Experimental Study on Mineral Dissolution and Carbonation Efficiency Applied to pH-Swing Mineral Carbonation for Improved CO2 Sequestration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. pH Swing Process
2.2.1. Dissolution of Minerals
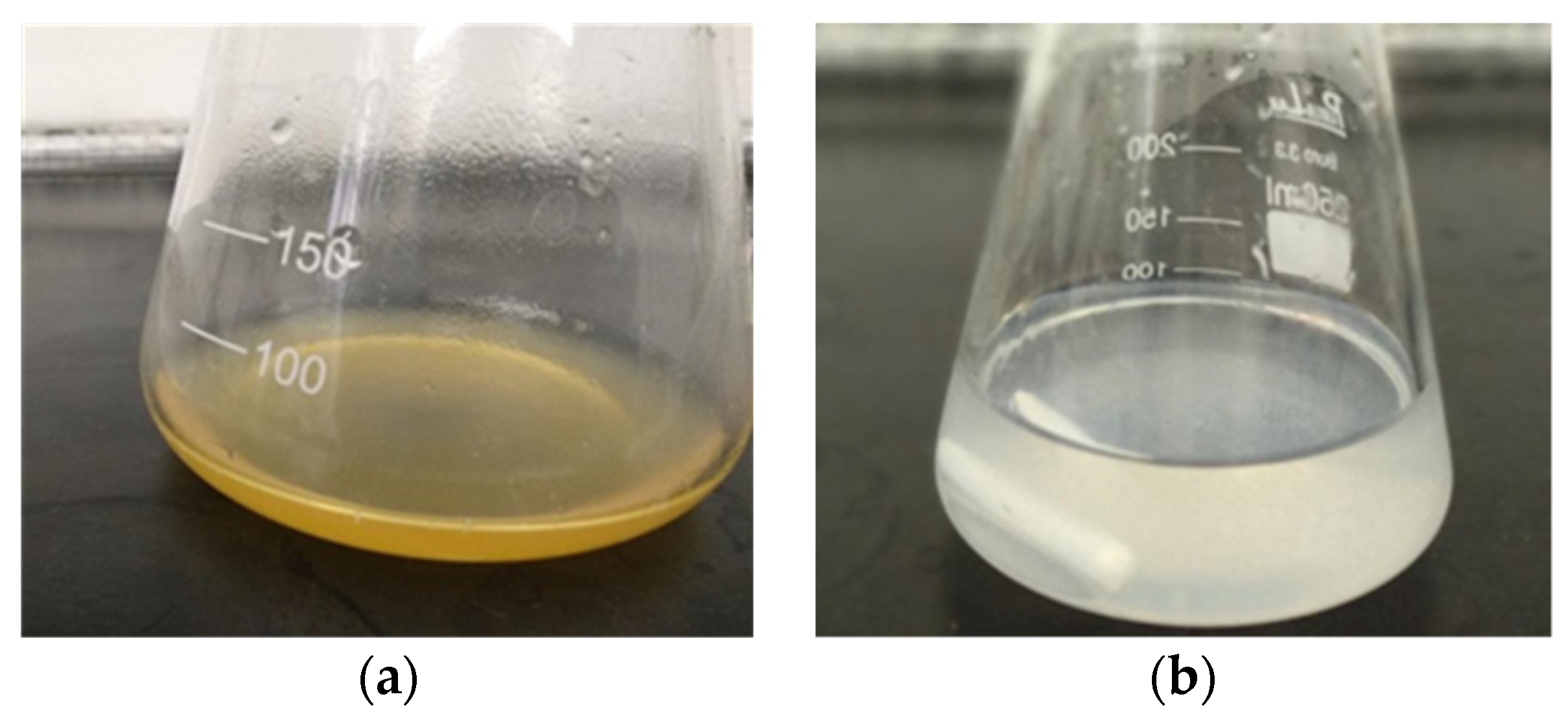
2.2.2. Purification
2.2.3. Mineral Carbonation
2.3. Experimental Design: Dissolution Stage
3. Results and Discussion
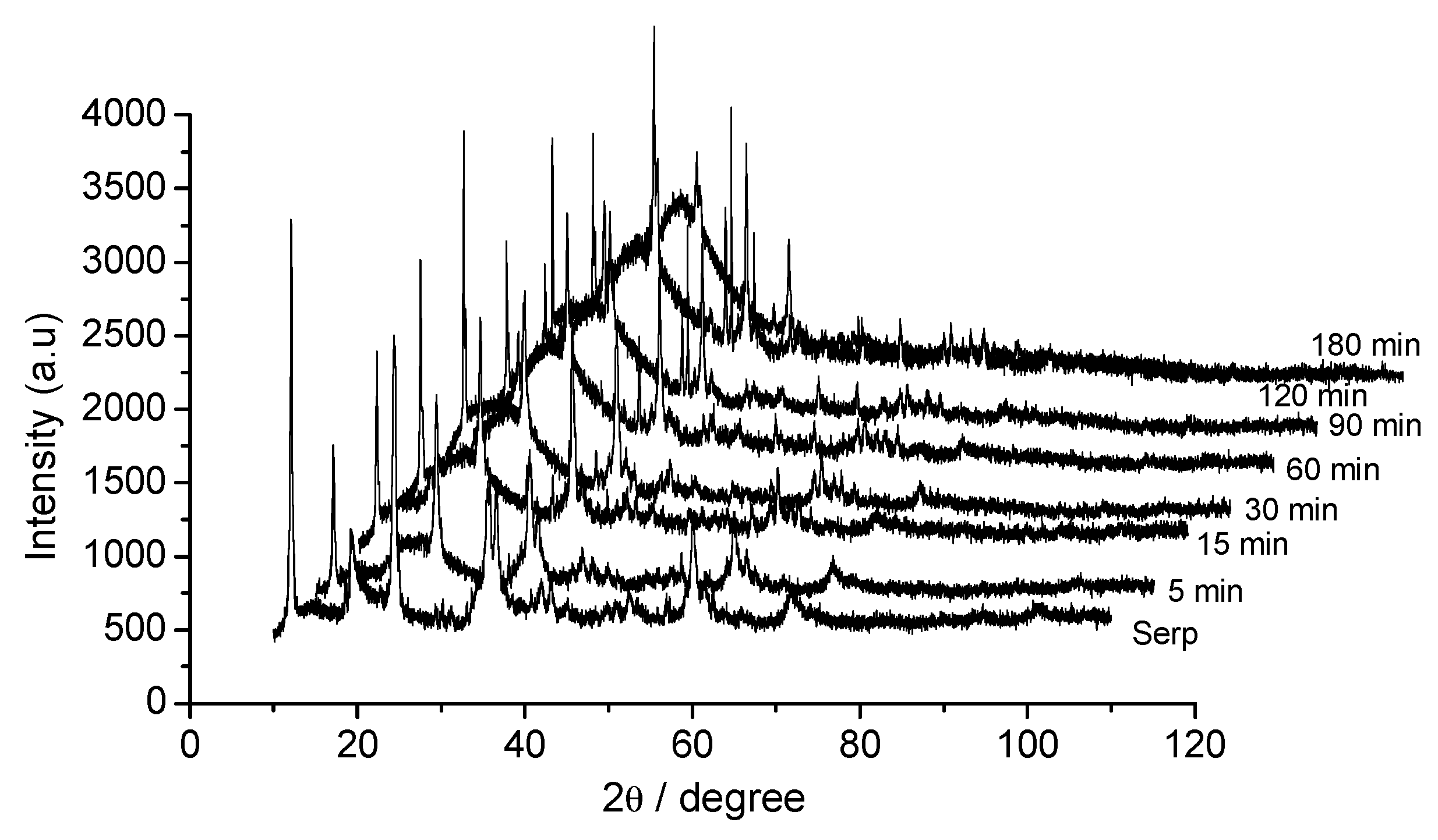
3.1. Dissolution Step
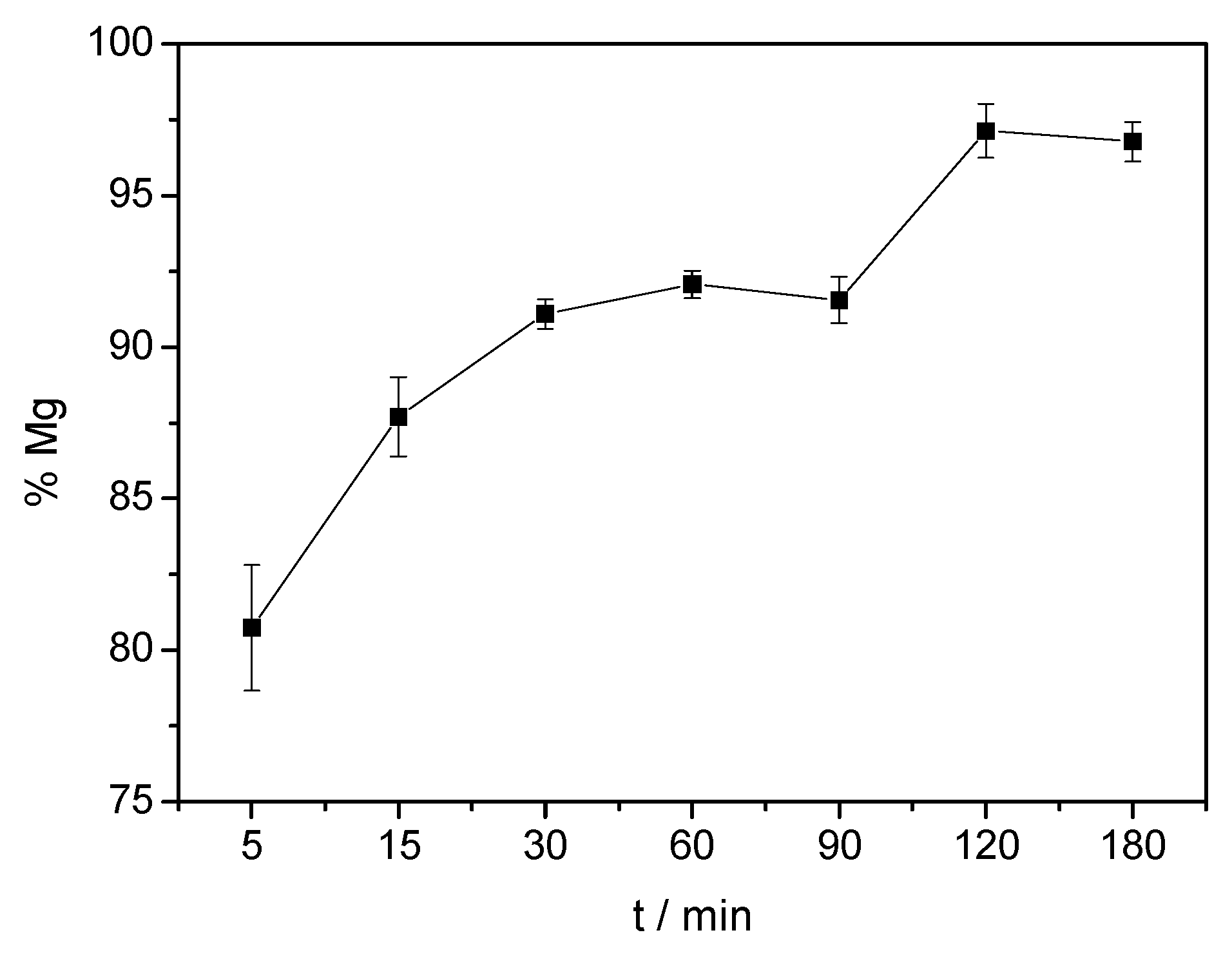
3.2. Effect of Time on Acid Dissolution
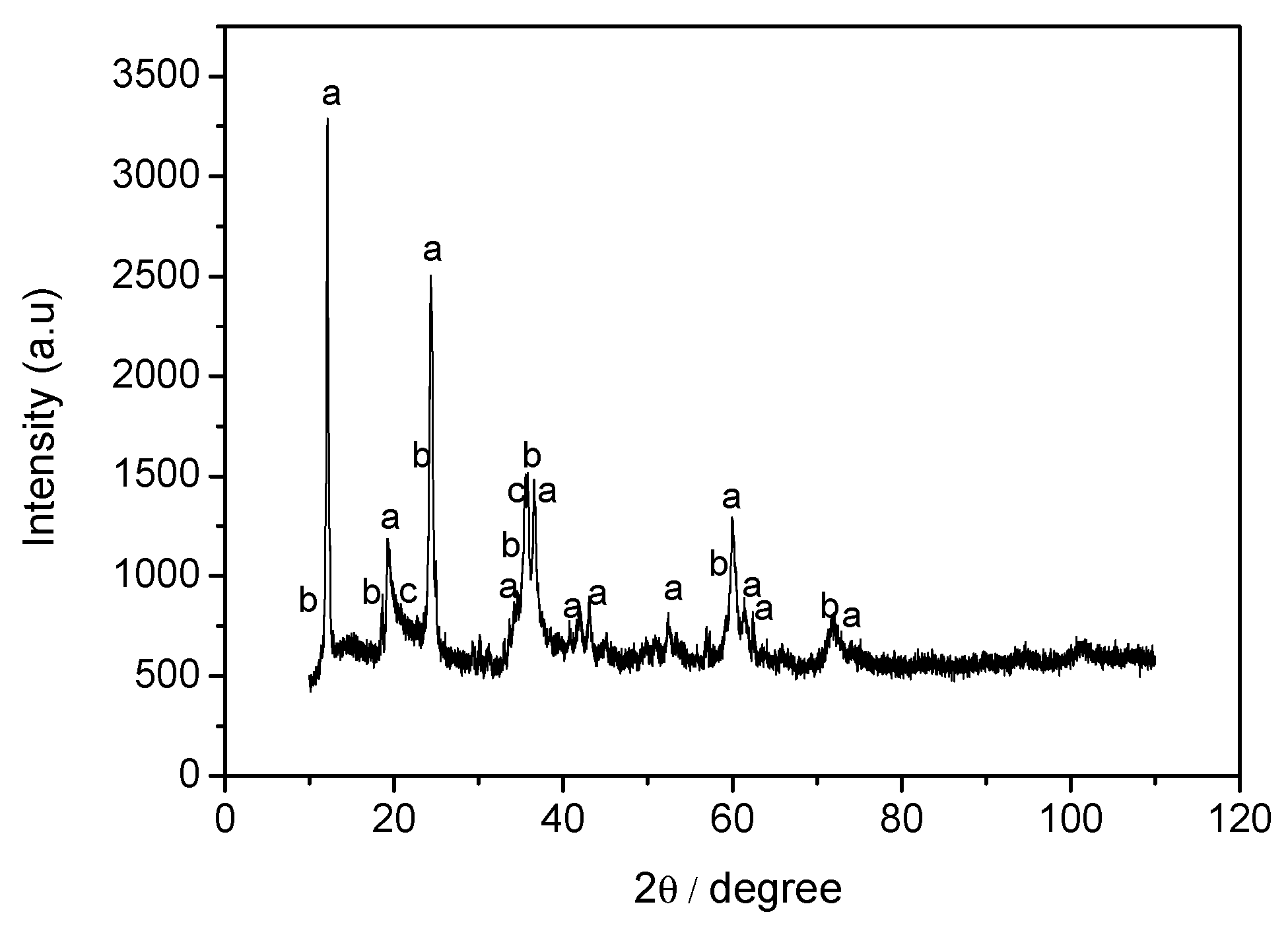
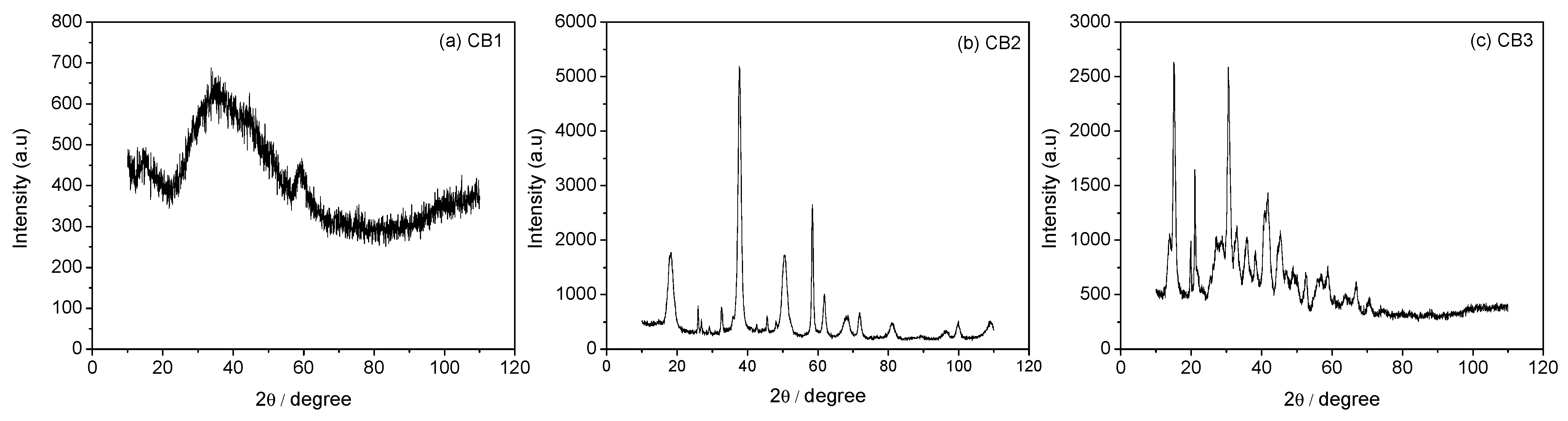
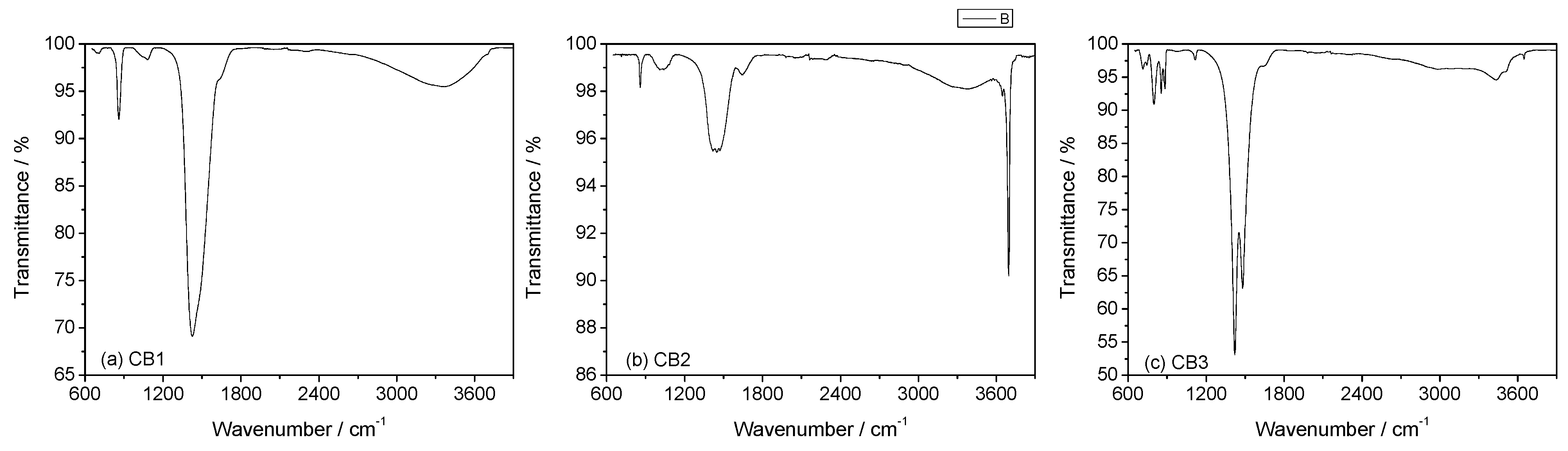
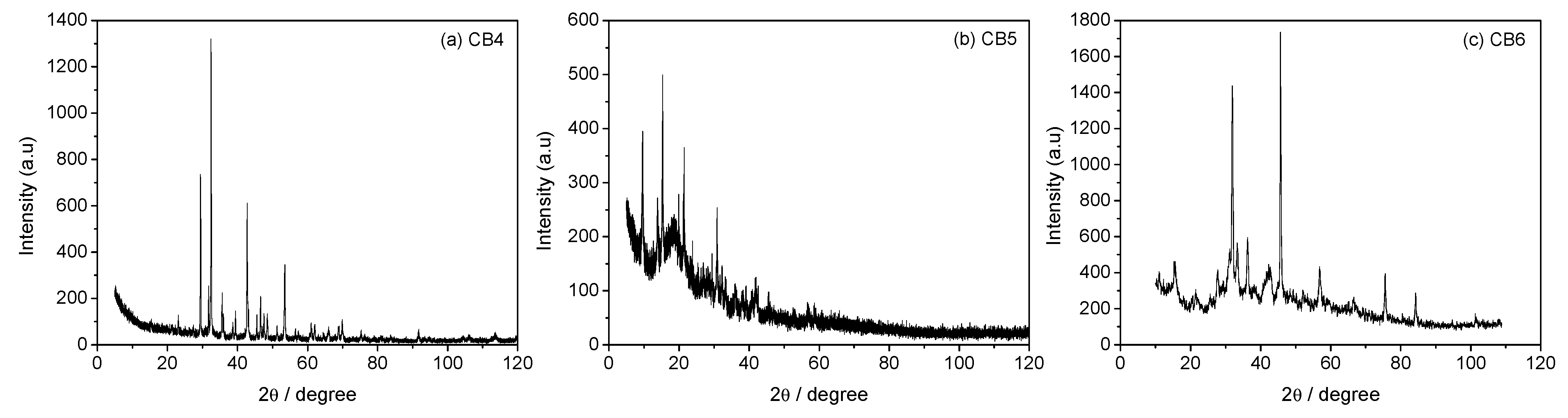
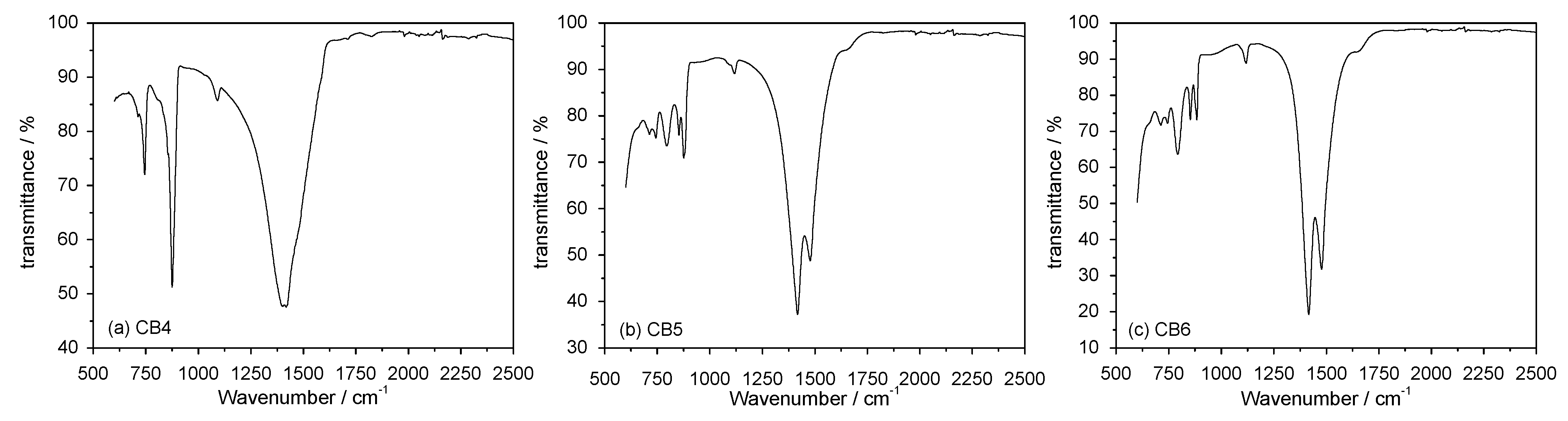
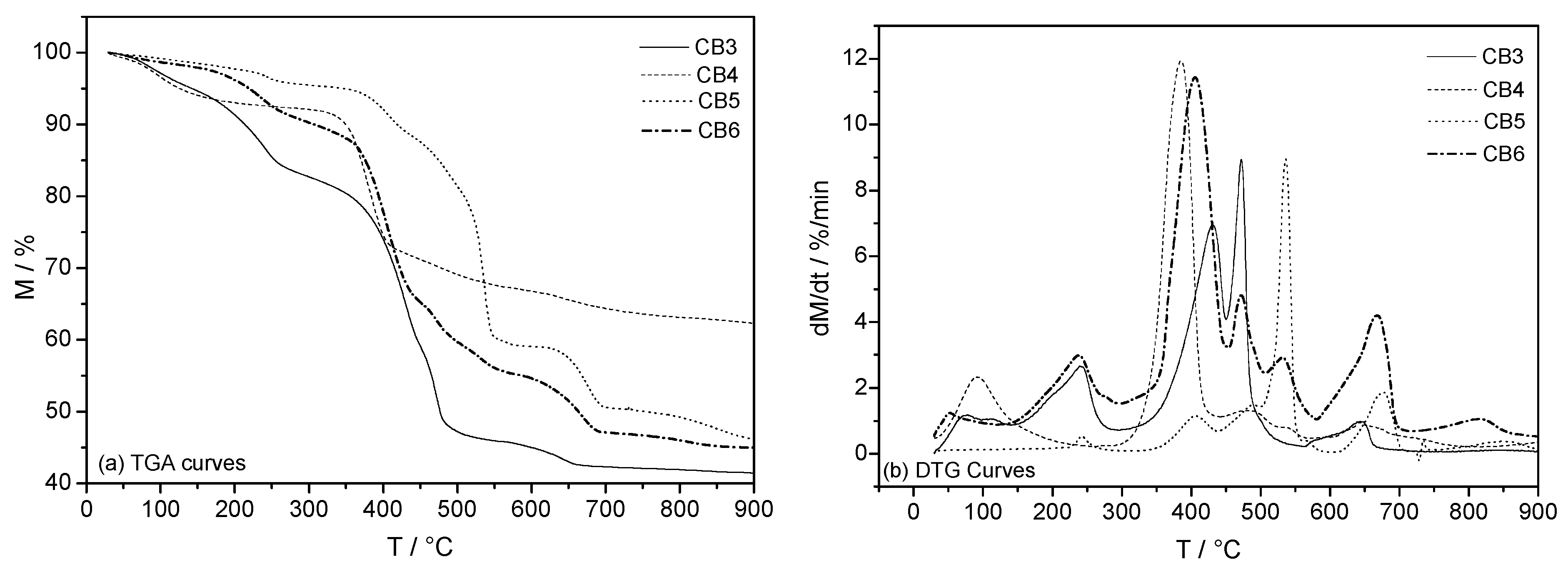
3.3. Mineral Carbonation Step
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mac Dowell, N.; Fennell, P.S.; Shah, N.; Maitland, G.C. The Role of CO2 Capture and Utilization in Mitigating Climate Change. Nat. Clim. Chang. 2017, 7, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Renforth, P. The Negative Emission Potential of Alkaline Materials. Nat. Commun. 2019, 10, 1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanniche, M.; Gros-Bonnivard, R.; Jaud, P.; Valle-Marcos, J.; Amann, J.M.; Bouallou, C. Pre-Combustion, Post-Combustion and Oxy-Combustion in Thermal Power Plant for CO2 Capture. Appl. Therm. Eng. 2010, 30, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Kheirinik, M.; Ahmed, S.; Rahmanian, N. Comparative Techno-Economic Analysis of Carbon Capture Processes: Pre-Combustion, Post-Combustion, and Oxy-Fuel Combustion Operations. Sustainability 2021, 13, 13567. [Google Scholar] [CrossRef]
- Galina, N.R.; Arce, G.L.A.F.; Ávila, I. Evolution of Carbon Capture and Storage by Mineral Carbonation: Data Analysis and Relevance of the Theme. Miner. Eng. 2019, 142, 105879. [Google Scholar] [CrossRef]
- Neeraj; Yadav, S. Carbon Storage by Mineral Carbonation and Industrial Applications of CO2. Mater. Sci. Energy Technol. 2020, 3, 494–500. [Google Scholar] [CrossRef]
