Development of Microencapsulation-Hybrid Jig Separation Technique as a Clean Coal Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Characterization
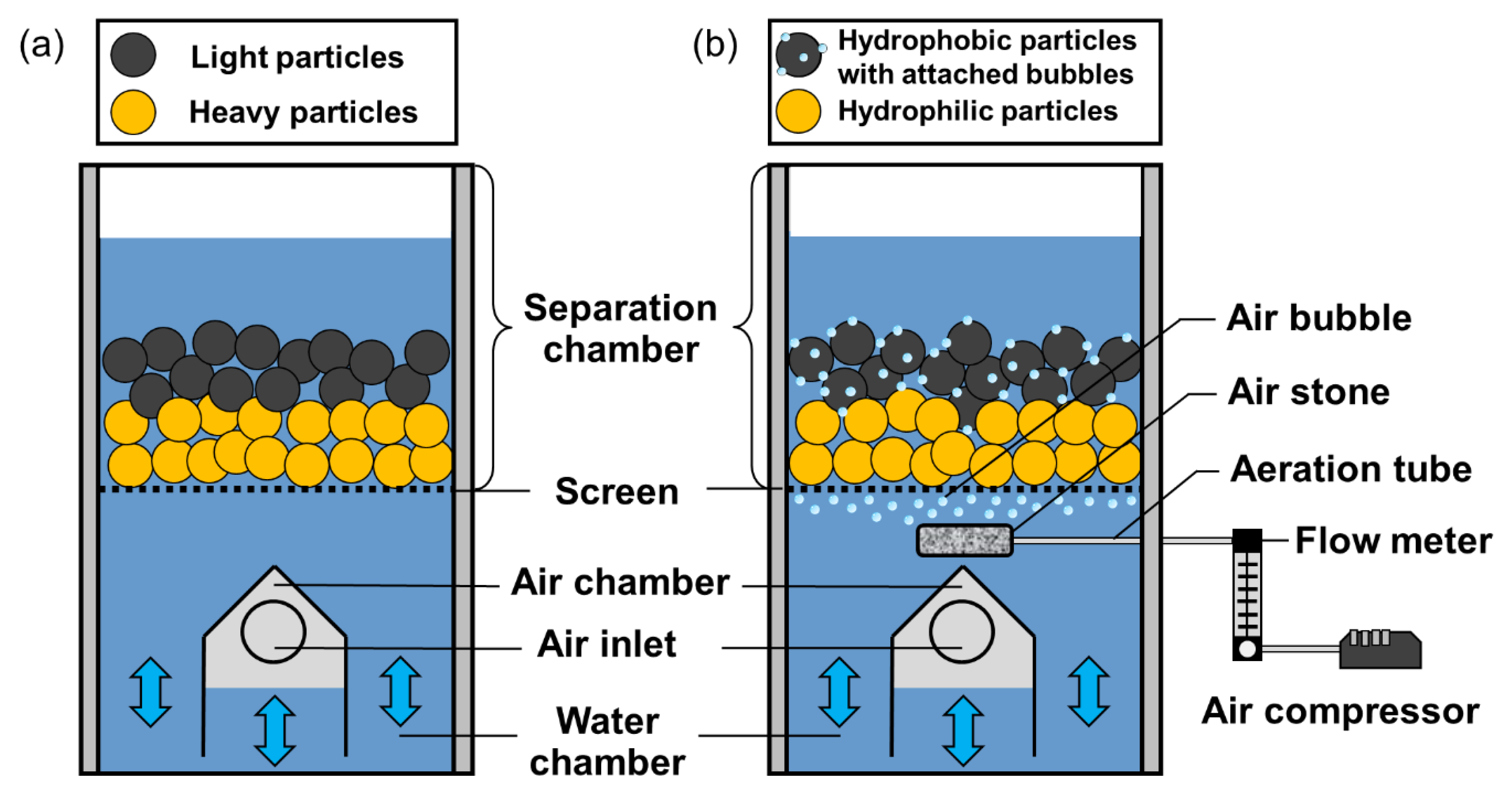
2.2. Jig Separation Experiments
2.3. Hybrid Jig Separation Experiments
2.4. Microencapsulation Experiments
3. Results and Discussion
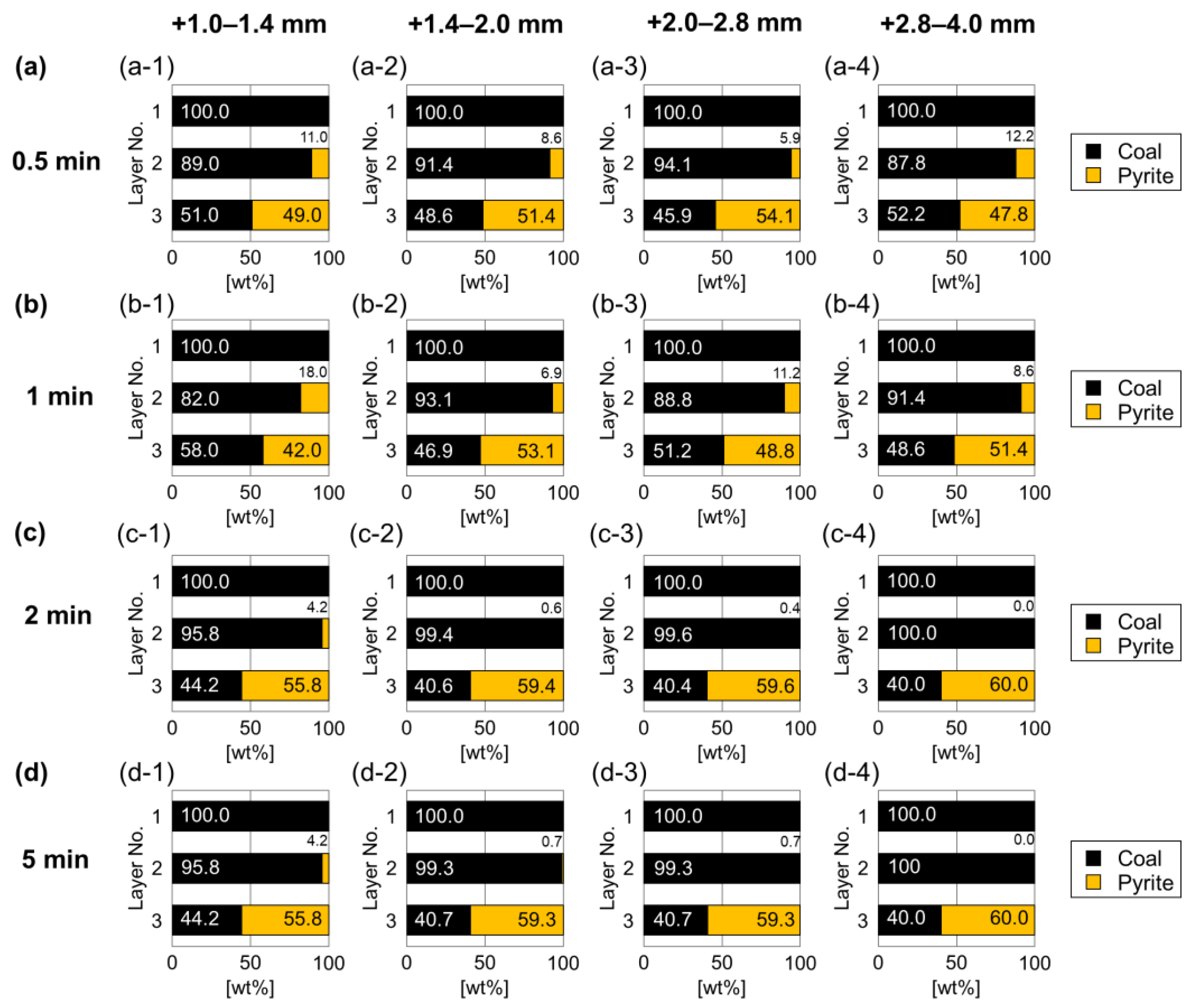
