Hydrogen Production by Steam Reforming of Pyrolysis Oil from Waste Plastic over 3 wt.% Ni/Ce-Zr-Mg/Al2O3 Catalyst
Abstract
:1. Introduction
2. Materials and Methods
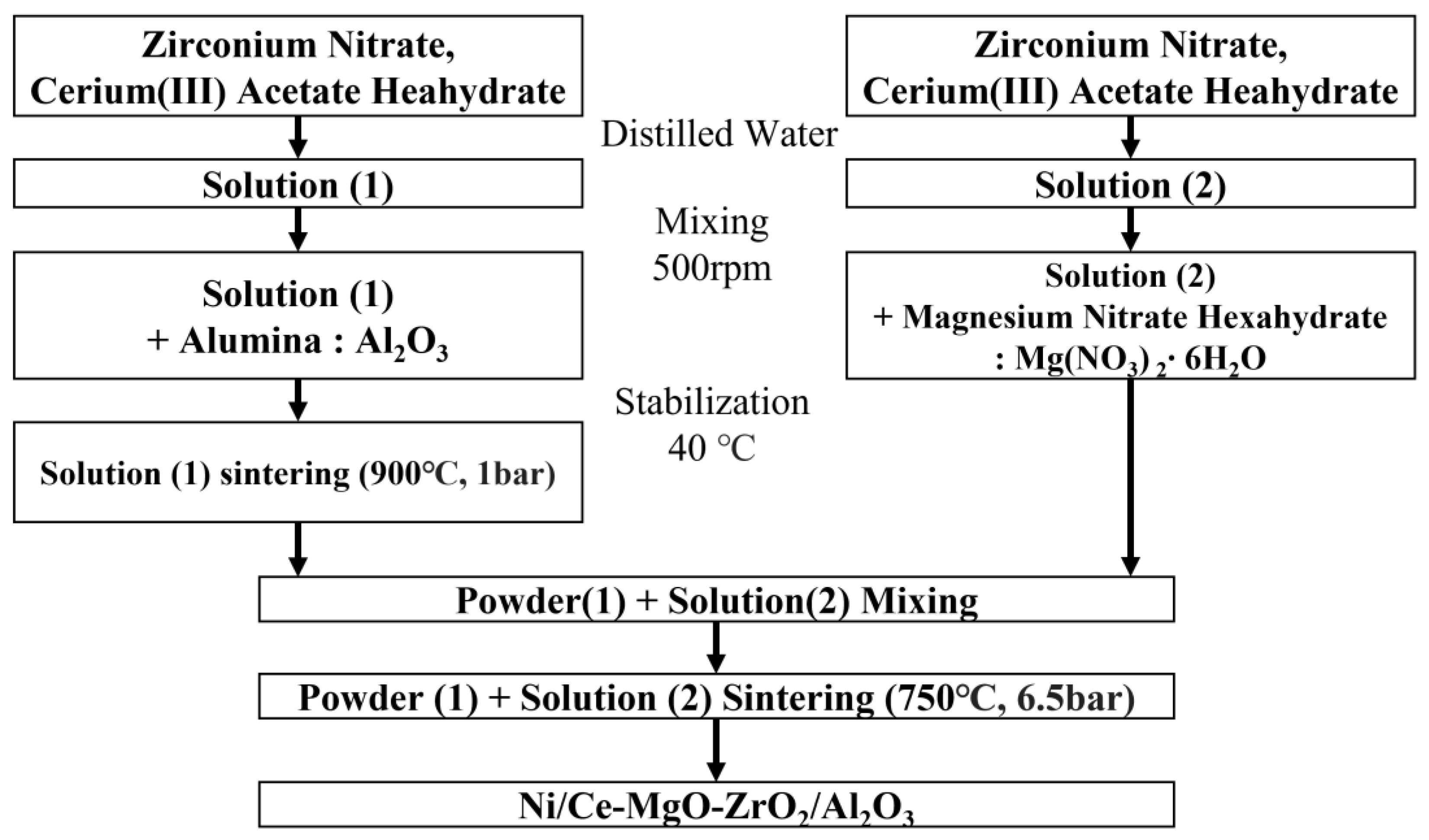
2.1. Catalyst Preparation
2.2. Catalyst Analysis
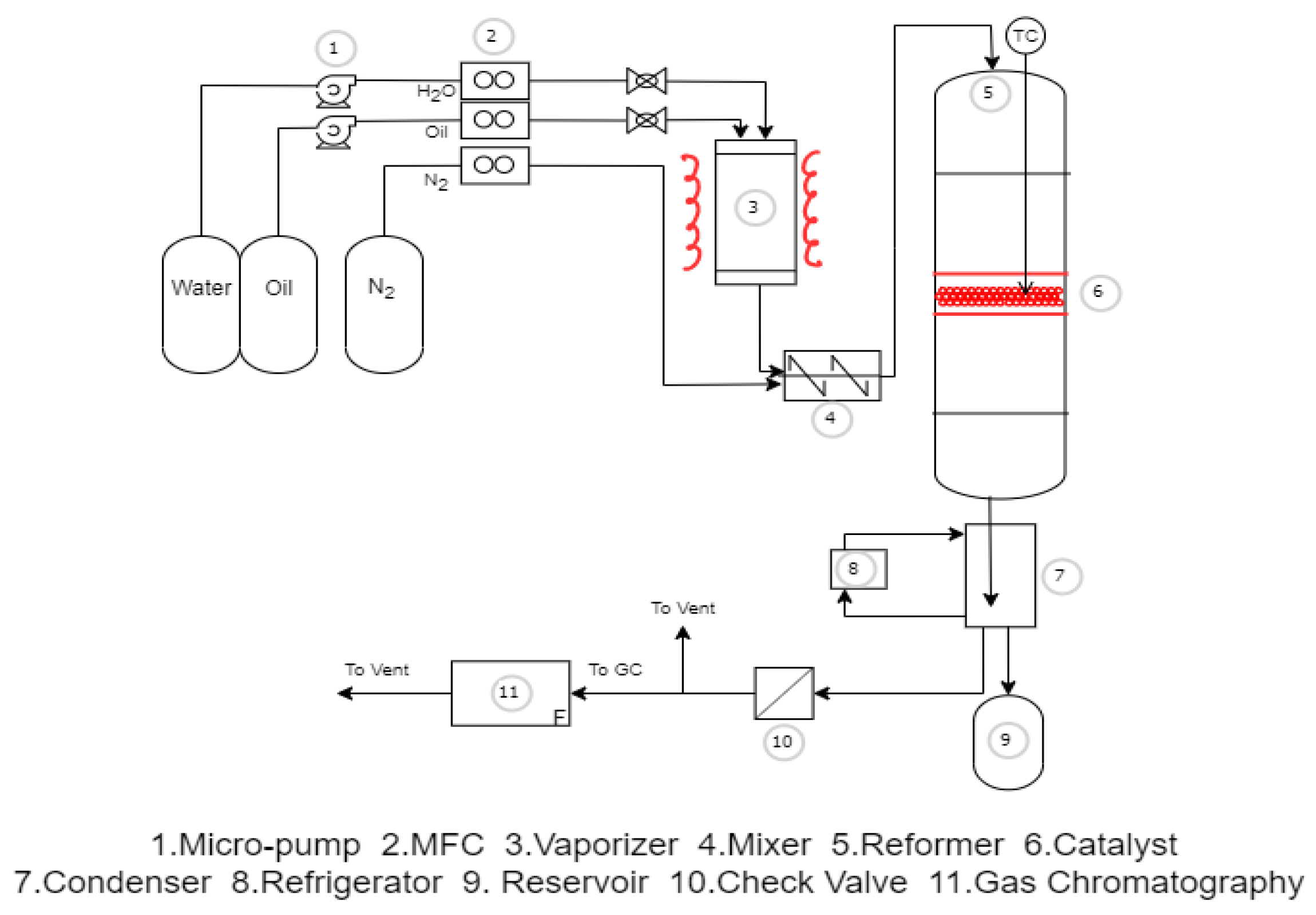
2.3. Experimental Method and Procedures
3. Results and Discussion
3.1. Catalyst Characterization
3.1.1. Specific Surface Area
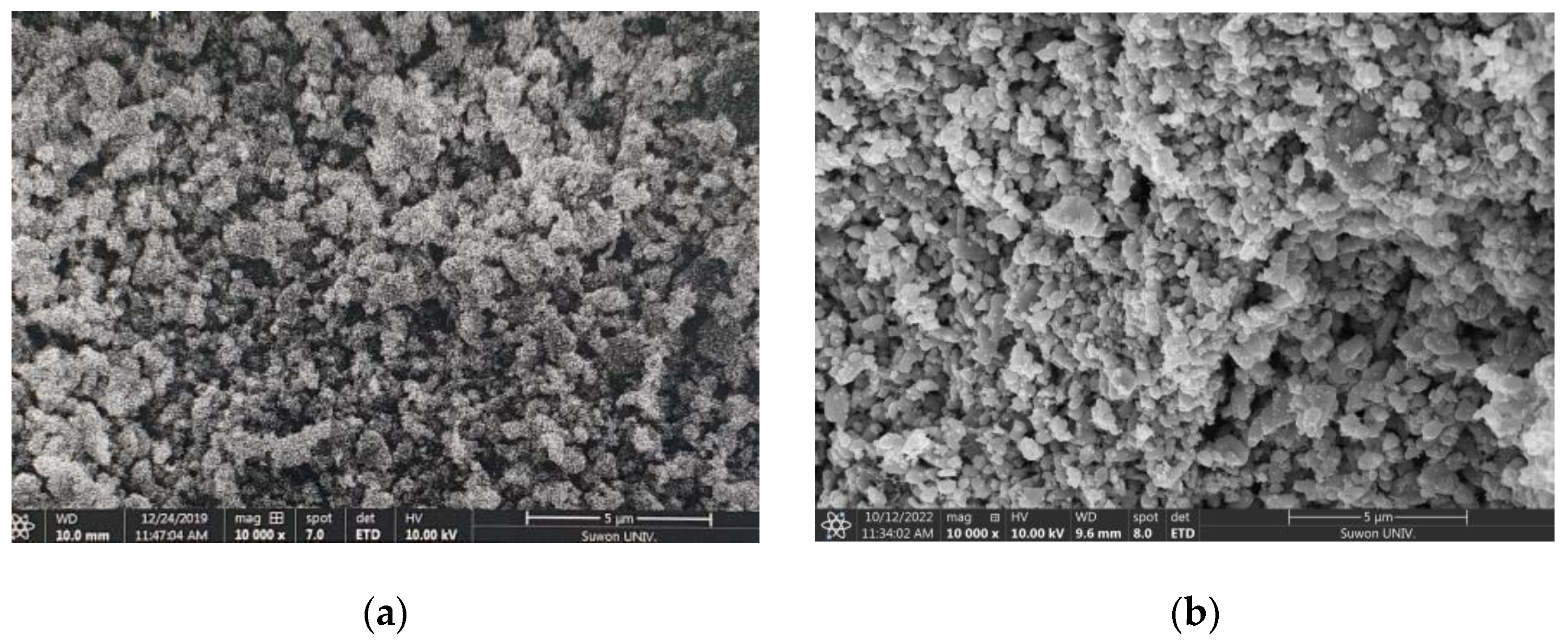
3.1.2. Scanning Electron Microscopy
3.1.3. X-ray Diffraction
3.1.4. X-ray Photoelectron Spectroscopy
3.1.5. Thermogravimetric Analysis
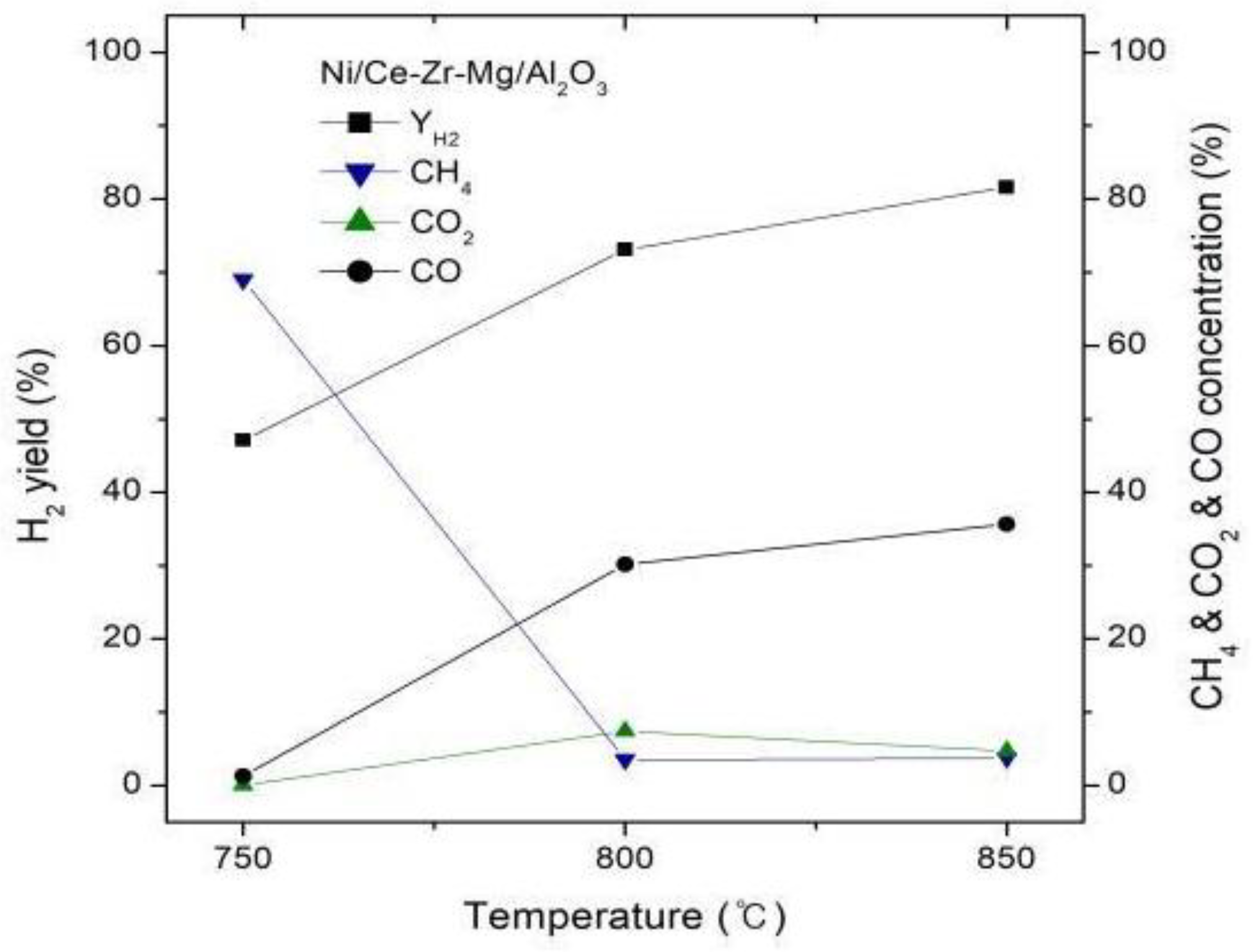
3.2. Effect of Temperature on Hydrogen Yield
3.3. Effect of Space Velocity on Hydrogen Yield
3.4. Effect of S/C Ratio on Hydrogen Yield
3.5. Effect of Oxygen and Nitrogen Addition on Hydrogen Yield
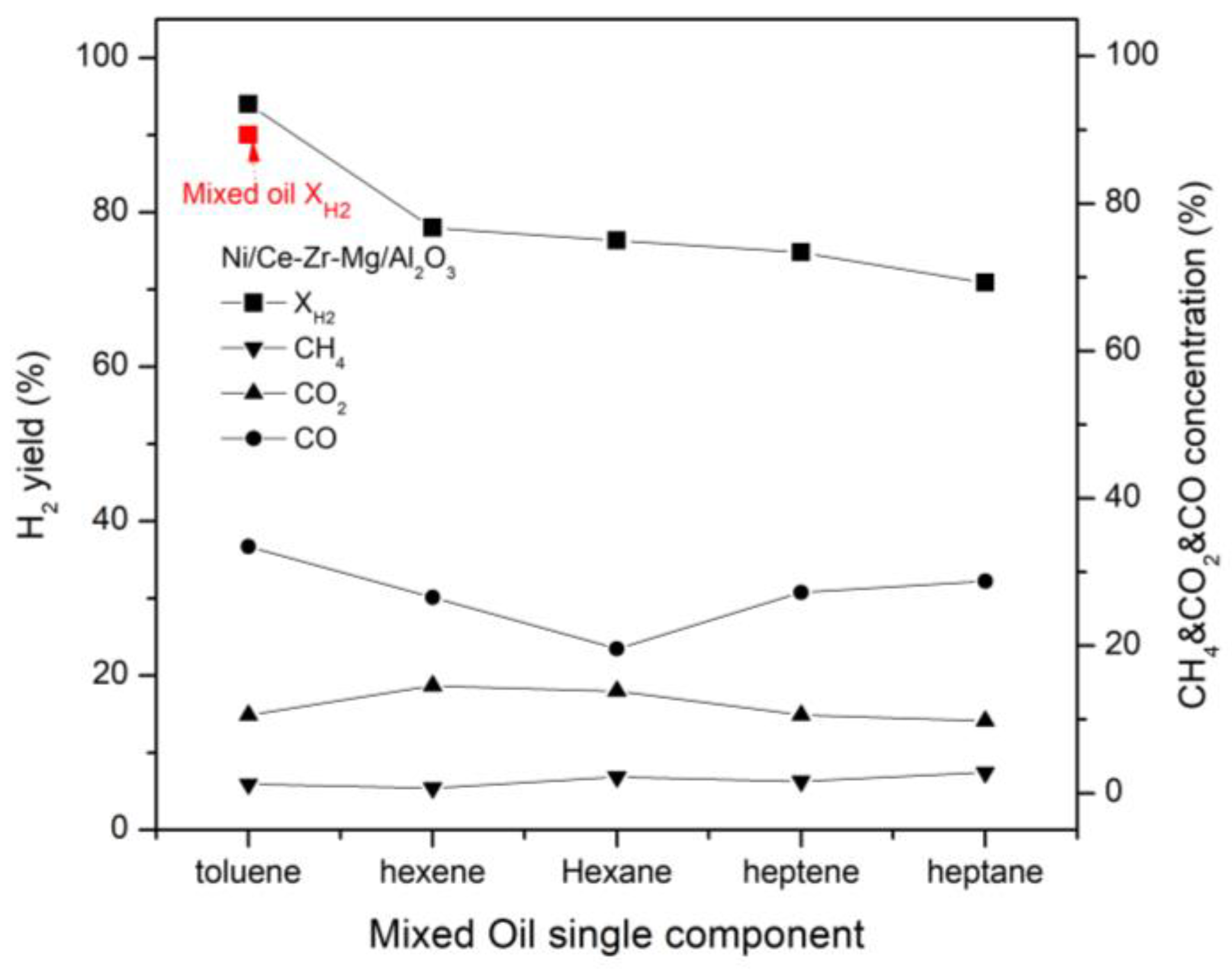
3.6. Effect of Single Components on Hydrogen Yield
3.7. The Durability and Activity Test of the Catalyst
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jo, J.; Shin, D.; Kim, Y. Pyrolysis of Plastic Waste: Current Status and Policy Tasks; KEI Policy Report; Korea Environment Institute: Sejong, Republic of Korea, 2022. [Google Scholar]
- Kim, Y.; Lim, S.; Kim, J.; Jae, J. Recent Research Trend in the Catalytic Pyrolysis of Waste Plastics for the Production of Renewable Fuels and Chemicals. KIC News 2021, 24, 10–21. [Google Scholar]
- Shent, H.; Pugh, R.J.; Forssberg, E. A review of plastics waste recycling and the flotation of plastics. Resour. Conserv. Recycl. 1999, 25, 85–109. [Google Scholar] [CrossRef]
- Samsung Securities Co. Nature Climate Change; Report; Samsung Securities Co., Ltd.: Seoul, Republic of Korea, 2021. [Google Scholar]
