Development of Manganese-Coated Graphite Electrode in a Dual-Chambered Fuel Cell for Selenite Removal and Bio-Electricity Generation from Wastewater Effluent by Bacillus cereus
Abstract
1. Introduction
2. Material and Method
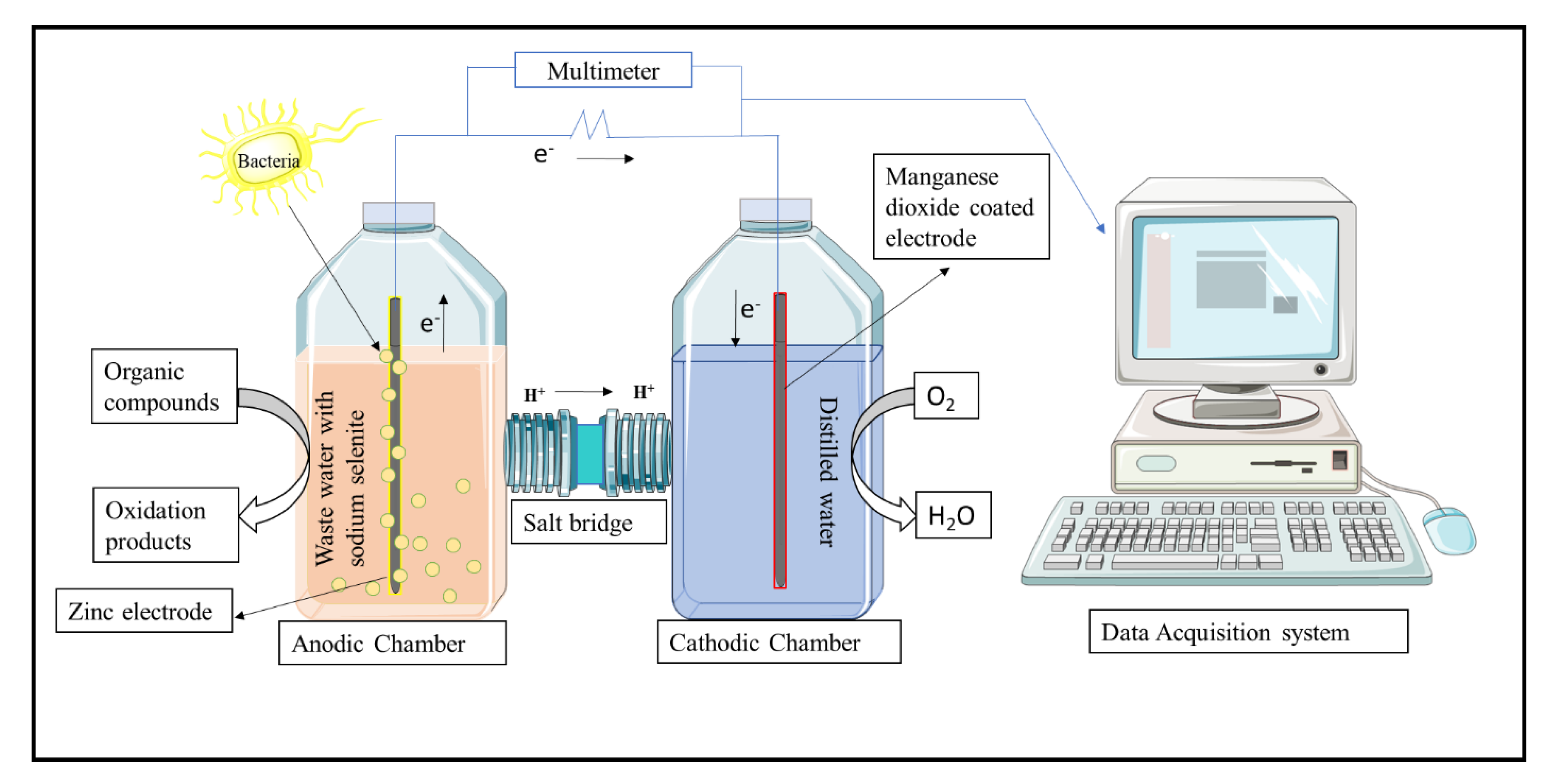
2.1. Design of the Microbial Fuel Cell Setup
2.2. Preparation of Cathodic Material
2.3. Preparation of Anodic and Cathodic Chamber
2.4. Electrochemical Measurement and Analysis
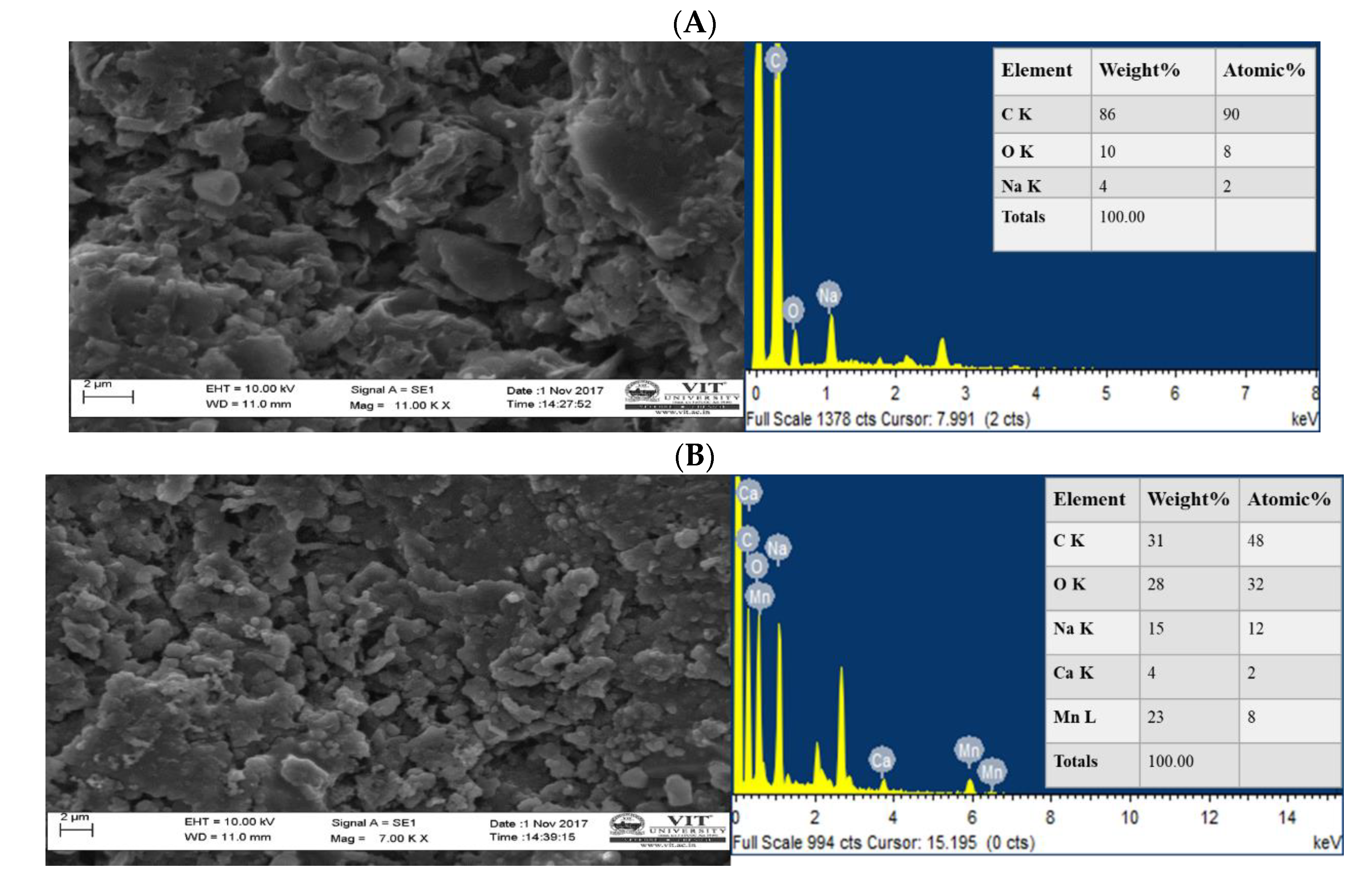
2.5. Morphological Analysis
3. Results and Discussion
3.1. Morphological Characterization of Manganese Dioxide (MnO2) Electrode
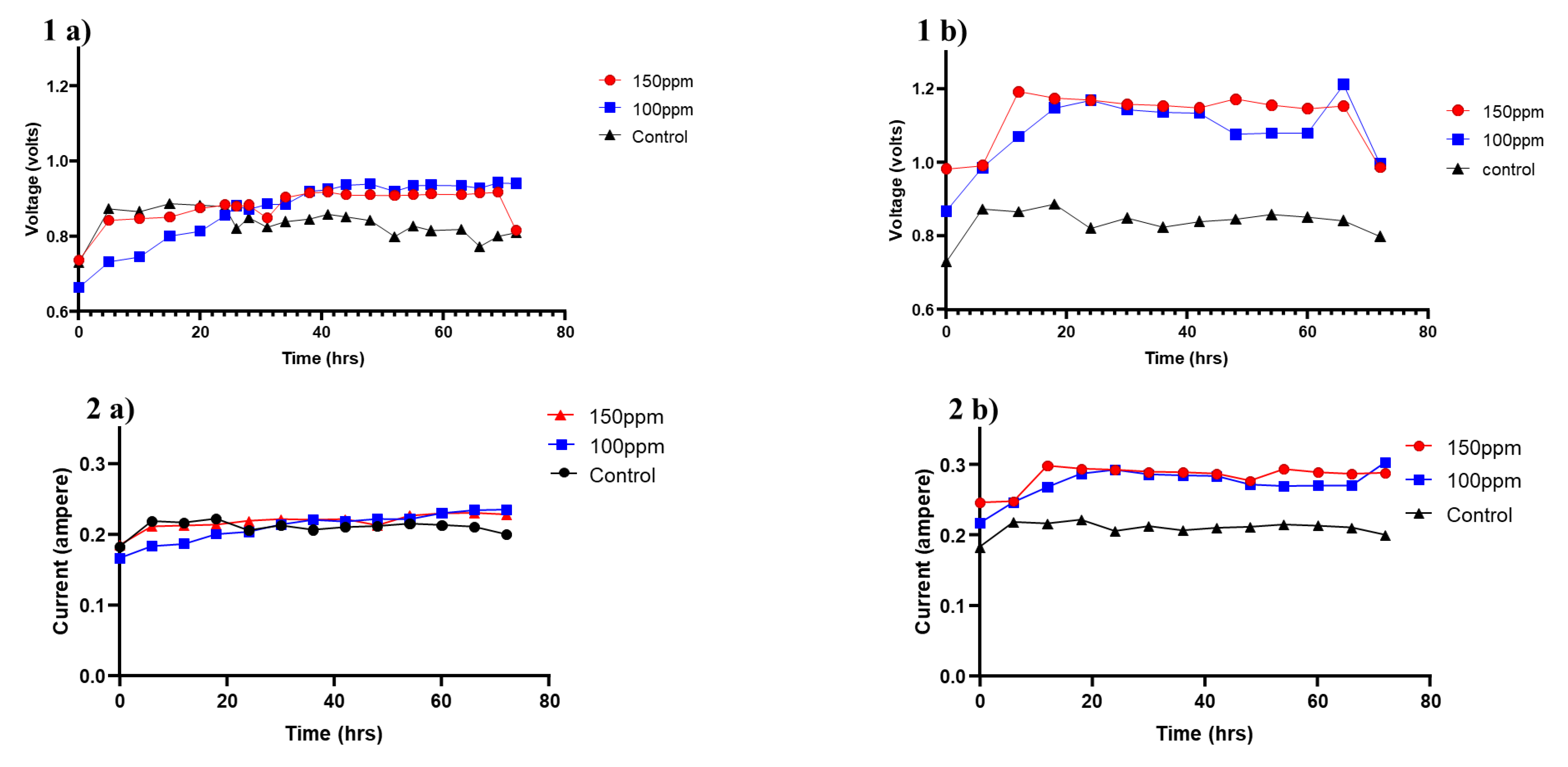
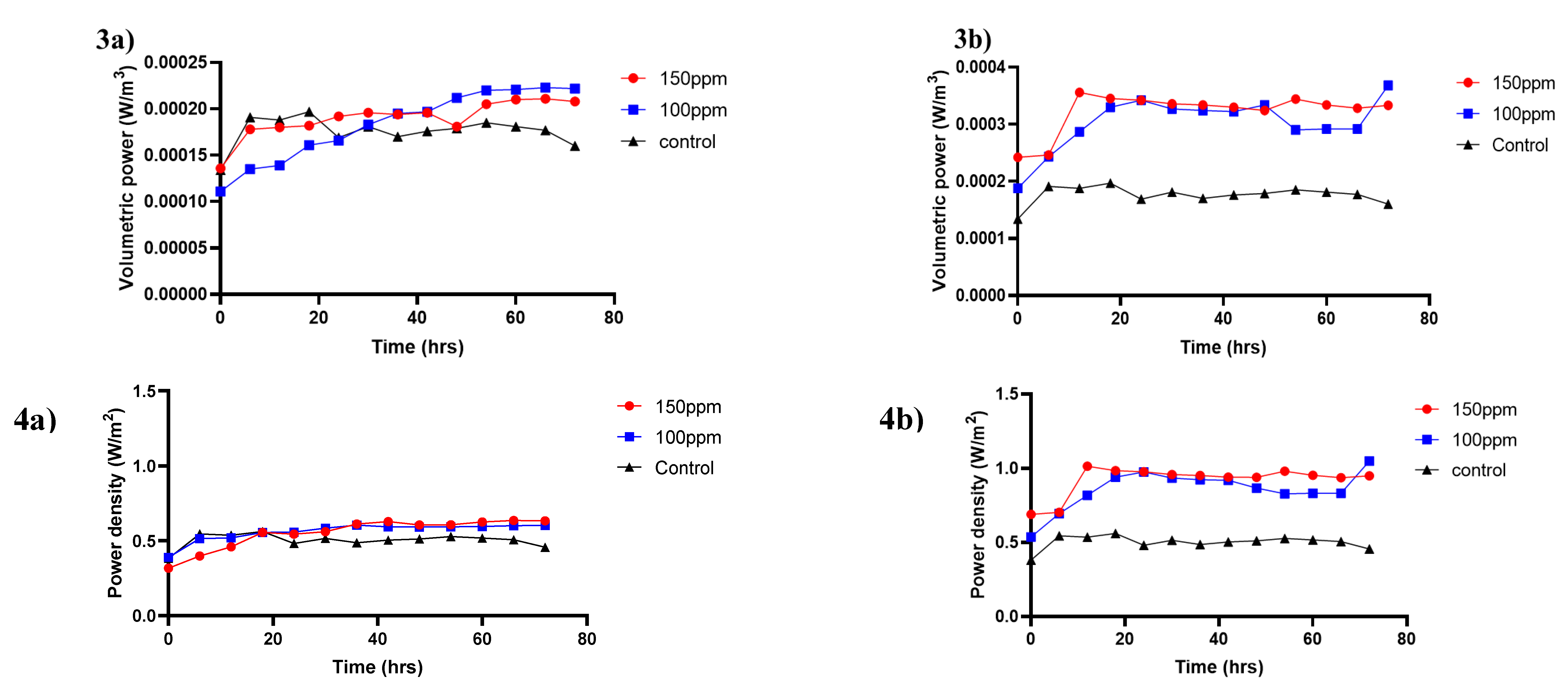
3.2. Effect of Selenite Concentration on Bioelectricity Generation
3.3. Selenite and Chemical Oxygen Demand Removal Efficiency
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hamilton, S. Review of selenium toxicity in the aquatic food chain. Sci. Total Environ. 2004, 326, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.C.; Nancharaiah, Y.V.; Van Hullebusch, E.D.; Lens, P.N.L. Selenium: Environmental significance, pollution, and biological treatment technologies. Biotechnol. Adv. 2016, 34, 886–907. [Google Scholar] [CrossRef]
- Rovira, M.; Giménez, J.; Martínez, M.; Martínez-Lladó, X.; De Pablo, J.; Martí, V.; Duro, L. Sorption of selenium(IV) and selenium(VI) onto natural iron oxides: Goethite and hematite. J. Hazard. Mater. 2008, 150, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Lemly, A.D. Ecosystem Recovery Following Selenium Contamination in a Freshwater Reservoir. Ecotoxicol. Environ. Saf. 1997, 36, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Ike, M.; Kashiwa, M.; Hashimoto, R.; Soda, S. Laboratory-scale continuous reactor for soluble selenium removal using selenate-reducing bacterium, Bacillus sp. SF-1. Biotechnol. Bioeng. 2002, 80, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, Z.; Wu, J.; Zhang, Y.; Li, M.; Lin, Z.Q.; Bañuelos, G. Simultaneous removal of selenite and electricity production from Se-laden wastewater by constructed wetland coupled with microbial fuel cells. Selenium Environ. Hum. Health 2014, 212, 212–214. [Google Scholar] [CrossRef]
