Abstract
The principle of sustainable development imposes an obligation on societies to manage natural resources rationally and to care for the quality of the environment, by, among other things, reducing CO2 emissions. Alternative ways of stabilising building substrates by increasing their shear strength (cu) are increasingly being sought. This paper presents how microorganisms can influence cu and thus the load-bearing capacity of building substrates. Tests were performed in a triaxial compression apparatus in three variants. The first variant of testing was carried out on cemented soil samples, which were cemented in situ. The next two series of tests were performed on reconstructed samples, i.e., natural soil and soil inoculated with a solution of Sporosarcina pasteurii bacteria. The results obtained show that carbonate cementation increases the shear strength of the soil; in addition, this biomineralization-induced cementation gives higher cu results than natural carbonate cementation.
1. Introduction
The development of an economy based on knowledge and innovation has been observed in Europe for several years. It goes hand in hand with reinforcing the requirements for environmental protection to primarily promote eco-innovation. At the same time, we are witness to a substantial reduction in the consumption of natural resources through a change in the energy and material consumption of products and an increase in the recirculation of raw materials and purchased goods.
The production of the most important building material of the 20th century, Portland cement, is associated with significant environmental pollution and energy issues since its production takes place at very high temperatures of 1400–1500 °C [1]. Significant amounts of carbon dioxide and highly toxic nitric oxide are emitted into the atmosphere during the production process [2,3]. Hence, alternative solutions that would lead to the reduction in the use of Portland cement and mineral resources, which are becoming increasingly scarce, are being searched for [4,5,6].
In the meantime, the development of the civilization makes it necessary to erect various buildings or communication routes in areas with complex geotechnical conditions, including land with insufficient load-bearing capacity. This requires the use of appropriate technologies to strengthen the subsoil with low bearing capacity. It is very difficult to adopt a simple, unambiguous classification of soil reinforcement methods. As a classification criterion, the following may be used: reinforcement technology, materials used, depth of interference in the subsoil or the final reinforcement effect. A number of techniques (technologies) for surface and deep stabilization are based on subsoil stabilization by, e.g., introducing hydraulic binders or a mixture of hydraulic binders and aggregate in order to improve subsoil parameters [7,8,9,10,11,12]. The most commonly used hydraulic binders are cement, fly ash and lime. These methods are very effective, but unfriendly to the environment. For stabilization, substances containing acrylamides, lignosulfonates or polyurethane are used, although they are toxic compounds with an adverse impact on the environment [13]. In addition to soil and groundwater pollution, undesirable effects of chemical stabilization also include excessive reduction in groundwater permeability, increase in the pH value of groundwater and disturbance of the ecosystem [14].
Worldwide cement production accounts for about 5–7% of global anthropogenic emissions of CO2 [3,14,15]. In addition to ecological aspects, disadvantages of methods based on the use of hydraulic binders are their cost and the need to combine surface and deep stabilization, which additionally generates higher investment costs.
Several methods are available on the market as alternatives to traditional hydraulic binders, e.g., electro-osmotic soil reinforcement [16,17] or the increasing development of inorganic polymer (geopolymer) technologies [15,18,19,20]. It is estimated that the synthesis of geopolymers is a much less energy-intensive process than the production of Portland cement and causes the emission of four to eight times less carbon dioxide [15,21]. Another example of subsoil reinforcement is the use of low-pressure injection of silica solution to stabilize liquefiable soils [22,23].
At the same time, the awareness of environmental care is constantly growing around the world, hence the increase in the trend of searching for substitutes for common stabilization methods to limit their impact on the environment.
Carbonate cementation, caused by bacteria of the Bacillus strain [24], may become an alternative to traditional cementation. This type of biocementation can be an alternative to surface stabilization of the subsoil by improving its mechanical properties [25,26,27,28].
Due to the fact that an intensive civilization development increasingly causes the need to build engineering structures in areas with transitional soils, which in some conditions are low-bearing soils, our research was carried out on silty soils [29]. In addition, the selection of soils is confirmed by the observations of [26] regarding the better effect of biomineralization in fine-grained soils. The main aim of current investigation was to prove the significant role of natural or artificially induced biocementation, as an environment-friendly technology for soil improvement. In order to verify the impact of carbonate cementation on improving soil shear strength, the tests were carried out in a triaxial compression apparatus on three types of soil samples. All samples were characterized by a similar granulometric composition and condition. In order to highlight the role of bacteria in improving the mechanical properties of soils, the research was carried out in three research series. The first series was conducted on samples of naturally cemented soil, P-I [30]. The second series of tests were carried out on natural model samples, i.e., not cemented (P-II), and the third one on reconstructed samples prepared from soil inoculated with Sporosarcina pasteurii (P-III). Finally, the results are discussed within the context of designing shallow foundations.
2. Existing Research Findings—Searching for Alternatives and Pro-Ecological Methods for Soil Stabilization
Calcium carbonate (CaCO3) is one of the most widespread chemical compounds—it makes up about 4% of the composition of the Earth’s crust, forming geological and oceanic sediments and biominerals [31,32,33]. It is an essential component of many rocks, such as limestone, chalk, marble, dolomite and travertine, as well as being found in bones and teeth, the external skeletons of crustaceans, snail shells, corals, pearls and shells.
Calcium carbonate is of great practical importance—for centuries it has been used as a building material. Today it is used in a variety of industrial and agricultural applications, as well as in medicine and cosmetology, and in a newly developing field—biomineralization for engineering purposes [31,32,33,34,35].
Precipitation of calcium carbonate in nature occurs chemically due to changes in environmental parameters, but a significant part of it is formed biogenically during biomineralization with the participation of living organisms. Calcium carbonate can occur in two varieties, as calcite or aragonite. These are minerals with identical chemical composition, but their different spatial structure causes them to have different physical and chemical properties. Other examples of anaerobic oxidation of methane in organic-rich ecosystems and the intense production rates of calcite, aragonite and siderite were presented by Valenzuela et al. and Deb et al. [35,36].
The most common research for engineering applications focuses on simple, fast and efficient methods—such as a method based on the reaction of urea hydrolysis (ureolysis), which uses, bacteria that exhibit ureolytic activity. Examples of such bacteria are Bacillus bacteria.
Bacteria of the Bacillus strain that were used in the present study are widespread in nature. They are isolated from soil, or fresh and salt water, but also from the digestive tract of animals or perishable food. The Bacillus strain contains Gram-positive bacteria with the ability to form highly resistant spore forms—endospores. These forms allow bacteria to survive in unfavourable environmental conditions, often at extreme temperatures, and in environments deficient in nutrients and water. In addition, they spread in nature, even over long distances. The Bacillus strain is characterized by high growth rates and an efficient system allowing for the synthesis of up to 20 to 25 g·L−1 of products [37]. Moreover, most of them are species safe for humans and animals (they have the status of ‘generally recognized as safe’).
So far, scientists have undertaken research on the use of Bacillus bacteria in construction as a binder for sealing structural cracks to improve the endurance of structures, including the phenomenon of so-called ‘self-healing’ of cement composites, or increasing the compressive strength of concrete [38].
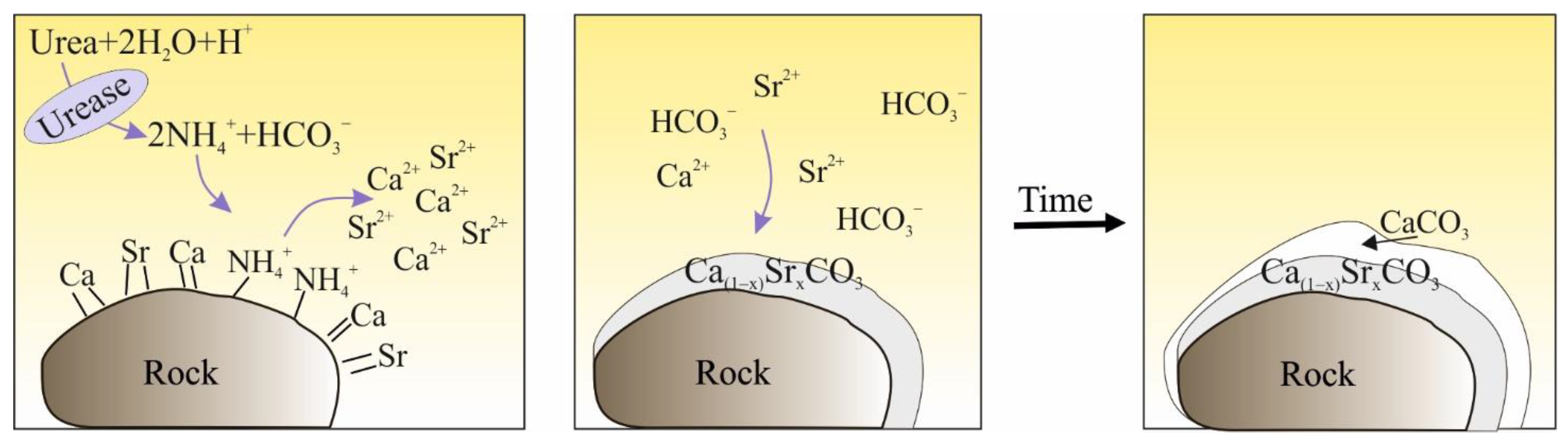
The possibility of using natural microbiological processes to create a cement matrix of the reinforced subsoil attracts attention of many researchers [25,27,39,40]. Biostabilization is a method focusing on microbiologically induced precipitation of calcium carbonate using urea hydrolysis [41,42,43,44,45,46,47,48,49]. This process takes place thanks to the presence of strains of urolithic bacteria, such as Sporosarcina pasteurii (S. pasteurii), which have the ability to precipitate calcium carbonate on their external cell walls [25,46,50]. This is due to the urease enzyme, which leads to the hydrolysis of urea contained in the medium [51]. After the formation of carbonate ‘battleships’, the bacteria die, while calcium carbonate remains, creating carbonate connections between grains [52] (Figure 1), thus strengthening the material.

Figure 1.
Carbonate joints between sand grains [53].
The above microbiological process of obtaining calcite precipitation is often described using the abbreviation MICP (microbially induced calcite precipitation) [53]. Chemically, the process is as follows (1)–(2):
The bacterium Sporosarcina pasteurii (S. pasteurii) attracts Ca2+ ions, which guarantee the proper function of the bacterial life processes (1). The amount of ions that will not be used in metabolic processes precipitates on the outer surface of the cell wall. After the addition of a medium containing, among others, urea, its hydrolysis takes place—urea is hydrolysed to ammonium and carbon dioxide (assuming the ionic forms and (2)) [54]. The reaction of calcium ions deposited on the cell wall (Ca2+) with the ion leads to the precipitation of CaCO3 [46,52,55], and thus to the cementation of the soil (3). At the same time, ammonium ions (NH4+) increase the pH value of the environment, improving the precipitation efficiency (Figure 2) [14,56].
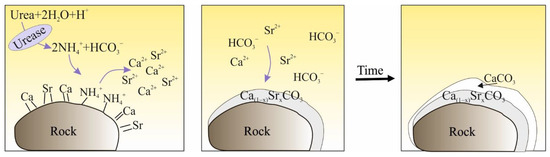
Figure 2.
An outline of the precipitation of carbonate coatings [57].
