Solar-Thermal-Chemical Integrated Design of a Cavity-Type Solar-Driven Methane Dry Reforming Reactor
Abstract
:1. Introduction
2. Mathematical Model and Optimization Method
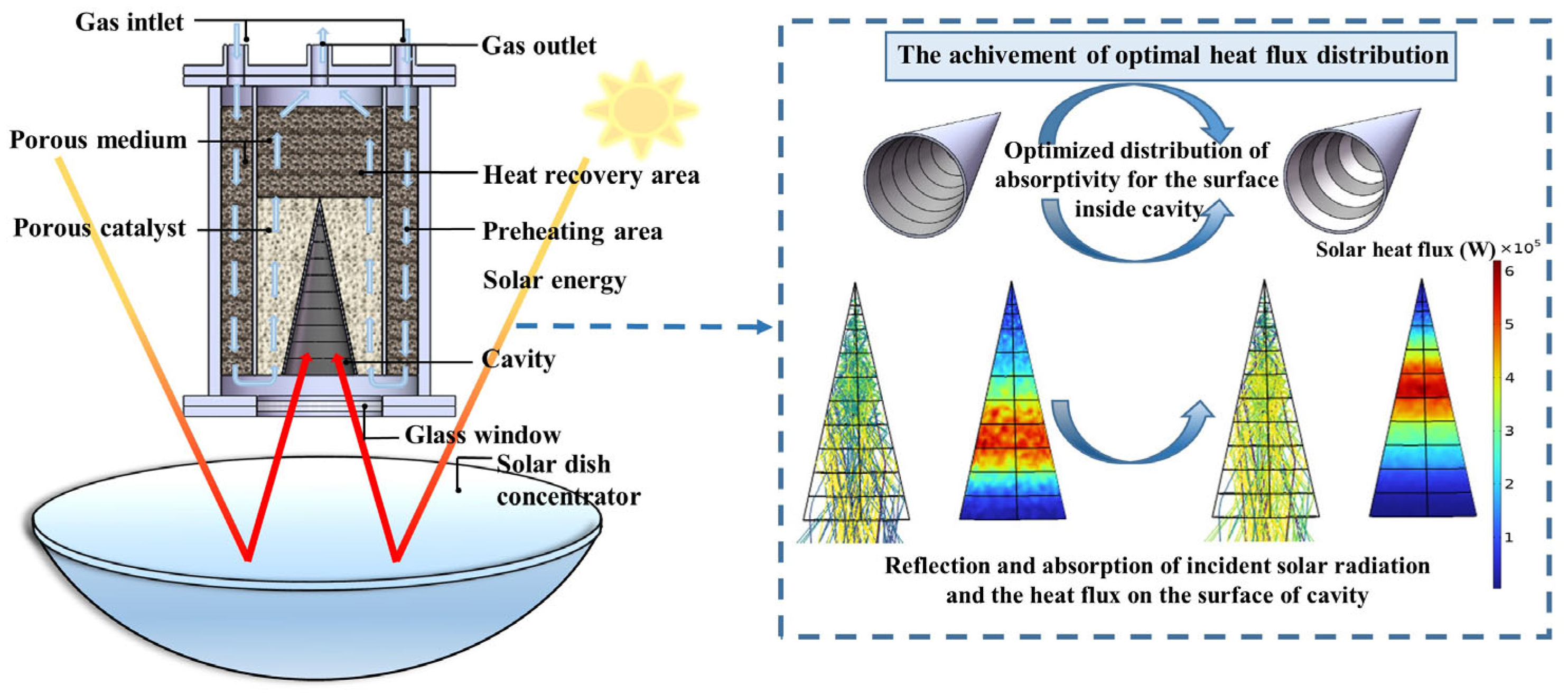
2.1. Model Description
2.2. Mathematical Model
2.2.1. Mass Conservation Equation
2.2.2. Momentum Conservation Equation
2.2.3. Energy Conservation Equation
2.2.4. Species Conservation Equation
2.2.5. Reaction Kinetics
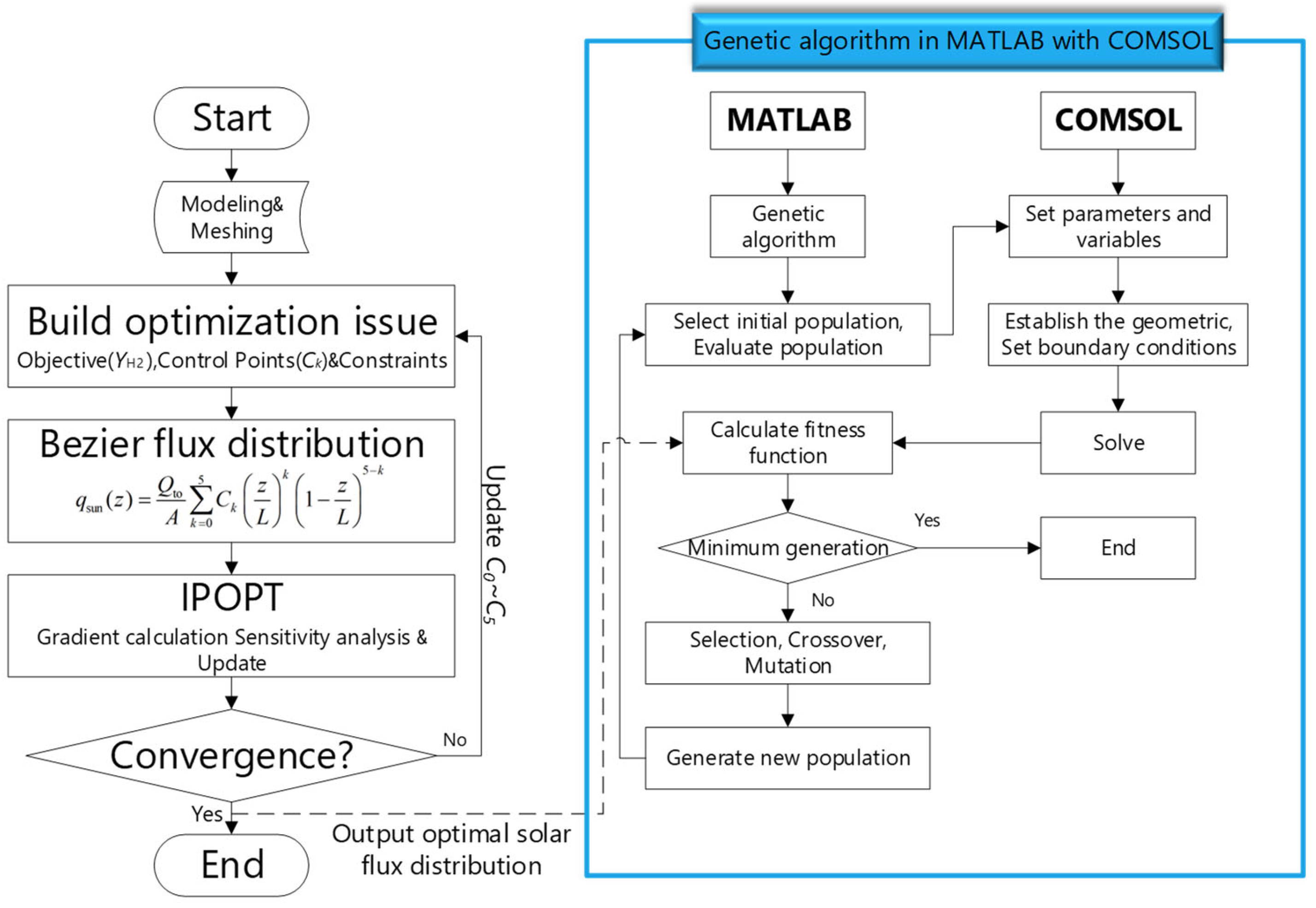
2.3. Optimization Method for Solar Flux and Absorptivity
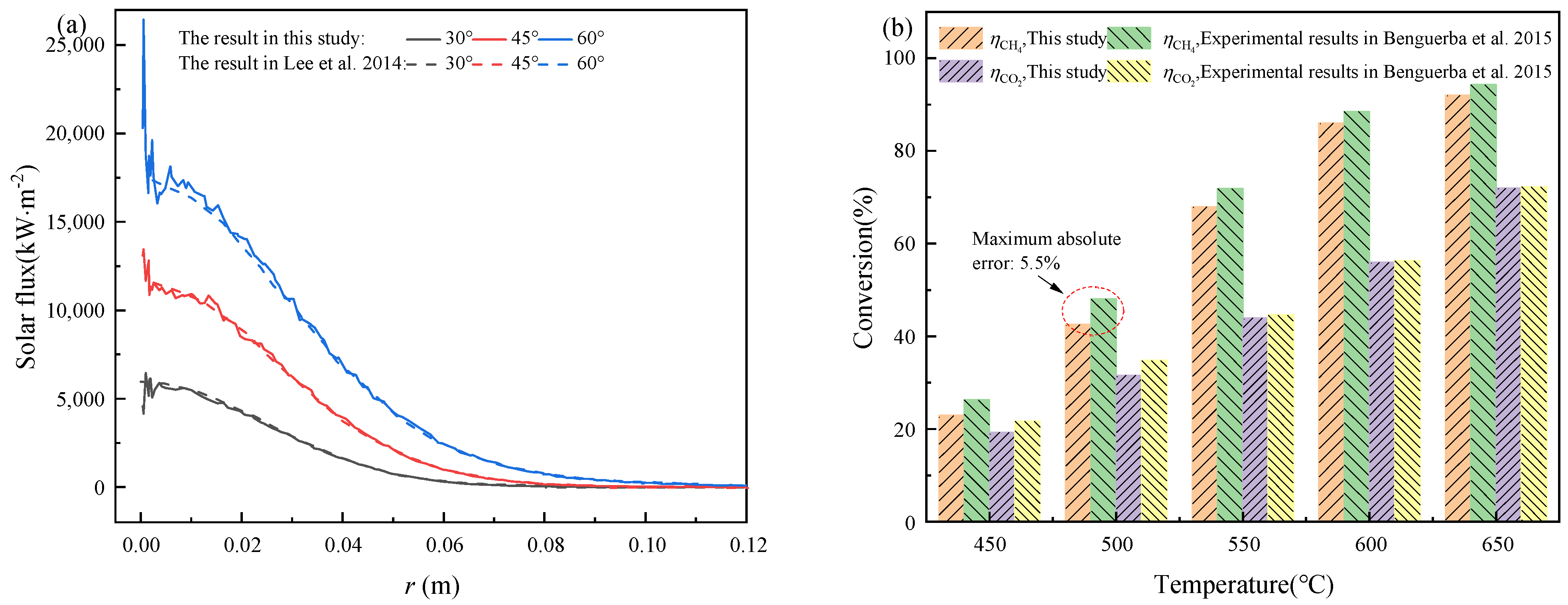
3. Model Validation
4. Results and Discussion
4.1. Analysis of Optimization Results and Comprehensive Performance of Cases
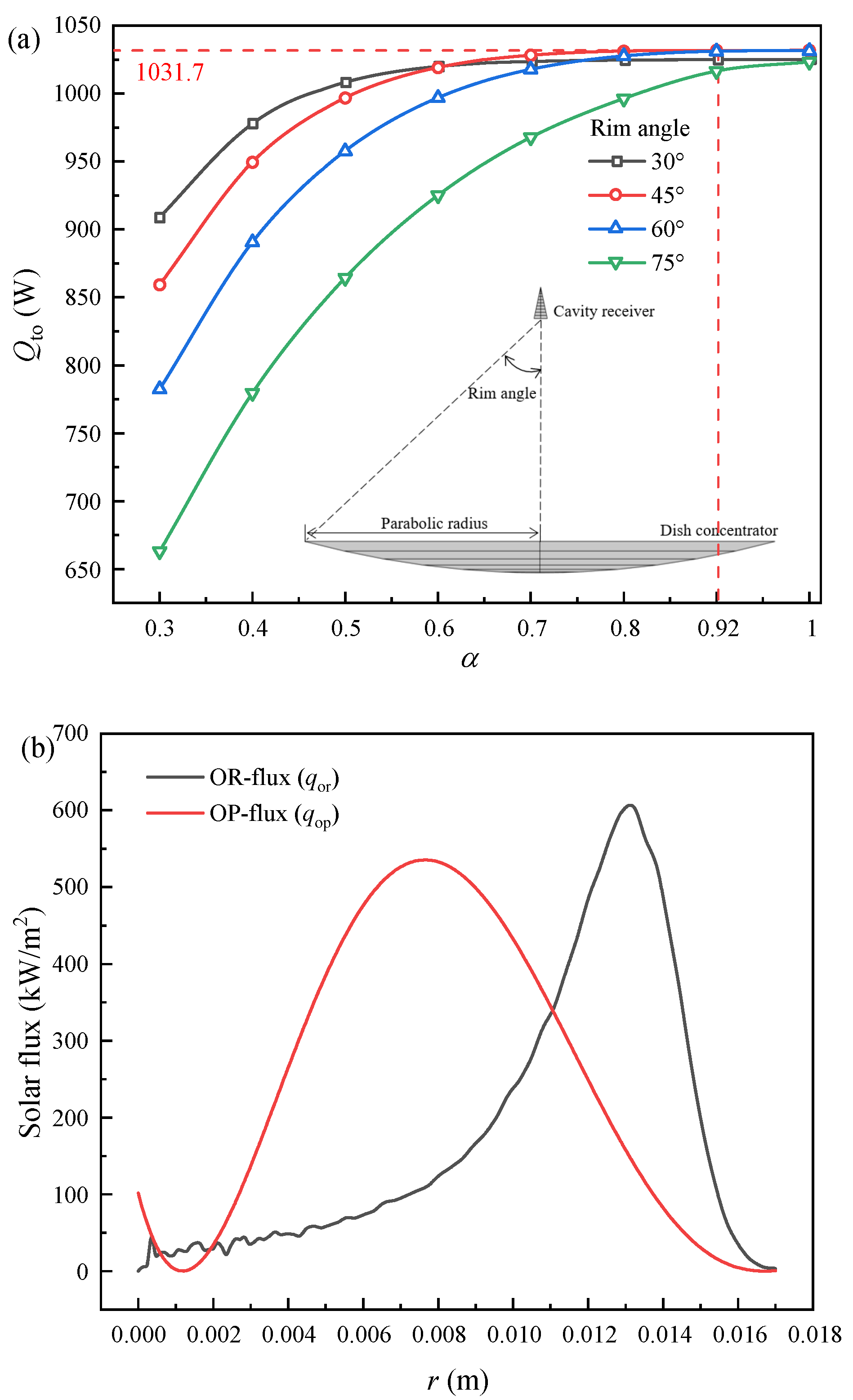
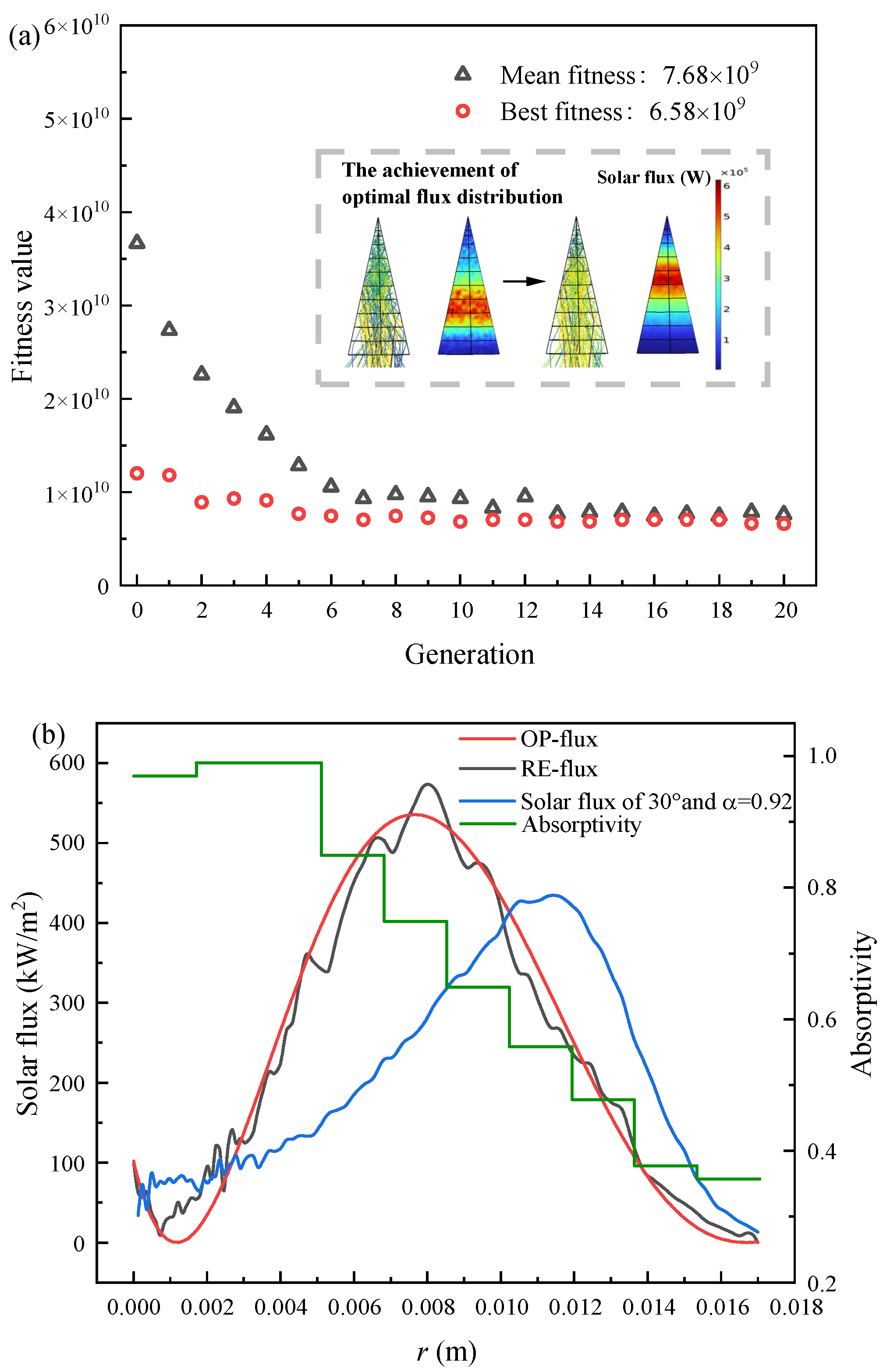
4.1.1. Optimal Flux Distribution Optimization Results
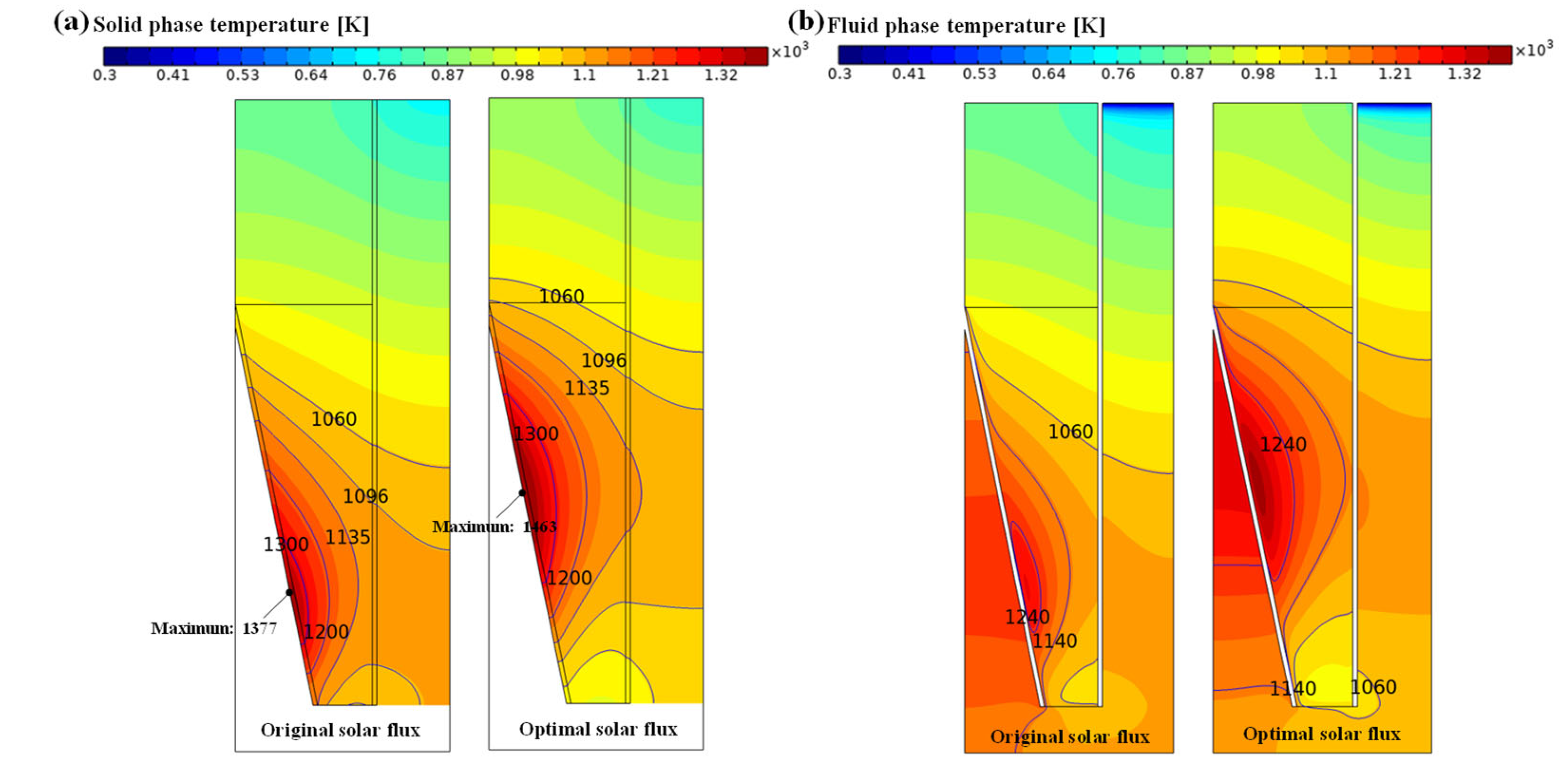
4.1.2. Analysis of Solid–Liquid Phase Temperature Field Distribution
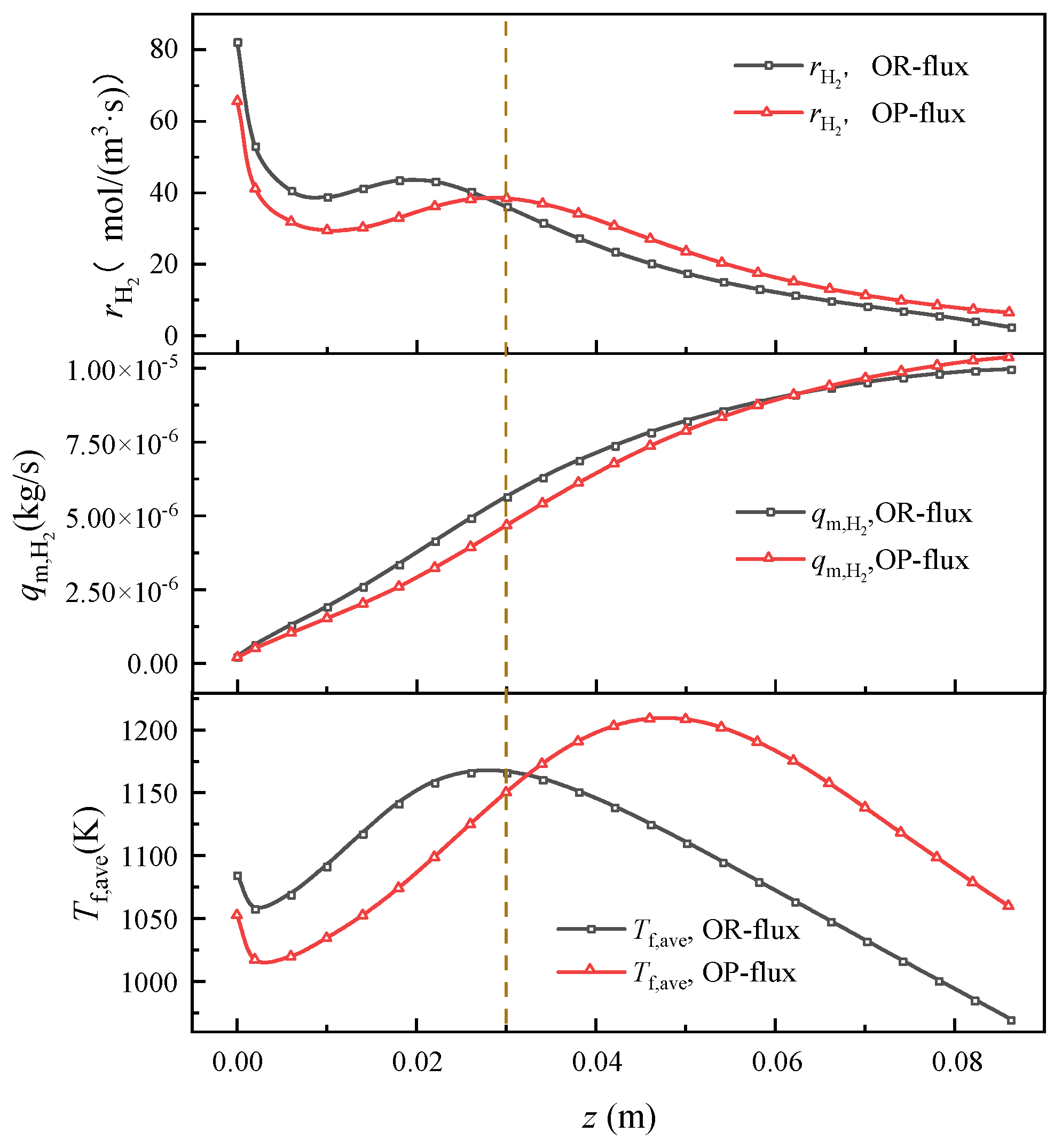
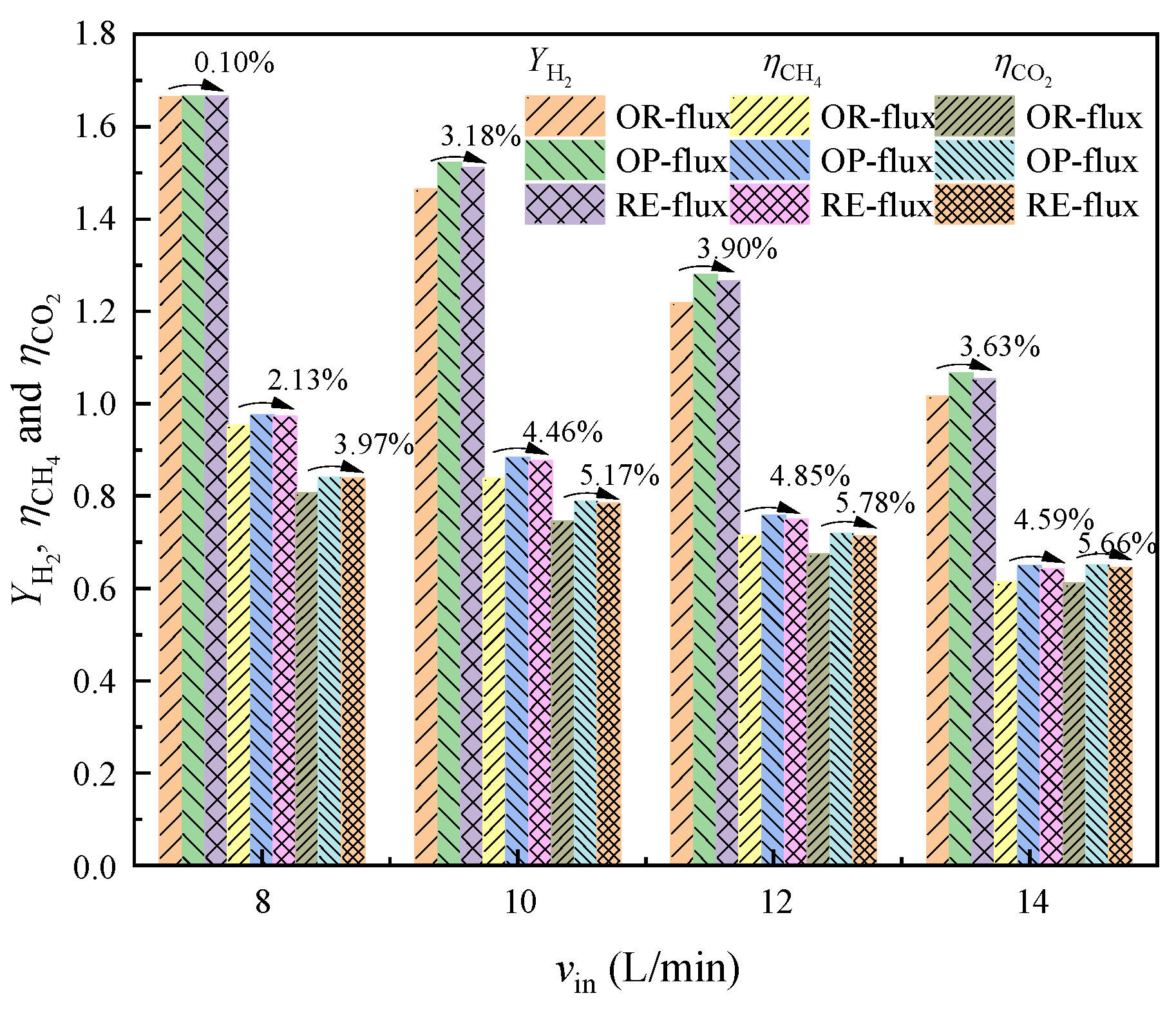
4.1.3. Comparison and Analysis of Hydrogen Production
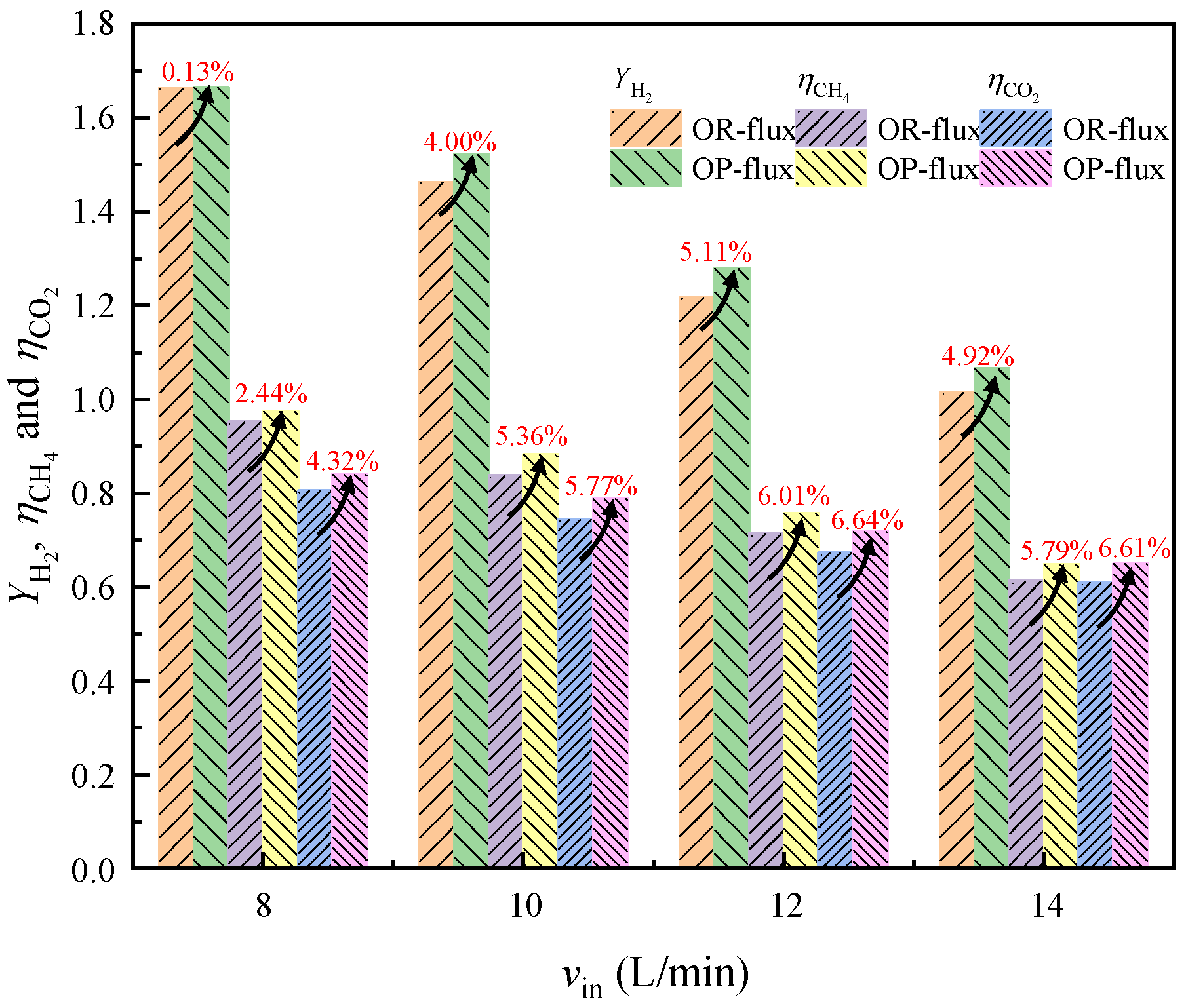
4.1.4. Performance Comparison under Different Inlet Volume Flow Rates
4.2. Achievement of Optimal Solar Flux Distribution
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Cp | specific heat (J·kg−1·K−1) |
| dp | aperture size (m) |
| Dij | binary diffusivity coefficient (m2·s−1) |
| Fi | mass flow of the species i (kg·s−1) |
| hv | local volumetric heat transfer coefficient (W·m−3·K−1) |
| enthalpy change of the reaction (kJ·mol−1) | |
| I | radiation intensity (MW·m−2·sr−1) |
| k | reaction rate constant |
| K | adsorption constant |
| Kp | equilibrium constant for reaction |
| L | length of reactor (m) |
| mi | mole fraction of species i |
| M | mole weight (kg·mol−1) |
| p | pressure (Pa) |
| Pi | partial pressure of species i (bar) |
| q | energy flux (MW·m−2) |
| Q | energy (W) |
| R | universal gas constant (J·mol−1·K−1) |