- Holmes, A.B.; Gu, F.X. Emerging nanomaterials for the application of selenium removal for wastewater treatment. Environ. Sci. Nano 2016, 3, 982–996. [Google Scholar] [CrossRef]
- Astratinei, V.; Van Hullebusch, E.; Lens, P. Bioconversion of Selenate in Methanogenic Anaerobic Granular Sludge. J. Environ. Qual. 2006, 35, 1873–1883. [Google Scholar] [CrossRef]
- Adeniran, J.A.; Huberts, R.; De-Koker, J.J.; Arotiba, O.A.; Olorundare, O.F.; Van-Zyl, E.; Du-Plessis, S.C. Energy generation from domestic wastewater using sandwich dual-chamber microbial fuel cell with mesh current collector cathode. Int. J. Environ. Sci. Technol. 2016, 13, 2209–2218. [Google Scholar] [CrossRef]
- Aelterman, P.; Rabaey, K.; Clauwaert, P.; Verstraete, W. Microbial fuel cells for wastewater treatment. Water Sci. Technol. 2006, 54, 9–15. [Google Scholar] [CrossRef]
- Reddy, T.V.; Kavya, C.; Krishna Prasad Reddy, K.; Srinivas Naik, K.; Burgula, S. Dual Chambered Microbial Fuel Cell For Bioelectricity Generation From Environmental Samples. Int. J. Sci. Res. Publ. 2019, 9, p8996. [Google Scholar] [CrossRef]
- Chellamuthu, T.; Ward, M.; Nealson, K. Removal of Selenite with Microbial Fuel Cells Utilizing Shewanella Oneidensis MR-1. In Proceedings of the 2011 AIChE Annual Meeting, Minneapolis, MN, USA, 16–21 October 2011. [Google Scholar]
- Catal, T.; Liu, H.; Bermek, H. Selenium induces manganese-dependent peroxidase production by the white-rot fungus bjerkandera adusta (Willdenow) P. Karsten. Biol. Trace Elem. Res. 2008, 123, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Kusmayadi, A.; Leong, Y.K.; Yen, H.W.; Huang, C.Y.; Dong, C.D.; Chang, J.S. Microalgae-microbial fuel cell (mMFC): An integrated process for electricity generation, wastewater treatment, CO2 sequestration and biomass production. Int. J. Energy Res. 2020, 44, 9254–9265. [Google Scholar] [CrossRef]