Biocementation is a process occurring naturally in the environment and is associated with the deposition of sandy formations. Examples of the occurrence of the biocementation process can be found, among others, in the Pinnacles desert in Western Australia and in the vicinity of East Cliff in England [53]. The naturalness of this phenomenon is the most important reason why scientists are so interested in using the metabolic processes of bacteria. Thanks to bacteria, we can obtain a natural binder in an environmentally friendly way.
Dhami et al. and Burbank et al. [25,46] presented the results of research in which they used natural microbiological processes to cement loose sands. Microbiologically, precipitation of calcite (MICP) was achieved using Bacillus pasteurii microorganisms, aerobic bacteria ubiquitous in the environment, including in soil. The test results of MICP-cemented samples and gypsum-cemented samples were evaluated in a non-destructive manner by measuring the shear wave velocity using bender elements, and then determining their strength parameters using isotropically consolidated undrained compression (CIUC) triaxial tests. A series of triaxial tests (CIUC) indicated that MICP-treated specimen exhibit behaviour similar to that of gypsum cement specimen, which represent typical cement sand behaviour. Studies using SEM confirmed the formation of a cement matrix in the form of a calcite forming a bond where particles meet. In turn, X-ray composition mapping confirmed that the observed cement bonds consist of calcite.
The mentioned bacteria were successfully used to repair the cracked surface of soil [58] or to reduce the swelling pressure of clay [59]. Mujah et al. and Canakci et al. [52,60] showed that the production of carbonate joints between grains of biostabilized soil reduces its porosity, which results in much lower hydraulic permeability. Libudzisz et al. [54] also noticed a decrease in the porosity of non-cohesive soil in their research, and the porosity values at the maximum content of CaCO3 were reduced by up to 90% compared to natural soil.
In their research on the possibility of using the biostabilization method in the reinforcement of silty soils, they noted that this process increases stiffness and shear strength [27]. Thanks to the conducted research, it was also noted that the Sporosarcina pasteurii strain has a high resistance to dynamic incidents, which often appear, for example, on construction sites of earthworks.
Harianto et al. [61] focused on shear strength and consolidation of sandy soil inoculated with bacteria in their research. Thanks to biostabilization, they obtained hardened sand blocks and the tested shear strength increased in relation to the strength of natural soil. The measurement of consolidation of samples showed that in the stress range of 10–50 kPa, there was a clear difference between the values of the porosity index of soil with bacteria and natural soil. In the case of the test sample, the porosity index value decreased from 2.2 to 2.0, while in samples with bacteria this value decreased from 2.5 to 2.1. Van Paassen and Biogrout [62] investigated the impact of biostabilization on cohesive soil, namely sandy clay. Based on the results, they reported that under the influence of bacteria, there was also an increase in the strength of the soil and a decrease in its permeability resulting in an increase in its load-bearing capacity. Cheng and Cord-Ruwisch, as well as Robertson [63,64] concluded that there is a significant increase in the strength of the treated soils.
Another study [56] focused on determining the optimal conditions for the biocementation process in soil. For testing, they used silty clay inoculated with bacteria, which they tested for shear strength at various temperatures. Based on the obtained results, they reported that the compressive strength increased systematically with the increase in soil pH, and the highest value was obtained at 40 °C.
3. Materials and Methods
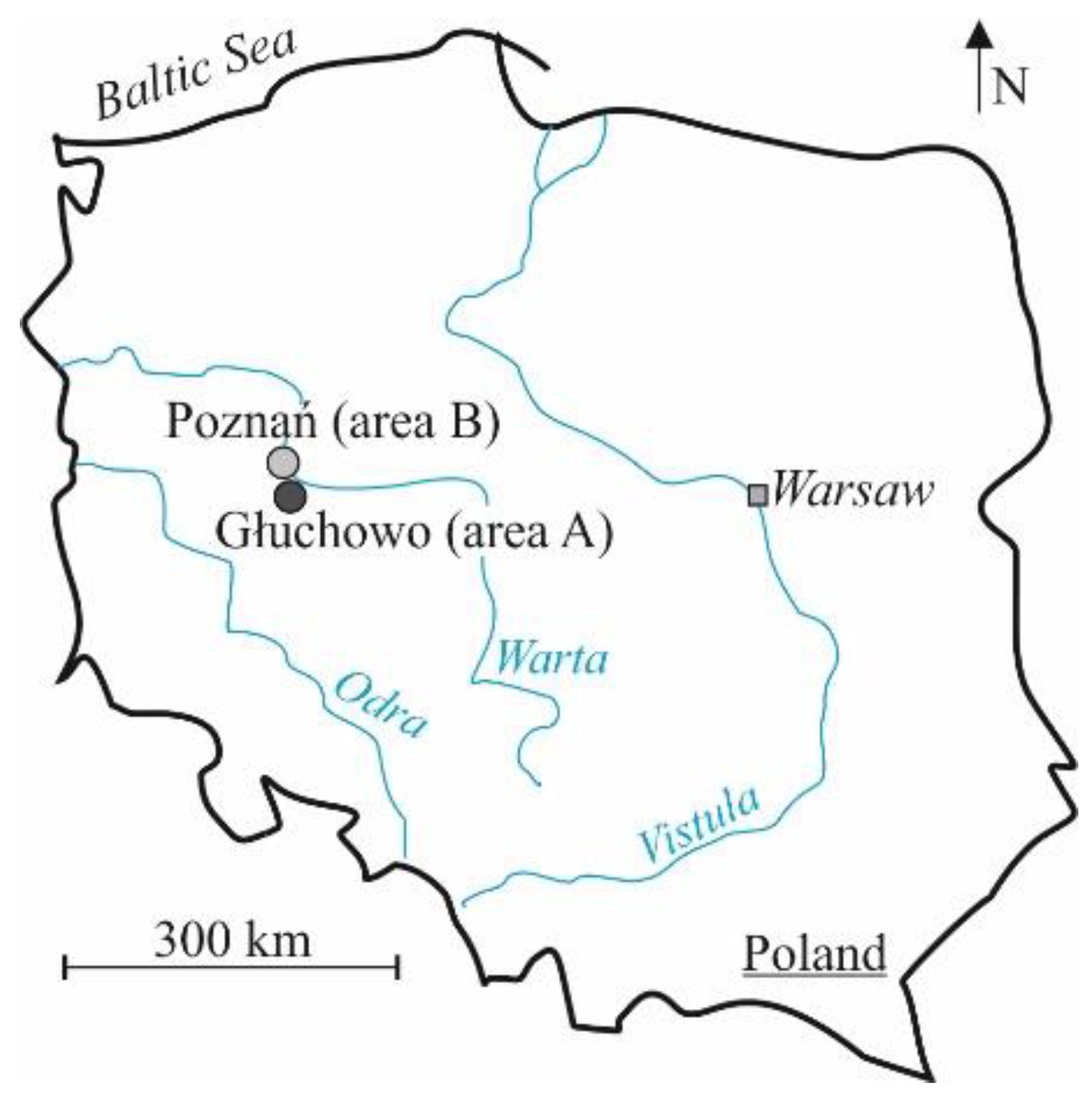
The tests were carried out on soils located in two research sites. The first area is located in the area of Głuchów (area A), about 15 km south-west of the centre of Poznań, and the second research site is located in the south-eastern part of Poznań (area B) (Figure 3).
Figure 3.
Location of research sites.
The main purpose of the conducted research was to compare the shear strength cu of undrained soil, i.e., natural silty soils (area B), and of cemented silty soils (area A) with silty soils subjected to biocement (soils from area B + Sporosarcina pasteurii). The research work was carried out in four stages. In the first stage, cone penetration tests (CPTU) were carried out in area A, which were used to assess the effect of preconsolidation [65,66,67,68] and, above all, to evaluate the shear strength of the soil [69].
The undrained shear strength from the CPTU test was determined from formula 3. On the basis of other research [70], a coefficient of Nkt = 15.5 for the zone of cemented soils and Nkt in range of 7 to 10, in the zone without cementation was adopted for the calculations.
where:
- qn—net cone resistance,
- Nkt—empirical coefficient, dependent on soil characteristics.
Using a Mostap sampler, samples with intact structure were taken for testing in a triaxial compression apparatus. In addition, samples with natural moisture content were taken from area A and B, and were used to determine physical properties and make reconstructed samples.
In the second stage, tests were carried out in a Proctor apparatus [71] to determine the optimum moisture content (wopt), i.e., the moisture content at which a given soil can be most compacted. Based on the results obtained, a series of model samples were prepared. The tests in the Proctor apparatus constituted the reference test for making model samples.
The third stage of the research involved the preparation of model samples, i.e., natural soil samples (P-II) and samples inoculated with bacteria Sporosarcina pasteurii (P-III). For this purpose, the cultivation of the Sporosarcina pasteurii bacteria was performed on liquid nutrient broth with shaking. The culture was enriched with calcium cations (Ca2+) in the form of CaCl2·2H2O [72]. The solution with bacteria was added in small amounts, spraying the soil, and then thoroughly mixed. As a result, a material with a moisture content similar to the optimal one was obtained [27,30], from which samples in the shape of a cylinder with a height of 80 mm and a diameter of 38 mm were formed. The formation of model samples without bacteria was carried out in a similar way (by adding water instead of a suspension of bacteria), i.e., they were compacted mechanically at a humidity close to optimal with a compacting device.
