Aromatic Clusters and Hydrogen Storage
Abstract
:1. Introduction
2. Theoretical Background
3. Computational Details
4. Atomic Clusters
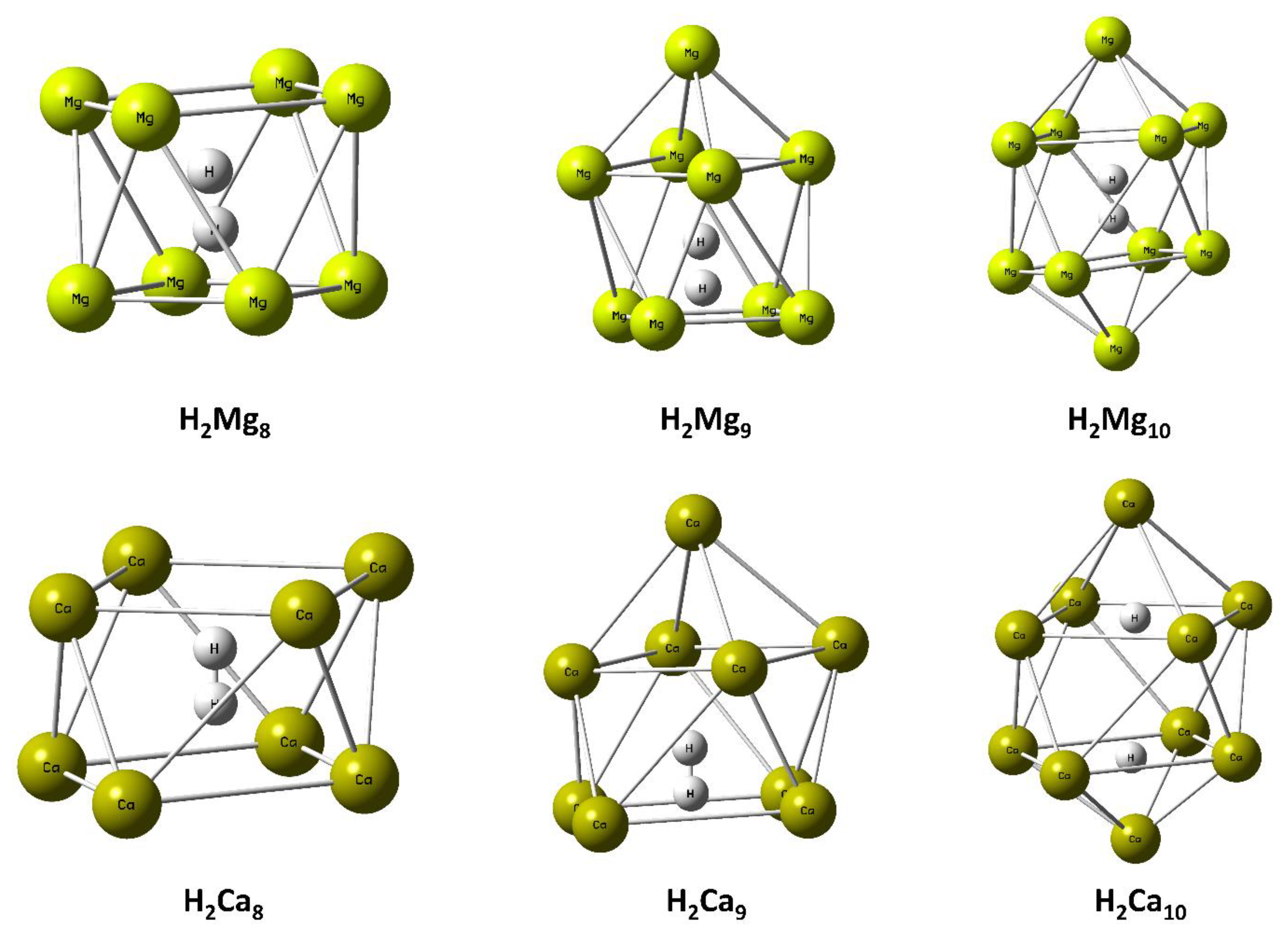
Mg and Ca Clusters
5. Ionic Clusters
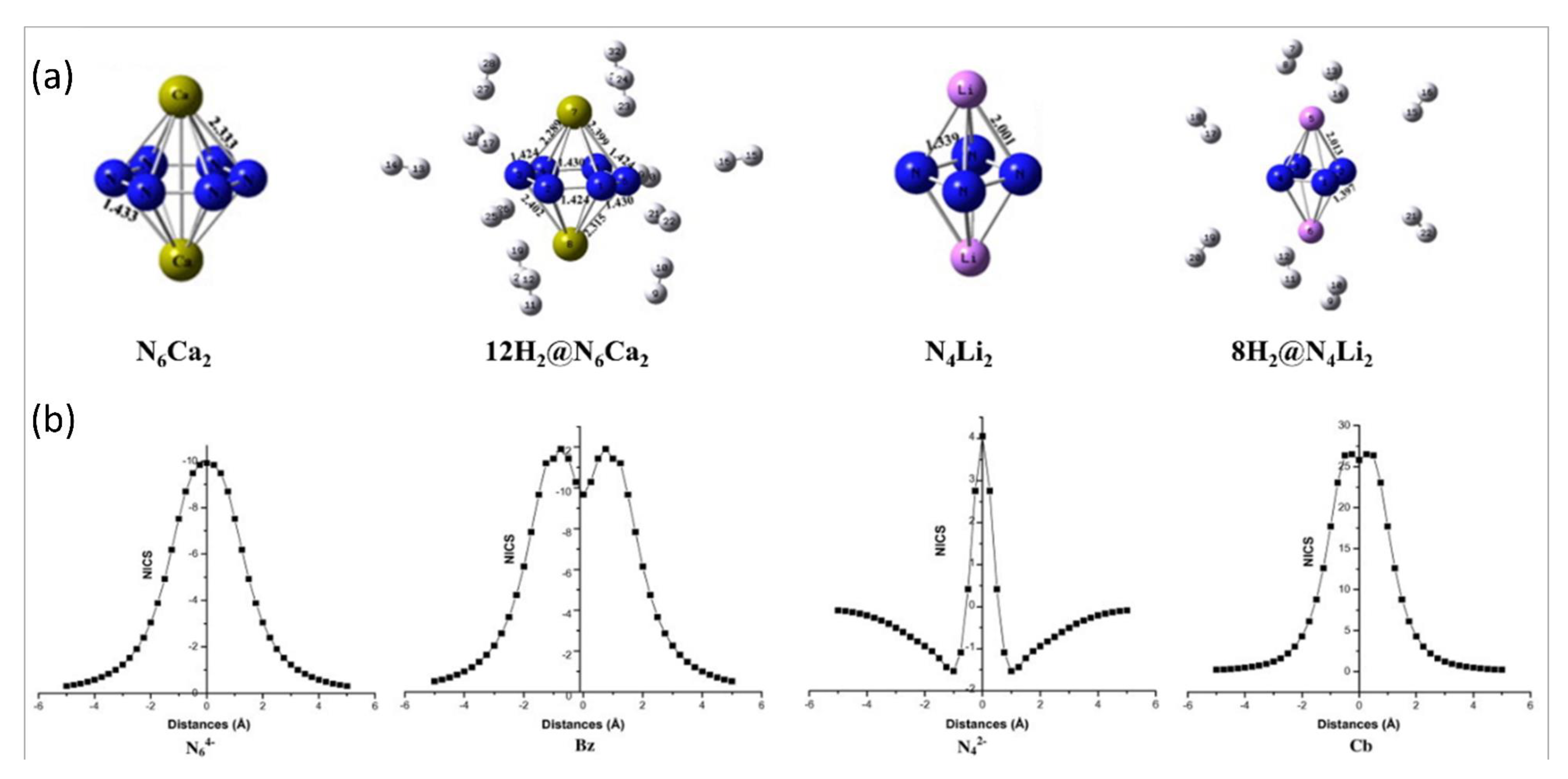
5.1. N4Li2 and N6Ca2 Clusters
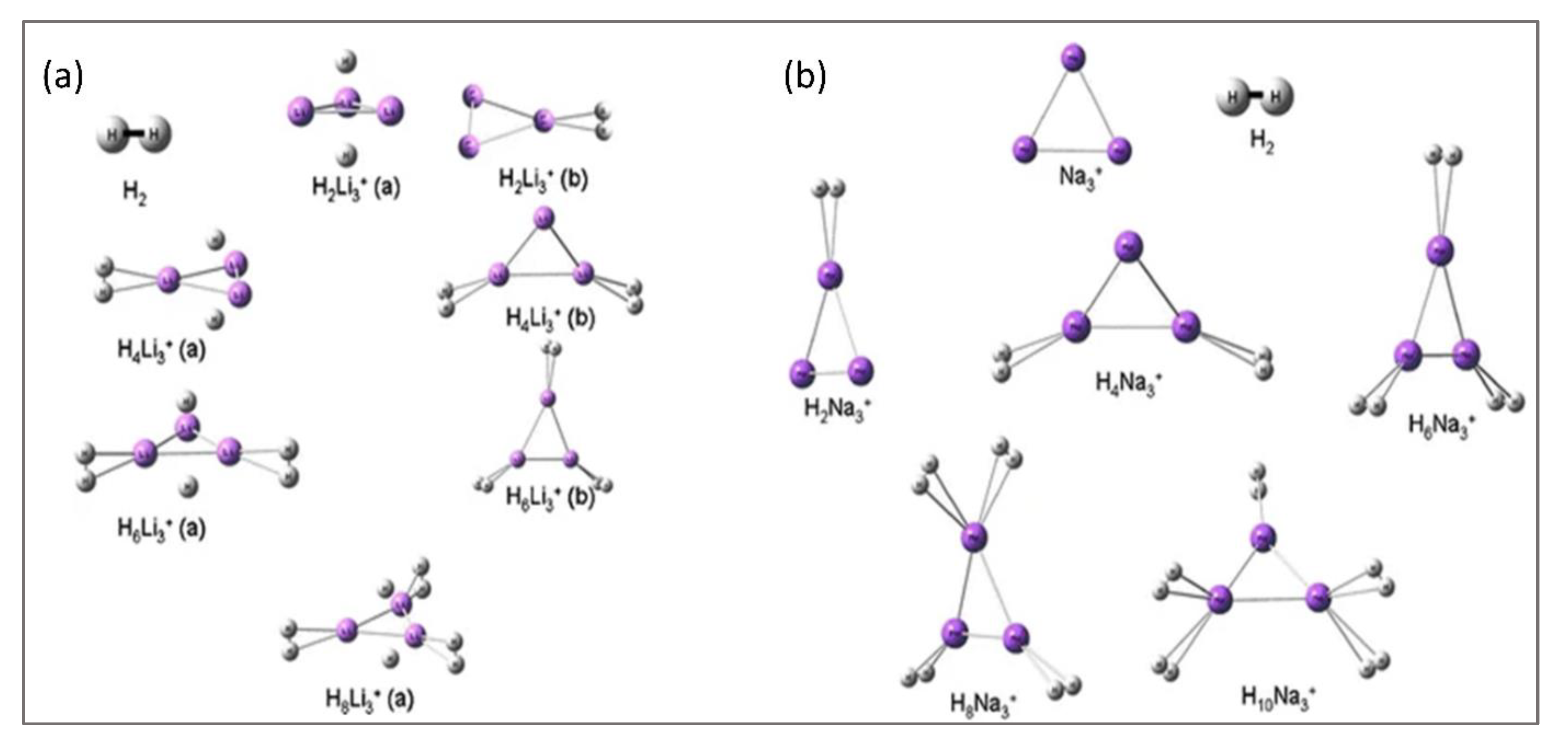
5.2. Li3+ and Na3+ Ions
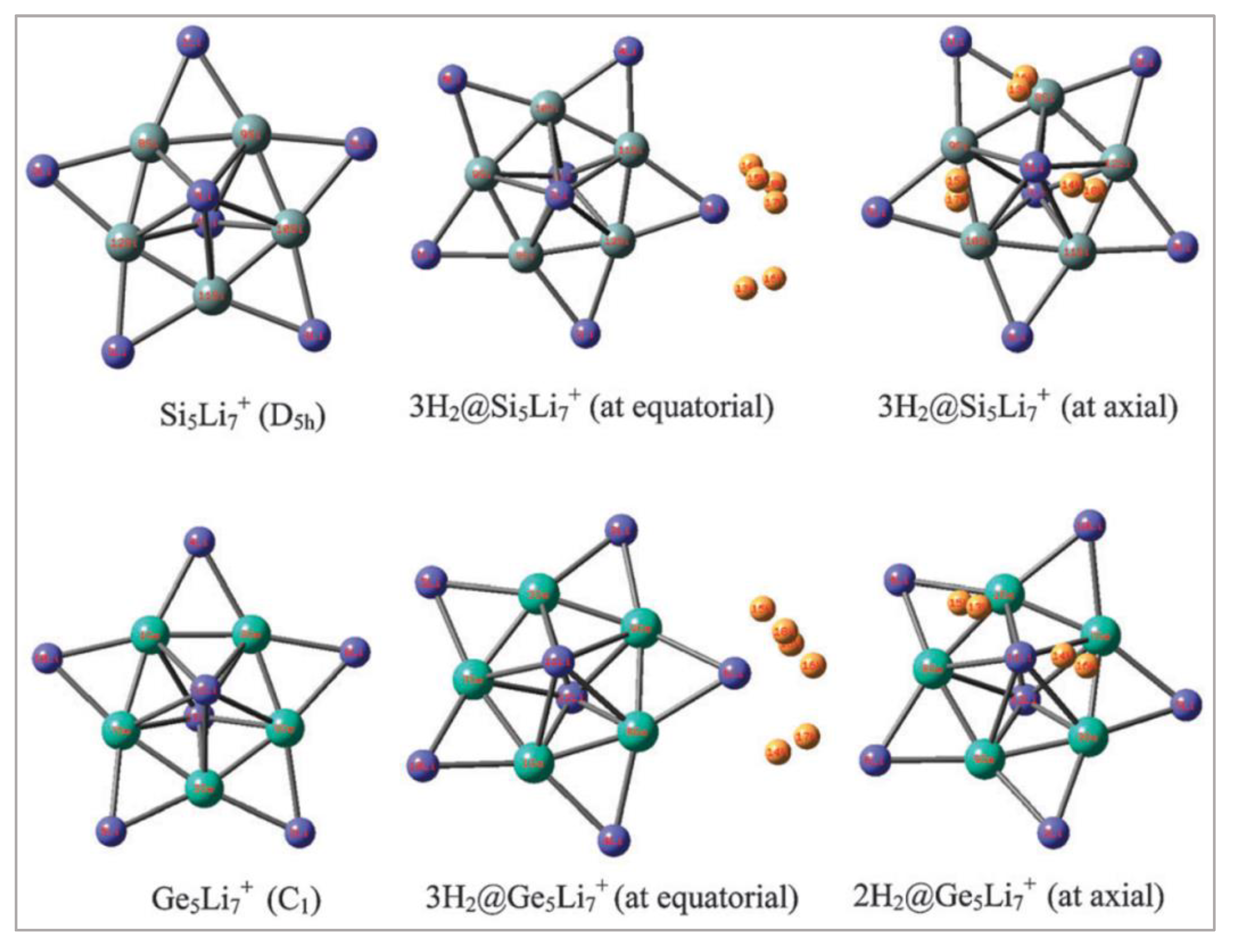