- Munoz-Cupa, C.; Hu, Y.; Xu, C.; Bassi, A. An overview of microbial fuel cell usage in wastewater treatment, resource recovery and energy production. Sci. Total Environ. 2021, 754, 142429. [Google Scholar] [CrossRef]
- Catal, T.; Bermek, H.; Liu, H. Removal of selenite from wastewater using microbial fuel cells. Biotechnol. Lett. 2009, 31, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, K.K.; Kumar, V.; Vlaskin, M.S.; Sharma, N.; Rautela, I.; Nanda, M.; Arora, N.; Singh, A.; Chauhan, P.K. Microalgae fuel cell for wastewater treatment: Recent advances and challenges. J. Water Process Eng. 2020, 38, 101549. [Google Scholar] [CrossRef]
- Liu, C.H.; Lee, S.K.; Ou, I.C.; Tsai, K.J.; Lee, Y.; Chu, Y.H.; Liao, Y.T.; Liu, C. Te Essential factors that affect bioelectricity generation by Rhodopseudomonas palustris strain PS3 in paddy soil microbial fuel cells. Int. J. Energy Res. 2021, 45, 2231–2244. [Google Scholar] [CrossRef]
- Suslick, K.S. Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: New York, NY, USA, 1998; Volume 26, pp. 517–541. [Google Scholar]
- Madan, G.S. Chands Success Guide (Q&A) Inorganic Chemistry; S. Chand Publishing: New Delhi, India, 2005; ISBN 8-12-191857-X. [Google Scholar]
- Anwer, A.H.; Khan, M.D.; Khan, N.; Nizami, A.S.; Rehan, M.; Khan, M.Z. Development of novel MnO2 coated carbon felt cathode for microbial electroreduction of CO2 to biofuels. J. Environ. Manag. 2019, 249, 109376. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Zhuang, L.; Li, W.; Zhou, S.; Zhang, J. Manganese dioxide as an alternative cathodic catalyst to platinum in microbial fuel cells. Biosens. Bioelectron. 2009, 24, 2825–2829. [Google Scholar] [CrossRef]
- Sevda, S.; Sreekrishnan, T.R. Effect of salt concentration and mediators in salt bridge microbial fuel cell for electricity generation from synthetic wastewater. J. Environ. Sci. Health 2012, 47, 878–886. [Google Scholar] [CrossRef]