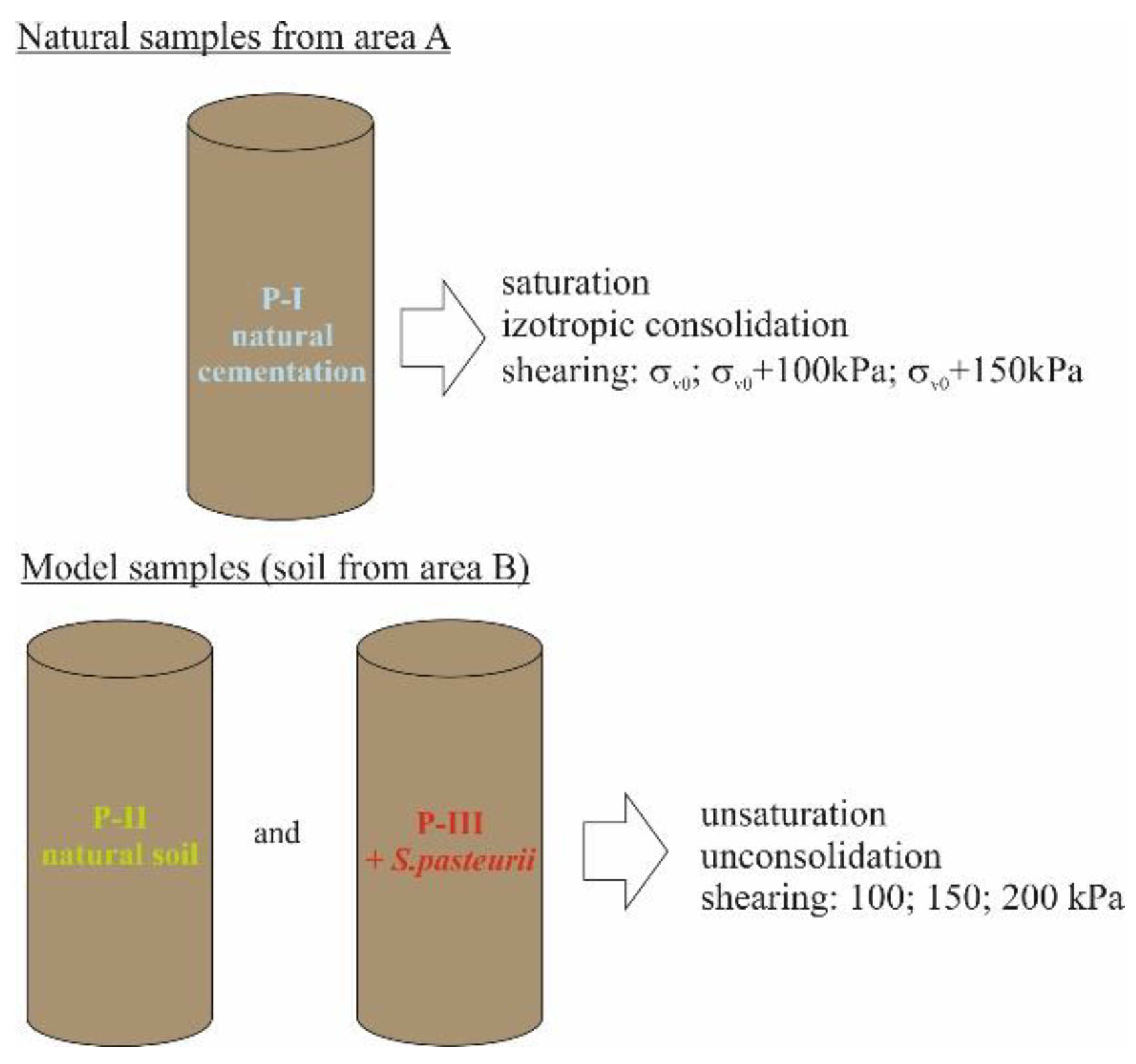
The final stage of the research aimed at verifying the changes in shear strength caused by carbonate cementation. The tests were carried out in a triaxial compression apparatus (TXT) [73,74] in three test series: on natural samples from area A (P-I), in which the occurrence of natural carbonate cementation was found [30,75], and on model samples formed via a special compaction of soils (LSC) (area B: P-II and P-III). The model samples consisted of natural soil from area B (P-II) and of the same soil inoculated with Sporosarcina pasteurii (P-III). A schematic of the test procedure in the TXT apparatus is shown in Figure 4.
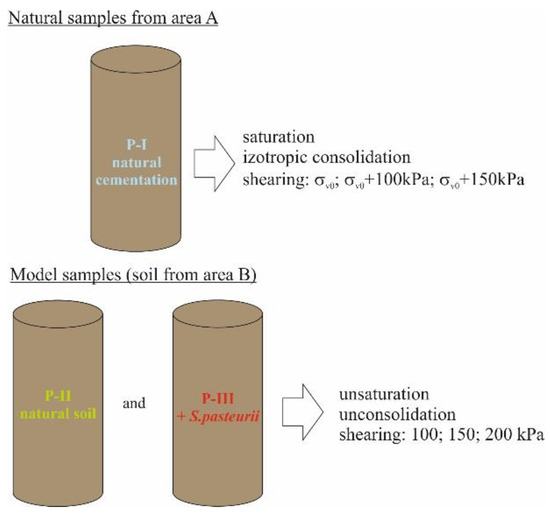
Figure 4.
Procedure adopted for triaxial testing.
4. Test Results
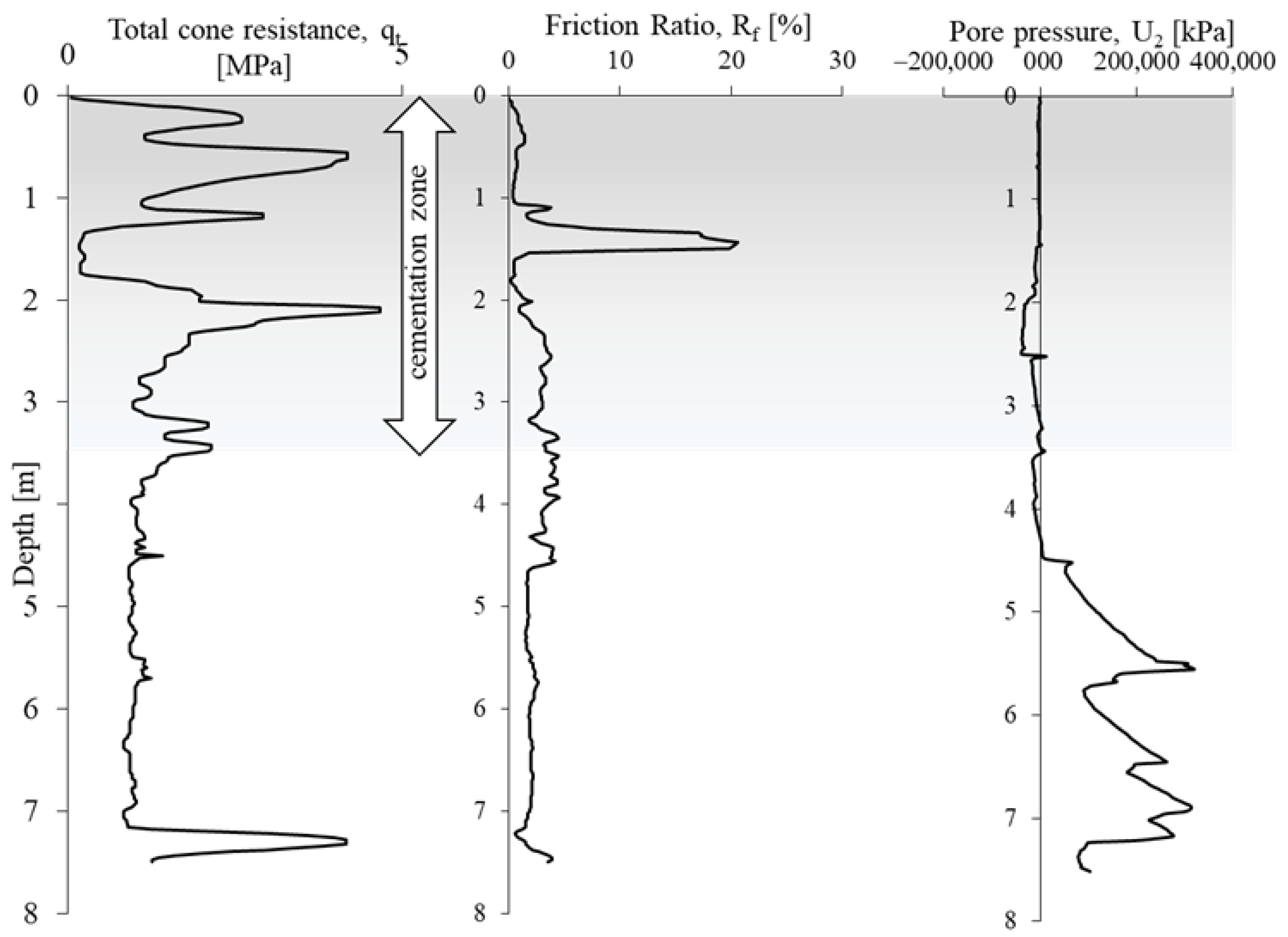
The obtained CPTU results (Figure 5) were used to evaluate the undrained shear strength cu [69] and identify the cementation zone of the tested soils from area A [65,66,67,68].
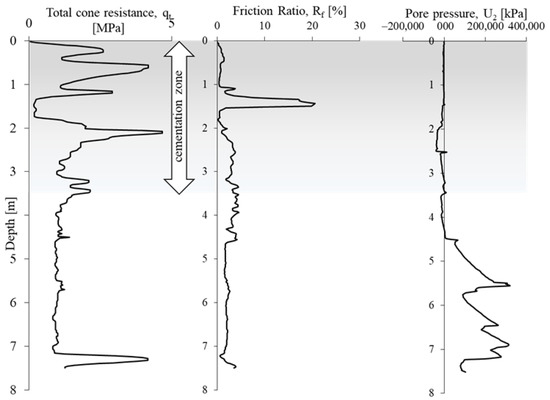
Figure 5.
Examples of in situ tests on the researched area A.
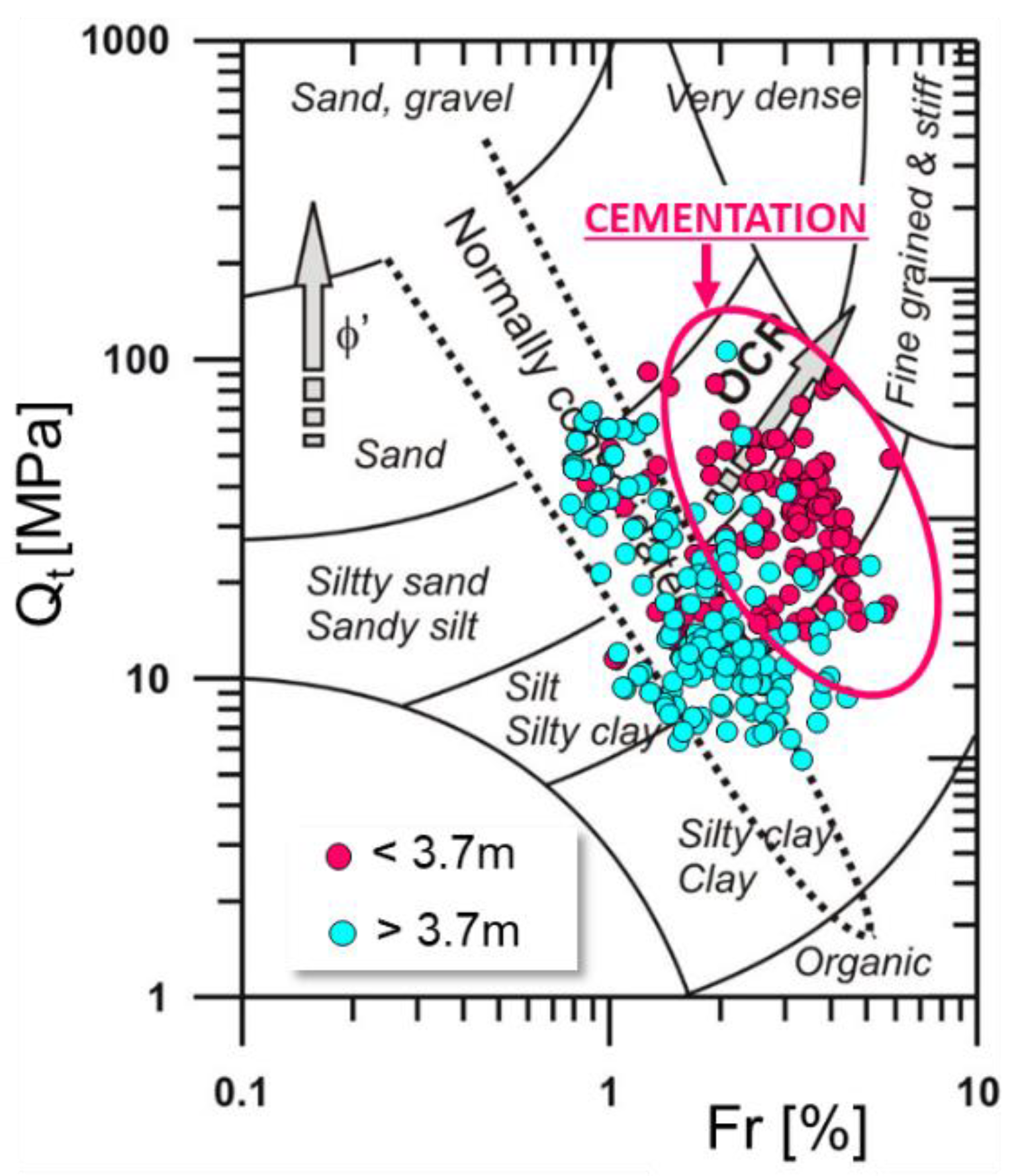
The obtained CPTU test results (Figure 5) were plotted on Robertson’s [65] classification system (Figure 6). The studied sediments are located in the group of silty soils. A variation in the degree of preconsolidation was observed in the studied soils (area A) (Figure 6).