5.3. M5Li7+ (M = C, Si, Ge) Clusters
6. Cage-like Clusters
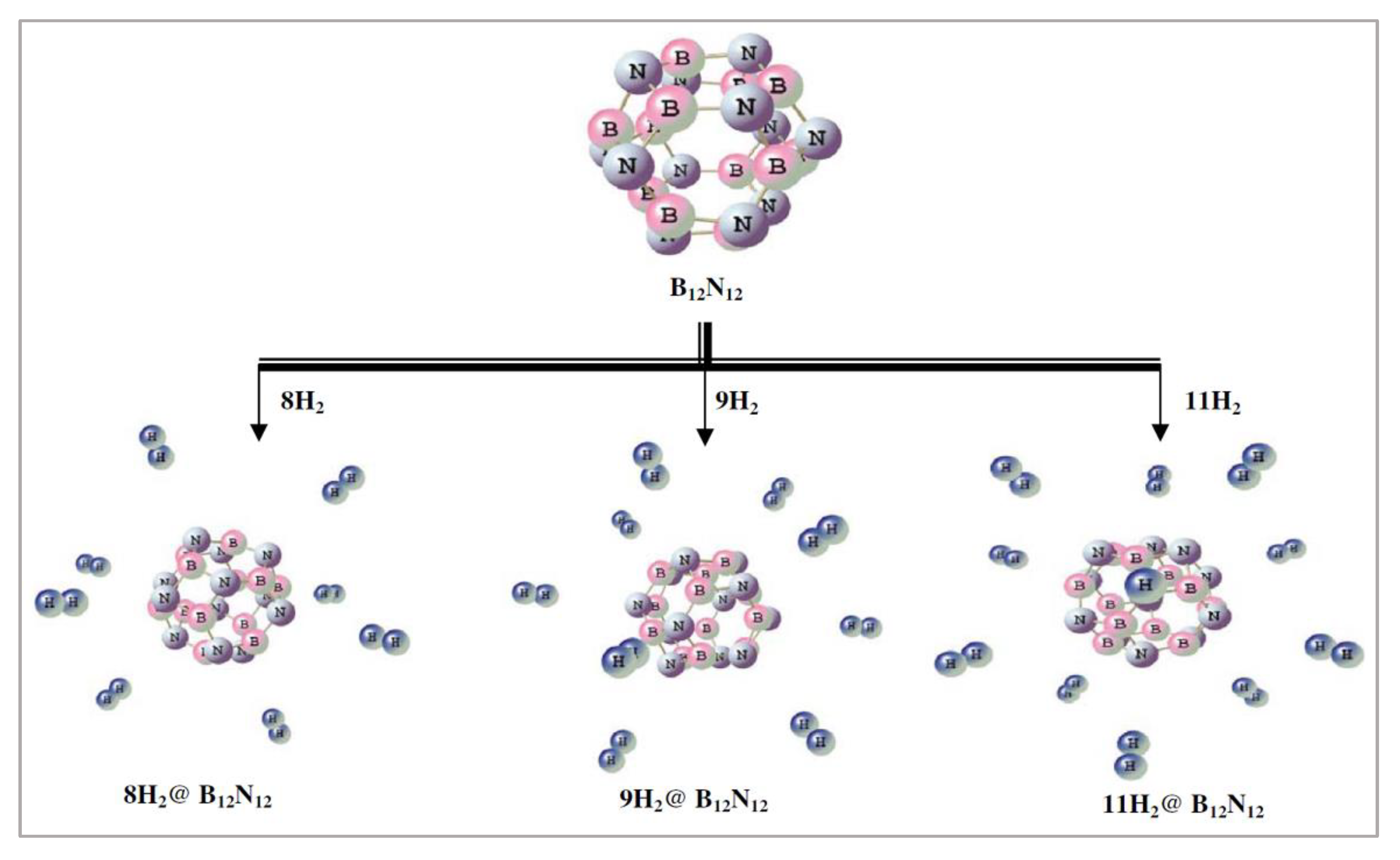
6.1. B12N12 Cage
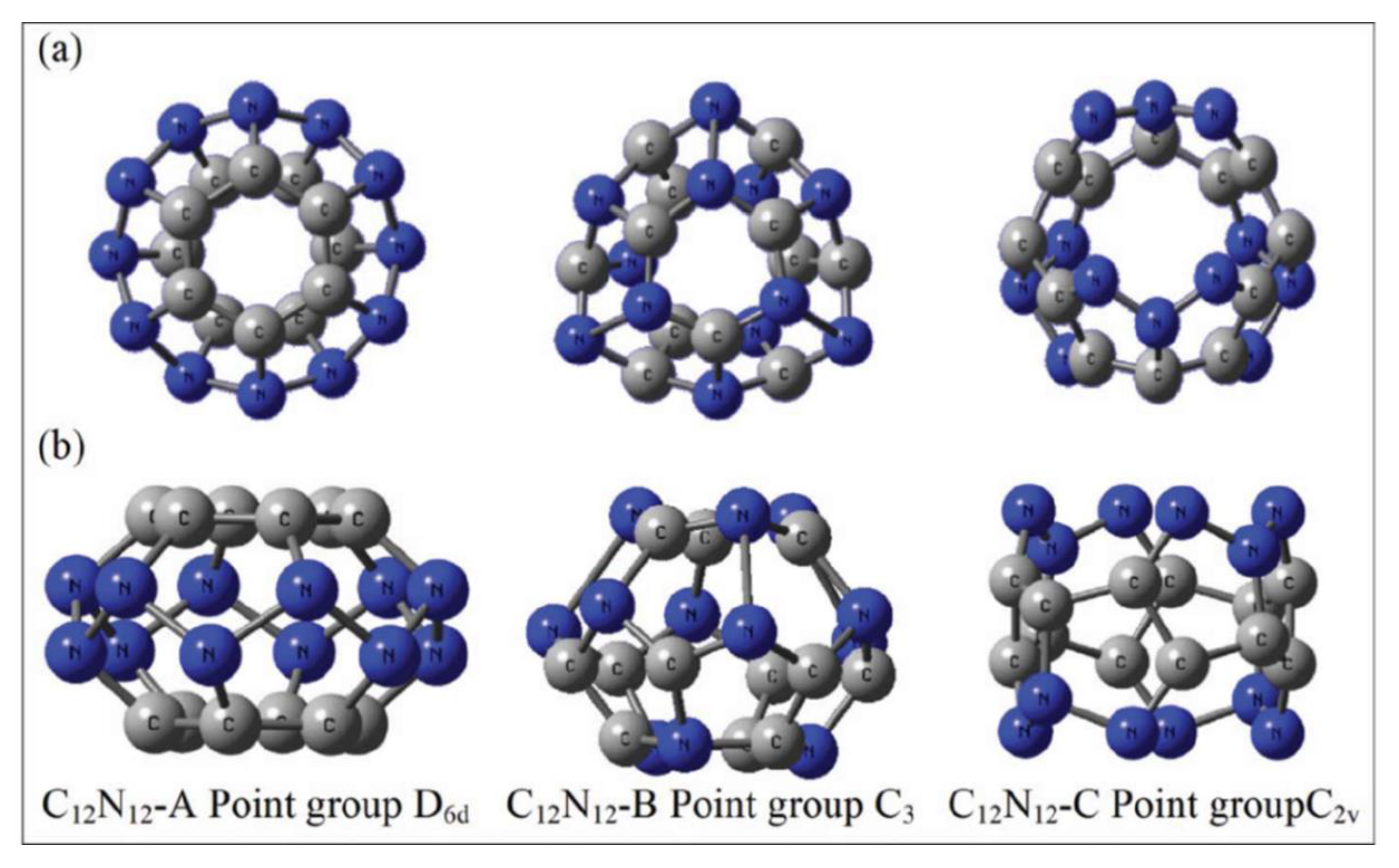
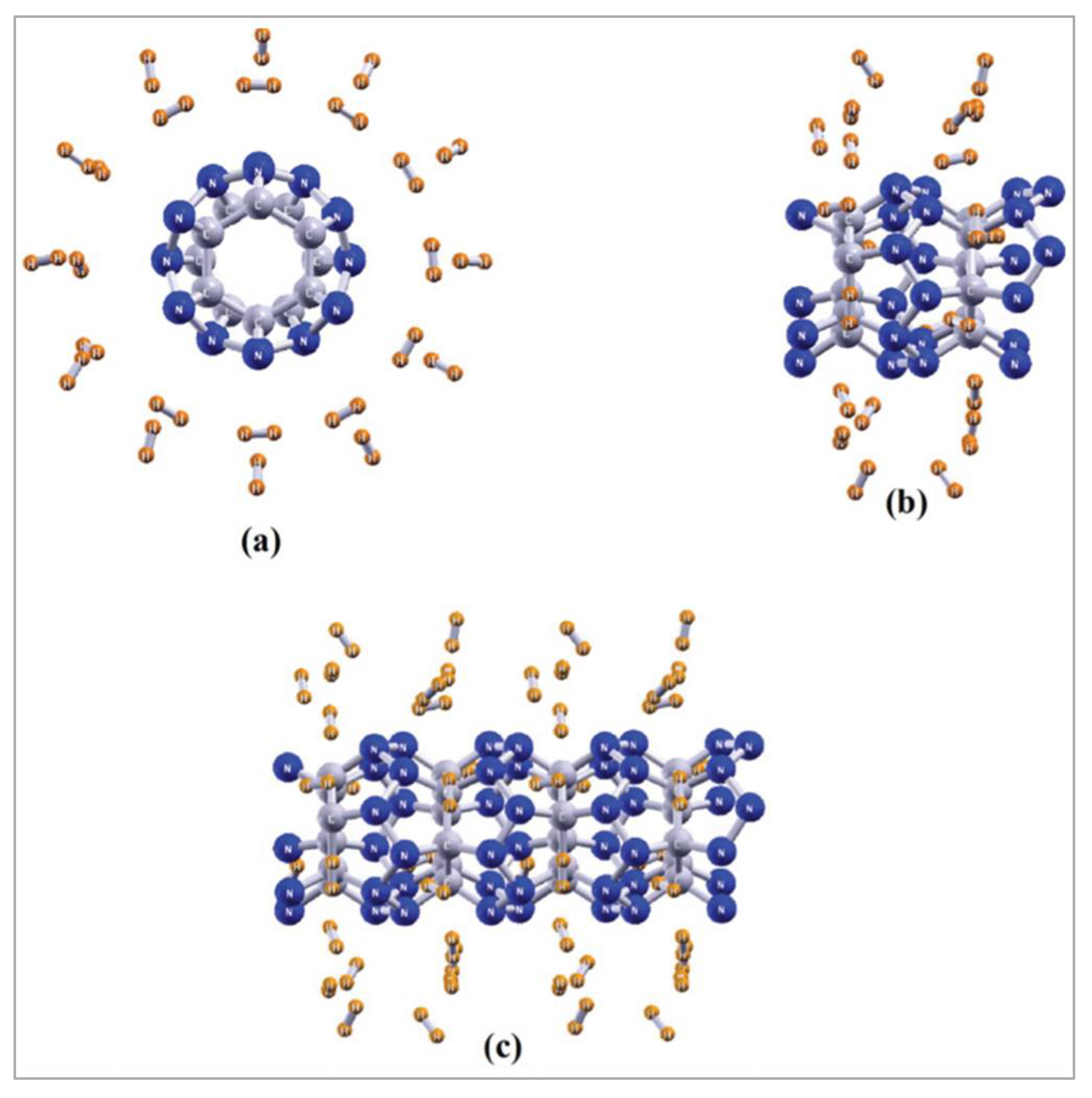
6.2. C12N12 Cage
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen storage for mobility: A review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef] [Green Version]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. IPCC 2013: Summary for Policymakers. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Walsh, B.S.; Parratt, S.R.; Hoffmann, A.A.; Atkinson, D.; Snook, R.R.; Bretman, A.; Price, T.A.R. The impact of climate change on fertility. Trends Ecol. Evol. 2019, 34, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Raymundo, R.; Asseng, S.; Robertson, R.; Petsakos, A.; Hoogenboom, G.; Quiroz, R.; Hareau, G.; Wolf, J. Climate change impact on global potato production. Eur. J. Agron. 2018, 100, 87–98. [Google Scholar] [CrossRef]
- Mazdiyasni, O.; Kouchak, A.A. Substantial increase in concurrent droughts and heatwaves in the United States. Proc. Natl. Acad. Sci. USA 2015, 112, 11484–11489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Environmental Protection Agency. Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2015. 2017. Available online: https://www.epa.gov/sites/default/files/2017-02/documents/2017_complete_report.pdf (accessed on 8 February 2023).
- BP Statistical Review of World Energy. 2018. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2018-full-report.pdf (accessed on 8 February 2023).