- Gnana Sundara Raj, B.; Asiri, A.M.; Qusti, A.H.; Wu, J.J.; Anandan, S. Sonochemically synthesized MnO2 nanoparticles as electrode material for supercapacitors. Ultrason. Sonochem. 2014, 21, 1933–1938. [Google Scholar] [CrossRef]
- Wang, L.; Asheim, K.; Vullum, P.E.; Svensson, A.M.; Vullum-Bruer, F. Sponge-like porous manganese(II,III) oxide as a highly efficient cathode material for rechargeable magnesium ion batteries. Chem. Mater. 2016, 28, 6459–6470. [Google Scholar] [CrossRef]
- Çek, N. Examination of Zinc Electrode Performance in Microbial Fuel Cells. Gazi Univ. J. Sci. 2017, 30, 395–402. [Google Scholar]
- Abourached, C. Microbial Fuel Cell for Wastewater Treatment: Heavy Metal Removal, Sewage Sludge Treatment, and its Potential Application in Wastewater Reuse in Irrigation. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 2014. [Google Scholar]
- Chaijak, P.; Lertworapreecha, M.; Changkit, N.; Sola, P. Electricity generation from hospital wastewater in microbial fuel cell using radiation tolerant bacteria. Biointerface Res. Appl. Chem. 2022, 12, 5601–5609. [Google Scholar] [CrossRef]
- Verma, M.; Mishra, V. Bioelectricity Generation by Microbial Degradation of Banana Peel Waste Biomass in a Dual-Chamber S. Cerevisiae-Based Microbial Fuel Cell. SSRN Electron. J. 2022, 168, 106677. [Google Scholar] [CrossRef]
- Lee, J.-H.; Han, J.; Choi, H.; Hur, H.-G. Effects of temperature and dissolved oxygen on Se (IV) removal and Se (0) precipitation by Shewanella sp. HN-41. Chemosphere 2007, 68, 1898–1905. [Google Scholar] [CrossRef] [PubMed]
- Sukkasem, C.; Xu, S.; Park, S.; Boonsawang, P.; Liu, H. Effect of nitrate on the performance of single chamber air cathode microbial fuel cells. Water Res. 2008, 42, 4743–4750. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.V.; Saravanan, R.; Raghavulu, S.V.; Mohanakrishna, G.; Sarma, P.N. Bioelectricity production from wastewater treatment in dual chambered microbial fuel cell (MFC) using selectively enriched mixed microflora: Effect of catholyte. Bioresour. Technol. 2008, 99, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Halim, M.A.; Rahman, M.O.; Ibrahim, M.; Kundu, R.; Biswas, B.K. Effect of Anolyte pH on the Performance of a Dual-Chambered Microbial Fuel Cell Operated with Different Biomass Feed. J. Chem. 2021, 2021, 5465680. [Google Scholar] [CrossRef]