| ri | chemical reaction rate of the i-th reaction (mol·m−3·s−1) |
| hydrogen production rate (mol·m−3·s−1) | |
| T | temperature (K) |
| velocity vector (m·s−1) | |
| hydrogen yield | |
| z | axial coordinate (m) |
| Greeks | |
| absorptivity | |
| emissivity | |
| energy efficiency or reactant conversion | |
| extinction coefficient of porous media (m−1) | |
| thermal conductivity (W·m−1·K−1) | |
| dynamic viscosity (Pa·s) | |
| density (kg·m−3) | |
| Stefan-Boltzmann constant (W·m−2·K−4) | |
| reflectivity | |
| porosity | |
| mass fraction | |
| absorptivity | |
| Superscripts and subscripts | |
| a | actual value |
| ch | chemical storage energy |
| cat | catalyst |
| eff | effective value |
| f | fluid |
| i, j | species (CH4, H2O, H2, CO, CO2) |
| in | inlet |
| max | maximum value |
| out | outlet |
| so | solar energy |
| s | solid |
| se | sensible thermal energy |
| Abbreviations | |
| DRM | dry reforming of methane |
References
- Wen, H.; Liang, W.; Lee, C.-C. China’s progress toward sustainable development in pursuit of carbon neutrality: Regional differences and dynamic evolution. Environ. Impact Assess. Rev. 2023, 98, 106959. [Google Scholar] [CrossRef]
- Gunnarsdottir, I.; Davidsdottir, B.; Worrell, E.; Sigurgeirsdottir, S. Sustainable energy development: History of the concept and emerging themes. Renew. Sustain. Energy Rev. 2021, 141, 110770. [Google Scholar] [CrossRef]
- Anam, M.Z.; Bari, A.B.M.M.; Paul, S.K.; Ali, S.M.; Kabir, G. Modelling the drivers of solar energy development in an emerging economy: Implications for sustainable development goals. Resour. Conserv. Recycl. Adv. 2022, 13, 200068. [Google Scholar] [CrossRef]
- He, C.; Wu, S.; Wang, L.; Zhang, J. Recent advances in photo-enhanced dry reforming of methane: A review. J. Photochem. Photobiol. C Photochem. Rev. 2022, 51, 100468. [Google Scholar] [CrossRef]
