A Study on Elemental Sulfur Equilibrium Content in Mixtures of Methane, Carbon Dioxide, and Hydrogen Sulfide under Conditions of Natural Gas Pipeline Transmission
Abstract
:1. Introduction
2. Modeling and Analysis
2.1. Model Assumption
2.2. Model Study on the Solution Mechanism of Elemental Sulfur in High-Sulfur-Content Natural Gas under Gathering Conditions
2.2.1. Physical Solution Mechanism Model of Elemental Sulfur
2.2.2. Chemical Solution Mechanism Model of Elemental Sulfur
2.3. Calculation Process of Important Parameters
2.3.1. Important Parameters in Physical Dissolution
- (1)
- Calculation of the molar volume of solid-phase sulfur
- (2)
- Calculation of elemental sulfur saturation vapor pressure
- (3)
- Calculation of the elemental sulfur gas phase fugacity coefficient
2.3.2. Important Parameters in Chemical Solution
- (1)
- The gas-phase fugacity coefficient of H2S
- (2)
- The gas-phase fugacity coefficient of H2S9
- (3)
- The standard chemical potentials , , and for H2S9, S8, and H2S
3. Results and Discussion
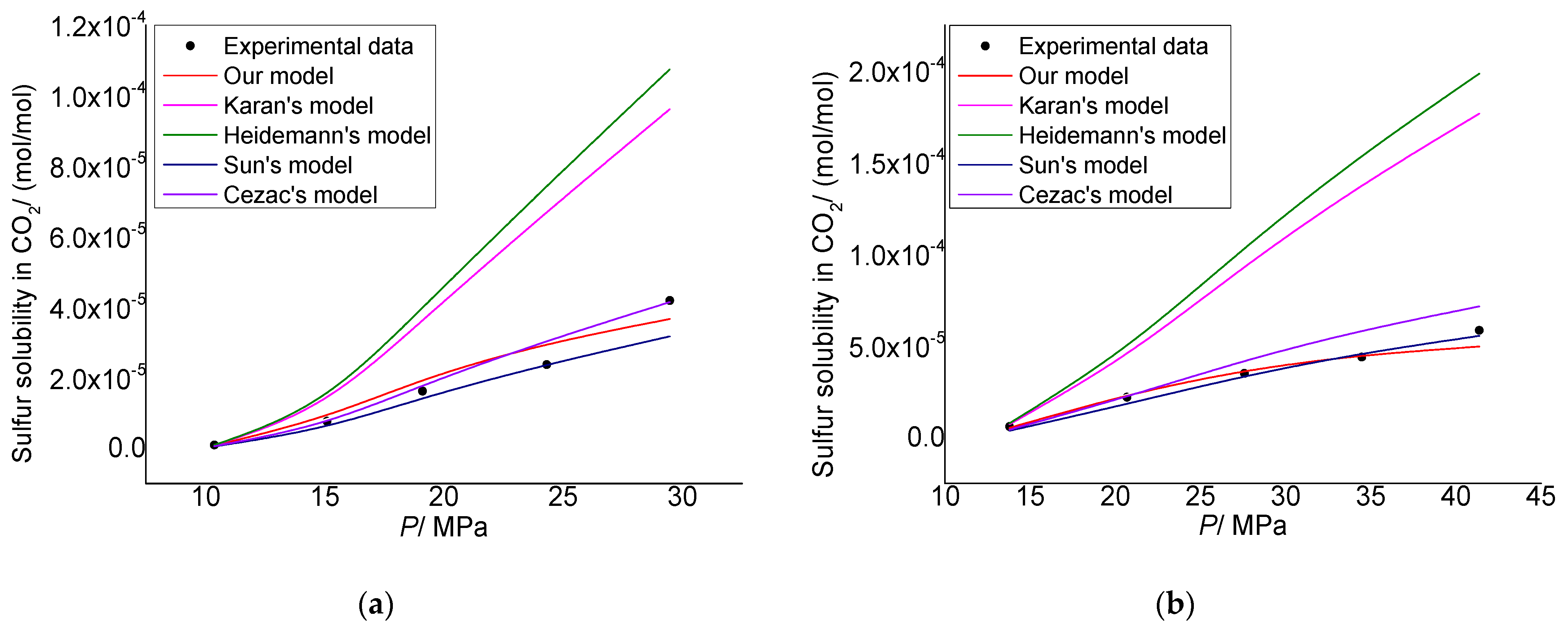
3.1. Model Verification
3.2. Calculated Case Description
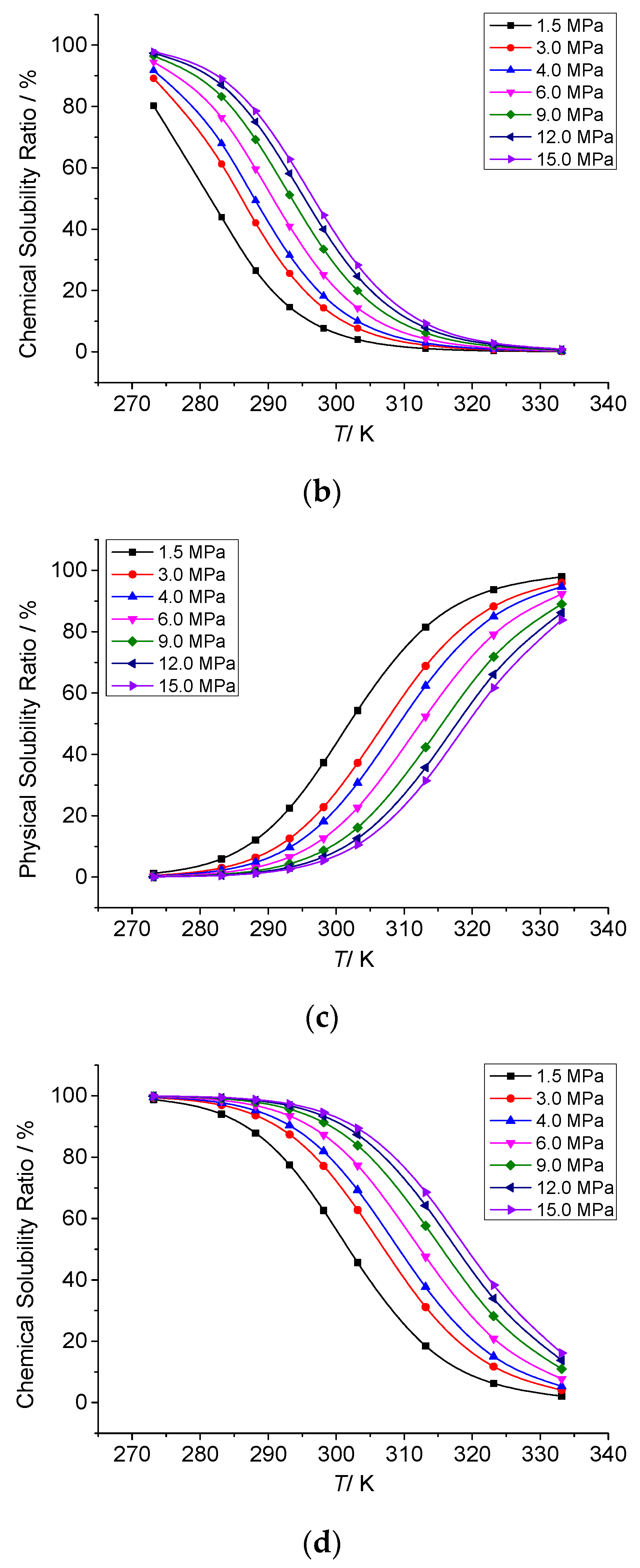
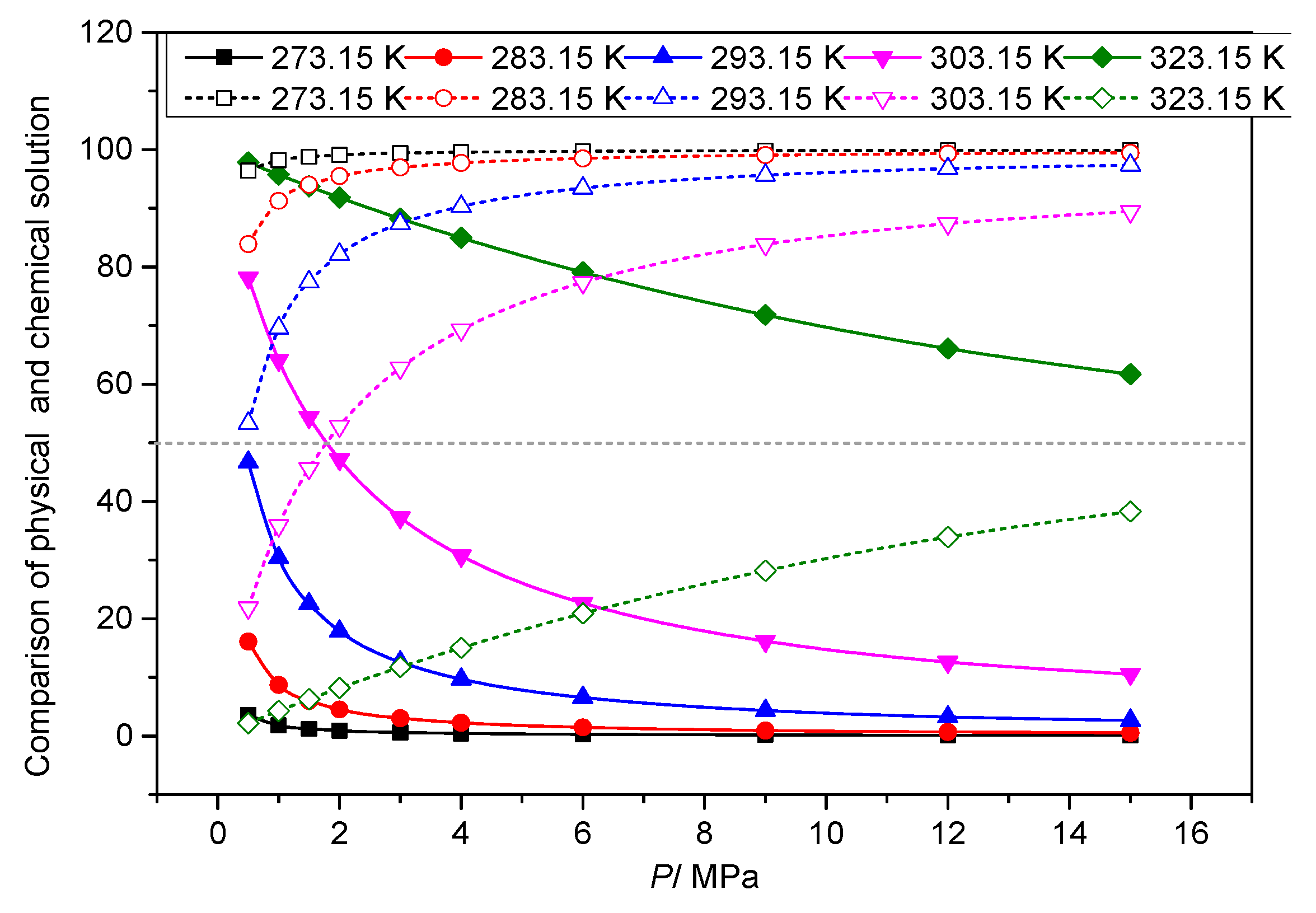
3.3. Analysis of the Effect of Temperature on the Solution Mechanism of Elemental Sulfur in High-Sulfur-Content Natural Gas
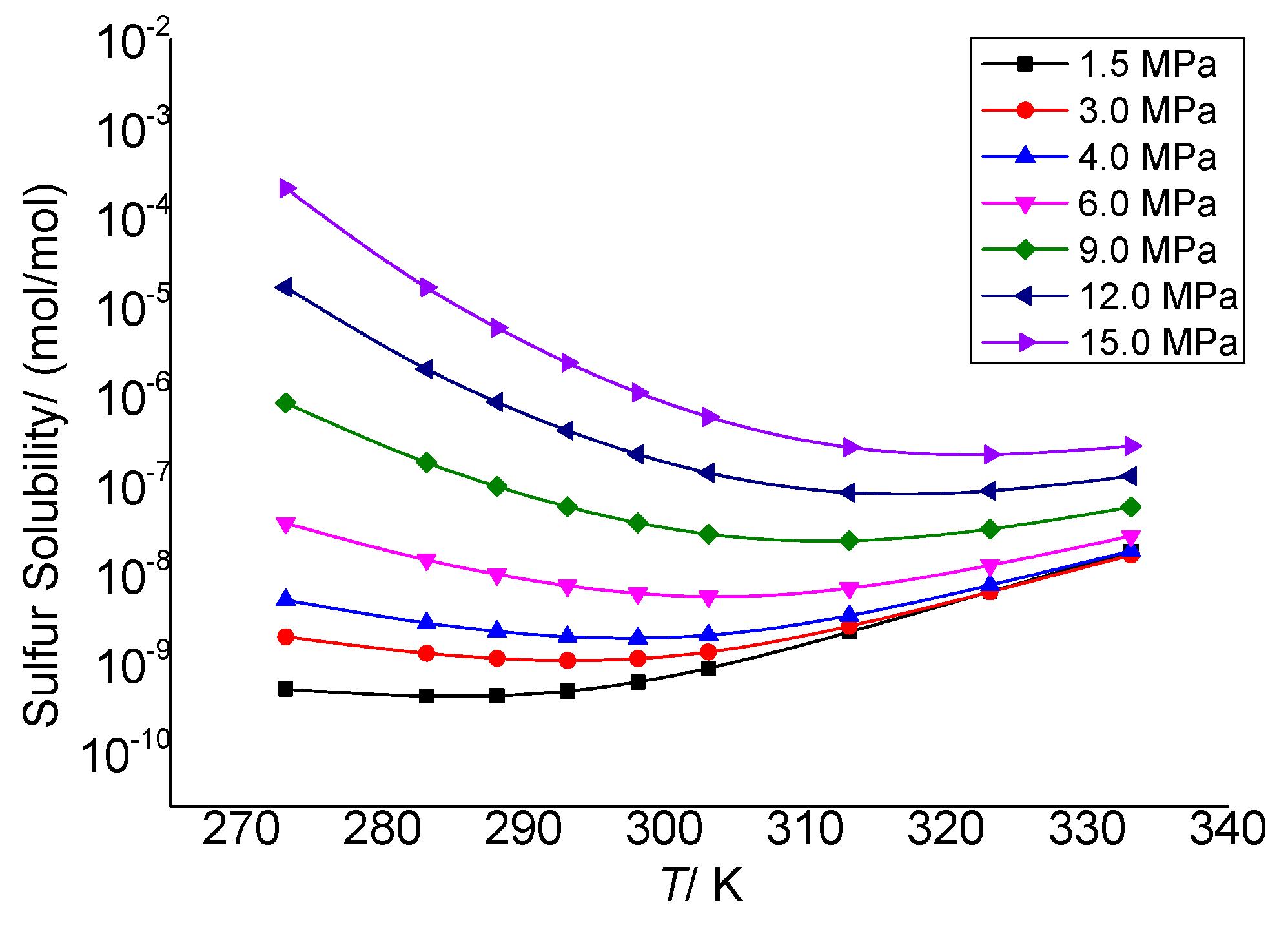
3.3.1. The Effect of Temperature on the Total Solubility of Elemental Sulfur
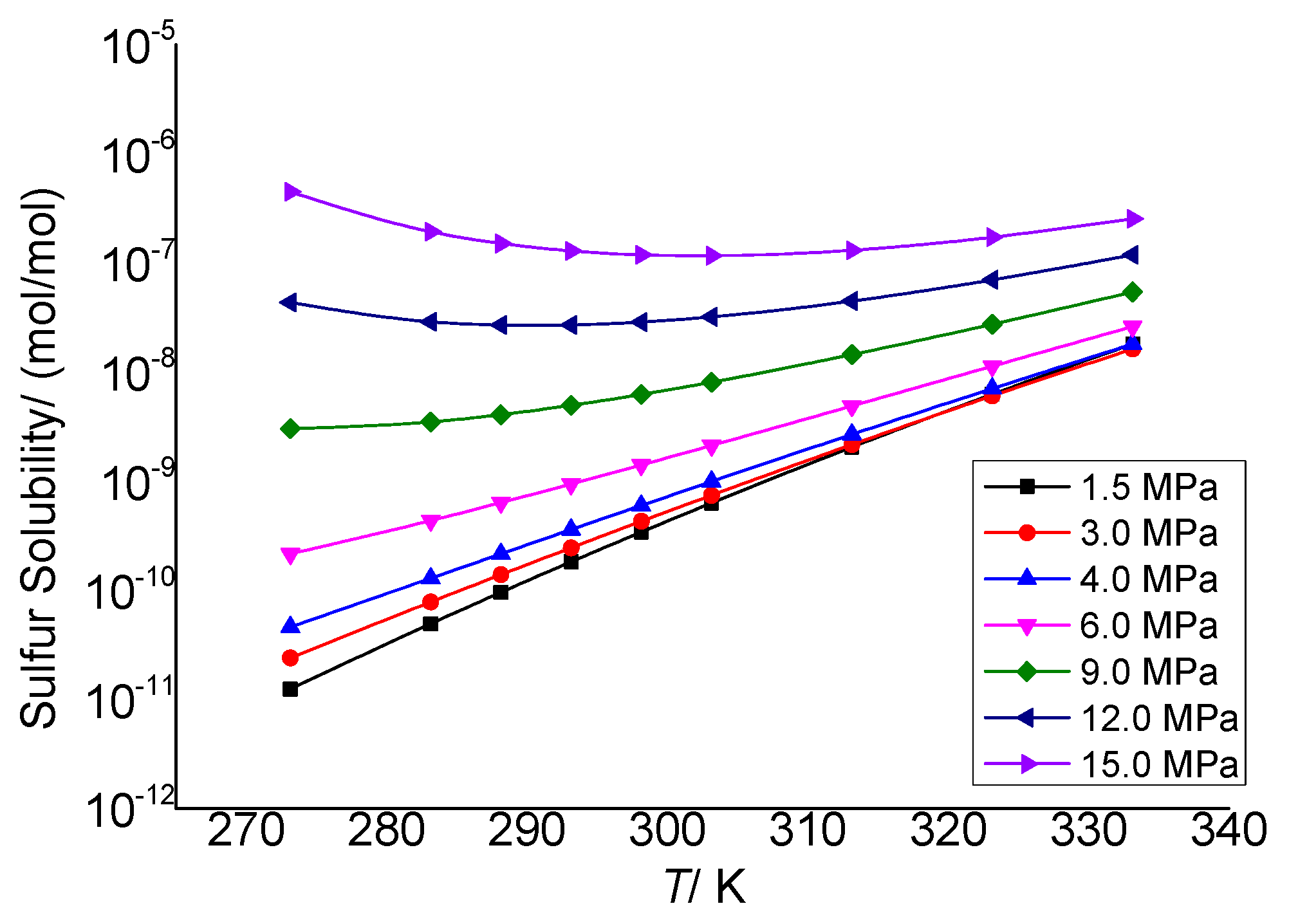
