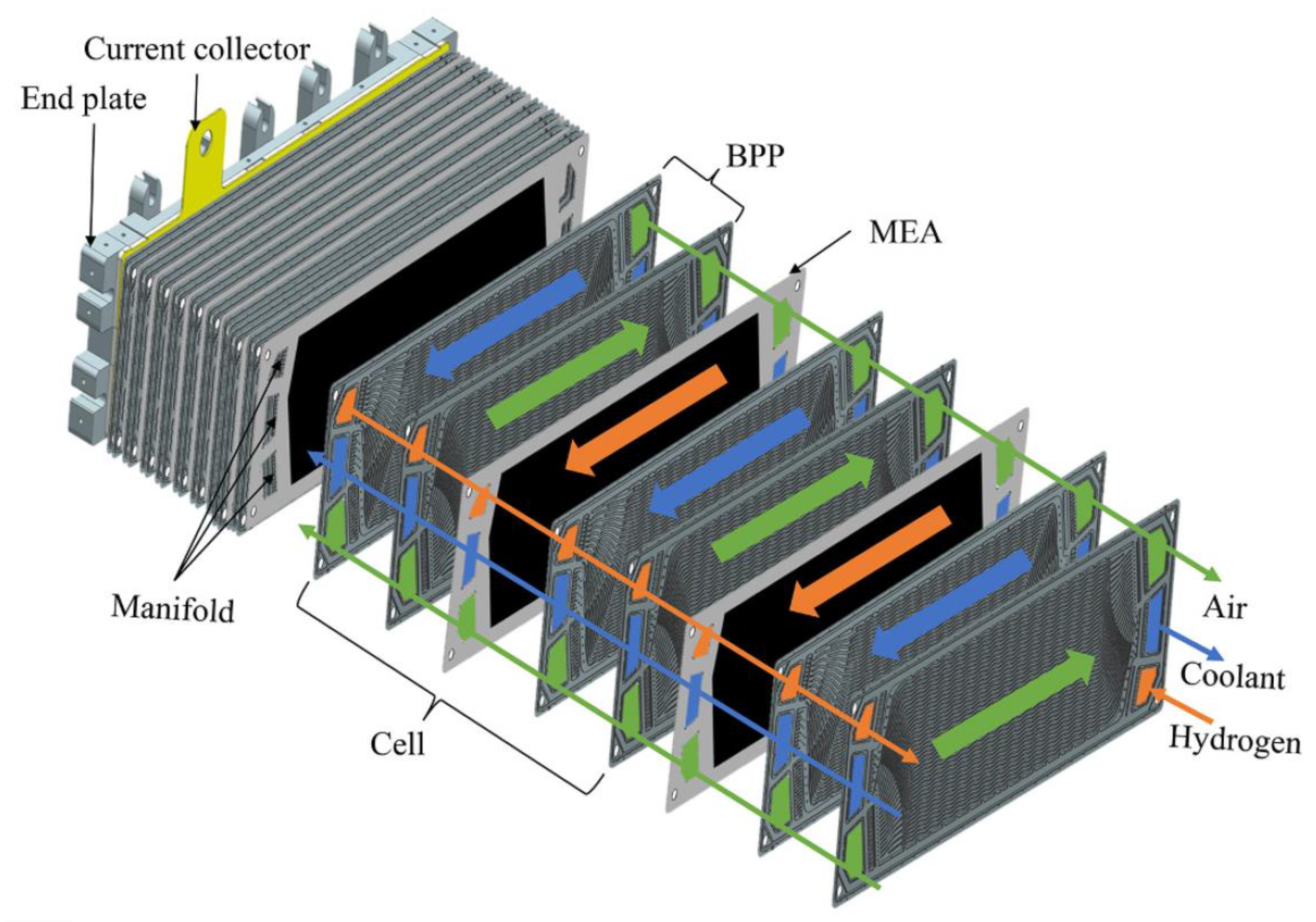
2.1. Stack
2.2. Bipolar Plate
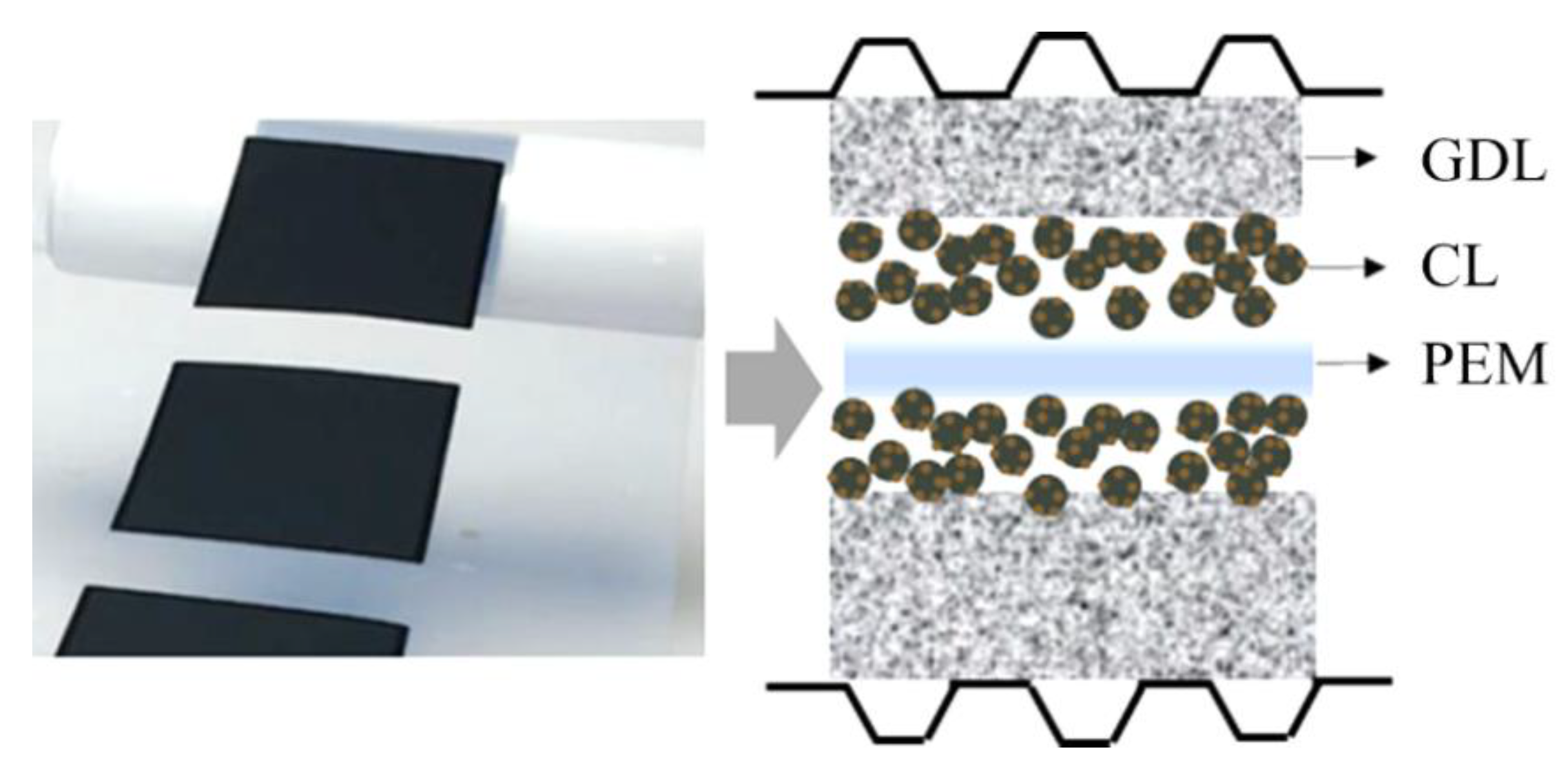
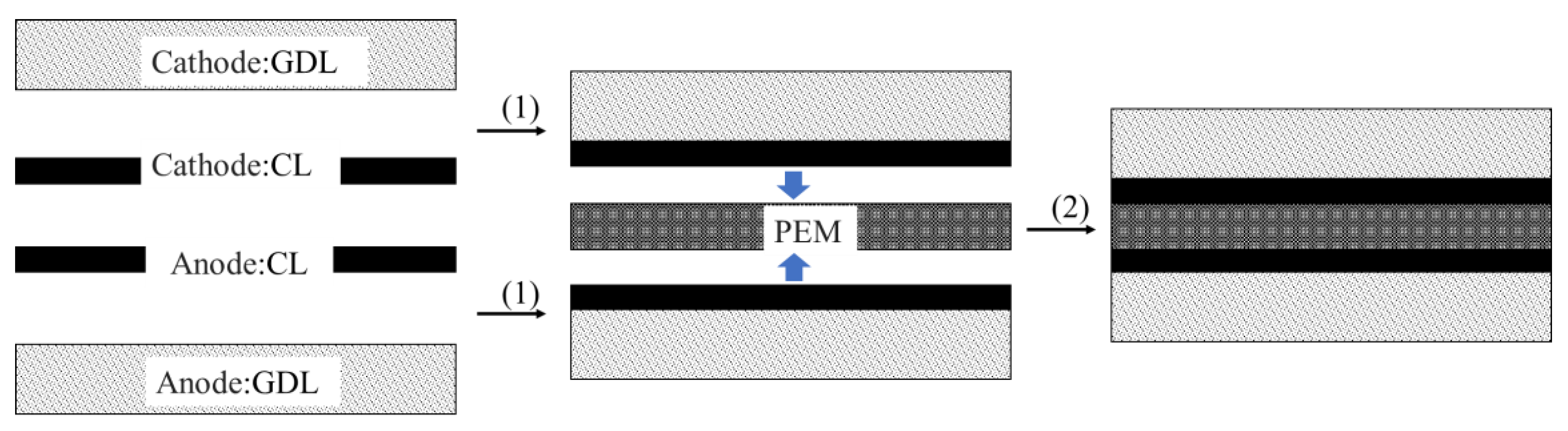
2.3. Membrane Electrode Assembly
2.4. Proton Exchange Membrane
- (a)
- High proton conductivity.
- (b)
- Good chemical stability to improve the cell service life.
- (c)
- Good thermal stability, not prone to degradation at high temperatures.
- (d)
- Good mechanical performance to ensure negligible or no morphological changes when switching between dry and wet states during operation of the fuel cell.
- (e)
- High utilization rate of raw materials, low gas permeability, and low point permeability coefficient of water.
- (f)
- Low price.
- (g)
- Good formability.
2.5. Catalyst Layer
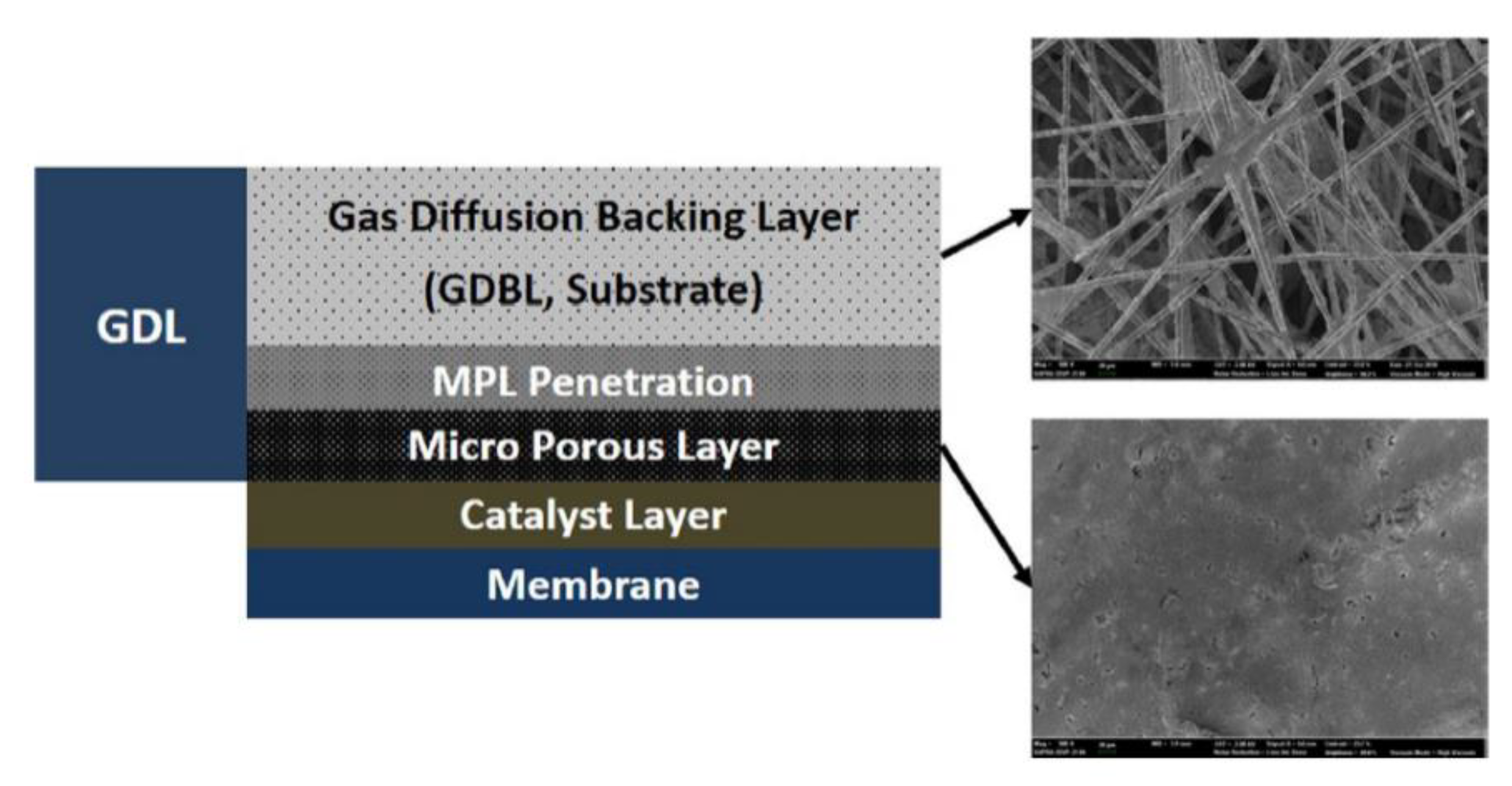
2.6. Gas Diffusion Layer
2.7. Air Compressor