- Su, B.; Wang, Y.; Xu, Z.; Han, W.; Jin, H.; Wang, H. Novel ways for hydrogen production based on methane steam and dry reforming integrated with carbon capture. Energy Convers. Manag. 2022, 270, 116199. [Google Scholar] [CrossRef]
- Wang, Z.; Han, W.; Zhang, N.; Gan, Z.; Sun, J.; Jin, H. Energy level difference graphic analysis method of combined cooling, heating and power systems. Energy 2018, 160, 1069–1077. [Google Scholar] [CrossRef]
- Ling, Y.; Li, W.; Jin, J.; Yu, Y.; Hao, Y.; Jin, H. A spectral-splitting photovoltaic-thermochemical system for energy storage and solar power generation. Appl. Energy 2020, 260, 113631. [Google Scholar] [CrossRef]
- Agrafiotis, C.; von Storch, H.; Roeb, M.; Sattler, C. Solar thermal reforming of methane feedstocks for hydrogen and syngas production—A review. Renew. Sustain. Energy Rev. 2014, 29, 656–682. [Google Scholar] [CrossRef]
- Villafán-Vidales, H.I.; Arancibia-Bulnes, C.A.; Riveros-Rosas, D.; Romero-Paredes, H.; Estrada, C.A. An overview of the solar thermochemical processes for hydrogen and syngas production: Reactors, and facilities. Renew. Sustain. Energy Rev. 2017, 75, 894–908. [Google Scholar] [CrossRef]
- Sheu, E.J.; Mokheimer, E.M.A.; Ghoniem, A.F. A review of solar methane reforming systems. Int. J. Hydrogen Energy 2015, 40, 12929–12955. [Google Scholar] [CrossRef] [Green Version]
- Kribus, A.; Gray, Y.; Grijnevich, M.; Mittelman, G.; Mey-Cloutier, S.; Caliot, C. The promise and challenge of solar volumetric absorbers. Sol. Energy 2014, 110, 463–481. [Google Scholar] [CrossRef]
- Gomez-Garcia, F.; González-Aguilar, J.; Olalde, G.; Romero, M. Thermal and hydrodynamic behavior of ceramic volumetric absorbers for central receiver solar power plants: A review. Renew. Sustain. Energy Rev. 2016, 57, 648–658. [Google Scholar] [CrossRef]
- Wang, P.; Li, J.B.; Xu, R.N.; Jiang, P.X. Non-uniform and volumetric effect on the hydrodynamic and thermal characteristic in a unit solar absorber. Energy 2021, 225, 120130. [Google Scholar] [CrossRef]