- Halim, A. Study of the Effect of pH on the Performance of Microbial Fuel Cell for Generation of Bioelectricity. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Chaudhari, D.; Dubey, H.; Kshirsagar, D.; Jadhav, V. Influence of microbial fuel cell with porous anode on voltage generation, chemical oxygen demand, chloride content and total dissolved solids. Water Sci. Technol. 2020, 82, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Singer, S.; Tong, Y.; Kimbell, L.; Anderson, E.; Hughes, M.; Zitomer, D.; McNamara, P. Characteristics and applications of biochars derived from wastewater solids. Renew. Sustain. Energy Rev. 2018, 90, 650–664. [Google Scholar] [CrossRef]
- Dinh, K.L.; Wang, C.T.; Dai, H.N.; Tran, V.M.; Le, M.L.P.; Saladaga, I.A.; Lin, Y.A. Lactate and acetate applied in dual-chamber microbial fuel cells with domestic wastewater. Int. J. Energy Res. 2021, 45, 10655–10666. [Google Scholar] [CrossRef]
- Hirooka, K.; Ichihashi, O.; Takeguchi, T. Sodium cobalt oxide as a non-platinum cathode catalyst for microbial fuel cells. Sustain. Environ. Res. 2018, 28, 322–325. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, L. A microbial fuel cell system with manganese dioxide/titanium dioxide/graphitic carbon nitride coated granular activated carbon cathode successfully treated organic acids industrial wastewater with residual nitric acid. Bioresour. Technol. 2020, 304, 122992. [Google Scholar] [CrossRef] [PubMed]
- Gnana kumar, G.; Awan, Z.; Suk Nahm, K.; Stanley Xavier, J. Nanotubular MnO2/graphene oxide composites for the application of open air-breathing cathode microbial fuel cells. Biosens. Bioelectron. 2014, 53, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Liang, B.; Zhong, M.; Li, K.; Qi, Y. Activated carbon-supported multi-doped graphene as high-efficient catalyst to modify air cathode in microbial fuel cells. Electrochim. Acta 2019, 304, 360–369. [Google Scholar] [CrossRef]