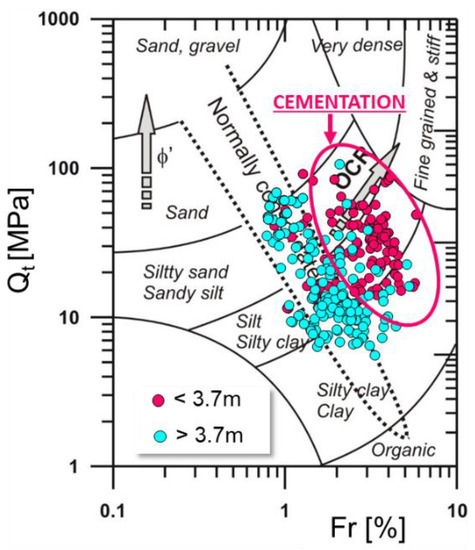
Figure 6.
Location of soil from area A on a Robertson diagram [65].
In the upper layer of the profile, up to a depth of about 3.70 m below ground level, the soils are located in the zone of preconsolidated soils (cementation zone), while soils from a depth of less than 3.70 m occupy a zone below the line of normal consolidation (non-cementation zone). Therefore, it can be concluded that the localization of normally consolidated soils at the boundary between the zone of normal consolidation and preconsolidation may be due to factors other than overloading.
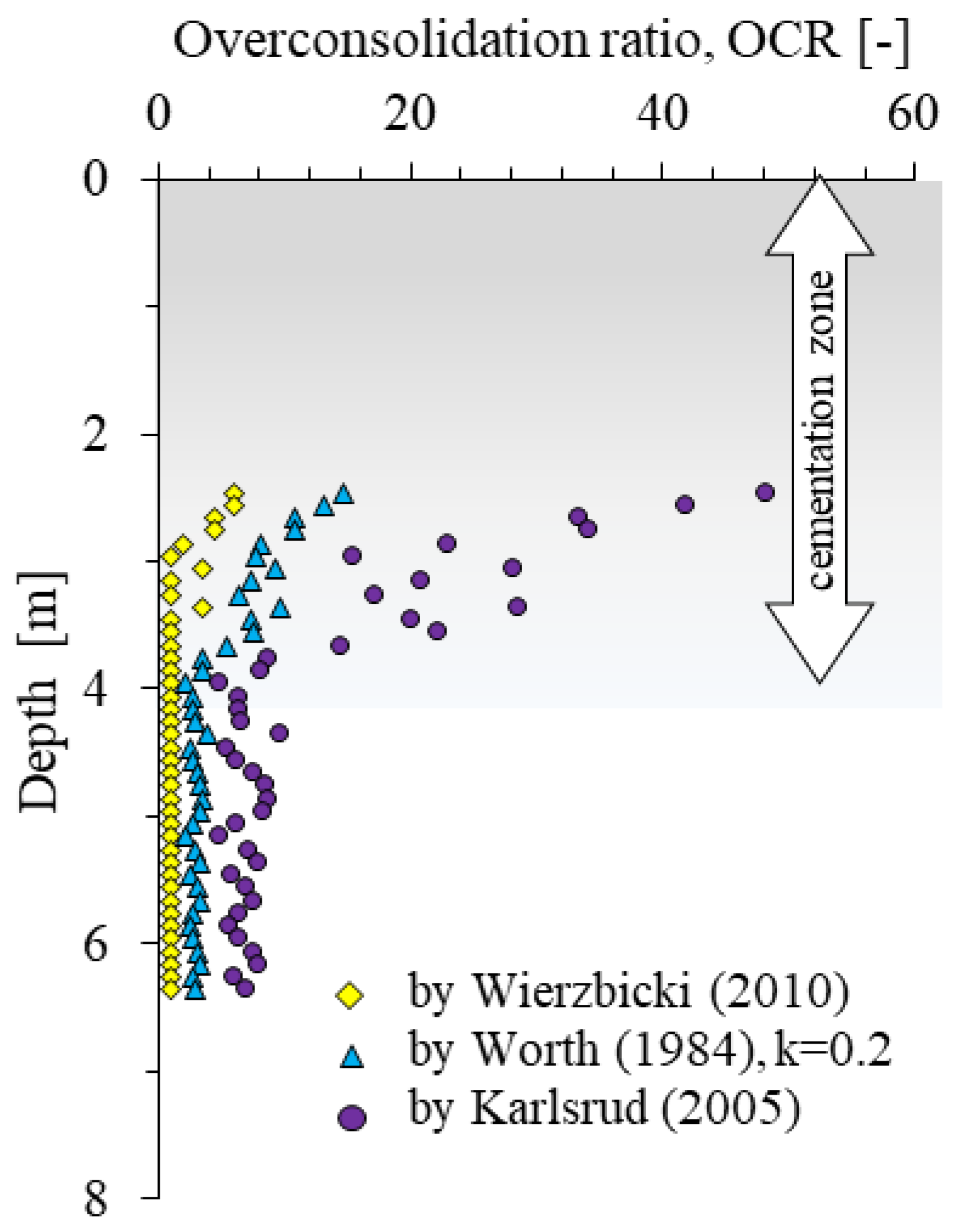
In addition, the identification of the cementation zone was carried out by determining the preconsolidation coefficient OCR (Figure 7). Each of the methods used [66,67,68] indicates the effect of pre-consolidation (OCR > 1) to a depth of about 3.7 m.
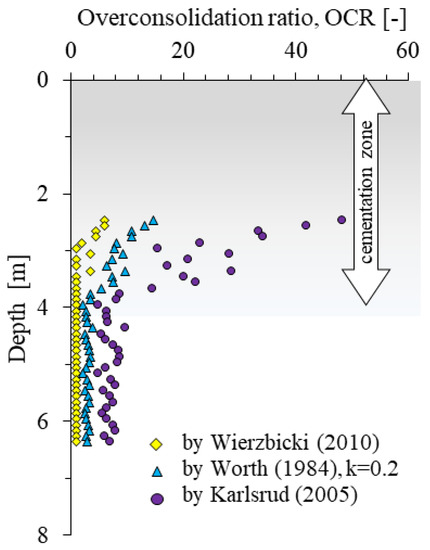
Figure 7.
Overconsolidation-ratio values determined by the Wierzbicki [66], Worth [67] and Karlsrud [68] formulas based on CPTU test.
The analysis of the obtained results (Figure 5) makes it possible to determine the presence of the effect of preconsolidation in the top part of the soil from the tested area A (Figure 7). In order to check whether the studied soils are preconsolidated or quasi preconsolidated in the strict sense, the verification proposed by Wierzbicki [66] of the ratio of OCRFs/OCRQt values was carried out. The tested soils gave values below 2, which, according to [66] may indicate a significant impact of a quasi preconsolidation processes, not connected to classic overloaded subsoil. It should be emphasized that this fact is confirmed by geological knowledge about the studied areas [75,76].
The presence of the preconsolidation effect in the upper part of the profile in the analysed case can be explained by the impact of the change in groundwater level and the formation of carbonate cementation.
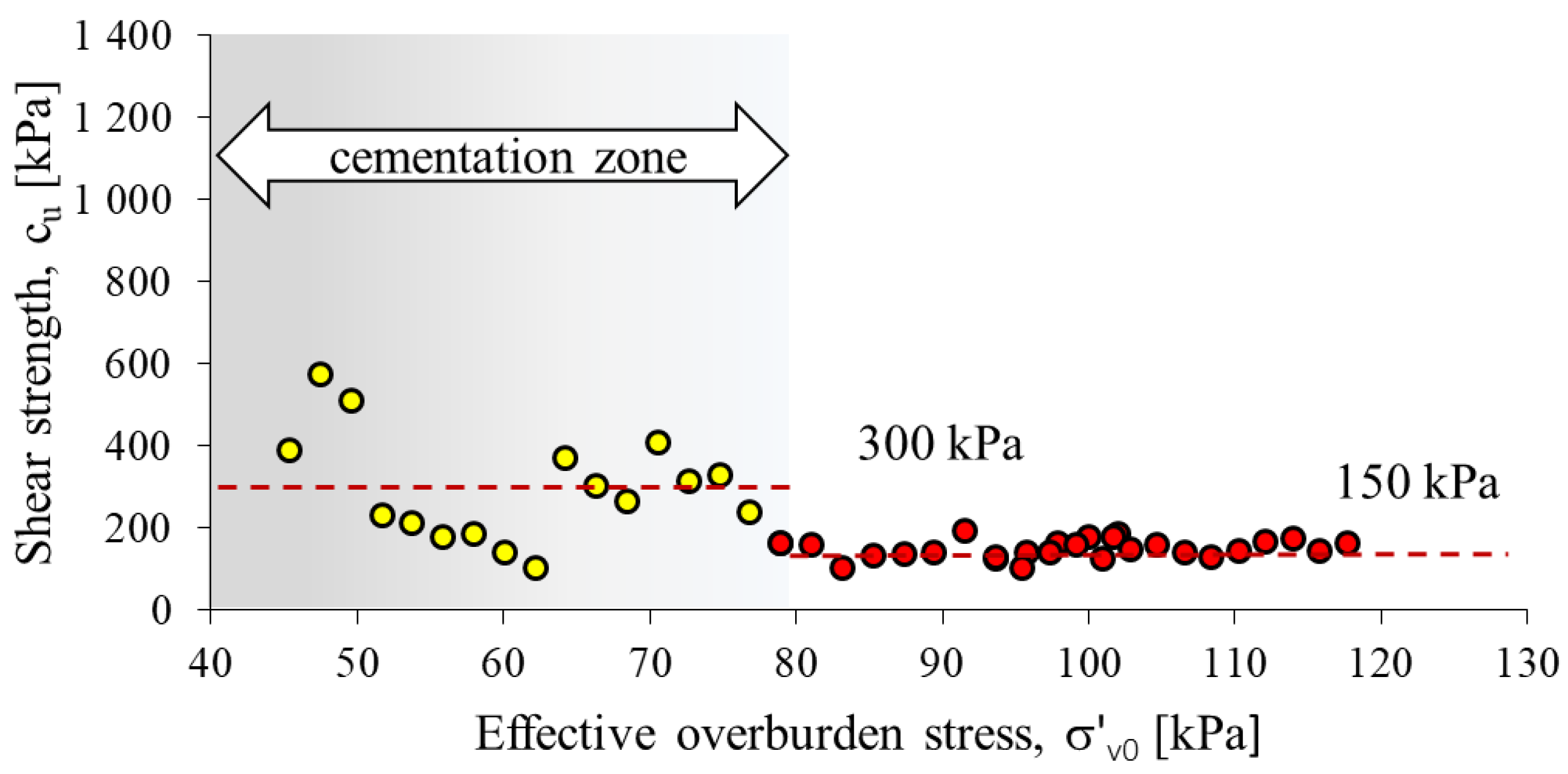
The undrained shear strength from the CPTU test was determined using formula 4 (Figure 8). The calculated average value of shear strength cu in the cementation zone is 300 kPa, while outside the zone it is 148 kPa. The cementation zone, due to the course of this process, is very heterogeneous. The part of the substrate outside the cementation zone is more homogeneous, the average cu value is 150 kPa. The heterogeneity of the cementation zone is also confirmed by the large variation in cone resistance with increasing depth in this part of the profile (Figure 5).