3.3.2. The Effect of Temperature on the Physical Solubility of Elemental Sulfur
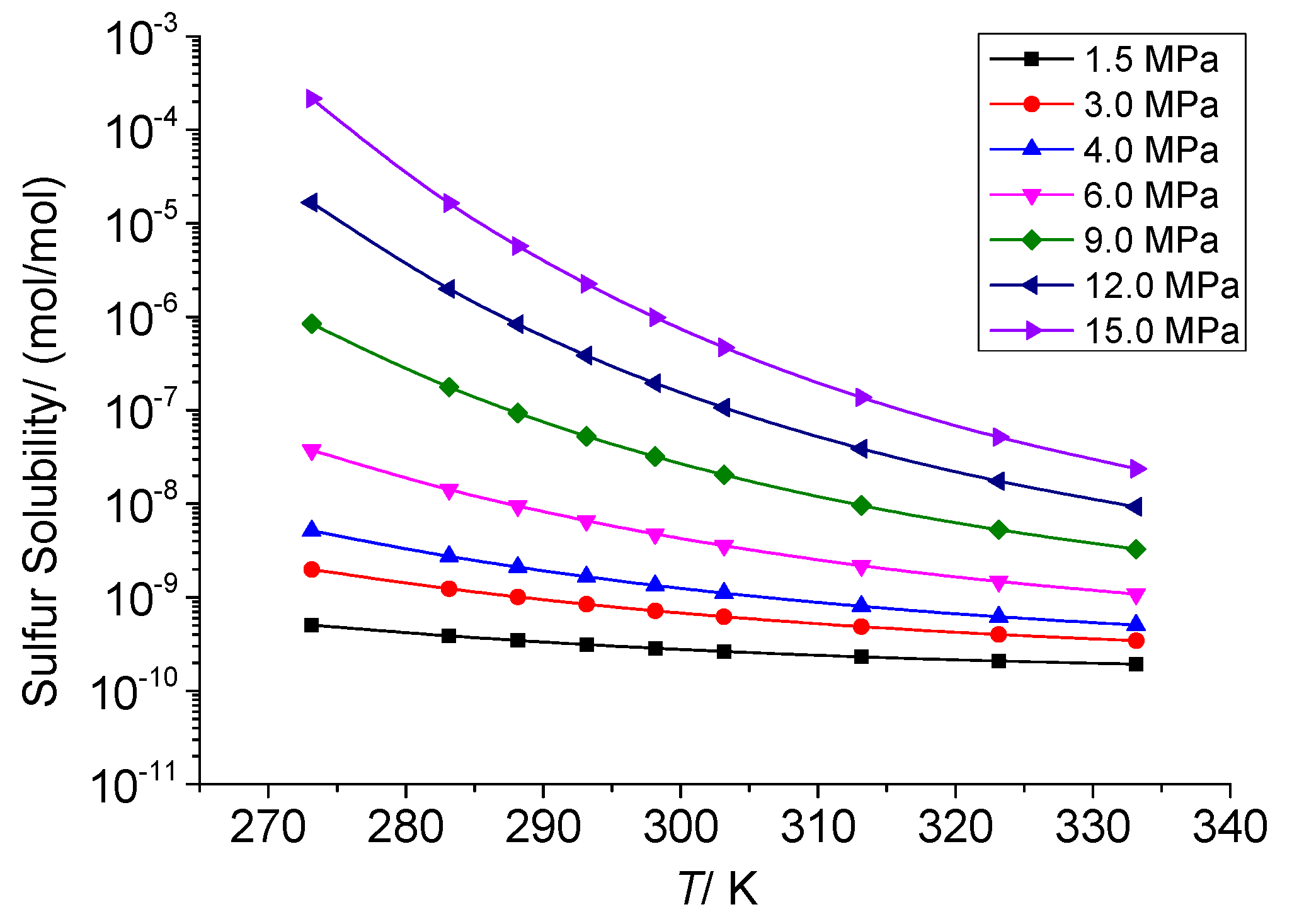
3.3.3. The Effect of Temperature on the Chemical Solubility of Elemental Sulfur
3.3.4. Analysis of the Effect of Temperature on the Solution Mechanism of Elemental Sulfur
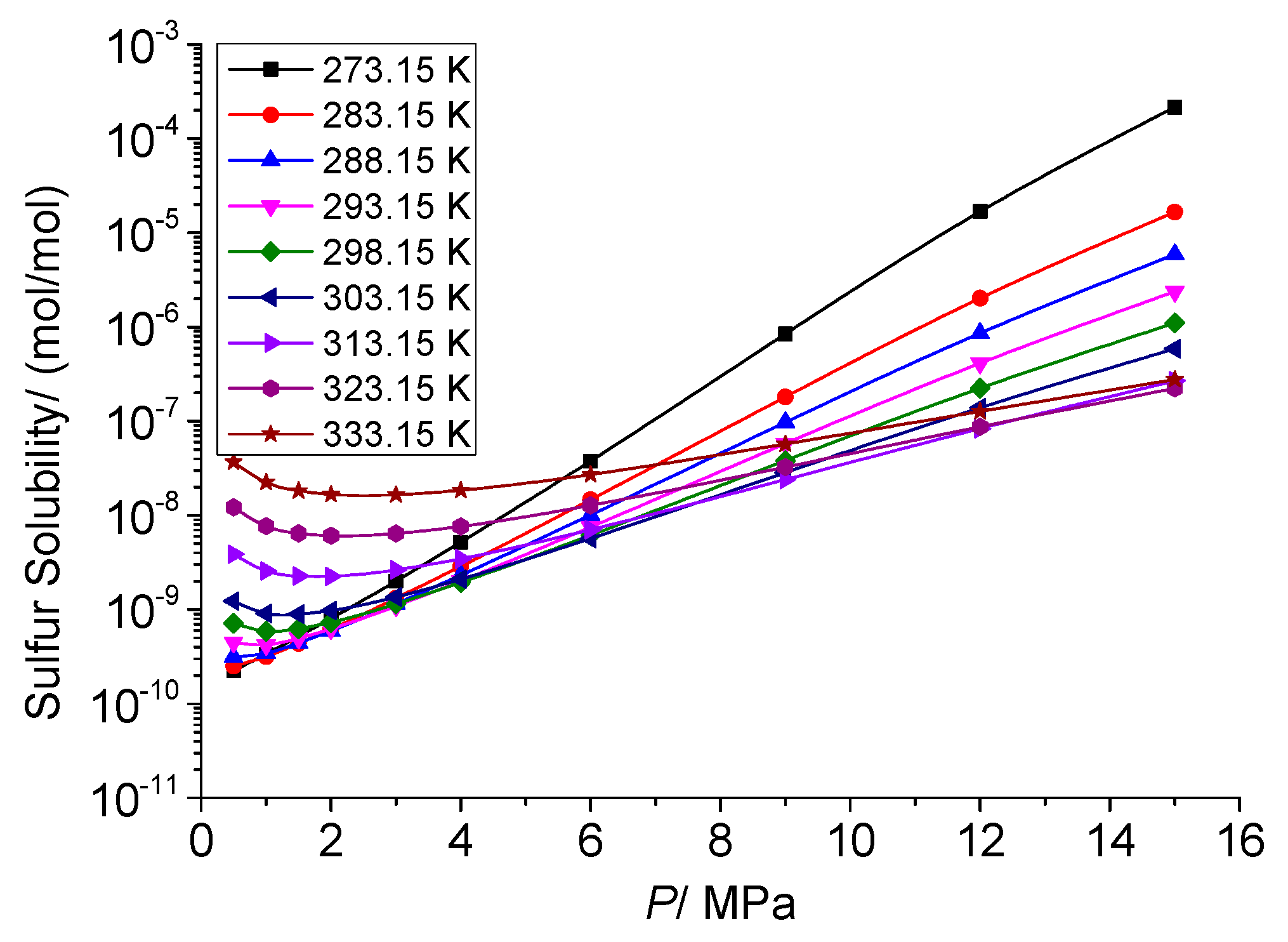
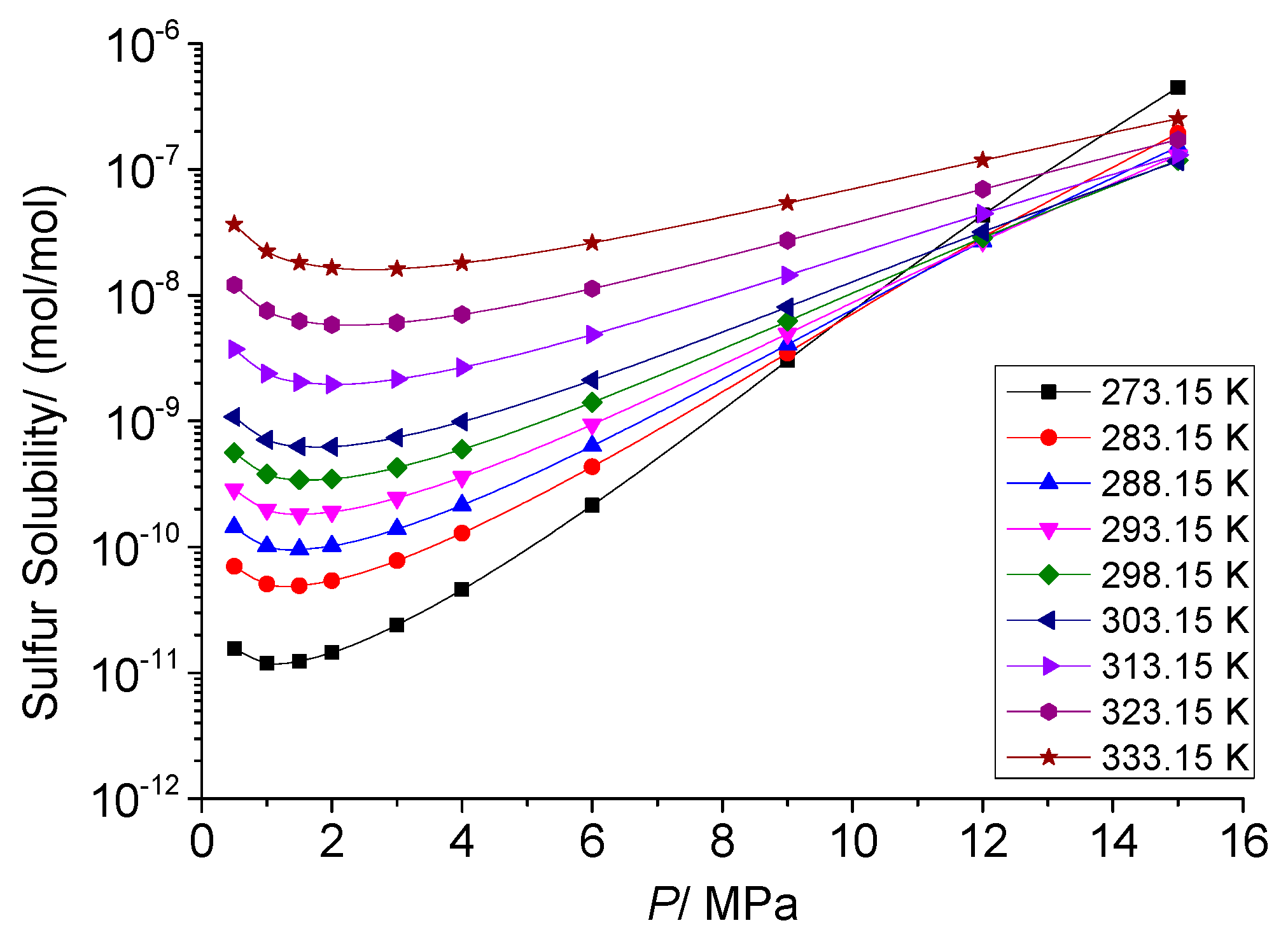
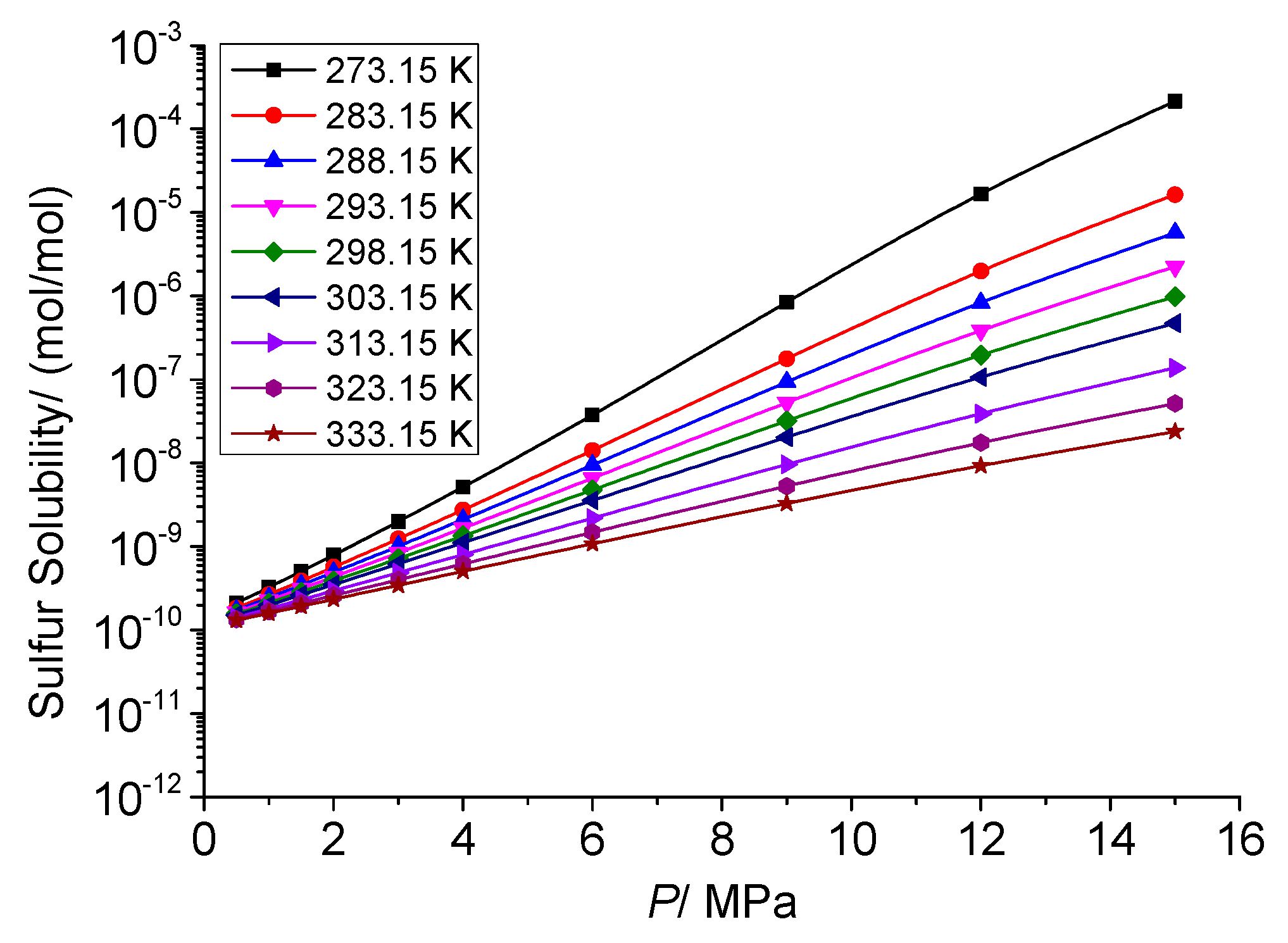
3.4. Analysis of the Effect of Pressure on the Solution Mechanism of Elemental Sulfur in High-Sulfur-Content Natural Gas
3.4.1. Analysis of the Effect of Pressure on the Total Solubility of Elemental Sulfur
3.4.2. Analysis of the Effect of Pressure on the Physical Solubility of Elemental Sulfur
3.4.3. Analysis of the Effect of Pressure on the Chemical Solubility of Elemental Sulfur
3.4.4. Analysis of the Effect of Pressure on the Dissolution Mechanism of Elemental Sulfur
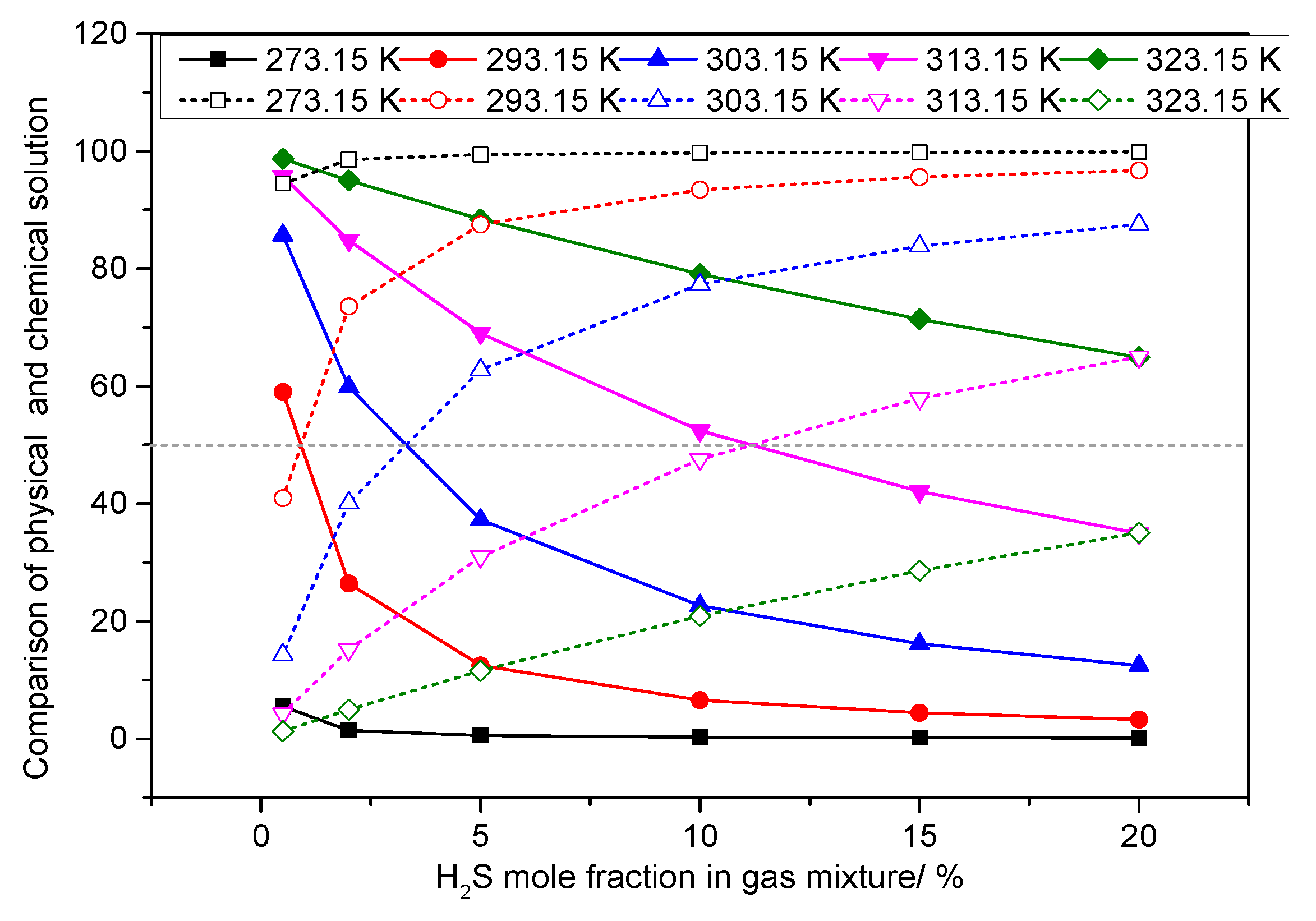
3.5. Analysis of the Effect of H2S Concentration on the Dissolution Mechanism of Elemental Sulfur in High-Sulfur-Bearing Natural Gas
- (1)
- Temperature of 293.15 K
- (2)
- Pressure of 6.0 MPa
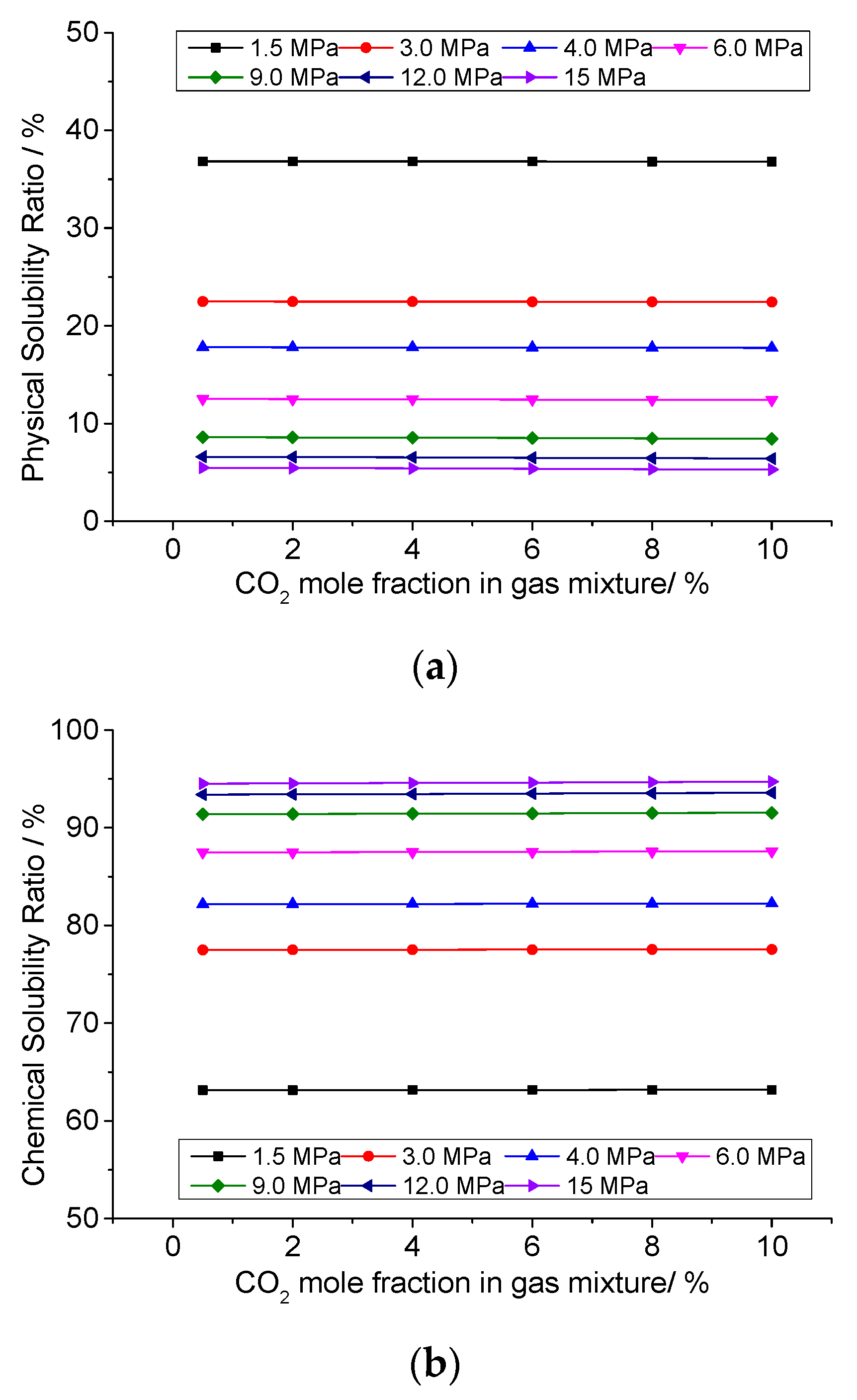
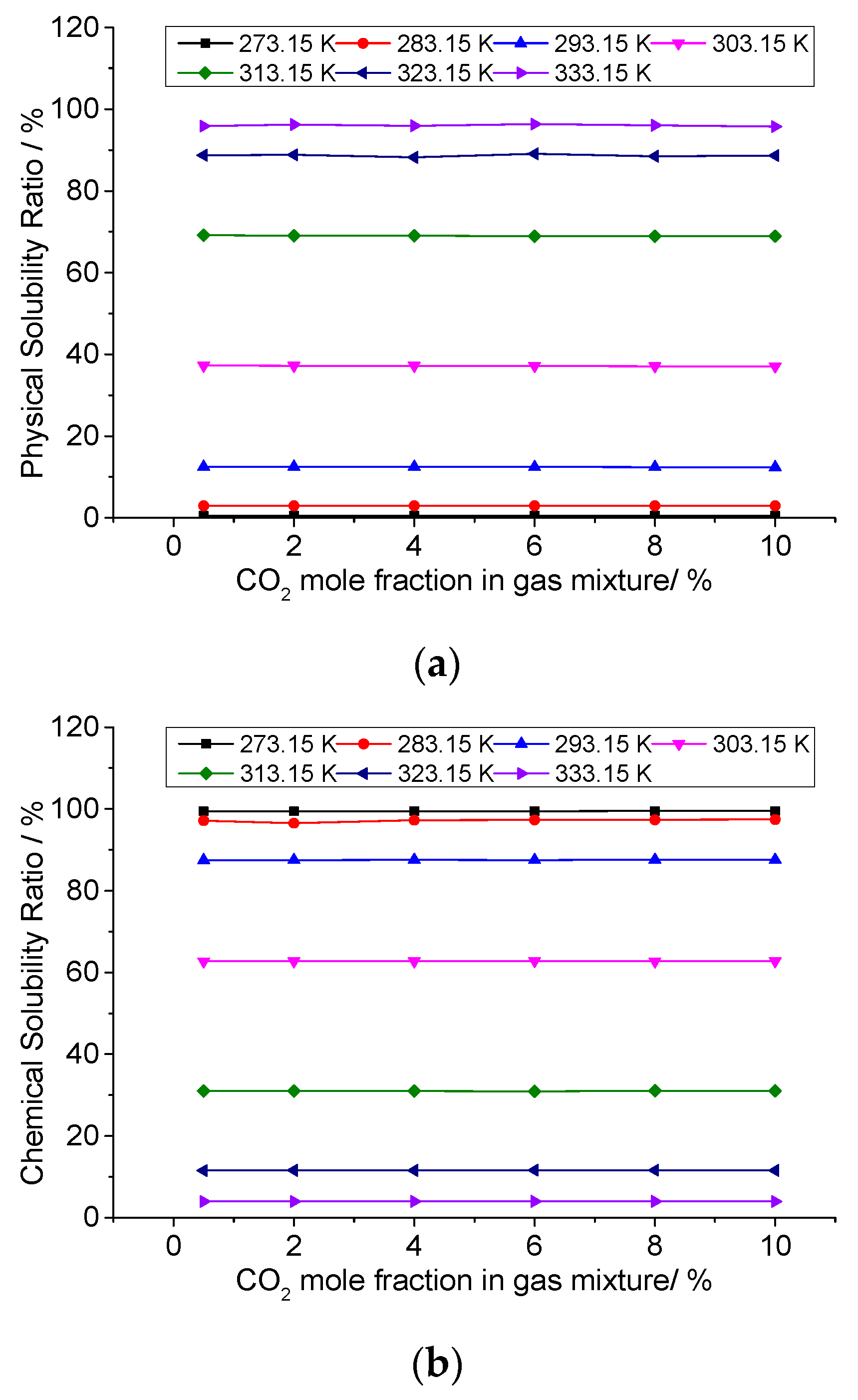
3.6. Analysis of the Effect of CO2 Concentration on the Solution Mechanism of Elemental Sulfur in High-Sulfur-Content Natural Gas
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pack, D.J. Elemental Formation in Natural Gas Transmission Pipelines. Ph.D. Thesis, The University of Western Australia, Perth, Australia, 2005. [Google Scholar]