- Zhang, S.; Su, W.; Wei, Y.; Liu, J.; Li, K. Mesoporous MnO2 structured by ultrathin nanosheet as electrocatalyst for oxygen reduction reaction in air-cathode microbial fuel cell. J. Power Sources 2018, 401, 158–164. [Google Scholar] [CrossRef]
- Yang, W.; Chata, G.; Zhang, Y.; Peng, Y.; Lu, J.E.; Wang, N.; Mercado, R.; Li, J.; Chen, S. Graphene oxide-supported zinc cobalt oxides as effective cathode catalysts for microbial fuel cell: High catalytic activity and inhibition of biofilm formation. Nano Energy 2019, 57, 811–819. [Google Scholar] [CrossRef]
- Divya Priya, A.; Deva, S.; Shalini, P.; Pydi Setty, Y. Antimony-tin based intermetallics supported on reduced graphene oxide as anode and MnO2@rGO as cathode electrode for the study of microbial fuel cell performance. Renew. Energy 2020, 150, 156–166. [Google Scholar] [CrossRef]
- Yan, Y.; Hou, Y.; Yu, Z.; Tu, L.; Qin, S.; Lan, D.; Chen, S.; Sun, J.; Wang, S. B-doped graphene quantum dots implanted into bimetallic organic framework as a highly active and robust cathodic catalyst in the microbial fuel cell. Chemosphere 2022, 286, 131908. [Google Scholar] [CrossRef] [PubMed]
- Nancharaiah, Y.V.; Venkata Mohan, S.; Lens, P.N.L. Metals removal and recovery in bioelectrochemical systems: A review. Bioresour. Technol. 2015, 195, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Narasingarao, P.; Häggblom, M.M. Identification of anaerobic selenate-respiring bacteria from aquatic sediments. Appl. Environ. Microbiol. 2007, 73, 3519–3527. [Google Scholar] [CrossRef] [PubMed]
- Rege, M.A.; Yonge, D.R.; Mendoza, D.P.; Petersen, J.N.; Bereded-Samuel, Y.; Johnstone, D.L.; Apel, W.A.; Barnes, J.M. Selenium reduction by a denitrifying consortium. Biotechnol. Bioeng. 1999, 62, 479–484. [Google Scholar] [CrossRef]