- Veetil, S.P.; Hitch, M. Recent Developments and Challenges of Aqueous Mineral Carbonation: A Review. Int. J. Environ. Sci. Technol. 2020, 17, 4359–4380. [Google Scholar] [CrossRef]
- Olajire, A.A. A Review of Mineral Carbonation Technology in Sequestration of CO2. J. Pet. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Azdarpour, A.; Asadullah, M.; Mohammadian, E.; Hamidi, H.; Junin, R.; Karaei, M.A. A Review on Carbon Dioxide Mineral Carbonation through PH-Swing Process. Chem. Eng. J. 2015, 279, 615–630. [Google Scholar] [CrossRef]
- Hills, C.D.; Tripathi, N.; Carey, P.J. Mineralization Technology for Carbon Capture, Utilization, and Storage. Front. Energy Res. 2020, 8, 1–14. [Google Scholar] [CrossRef]
- Vieira, K.R.M.; Arce, G.L.A.F.; Luna, C.M.R.; Facio, V.O.; Carvalho, J.A.; Neto, T.G.S.; Ávila, I. Understanding the Acid Dissolution of Serpentinites (Tailings and Waste Rock) for Use in Indirect Mineral Carbonation. South African J. Chem. Eng. 2022, 40, 154–164. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A Review of Mineral Carbonation Technologies to Sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobicki, E.R.; Liu, Q.; Xu, Z.; Zeng, H. Carbon Capture and Storage Using Alkaline Industrial Wastes. Prog. Energy Combust. Sci. 2012, 38, 302–320. [Google Scholar] [CrossRef]
- Kemache, N.; Pasquier, L.; Cecchi, E.; Mouedhen, I.; Blais, J.; Mercier, G. Aqueous Mineral Carbonation for CO2 Sequestration: From Laboratory to Pilot Scale. Fuel Process. Technol. 2017, 166, 209–216. [Google Scholar] [CrossRef]
- Benhelal, E.; Imran, M.; Rayson, M.S.; Prigge, J.; Molloy, S.; Brent, F.; Cote, A.; Stockenhuber, M.; Kennedy, E.M. Study on Mineral Carbonation of Heat Activated Lizardite at Pilot and Laboratory Scale. J. CO2 Util. 2018, 26, 230–238. [Google Scholar] [CrossRef]
- Sanna, A.; Dri, M.; Maroto-Valer, M. Carbon Dioxide Capture and Storage by PH Swing Aqueous Mineralisation Using a Mixture of Ammonium Salts and Antigorite Source. Fuel 2013, 114, 153–161. [Google Scholar] [CrossRef]