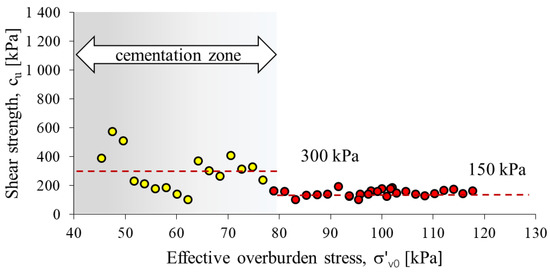
Figure 8.
A comparison of shear strength values from CPTU for recommended values of Nkt.
In addition to in situ testing, laboratory tests were performed to determine the physical properties of the tested soils (from area A and B) and the shear strength of the soils. The granulometric composition of soils from both areas is similar, they are saclSi (clayey/sandy silt) (according to [77]). In the tested soil samples, the leading fraction is the silty fraction, with the content of 69–76% on average. In terms of consistency, these are soils between stiff and plastic consistency. On the other hand, soils from area A are characterized by a calcium carbonate content, higher by 4.9–8.9% (on average by approx. 5%).
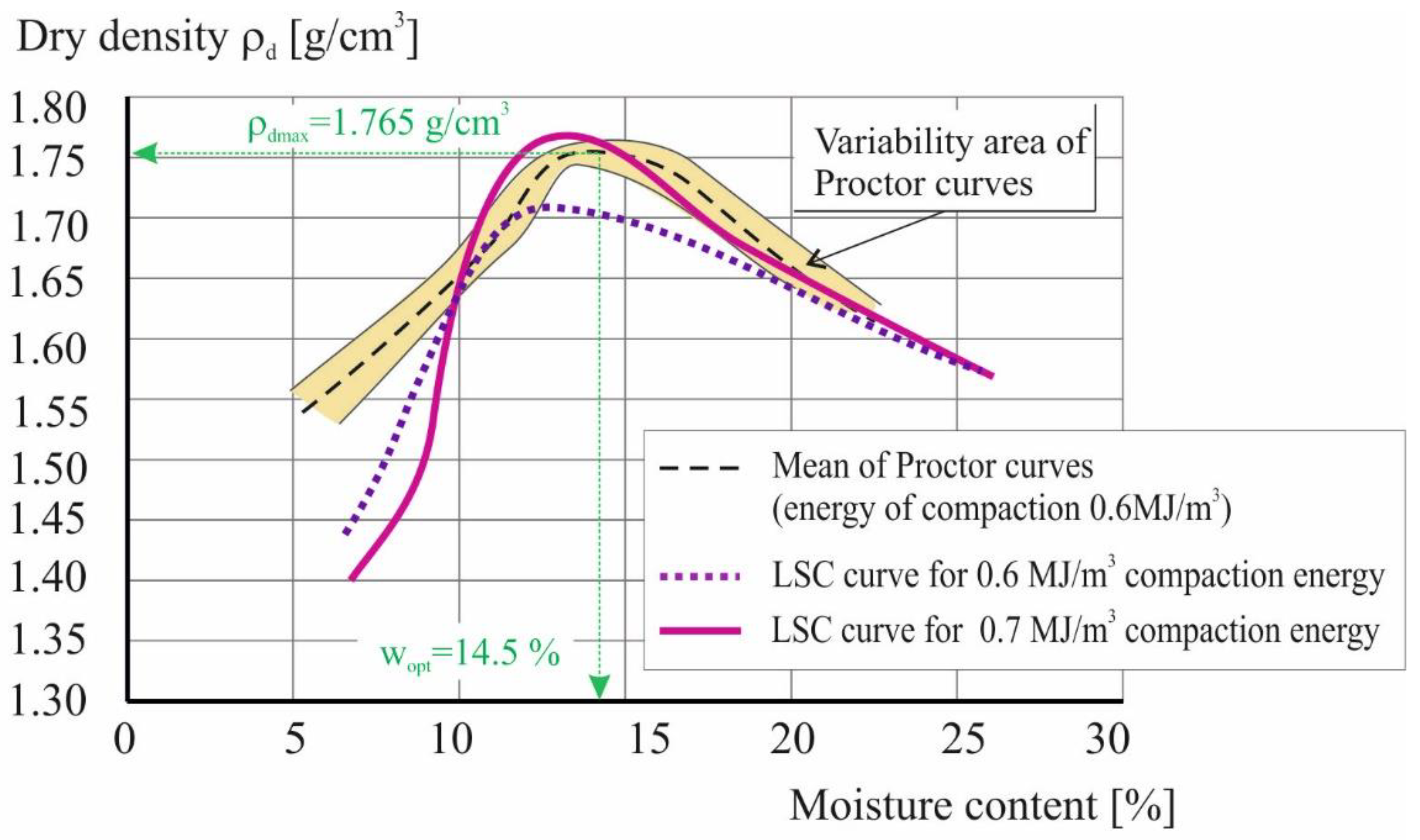
The second stage of the study made it possible to develop a procedure for compacting the soil in a laboratory soil compactor (LSC). For this purpose, 10 repetitions of the standard Proctor [71] test of the material without the addition of bacteria were performed, determining the range of compaction achieved (Figure 9). On this basis, the average value of the maximum volumetric density of the tested soil was determined to be 1.765 g/cm3 (ρdmax) at an optimum moisture content of 14.5% (wopt). A series of tests were then carried out in a laboratory compaction device using different values of compaction energy.
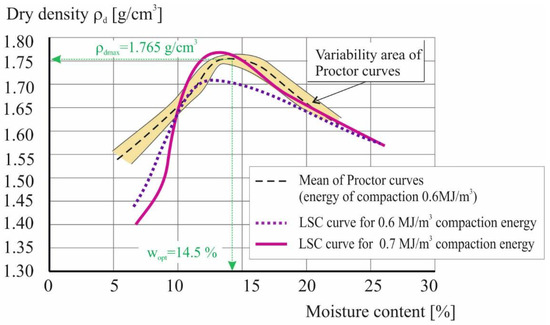
Figure 9.
Soil compaction curves in standard Proctor test and laboratory soil compactor.
As can be seen in Figure 9, gaining the same density as in the Proctor apparatus required an increase in compaction energy by almost 20% (up to 0.7 MJ/m3). This can be explained by the effect of scale; the volume of the cylinder in the Proctor apparatus is 1000 cm3. In contrast, the LSC obtains samples ready for testing in the triaxial compression apparatus in a volume of 74 cm3. At the same time however, increasing the compaction energy results in the maximum density being obtained at approximately 2.5% lower soil moisture.
Following these results, the next stage of testing was carried out by compacting both natural and bacteria-inoculated soil at a near optimal moisture content using a compaction energy of 0.7MJ/m3.
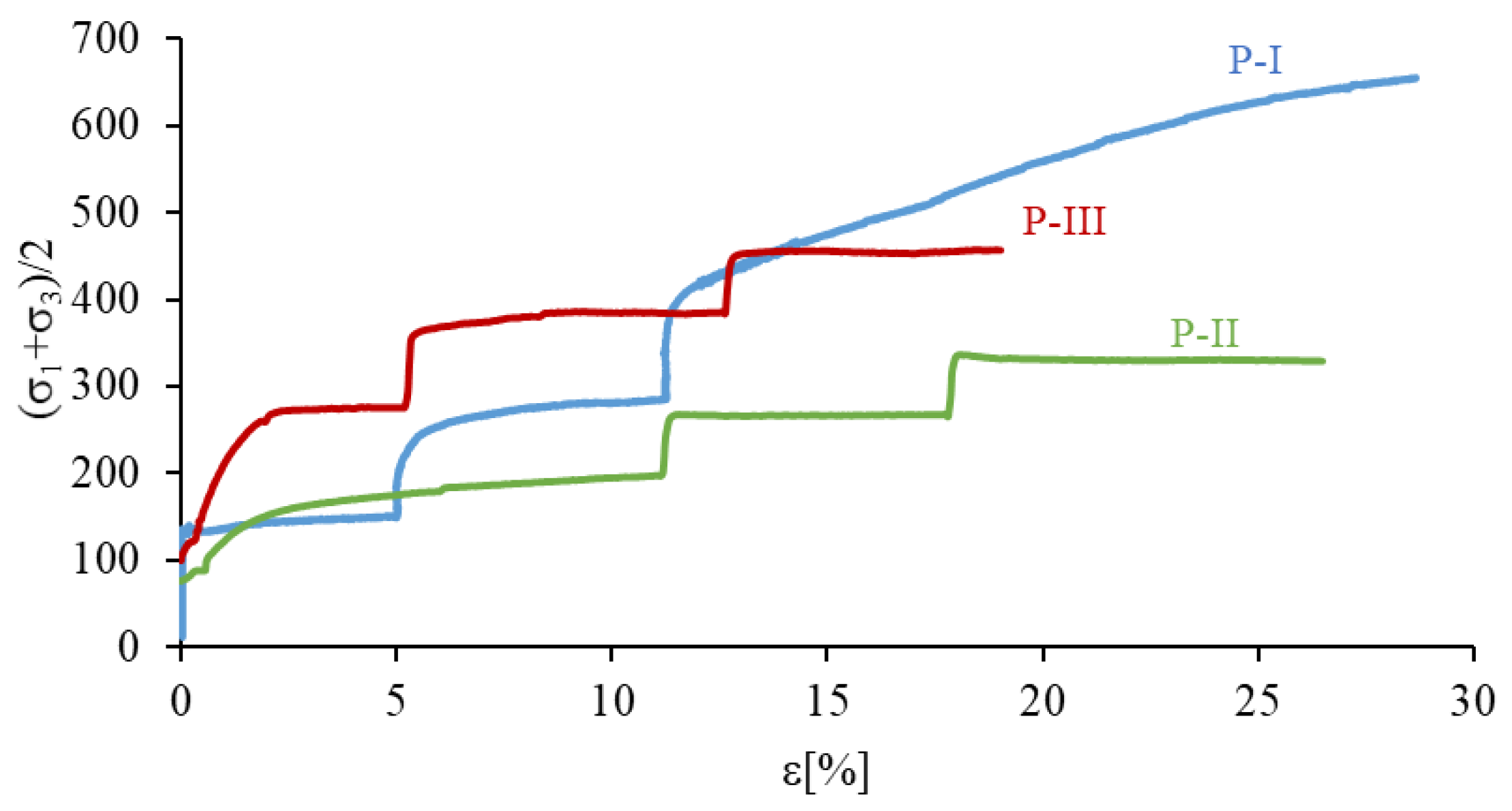
The results of TXT strength tests are presented in p’–ε diagrams, in the form of stress paths (Figure 10). The course of these paths allows for the assessment of changes in the value of the deviator stress in relation to the sample deformation ε, and as a result, the determination of the strength characteristics of the soil. The obtained results show a significant impact of the natural cementation, caused by natural processes occurring in nature (sample P-I), as well the cementation caused by bacterial activity (sample P-III) on the shear strength of the tested soil. In the case of the cemented sample (P-I), the initial path (to ε = 5%) is similar to the stress path of a reconstructed sample inoculated with bacteria (P-III), i.e., they were destroyed at similar deformation values. This is particularly evident in the first stage of the study, when the destruction of the P-I and P-III samples occurred at about 5% deformation. At the same time, it should be noted that the destruction of the biostabilized sample (P-III) occurred at significantly higher deviator stresses, about 120 kPa higher than a naturally cemented sample (P-I). At a further increase in the deviator stress, generated in the subsequent steps of the TXT test, a greater strength of cemented samples (P-I and P-III) than the natural reconstructed sample (P-II) was observed. It is worth noting that the destruction of a reconstructed sample (P-II) devoid of cementation is not brittle and occurs with significant deformations.