- Samsudeen, N.; Sharma, A.; Radhakrishnan, T.K.; Matheswaran, M. Performance investigation of multi-chamber microbial fuel cell: An alternative approach for scale up system. J. Renew. Sustain. Energy 2015, 7, 043101. [Google Scholar] [CrossRef]
| Content | Values and Units |
|---|---|
| pH | 6.8 |
| Suspended solids | 100 to 150 mg/L |
| Total dissolved solids | 16 mg/L |
| BOD | 100–180 mg/L |
| COD | 250 mg/L |
| Oil and grease | 10–100 mg/L |
| S No. | Cathode Material | Type of Cathode | Power Density (Wm−2) | Reference |
|---|---|---|---|---|
| 1. | NaCo2O4 carbon cathode | Air-cathode | 0.6 | [38] |
| 2. | Manganese dioxide/titanium dioxide/graphitic carbon nitride coated granular activated carbon cathode | - | 1.17 | [39] |
| 3. | MnO2 nanotubes/graphene oxide nanocomposite modified cathode | Air-breathing cathode | 3.359 | [40] |
| 4. | Fe-Ag-N multi-doped graphene Cathode | Air-cathode | 1.96 | [41] |
| 5. | α-MnO2 Nanosheet | Air-cathode | 1.671 | [42] |
| 6. | Graphene oxide-supported zinc cobalt oxide cathode | - | 0.773 | [43] |
| 7. | MnO2@rGO Cathode | - | 0.008 | [44] |
| 8. | BGQDs/MOF-15 cathode | - | 0.703 | [45] |
| 9. | Manganese dioxide coated graphite cathode | - | 1.29 W/m2 | This study |
| S. No | Operating Conditions | Coating on Electrode | Maximum Voltage (V) | Maximum Power Density (W/m2) | % Difference in Voltage Production Compared to Coated and Uncoated | % Difference in Power Density Compared to Coated and Uncoated |
|---|---|---|---|---|---|---|
| 1 | Without wastewater-100 ppm (Control) | Uncoated | 0.874 | 0.55 | - | - |
| 2 | Without wastewater-100 ppm (Control) | Coated | 1.070 | 0.82 | 19.6 | 27 |
| 3 | Without wastewater-150 ppm (Control) | Uncoated | 0.67 | 0.32 | - | |
| 4 | Without wastewater-150 ppm (Control) | Coated | 0.88 | 0.56 | 21 | 24 |
| 5 | With wastewater-100 ppm | Uncoated | 0.95 | 0.64 | - | |
| 6 | With wastewater-100 ppm | Coated | 1.22 | 1.1 | 27 | 46 |
| 7 | With wastewater-150 ppm | Uncoated | 0.92 | 0.60 | - | |
| 8 | With wastewater-150 ppm | Coated | 1.35 | 1.29 | 43 | 69 |
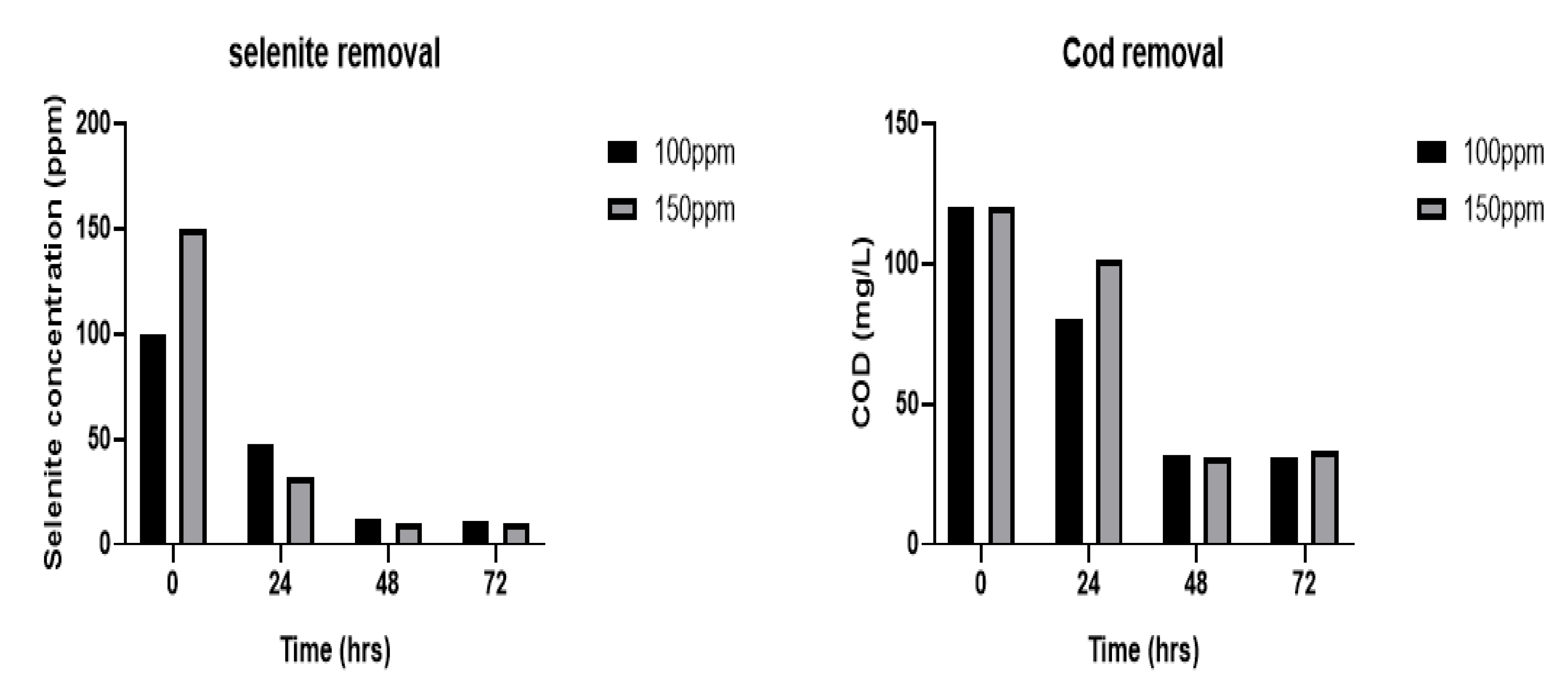