- Arce Ferrufino, G.L.A.; Okamoto, S.; Dos Santos, J.C.; de Carvalho, J.A.; Avila, I.; Romero Luna, C.M.; Gomes Soares Neto, T. CO2 Sequestration by PH-Swing Mineral Carbonation Based on HCl/NH4OH System Using Iron-Rich Lizardite 1T. J. CO2 Util. 2018, 24, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Teir, S.; Kuusik, R.; Fogelholm, C.-J.; Zevenhoven, R. Production of Magnesium Carbonates from Serpentinite for Long-Term Storage of CO2. Int. J. Miner. Process. 2007, 85, 1–15. [Google Scholar] [CrossRef]
- Hemmati, A.; Shayegan, J.; Bu, J.; Yeo, T.Y.; Sharratt, P. Process Optimization for Mineral Carbonation in Aqueous Phase. Int. J. Miner. Process. 2014, 130, 20–27. [Google Scholar] [CrossRef]
- Park, A.-H.H.A.; Fan, L.-S.S. CO2 Mineral Sequestration: Physically Activated Dissolution of Serpentine and PH Swing Process. Chem. Eng. Sci. 2004, 59, 5241–5247. [Google Scholar] [CrossRef]
- Wang, X.; Maroto-valer, M.M. Dissolution of Serpentine Using Recyclable Ammonium Salts for CO2 Mineral Carbonation. Fuel 2011, 90, 1229–1237. [Google Scholar] [CrossRef]
- Teir, S.; Revitzer, H.; Eloneva, S.; Fogelholm, C.-J.J.; Zevenhoven, R. Dissolution of Natural Serpentinite in Mineral and Organic Acids. Int. J. Miner. Process. 2007, 83, 36–46. [Google Scholar] [CrossRef]
- Yuen, Y.T.; Sharratt, P.N.; Jie, B. Carbon Dioxide Mineralization Process Design and Evaluation: Concepts, Case Studies, and Considerations. Environ. Sci. Pollut. Res. 2016, 23, 22309–22330. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Maroto-Valer, M.M. Optimization of Carbon Dioxide Capture and Storage with Mineralisation Using Recyclable Ammonium Salts. Energy 2013, 51, 431–438. [Google Scholar] [CrossRef]
- Dri, M.; Sanna, A.; Maroto-Valer, M.M. Mass and Energy Balance of NH4-Salts PH Swing Mineral Carbonation Process Using Steel Slag. Energy Procedia 2014, 63, 6544–6547. [Google Scholar] [CrossRef] [Green Version]
- Teir, S. Fixation of Carbon Dioxide by Producing Carbonates from Minerals and Steelmaking Slags; Helsinki University of Technology: Espoo, Finland, 2008. [Google Scholar]