- Zuttel, A. Hydrogen storage methods. Naturwissenschaften 2004, 91, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Lubitz, W.; Tumas, W. Hydrogen: An overview. Chem. Rev. 2007, 107, 3900–3903. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, O.A.; Latiff, A.A.A.; Saphira, M.R.; Daud, Z.; Ismail, N.; Ahsan, A.; Aziz, N.A.A.; Al-Gheethi, A.; Kumar, V.; Fadilat, A.; et al. Principles and mechanism of adsorption for the effective treatment of palm oil mill effluent for water reuse. In Nanotechnology in Water and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–33. [Google Scholar]
- Tarasov, B.P.; Lototskii, M.V.; Yartys, V.A. Problem of hydrogen storage and prospective uses of hydrides for hydrogen accumulation. Russ. J. Gen. Chem. 2007, 77, 694–711. [Google Scholar] [CrossRef]
- Mondal, S.; Das, P.; Giri, S. Hydrogen Trapping Potential of a Few Novel Molecular Clusters and Ions. In Atomic Clusters with Unusual Structure, Bonding and Reactivity: Theoretical Approaches, Computational Assessment and Applications; Chattaraj, P.K., Pan, S., Merino, G., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Hubner, O.; Glolss, A.; Fichtner, M.; Klopper, W. On the interaction of dihydrogen with aromatic systems. J. Phys. Chem. A 2004, 108, 3019–3023. [Google Scholar] [CrossRef]
- Srinivasu, K.; Chandrakumar, K.R.S.; Ghosh, S.K. Computational investigation of hydrogen adsorption by alkali metal doped organic molecules: Role of aromaticity. Chem. Phys. Chem. 2009, 10, 427–435. [Google Scholar] [CrossRef]
- Bodrenko, I.V.; Avdeenkov, A.V.; Bessarabov, D.G.; Bibikov, A.V.; Nikolaev, A.V.; Taran, M.D.; Tkalya, E.V. Hydrogen storage in aromatic carbon ring based molecular materials decorated with alkali or alkali-earth metals. J. Phys. Chem. C 2012, 116, 25286–25292. [Google Scholar] [CrossRef]
- Giri, S.; Chakraborty, A.; Chattaraj, P.K. Potential use of some metal clusters as hydrogen storage materials—A conceptual DFT approach. J. Mol. Model. 2011, 17, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Duley, S.; Giri, S.; Sathymurthy, N.; Islas, R.; Merino, G.; Chattaraj, P.K. Aromaticity and hydrogen storage capability of planar N64− and N42− rings. Chem. Phys. Lett. 2011, 506, 315–320. [Google Scholar] [CrossRef]
- Pan, S.; Merino, G.; Chattaraj, P.K. The hydrogen trapping potential of some Li-doped star-like clusters and super-alkali systems. Phys. Chem. Chem. Phys. 2012, 14, 10345–10350. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Chakraborty, A.; Chattaraj, P.K. Stability and aromaticity of nH2@B12N12 (n = 1–12) clusters. Nano Rev. 2011, 2, 5767. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Srinivasu, K.; Ghosh, S.K.; Chattaraj, P.K. Isomers of C12N12 as potential hydrogen storage materials and the effect of the electric field therein. RSC Adv. 2013, 3, 6991–7000. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Geerlings, P.; De Proft, F.; Langenaeker, W. Conceptual density functional theory. Chem. Rev. 2003, 103, 1793–1874. [Google Scholar] [CrossRef]
- Chattaraj, P.K. Chemical Reactivity Theory: A Density Functional View; Taylor and Francis/CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Cohen, A.J.; Mori-Sanchez, P.; Yang, W. Challenges for density functional theory. Chem. Rev. 2012, 112, 289–320. [Google Scholar] [CrossRef]
- Chakraborty, D.; Chattaraj, P.K. Conceptual density functional theory based electronic structure principles. Chem. Sci. 2021, 12, 6264–6279. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Chattaraj, P.K. Stability and structural dynamics of Be32−cluster. Chem. Phys. Lett. 2014, 593, 128–131. [Google Scholar] [CrossRef]
- Chattaraj, P.K. Electronegativity and hardness: A density functional treatment. J. Indian Chem. Soc. 1992, 69, 173–183. [Google Scholar]
- Chattaraj, P.K.; Roy, D.R. Update 1 of: Electrophilicity index. Chem. Rev. 2007, 107, PR46–PR74. [Google Scholar] [CrossRef]
- Chattaraj, P.K.; Sarkar, U.; Roy, D.R. Electrophilicity index. Chem. Rev. 2006, 106, 2065–2091. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic population analysis on LCAO–MO molecular wave functions I. J. Chem. Phys. 1955, 23, 1833. [Google Scholar] [CrossRef] [Green Version]
- Parr, R.G.; Donnelly, R.A.; Levy, M.; Palke, W.E. Electronegativity: The density functional viewpoint. J. Chem. Phys. 1978, 68, 3801. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density functional approach to the frontier-electron theory of chemical reactivity. J. Am. Chem. Soc. 1984, 106, 4049–4050. [Google Scholar] [CrossRef]
- Pearson, R.G. Chemical Hardness: Applications from Molecules to Solids; Wiley-VCH: Weinheim, Germany, 1997. [Google Scholar]
- Koopmans, T.A. Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica 1933, 1, 104–113. [Google Scholar] [CrossRef]
- Yang, W.; Mortier, W.J. The use of global and local molecular parameters for the analysis of the gas-phase basicity of amines. J. Am. Chem. Soc. 1986, 108, 5708–5711. [Google Scholar] [CrossRef]
- Pearson, R.G. Recent advances in the concept of hard and soft acids and bases. J. Chem. Educ. 1999, 64, 561–567. [Google Scholar] [CrossRef]
- Pearson, R.G. The principle of maximum hardness. Acc. Chem. Res. 1993, 26, 250–255. [Google Scholar] [CrossRef]
- Parr, R.G.; Chattaraj, P.K. Principle of maximum hardness. J. Am. Chem. Soc. 1991, 113, 1854–1855. [Google Scholar] [CrossRef]