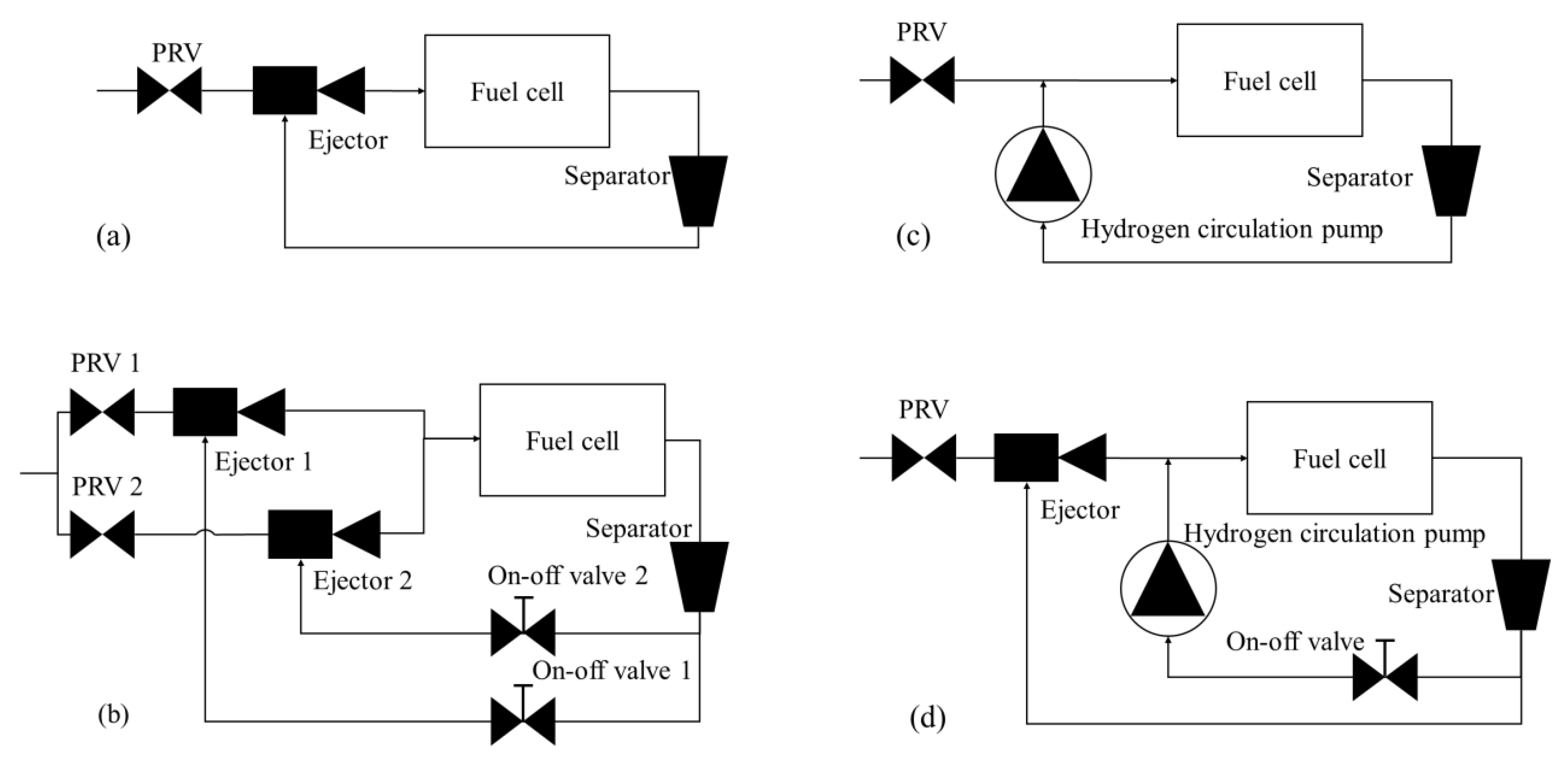
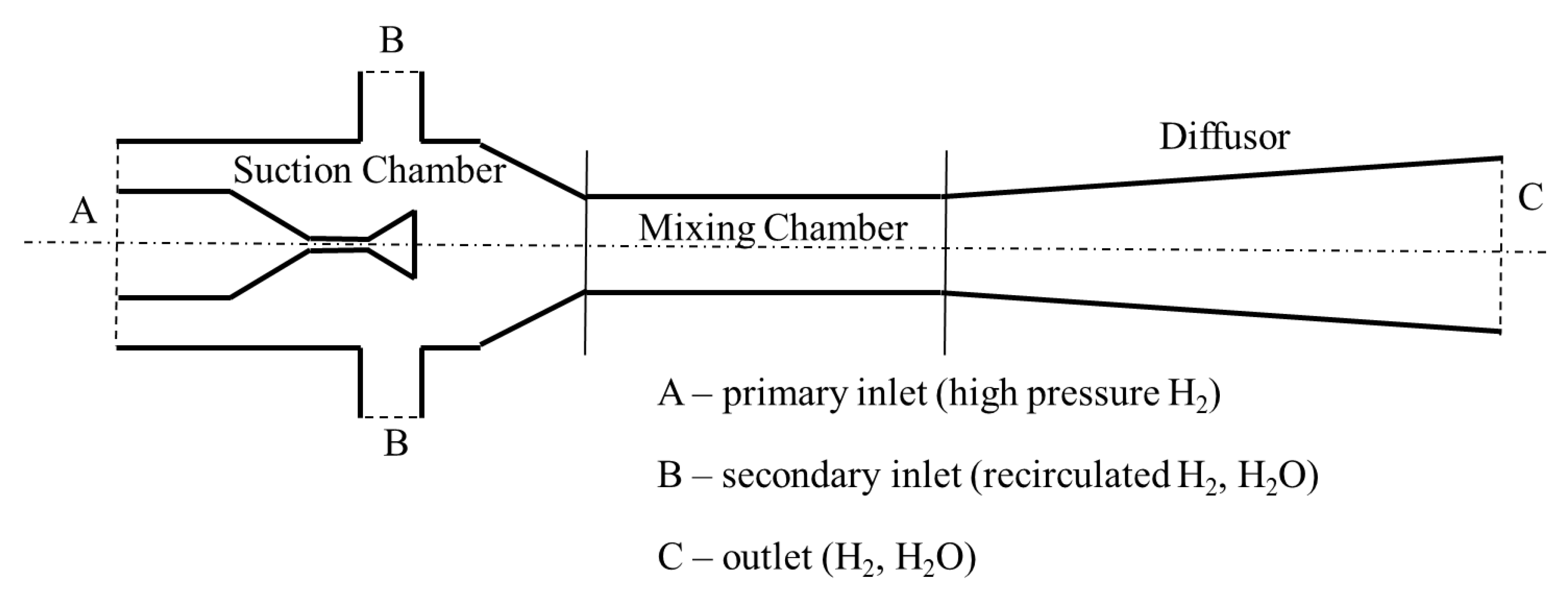
2.8. Hydrogen Circulation System
3. Problems and Suggestions of Industry Development in China
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| Al | aluminum |
| BPP | bipolar plate |
| CCM | catalyst coated membrane |
| CL | catalyst layer |
| Cu | copper |
| DOE | the US Department of Energy |
| FCHEA | the US Fuel Cell and Hydrogen Energy Association |
| FCH-JU | the Fuel Cells and Hydrogen Joint Undertaking |
| ICR | interfacial contact resistance |
| GDB | gas diffusion backing |
| GDE | gas diffusion electron |
| GDL | gas diffusion layer |
| MEA | membrane electrode assembly |
| METI | the Ministry of Economy, Trade, and Industry |
| M-N-C | metal–nitrogen–carbon |
| MPL | microporous layer |
| Ni | nickel |
| ORR | oxygen reduction reaction |
| PBI | polybenzimidazole |
| PEM | proton exchange membrane |
| PI | polyimide |
| PPS/PES/PEK/PKS | polyarylether polymers |
| PFSA | perfluorosulfonic acid |
| PRV | pressure regulating valve |
| Pt | platinum |
| PTFE | polytetrafluoroethylene |
| R&D | research and development |
| SS | stainless steel |
| Ti | titanium |
References
- Zhu, L.; Hu, L.; Yüksel, S.; Dinçer, H.; Karakuş, H.; Ubay, G.G. Analysis of Strategic Directions in Sustainable Hydrogen Investment Decisions. Sustainability 2020, 12, 4581. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, H. Review of the Development of First-Generation Redox Flow Batteries: Iron-Chromium System. ChemSusChem 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- The Strategic Road Map for Hydrogen and Fuel Cells; Hydrogen and Fuel Cell Strategy Council: Danbury, CT, USA, 2019.