- Rashid, M.I.; Benhelal, E.; Farhang, F.; Oliver, T.K.; Stockenhuber, M.; Kennedy, E.M. Application of a Concurrent Grinding Technique for Two-Stage Aqueous Mineral Carbonation. J. CO2 Util. 2020, 42, 101347. [Google Scholar] [CrossRef]
- Bu, J.; Bai, P.; Sharratt, P. Carbon Dioxide Capture with Regeneration of Salt. 2012. [Google Scholar]
- Arce, G.L.A.F.; Soares Neto, T.G.; Ávila, I.; Luna, C.M.R.; Carvalho, J.A. Leaching optimization of mining wastes with lizardite and brucite contents for use in indirect mineral carbonation through the pH swing method. J. Clean. Prod. 2017, 141, 1324–1336. [Google Scholar] [CrossRef] [Green Version]
- Stokreef, S.; Sadri, F.; Stokreef, A.; Ghahreman, A. Mineral Carbonation of Ultramafic Tailings: A Review of Reaction Mechanisms and Kinetics, Industry Case Studies, and Modelling. Clean. Eng. Technol. 2022, 8, 100491. [Google Scholar] [CrossRef]
- Sanna, A.; Gaubert, J.; Maroto-valer, M.M. Alternative Regeneration of Chemicals Employed in Mineral Carbonation towards Technology Cost Reduction. Chem. Eng. J. 2016, 306, 1049–1057. [Google Scholar] [CrossRef] [Green Version]
- Hitch, M.; Dipple, G.M. Economic Feasibility and Sensitivity Analysis of Integrating Industrial-Scale Mineral Carbonation into Mining Operations. Miner. Eng. 2012, 39, 268–275. [Google Scholar] [CrossRef]
- Zhang, N.; Chai, Y.E.; Santos, R.M.; Šiller, L. Advances in Process Development of Aqueous CO2 Mineralisation towards Scalability. J. Environ. Chem. Eng. 2020, 8, 104453. [Google Scholar] [CrossRef]
- Bi, R.; Chen, C.; Tang, J.; Jia, X.; Xiang, S. Two-Level Optimization Model for Water Consumption Based on Water Prices in Eco-Industrial Parks. Resour. Conserv. Recycl. 2019, 146, 308–315. [Google Scholar] [CrossRef]
- Sanna, A.; Steel, L.; Maroto-Valer, M.M. Carbon Dioxide Sequestration Using NaHSO4 and NaOH: A Dissolution and Carbonation Optimisation Study. J. Environ. Manag. 2017, 189, 84–97. [Google Scholar] [CrossRef]