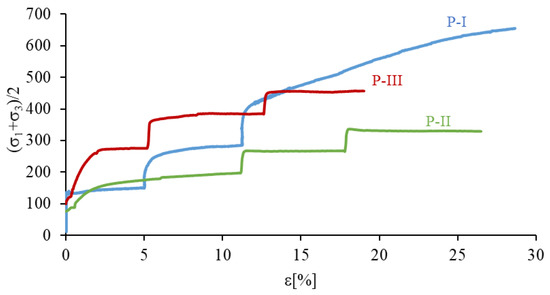
Figure 10.
The comparison of shear strength results for analysed soils.
5. Discussion
The results of the current research prompted us to consider a practical problem: to what extent does cementation with calcium carbonate, in particular caused by bacteria Sporosarcina pasteurii, improve the bearing capacity of the subsoil?
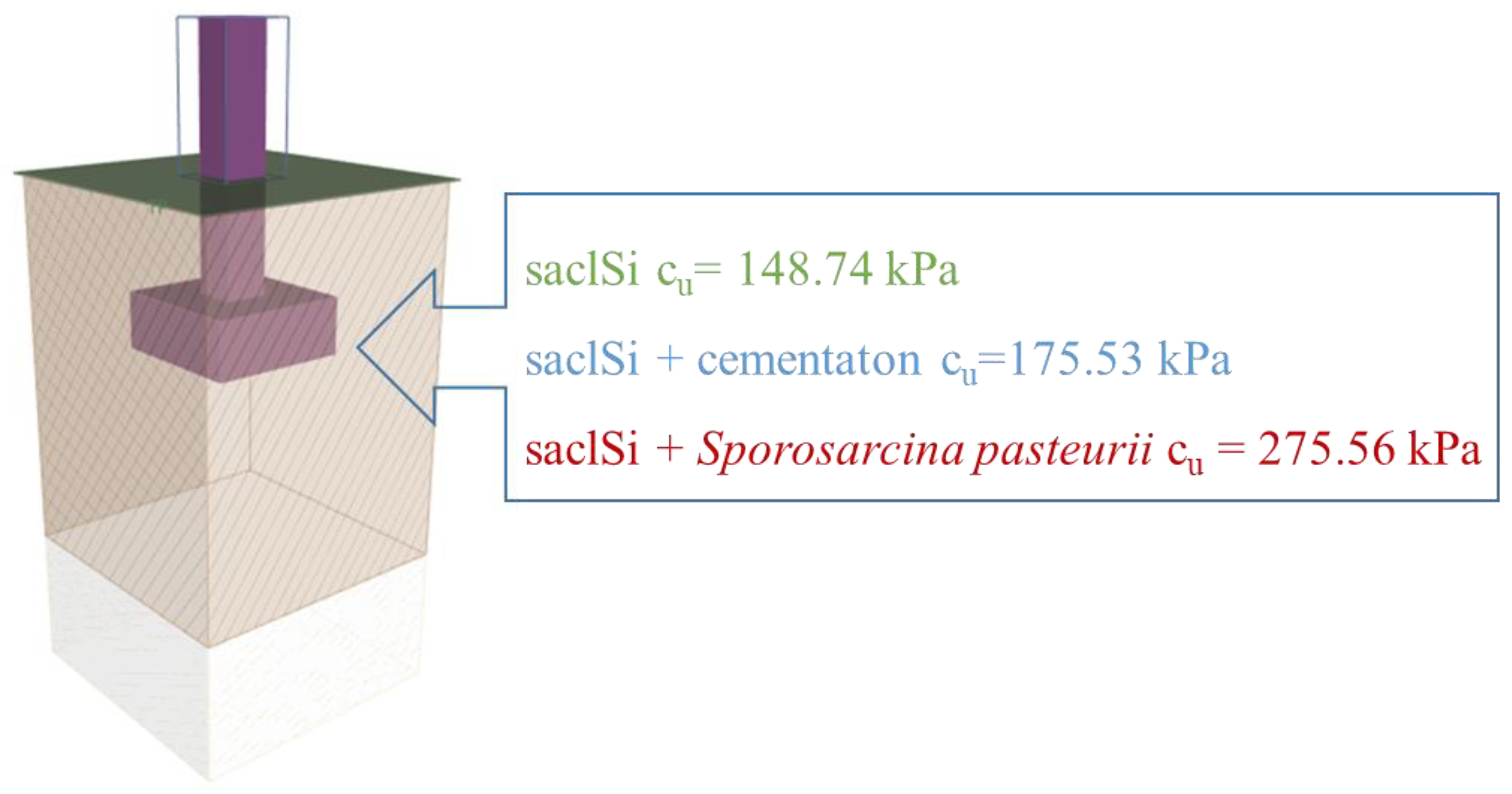
In order to verify the impact of cementation on the bearing capacity of the subsoil, an analysis of the direct foundation, i.e., on the subsoil made of the analysed soils was carried out. The GEO5 program—Shallow Foundations (License No. 6792/4) was used for the analysis. The analysis was carried out for undrained conditions (according to ref. [78], for the calculation approach 2). The foundation footing was designed for three soil profiles (i.e., natural soil saclSi; saclSi + natural cementation and saclSi + Sporosarcina pasteurii), for which the undrained shear strength cu was assumed from tests in the triaxial compression apparatus (Figure 11). The geometry of the footing was adopted as a 1.0 m x 1.0 m square, set at a depth of 1.2 m.
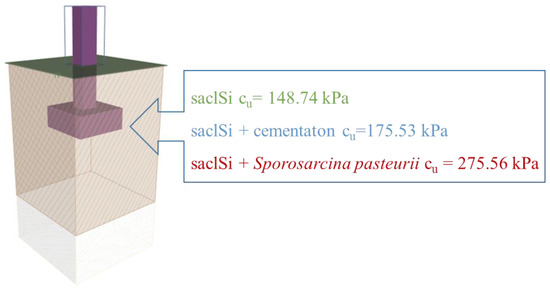
Figure 11.
The analysed direct foundation together with the shear strength values of the analysed soils.
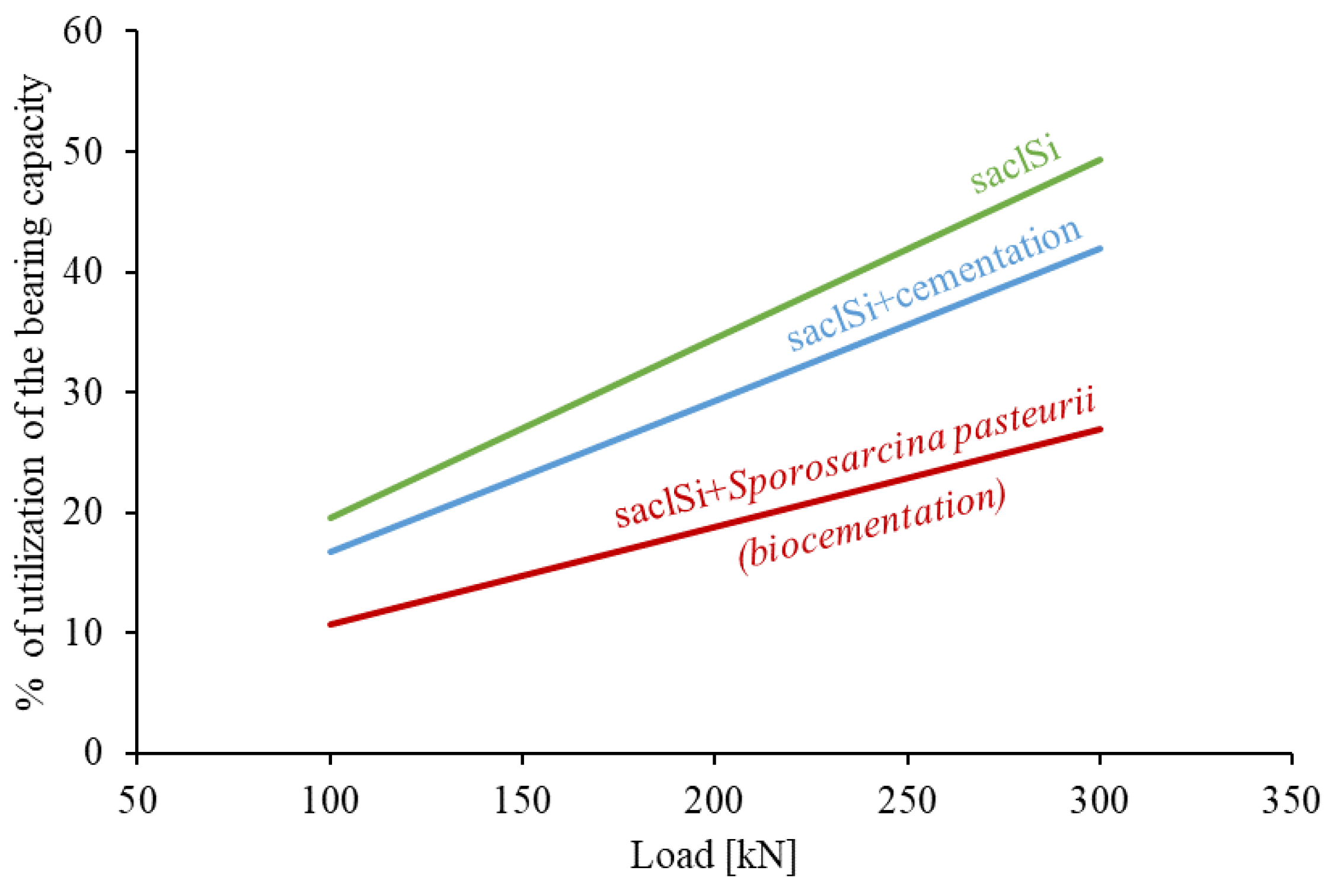
The analysis shows that cementation causes a noticeable increase in the bearing capacity of the subsoil (Figure 12). For example, with a load of 200 kN, the percentage of utilization of the bearing capacity of a subsoil built of saclSi is 34.5%, for naturally cemented soil 29.3%, and for biostabilized soil 18.8%. However, only cementation carried out intentionally, i.e., by adding Sporosarcina pasteurii, increases the bearing capacity by 50% (Figure 12). Such treatments (i.e., the use of biocementation) can therefore significantly reduce the costs of construction investment, while limiting CO2 emissions into the environment.
