Emission Mitigation by Aluminum-Silicate-Based Fuel Additivation of Wood Chips with Kaolin and Kaolinite
Abstract
1. Introduction
2. Material and Methods
2.1. Wood Chips
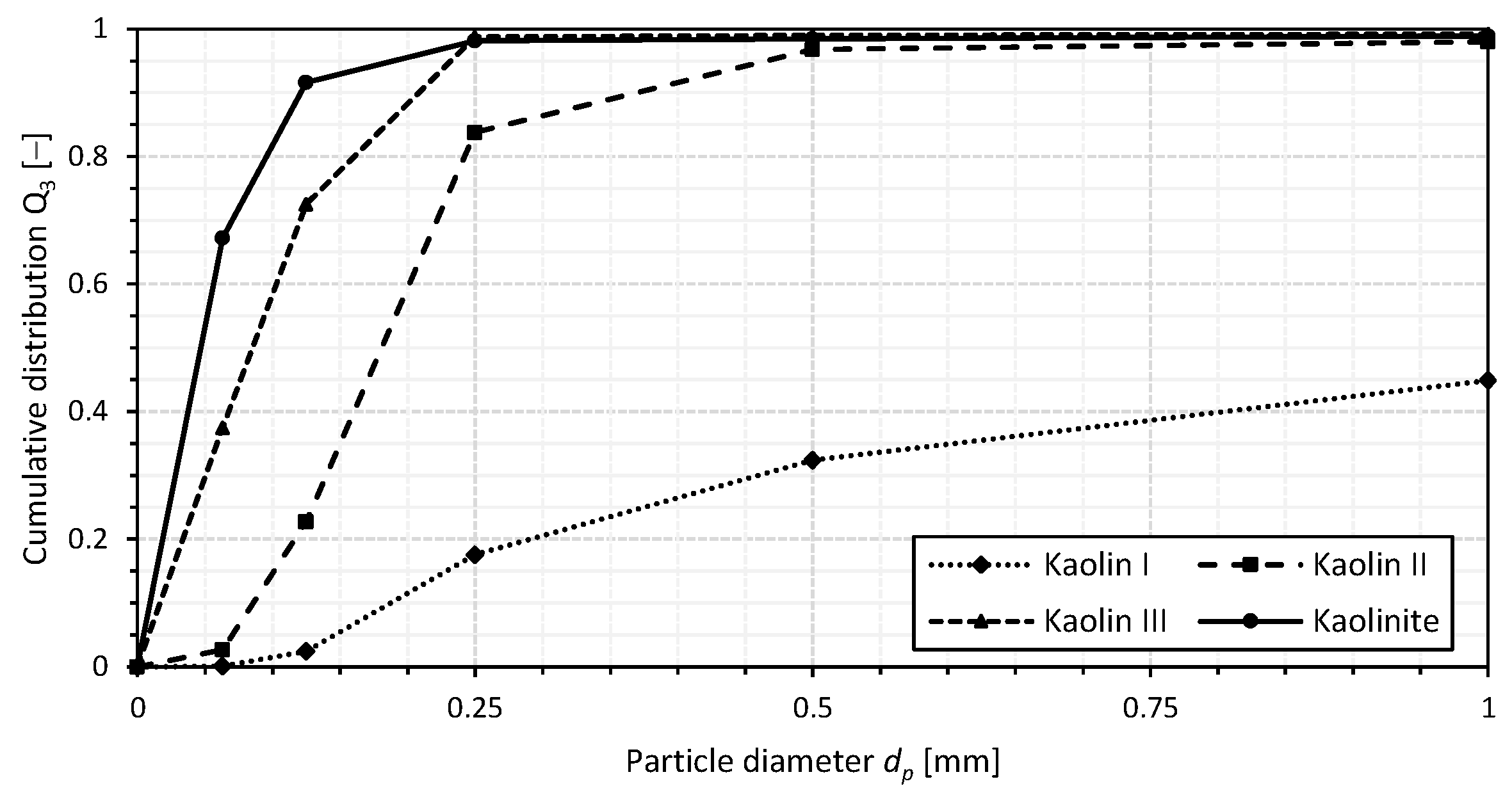
2.2. Additives
2.3. Combustion Plant
2.4. Emissions
2.4.1. Total Particulate Matter
Potassium
Ultrafine Particulate Matter
2.4.2. Carbon Monoxide