- Agora Energiewende and Wuppertal Institute. Breakthrough Strategies for Climate-Neutral Industry in Europe: Policy and Technology Pathways for Raising EU Climate Ambition. 2021. Available online: https://www.agora-energiewende.de/en/publications/breakthrough-strategies-for-climate-neutralindustry-in-europe-study/ (accessed on 26 May 2021).
- Nabgan, W.; Nabgan, B.; Abdullah, T.A.T.; Ngadi, N.; Jalil, A.A.; Nordin, A.H.; Latif, N.A.F.A.; Othman, N.F.H. Hydrogen Production from Catalytic Polyethylene Terephthalate Waste Reforming Reaction, an overview. Catal. Sustain. Energy 2020, 7, 45–64. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Lavoie, J.M. Review on dry reforming of methane, a potentially more environmentally-friendly approach to the increasing natural gas exploitation. Front. Chem. 2014, 2, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifianjazi, F.; Esmaeilkhanian, A.; Bazli, L.; Eskandarinezhad, S.; Khaksar, S.; Shafiee, P.; Yusuf, M.; Abdullah, B.; Salahshour, P.; Sadeghi, F. A review on recent advances in dry reforming of methane over Ni- and Co-based nanocatalysts. Int. J. Hydrog. Energy 2022, 47, 42213–42233. [Google Scholar] [CrossRef]
- Pawelczyk, E.; Wysocka, I.; Ģebicki, J. Pyrolysis Combined with the Dry Reforming of Waste Plastics as a Potential Method for Resource Recovery—A Review of Process Parameters and Catalysts. Catalysts 2022, 12, 362. [Google Scholar] [CrossRef]
- Farooqi, A.S.; Yusuf, M.; Ishak, M.A.I.; Zabidi, N.A.M.; Saidur, R.; Khan, A. Combined H2O and CO2 Reforming of CH4 Over Ca Promoted Ni/Al2O3 Catalyst: Enhancement of Ni-CaO Interactions. In Advances in Material Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2021; pp. 220–229. [Google Scholar]