- Liu, E.; Li, D.; Li, W.; Liao, Y.; Qiao, W.; Liu, W.; Azimi, M. Erosion simulation and improvement scheme of separator blowdown system—A case study of Changning national shale gas demonstration area. J. Nat. Gas Sci. Eng. 2021, 88, 103856. [Google Scholar] [CrossRef]
- Serin, J.P.; Cézac, P.; Broto, F.; Mouton, G. Modelling of sulphur deposition in natural gas. Comput. Aided Chem. Eng. 2005, 20, 799–804. [Google Scholar]
- Liu, E.; Peng, Y.; Peng, S.; Yu, B.; Chen, Q. Research on low carbon emission optimization operation technology of natural gas pipeline under multi-energy structure. Pet. Sci. 2022, 19, 3046–3058. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Q. A new prediction model of elemental sulfur solubility in sour gas mixtures. J. Nat. Gas Sci. Eng. 2016, 31, 98–107. [Google Scholar] [CrossRef]
- Li, C.; Liu, G.; Peng, Y. Predicting sulfur solubility in hydrogen sulfide, carbon dioxide, and methane with an improved thermodynamic model. RSC Adv. 2018, 8, 16069–16081. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Liu, G.; Jia, W. A review of elemental sulfur deposition in high sulfur-content natural gas transmission pipeline. Chin. Sci. Bull. 2018, 63, 816–827. (In Chinese) [Google Scholar] [CrossRef] [Green Version]
- Kennedy, H.T.; Wieland, D.R. Equilibrium in the methane-carbon dioxide-hydrogen sulfide-sulfur system. Trans. AIME 1960, 219, 166–169. [Google Scholar] [CrossRef]