2.5. Ashes
3. Results and Discussion
3.1. Emissions
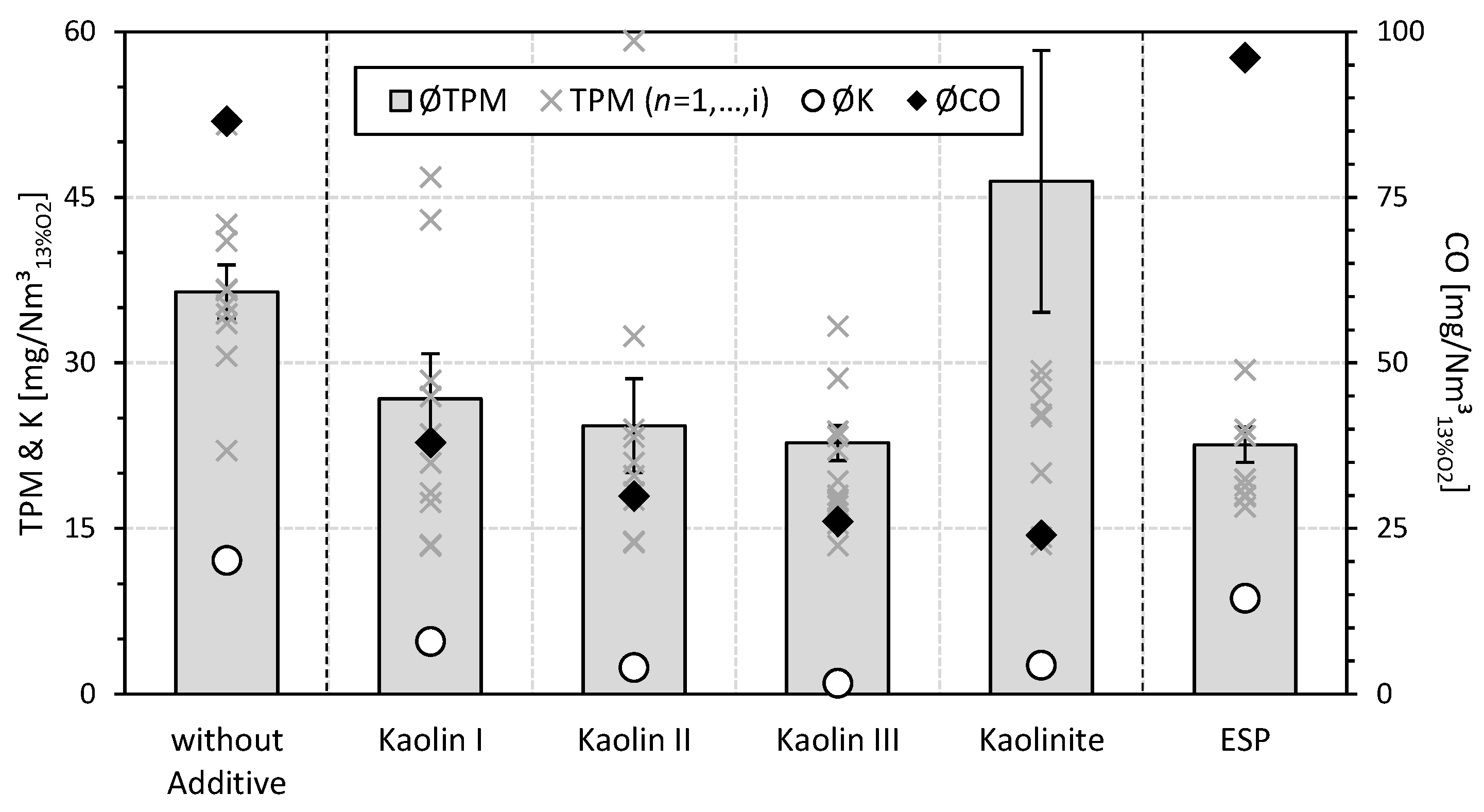
3.1.1. Total Particulate Matter
Potassium
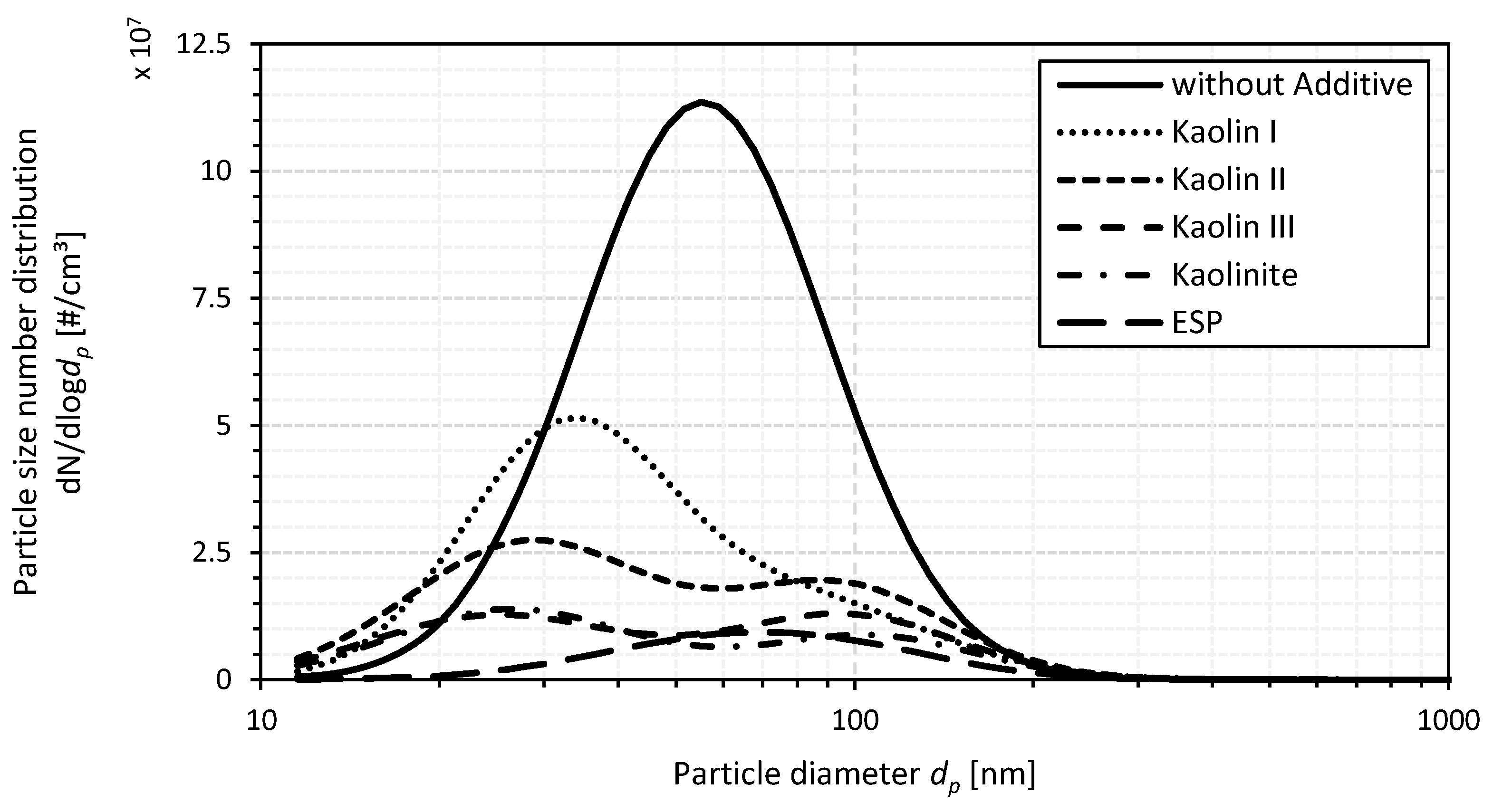
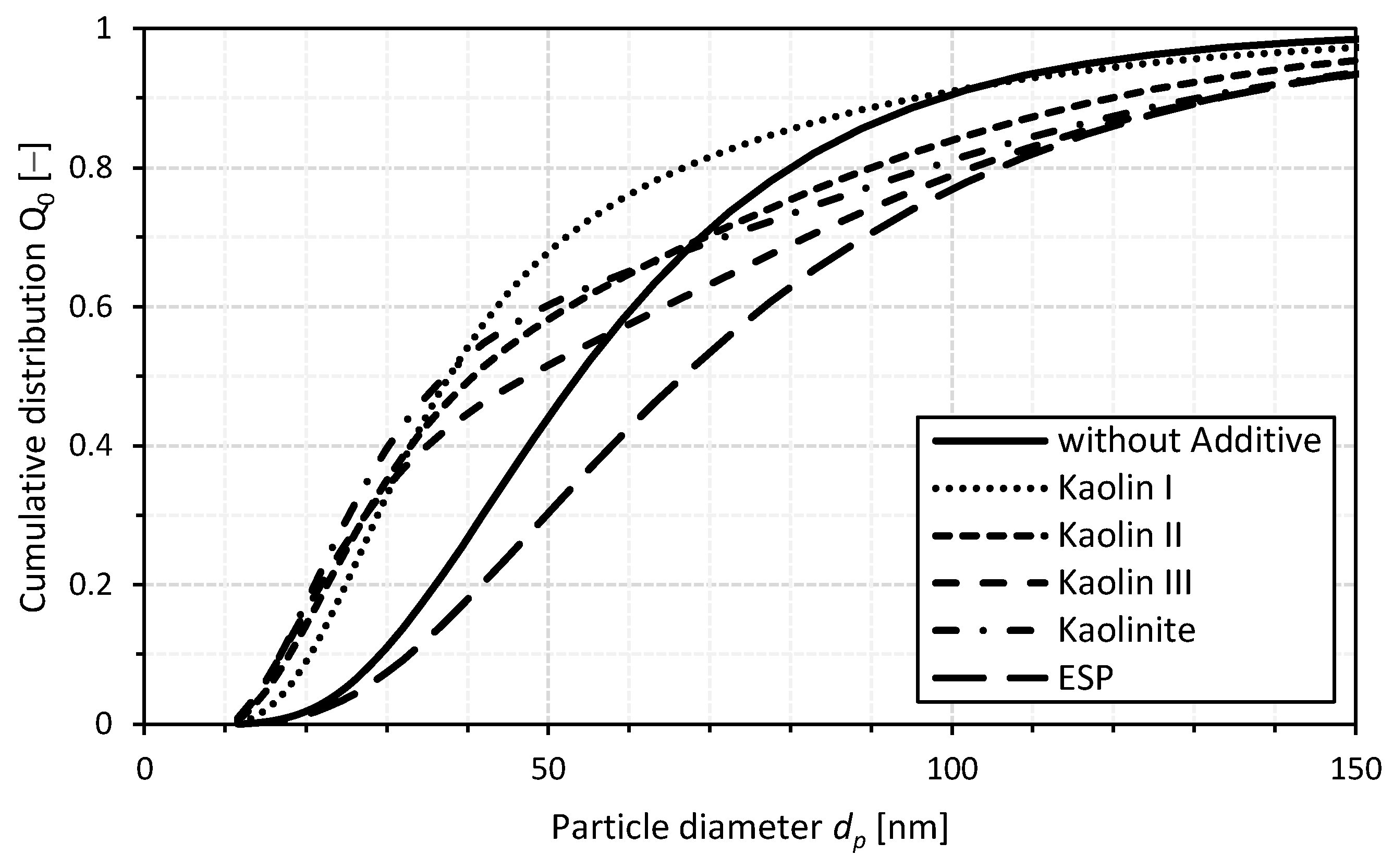
Ultrafine Particulate Matter
3.1.2. Carbon Monoxide
3.2. Ashes
4. Conclusions
- ▪
- As a result of a reduction in the mean particle size of the aluminum-silicate-based additive kaolin, in addition to the increasing mitigation of TPM emissions, a progressive decrease in the K emissions, as well as in the CO emissions, occurs during the fuel additivation of wood chips compared to the non-additivated reference case without ESP. Thus, the lowest absolute TPM emissions (23 mg/Nm3), K emissions (1 mg/Nm3) and CO emissions (26 mg/Nm3) are obtained for the aluminum-silicate-based fuel additivation of the wood chips, with the kaolin demonstrating the smallest mean particle size (i.e., Kaolin III). Consequently, the aluminum-silicate-based fuel additivation can be regarded as transferable and implementable for real small-scale wood chip combustion.
