Microbial Fuel Cells as a Promising Power Supply for Implantable Medical Devices
Abstract
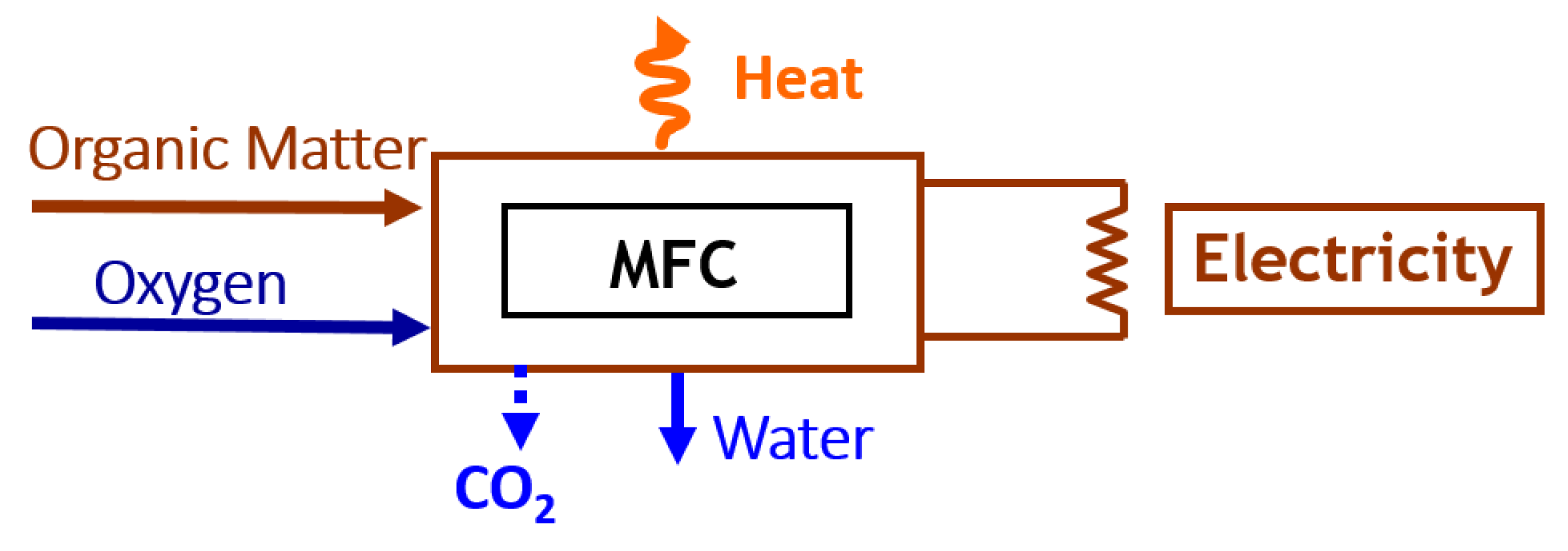
1. Background
2. Microbial Fuel Cells for IMDs
3. Microbial Fuel Cells for IMDs Challenges and Perspectives
- (1)
- Low power outputs
- (2)
- Biocompatibility
- (3)
- High cost
- (4)
- Low durability/lifetime
4. Summary
Funding
Conflicts of Interest
References
- Joung, Y.-H. Development of implantable medical devices: From an engineering perspective. Int. Neurourol. J. 2013, 17, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Kiley, M.L.; Anthony, F.; Young, C.; Brar, S.; Kwaku, K. Multi-center, community-based cardiac implantable electronic devices registry: Population, device utilization, and outcomes. J. Am. Heart Assoc. 2016, 5, e002798. [Google Scholar] [CrossRef]
- Raatikainen, M.J.; Arnar, D.O.; Merkely, B.; Camm, A.J.; Hindricks, G. Access to and clinical use of cardiac implantable electronic devices and interventional electrophysiological procedures in the European Society of Cardiology Countries, 2016 report from the European Heart Rhythm Association. Europace 2016, 18, 1–79. [Google Scholar] [CrossRef] [PubMed]
- Amar, A.B.; Kouki, A.B.; Cao, H. Power Approaches for Implantable Medical Devices. Sensors 2015, 15, 28889–28914. [Google Scholar] [CrossRef] [PubMed]
- Zebda, A.; Alcaraz, J.-P.; Vadgama, P.; Shleev, S.; Minteer, S.D.; Boucher, F.; Cinquin, P.; Martin, D.K. Challenges for successful implantation of biofuel cells. Bioelectrochemistry 2018, 124, 57–72. [Google Scholar] [CrossRef]
- Orhan, S. Power Sources for implantable medical devices. Device Technol. Appl. Electron. 2002, 18, 76–79. [Google Scholar]
- Wang, Y.; Wang, H.; Xuan, J.; Leung, D.Y.C. Powering future body sensor network systems: A review of power sources. Biosens. Bioelectron. 2020, 166, 112410. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, F.; Xu, L.; Leung, P.; Yang, C.; Li, H. The applications and prospect of fuel cells in medical field: A review. Renew. Sust. Energy Rev. 2017, 67, 574–580. [Google Scholar] [CrossRef]
- Vilas Boas, J.; Oliveira, V.B.; Pinto, A.M.F.R.; Simões, M. Fuel Cell Bioreactors. In Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 464–478. [Google Scholar]
- Rasouli, M.; Phee, L.S.J. Energy sources and their development for application in medical devices. Expert Rev. Med. Devic. 2010, 7, 693–709. [Google Scholar] [CrossRef]
- Nishaa, V.; Soumya; Spoorthi, B.V.; Desai, V.S.; Meda, U.S. Powering Implantable Medical Devices with Biological Fuel Cells. ECS Trans. 2022, 107, 19197–19215. [Google Scholar]
- Singh, R.; Kaur, N.; Singh, M. Bio-compatible bio-fuel cells for medical devices. Mater. Today-Proc. 2021, 44, 242–249. [Google Scholar] [CrossRef]
- Ibrahim, R.; Shaari, N.; Aman, A.H.M. Bio-fuel cell for medical device energy system: A Review. Int. J. Energy Res. 2021, 45, 14245–14273. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Y.; Kjøniksen, A.-L.; Zhou, X.; Zhou, X. Wearable Biofuel Cells: Advances from Fabrication to Application. Adv. Funct. Mater. 2021, 31, 2103976. [Google Scholar] [CrossRef]
- Justin, G.A.; Zhang, Y.; Cui, X.T.; Bradberry, C.W.; Sun, M.; Sclabassi, R.J. A metabolic biofuel cell: Conversion of human leukocyte metabolic activity to electrical currents. J. Biol. Eng. 2011, 5, 5–9. [Google Scholar] [CrossRef]
- Zhang, J.L.; Wang, Y.H.; Huang, K.; Huang, K.J.; Jiang, H.; Wang, X.M. Enzyme-based biofuel cells for biosensors and in vivo power supply. Nano Energy 2021, 84, 105853. [Google Scholar] [CrossRef]
- Potter, M.C. Electrical Effects Accompanying the Decomposition of Organic Compounds. Proc. R. Soc. B 1911, 84, 260–276. [Google Scholar]