- Bona, S.; Guillén, P.; Alcalde, J.G.; García, L.; Bilbao, R. Toluene steam reforming using coprecipitated Ni/Al catalysts modified with lanthanum or cobalt. Chem. Eng. J. 2008, 137, 587–597. [Google Scholar] [CrossRef]
- Kontchouo, F.M.B.; Gao, Z.; Xianglin, X.; Wang, Y.; Sun, Y.; Zhang, S.; Hu, X. Steam reforming of n-hexane and toluene: Understanding impacts of structural difference of aliphatic and aromatic hydrocarbons on their coking behaviours. J. Environ. Chem. Eng. 2021, 9, 106383. [Google Scholar] [CrossRef]
- Rostrupnielsen, J.R.; Hansen, J.H.B. CO2 reforming of methane over transition metals. J. Catal. 1993, 144, 38–49. [Google Scholar] [CrossRef]
- Freni, S.; Cavallaro, S.; Mondello, N.; Spadaro, L.; Frusteri, F. Production of hydrogen for MC fuel cell by steam reforming of ethanol over MgO supported Ni and Co catalysts. Catal. Commun. 2003, 4, 259–268. [Google Scholar] [CrossRef]
- Tuza, P.V.; Manfro, R.L.; Ribeiro, N.F.; Souza, M.M. Production of renewable hydrogen by aqueous-phase reforming of glycerol over Ni–Cu catalysts derived from hydrotalcite precursors. Renew. Energy 2013, 50, 408–414. [Google Scholar] [CrossRef]
- García-Diéguez, M.; Pieta, I.S.; Herrera, M.C.; Larrubia, M.A.; Alemany, L.J. Nanostructured Pt- and Ni-based catalysts for CO2-reforming of methane. J. Catal. 2010, 270, 136–145. [Google Scholar] [CrossRef]
- Davda, R.; Shabaker, J.; Huber, G.; Cortright, R.; Dumesic, J.A. A review of catalytic issues and process conditions for renewable hydrogen and alkanes by aqueous-phase reforming of oxygenated hydrocarbons over supported metal catalysts. Appl. Catal. B Environ. 2005, 56, 171–186. [Google Scholar] [CrossRef]