- Du, S.; Xia, T.; He, Y.-L.; Li, Z.-Y.; Li, D.; Xie, X.-Q. Experiment and optimization study on the radial graded porous volumetric solar receiver matching non-uniform solar flux distribution. Appl. Energy 2020, 275, 115343. [Google Scholar] [CrossRef]
- Kasaeian, A.; Barghamadi, H.; Pourfayaz, F. Performance comparison between the geometry models of multi-channel absorbers in solar volumetric receivers. Renew. Energy 2017, 105, 1–12. [Google Scholar] [CrossRef]
- Li, J.B.; Wang, P.; Liu, D.Y. Optimization on the gradually varied pore structure distribution for the irradiated absorber. Energy 2022, 240, 122787. [Google Scholar] [CrossRef]
- Capuano, R.; Fend, T.; Stadler, H.; Hoffschmidt, B.; Pitz-Paal, R. Optimized volumetric solar receiver: Thermal performance prediction and experimental validation. Renew. Energy 2017, 114, 556–566. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Wang, F.; Cheng, Z.; Liang, H.; Dong, Y.; Chen, X. Numerical analysis of the biomimetic leaf-type hierarchical porous structure to improve the energy storage efficiency of solar driven steam methane reforming. Int. J. Hydrogen Energy 2021, 46, 17653–17665. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, X.; Wang, F.; Yang, L.; Dong, Y.; Shuai, Y. Thermochemical analysis of dry methane reforming hydrogen production in biomimetic venous hierarchical porous structure solar reactor for improving energy storage. Int. J. Hydrogen Energy 2021, 46, 7733–7744. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, B.; Zhu, Q.; Gao, K.; Sun, N.; Tian, C.; Wang, J.; Zheng, H.; Wang, X.; Dang, C.; et al. Highly efficient solar-driven CO2 reforming of methane via concave foam reactors. Energy 2022, 261, 125141. [Google Scholar] [CrossRef]
- Bellos, E.; Bousi, E.; Tzivanidis, C.; Pavlovic, S. Optical and thermal analysis of different cavity receiver designs for solar dish concentrators. Energy Convers. Manag. X 2019, 2, 100013. [Google Scholar] [CrossRef]
