Hydrocarbon Generation Mechanism of Mixed Siliciclastic–Carbonate Shale: Implications from Semi–Closed Hydrous Pyrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Characterization
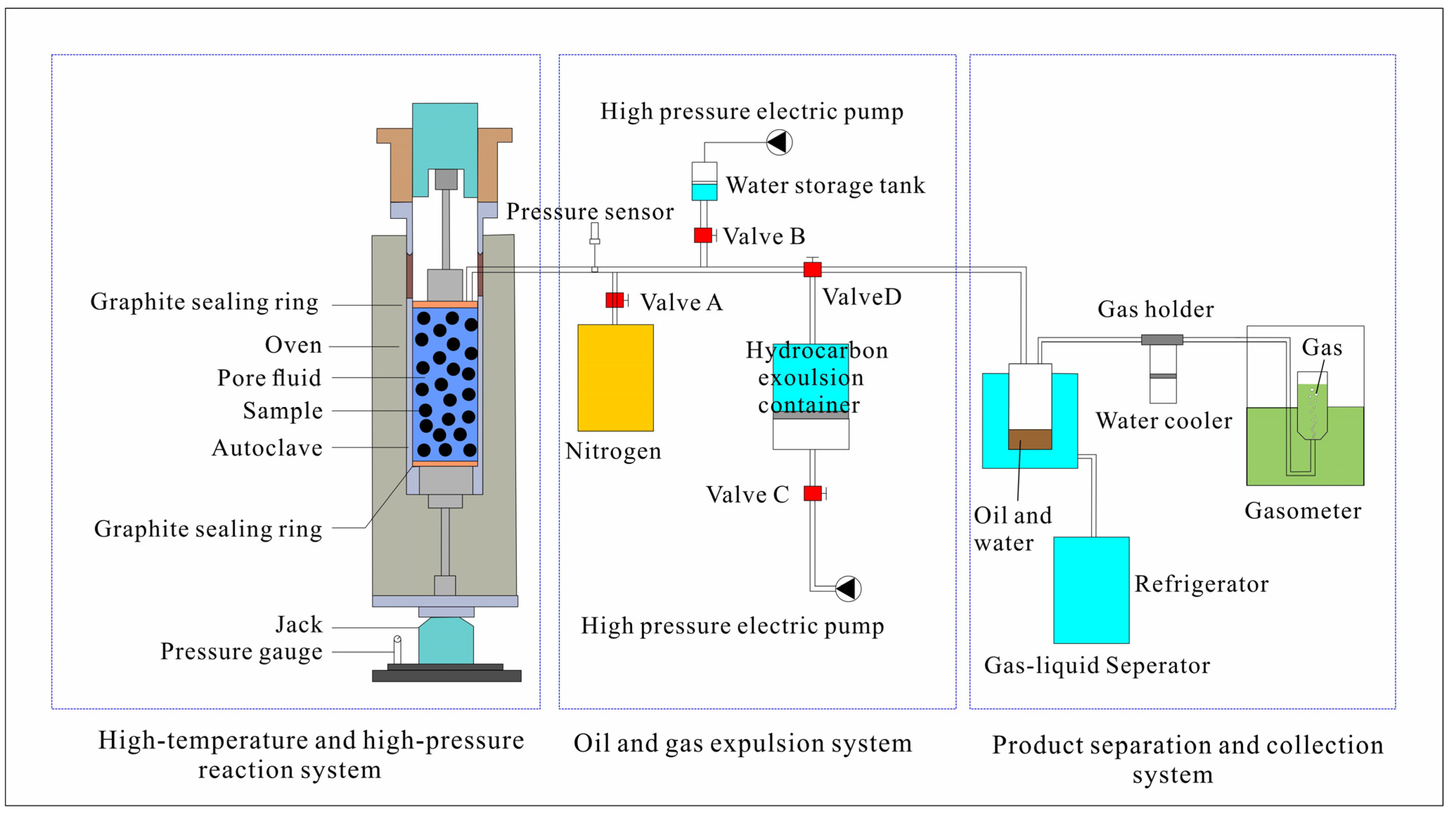
2.2. Semi–Closed Pyrolysis Experiments
2.3. Product Analysis
2.3.1. Gas Composition and Quantities
2.3.2. Oil (Expelled Oil) and Bitumen (Retained Oil)
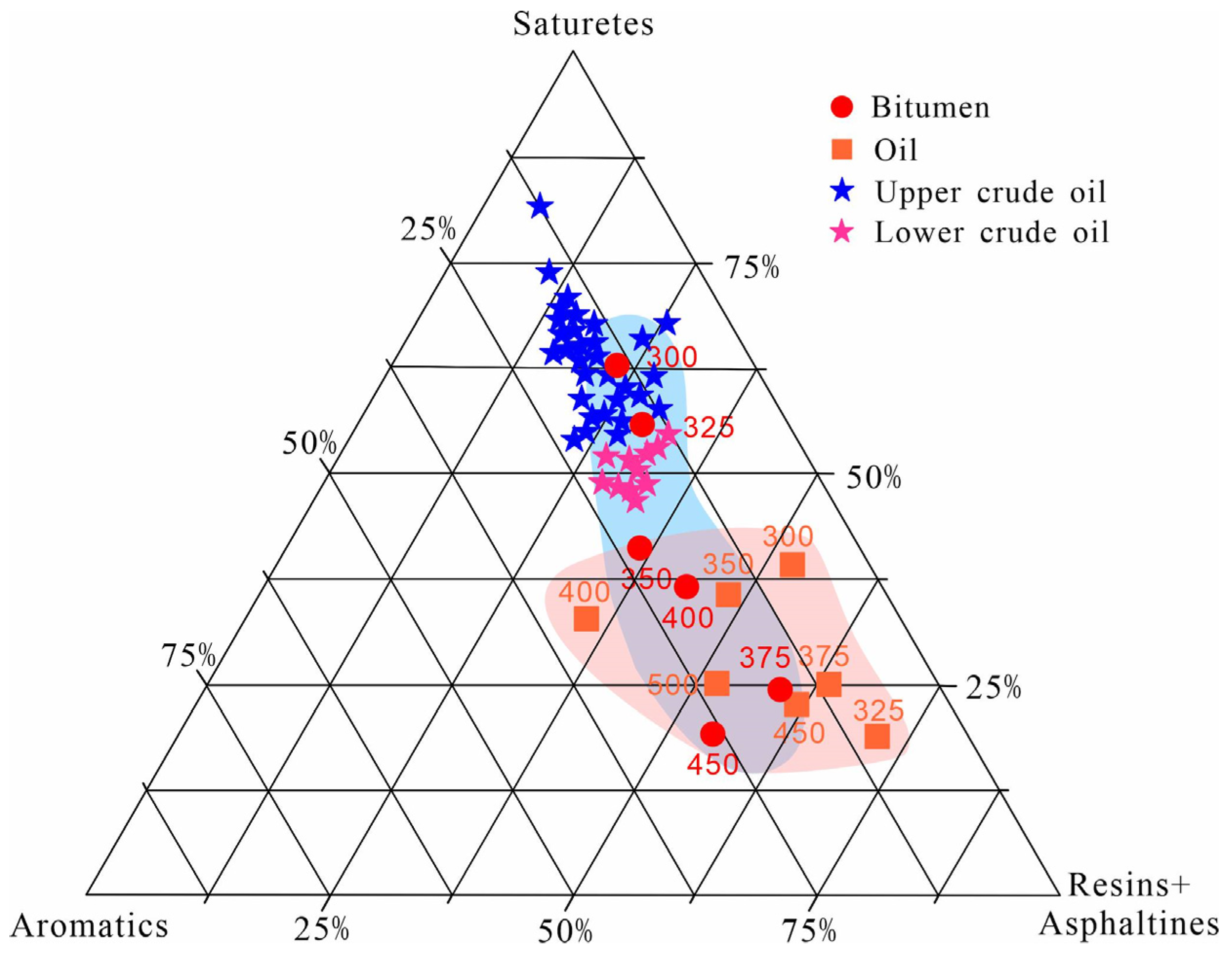
2.3.3. Fractional Composition of Oil and Bitumen
3. Results