- Ross, J.R.H. Heterogeneous Catalysis: Fundamentals and Applications. Angew. Chem. 2012, 51, 5289. [Google Scholar] [CrossRef]
- Abdullah, T.A.T.; Nabgan, W.; Kamaruddin, M.J.; Mat, R.; Johari, A.; Ahmad, A. Hydrogen production from acetic acid steam reforming over bimetallic Ni-Co on La2O3 catalyst—Effect of the catalyst dilution. Appl. Mech. Mat. 2014, 493, 39–44. [Google Scholar] [CrossRef]
- Akbari, E.; Mehdi, S.; Rezea, A.M. CeO2 promoted Ni-MgO-Al2O3 nanocatalysts for carbon dioxide reforming of methane. J. CO2 Util. 2018, 24, 128–138. [Google Scholar] [CrossRef]
- Ray, K.; Bhardwaj, R.; Singh, B.; Deo, G. Developing descriptors for CO2 methanation and CO2 reforming of CH4 over Al2O3 supported Ni and low-cost Ni based alloy catalysts. Phys. Chem. Chem. Phys. 2018, 20, 15939–15950. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Graca, I.; Bebiano, S.S.; Lopes, J.M.; Henriques, C. Micro-and mesoporous supports for CO2 methanation catalysts: A comparison between SBA-15, MCM-41 and USY zeolite. Chem. Eng. Sci. 2018, 175, 72–83. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Li, L.; Zhang, X.; Wang, Z.; Wang, X.; Peng, H. Design of Ni-ZrO2-SiO2 catalysts with ultra-high sintering and coking resistance for dry reforming of methane to prepare syngas. J. CO2 Util. 2018, 27, 297–307. [Google Scholar] [CrossRef]
- Li, H.; Wang, J. Study on CO2 reforming of methane to syngas over Al2O3-ZrO2 supported Ni catalysts prepared via a direct sol-gel process. J. Chem. Eng. Sci. 2004, 59, 4861–4867. [Google Scholar] [CrossRef]
- Löfberg, A.; Guerrero-Caballero, J.; Kane, T.; Rubbens, A.; Jalowiecki-Duhamel, L. Ni/CeO2 based catalysts as oxygen vectors for the chemical looping dry reforming of methane for syngas production. Appl. Catal. B Environ. 2017, 212, 159–174. [Google Scholar] [CrossRef]