- Hydrogen Roadmap Europe: A Sustainable Pathway for the European Energy Transition; Fuel Cells and Hydrogen Joint Undertaking: Danbury, CT, USA, 2019.
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef]
- Gröger, O.; Hubert, A. Gasteiger and Jens-Peter Suchsland. Review-Electromobility: Batteries or Fuel Cells? J. Electrochem. Soc. 2015, 162, A2605–A2622. [Google Scholar]
- Wang, F.; Harindintwali, J.D.; Yuan, Z.; Wang, M.; Li, S.; Yin, Z.; Huang, L.; Fu, Y.; Li, L.; Chang, S.X.; et al. Technologies and perspectives for achieving carbon neutrality. Resour. Conserv. Recycl. 2022, 2, 176. [Google Scholar] [CrossRef] [PubMed]
- Pollet, B.G.; Staffell, I.; Shang, J.L. Current status of hybrid, battery and fuel cell electric vehicles: From electrochemistry to market prospects. Electrochim. Acta 2012, 84, 235–249. [Google Scholar] [CrossRef]
- Whiston, M.M.; Lima Azevedo, I.M.; Litster, S.; Samaras, C.; Whitefoot, K.S.; Whitacre, J.F. Hydrogen Storage for Fuel Cell Electric Vehicles: Expert Elicitation and a Levelized Cost of Driving Model. Environ. Sci. Technol. 2020, 55, 553–562. [Google Scholar] [CrossRef]
- Baba, M.A.; Labbadi, M.; Cherkaoui, M.; Maaroufi, M. Fuel cell electric vehicles: A review of current power electronic converters Topologies and technical challenges. IOP Conf. Ser. Earth Environ. Sci. 2021, 785, 012011. [Google Scholar] [CrossRef]
- Available online: https://www.miit.gov.cn/xwdt/szyw/art/2020/art_4390362916324365a260ed97d7558f18.html (accessed on 4 January 2023).
- Available online: http://jjs.mof.gov.cn/zhengcefagui/202009/t20200918_3591168.htm (accessed on 4 January 2023).
- Lf, A.; Zt, A.; Shc, B. Recent development of hydrogen and fuel cell technologies: A review. Energy Rep. 2021, 7, 8421–8446. [Google Scholar]
- Qiu, D.; Peng, L.; Yi, P.; Lehnert, W.; Lai, X. Review on proton exchange membrane fuel cell stack assembly: Quality evaluation, assembly method, contact behavior and process design. Renew. Sustain. Energy Rev. 2021, 152, 111660. [Google Scholar] [CrossRef]
- Qiu, Y.; Zeng, T.; Zhang, C.; Wang, G.; Wang, Y.; Hu, Z.; Meng, Y.; Wei, Z. Progress and challenges in multi-stack fuel cell system for high power applications:architecture and energy management. Green Energy Intell. Transp. 2023, 100068. [Google Scholar] [CrossRef]
- Available online: https://www.shpt.com/pc/productDetail.html?p=29 (accessed on 4 January 2023).
- Available online: https://baijiahao.baidu.com/s?id=1746112652127783625&wfr=spider&for=pc (accessed on 4 January 2023).
- Available online: http://www.sl-power.com/FCStack/index_154.aspx (accessed on 4 January 2023).
- Available online: https://www.sinosynergypower.com/product/40.html (accessed on 4 January 2023).
- Available online: https://www.h-rise.com/Products_208.html (accessed on 4 January 2023).
- Silva, R.; Franchi, D.; Leone, A.; Pilloni, L.; Masci, A.; Pozio, A. Surface conductivity and stability of metallic bipolar plate materials for polymer electrolyte fuel cells. Electrochim. Acta 2006, 51, 3592–3598. [Google Scholar] [CrossRef]
- Taherian, R.; Nasr, M. Performance and material selection of nanocomposite bipolar plate in proton exchange membrane fuel cells. Int. J. Energy Res. 2014, 38, 94–105. [Google Scholar] [CrossRef]
- Peker, M.F.; Cora, Ö.N.; Koç, M. Investigations on the variation of corrosion and contact resistance characteristics of metallic bipolar plates manufactured under long-run conditions. Int. J. Hydrogen Energy 2011, 36, 15427–15436. [Google Scholar]
- Li, X.; Sabir, I. Review of bipolar plates in PEM fuel cells: Flow-field designs. Int. J. Hydrogen Energy 2005, 30, 359–371. [Google Scholar] [CrossRef]
- Antunes, R.A.; Oliveira, M.C.L.; Ett, G.; Ett, V. Corrosion of metal bipolar plates for PEM fuel cells: A review. Int. J. Hydrogen Energy 2010, 35, 3632–3647. [Google Scholar] [CrossRef]
- US Department of Energy. Fuel Cell Technical Ream Roadmap; US Department of Energy: Washington, DC, USA, 2013.