- Chamorro, E.; Chattaraj, P.K.; Fuentealba, P. Variation of the electrophilicity index along the reaction path. J. Phys. Chem. A 2003, 107, 7068–7072. [Google Scholar] [CrossRef]
- Parthasarathi, R.; Elango, M.; Subramanian, V.; Chattaraj, P.K. Variation of electrophilicity during molecular vibrations and internal rotations. Theor. Chem. Acc. 2005, 113, 257–265. [Google Scholar] [CrossRef]
- Chattaraj, P.K.; Gutierrez-Oliva, S.; Jaque, P.; Toro-Labbé, A. Towards understanding the molecular internal rotations and vibrations and chemical reactions through the profiles of reactivity and selectivity indices: An ab initio SCF and DFT study. Mol. Phys. 2003, 101, 2841–2853. [Google Scholar] [CrossRef]
- Garza, J.; Vargas, R.; Cedillo, A.; Galván, M.; Chattaraj, P.K. Comparison between the frozen core and finite differences approximations for the generalized spin-dependent global and local reactivity descriptors in small molecules. Theor. Chem. Acc. 2006, 115, 257–266. [Google Scholar] [CrossRef]
- Faraday, M.X.X. On new compounds of carbon and hydrogen, and on certain other products obtained during the decomposition of oil by heat. Phil. Trans. R. Soc. 1825, 115, 440–466. [Google Scholar] [CrossRef] [Green Version]
- Kekulè, A. Sur la constitution des substances aromatiques. Bull. Soc. Chim. 1865, 3, 98–100. [Google Scholar]
- Kekulè, A. Untersuchungen uber aromatische verbindungen. Ann. Chem. Pharm. 1866, 137, 129–197. [Google Scholar]
- Kekulè, A. Lehrbuch der Organische Chemie Band 2; Verlag Ferdinand Enke: Erlangen, Germany, 1866; pp. 493–741. [Google Scholar]
- Kekulè, A. Ueber einige condensationsproducte des aldehyds. Liebigs Ann. Chem. 1872, 162, 77–124. [Google Scholar] [CrossRef] [Green Version]
- Giambiagi, M.; De Giambiagi, M.S.; Mundim, K.C. Definition of a multicenter bond index. Struct. Chem. 1990, 1, 423–427. [Google Scholar] [CrossRef]
- Sannigrahi, A.B.; Kar, T. Three-center bond index. Chem. Phys. Lett. 1990, 173, 569–572. [Google Scholar] [CrossRef]
- Matito, E.; Solà, M.; Salvador, P.; Duran, M. Electron sharing indexes at the correlated level. Application to aromaticity calculations. Faraday Discuss. 2007, 135, 325–345. [Google Scholar] [CrossRef] [Green Version]
- Fulton, R.L.; Mixon, S.T. Comparison of covalent bond indexes and sharing indexes. J. Phys. Chem. 1993, 97, 7530–7534. [Google Scholar] [CrossRef]
- Fulton, R.L. Sharing of electrons in molecules. J. Phys. Chem. 1993, 97, 7516–7529. [Google Scholar] [CrossRef]
- Mayer, I. Charge, Bond Order, and Valence in the ab initio SCF Theory. Chem. Phys. Lett. 1983, 97, 270–274. [Google Scholar] [CrossRef]
- Mayer, I. On bond orders and valences in the Ab initio quantum chemical theory. Int. J. Quantum Chem. 1986, 29, 73–84. [Google Scholar] [CrossRef]
- Mayer, I. Bond orders and valences from ab initio wave functions. Int. J. Quantum Chem. 1986, 29, 477–483. [Google Scholar] [CrossRef]
- Mayer, I. Bond order and valence: Relations to Mulliken’s population analysis. Int. J. Quantum Chem. 1984, 26, 151–154, Addendum Int. J. Quantum Chem. 1985, 28, 419–419. [Google Scholar] [CrossRef]
- Fradera, X.; Austen, M.A.; Bader, R.F.W. The Lewis Model and Beyond. J. Phys. Chem. A 1999, 103, 304–314. [Google Scholar] [CrossRef]
- Bader, R.F.W.; Stephens, M.E. Spatial localization of the electronic pair and number distributions in molecules. J. Am. Chem. Soc. 1975, 97, 7391–7399. [Google Scholar] [CrossRef]
- Poater, J.; Fradera, X.; Duran, M.; Sola, M. An Insight into the Local Aromaticities of Polycyclic Aromatic Hydrocarbons and Fullerenes. Chem. Eur. J. 2003, 9, 1113–1122. [Google Scholar] [CrossRef]
- Matito, E.; Duran, M.; Solà, M. The aromatic fluctuation index (FLU): A new aromaticity index based on electron delocalization. J. Chem. Phys. 2005, 122, 014109, Erratum in J. Chem. Phys. 2006, 125, 059901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giambiagi, M.S.; Giambiagi, M.; Fortes, M.S. Multicenter bonds, bond valence and bond charge apportionment. J. Mol. Struct. Theochem 1997, 391, 141–150. [Google Scholar] [CrossRef]
- Giambiagi, M.; De Giambiagi, M.S.; Dos Santos Silva, C.D.; De Figuereido, A.P. Multicenter bond indices as a measure of aromaticity. Phys. Chem. Chem. Phys. 2000, 2, 3381–3392. [Google Scholar] [CrossRef]
- Bultinck, P.; Ponec, R.; Van Damme, S. Multicenter bond indices as a new measure of aromaticity in polycyclic aromatic hydrocarbons. J. Phys. Org. Chem. 2005, 18, 706–718. [Google Scholar] [CrossRef]
- Bultinck, P.; Fias, S.; Ponec, R. Local Aromaticity in Polycyclic Aromatic Hydrocarbons: Electron Delocalization versus Magnetic Indices. Chem.-Eur. J. 2006, 12, 8813–8818. [Google Scholar] [CrossRef]
- Bultinck, P.; Ponec, R.; Carbo-Dorca, R. Aromaticity in linear polyacenes: Generalized population analysis and molecular quantum similarity approach. J. Comput. Chem. 2007, 28, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Bultinck, P.; Rafat, M.; Ponec, R.; Gheluwe, B.V.; Carbo-Dorca, R.; Popelier, P.J. Electron Delocalization and Aromaticity in Linear Polyacenes: Atoms in Molecules Multicenter Delocalization Index. J. Phys. Chem. A 2006, 110, 7642–7648. [Google Scholar] [CrossRef]
- Ponec, R.; Bultinck, P.; Saliner, A.G. Multicenter Bond Indices as a New Means for the Quantitative Characterization of Homoaromaticity. J. Phys. Chem. A 2005, 109, 6606–6609. [Google Scholar] [CrossRef] [PubMed]