- Yan, X.; Hu, T.; Liu, P.; Li, S.; Zhao, A.; Zhang, Q.; Jiao, W.; Chen, S.; Wang, P.; Lu, J.; et al. Highly efficient and stable Ni/CeO2-SiO2 catalyst for dry reforming of methane: Effect of interfacial structure of Ni/CeO2 on SiO2. Appl. Catal. B Environ. 2019, 246, 221–231. [Google Scholar] [CrossRef]
- Sengupta, S.; Deo, G. Modifying alumina with CaO or MgO in supported Ni and Ni-Co catalysts and its effect on dry reforming of CH4. J. CO2 Util. 2015, 10, 62–77. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Arafat, Y.; Atia, H.; Ibrahim, A.; Luu, Q.; Ha, M.; Schneuder, M.; M-Pohl, M.; Fakeeha, A.H. CO2 reforming of methane to produce syngas over Co-Ni/SBA-15-Catalysts: Effect of support modifiers (Mg, La and Sc) on catalytic stability. J. CO2 Util. 2017, 21, 395–404. [Google Scholar] [CrossRef]
- Feng, X.; Feng, J.; Li, W. Insight into MgO promoter with low concentration for the carbon deposition resistance of Ni-based catalysts in the CO2 reforming of CH4. Chin. J. Catal. 2018, 39, 88–98. [Google Scholar] [CrossRef]
- Hu, D.; Liu, C.; Li, L.; Lv, K.L.; Zhang, Y.H.; Li, J.L. Carbon dioxide reforming of methane over nickel catalysts supported on TiO2(001) nanosheets. Int. J. Hydrog. Energy 2018, 43, 21345–21354. [Google Scholar] [CrossRef]
- Ahmed, S.; Aitani, A.; Rahman, F.; Al-Dawood, A.; Al-Muhaish, F. Decomposition of hydrocarbons to hydrogen and carbon. Appl. Catal. A 2009, 359, 1–24. [Google Scholar] [CrossRef]
- Wang, L.; Li, D.; Koike, M.; Watanabe, H.; Xu, Y.; Nakagawa, Y.; Tomishige, K. Catalytic performance and characterization of Ni-Co catalysts for the steam reforming of biomass tar to synthesis gas. Fuel 2013, 112, 654–661. [Google Scholar] [CrossRef]