- Sedighi, M.; Taylor, R.A.; Lake, M.; Rose, A.; Izadgoshasb, I.; Vasquez Padilla, R. Development of a novel high-temperature, pressurised, indirectly-irradiated cavity receiver. Energy Convers. Manag. 2020, 204, 112175. [Google Scholar] [CrossRef]
- Huang, H.; Lin, M. Optimization of solar receivers for high-temperature solar conversion processes: Direct vs. Indirect illumination designs. Appl. Energy 2021, 304, 117675. [Google Scholar] [CrossRef]
- Sánchez-González, A.; Santana, D. Solar flux distribution on central receivers: A projection method from analytic function. Renew. Energy 2015, 74, 576–587. [Google Scholar] [CrossRef]
- Yan, J.; Peng, Y.; Cheng, Z. Optimization of a discrete dish concentrator for uniform flux distribution on the cavity receiver of solar concentrator system. Renew. Energy 2018, 129, 431–445. [Google Scholar] [CrossRef]
- Fang, J.; Tu, N.; Wei, J.; Du, X. Effects of surface optical and radiative properties on the thermal performance of a solar cavity receiver. Sol. Energy 2018, 171, 157–170. [Google Scholar] [CrossRef]
- Chen, F.; Li, M.; Hassanien Emam Hassanien, R.; Luo, X.; Hong, Y.; Feng, Z.; Ji, M.; Zhang, P. Study on the Optical Properties of Triangular Cavity Absorber for Parabolic Trough Solar Concentrator. Int. J. Photoenergy 2015, 2015, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Xiao, G.; Yang, T.; Ni, D.; Cen, K.; Ni, M. A model-based approach for optical performance assessment and optimization of a solar dish. Renew. Energy 2017, 100, 103–113. [Google Scholar] [CrossRef]
- Tu, N.; Wei, J.; Fang, J. Numerical investigation on uniformity of heat flux for semi-gray surfaces inside a solar cavity receiver. Sol. Energy 2015, 112, 128–143. [Google Scholar] [CrossRef]
- Wang, F.; Shuai, Y.; Yuan, Y.; Yang, G.; Tan, H. Thermal stress analysis of eccentric tube receiver using concentrated solar radiation. Sol. Energy 2010, 84, 1809–1815. [Google Scholar] [CrossRef]