- Roof, J.G. Solubility of sulfur in hydrogen sulfide and in carbon disulfide at elevated temperature and pressure. Soc. Pet. Eng. J. 1971, 11, 272–276. [Google Scholar] [CrossRef]
- Smith, J.J.; Jensen, D.; Meyer, B. Liquid hydrogen sulfide in contact with sulfur. J. Chem. Eng. Data 1970, 15, 144–146. [Google Scholar] [CrossRef]
- Swift, S.C.; Manning, F.S.; Thompson, R.E. Sulfur-bearing capacity of hydrogen sulfide gas. Soc. Pet. Eng. J. 1976, 16, 57–64. [Google Scholar] [CrossRef]
- Brunner, E.; Woll, W. Solubility of sulfur in hydrogen sulfide and sour gases. Soc. Pet. Eng. J. 1980, 20, 377–384. [Google Scholar] [CrossRef]
- Brunner, E.; Place, M.C.; Woll, W.H. Sulfur solubility in sour gas. J. Pet. Technol. 1988, 40, 1587–1592. [Google Scholar] [CrossRef]
- Migdisov, A.A.; Suleimenov, O.M.; Alekhin, Y.V. Experimental study of polysulfane stability in gaseous hydrogen sulfide. Geochim. Cosmochim. Acta 1998, 62, 2627–2635. [Google Scholar] [CrossRef]
- Sun, C.Y.; Chen, G.J. Experimental and modeling studies on sulfur solubility in sour gas. Fluid Phase Equilib. 2003, 214, 187–195. [Google Scholar] [CrossRef]
- Serin, J.P.; Jay, S.; Cézac, P.; Contamine, F.; Mercadier, J.; Arrabie, C.; Legros-Adrian, J.M. Experimental studies of solubility of elemental sulphur in supercritical carbon dioxide. J. Supercrit. Fluids 2010, 53, 12–16. [Google Scholar] [CrossRef]
- Cloarec, E.; Serin, J.P.; Cézac, P.; Contamine, F.; Mercadier, J.; Louvat, A.; Kim, U. Experimental studies of solubility of elemental sulfur in methane at 363.15 K for pressure ranging from (4 to 25) MPa. J. Chem. Eng. Data 2012, 57, 1222–1225. [Google Scholar] [CrossRef]