3.1. Effects of Particle Size and Separation Time on Jig Separation
3.2. Effects of Particle Size and Separation Time on the Hybrid Jig Separation
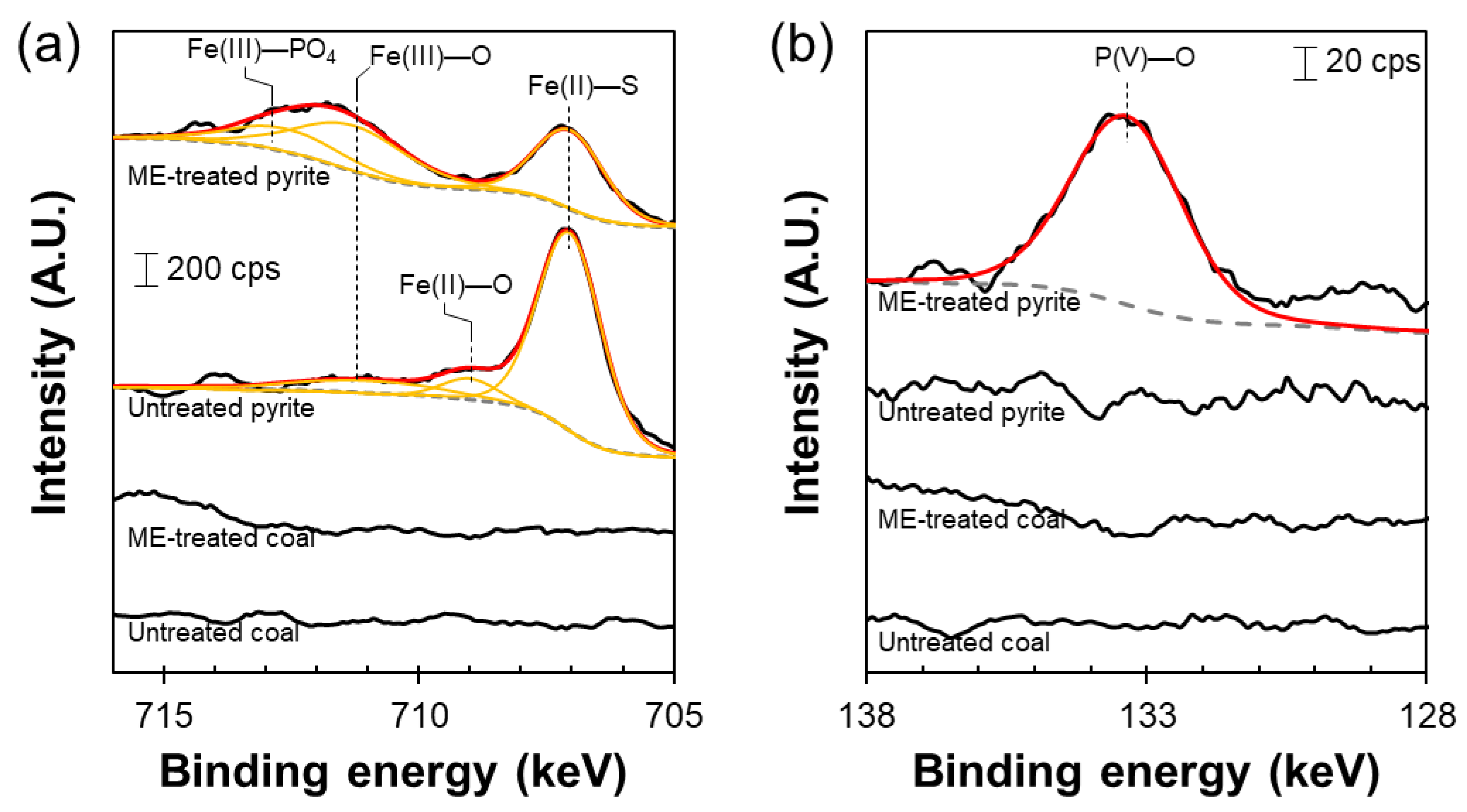
3.3. Effects of Microencapsulation as Pretreatment on Hybrid Jig Separation
4. Conclusions
- For the conventional jig, most of coal and pyrite could be recovered in the top and bottom layers, respectively. In the middle layer, no pyrite was present for +2.8–4.0 mm size fraction, only a few pyrite (less than 1%) was found in +1.4–2.0 and +2.0–2.8 mm size fractions, and 4.2% pyrite remained for +1.0–1.4 mm size fraction, indicating that separation is more difficult when particle size is reduced.