- Oliveira, V.B.; Simões, M.; Melo, L.F.; Pinto, A.M.F.R. Overview on the developments of microbial fuel cells. Biochem. Eng. J. 2013, 73, 53–64. [Google Scholar] [CrossRef]
- Kumar, S.S.; Kumar, V.; Kumar, R.; Malyan, S.K.; Pugazhendhi, A. Microbial fuel cells as a sustainable platform technology for bioenergy, biosensing, environmental monitoring, and other low power device applications. Fuel 2019, 255, 115682. [Google Scholar] [CrossRef]
- Vilas Boas, J.; Oliveira, V.B.; Simões, M.; Pinto, A.M.F.R. Review on microbial fuel cells applications, developments and costs. J. Environ. Manag. 2022, 307, 114525. [Google Scholar] [CrossRef]
- Sun, M.; Justin, G.A.; Roche, P.A.; Zhao, J.Z.J.; Wessel, B.L.; Zhang, Y.Z.Y.; Sclabassi, R.J. Passing data and supplying power to neural implants. IEEE Eng. Med. Biol. 2006, 25, 39–46. [Google Scholar]
- Chiao, M.; Lin, L.; Lam, K.-B. Implantable, Miniaturized Microbial Fuel Cell. U.S. Patent US7,160,637B2, 9 January 2007. [Google Scholar]
- Siu, C.-P.-B.; Mu, C. A Microfabricated PDMS Microbial Fuel Cell. J. Microelectromech. Syst. 2008, 17, 1329–1341. [Google Scholar] [CrossRef]
- Han, Y.; Yu, C.; Liu, H. A microbial fuel cell as power supply for implantable medical devices. Biosens. Bioelectron. 2010, 25, 2156–2160. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Jia, B.; Yu, C.; Dong, W.; Du, F.; Liu, H. Microbial fuel cell as power supply for implantable medical devices: A novel configuration design for simulating colonic environment. Biosens. Bioelectron. 2013, 41, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Lee, H.S.; Chae, J. Miniaturizing microbial fuel cells for potential portable power sources: Promises and challenges. Microfluid. Nanofluid. 2012, 13, 353–381. [Google Scholar] [CrossRef]
- Choi, S. Microscale microbial fuel cells: Advances and challenges. Biosens. Bioelectron. 2015, 69, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Parkhey, P.; Sahu, R. Microfluidic microbial fuel cells: Recent advancements and future prospects. Int. J. Hydrogen Energy 2021, 46, 3105–3123. [Google Scholar] [CrossRef]
- Mardanpour, M.M.; Yaghmaei, S. Characterization of a microfluidic microbial fuel cell as a power generator based on a nickel electrode. Biosens. Bioelectron. 2016, 79, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.Y.; Ahn, Y.; Schröder, U. Parylene C-coated PDMS-based microfluidic microbial fuel cells with low oxygen permeability. J. Power Sources 2018, 398, 209–214. [Google Scholar] [CrossRef]
- Mousavi, M.R.; Ghasemi, S.; Sanaee, Z.; Nejad, Z.G.; Mardanpour, M.M.; Yaghmaei, S.; Ghorbanzadeh, M. Improvement of the microfluidic microbial fuel cell using a nickel nanostructured electrode and microchannel modifications. J. Power Sources 2019, 437, 226891. [Google Scholar] [CrossRef]
- Pinto, A.M.F.R.; Oliveira, V.B.; Falcão, D.S. Direct Alcohol Fuel Cells for Portable Applications: Fundamentals, Engineering and Advances, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-811849-8. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, V.B. Microbial Fuel Cells as a Promising Power Supply for Implantable Medical Devices. Energies 2023, 16, 2647. https://doi.org/10.3390/en16062647
Oliveira VB. Microbial Fuel Cells as a Promising Power Supply for Implantable Medical Devices. Energies. 2023; 16(6):2647. https://doi.org/10.3390/en16062647
Chicago/Turabian StyleOliveira, Vânia B. 2023. "Microbial Fuel Cells as a Promising Power Supply for Implantable Medical Devices" Energies 16, no. 6: 2647. https://doi.org/10.3390/en16062647
APA StyleOliveira, V. B. (2023). Microbial Fuel Cells as a Promising Power Supply for Implantable Medical Devices. Energies, 16(6), 2647. https://doi.org/10.3390/en16062647