- Wang, K.; He, Y.-L.; Li, P.; Li, M.-J.; Tao, W.-Q. Multi-objective optimization of the solar absorptivity distribution inside a cavity solar receiver for solar power towers. Sol. Energy 2017, 158, 247–258. [Google Scholar] [CrossRef]
- Tang, X.; Yang, W.; Dai, Z.; Yang, Y. Inverse Design of Local Solar Flux Distribution for a Solar Methanol Reforming Reactor Based on Shape Optimization. Front. Energy Res. 2022, 10, 881822. [Google Scholar] [CrossRef]
- Tang, X.Y.; Yang, W.W.; Yang, Y.; Jiao, Y.H.; Zhang, T. A design method for optimizing the secondary reflector of a parabolic trough solar concentrator to achieve uniform heat flux distribution. Energy 2021, 229, 120749. [Google Scholar] [CrossRef]
- Dai, Z.-Q.; Yang, Y.-J.; Yang, W.-W.; Rong, L.; Tang, X.-Y. A thermochemical reactor design with better thermal management and improved performance for methane/carbon dioxide dry reforming. Int. J. Hydrogen Energy 2022, 47, 34794–34809. [Google Scholar] [CrossRef]
- Wu, Z.; Caliot, C.; Flamant, G.; Wang, Z. Coupled radiation and flow modeling in ceramic foam volumetric solar air receivers. Sol. Energy 2011, 85, 2374–2385. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, C.; Zhang, X.; Zhang, J. Flow and radiation heat transfer of a nanofluid over a stretching sheet with velocity slip and temperature jump in porous medium. J. Frankl. Inst. 2013, 350, 990–1007. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Z. Fully coupled transient modeling of ceramic foam volumetric solar air receiver. Sol. Energy 2013, 89, 122–133. [Google Scholar] [CrossRef]
- Wang, F.; Tan, J.; Yong, S.; Tan, H.; Chu, S. Thermal performance analyses of porous media solar receiver with different irradiative transfer models. Int. J. Heat Mass Transf. 2014, 78, 7–16. [Google Scholar] [CrossRef]
- Lee, B.; Yun, S.-W.; Kim, S.; Heo, J.; Kim, Y.-T.; Lee, S.; Lim, H. CO2 reforming of methane for H2 production in a membrane reactor as CO2 utilization: Computational fluid dynamics studies with a reactor geometry. Int. J. Hydrogen Energy 2019, 44, 2298–2311. [Google Scholar] [CrossRef]