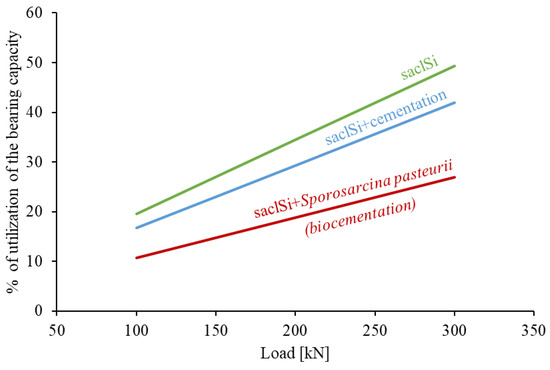
Figure 12.
Percentage of utilization of vertical bearing capacity at different values of subsoil loads.
6. Conclusions
The conducted research indicates the possibility of using carbonate biocementation as an alternative method of strengthening the load-bearing subsoil.
Soil biocementation has a significant impact on improving the strength of the subsoil, increasing its bearing capacity by up to 50%.
Based on publicly available knowledge, it can be assumed that strengthening the subsoil by using biocementation, i.e., using bacteria and their cementing capabilities, would definitely reduce the amount of CO2 emitted to the atmosphere. In addition, microbiologically produced calcium carbonate is harmless to the environment, compared to hydraulic mixtures used in construction.
The use of biomineralization as a method of subsoil reinforcement also creates the possibility of reducing the amount of certain waste (such as bio-waste, cement kiln silt and dairy waste) by using it as a secondary raw material, source of urea, calcium and nutrients for bacteria. Such a possibility significantly reduces the costs of the method and makes this method of soil stabilization more attractive.
Author Contributions
Conceptualization, K.S. and J.W.; methodology, K.S.; software, J.W.; validation, K.S. and J.W.; formal analysis, A.S.-G.; investigation, K.S. and B.K.; resources, K.S.; data curation, B.K.; writing—original draft preparation, K.S. and J.W.; writing—review and editing, K.S. and J.W.; visualization, B.K.; supervision, A.S.-G. and B.K.; project administration, A.S.-G.; funding acquisition, A.S.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This work is a part of project the “Excellence Initiative—Research University” Adam Mickiewicz University in Poznań. Project no. 038/04/NP/0023.
Data Availability Statement
Not applicable.
Acknowledgments
The publication was co-financed within the framework of the Ministry of Science and Higher Education programme as “Regional Initiative Excellence” in the years 2019–2022, Project No. 005/RID/2018/19.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mian, A.; Bendig, M.; Piazzesi, G.; Manente, G.; Lazzaretto, A.; Maréchal, F. Energy Integration in the cement industry. Comput. Aided Chem. Eng. 2013, 32, 349–354. [Google Scholar] [CrossRef]
- Ali, M.B.; Saidur, R.; Hossain, M.S. A review on emission analysis in cement industries. Renew. Sust. Energ. Rev. 2011, 15, 2252–2261. [Google Scholar] [CrossRef]
- Vatopoulos, K.; Tzimas, E. Assessment of CO2 capture technologies in cement manufacturing process. J. Clean. Prod. 2012, 32, 251–261. [Google Scholar] [CrossRef]
- Petroleum, B. Statistical Review of World Energy; BP: London, UK, 2009. [Google Scholar]
- Bremner, T. Environmental aspects of concrete: Problems and solutions. In Proceedings of the 1-st All-Russian Conference on Concrete and Reinforced Concrete, Moscow, Russia, 9–14 September 2001. [Google Scholar]
- Worrell, E.; Price, L.; Martin, N.; Hendriks, C.; Meida, L.O. Carbon dioxide emissions from the global cement industry. Annu. Rev. Energy Environ. 2001, 26, 303–329. [Google Scholar] [CrossRef]
- Sherwood, P. Soil stabilization with cement and lime. In State of the Art Review; Transport Research Laboratory, HMSO: London, UK, 1993. [Google Scholar]
- Åhnberg, H.; Holm, G. Stabilization of Some Swedish Organic Soils with Different Types of Binder in Proceeding of Dry Mix Methods for Deep Soil Stabilization; Bredenberg, H., Ed.; Routledge: London, UK, 1999; pp. 101–108. [Google Scholar]
- EuroSoilStab. Development of Design and Construction Methods to Stabilize Soft Organic Soils: Design Guide for Soft Soil Stabilization; CT97-0351; European Commission, Industrial and Materials Technologies Programme (Rite-EuRam III): Brussels, Belgium, 2002. [Google Scholar]
- Maher, A.; Bennert, T.; Jafari, F.; Doglas, W.S.; Gucunski, N. Geotechnical Properties of Stabilized Dredged Material from New York-New Jersey Harbor. J. Transp. Res. Board 2004, 1874, 86–96. [Google Scholar] [CrossRef]
- Massarsch, K.R.; Topolnicki, M. Regional Report: European Practice of Soil Mixing Technology. In Proceedings of the International Conference on Deep Mixing Best Practice and Recent Advances, Stockholm, Sweden, 23–25 May 2005. [Google Scholar]
- Yasui, S.; Yokozawa, K.; Yasuoka, N.; Kondo, H. Recent Technical Trends in Dry Mixing (DJM) in Japan. In Proceedings of the International Conference on Deep Mixing-Best Practice and Recent Advances, Stockholm, Sweden, 23–25 May 2005. [Google Scholar]
- Achal, V.; Kawasaki, S. Biogrout: A Novel Binding Material for Soil Improvement and Concrete Repair. Front. Microbiol. 2016, 7, 314. [Google Scholar] [CrossRef]
- Krajewska, B.; Raczak, K. Podstawy i możliwości wykorzystania procesu biomineralizacji węglanu wapnia. Ochr. Sr. 2019, 41, 31–37. [Google Scholar]
- Provis, L.; van Deventer, J.S.J. Geopolymers: Structure, Processing, Properties and Industrial Applications; CRS Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Chien, S.; Chang-Yu, C.; Fu-Chen, O.T. Soil improvement using electro-osmotic chemical treatment with harmonic waves. In Geotechnical and Geophysical Site Characterization 4; Coutinho, M., Ed.; Taylor and Francis Group: London, UK, 2013. [Google Scholar]
- Otsuki, N.; Yodsudjai, N.W.; Nishida, T. Feasibility study on soil improvement using electrochemical technique. Constr. Build. Mater. 2007, 21, 1046–1051. [Google Scholar] [CrossRef]
- Chang, I.; Im, J.; Shin, H.D.; Cho, G.C. Biochemical soil treatment for erosion control against desertification. In Geotechnical Engineering for Infrastructure and Development: XVI European Conference on Soil Mechanics and Geotechnical Engineering; Winter, M.G., Smith, D.M., Eldred, P.J.L., Eds.; ICE Publishing: London, UK, 2015; pp. 2687–2692. [Google Scholar]
- Iqbal, D.; Wong, L.; Kong, S. Bio-Cementation in Construction Materials: A Review. Materials 2021, 14, 2175. [Google Scholar] [CrossRef] [PubMed]
- Alhawat, M.; Ashour, A.; Yildirim, G.; Aldemir, A.; Sahmaran, M. Properties of geopolymers sourced from construction and demolition waste: A review. J. Build. Eng. 2022, 50, 104104. [Google Scholar] [CrossRef]
- Uliasz-Bocheńczyk, A.; Mokrzycki, E. Emisja Dwutlenku Węgla w Przemyśle Cementowym, Polityka Energetyczna; Instytut GSMiE PAN: Krakow, Poland, 2003; Volume 6, pp. 367–375. [Google Scholar]
- Agapoulaki, G.I.; Papdimitriou, A.G.; Kandris, K.; Pantazidou, M. Permeation potential of colloidal silica for passive stabilization of liquefiable soils. In Geotechnical Engineering for Infrastructure and Development: XVI European Conference on Soil Mechanics and Geotechnical Engineering; Winter, M.G., Smith, D.M., Eldred, P.J.L., Eds.; ICE Publishing: London, UK, 2015; pp. 2201–2206. [Google Scholar]
- Gallagher, P.M. Passive Site Remediation for Mitigation of Liquefaction Risk. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2000. [Google Scholar]
- Chiet, K.T.P.; Kassim, K.A.; Chong, S.Y. Biocementation Potential of Tropical Residue Soil Infused with Facultative Anaerobe Bacteria. Appl. Mech. Mater. 2015, 773–774, 1412–1416. [Google Scholar] [CrossRef]
- DeJong, J.T.; Fritzges, M.B.; Nüsslein, K. Microbially induced cementation to control sand response to undrained shear. J. Geotechn. Geoenviron. Eng. ASCE 2006, 11, 1381–1392. [Google Scholar] [CrossRef]
- Dhami, N.K.; Reddy, S.; Mukherje, A. Significant indicators for biomineralization in sand of varying grain size. Constr. Build. Mater. 2016, 104, 198–207. [Google Scholar] [CrossRef]
- Grabiec, A.M.; Starzyk, J.; Stefaniak, K.; Wierzbicki, J.; Zawal, D. On possibility of improvement of compacted silty soils using biodeposition method. Constr. Build. Mater. 2017, 138, 134–140. [Google Scholar] [CrossRef]
- Indriani, A.M.; Harianto, T.; Djamaluddin, A.R.; Arsyad, A. Study on Bio-cementation of Ex-coal Mining Soil as a Road Construction Material. In Advances in Sustainable Construction and Resource Management. Lecture Notes in Civil Engineering; Hazarika, H., Madabhushi, G.S.P., Yasuhara, K., Bergado, D.T., Eds.; Springer: Singapore, 2021; p. 144. [Google Scholar] [CrossRef]
- Kezdi, A.; Ladanyi, J.; Kabai, J. Compaction of transition soils. In Proceedings of the 4th International Conference on Soil Mechanics, Budapest, Hungary, 12–15 October 1971; pp. 177–185. [Google Scholar]
- Stefaniak, K. Wybrane Osady Aluwialne Jako Podłoże Budowlane i Materiał do Budowli Ziemnych. Ph.D. Thesis, Uniwersytet Przyrodniczy w Poznaniu, Poznań, Poland, 2014. [Google Scholar]
- Zhu, T.; Dittrich, M. Carbonate precipitation through microbial activities in natural environment and their potential in biotechnology: A review. Front. Bioeng. Biotech. 2016, 4, 4. [Google Scholar] [CrossRef]
- Chaparro-Acuña, S.P.; Becerra-Jiménez, M.B.; Martínez-Zambrano, J.J.; Rojas-Sarmiento, H.A. Soil bacteria that precipitate calcium carbonate: Mechanism and applications of the process. Acta Agron. 2018, 67, 277–288. [Google Scholar] [CrossRef]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Biomineralization of calcium carbonates and their engineered applications. Front. Microbiol. 2013, 4, 314. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, V.; Chu, J. Applications of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev. Environ. Sci. Biotechnol. 2008, 7, 139–153. [Google Scholar] [CrossRef]