- For hybrid jig separation without microencapsulation, the top and bottom layers contain clean coal and pyrite, respectively, similar to the conventional jig. However, the required time for separation in the hybrid jig (0.5 min) was shorter than in the conventional jig (2 min).
- Higher pyrite content was found in the middle layer of hybrid jig especially in the +1.0–1.4 size fraction, suggesting that at <1.4 mm separation of pyrite and coal was less efficient.
- The worst results were found with +1.0–1.4 mm fraction and longer separation time, which could be attributed to bubble-induced agglomeration of fine, hydrophobic coal and pyrite.
- Microencapsulation-hybrid jig separation dramatically shortened the separation time and improved the separation efficiency because of the selective formation of hydrophilic iron phosphate coating on pyrite.
- For processing coals containing finely dispersed pyrite, the size reduction to achieve a sufficient liberation of coal from pyrite is necessary. In contrast to conventional jig, microencapsulation-hybrid jigs can produce low S coal from coal/pyrite mixture with a size fraction of +1.0–1.4 mm. Thus, micro-encapsulation-hybrid jig will contribute to the processing of coals that is difficult to be cleaned by conventional jig. In addition, pyrite is covered with a surface protective coating that will contribute to the suppression of AMD formation caused by pyrite oxidation.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phengsaart, T.; Srichonphaisan, P.; Kertbundit, C.; Soonthornwiphat, N.; Sinthugoot, S.; Phumkokrux, N.; Juntarasakul, O.; Maneeintr, K.; Numprasanthai, A.; Park, I.; et al. Conventional and recent advances in gravity separation technologies for coal cleaning: A systematic and critical review. Heliyon 2023, 9, e13083. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, J.S.; Marley, N.A. The impacts of combustion emissions on air quality and climate—From coal to biofuels and be-yond. Atmos. Environ. 2009, 43, 23–36. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, P.; Cheng, H.; Liu, Q. Thermal phase transition of pyrite from coal. J. Therm. Anal. Calorim. 2015, 134, 2391–2396. [Google Scholar] [CrossRef]
- Park, I.; Hong, S.; Jeon, S.; Ito, M.; Hiroyoshi, N. Flotation separation of chalcopyrite and molybdenite assisted by microencapsulation using ferrous and phosphate ions: Part I. Selective coating formation. Metals 2020, 10, 1667. [Google Scholar] [CrossRef]
- Guan, G. Clean coal technologies in Japan: A review. Chin. J. Chem. Eng. 2017, 25, 689–697. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, S.; Li, Y. Coal liquefaction technologies—Development in China and challenges in chemical reaction engineering. Chem. Eng. Sci. 2010, 65, 12–17. [Google Scholar] [CrossRef]
- Minchener, A.J. Coal gasification for advanced power generation. Fuel 2005, 84, 2222–2235. [Google Scholar] [CrossRef]
- Sen, S. An overview of clean coal technologies I: Pre-combustion and post-combustion emission control. Energy Sources B Econ. Plan. Policy 2010, 5, 261–271. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Z.; Zhang, B.; Sun, J.; Zhang, L.; Zhang, T.; Xu, H.; Bei, N.; Tian, J.; Wang, Q.; et al. Emission reduction effect on PM2.5, SO2 and NOx by using red mud as additive in clean coal briquetting. Atmos. Environ. 2020, 223, 117203. [Google Scholar] [CrossRef]