3.1. Overall Mass Balance Calculation
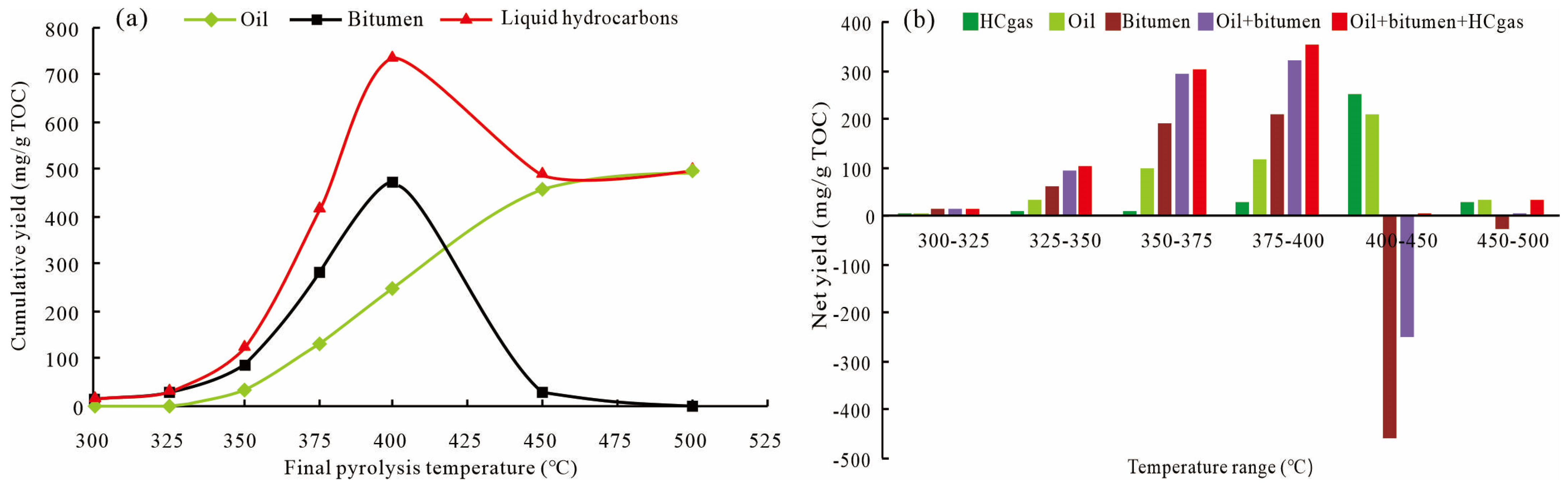
3.2. Yields of Liquid Hydrocarbons
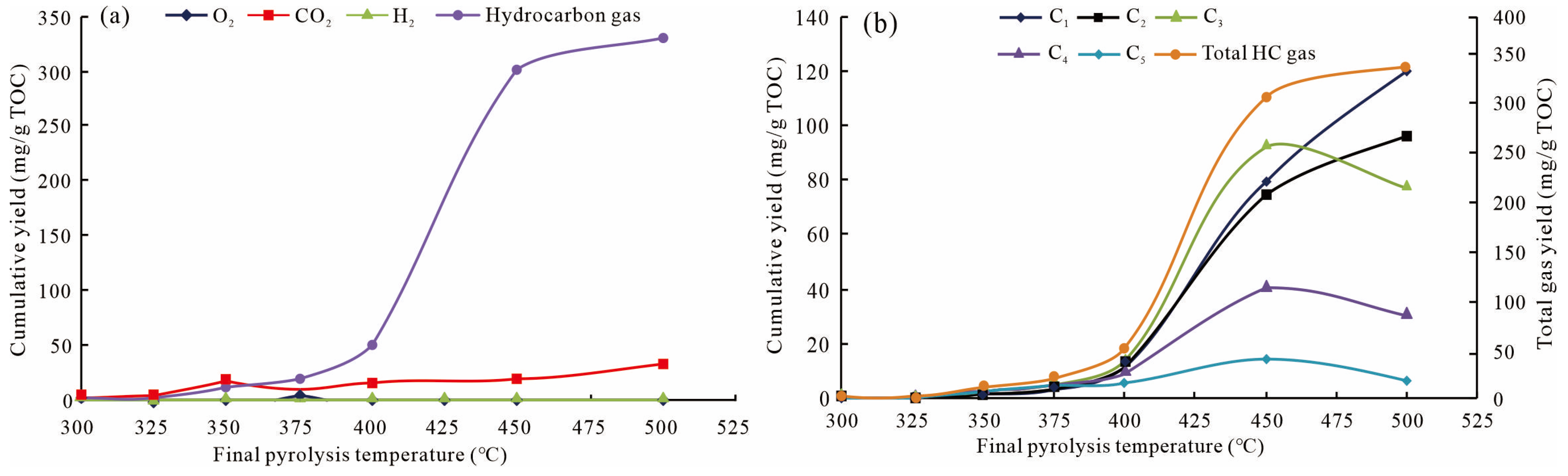
3.3. Product Yields of Gas
4. Discussion
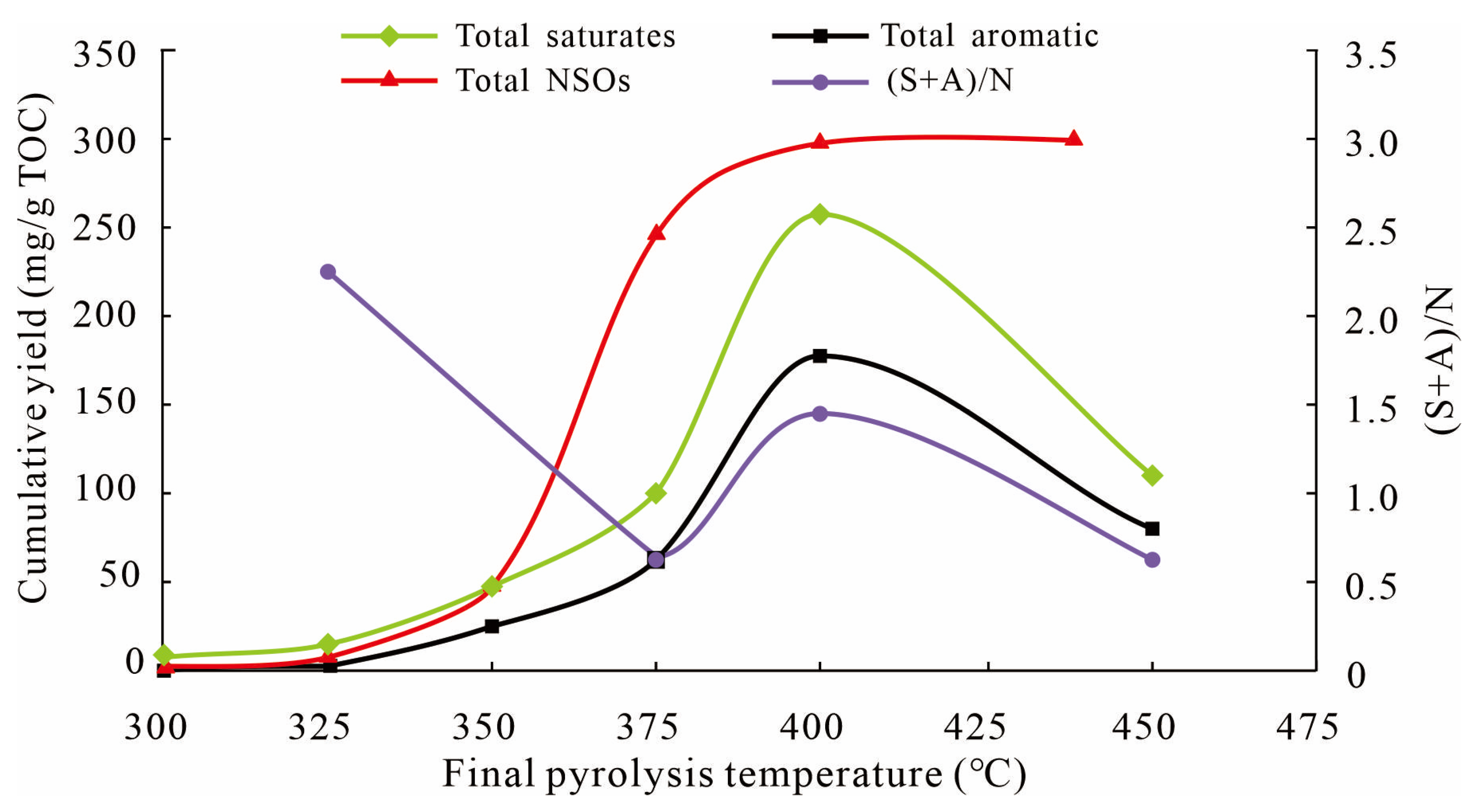
4.1. Characteristics of Liquid Hydrocarbon Generation, Expulsion, and Retention
4.2. Kerogen Decomposition Process and Oil Generation Mechanism
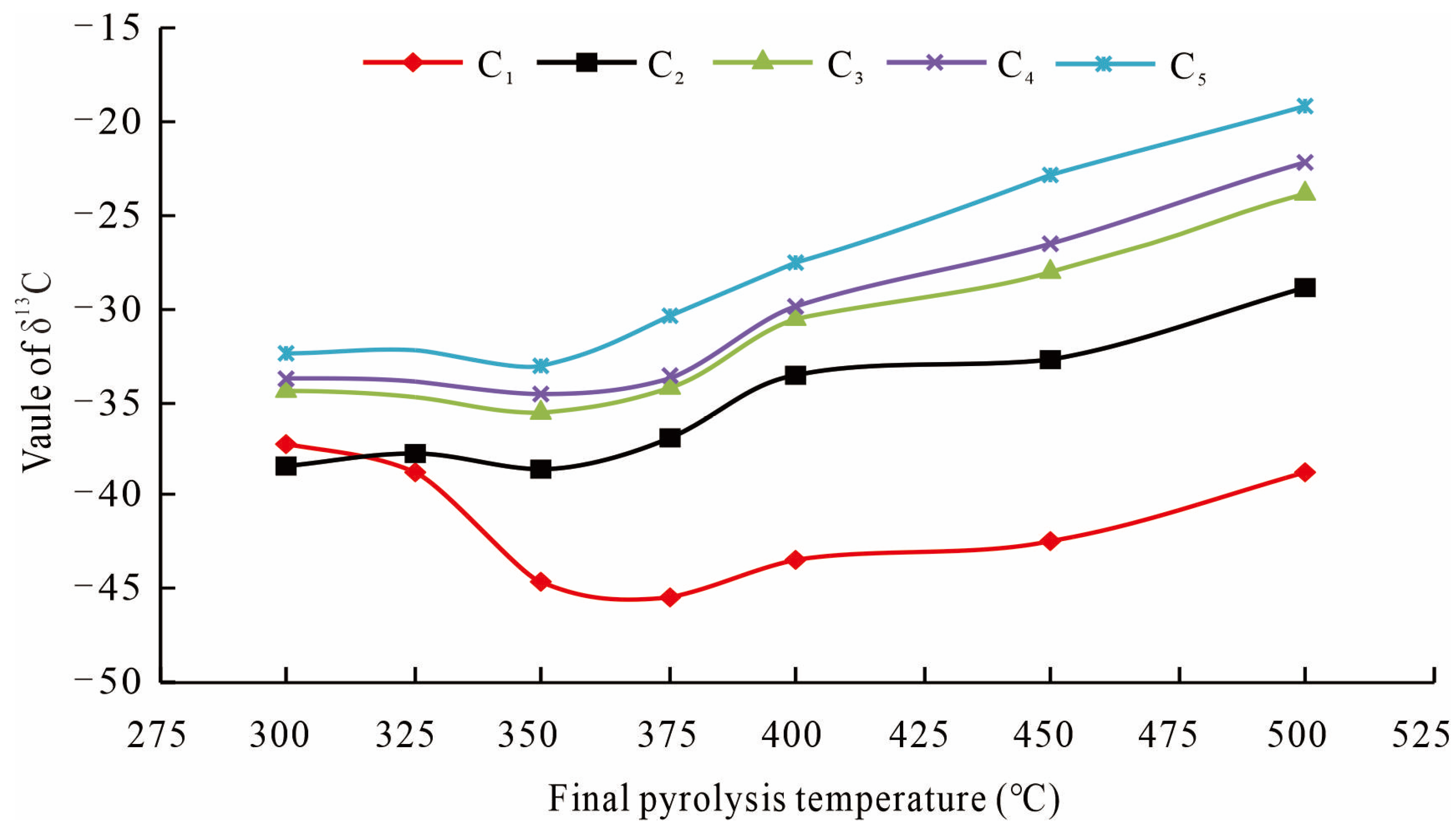
4.3. Implications for Shale Gas Genesis
4.4. Hydrocarbon Expulsion Efficiency and Producible Shale Oil Assessment
5. Conclusions
- (1)
- The hydrocarbon generation stage of mixed siliciclastic–carbonate shale from Lucaogou Formation can be divided into the kerogen cracking stage (300–350 °C), the peak oil generation stage (350–400 °C), the wet gas generation stage (400–450 °C), and the hydrocarbon gas secondary cracking stage (450–500 °C). The liquid hydrocarbon yield (oil + bitumen) reached at a peak of 720.42 mg/g TOC at 400 °C.