- Hermann, A.; Chaudhuri, T.; Spagnol, P. Bipolar plates for PEM fuel cells: A review. Int. J. Hydrogen Energy 2005, 30, 1297–1302. [Google Scholar] [CrossRef]
- Guo, N.; Leu, M. Effect of different graphite materials on the electrical conductivity and flexural strength of bipolar plates fabricated using selective laser sintering. Int. J. Hydrogen Energy 2012, 37, 3558–3566. [Google Scholar] [CrossRef]
- Mathur, R.; Dhakate, S.; Gupta, D.; Dhami, T.; Aggarwal, R. Effect of different carbon fillers on the properties of graphite composite bipolar plate. J. Mater. Process. Technol. 2008, 203, 184–192. [Google Scholar] [CrossRef]
- Ji, S.; Hwang, Y.-S.; Park, T.; Lee, Y.H.; Paek, J.Y.; Chang, I.; Lee, M.H.; Cha, S.W. Graphite foil based assembled bipolar plates for polymer electrolyte fuel cells. Int. J. Precis. Eng. Manuf. 2012, 13, 2183–2186. [Google Scholar] [CrossRef]
- Wilson, M.; Busick, D. Composite Bipolar Plate for Electrochemical Cells. U.S. Patent US6248467B1, 19 June 2001. [Google Scholar]
- Bisaria, M.K.; Andrin, P.; Abdou, M.; Cai, Y. Injection Moldable Conductive Aromatic Thermoplastic Liquid Crystalline Polymeric Compositions. U.S. Patent US6379795, 30 April 2002. [Google Scholar]
- Wu, S.; Yang, W.; Yan, H.; Zuo, X.; Cao, Z.; Li, H.; Shi, M.; Chen, H. A review of modified metal bipolar plates for proton exchange membrane fuel cells. International. J. Hydrogen Energy 2021, 46, 8672–8701. [Google Scholar] [CrossRef]
- Karimi, S.; Fraser, N.; Roberts, B.; Foulkes, F.R. A Review of Metallic Bipolar Plates for Proton Exchange Membrane Fuel Cells: Materials and Fabrication Methods. Adv. Mater. Sci. Eng. 2012, 2012, 1–22. [Google Scholar] [CrossRef]
- Chen, B.; Ke, W.; Luo, M.; Wang, J.; Tu, Z.; Pan, M.; Zhang, H.; Liu, X.; Liu, W. Operation characteristics and carbon corrosion of PEMFC (Proton exchange membrane fuel cell) with dead-ended anode for high hydrogen utilization. Energy 2015, 91, 799–806. [Google Scholar] [CrossRef]
- Jin, J.; Hu, M.; Zhao, X. Investigation of incorporating oxygen into TiN coating to resist high potential effects on PEMFC bipolar plates in vehicle applications. Int. J. Hydrogen Energy 2020, 45, 23310–23326. [Google Scholar] [CrossRef]
- Mani, S.; Srinivasan, A.; Rajendran, N. Effect of nitrides on the corrosion behaviour of 316L SS bipolar plates for Proton Exchange Membrane Fuel Cell (PEMFC). Int. J. Hydrogen Energy 2015, 40, 3359–3369. [Google Scholar] [CrossRef]
- Chen, B.; Wang, J.; Yang, T.; Cai, Y.; Pan, M.; Tu, Z.; Zhang, C.; Chan, S.H.; Yu, Y. Mitigation studies of carbon corrosion by optimizing the opening size of the cathode outlet in a proton exchange membrane fuel cell with dead-ended anode. Energy Convers. Manag. 2016, 119, 60–66. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Lu, Z.; Wang, L.; Li, W. Preparation and performances of electrically conductive Nb-doped TiO2 coatings for 316 stainless steel bipolar plates of proton exchange membrane fuel cells. Corros. Sci. 2018, 142, 249.e57. [Google Scholar] [CrossRef]
- Barranco, J.; Barreras, F.; Lozano, A.; Maza, M. Influence of CrN-coating thickness on the corrosion resistance behaviour of aluminium-based bipolar plates. J. Power Sources 2011, 196, 4283.e9. [Google Scholar] [CrossRef]
- Cheng, X.; Shi, Z.; Glass, N.; Zhang, L.; Zhang, J.; Song, D.; Liu, Z.-S.; Wang, H.; Shen, J. A review of PEM hydrogen fuel cell contamination: Impacts, mechanisms, and mitigation. J. Power Sources 2007, 165, 739.e56. [Google Scholar] [CrossRef]
- Yun, Y.H. Deposition of gold-titanium and gold-nickel coatings on electropolished 316L stainless steel bipolar plates for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2010, 35, 1713–1718. [Google Scholar] [CrossRef]
- Bagherzadeh, M.; Jaberinia, F. Electrochemical study of Monel alloy corrosion in hydrochloric acid solution and pyrrolidine dithiocarboxylate self-assembled monolayers as its corrosion protector. J. Alloys Compd. 2018, 750, 677–686. [Google Scholar] [CrossRef]
- Yao, K.; Adams, D.; Hao, A.; Zheng, J.P.; Liang, Z.; Nguyen, N. Highly Conductive and Strong Graphite-Phenolic Resin Composite for Bipolar Plate Applications. Energy Fuels 2017, 31, 14320–14331. [Google Scholar] [CrossRef]
- Li, W.; Jing, S.; Wang, S.; Wang, C.; Xie, X. Experimental investigation of expanded graphite/phenolic resin composite bipolar plate – ScienceDirect. Int. J. Hydrogen Energy 2016, 41, 16240–16246. [Google Scholar] [CrossRef]
- Him, J.; Lee, D. Development of composite-metal hybrid bipolar plates for PEM fuel cells. Int. J. Hydrogen Energy 2012, 37, 12504–12512. [Google Scholar]
- Lin, S.Y.; Chang, M. Effect of microporous layer composed of carbon nanotube and acetylene black on polymer electrolyte membrane fuel cell performance. Int. J. Hydrogen Energy 2015, 40, 7879–7885. [Google Scholar] [CrossRef]
- Litster, S.; Mclean, G. PEM fuel cell electrodes. J. Power Sources 2004, 130, 61–76. [Google Scholar] [CrossRef]
- Fu, X.; Gao, R.; Jiang, G.; Li, M.; Li, S.; Luo, D.; Hu, Y.; Yuan, Q.; Huang, W.; Zhu, N.; et al. Evolution of atomic-scale dispersion of FeNx in hierarchically porous 3D air electrode to boost the interfacial electrocatalysis of oxygen reduction in PEMFC. Nano Energy 2021, 83, 105734. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, P.K. Advances in the high performance polymer electrolyte membranes for fuel cells. Chem. Soc. Rev. 2012, 41, 2382–2394. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; Liu, X.; Wu, W.-T. Modeling of PEM fuel cell with thin MEA under low humidity operating condition. Appl. Energy 2019, 242 Pt 1285-176, 1513–1527. [Google Scholar] [CrossRef]
- Ticianelli, E.A. Methods to Advance Technology of Proton Exchange Membrane Fuel Cells. J. Electrochem. Soc. 1988, 135, 2209–2214. [Google Scholar] [CrossRef]
- Murphy, O.J.; Hitchens, G.D.; Manko, D.J. High power density proton-exchange membrane fuel cells. J. Power Sources 1994, 47, 353–368. [Google Scholar] [CrossRef]
- Ticianelli, E.A.; Derouin, C.R.; Srinivasan, S. Localization of platinum in low catalyst loading electrodes to to attain high power densities in SPE fuel cells. J. Electroanal. Chem. Interfacial Electrochem. 1988, 251, 275–295. [Google Scholar] [CrossRef]