- Eslamimanesh, A.; Mohammadi, A.H.; Richon, D. Thermodynamic consistency test for experimental data of sulfur content of hydrogen sulfide. Ind. Eng. Chem. Res. 2011, 50, 3555–3563. [Google Scholar] [CrossRef]
- Cézac, P.; Serin, J.P.; Mercadier, J.; Mouton, G. Modelling solubility of solid sulphur in natural gas. Chem. Eng. J. 2007, 133, 283–291. [Google Scholar] [CrossRef]
- Roberts, B.E. The effect of sulfur deposition on gaswell inflow performance. SPE Reserv. Eng. 1997, 12, 118–123. [Google Scholar] [CrossRef]
- Yang, X. A Study on the Special Phase Behavior of Multiphase Fluid in Gas Reservoirs with High Sulfur Content and Formation Damage Caused by the Sulfur Deposition. Ph.D. Thesis, Southwest Petroleum University, Chengdu, China, 2006. (In Chinese). [Google Scholar]
- Gu, M.X.; Li, Q.; Zhou, S.Y.; Chen, W.D.; Guo, T.M. Experimental and modeling studies on the phase behavior of high H2S-content natural gas mixtures. Fluid Phase Equilib. 1993, 82, 173–182. [Google Scholar] [CrossRef]
- Gu, M.X.; Li, Q.; Zhou, S.Y.; Chen, W.D.; Guo, T.M. Solubility of solid sulfur in super/near-critical H2S-containing sour fluid mixtures (II) the thermodynamic model. CIESC J. 1993, 44, 321–327. (In Chinese) [Google Scholar]
- Karan, K.; Heidemann, R.A.; Behie, L.A. Sulfur solubility in sour gas: Predictions with an equation of state model. Ind. Eng. Chem. Res. 1998, 37, 1679–1684. [Google Scholar] [CrossRef]