- Daval, D.; Hellmann, R.; Martinez, I.; Gangloff, S.; Guyot, F. Lizardite Serpentine Dissolution Kinetics as a Function of PH and Temperature, Including Effects of Elevated PCO2. Chem. Geol. 2013, 351, 245–256. [Google Scholar] [CrossRef]
- Teir, S.; Eloneva, S.; Fogelholm, C.-J.; Zevenhoven, R. Carbonation of Minerals and Industrial By-Products for CO2 Sequestration. In Proceedings of the 3rd International Green Energy Conference, Västeras, Sweden, 18–20 June 2007. [Google Scholar]
- Brown, T.L.; LeMay, E.; Bursten, B. Química: A Ciência Central, 9th ed.; Pearson Prentice Hall: São Paulo, Brazil, 2005; ISBN 85-87918-42-7. [Google Scholar]
- Kola, A.K.; Mekala, M.; Goli, V.R. Experimental Design Data for the Biosynthesis of Citric Acid Using Central Composite Design Method. Data Br. 2017, 12, 234–241. [Google Scholar] [CrossRef]
- Venkatesan, L.; Harris, A.; Greyling, M. Optimisation of Air Rate and Froth Depth in Flotation Using a CCRD Factorial Design—PGM Case Study. Miner. Eng. 2014, 66–68, 221–229. [Google Scholar] [CrossRef]
- Mortari, D.A.; Ávila, I.; Crnkovic, P.M. Response Surface Methodology Applied to the Evaluation of the SO2 Sorption Process in Two Brazilian Limestones. Energy Fuels 2013, 27, 2890–2898. [Google Scholar] [CrossRef]
- Bösiger, P.; Richard, I.M.T.; Le Gat, L.; Michen, B.; Schubert, M.; Rossi, R.M.; Fortunato, G. Application of Response Surface Methodology to Tailor the Surface Chemistry of Electrospun Chitosan-Poly(Ethylene Oxide) Fibers. Carbohydr. Polym. 2018, 186, 122–131. [Google Scholar] [CrossRef]
- Sanna, A.; Wang, X.; Lacinska, A.; Styles, M.; Paulson, T.; Maroto-Valer, M.M. Enhancing Mg Extraction from Lizardite-Rich Serpentine for CO2 Mineral Sequestration. Miner. Eng. 2013, 49, 135–144. [Google Scholar] [CrossRef]
- Arce, G.L.A.F.; Neto, T.G.S.; Ávila, I.; Luna, C.M.R.; dos Santos, J.C.; Carvalho, J.A. Influence of Physicochemical Properties of Brazilian Serpentinites on the Leaching Process for Indirect CO2 Mineral Carbonation. Hydrometallurgy 2017, 169, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Hemmati, A.; Shayegan, J.; Sharratt, P.; Yeo, T.Y.; Bu, J. Solid Products Characterization in a Multi-Step Mineralization Process. Chem. Eng. J. 2014, 252, 210–219. [Google Scholar] [CrossRef]