- Raistrick, I.D. Modified gas diffusion electrode for proton exchange membrane fuel cells. In Proceedings of the Symposium on Diaphragms, Separators, and Ion-Exchange Membranes, Boston, MA, USA, 1 October 1986; Volume 86-14, pp. 172–178. [Google Scholar]
- Wilson, M.S.; Gottesfeld, S. Thin-film catalyst layers for polymer electrolyte fuel cell electrodes. J. Appl. Electrochem. 1992, 22, 1–7. [Google Scholar] [CrossRef]
- Sassin, M.B.; Garsany, Y.; Gould, B.D.; Swider-Lyons, K.E. Fabrication Method for Laboratory-Scale High-Performance Membrane Electrode Assemblies for Fuel Cells. Anal. Chem. 2016, 89, 511. [Google Scholar] [CrossRef]
- Mehta, V.; Cooper, J.S. Review and analysis of PEM fuel cell design and manufacturing. J. Power Sources 2003, 114, 32–53. [Google Scholar] [CrossRef]
- Lobato, J.; Rodrigo, M.A.; Linares, J.J. Effect of the catalytic ink preparation method on the performance of high temperature polymer electrolyte membrane fuel cells. J. Power Sources 2006, 157, 284–292. [Google Scholar] [CrossRef]
- Antoine, O.; Bultel, Y.; Ozil, P.; Durand, R. Catalyst gradient for cathode active layer of proton exchange membrane fuel cell. Electrochim. Acta 2000, 45, 4493–4500. [Google Scholar] [CrossRef]
- Debe, M.K. Tutorial on the Fundamental Characteristics and Practical Properties of Nanostructured Thin Film (NSTF) Catalysts. J. Electrochem. Soc. 2013, 160, F522–F534. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Ozden, A.; Li, X.; Hamdullahpur, F. A novel membrane electrode assembly design for proton exchange membrane fuel cells: Characterization and performance evaluation - ScienceDirect. Electrochim. Acta 2019, 299, 809–819. [Google Scholar] [CrossRef]
- Cha, D.; Jeon, S.W.; Yang, W.; Kim, D.; Kim, Y. Comparative performance evaluation of self-humidifying PEMFCs with short-side-chain and long-side-chain membranes under various operating conditions. Energy 2018, 150, 320–328. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, B.; Shu, P.; Luo, M.; Xie, C.; Quan, S.; Tu, Z.; Yu, Y. Evaluation of performance enhancement by condensing the anode moisture in a proton exchange membran fuel cell stack. Appl. Therm. Eng. 2017, 120, 115–120. [Google Scholar] [CrossRef]
- García-Salaberri, P.; Sánchez, D.; Boillat, P.; Vera, M.; Friedrich, K. Hydration and dehydration cycles in polymer electrolyte fuel cells operated with wet anode and dry cathode feed: A neutron imaging and modeling study. J. Power Sources 2017, 359, 634–655. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid Ionomers. Chem. Rev. 2017, 117, 987. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yi, B.; Xing, D.; Liu, F.; Shao, Z.; Fu, Y.; Zhang, H. Degradation mechanism of polystyrene sulfonic acid membrane and application of its composite membranes in fuel cells. Phys. Chem. Chem. Phys. 2002, 5, 611–615. [Google Scholar] [CrossRef]
- Kerres, J.A.; Xing, D.; Schnberger, F. Comparative investigation of novel PBI blend ionomer membranes from nonfluorinated and partially fluorinated poly arylene ethers. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 2311–2326. [Google Scholar] [CrossRef]
- Genies, C.; Mercier, R.; Sillion, B.; Cornet, N.; Gebel, G.; Pineri, M. Soluble sulfonated naphthalenic polyimides as materials for proton exchange membranes. Polymer 2001, 42, 359–373. [Google Scholar] [CrossRef]
- Genies, C.; Mercier, R.; Sillion, B.; Petiaud, R.; Cornet, N.; Gebel, G.; Pineri, M. Stability study of sulfonated phthalic and naphthalenic polyimide structures in aqueous medium. Polymer 2001, 42, 5097–5105. [Google Scholar] [CrossRef]
- Available online: http://www.dyfhem.cn/cn/ProductInfo.aspx?Id=10065 (accessed on 4 January 2023).
- Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-polymer-electrolyte-membrane-fuel-cell-components (accessed on 4 January 2023).
- Tang, L.; Yu, L.; Zhang, K.; Zhu, Y.; Zhu, Y.; Yang, S. Research Progress on Proton Exchange Membrane Fuel Cell Catalysts. Automotive Digest 2020, 1, 1–7. (In Chinese) [Google Scholar]
- Markovic, N.M.; Schmidt, T.J.; Stamenkovic, V.; Ross, P.N. Oxygen reduction reaction on Pt and Pt bimetallic surfaces: A selective review. Fuel Cells 2001, 1, 105–116. [Google Scholar] [CrossRef]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D.; et al. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef]
- Marković, N.; Adžić, R.; Cahan, B.; Yeager, E. Structural effects in electrocatalysis: Oxygen reduction on platinum low index single- crystal surfaces in perchloric acid solutions. J. Electroanal. Chem. 1994, 377, 249–259. [Google Scholar] [CrossRef]
- Hitotsuyanagi, A.; Nakamura, M.; Hoshi, N. Structural effects on the activity for the oxygen reduction reaction on n(111)-(100) series of Pt: Correlation with the oxide film formation. Electrochim. Acta 2012, 82, 512–516. [Google Scholar] [CrossRef]
- Maciá, M.; Campiña, J.; Herrero, E.; Feliu, J. On the kinetics of oxygen reduction on platinum stepped surfaces in acidic media. J. Electroanal. Chem. 2004, 564, 141–150. [Google Scholar] [CrossRef]
- Antolini, E.; Giorgi, L.; Pozio, A.; Passalacqua, E. Influence of Nafion loading in the catalyst layer of gas-diffusion electrodes for PEFC. J. Power Sources 1999, 77, 136–142. [Google Scholar] [CrossRef]
- Henry, P.; Guétaz, L.; Pélissier, N.; Jacques, P.A.; Escribano, S. Structural and chemical analysis by transmission electron microscopy of Pt- Ru membrane precipitates in proton exchange membrane fuel cell aged under reformate. J. Power Sources 2015, 275, 312–321. [Google Scholar] [CrossRef]
- Aslam, U.; Linic, S. Addressing challenges and scalability in the synthesis of thin uniform metal shells on large metal nanoparticle cores: Case study of Ag- Pt core- shell nanocubes. ACS Appl. Mater. Interfaces 2017, 9, 43127–43132. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, Z.; Cao, L.; Chen, Y.; Zhu, E.; Lin, Z.; Li, M.; Yan, A.; Zettl, A.; Wang, Y.M.; et al. High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction. Science 2015, 348, 1230–1234. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Sultan, S.; Myung, C.W.; Yoon, T.; Li, N.; Ha, M.; Harzandi, A.M.; Park, H.J.; Kim, D.Y.; Chandrasekaran, S.S.; et al. Multicomponent electrocatalyst with ultralow Pt loading and high hydrogen evolution activity. Nat. Energy 2018, 3, 773–782. [Google Scholar] [CrossRef]