- Heidemann, R.A.; Phoenix, A.V.; Karan, K.; Behie, L.A. A chemical equilibrium equation of state model for elemental sulfur and sulfur-containing fluids. Ind. Eng. Chem. Res. 2001, 40, 2160–2167. [Google Scholar] [CrossRef]
- Guo, X.; Du, Z. EOS-Related mathematical model to predict sulfur deposition and cost-effective approach of removing sulfides from sour natural gas. In Proceedings of the Production and Operations Symposium, Oklahoma City, OK, USA, 31 March–3 April 2007. [Google Scholar]
- Cézac, P.; Serin, J.P.; Reneaume, J.M.; Mercadier, J.; Mouton, G. Elemental sulphur deposition in natural gas transmission and distribution networks. J. Supercrit. Fluids 2008, 44, 115–122. [Google Scholar] [CrossRef]
- Serin, J.P.; Cézac, P. Three thermodynamic paths to describe solid fugacity: Application to sulphur precipitation from supercritical natural gas. J. Supercrit. Fluids 2008, 46, 21–26. [Google Scholar] [CrossRef]
- Jay, S.; Cezac, P.; Serin, J.P.; Contamine, F.; Martin, C.; Mercadier, J. Solubility of elemental sulfur in toluene between (267.15 and 313.15) K under atmospheric pressure. J. Chem. Eng. Data 2009, 54, 3238–3241. [Google Scholar] [CrossRef]
- Li, C.; Liu, G.; Peng, Y. Solution Mechanism of Elemental Sulfur in Hydrogen Sulfide under Conditions of Natural Gas Transmission. Ind. Eng. Chem. Res. 2018, 58, 440–447. [Google Scholar] [CrossRef]