- Tutolo, B.M.; Luhmann, A.J.; Tosca, N.J.; Seyfried, W.E. Serpentinization as a Reactive Transport Process: The Brucite Silicification Reaction. Earth Planet. Sci. Lett. 2018, 484, 385–395. [Google Scholar] [CrossRef]
- Hollingbery, L.A.; Hull, T.R. The Thermal Decomposition of Natural Mixtures of Huntite and Hydromagnesite. Thermochim. Acta 2012, 528, 45–52. [Google Scholar] [CrossRef]
- Winnefeld, F.; Epifania, E.; Montagnaro, F.; Gartner, E.M. Further Studies of the Hydration of MgO-Hydromagnesite Blends. Cem. Concr. Res. 2019, 126, 105912. [Google Scholar] [CrossRef]
- Azdarpour, A.; Asadullah, M.; Mohammadian, E.; Junin, R.; Hamidi, H.; Manan, M.; Rafizan, A.; Daud, M. Mineral Carbonation of Red Gypsum via PH-Swing Process: Effect of CO2 Pressure on the Efficiency and Products Characteristics. Chem. Eng. J. 2015, 264, 425–436. [Google Scholar] [CrossRef]
- Herring, A.; King, P.L.; Saadatfar, M.; Mahdini, F.; Kemis Yahyah, A.M.; Andò, E. 3D Microstructure Controls on Mineral Carbonation. J. CO2 Util. 2021, 47, 101494. [Google Scholar] [CrossRef]
- Maroto-Valer, M.M.; Fauth, D.J.; Kuchta, M.E.; Zhang, Y.; Andrésen, J.M. Activation of Magnesium Rich Minerals as Carbonation Feedstock Materials for CO2 Sequestration. Fuel Process. Technol. 2005, 86, 1627–1645. [Google Scholar] [CrossRef]
- Krevor, S.C.; Lackner, K.S. Enhancing Process Kinetics for Mineral Carbon Sequestration. Energy Procedia 2009, 1, 4867–4871. [Google Scholar] [CrossRef] [Green Version]
- Pasquier, L.C.; Mercier, G.; Blais, J.F.; Cecchi, E.; Kentish, S. Technical & Economic Evaluation of a Mineral Carbonation Process Using Southern Québec Mining Wastes for CO2 Sequestration of Raw Flue Gas with by-Product Recovery. Int. J. Greenh. Gas Control 2016, 50, 147–157. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, R.; Geerlings, H.; Bi, J. Mg-Silicate Carbonation Based on an HCl- and NH 3-Recyclable Process: Effect of Carbonation Temperature. Chem. Eng. Technol. 2012, 35, 525–531. [Google Scholar] [CrossRef]