- Topper, J.M.; Cross, P.J.I.; Goldthorpe, S.H. Clean coal technology for power and cogeneration. Fuel 1994, 73, 1056–1063. [Google Scholar] [CrossRef]
- Hutson, N.D.; Krzyzynska, R.; Srivastava, R.K. Simultaneous removal of SO2, NOx, and Hg from coal flue gas using a NaClO2-enhanced wet scrubber. Ind. Eng. Chem. Res. 2008, 47, 5825–5831. [Google Scholar] [CrossRef]
- Maneeintr, K.; Henni, A.; Idem, R.O.; Tontiwachwuthikul, P.; Wee, A.G.H. Physical and transport properties of aqueous amino alcohol solutions for CO2 capture from flue gas streams. Process. Saf. Environ. Prot. 2008, 86, 291–295. [Google Scholar] [CrossRef]
- Maneeintr, K.; Idem, R.O.; Tontiwachwuthikul, P.; Wee, A.G.H. Comparative mass transfer performance studies of CO2 absorption into squeous solutions of DEAB and MEA. Ind. Eng. Chem. Res. 2010, 49, 2857–2863. [Google Scholar] [CrossRef]
- Maneeintr, K.; Photien, K.; Charinpanitkul, T. Mixture of MEA/2-MAE for effective CO2 capture from flue gas stream. Chem. Eng. Trans. 2018, 63, 229–234. [Google Scholar] [CrossRef]
- Noda, N.; Makino, H. Influence of operating temperature on performance of electrostatic precipitator for pulverized coal combustion boiler. Adv. Powder Technol. 2010, 21, 495–499. [Google Scholar] [CrossRef]
- Yoosook, H.; Maneeintr, K. CO2 geological storage coupled with water alternating gas for enhanced oil recovery. Chem. Eng. Trans. 2018, 63, 217–222. [Google Scholar] [CrossRef]
- Zhao, L.; Li, C.; Zhang, X.; Zeng, G.; Zhang, J.; Xie, Y. A review on oxidation of elemental mercury from coal-fired flue gas with selective catalytic reduction catalysts. Catal. Sci. Technol. 2015, 5, 3459–3472. [Google Scholar] [CrossRef]
- Lejre, K.H.L.; Glarborg, P.; Christensen, H.; Mayer, S.; Kiil, S. Experimental investigation and mathematical modeling of the reaction between SO2(g) and CaCO3(s)-containing micelles in lube oil for large two-stroke marine diesel engines. Chem. Eng. J. 2020, 388, 124188. [Google Scholar] [CrossRef]
- Pyka, I.; Wierzchowski, K. Influence of equal settling particles on fine coal separation in water-only cyclones. Int. J. Coal Prep. Util. 2019, 42, 410–420. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, J.; Yin, W.; Yang, H.; Wei, L. Numerical study of gas-solid flow in a coal beneficiation fluidized bed using kinetic theory of granular flow. Fuel Process. Technol. 2013, 111, 29–41. [Google Scholar] [CrossRef]
- Shahzad, M.; Ali, Z. Short communication cleaning of Dalwal-Punjab coal by using shaking table. Pak. J. Sci. Ind. Res. 2018, 61, 56–58. [Google Scholar] [CrossRef]
- Shahzad, M.; Ali, Z. A simple and efficient process for small-scale cleaning of poorly liberated, difficult-to-wash low-rank coal. Energy Sources A Recovery Util. Environ. Eff. 2019, 1–14. [Google Scholar] [CrossRef]
- Chalavadi, G.; Singh, R.K.; Sharma, M.; Das, A. Recovery of combustibles from fine coal through controlled air fluidization over inclined deck. Sep. Sci. Technol. 2015, 50, 2032–2040. [Google Scholar] [CrossRef]
- Chalavadi, G.; Singh, R.K.; Sharma, M.; Singh, R. Development of a generalized strategy for dry beneficiation of fine coal over a vibrating inclined deck. Int. J. Coal Prep. Util. 2016, 36, 10–27. [Google Scholar] [CrossRef]
- Mohanty, M.; Zhang, B.; Wang, H.; Mahajan, A.; Akbari, H.; Bashir, Z.; Ramamoorthy, S.; Hirschi, J. Development and demonstration of an automation and control system for coal spirals. Int. J. Coal Prep. Util. 2014, 34, 157–171. [Google Scholar] [CrossRef]