- Li, N.; Ren, B.; He, Y.; Guo, D.; Jiang, M. Analysis on Causes of Sulfur Deposition in Puguang Gas Field Surface Gathering and TransportationSystem and Countermeasures. Nat. Gas Oil 2012, 30, 8–10. (In Chinese) [Google Scholar]
- Wang, Y. Investigation on Sulfur Deposition of the Natural Gas Gathering System. Master’s Thesis, China University of Petroleum (EastChina), Qingdao, China, 2013. (In Chinese). [Google Scholar]
- Li, L. Research on Prediction Models of Phase State and Dynamics for Well Bore Sulfur Deposition in Gas Reservoir with High Sulfur Content. Master’s Thesis, Southwest Petroleum University, Chengdu, China, 2012. (In Chinese). [Google Scholar]
- Smith, J.M.; Ness, H.; Abbott, M.M. Introduction to Chemical Engineering Thermodynamics; McGraw-Hill: New York, NY, USA, 2005; pp. 371–372. [Google Scholar]
- Shuai, X.; Meisen, A. New correlations predict physical properties of elemental sulfur. Oil Gas J. 1995, 93, 50–55. [Google Scholar]
- Peng, D.Y.; Robinson, D.B. A new two-constant equation of state. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Heidemann, R.A.; Prausnitz, J.M. A van der Waals-type equation of state for fluids with associating molecules. Proc. Natl. Acad. Sci. USA 1976, 73, 1773–1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Components | /MPa | /K | |
|---|---|---|---|
| S8 | 5.0 | 1025.0 | 0.4439 |
| H2S | 8.963 | 373.5 | 0.094 |
| CO2 | 7.383 | 304.2 | 0.224 |
| CH4 | 4.599 | 190.6 | 0.012 |
| Constants | c1 × 10−3 | c2 | c3 | c4 × 103 | c5 × 106 | c6 × 109 |
|---|---|---|---|---|---|---|
| H2S | −3.36061 | −1.40815 | −11.7803 | −7.81173 | 4.85597 | −1.68973 |