- (2)
- Oil and bitumen shared the same group fractions, but there was a clear separation with no overlap between oil and bitumen in the initial stage of pyrolysis (300 and 325 °C). The group fractions of bitumen overlapped with those of crude oil from the upper section of Lucaogou Formation, and bitumen was more enriched with saturates than their corresponding oil. These results support the reservoir–forming model of “self–generation and self–preservation” of the shale of Lucaogou Formation. It also shows that the semi–closed hydrous thermal simulation experiment could reliably simulate the hydrocarbon generation process for site conditions.
- (3)
- In the initial stage of pyrolysis (300–350 °C), due to the joint adsorption of shale organic matter and pores, the hydrocarbon expulsion efficiency of shale was relatively low, with an average of 13%. The source rock reached the hydrocarbon explosion threshold (average HEE = 36%) at 350–400 °C and then rose sharply to 97% at 450 °C. These results mean that considerable amounts of oil cannot be discharged from shale until the temperature reaches 450 °C.
- (4)
- The producible oil was positively correlated with total liquid hydrocarbons, showing an arched evolution trend with the increase in the pyrolysis temperature and reaching a maximum of 512.46 mg/g TOC at 400 °C. After comprehensive consideration of the generated yield and hydrocarbon expulsion efficiency. Therefor, 400 °C is considered to be the most suitable temperature for fracturing technology.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lei, Y.; Zhijun, J.I.N. Global shale oil development and prospects. China Pet. Explor. 2019, 24, 553. [Google Scholar]
- Zou, C.; Yang, Z.; Cui, J.; Zhu, R.; Hou, L.; Tao, S.; Yuan, X.; Wu, S.; Lin, S.; Wang, L.; et al. Formation mechanism, geological characteristics and development strategy of nonmarine shale oil in China. Petrol. Explor. Dev. 2013, 40, 15–27. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, S.; Hou, L.; Tao, Y.; Li, X.; Guo, B.; Yang, Z. Types and resource potential of continental shale oil in China and its boundary with tight oil. Pet. Explor. Dev. 2020, 47, 1–11. [Google Scholar] [CrossRef]
- Shao, X.H.; Pang, X.Q.; Li, M.W.; Li, Z.M.; Zhao, Y. Hydrocarbon generation from lacustrine shales with retained oil during thermal maturation. Pet. Sci. 2020, 17, 1478–1490. [Google Scholar] [CrossRef]
- Hill, R.J.; Zhang, E.; Katz, B.J.; Tang, Y. Modeling of gas generation from the Barnett shale, Fort Worth Basin, Texas. AAPG Bull. 2007, 91, 501–521. [Google Scholar] [CrossRef]
- Ritter, U. Fractionation of petroleum during expulsion from kerogen. J. Geochem. Explor. 2003, 78, 417–420. [Google Scholar] [CrossRef]
- Li, T.; Jiang, Z.; Xu, C.; Liu, B.; Liu, G.; Wang, P.; Li, X.; Chen, W.; Ning, C.; Wang, Z. Effect of pore structure on shale oil accumulation in the lower third member of the Shahejie formation, Zhanhua Sag, eastern China: Evidence from gas adsorption and nuclear magnetic resonance. Mar. Pet. Geol. 2017, 88, 932–949. [Google Scholar] [CrossRef]
- Sun, L.; He, W.; Feng, Z.; Zeng, H.; Jiang, H.; Pan, Z. Shale Oil and Gas Generation Process and Pore Fracture System Evolution Mechanisms of the Continental Gulong Shale, Songliao Basin, China. Energy Fuels 2022, 36, 6893–6905. [Google Scholar] [CrossRef]
- Seewald, J.S. Aqueous geochemistry of low molecular weight hydrocarbons at elevated temperatures and pressures: Constraints from mineral buffered laboratory experiments. Geochim. Et Cosmochim. Acta 2001, 65, 1641–1664. [Google Scholar] [CrossRef]
- Seewald, J.S. Organic–inorganic interactions in petroleum–producing sedimentary basins. Nature 2003, 426, 327–333. [Google Scholar] [CrossRef]
- Pan, C.; Jiang, L.; Liu, J.; Zhang, S.; Zhu, G. The effects of calcite and montmorillonite on oil cracking in confined pyrolysis Experiments. Org. Geochem. 2010, 41, 611–626. [Google Scholar] [CrossRef]
- Xi, K.; Cao, Y.C.; Zhu, R.K.; Shao, Y.; Xue, X.; Wang, X.; Gao, Y.; Zhang, J. Rock types and characteristics of tight oil reservoir in Permian Lucaogou Formation, Jimusaer sag. Acta Petrol. Sin. 2015, 36, 1495–1507, (In Chinese with English abstract). [Google Scholar]
- Wu, H.; Hu, W.; Cao, J.; Wang, X.; Wang, X.; Liao, Z. A unique lacustrine mixed dolomitic–clastic sequence for tight oil reservoir within the middle Permian Lucaogou Formation of the Junggar Basin, NW China: Reservoir characteristics and origin. Mar. Pet. Geol. 2016, 76, 115–132. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, G.; Cao, Z.; Tao, S.; Felix, M.; Kong, Y.; Zhang, Y. Characteristics and formation mechanism of multi–source mixed sedimentary rocks in a saline lake, a case study of the Permian Lucaogou Formation in the Jimusaer Sag, northwest China. Mar. Pet. Geol. 2019, 102, 704–724. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, J.; Tao, K.; Li, E.; Ma, C.; Shi, C. Reevaluating the source and accumulation of tight oil in the middle Permian Lucaogou Formation of the Junggar Basin, China. Mar. Pet. Geol. 2020, 117, 104384. [Google Scholar] [CrossRef]