- Yu, X.; Ye, S. Recent advances in activity and durability enhancement of Pt/C catalytic cathode in PEMFC- Part II: Degradation mechanism and durability enhancement of carbon supported platinum catalyst. J. Power Sources 2007, 172, 145–154. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, G.; Gauquelin, N.; Chen, N.; Zhou, J.; Yang, S.; Chen, W.; Meng, X.; Geng, D.; Banis, M.N.; et al. Single- atom catalysis using Pt/graphene achieved through atomic layer deposition. Sci. Rep. 2013, 3, 9. [Google Scholar] [CrossRef]
- Chen, S.; Wei, Z.; Qi, X.; Dong, L.; Guo, Y.-G.; Wan, L.; Shao, Z.; Li, L. Nanostructured polyaniline- decorated Pt/C@PANI core- shell catalyst with enhanced durability and activity. J. Am. Chem. Soc. 2012, 134, 13252–13255. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Pt-low-loading or Pt-free Catalyts for the Oxygen Reduction Reaction in Fuel Cells. Ph.D. Thesis, Chongqing University, Chong Qing, China, 2017. [Google Scholar]
- Bezerra, C.W.; Zhang, L.; Lee, K.; Liu, H.; Marques, A.L.; Marques, E.P.; Wang, H.; Zhang, J. A review of Fe-N/C and Co-N/C catalysts for the oxygen reduction reaction. Electrochim. Acta 2008, 53, 4937–4951. [Google Scholar] [CrossRef]
- Hu, M.Y.; Jensen, J.O.; Zhang, W.; Cleemann, L.N.; Xing, W.; Bjerrum, N.J.; Li, Q. Hollow spheres of iron carbide nanoparticles encased in graphitic layers as oxygen reduction catalysts. Angew. Chem. Int. Ed. 2014, 53, 3675–3679. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lee, W.J.; Kim, S.O.; Kim, Y.-H. Theory, synthesis, and oxygen reduction catalysis of Fe-porphyrin-like carbon nanotube. Phys. Rev. Lett. 2011, 106, 175502. [Google Scholar] [CrossRef]
- Deng, D.; Yu, L.; Chen, X.; Wang, G.; Jin, L.; Pan, X.; Deng, J.; Sun, G.; Bao, X. Iron encapsulated within pod-like carbon nanotubes for oxygen reduction reaction. Angew. Chem. Int. Ed. 2013, 52, 371–375. [Google Scholar] [CrossRef]
- Park, J.; Oh, H.; Ha, T.; Lee, Y.I.; Min, K. A review of the gas diffusion layer in proton exchange membrane fuel cells: Durability and degradation. Appl. Energy 2015, 155, 866–880. [Google Scholar] [CrossRef]
- Lapicque, F.; Belhadj, M.; Bonnet, C.; Pauchet, J.; Thomas, Y. A critical review on gas diffusion micro and macroporous layers degradations for improved membrane fuel cell durability. J. Power Sources 2016, 336, 40–53. [Google Scholar] [CrossRef]
- Rofaiel, A.; Ellis, J.; Challa, P.; Bazylak, A. Heterogeneous through-plane distributions of polytetrafluoroethylene in polymer electrolyte membrane fuel cell gas diffusion layers. J. Power Sources 2012, 201, 219–225. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, M.J.; Cui, T.; Shimpalee, S.; Seraphin, S.; Duong, B.; Van Zee, J. Effect of microporous layer on MacMullin number of carbon paper gas diffusion layer. J. Power Sources 2012, 207, 91–100. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Falcão, D.; Oliveira, V.; Pinto, A. Experimental study on the membrane electrode assembly of a proton exchange membrane fuel cell: Effects of microporous layer, membrane thickness and gas diffusion layer hydrophobic treatment. Electrochim Acta 2017, 224, 337–345. [Google Scholar] [CrossRef]
- Yuan, W.; Tang, Y.; Yang, X.; Wan, Z. Porous metal materials for polymer electrolyte membrane fuel cells-a review. Appl. Energy 2012, 94, 309–329. [Google Scholar] [CrossRef]
- Ozden, A.; Shahgaldi, S.; Li, X.; Hamdullahpur, F. A review of gas diffusion layers for proton exchange membrane fuel cells—With a focus on characteristics, characterization techniques, materials and designs. Prog. Energy Combust. Sci. 2019, 74, 50–102. [Google Scholar] [CrossRef]
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z.; et al. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, S.; Lin, C. Performance Improvement of Fuel Cell Systems Based on Turbine Design and Supercharging System Matching. Appl. Therm. Eng. 2020, 180, 115806. [Google Scholar] [CrossRef]
- Yan, W.-M.; Chen, C.-Y.; Mei, S.-C.; Soong, C.-Y.; Chen, F. Effects of operating conditions on cell performance of PEM fuel cells with conventional or interdigitated flow field. J. Power Sources 2006, 162, 1157–1164. [Google Scholar] [CrossRef]
- Sun, H.; Liu, H.; Guo, L.J. PEM fuel cell performance and its two-phase mass transport. J. Power Sources 2005, 143, 125–135. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Wan, Y. Performance improvement of centrifugal compressors for fuel cell vehicles using the aerodynamic optimization and data mining methods. Int. J. Hydrogen Energy 2020, 45, 11276–11286. [Google Scholar] [CrossRef]
- Zhang, Y.; Bao, P.; Wan, Y.; Xu, S. Modeling and analysis of air supply system of polymer electrolyte membrane fuel cell system. Energy Procedia 2017, 142, 1053–1058. [Google Scholar] [CrossRef]
- Ahluwalia, R.; Wang, X.; Kwon, J.; Rousseau, A.; Kalinoski, J.; James, B.; Marcinkoski, J. Performance and cost of automotive fuel cell systems with ultra-low platinum loadings. J. Power Sources 2011, 196, 4619–4630. [Google Scholar] [CrossRef]
- Ahluwalia, R.K.; Wang, X.; Kumar, R. Fuel Cells Systems Analysis 2011. DOE Hydrogen and Fuel Cells Program Review; US Department of Energy: Washington, DC, USA, 2011.
- Ahluwalia, R.K.; Wang, X. Fuel Cells Systems Analysis 2014; DOE Hydrogen and Fuel Cells Program Review; US Department of Energy: Washington, DC, USA, 2014.
- Ahluwalia, R.K.; Wang, X.; Kumar, R. Fuel Cells Systems Analysis 2015, DOE Hydrogen and Fuel Cells Program Review; US Department of Energy: Washington, DC, USA, 2015.
- Blunier, B.; Miraoui, A. Proton exchange membrane fuel cell air management in automotive applications. J. Fuel Cell Sci. Technol. 2010, 7, 041007. [Google Scholar] [CrossRef]
- Drive US. Fuel Cell Technical Team Roadmap; US DRIVE Partnership: New York, NY, USA, 2013.
- Sang, J.; Venturi, M.; Bocksch, R. NVH-Challenges of Air Supply Subsystems for Automotive Fuel Cell Applications. SAE Int. J. Engines 2009, 1, 258–266. [Google Scholar]
- Available online: https://www.snowkey.com/cn/product/air_compressor/4470def64bed45d9ac953579244d3771.html (accessed on 4 January 2023).