| Types of Carbonation | Advantage | Disadvantage | Application |
|---|---|---|---|
| pH-Swing | Shorter reaction time; greater efficiency | Two or more reactors; regeneration of chemical additives; large water consumption in regeneration stages | It cannot be currently applied due to its large energy consumption in additive regeneration stages |
| Aqueous Mineral Carbonation | A single reactor | Additive regeneration; non-reusable chemical additives; impure carbonates | Difficult applicability due to the level of complexity to regenerate used additives |
| Gas–Solid Mineral Carbonation | A single reactor; simple process | Very slow kinetics | Non-applicable due to very slow reaction rates |
| Oxides | XRF Concentration (%) | Elements | ICP-OES * Concentration (%) |
|---|---|---|---|
| MgO | 37.09 | Mg | 23 |
| Al2O3 | 1.62 | Al | 0.35 |
| SiO2 | 44.23 | Si | 8.4 |
| CaO | 2.79 | Ca | 279.5 |
| Cr2O3 | 0.84 | Cr | n.d |
| Fe2O3 | 12.82 | Fe | 4.49 |
| NiO | 0.58 | Ni | n.d |
| Experiments | Temperature | Pressure | CNaOH | pH |
|---|---|---|---|---|
| CB1 | 70 | 1 atm | 50% | 11 |
| CB2 | 90 | 1 atm | 50% | 11 |
| CB3 | 90 | 1 atm | solid (2.5 g) | 11 |
| CB4 | 70 | 100 bar | solid (0.8 g) | 11 |
| CB5 | 90 | 100 bar | solid (0.8 g) | 11 |
| CB6 | 90 | 150 bar | solid (0.8 g) | 11 |
| Factors | Description | Level | ||||
|---|---|---|---|---|---|---|
| −1 | 0 | +1 | ||||
| x1 | Temperature, T (°C) | 30 | 40 | 65 | 90 | 100 |
| x2 | HCl concentration, CHCl (M) | 1 | 1.44 | 2.5 | 3.5 | 4 |
| Experiments | Coded Factors | Numeric Factors | Response | ||
|---|---|---|---|---|---|
| x1 | x2 | T (°C) | CHCl (M) | % Mg | |
| 1 | −1 | −1 | 40 | 1.4 | 61 |
| 2 | +1 | −1 | 90 | 1.4 | 79 |
| 3 | −1 | +1 | 40 | 3.5 | 34 |
| 4 | +1 | +1 | 90 | 3.5 | 91 |
| 5 | − | 0 | 30 | 2.5 | 29 |
| 6 | 0 | + | 65 | 4 | 84 |
| 7 | 0 | 100 | 2.5 | 96 | |
| 8 | 0 | − | 65 | 1 | 82 |
| 9 | 0 | 0 | 65 | 2.5 | 85 |
| 10 | 0 | 0 | 65 | 2.5 | 85 |
| 11 | 0 | 0 | 65 | 2.5 | 85 |
| 12 | 0 | 0 | 65 | 2.5 | 85 |