- Zhang, H.; Shuai, Y.; Lougou, B.G.; Jiang, B.; Wang, F.; Cheng, Z.; Tan, H. Effects of multilayer porous ceramics on thermochemical energy conversion and storage efficiency in solar dry reforming of methane reactor. Appl. Energy 2020, 265, 114799. [Google Scholar] [CrossRef]
- Richardson, J.T.; Paripatyadar, S.A. Carbon dioxide reforming of methane with supported rhodium. Appl. Catal. 1990, 61, 293–309. [Google Scholar] [CrossRef]
- Snoeck, J.-W.; Froment, G.F.; Fowles, M. Kinetic Study of the Carbon Filament Formation by Methane Cracking on a Nickel Catalyst. J. Catal. 1997, 169, 250–262. [Google Scholar] [CrossRef]
- Tsai, C.-Y. Design of free-form trough reflector for solar thermal concentrator system based on quadratic Bézier curves. Opt. Commun. 2022, 511, 128024. [Google Scholar] [CrossRef]
- Tang, X.-Y.; Dou, P.-Y.; Dai, Z.-Q.; Yang, W.-W. Structural design and analysis of a solar thermochemical reactor partially filled with phase change material based on shape optimization. Sol. Energy 2022, 236, 613–625. [Google Scholar] [CrossRef]
- Duraklı, Z.; Nabiyev, V. A new approach based on Bezier curves to solve path planning problems for mobile robots. J. Comput. Sci. 2022, 58, 101540. [Google Scholar] [CrossRef]
- Xu, L.; Cao, M.; Song, B. A new approach to smooth path planning of mobile robot based on quartic Bezier transition curve and improved PSO algorithm. Neurocomputing 2022, 473, 98–106. [Google Scholar] [CrossRef]
- Biegler, L.T.; Zavala, V.M. Large-scale nonlinear programming using IPOPT: An integrating framework for enterprise-wide dynamic optimization. Comput. Chem. Eng. 2009, 33, 575–582. [Google Scholar] [CrossRef]
- Saad, Y.; Schultz, M.H. GMRES: A Generalized Minimal Residual Algorithm for Solving Nonsymmetric Linear Systems. SIAM J. Sci. Stat. Comput. 1986, 7, 856–869. [Google Scholar] [CrossRef] [Green Version]
- Schenk, O.; Gartner, K. Solving unsymmetric sparse systems of linear equations with PARDISO. Futur. Gener. Comp. Syst. 2004, 20, 475–487. [Google Scholar] [CrossRef]