- Chattaraj, P.K.; Roy, D.R.; Elango, M.; Subramanian, V. Chemical reactivity descriptor based aromaticity indices applied to Al42− and Al44− systems. J. Mol. Struct. Theochem 2006, 759, 109–110. [Google Scholar] [CrossRef]
- Roy, D.R.; Bultinck, P.; Subramanian, V.; Chattaraj, P.K. Bonding, reactivity and aromaticity in the light of the multicenter indices. J. Mol. Struct. Theochem 2008, 854, 35–39. [Google Scholar] [CrossRef]
- Kruszewski, J.; Krygowski, T.M. Definition of aromaticity basing on the harmonic oscillator model. Tetrahedron Lett. 1972, 36, 3839–3842. [Google Scholar] [CrossRef]
- Krygowski, T.M. Crystallographic studies of inter- and intramolecular interactions reflected in aromatic character of π-electron systems. J. Chem. Inf. Comput. Sci. 1993, 33, 70–78. [Google Scholar] [CrossRef]
- Krygowski, T.M.; Cyranski, M. Structural aspects of aromaticity. Chem. Rev. 2001, 101, 1385–1420. [Google Scholar] [CrossRef] [PubMed]
- Schleyer, P.v.R.; Maerker, C.; Dransfeld, A.; Jiao, H.; Hommes, N.J.R.v.E. Nucleus-independent chemical shifts: A simple and efficient aromaticity probe. J. Am. Chem. Soc. 1996, 118, 6317–6318. [Google Scholar] [CrossRef]
- Hirsch, A.; Chen, Z.; Jiao, H. Spherical aromaticity in Ih symmetrical fullerenes: The 2(N + 1)2 rule. Angew. Chem. Int. Ed. 2000, 39, 3915–3917. [Google Scholar] [CrossRef]
- Poater, J.; Solà, M. Open-shell spherical aromaticity: The 2N2 + 2N + 1 (with S = N + ½) rule. Chem. Commun. 2011, 47, 11647–11649. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 3.0, and 5.0.8; Semichem, Inc.: Shawnee, KS, USA, 2009. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.; Vreven, T., Jr.; Kudin, K.N.; Burant, J.C.N.; et al. Gaussian 03, Revision B.03; Gaussian, Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Schlegel, H.B.; Scalmani, G.; Barone, V.; Mennucci, B.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251. [Google Scholar] [CrossRef] [PubMed]
- Kokalj, A. Computer graphics and graphical user interfaces as tools in simulations of matter at the atomic scale. Comp. Mater. Sci. 2003, 28, 155–168. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Q.; Sun, Q.; Jena, P.; Chen, X.S. Electric field enhanced hydrogen storage on polarizable materials substrates. Proc. Natl. Acad. Sci. USA 2010, 107, 2801–2806. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Hwang, J.-Y.; Shi, S. Hydrogen storage in mesoporous metal oxides with catalyst and external electric field. J. Phys. Chem. C 2010, 114, 7178–7184. [Google Scholar] [CrossRef]
- Vogler, A.; Wright, R.E.; Kunkely, H. Photochemical reductive cis-elimination in cis-diazidobis(triphenylphosphane)platinum(II) evidence of the formation of bis(triphenylphosphane)platinum(0) and hexaazabenzene. Angew. Chem. Ind. Ed. Engl. 1980, 19, 717–718. [Google Scholar] [CrossRef]
- Chung, G.; Schmidt, M.W.; Gordon, M.S. An ab Initio study of potential energy surfaces for N8 isomers. J. Phys. Chem. A 2000, 104, 5647–5650. [Google Scholar] [CrossRef] [Green Version]
- Engelke, R. Ab initio correlated calculations of six nitrogen (N6) isomers. J. Phys. Chem. 1992, 96, 10789–10792. [Google Scholar] [CrossRef]
- Ha, T.-K.; Nguyen, M.T. The identity of the six nitrogen atoms (N6) species. Chem. Phys. Lett. 1992, 195, 179–183. [Google Scholar] [CrossRef]
- Strout, D.L. Acyclic N10 fails as a high energy density material. J. Phys. Chem. A 2002, 106, 816–818. [Google Scholar] [CrossRef]
- Perez-Peralta, N.; Contreras, M.; Tiznado, W.; Stewart, J.K.; Donald, J.; Merino, G. Stabilizing carbon-lithium stars. Phys. Chem. Chem. Phys. 2011, 13, 12975–12980. [Google Scholar] [CrossRef] [PubMed]
- Tiznado, W.; Perez-Peralta, N.; Islas, R.; Toro-Labbe, A.; Ugalde, J.M.; Merino, G. Designing 3-D Molecular Stars. J. Am. Chem. Soc. 2009, 131, 9426–9431. [Google Scholar] [CrossRef]
- Kuhn, A.; Sreeraj, P.; Pöttgen, R.; Wiemhöfer, H.-D.; Wilkening, M.; Heitjans, P. Li NMR Spectroscopy on Crystalline Li12Si7: Experimental Evidence for the Aromaticity of the Planar Cyclopentadienyl-Analogous Si56− Rings. Angew. Chem. Int. Ed. 2011, 50, 12099–12102. [Google Scholar] [CrossRef]
- Jena, N.K.; Srinivasu, K.; Ghosh, S.K. Computational investigation of hydrogen adsorption in silicon-lithium binary clusters. J. Chem. Sci. 2012, 124, 255–260. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, Q.; Jena, P. Storage of molecular hydrogen in B-N cage: Energetics and thermal stability. Nano Lett. 2005, 5, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.-H.; Deng, W.-Q.; Han, K.-L. Endohedral BN metallofullerene M@B36N36 complex as promising hydrogen storage materials. J. Phys. Chem. C 2008, 112, 12195–12200. [Google Scholar] [CrossRef]
- Cui, X.-Y.; Yang, B.-S.; Wu, H.-S. Ab initio investigation of hydrogenation of (BN)16: A comparison with that of (BN)12. J. Mol. Struct. (Theochem) 2010, 941, 144–149. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondal, S.; Chattaraj, P.K. Aromatic Clusters and Hydrogen Storage. Energies 2023, 16, 2833. https://doi.org/10.3390/en16062833
Mondal S, Chattaraj PK. Aromatic Clusters and Hydrogen Storage. Energies. 2023; 16(6):2833. https://doi.org/10.3390/en16062833
Chicago/Turabian StyleMondal, Sukanta, and Pratim Kumar Chattaraj. 2023. "Aromatic Clusters and Hydrogen Storage" Energies 16, no. 6: 2833. https://doi.org/10.3390/en16062833