- Hori, K.; Tsunekawa, M.; Ueda, M.; Hiroyoshi, N.; Ito, M.; Okada, H. Development of a new gravity separator for plastics—A hybrid-jig—. Mater. Trans. 2009, 50, 2844–2847. [Google Scholar] [CrossRef] [Green Version]
- Ito, M.; Saito, A.; Murase, N.; Phengsaart, T.; Kimura, S.; Tabelin, C.B.; Hiroyoshi, N. Development of suitable product recovery systems of continuous hybrid jig for plastic-plastic separation. Miner. Eng. 2019, 141, 105839. [Google Scholar] [CrossRef]
- Ito, M.; Takeuchi, M.; Saito, A.; Murase, N.; Phengsaart, T.; Tabelin, C.B.; Hiroyoshi, N.; Tsunekawa, M. Improvement of hybrid jig separation efficiency using wetting agents for the recycling of mixed-plastic wastes. J Mater. Cycles Waste Manag. 2019, 21, 1376–1383. [Google Scholar] [CrossRef]
- Ito, M.; Takeuchi, M.; Saito, A.; Murase, N.; Phengsaart, T.; Kimura, S.; Kitajima, N.; Tabelin, C.B.; Hiroyoshi, N. Estimation of hybrid jig separation efficiency using a modified concentration criterion based on apparent densities of plastic particles with attached bubbles. J. Mater. Cycles Waste Manag. 2020, 22, 2071–2080. [Google Scholar] [CrossRef]
- Jeon, S.; Ito, M.; Tabelin, C.B.; Pongsumrankul, R.; Tanaka, S.; Kitajima, N.; Saito, A.; Park, I.; Hiroyoshi, N. A physical separation scheme to improve ammonium thiosulfate leaching of gold by separation of base metals in crushed mobile phones. Miner. Eng. 2019, 138, 168–177. [Google Scholar] [CrossRef]
- Hornn, V.; Ito, M.; Shimada, H.; Tabelin, C.B.; Jeon, S.; Park, I.; Hiroyoshi, N. Agglomeration–Flotation of Finely Ground Chalcopyrite using Emulsified Oil Stabilized by Emulsifiers: Implications for Porphyry Copper Ore Flotation. Metals 2020, 10, 912. [Google Scholar] [CrossRef]
- Seng, S.; Tabelin, C.B.; Makino, Y.; Chea, M.; Phengsaart, T.; Park, I.; Hiroyoshi, N.; Ito, M. Improvement of flotation and suppression of pyrite oxidation using phosphate-enhanced galvanic microencapsulation (GME) in a ball mill with steel ball media. Miner. Eng. 2019, 143, 105931. [Google Scholar] [CrossRef]
- Park, I.; Hong, S.; Jeon, S.; Ito, M.; Hiroyoshi, N. Flotation separation of chalcopyrite and molybdenite assisted by microencapsulation using ferrous and phosphate ions: Part II. Flotation. Metals 2020, 11, 439. [Google Scholar] [CrossRef]
- Duba, A.G. Electrical conductivity of coal and coal char. Fuel 1977, 56, 441–443. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, D.; Wang, Y.; Zhong, X.; Tang, X.; Xi, D. Electrical resistivity of coal-bearing rocks under high temperature and the detection of coal fires using electrical resistance tomography. Geophys. J. Int. 2016, 204, 1316–1331. [Google Scholar] [CrossRef]
- Ferrer, I.J.; Nevskala, D.M.; de las Heras, C.; Sanchez, C. About the band gap nature of FeS2 as determined from optical and photoelectrochemical measurements. Solid State Commun. 1990, 74, 913–916. [Google Scholar] [CrossRef]
- McKibben, M.A.; Barnes, H.L. Oxidation of pyrite in low temperature acidic solutions: Rate laws and surface textures. Geochim. Cosmochim. Acta 1986, 50, 1509–1520. [Google Scholar] [CrossRef]
- Chou, C. Sulfur in coals: A review of geochemistry and origins. Int. J. Coal Geol. 2012, 100, 1–13. [Google Scholar] [CrossRef]