- You, J.; Lee, K.J. The experimental investigation and data–driven modeling for thermal decomposition kinetics of Green River Shale. Fuel 2022, 320, 123899. [Google Scholar] [CrossRef]
- Kuang, W.; Lu, M.; Yeboah, I.; Qian, G.; Duan, X.; Yang, J. A comprehensive kinetics study on non–isothermal pyrolysis of kerogen from green river oil shale. Chem. Eng. J. 2018, 377, 120275. [Google Scholar] [CrossRef]
- Hillier, J.L.; Fletcher, T.H.; Solum, M.S.; Pugmire, R.J. Characterization of macromolecular structure of pyrolysis products from a Colorado Green River oil shale. Ind. Eng. Chem. Res. 2013, 52, 15522–15532. [Google Scholar] [CrossRef]
- Le Doan, T.V.; Bostrom, N.W.; Burnham, A.K.; Kleinberg, R.L.; Pomerantz, A.E.; Allix, P. Green River oil shale pyrolysis: Semi–open conditions. Energy Fuels 2013, 27, 6447–6459. [Google Scholar] [CrossRef]
- Burnham, A.K. Kinetic models of vitrinite, kerogen, and bitumen reflectance. Org. Geochem. 2019, 131, 50–59. [Google Scholar] [CrossRef]
- Lewan, M.D.; Roy, S. Role of water in hydrocarbon generation from Type–I kerogen in Mahogany oil shale of the Green River Formation. Org. Geochem. 2011, 42, 31–41. [Google Scholar] [CrossRef]
- Bohacs, K.M.; Carroll, A.R.; Neal, J.E.; Mankiewicz, P.J. Lake–basin type, sourcepotential, and hydrocarbon character: An integrated–sequence–stratigraphice geochemical framework. In Aapg Studies in Geology; Lake Basins Through Space and Time; Gierlowski–Kordesch, E.H., Kelts, K.R., Eds.; AAPG: Tulsa, OK, USA, 2000; Volume 46, p. 3e34. [Google Scholar]
- Burnett, A.P.; Soreghan, M.J.; Scholz, C.A.; Brown, E.T. Tropical East African climate change and its relation to global climate: A record from Lake Tanganyika, tropical East Africa, over the past 90þ kyr. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 303, 155e167. [Google Scholar] [CrossRef]
- Spigolon, A.L.; Lewan, M.D.; De Barros Penteado, H.L.; Coutinho, L.F.C.; Mendonça Filho, J.G. Evaluation of the petroleum composition and quality with increasing thermal maturity as simulated by hydrous pyrolysis: A case study using a Brazilian source rock with Type I kerogen. Org. Geochem. 2015, 83, 27–53. [Google Scholar] [CrossRef]
- Korkmaz, S.; Kara–G€ulbay, R.; Iztan, Y.H. Organicgeochemistry of the Lower Cretaceous black shales and oilseep in the Sinop Basin, northern Turkey: An oil–sourcerock correlation study. Mar. Pet. Geol. 2013, 43, 272–283. [Google Scholar] [CrossRef]
- Ma, Z.; Tan, J.; Zheng, L.; Shen, B.; Wang, Z.; Shahzad, A. Evaluating gas generation and preservation of the Wufeng–Longmaxi Formation shale in southeastern Sichuan Basin, China: Implications from semiclosed hydrous pyrolysis. Mar. Pet. Geol. 2021, 129, 105102. [Google Scholar] [CrossRef]
- Ma, Z.; Zheng, L.; Xu, X.; Bao, F.; Yu, X. Thermal simulation experiment of organic matter–rich shale and implication for organic pore formation and evolution. Pet. Res. 2017, 2, 347–354. [Google Scholar] [CrossRef]
- Fang, S.; Xu, H.; Song, Y.; Li, J.; Liu, L.; Zhang, J. Characteristics and evolution of the composite petroleum system in Jimsar Depression, eastern Junggar Basin. Acta Geosci. Sin. 2005, 26, 259–264, (In Chinese with English abstract). [Google Scholar]
- Qiu, N.; Yang, H.; Wang, X. Tectonothermal evolution in the Junggar Basin. Chin. J. Geol. 2002, 37, 423–429, (In Chinese with English abstract). [Google Scholar]
- Wu, Y.; Zhang, Z.; Sun, L.; Li, Y.; He, C.; Ji, L.; Su, L.; Zhang, D. Hydrocarbon generation and potential in continental organic–rich shales at the highly–mature stage, as determined by hydrous pyrolysis under supercritical conditions. Int. J. Coal Geol. 2018, 187, 83–93. [Google Scholar] [CrossRef]
- Stainforth, J.G. Practical kinetic modeling of petroleum generation and expulsion. Mar. Pet. Geol. 2009, 26, 552–572. [Google Scholar] [CrossRef]
- Tang, Y.; Perry, J.; Jenden, P.; Schoell, M. Mathematical modeling of stable carbon isotope ratios in natural gases. Geochim. Cosmochim. Acta 2000, 64, 2673–2687. [Google Scholar] [CrossRef]
- He, K.; Zhang, S.; Mi, J.; Zhang, W. The evolution of chemical groups and isotopic fractionation at different maturation stages during lignite pyrolysis. Fuel 2018, 211, 492–506. [Google Scholar] [CrossRef]
- Liu, P.; Wang, X.; Meng, Q.; Wang, X.; Zhang, L.; Liu, C. Simulation of shale gas generation by using different experimental systems: A case study from Chang7 shale in the Ordos Basin. J. Nat. Gas Sci. Eng. 2018, 49, 169–178. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, Q.; Jiang, C.; Liu, X.; Reyes, J.; Mort, A. Source rock characteristics and Rock–Eval–based hydrocarbon generation kinetic models of the lacustrine Chang–7 Shale of Triassic Yanchang Formation, Ordos Basin, China. Int. J. Coal Geol. 2017, 182, 52–65. [Google Scholar] [CrossRef]