- Sugawara, T.; Kanazawa, T.; Imai, N.; Tachibana, Y. Development of Motorized Turbo Compressor for Clarity Fuel Cell. In Proceedings of the Wcx™ 17: Sae World Congress Experience, Detroit, MI, USA, 4–6 April 2017. [Google Scholar]
- Chen, H.; Pei, P.; Song, M. Lifetime prediction and the economic lifetime of Proton Exchange Membrane fuel cells. Appl. Energy 2015, 142, 154–163. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, J.; Liu, H.; Yan, C.; Hou, Y.; He, Q.; Zhang, J.; Hissel, D. Anode purge management for hydrogen utilization and stack durability improvement of PEM fuel cell systems. Appl. Energy 2020, 275, 115110. [Google Scholar] [CrossRef]
- Badami, M.; Mura, R. Theoretical model with experimental validation of a regenerative blower for hydrogen recirculation in a PEM fuel cell system. Energy Convers. Manag. 2010, 51, 553–560. [Google Scholar] [CrossRef]
- Hou, J.; Yang, M.; Zhang, J. Active and passive fuel recirculation for solid oxide and proton exchange membrane fuel cells. Renew. Energy 2020, 155, 1355–1371. [Google Scholar] [CrossRef]
- Kim, M.; Sohn, Y.-J.; Cho, C.-W.; Lee, W.-Y.; Kim, C.-S. Customized design for the ejector to recirculate a humidified hydrogen fuel in a submarine PEMFC. J. Power Sources 2008, 176, 529–533. [Google Scholar] [CrossRef]
- Dadvar, M.; Afshari, E. Analysis of design parameters in anodic recirculation system based on ejector technology for PEM fuel cells: A new approach in designing. Int. J. Hydrogen Energy 2014, 39, 12061–12073. [Google Scholar] [CrossRef]
- Morishima, S.; Suzuki, M. Fuel cell system working to control supply pressure of fuel accurately. U.S. Patent US7105243B2, 12 September 2006. [Google Scholar]
- Sugawara, T.; Kizaki, S.; Nuiya, Y. Variable flow-rate ejector and fuel cell system having the same. U.S. Patent US6858340B2, 22 February 2005. [Google Scholar]
- Blaszczyk, J.; Fleck, W.; Paterson, P.; Paterson, P.L. Fuel cell system with fluid stream recirculation. U.S. Patent US7309537B2, 18 December 2007. [Google Scholar]
- Park, M.; Kim, Y.; Kang, S. Integrated hydrogen recirculation blower for fuel cell vehicle. U.S. Patent US8113791B2, 14 February 2012. [Google Scholar]
- James, B.; Spisak, A.; Colella, W. Design for manufacturing and assembly cost estimate methodology for transportation fuel cell systems. J. Manuf. Sci. Eng. 2014, 136, 024503. [Google Scholar] [CrossRef]
- Ahluwalia, R.; Wang, X. Fuel cell systems for transportation: Status and trends. J. Power Sources 2008, 177, 167–176. [Google Scholar] [CrossRef]




| Enterprise Name | SHPT | Sunrise Power | SinoFuelCell | Sinosynergy | H-RISE |
|---|---|---|---|---|---|
| Rated power /kW | 163 [16] | 200 [17] | 150 [18] | 178 [19] | 170 [20] |
| Power density /kW/L | 4.2 [16] | 6.0 [17] | 4.0 [18] | 2.9 [19] | 5.5 [20] |
| Cold start temperature /°C | −30 [16] | −40 [17] | −35 [18] | −35 [19] | −30 [20] |
| Life /h | 15,000 [16] | 20,000 [17] | 15,000 [18] | 30,000 [19] | 15,000 [20] |
| Plate type | Metal [16] | Metal [17] | Metal | Graphite [19] | Metal |
| Characteristic | Units | 2020 Target |
|---|---|---|
| Plate Cost | $/kW | 3 |
| Plate weight | kg/kW | 0.4 |
| Plate H2 permeation coefficient | Std cm3/(s cm2Pa)@ 80 °C, 3 atm 100% RH | 1.3 × 10−14 |
| Corrosion, anode | μA/cm2 | 1 |
| Corrosion, cathode | μA/cm2 | 1 |
| Electrical conductivity | S/cm | 100 |
| Areal specific resistance | Ohm-cm2 | 0.01 |
| Flexural strength | MPa | 25 |
| Forming elongation | % | 40 |
| Number | Enterprise Name | Product Range | Nationality |
|---|---|---|---|
| 1 | Dupont | Nafion | U.S.A |
| 2 | Gore | - | U.S.A |
| 3 | 3M | - | U.S.A |
| 4 | Mitsubishi | Aciplex F800 | Japan |
| 5 | AGC | Flemion F4000 | Japan |
| 6 | Dongyue Federation | DF988; DF2801 | China |
| Enterprise | Toyota | Hyundai | Nissan | GE | Mercedes-Benz | SAICMOTOR |
|---|---|---|---|---|---|---|
| Stake Power/kW | 114 | 100 | 90 | 92 | 100 | 40 |
| Pt Used/g | 20 | 40 | 30 | 30 | 20 | - |
| Durability/h | >5000 | 5500 | - | 5500 | >5000 | 2000 |
| Type | Advantages | Disadvantages |
|---|---|---|
| Pt alloy CL | Enhanced activity and stability | Prone to agglomerate during preparation |
| Surface controlled Pt CL | Enhanced activity | Changeable morphological structure and reduced stability |
| Transition metal CL | Enhanced activity and reduced cost | Unstable in acid electrolytes |
| Nonmetallic CL | Reduced cost | Unknown activity and stability |
| Type | Screw Type | Root Type | Centrifugal Type | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Double Screw | Four-Lobe Roots | Six-Lobe Roots | Single-Stage Centrifugal | Two-Stage Centrifugal | |||||
| Enterprise | Snowman Group | UQM | Toyota Industries | Liebherr | Easyland | Garrett | Liebherr | ZCJSD | Xeca Turbo |
| Maximum pressure ratio | 2.8 [112] | 1.8 * | 2.4 | 2.7 * | 2.1 * | 4 [113] | 3.7 * | 3.4 * | 2.8 * |
| Rated flow/g∙s−1 | 100 [112] | 150 * | 100 | 120 * | 68 * | 125 [113] | 140 * | 114 * | 144 * |
| Maximum aerodynamic efficiency/% | – | 71 * | – | 75 * | 80 * | 70 (including the motor and controller) [113] | – | – | – |
| Maximum rotating speed/r∙min−1 | 24,000 [112] | 18,000 * | 12,500 | 78,000 * | 100,000 * | 100,000 [113] | 85,000 * | 95,000 * | 95,000 * |
| Maximum steady power/kW | – | 14 * | 20 | 22 * | 10 * | 20 [113] | 25 * | 25 * | 22 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, J.; Yao, Z. Recent Development of Fuel Cell Core Components and Key Materials: A Review. Energies 2023, 16, 2099. https://doi.org/10.3390/en16052099
Zhang Y, Wang J, Yao Z. Recent Development of Fuel Cell Core Components and Key Materials: A Review. Energies. 2023; 16(5):2099. https://doi.org/10.3390/en16052099
Chicago/Turabian StyleZhang, Yuemeng, Jia Wang, and Zhanhui Yao. 2023. "Recent Development of Fuel Cell Core Components and Key Materials: A Review" Energies 16, no. 5: 2099. https://doi.org/10.3390/en16052099
APA StyleZhang, Y., Wang, J., & Yao, Z. (2023). Recent Development of Fuel Cell Core Components and Key Materials: A Review. Energies, 16(5), 2099. https://doi.org/10.3390/en16052099