- Dilrukshi, R.A.N.; Kawasaki, S. Effective use of plant derived urease in the field of geoenvironmental/geotechnical engineering. Int. J. Civ. Environ. Eng. 2016, 6, 207. [Google Scholar] [CrossRef]
- Valenzuela, E.I.; Avendaño, K.A.; Balagurusamy, N.; Arriaga, S.; Nieto-Delgado, C.; Thalasso, F.; Cervantes, F.J. Electron shuttling mediated by humic substances fuels anaerobic methane oxidation and carbon burial in wetland sediments. Sci. Total Environ. 2019, 650, 2674–2684. [Google Scholar] [CrossRef] [PubMed]
- Deb, P.; Talukdar, S.A.; Mohsina, K.; Sarker, P.K.; Sayem, S.M.A. Production and partial characterization of extracellular amylase enzyme from Bacillus amyloliquefaciens P-001. SpringerPlus 2013, 2, 154. [Google Scholar] [CrossRef]
- Grabiec, A.M.; Klama, J.; Zawal, D.; Krupa, D. Modification of recycled concrete aggregate by calcium carbonate biodeposition. Constr. Build. Mater. 2012, 34, 145–150. [Google Scholar] [CrossRef]
- Gomez, M.G.; Graddy, C.M.R.; DeJong, J.T.; Nelson, D.C.; Tsesarsky, M. Stimulation of Native Microorganisms for Biocementation in Samples Recovered from Field-Scale Treatment Depths. J. Geotech. Geoenviron. 2018, 144, 04017098. [Google Scholar] [CrossRef]
- Eberemu, A.O.; Onah, M.; Osinub, K.J. Effect of curing method on unconfined compressive strength of silty sand treated with Bacillus Megaterium. IOP Conf. Ser.: Mater. Sci. Eng. 2021, 1036, 012032. [Google Scholar] [CrossRef]
- Douglas, S.; Beveridge, T.J. Mineral formation by bacteria in natural Communities. FEMS Microb. Ecol. 1998, 26, 79–88. [Google Scholar] [CrossRef]
- Benini, S.; Rypniewski, W.R.; Wilson, K.S.; Miletti, S.; Ciurli, S.; Mangani, S. A new proposal for urease mechanism based on the crystal structures of the native and inhibited enzyme from Bacillus pasteurii: Why urea hydrolysis costs two nickels. Structure 1999, 7, 205–216. [Google Scholar] [CrossRef]
- Warthmann, R.; van Lith, Y.; Vasconcelos, C.; McKenzie, J.A.; Karpoff, A.M. Bacterially induced dolomite precipitation in anoxic culture experiments. Geology 2000, 28, 1091–1094. [Google Scholar] [CrossRef]
- Roden, E.E.; Leonardo, M.R.; Ferris, F.G. Immobilization of strontium during iron biomineralization coupled to dissimilatory hydrous ferric oxide reduction. Geochim. Cosmochim. Acta. 2002, 66, 2823–2839. [Google Scholar] [CrossRef]
- Karatas, I.; Kavazanjian, E., Jr.; Rittmann, B.E. Microbially induced precipitation of calcite using Pseudomonas denitrificans. In Proceedings of the 1st International Conference on Biogeotechnical Engineering, Delft, The Netherlands, 23–25 June 2008. [Google Scholar]
- Dejong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Biomediated soil improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- Burbank, M.; Weaver, T.; Lewis, R.; Williams, T.; Williams, B.; Crawford, R. Geotechnical Tests of Sands Following Bioinduced Calcite Precipitation Catalyzed by Indigenous Bacteria. J. Geotech. Geoenviron. 2013, 139, 928–936. [Google Scholar] [CrossRef]
- Hamdan, N.; Kavazanjian, E., Jr.; Rittmann, B.E.; Karatas, I. Carbonate mineral precipitation for soil improvement through microbial denitrification. In Proceedings of the Geo Frontiers 2011: Advances in Geotechnical Engineering, Dallas, TX, USA, 13–16 March 2011; ASCE Geotechnical Special Publication: Reston, VA, USA, 2011; Volume 211, pp. 3925–3934. [Google Scholar]
- Weaver, T.; Burbank, M.; Lewis, R.; Lewis, A.; Crawford, R.; Williams, B. Bio-induced calcite, iron, and manganese precipitation for geotechnical engineering applications. In Proceedings of the Geo Frontiers 2011: Advances in Geotechnical Engineering, Dallas, TX, USA, 13–16 March 2011; ASCE Geotechnical Special Publication: Reston, VA, USA, 2011; Volume 211, pp. 3975–3983. [Google Scholar]
- Bang, S.S.; Galinat, J.K.; Ramakrishnan, V. Calcite precipitation induced by polyurethane-immobilized Sporosarcina pasteurii. Enzym. Microb. Technol. 2001, 28, 404–409. [Google Scholar] [CrossRef]
- Okwadha, G.D.O.; Li, J. Optimum conditions for microbial carbonate precipitation. Chemosphere 2010, 81, 1143–1148. [Google Scholar] [CrossRef]
- Carmona, J.P.S.F.; Venda Oliviera, P.J.; Lemos, L.J.L. Biostabilization of a Sandy Soil Using Enzymatic Calcium Carbonate Precipitation. In Proceedings of the 3rd International Conference on Transportation Geotechnics, University of Coimbra, Coimbra, Portugal, 4–7 September 2016. [Google Scholar]
- Mujah, D.; Shahin, M.A.; Cheng, L. State-of-the-Art. Review of Biocementation by Microbially Iduced Calcite Precipitation (MICP) for Soil Stabilization. Geomicrobiol. J. 2016, 8, 1–14. [Google Scholar]
- Whiffin, V.S.; van Paassen, L.A.; Harkes, M.P. Microbial carbonate precipitation as soil improvement technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Libudzisz, Z.; Kowal, K.; Żakowska, Z. Mikrobiologia Techniczna; Naukowe, P.W.N.W., Ed.; Mikroorganizmy w Biotechnologii, Ochronie Środowiska i Produkcji Żywności: Warsow, Poland, 2008; Volume 2. [Google Scholar]
- Keykha, H.A.; Asadi, A.; Zareian, M. Environmental Factors Affecting the Compressive Strength of Microbiologically Induced Calcite Precipitation-Treated Soil. Geomicrobiol. J. 2017, 34, 889–894. [Google Scholar] [CrossRef]
- Available online: www.geochemicaltranactions.com (accessed on 1 December 2022).
- Hartbottle, M.J.; Lam, M.T.; Botusharova, S.P.; Gardner, D.R. Self-healing soil: Biomimetic engineering of geotechnical structures to respond to damage. In Proceedings of the 7th 397 International Congress on Environmental Geotechnics, Melbourne, Australia, 10 November 2014. [Google Scholar]
- Janssen, J.; Di Emidio, G.; Verasteguiflores, R.D.; Bezuijen, A. Hydraulic conductivity and g pressure of GCLs using polymer treated clays to high concentration CaCl2 solutions. In Proceedings of the Geotechnical Engineering for Infrastructure and Development: XVI European Conference on Soil Mechanics and Geotechnical Engineering, Edinburgh, UK, 13–18 September 2015; Winter, M.G., Smith, D.M., Eldred, P.J.L., Eds.; ICE Publishing: London, UK, 2015; pp. 2687–2692. [Google Scholar]
- Qabany, A.; Soga, K. Effect of chemical treatment used in MICP on engineering properties of cemented soils. Géotechnique 2013, 63, 331–339. [Google Scholar] [CrossRef]
- Canakci, H.; Sidik, W.; Kilic, I.H. Bacterail Calcium Carbonate Precipitation in Peat. Arab. J. Sci. Eng. 2015, 40, 2251–2260. [Google Scholar] [CrossRef]
- Harianto, T.; Hamzah, S.; Nur, S.H.; Abdurrahman, F.; Latief, R.U.; Fadliah, I.; Waleman, A. Biogrouting Stabilization on Marine Sandy Clay Soil. In Proceedings of the International Conference on Asian and Pacific Coasts (APAC), Bali, Indonesia, 24–26 September 2013. [Google Scholar]
- van Paassen, L. Biogrout, Ground Improvement by Microbially Induced Carbonate Precipitation. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2009. [Google Scholar]
- Cheng, L.; Cord-Ruwisch, R. In situ soil cementation with ureolytic bacteria by surfacepercolation. Ecol. Eng. 2012, 42, 64–72. [Google Scholar] [CrossRef]
- Robertson, P.K. Soil classification using the cone penetration test. Can. Geotech. J. 1990, 27, 151–158. [Google Scholar] [CrossRef]
- Wierzbicki, J. Ocena Prekonsolidacji Podłoża Metodami In-Situ w Aspekcie Jego Genezy. Ph.D. Thesis, Uniwersytet Przyrodniczy w Poznaniu, Poznań, Poland, 2010. [Google Scholar]
- Worth, C.P. The interpretation of in-situ soil tests. Geotechnique 1984, 34, 449–489. [Google Scholar]
- Karlsrud, K.; Lunne, T.; Kort, D.A.; Strandvik, S. CPTU correlations for clays. In Proceedings of the 16th ICSSMGE, Osaka, Japan, 12–16 September 2005; Millpress: Rotterdam, The Netherlands, 2005; pp. 693–702. [Google Scholar]
- Lunne, T.; Robertson, P.K.; Powell, J.J.M. Cone Penetration Testing in Geotechnical Practice; Blackie Academic EF Spon/Routledge Publishers: New York, NY, USA, 1997. [Google Scholar]
- Stefaniak, K. Assessment of shear strength in silty soils. Stud. Geotech. Mech. 2015, 37, 51–55. [Google Scholar] [CrossRef]
- PN-EN 13286-2 2010; Unbound and Hydraulically Bound Mixtures. Part 2: Test Methods for Laboratory Reference Density and Water Content. Proctor Compaction. CEN: Brussels, Belgium, 2010.
- Chunxiang, Q.; Jianyun, W.; Ruixing, W.; Liang, C. Corrosion protection of cement-based building materials by surface deposition of CaCO3 by Bacillus pasteurii. Mater. Sci. Eng. 2009, C 29, 1273–1280. [Google Scholar] [CrossRef]
- ISO 17892-9; Geoetchnical Investigation and Testing. Laboratory Testing of Soil. Part 8: Unconsolidated Undrained Triaxial Test. ISO: Geneva, Switzerland, 2018.
- ISO 17892-9; Geoetchnical Investigation and Testing. Laboratory Testing of Soil. Part 9: Consolidated Triaxial Compression Tests on Water Saturated Soils. ISO: Geneva, Switzerland, 2018.
- Stefaniak, K. Analysis of overconsolidation effect in alluvial subsoil using CPTU and DMT. In Advances in Soil Mechanics and Geotechnical Engineering, 2: Proceedings of the 5th International Young Geotechnical Engineers’ Conference; Millpress: Paris, France, 2013; pp. 383–386. [Google Scholar]
- Radaszewski, R.; Stefaniak, K. Oedometric tests of cohesive soils—Testing methods and their results. Archit. Civ. Eng. Environ. 2015, 8, 53–60. [Google Scholar]
- ISO 14688-2:2018; Geotechnical Investigation and Testing. Identification and Classification of Soil. Part 2: Principles for a Classification. ISO: Geneva, Switzerland, 2018.
- EC 7-1 EN 1997-1:2003; Design of Geotechnical Structures. Part 1: General Rules. EC Directive: Brussels, Belgium, 2003.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).