| 13 | 0 | 0 | 65 | 2.5 | 85 |
| Variable | Sum of Squares | df | Mean Square | F | p-Value | R2 |
|---|---|---|---|---|---|---|
| x1 | 3549.94 | 1 | 3549.94 | 135.85 | 0 | n.d |
| x2 | 22 | 1 | 22 | 0.84 | 0.389 | n.d |
| x12 | 1118.2 | 1 | 1118.2 | 42.79 | 0 | n.d |
| x22 | 44.65 | 1 | 44.65 | 1.71 | 0.232 | n.d |
| x1x2 | 386.32 | 1 | 386.32 | 14.78 | 0.006 | n.d |
| Model | 5081.94 | 5 | 1016.39 | 38.89 | 0 | 96.53% |
| Residue | 182.92 | 7 | 26.13 | n.d | n.d | |
| Total | 5264.86 | 12 | 438.73 | n.d | n.d |
| Experiments | Product | Efficiency |
|---|---|---|
| CB1 | Amorphous u.d. | n.d |
| CB2 | Mg (OH)2 | n.d |
| CB3 | Hydromagnesite | 66% |
| CB4 | Magnesite (MgCO3) | 78% |
| CB5 | Hydromagnesite | 76% |
| CB6 | Hydromagnesite | 90% |
| Process Type | System | t (min) | T (°C) | p (Bar) | Reference | |
|---|---|---|---|---|---|---|
| pH swing | MgCl2-CO2-NaOH-H2O | 30 | 90 | 100 | 76% | This study |
| 150 | 90% | |||||
| pH swing | MgCl2-CO2-NH3-H2O | 60 | 70 | 10 | 43.5% | [51] |
| 20 | 60.9% | |||||
| 30 | 66.7% | |||||
| 60 | 68.6% | |||||
| pH Swing * | MgSO4-NaOH-CO2-H2O | 10 | 20 | 40 | 55% | [52] |
| Aqueous carbonation * | EDTA | 420 | 120 | 20 | 80% | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galina, N.R.; Arce, G.L.A.F.; Maroto-Valer, M.; Ávila, I. Experimental Study on Mineral Dissolution and Carbonation Efficiency Applied to pH-Swing Mineral Carbonation for Improved CO2 Sequestration. Energies 2023, 16, 2449. https://doi.org/10.3390/en16052449
Galina NR, Arce GLAF, Maroto-Valer M, Ávila I. Experimental Study on Mineral Dissolution and Carbonation Efficiency Applied to pH-Swing Mineral Carbonation for Improved CO2 Sequestration. Energies. 2023; 16(5):2449. https://doi.org/10.3390/en16052449
Chicago/Turabian StyleGalina, Natália R., Gretta L. A. F. Arce, Mercedes Maroto-Valer, and Ivonete Ávila. 2023. "Experimental Study on Mineral Dissolution and Carbonation Efficiency Applied to pH-Swing Mineral Carbonation for Improved CO2 Sequestration" Energies 16, no. 5: 2449. https://doi.org/10.3390/en16052449