- Dil, E.A.; Ghaedi, M.; Ghaedi, A.M.; Asfaram, A.; Goudarzi, A.; Hajati, S.; Soylak, M.; Agarwal, S.; Gupta, V.K. Modeling of quaternary dyes adsorption onto ZnO–NR–AC artificial neural network: Analysis by derivative spectrophotometry. J. Ind. Eng. Chem. 2016, 34, 186–197. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; Wiley-Interscience: New York, NY, USA, 1996. [Google Scholar]
- Descostes, M.; Mercier, F.; Thromat, N.; Beaucaire, C.; Gautier-Soyer, M. Use of XPS in the determination of chemical environment and oxidation state of iron and sulfur samples: Constitution of a data basis in binding energies for Fe and S reference compounds and applications to the evidence of surface species of an oxidized pyrite in a carbonate medium. Appl. Surf. Sci. 2000, 165, 288–302. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Z.; Wang, L.; Yu, L.; Chen, J.; Liang, Z.; Wang, R. A joint experimental and theoretical study on the effect of manganese doping on the structural, electrochemical and physical properties of lithium iron phosphate. RSC Adv. 2014, 4, 51609–51614. [Google Scholar] [CrossRef]
- Zeng, L.; Li, X.; Shi, Y.; Qi, Y.; Huang, D.; Tadé, M.; Wang, S.; Liu, S. FePO4 based single chamber air-cathode microbial fuel cell for online monitoring levofloxacin. Biosens. Bioelectron. 2017, 91, 367–373. [Google Scholar] [CrossRef] [PubMed]



| Purity [%] | Recovery [%] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Separation Time [min] | 0.5 | 5.0 | 0.5 | 5.0 | ||||||||
| Layer No. | 1 | 1 + 2 | 1 + 2 + 3 | 1 | 1 + 2 | 1 + 2 + 3 | 1 | 1 + 2 | 1 + 2 + 3 | 1 | 1 + 2 | 1 + 2 + 3 |
| Jig | 100.0 | 94.5 | 80.0 | 100.0 | 97.9 | 80.0 | 41.7 | 78.8 | 100.0 | 41.7 | 81.6 | 100.0 |
| 100.0 | 98.5 | 80.0 | 100.0 | 86.5 | 80.0 | 41.7 | 82.1 | 100.0 | 41.7 | 72.1 | 100.0 | |
| 100.0 | 99.5 | 80.0 | 100.0 | 100.0 | 80.0 | 41.7 | 82.9 | 100.0 | 41.7 | 83.3 | 100.0 | |
| Hybrid jig w/o microencapsulation | 100.0 | 94.5 | 80.0 | 100.0 | 97.9 | 80.0 | 41.7 | 78.8 | 100.0 | 41.7 | 81.6 | 100.0 |
| 100.0 | 98.5 | 80.0 | 100.0 | 86.5 | 80.0 | 41.7 | 82.1 | 100.0 | 41.7 | 72.1 | 100.0 | |
| Hybrid jig w/ microencapsulation | 100.0 | 99.5 | 80.0 | 100.0 | 100.0 | 80.0 | 41.7 | 82.9 | 100.0 | 41.7 | 83.3 | 100.0 |
| 100.0 | 94.5 | 80.0 | 100.0 | 97.9 | 80.0 | 41.7 | 78.8 | 100.0 | 41.7 | 81.6 | 100.0 | |
| 100.0 | 98.5 | 80.0 | 100.0 | 86.5 | 80.0 | 41.7 | 82.1 | 100.0 | 41.7 | 72.1 | 100.0 | |
| 100.0 | 99.5 | 80.0 | 100.0 | 100.0 | 80.0 | 41.7 | 82.9 | 100.0 | 41.7 | 83.3 | 100.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phengsaart, T.; Park, I.; Pasithbhattarabhorn, J.; Srichonphaisarn, P.; Kertbundit, C.; Phumkokrux, N.; Juntarasakul, O.; Tabelin, C.B.; Hiroyoshi, N.; Ito, M. Development of Microencapsulation-Hybrid Jig Separation Technique as a Clean Coal Technology. Energies 2023, 16, 2432. https://doi.org/10.3390/en16052432
Phengsaart T, Park I, Pasithbhattarabhorn J, Srichonphaisarn P, Kertbundit C, Phumkokrux N, Juntarasakul O, Tabelin CB, Hiroyoshi N, Ito M. Development of Microencapsulation-Hybrid Jig Separation Technique as a Clean Coal Technology. Energies. 2023; 16(5):2432. https://doi.org/10.3390/en16052432
Chicago/Turabian StylePhengsaart, Theerayut, Ilhwan Park, Jirathpapol Pasithbhattarabhorn, Palot Srichonphaisarn, Chinawich Kertbundit, Nutthakarn Phumkokrux, Onchanok Juntarasakul, Carlito Baltazar Tabelin, Naoki Hiroyoshi, and Mayumi Ito. 2023. "Development of Microencapsulation-Hybrid Jig Separation Technique as a Clean Coal Technology" Energies 16, no. 5: 2432. https://doi.org/10.3390/en16052432