- Wu, H.; Hu, W.; Wang, Y.; Tao, K.; Tang, Y.; Cao, J.; Wang, X.; Kang, X. Depositional conditions and accumulation models of tight oils in the middle Permian Lucaogou Formation in Junggar Basin, northwestern China: New insights from geochemical analysis. AAPG Bull. 2021, 105, 2477–2518. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, G.; Xiang, B.; Wang, P.; Niu, G.; Niu, Z.; Li, C.; Wang, C. Geochemical characteristics of crude oil from a tight oil reservoir in the Lucaogou Formation, Jimusar sag, Junggar Basin. AAPG Bull. 2017, 101, 39–72. [Google Scholar] [CrossRef]
- Hu, T.; Pang, X.; Wang, X.; Pang, H.; Tang, L.; Pan, Z.; Wang, Y.; Shen, W.; Jiang, H.; Pang, Y. Source rock characteristics of Permian Lucaogou Formation in the Jimusar Sag, Junggar Basin, northwest China, and its significance on tight oil source and occurrence. Geol. J. 2017, 52, 624–645. [Google Scholar] [CrossRef]
- Hou, L.; Ma, W.; Luo, X.; Liu, J.; Liu, S.; Zhao, Z. Hydrocarbon generation–retention–expulsion mechanism and shale oil producibility of the permian lucaogou shale in the Junggar Basin as simulated by semi–open pyrolysis experiments. Mar. Pet. Geol. 2021, 125, 104880. [Google Scholar] [CrossRef]
- Ruble, T.E.; Lewan, M.D.; Philp, R.P. New insights on the Green River petroleum system in the Uinta basin from hydrous pyrolysis experiments. AAPG (Am. Assoc. Pet. Geol.) Bull. 2001, 85, 1333–1371. [Google Scholar] [CrossRef]
- Hou, L.; Ma, W.; Luo, X.; Liu, J. Characteristics and quantitative models for hydrocarbon generation–retention– production of shale under ICP conditions: Example from the Chang 7 member in the Ordos Basin. Fuel 2020, 279, 118497. [Google Scholar] [CrossRef]
- Faraj, B.; Jarvie, D. Producibility and commerciality of shale resource systems: Contrasting geochemical attributes of shale gas and shale oil systems. APPEA J. 2013, 53, 469. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, G.; Kong, Y.; Wang, C.; Niu, Z.; Zhang, J.; Geng, C.; Shan, X.; Zhang, J.; Wei, Z. Lacustrine tight oil accumulation characteristics: Permian lucaogou formation in jimusaer sag, junggar basin. Int. J. Coal Geol. 2016, 153, 37–51. [Google Scholar] [CrossRef]
- Behar, F.; Lorant, F.; Lewan, M.D. Role of NSO compounds during primary cracking of a Type II kerogen and a Type III lignite. Org. Geochem. 2008, 39, 1–22. [Google Scholar] [CrossRef]
- Behar, F.; Roy, S.; Jarvie, D. Artificial maturation of a Type I kerogen in closed system, mass balance and kinetic modeling. Org. Geochem. 2010, 41, 1235–1247. [Google Scholar] [CrossRef]
- Burnham, A.K. Global Chemical Kinetics of Fossil Fuels. How to Model Maturation and Pyrolysis; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Freund, H.; Walters, C.C.; Kelemen, S.R.; Siskin, M.; Gorbaty, M.L.; Curry, D.J.; Bence, A.E. Predicting oil and gas compositional yields via chemical structure–chemical yield modeling (CS–CYM): Part 1–Concepts and implementation. Org. Geochem. 2007, 38, 288–305. [Google Scholar] [CrossRef]
- Huang, Z.; Liang, T.; Zhan, Z.W.; Zou, Y.R.; Li, M.; Peng, P. Chemical structure evolution of kerogen during oil generation. Mar. Pet. Geol. 2018, 98, 422–436. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, J.; Yang, H.; Qi, L. Chemical Composition and Structural Characteristics of Oil Shales and Their Kerogens Using Fourier Transform Infrared (FTIR) Spectroscopy and Solid–State 13C Nuclear Magnetic Resonance (NMR). Energy Fuels 2016, 30, 6271–6280. [Google Scholar] [CrossRef]
- Mao, J.; Fang, X.; Lan, Y.; Schimmelmann, A.; Mastalerz, M.; Xu, L.; Schmidt–Rohr, K. Chemical and nanometer–scale structure of kerogen and its change during thermal maturation investigated by advanced solid–state 13C NMR spectroscopy. Geochim. Et Cosmochim. Acta 2010, 74, 2110–2127. [Google Scholar] [CrossRef]
- Ru, X.; Cheng, Z.; Song, L.; Wang, H.; Li, J. Experimental and computational studies on the average molecular structure of Chinese Huadian oil shale kerogen. J. Mol. Struct. 2012, 1030, 10–18. [Google Scholar] [CrossRef]
- Lis, G.P.; Mastalerz, M.; Schimmelmann, A.; Lewan, M.D.; Stankiewicz, B.A. FTIR absorption indices for thermal maturity in comparison with vitrinite reflectance R0 in type–II kerogens from Devonian black shales. Org. Geochem. 2005, 36, 1533–1552. [Google Scholar] [CrossRef]
- Burnham, A.K.; Pomerantz, A.E.; Gelin, F. Oil, bitumen, and other confusing concepts: What do lab experiments really tell us? AAPG Bull. 2018, 102, 653–669. [Google Scholar] [CrossRef]
- Ma, W.; Hou, L.; Luo, X.; Tao, S.; Guan, P.; Liu, J.; Lin, S. Role of bitumen and NSOs during the decomposition process of a lacustrine Type–II kerogen in semi–open pyrolysis system. Fuel 2020, 259, 116211. [Google Scholar] [CrossRef]
- Katz, B.; Lin, F. Lacustrine basin unconventional resource plays: Key differences. Mar. Pet. Geol. 2014, 56, 255–265. [Google Scholar] [CrossRef]