- Wang, L.; Li, D.; Koike, M.; Koso, S.; Nakagawa, Y.; Xu, Y.; Tomishige, K. Catalytic performance and characterization of Ni-Fe catalysts for the steam reforming of tar from biomass pyrolysis to synthesis gas. Appl. Catal. A 2011, 392, 248–255. [Google Scholar] [CrossRef]
- Chen, J.; Tamura, M.; Nakagawa, Y.; Okumura, K.; Tomishige, K. Promoting effect of trace Pd on hydrotalcite-derived Ni/Mg/Al catalyst in oxidative steam reforming of biomass tar. Appl. Catal. B 2015, 179, 412–421. [Google Scholar] [CrossRef]
- Guan, G.; Chen, G.; Kasai, Y.; Lim, E.W.C.; Hao, X.; Kaewpanha, M.; Abuliti, A.; Fushimi, C.; Tsutsumi, A. Catalytic steam reforming of biomass tar over iron- or nickel-based catalyst supported on calcined scallop shell. Appl. Catal. B 2012, 115–116, 159–168. [Google Scholar] [CrossRef]
- Achouri, I.E.; Abatzoglou, N.; Fauteux-Lefebvre, C.; Braidy, N. Diesel steam reforming: Comparison of two nickel aluminate catalysts prepared by wet-impregnation and co-precipitation. Catal. Today 2013, 207, 13–20. [Google Scholar] [CrossRef]
- Han, D.; Kim, Y.; Cho, W.; Baek, Y. Effect of Oxidants on Syngas Synthesis from Biogas over 3 wt % Ni-Ce-MgO-ZrO2/Al2O3. Catal. Energ. 2020, 13, 297. [Google Scholar]
- Li, H.; Ren, J.; Qin, X.; Qin, Z.; Lin, J.; Li, Z. Ni/SBA-15 catalysts for CO methanation: Effects of V, Ce, and Zr promoters. RSC Adv. 2015, 115, 96504–96517. [Google Scholar] [CrossRef]
- Tao, M.; Meng, X.; Lv, Y.; Bian, Z.; Xin, Z. Effect of impregnation solvent on Ni dispersion and catalytic properties of Ni/SBA-15 for CO methanation reaction. Fuel 2016, 130, 289–297. [Google Scholar] [CrossRef]