| S8 | 6.66384 | −16.1969 | 60.6753 | −7.37600 | 2.28741 | −0.368491 |
| H2S9 | −5.68656 | −71.6492 | 377.350 | 99.3619 | −49.2157 | 11.2677 |
| No. | Gas 1 | Gas 2 | Gas 3 | Gas 4 | Gas 5 | Gas 6 |
|---|---|---|---|---|---|---|
| Molar Content of Components/% | ||||||
| H2S | 0.5 | 2 | 5 | 10 | 15 | 20 |
| CO2 | 5 | 5 | 5 | 5 | 5 | 5 |
| CH4 | 94.5 | 93 | 90 | 85 | 80 | 75 |
| NO. | Gas 7 | Gas 8 | Gas 9 | Gas 10 | Gas 11 | Gas 12 |
|---|---|---|---|---|---|---|
| Molar Content of Components/% | ||||||
| H2S | 5 | 5 | 5 | 5 | 5 | 5 |
| CO2 | 0.5 | 2 | 4 | 6 | 8 | 10 |
| CH4 | 94.5 | 93 | 91 | 89 | 87 | 85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Hao, M.; Fan, S.; Li, C. A Study on Elemental Sulfur Equilibrium Content in Mixtures of Methane, Carbon Dioxide, and Hydrogen Sulfide under Conditions of Natural Gas Pipeline Transmission. Energies 2023, 16, 2466. https://doi.org/10.3390/en16052466
Liu G, Hao M, Fan S, Li C. A Study on Elemental Sulfur Equilibrium Content in Mixtures of Methane, Carbon Dioxide, and Hydrogen Sulfide under Conditions of Natural Gas Pipeline Transmission. Energies. 2023; 16(5):2466. https://doi.org/10.3390/en16052466
Chicago/Turabian StyleLiu, Gang, Mengqi Hao, Shishui Fan, and Changjun Li. 2023. "A Study on Elemental Sulfur Equilibrium Content in Mixtures of Methane, Carbon Dioxide, and Hydrogen Sulfide under Conditions of Natural Gas Pipeline Transmission" Energies 16, no. 5: 2466. https://doi.org/10.3390/en16052466