| Operating Condition | Initial Selenite Concentration (ppm) | Final Selenite Concentration (ppm) | % Selenite Removal | Initial COD (mg/L) | Final COD (mg/L) | % COD Removal |
|---|---|---|---|---|---|---|
| Without wastewater (Control) | 100 | 14 | 86 | - | - | - |
| Without wastewater (Control) | 150 | 9 | 91 | - | - | - |
| With wastewater and selenite | 100 | 9 | 91 | 120 | 31 | 74 |
| With wastewater and selenite | 150 | 7 | 93 | 120 | 33 | 73 |
| Time (h) | Final COD (mg/L) for Coated Electrode with 100 ppm of Selenite | Coulombic Efficiency Coated Electrode with 100 ppm of Selenite (%) | Final COD (mg/L) for Coated Electrode with 150 ppm of Selenite | Coulombic Efficiency Coated Electrode with 150 ppm of Selenite (%) |
|---|---|---|---|---|
| 24 | 80 | 49.29 | 101 | 20.7 |
| 48 | 32 | 44.61 | 31 | 59.16 |
| 72 | 31 | 64.71 | 33 | 70.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velayudhan, J.; Subramanian, S. Development of Manganese-Coated Graphite Electrode in a Dual-Chambered Fuel Cell for Selenite Removal and Bio-Electricity Generation from Wastewater Effluent by Bacillus cereus. Energies 2023, 16, 2880. https://doi.org/10.3390/en16062880
Velayudhan J, Subramanian S. Development of Manganese-Coated Graphite Electrode in a Dual-Chambered Fuel Cell for Selenite Removal and Bio-Electricity Generation from Wastewater Effluent by Bacillus cereus. Energies. 2023; 16(6):2880. https://doi.org/10.3390/en16062880
Chicago/Turabian StyleVelayudhan, Jayanthi, and Sangeetha Subramanian. 2023. "Development of Manganese-Coated Graphite Electrode in a Dual-Chambered Fuel Cell for Selenite Removal and Bio-Electricity Generation from Wastewater Effluent by Bacillus cereus" Energies 16, no. 6: 2880. https://doi.org/10.3390/en16062880
APA StyleVelayudhan, J., & Subramanian, S. (2023). Development of Manganese-Coated Graphite Electrode in a Dual-Chambered Fuel Cell for Selenite Removal and Bio-Electricity Generation from Wastewater Effluent by Bacillus cereus. Energies, 16(6), 2880. https://doi.org/10.3390/en16062880