- Vu, T.; Horsfield, B.; Mahlstedt, N.; Schenk, H.J.; Kelemen, S.R.; Walters, C.C. The structural evolution of organic matter during maturation of coals and its impact on petroleum potential and feedstock for the deep biosphere. Org. Geochem. 2013, 62, 17–27. [Google Scholar] [CrossRef]
- Hazra, B.; Karacan, C.Ö.; Tiwari, D.M.; Singh, P.K.; Singh, A.K. Insights from Rock–Eval analysis on the influence of sample weight on hydrocarbon generation from Lower Permian organic matter rich rocks, West Bokaro basin, India. Mar. Pet. Geol. 2019, 106, 160–170. [Google Scholar] [CrossRef]
- Hazra, B.; Singh, D.P.; Chakraborty, P.; Singh, P.K.; Sahu, S.G.; Adak, A.K. Using rock–eval S4T(peak) as thermal maturity proxy for shales. Mar. Pet. Geol. 2021, 127, 104977. [Google Scholar] [CrossRef]
- Karayigit, A.I.; Yerin, U.O.; Oskay, R.G.; Bulut, Y.; Cordoba, P. Enrichment and distribution of elements in the middle miocene coal seams in the orhaneli coalfield (nw turkey). Int. J. Coal Geol. 2021, 247, 103854. [Google Scholar] [CrossRef]
- Craddock, P.R.; Le Doan, T.V.; Bake, K.; Polyakov, M.; Charsky, A.M.; Pomerantz, A.E. Evolution of kerogen and bitumen during thermal maturation via semi–open pyrolysis investigated by infrared spectroscopy. Energy Fuels 2015, 29, 2197–2210. [Google Scholar] [CrossRef]
- Hou, L.; Ma, W.; Luo, X.; Tao, S.; Guan, P.; Liu, J. Chemical structure changes of lacustrine Type–II kerogen under semi–open pyrolysis as investigated by solid–state 13C NMR and FT–IR spectroscopy. Mar. Pet. Geol. 2020, 116, 104348. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Miao, X.; Gan, M.; Li, X. Geochemistry in geologic CO2 utilization and storage: A brief review. Adv. Geo–Energy Res. 2019, 3, 304–313. [Google Scholar] [CrossRef]
- Wu, H.; Hu, W.; Tang, Y.; Cao, J.; Wang, X.; Wang, Y.; Kang, X. The impact of organic fluids on the carbon isotopic compositions of carbonate–rich reservoirs: Case study of the Lucaogou Formation in the Jimusaer Sag, Junggar Basin, NW China. Mar. Pet. Geol. 2017, 85, 136–150. [Google Scholar] [CrossRef]
- Kotarba, M.J.; Lewan, M.D. Sources of natural gases in Middle Cambrian reservoirs in Polish and Lithuanian Baltic Basin as determined by stable isotopes and hydrous pyrolysis of Lower Palaeozoic source rocks. Chem. Geol. 2013, 345, 62–76. [Google Scholar] [CrossRef]
- Hill, R.J.; Tang, Y.; Kaplan, I.R. Insights into oil cracking based on laboratory experiments. Org. Geochem. 2003, 34, 1651–1672. [Google Scholar] [CrossRef]
- Burnham, A.K.; McConaghy, J.R. Semi–open pyrolysis of oil shale from the Garden Gulch Member of the Green River Formation. Energy Fuels 2014, 28, 7426–7439. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiang, C.; Liao, Y. Relationship between hydrocarbon gas generation and kerogen structural evolution revealed by closed system pyrolysis and quantitative Py–GC analysis of a type II kerogen. Energy Fuels 2020, 35, 251–263. [Google Scholar] [CrossRef]
- Lewan, M.D.; Henry, A.A. Gas: Oil Ratios for Source Rocks Containing Type–I, –II, –IIS, and–III Kerogens as Determined by Hydrous Pyrolysis; US Department of the Interior: Washion, DC, USA; US Geological Survey: Reston, VA, USA, 1999. [Google Scholar]
- Eseme, E.; Krooss, B.M.; Littke, R. Evolution of petrophysical properties of oil shales during high–temperature compaction tests: Implications for petroleum expulsion. Mar. Pet. Geol. 2013, 31, 110–124. [Google Scholar] [CrossRef]
- Bai, H.; Pang, X.; Kuang, L.; Pang, H.; Wang, X.; Jia, X.; Zhou, L.; Hu, T. Hydrocarbon expulsion potential of source rocks and its influence on the distribution of lacustrine tight oil reservoir, Middle Permian Lucaogou Formation, Jimsar Sag, Junggar Basin, Northwest China. J. Pet. Sci. Eng. 2017, 149, 740–755. [Google Scholar] [CrossRef]
- Zhou, L.; Pang, X.; Wu, L.; Kuang, L.; Pang, H.; Jiang, F.; Bai, H.; Peng, J.; Pan, Z.; Zheng, D. Petroleum generation and expulsion in middle Permian Lucaogou Formation, Jimusar Sag, Junggar Basin, northwest China: Assessment of shale oil resource potential. Geol. J. 2017, 52, 1032–1048. [Google Scholar] [CrossRef]
- Abrams, M.A.; Gong, C.; Garnier, C.; Sephton, M.A. A new thermal extraction protocol to evaluate liquid rich unconventional oil in place and in–situ fluid chemistry. Mar. Pet. Geol. 2017, 88, 659–675. [Google Scholar] [CrossRef]
- Zhou, F.; Su, H.; Liang, X.; Meng, L.; Yuan, L.; Li, X.; Liang, T. Integrated hydraulic fracturing techniques to enhance oil recovery from tight rocks. Pet. Explor. Dev. 2019, 46, 1065–1072. [Google Scholar] [CrossRef]
- Ritter, U. Solubility of petroleum compounds in kerogen: Implications for petroleum expulsion. Org. Geochem. 2003, 34, 319–326. [Google Scholar] [CrossRef]
- Jarvie, D.M. Shale Resource Systems for Oil and Gas: Part 2–Shale–Oil Resource Systems; American Association of Petroleum Geologists: Tulsa, OK, USA, 2012. [Google Scholar]