- Yusuf, M.; Farooqi, A.; Ying, Y.; Alam, M.; Hellgardt, K.; Abdullah, B. Syngas production employing nickel on aluminamagnesia supported catalyst via dry methane reforming. Materialwiss. Werkstoftech 2021, 52, 10901100. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Z.X.; Kan, T.; Zhu, X.F.; Li, Q.X. Hydrogen production by catalytic steam reforming of bio-oil, naphtha and CH4 over C12A7-Mg catalyst. Chin. J. Chem. Phys. 2006, 19, 190. [Google Scholar] [CrossRef]
- Kong, M.; Yang, Q.; Fei, J.; Zheng, X. Experimental study of Ni/MgO catalyst in carbon dioxide reforming of toluene, a model compound of tar from biomass gasification. Int. J. Hydrog. Energy 2012, 37, 13355–13364. [Google Scholar] [CrossRef]
- Yoon, S.J.; Choi, Y.C.; Lee, J.G. Hydrogen production from biomass tar by catalytic steam reforming. Energy Convers Manag. 2010, 51, 42–47. [Google Scholar] [CrossRef]
- Phongprueksathat, N.; Meeyoo, V.; Rirksomboon, T. Steam reforming of acetic acid for hydrogenproduction: Catalytic performances of Ni and Co supported on Ce0.75Zr0.25O2 catalysts. Int. J. Hydrog. Energy 2019, 44, 9359–9367. [Google Scholar] [CrossRef]
- Świerczyński, D.; Libs, S.; Courson, C.; Kiennemann, A. Steam reforming of tar from a biomass gasification process over Ni/olivine catalyst using toluene as a model compound. Appl. Catal. B Environ. 2007, 74, 211–222. [Google Scholar] [CrossRef]
- Gao, N.; Liu, S.; Han, Y.; Xing, C.; Li, A. Steam reforming of biomass tar for hydrogen production over NiO/ceramic foam catalyst. Int. J. Hydrog. Energy 2015, 40, 7983–7990. [Google Scholar] [CrossRef]
- Gao, N.; Wang, X.; Li, A.; Wu, C.; Yin, Z. Hydrogen production from catalytic steam reforming of benzene as tar model compound of biomass gasification. Fuel Process. Technol. 2016, 148, 380–387. [Google Scholar] [CrossRef]