- Lee, H. The geometric-optics relation between surface slope error and reflected ray error in solar concentrators. Sol. Energy 2014, 101, 299–307. [Google Scholar] [CrossRef]
- Benguerba, Y.; Dehimi, L.; Virginie, M.; Dumas, C.; Ernst, B. Modelling of methane dry reforming over Ni/Al2O3 catalyst in a fixed-bed catalytic reactor. Reac. Kinet. Mech. Cat. 2015, 114, 109–119. [Google Scholar] [CrossRef]
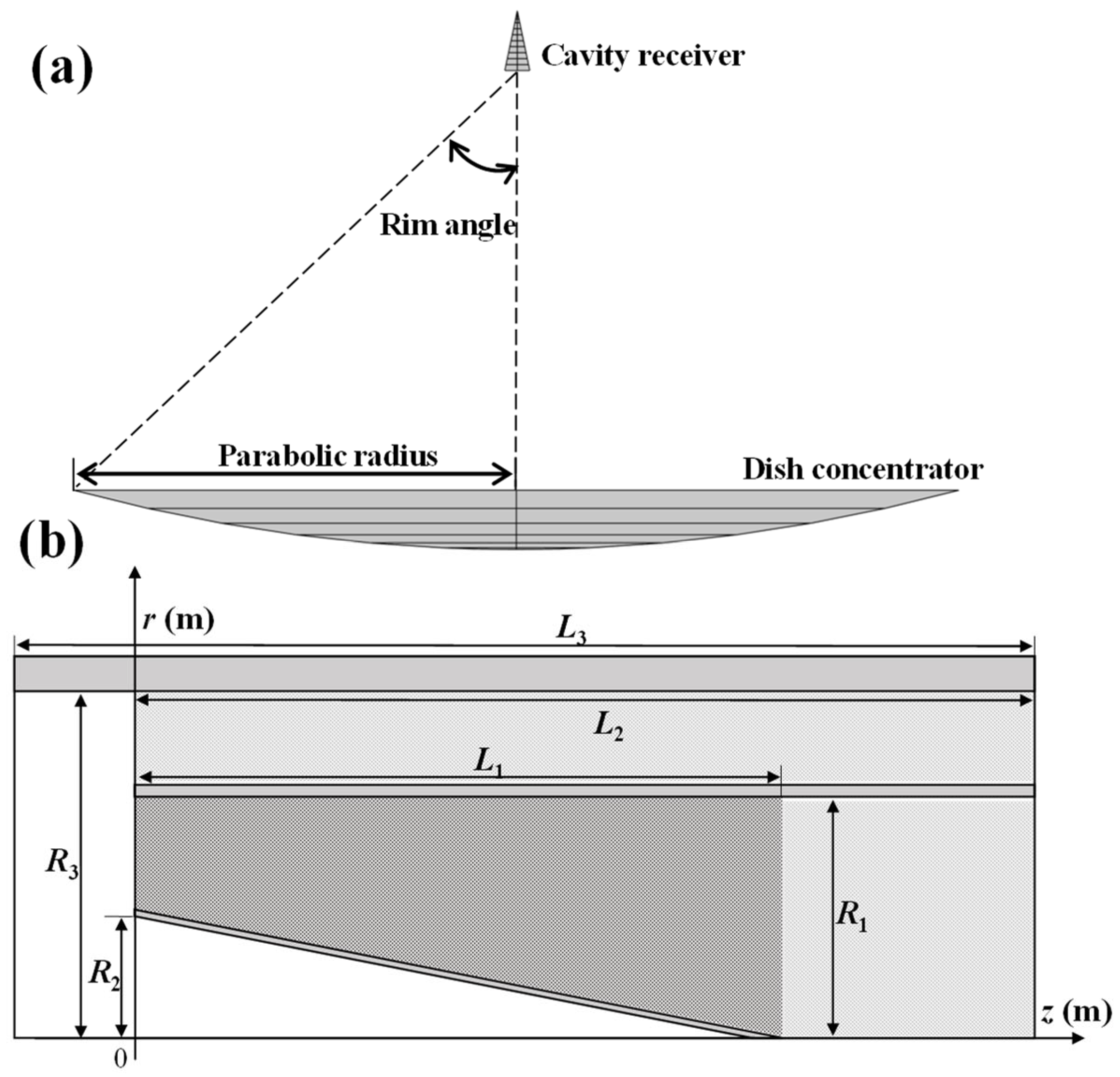

| Parameter | Values | Parameter | Value |
|---|---|---|---|
| Dish diameter | 1.20 (m) | R3 | 0.047 (m) |
| Surface reflectivity | 0.9 | dp | 0.003 (m) |
| Slope error | 2 (mrad) | L1 | 0.086 (m) |
| Solar half angle | 4.65 (mrad) | L2 | 0.13 (m) |
| R1 | 0.03 (m) | L3 | 0.14 (m) |
| R2 | 0.017 (m) | 0.8 |
| Parameter | Values | Parameter | Value |
|---|---|---|---|
| k1 | 1.29 × 106e−102,065/(RT) | 2.61 × 10−2e+37,641/(RT) | |
| k2 | 0.35 × 106e−81,030/(RT) | 2.60 × 10−2e+40,684/(RT) | |
| k3 | 6.95 × 103e−58,893/(RT) | 0.5771e+9262/(RT) | |
| 6.78 × 1014e−259,660/(RT) | 1.494e+6025/(RT) | ||
| 56.4971e−36,580/(RT) | 0.21e−567/(RT) | ||
| 2.98 × 105e−84,400/(RT) | 5.18 × 107e−133,210/(RT) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Z.-Q.; Ma, X.; Tang, X.-Y.; Zhang, R.-Z.; Yang, W.-W. Solar-Thermal-Chemical Integrated Design of a Cavity-Type Solar-Driven Methane Dry Reforming Reactor. Energies 2023, 16, 2781. https://doi.org/10.3390/en16062781
Dai Z-Q, Ma X, Tang X-Y, Zhang R-Z, Yang W-W. Solar-Thermal-Chemical Integrated Design of a Cavity-Type Solar-Driven Methane Dry Reforming Reactor. Energies. 2023; 16(6):2781. https://doi.org/10.3390/en16062781
Chicago/Turabian StyleDai, Zhou-Qiao, Xu Ma, Xin-Yuan Tang, Ren-Zhong Zhang, and Wei-Wei Yang. 2023. "Solar-Thermal-Chemical Integrated Design of a Cavity-Type Solar-Driven Methane Dry Reforming Reactor" Energies 16, no. 6: 2781. https://doi.org/10.3390/en16062781