- Li, M.; Chen, Z.; Ma, X.; Cao, T.; Li, Z.; Jiang, Q. A numerical method for calculating total oil yield using a single routine Rock–Eval program: A case study of the Eocene Shahejie Formation in Dongying Depression, Bohai Bay Basin, China. Int. J. Coal Geol. 2018, 191, 49–65. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, Z.; Jiang, S.; Chang, J.; Li, X.; Wang, X.; Zhu, L. Pore evolution and formation mechanism of organic–rich shales in the whole process of hydrocarbon generation: Study of artificial and natural shale samples. Energy Fuels 2019, 34, 332–347. [Google Scholar] [CrossRef]
- Curtis, M.E.; Cardott, B.J.; Sondergeld, C.H.; Rai, C.S. Development of organic porosity in the Woodford Shale with increasing thermal maturity. Int. J. Coal Geol. 2012, 103, 26–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, S.; Shen, C.; Liao, Z.; Xu, J.; Zhang, X. Factors influencing the evolution of shale pores in enclosed and semi–enclosed thermal simulation experiments, Permian Lucaogou Formation, Santanghu Basin, China. Mar. Pet. Geol. 2022, 135, 105421. [Google Scholar] [CrossRef]







| Whole–Rock Analysis | |
|---|---|
| Vitrinite reflectance (Ro; %) a | 0.6 |
| Total organic carbon (TOC; wt%) | 21.5 |
| S1 (mg/g TOC) b | 2 |
| S2 (mg/g TOC) b | 208.6 |
| Tmax (°C) b | 454 |
| HI (mg S2/g TOC) b | 970 |
| Quartz c | 82 |
| Feldspar c | 9.7 |
| Pyrite c | 0.9 |
| Calcite c | 2.2 |
| Dolomite c | 1.5 |
| Siderite c | 0.8 |
| Clay c | 1.8 |
| Liptinite (%) a | 89 |
| Vitrinite (%) a | 6.1 |
| Inertinite (%) a | 4.9 |
| Temperature (°C) | Simulation Depth (m) | Expulsion Temperature (°C) | Lithostatic Pressure (MPa) | Water Pressure (MPa) | Fluid Pressure Threshold (MPa) |
|---|---|---|---|---|---|
| 300 | 1650 | 57 | 40 | 17 | 4 |
| 325 | 1860 | 68 | 47 | 19 | 5 |
| 350 | 2340 | 73 | 58 | 23 | 6 |
| 375 | 2680 | 110 | 67 | 27 | 7 |
| 400 | 3250 | 126 | 81 | 33 | 8 |
| 450 | 4285 | 143 | 107 | 43 | 11 |
| 500 | 5900 | 185 | 148 | 59 | 15 |
| Temperature (°C) | Raw Rock (g) | Residual Rock (g) | Cumulative Oil Yield (mg/g TOC) | Cumulative Bitumen Yield (mg/g TOC) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sat | Aro | Res | Asph | Total | Sat | Aro | Res | Asph | Total | |||
| 300 | 55.3 | 54.9 | 0.29 | 0.06 | 0.24 | 0.15 | 0.73 | 8.98 | 1.99 | 1.67 | 1.64 | 14.29 |
| 325 | 56.7 | 56.4 | 0.17 | 0.08 | 0.37 | 0.27 | 0.89 | 15.66 | 4.16 | 5.05 | 3.16 | 28.03 |
| 350 | 56.4 | 55.4 | 11.73 | 5.37 | 8.94 | 6.89 | 32.92 | 36.47 | 19.8 | 25.79 | 6.46 | 88.52 |
| 375 | 51.5 | 48.7 | 33.24 | 15.03 | 44.54 | 40.43 | 133.15 | 67.98 | 47.37 | 98.71 | 66.17 | 280.23 |
| 400 | 46.7 | 39.4 | 81.32 | 80.09 | 52.03 | 34.89 | 248.33 | 171.75 | 95.37 | 120.05 | 84.92 | 472.09 |
| 450 | 49.9 | 37.3 | 104.21 | 72.1 | 113.13 | 170.07 | 459.51 | 5.34 | 7.24 | 8.33 | 6.86 | 27.77 |
| 500 | 49.5 | 36.0 | 124.74 | 112.81 | 118.74 | 139.99 | 494.28 | n.m | n.m | n.m | n.m | n.m |
| Temperature (°C) | Cumulative Gas Yield (mg/g TOC) | δ13CPDB (‰) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | CO2 | H2 | O2 | C1 | C2 | C3 | C4 | C5 | |
| 300 | 0.06 | 0.1 | 0.24 | 0.22 | 0.15 | 1.46 | 0 | 0.11 | −37.3 | −38.5 | −34.4 | −33.8 | −32.4 |
| 325 | 0.09 | 0.1 | 0.27 | 0.27 | 0.23 | 4.25 | 0 | 0.07 | −38.7 | −37.7 | −34.7 | −33.9 | −32.3 |
| 350 | 1.53 | 1.27 | 2.45 | 2.57 | 2.38 | 16 | 0.04 | 0.39 | −44.7 | −38.6 | −35.6 | −34.6 | −33.1 |
| 375 | 2.7 | 3.1 | 4.64 | 4.51 | 4.51 | 8.83 | 0.08 | 4.04 | −45.4 | −37 | −34.3 | −33.7 | −30.4 |
| 400 | 10.88 | 11.32 | 13.22 | 8.8 | 5.06 | 15.88 | 0..23 | 1.02 | −43.5 | −33.5 | −30.6 | −29.9 | −27.6 |
| 450 | 79.54 | 74.35 | 91.9 | 40.2 | 14.45 | 18.09 | 0.11 | 1.29 | −42.5 | −32.7 | −28.1 | −26.5 | −22.9 |
| 500 | 120.27 | 96.09 | 77.07 | 30.31 | 6.61 | 33.03 | 0.24 | 2.32 | −38.7 | −28.9 | −23.8 | −22.1 | −19.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Jin, J.; Liu, J.; Tan, J.; Chen, L.; Cui, H.; Ma, X.; Song, X. Hydrocarbon Generation Mechanism of Mixed Siliciclastic–Carbonate Shale: Implications from Semi–Closed Hydrous Pyrolysis. Energies 2023, 16, 3065. https://doi.org/10.3390/en16073065
Wang J, Jin J, Liu J, Tan J, Chen L, Cui H, Ma X, Song X. Hydrocarbon Generation Mechanism of Mixed Siliciclastic–Carbonate Shale: Implications from Semi–Closed Hydrous Pyrolysis. Energies. 2023; 16(7):3065. https://doi.org/10.3390/en16073065
Chicago/Turabian StyleWang, Jian, Jun Jin, Jin Liu, Jingqiang Tan, Lichang Chen, Haisu Cui, Xiao Ma, and Xueqi Song. 2023. "Hydrocarbon Generation Mechanism of Mixed Siliciclastic–Carbonate Shale: Implications from Semi–Closed Hydrous Pyrolysis" Energies 16, no. 7: 3065. https://doi.org/10.3390/en16073065
APA StyleWang, J., Jin, J., Liu, J., Tan, J., Chen, L., Cui, H., Ma, X., & Song, X. (2023). Hydrocarbon Generation Mechanism of Mixed Siliciclastic–Carbonate Shale: Implications from Semi–Closed Hydrous Pyrolysis. Energies, 16(7), 3065. https://doi.org/10.3390/en16073065