| Boiling Point (°C) | Concentration of Pyrolysis Oil (%) | Mixed Ratio of Model Oil (%) | |
|---|---|---|---|
| 1-Hexene | 63.4 | 1.55 | 11 |
| N-Hexane | 68.7 | 1.84 | 12 |
| 1-Heptene | 93.6 | 2.77 | 17 |
| N-Heptane | 98.5 | 2.17 | 12 |
| Toluene | 110.6 | 6.13 | 48 |
| Temperature (°C) | 750, 800, 850 |
| Pressure (atm) | 1 |
| GHSV (h−1) | 7600, 10,000, 15,000, 19,100 |
| S/C ratio | 0.6, 2, 3, 4 |
| BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (Å) | |
|---|---|---|---|
| Fresh catalyst | 2.94 | 0.001726 | 47.31 Å |
| Spent catalyst | 5.01 | 0.007327 | 56.45 Å |
| Metal | NiO | Ce | MgO | Zr | C * |
|---|---|---|---|---|---|
| Fresh (%) | 3.1 | 1.3 | 3.1 | 2.6 | 0 |
| Spent (%) | 2.7 | 1.1 | 2.7 | 2.4 | 5.8 |
| S/C | GHSV (h-1) | Reaction Material (mL/min) | H2 Yield (%) | |||
|---|---|---|---|---|---|---|
| Oil | H2O | O2 | N2 | |||
| 2 | 10,000 | 0.058 | 0.113 | 0 | 12 | 81.1 |
| 12 | 0 | 94.6 | ||||
| 0 | 0 | 90.1 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Shin, S.; Jung, H.; Cho, W.; Baek, Y. Hydrogen Production by Steam Reforming of Pyrolysis Oil from Waste Plastic over 3 wt.% Ni/Ce-Zr-Mg/Al2O3 Catalyst. Energies 2023, 16, 2656. https://doi.org/10.3390/en16062656
Han D, Shin S, Jung H, Cho W, Baek Y. Hydrogen Production by Steam Reforming of Pyrolysis Oil from Waste Plastic over 3 wt.% Ni/Ce-Zr-Mg/Al2O3 Catalyst. Energies. 2023; 16(6):2656. https://doi.org/10.3390/en16062656
Chicago/Turabian StyleHan, Danbee, Seungcheol Shin, Haneul Jung, Wonjun Cho, and Youngsoon Baek. 2023. "Hydrogen Production by Steam Reforming of Pyrolysis Oil from Waste Plastic over 3 wt.% Ni/Ce-Zr-Mg/Al2O3 Catalyst" Energies 16, no. 6: 2656. https://doi.org/10.3390/en16062656