- ▪
- Although the mean particle size of the impurity-free aluminum-silicate-based additive Kaolinite is smaller than for Kaolin III, the overall emission mitigation (i.e., TPM emissions (47 mg/Nm3), K emissions (3 mg/Nm3) and CO emissions (24 mg/Nm3)) for the fuel additivation of the wood chips with Kaolinite is worse than with Kaolin III. Hence, kaolin is to be given preference over kaolinite in the aluminum-silicate-based fuel additivation of wood chips.
- ▪
- Comparing the primary, fuel-side mitigation measure of aluminum-silicate-based fuel additivation (e.g., Kaolin III) and the secondary mitigation measure of ESP, both measures result in comparable reductions in absolute TPM emissions (23 mg/Nm3) regarding the non-additivated reference case without ESP (36 mg/Nm3). However, K emissions (9 mg/Nm3) and CO emissions (96 mg/Nm3) are higher for the ESP than for the use of 0.5 wt% Kaolin III. Consequently, fuel additivation of wood chips with kaolin offers the additional benefit of simultaneously reducing other air pollutants (e.g., CO) compared to the ESP, although the different chemical compositions of the TPM emissions make the combined utilization of the two mitigation measures very promising.
- ▪
- While the use of an ESP as a secondary emission mitigation measure tends to shift the particle size number distribution of the PM emissions to larger particle diameters during small-scale wood chip combustion, more small particles are emitted for fuel additivation using kaolin or kaolinite, respectively. Nevertheless, comparable reductions in the share of PM0.1 emissions in PM emissions can be achieved for both the aluminum-silicate-based fuel additivation using Kaolin III and the utilization of the ESP. Again, a combined use of the two mitigation measures appears to provide added value over considering them alone.
- ▪
- Finally, the ashes for the aluminum-silicate-based fuel additivation of the wood chips show that, in contrast to the non-additivated wood chips (i.e., without and with ESP), there is evidence of high-temperature stable ash incorporation of the ash- and PM-forming alkali element K through the addition of kaolin, or kaolinite, which in turn reduces the (inorganic) TPM emissions. Thus, aluminum-silicate-based fuel additivation of wood chips releases fewer TPM emissions overall, which could subsequently be potentially further reduced by the utilization of an ESP.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paletto, A.; Bernardi, S.; Pieratti, E.; Teston, F.; Romagnoli, M. Assessment of environmental impact of biomass power plants to increase the social acceptance of renewable energy technologies. Heliyon 2019, 5, e02070. [Google Scholar] [CrossRef]
- Vicente, E.D.; Alves, C.A. An overview of particulate emissions from residential biomass combustion. Atmos. Res. 2018, 199, 159–185. [Google Scholar] [CrossRef]
- Proskurina, S.; Sikkema, R.; Heinimö, J.; Vakkilainen, E. Five years left—How are the EU member states contributing to the 20% target for EU’s renewable energy consumption; the role of woody biomass. Biomass Bioenergy 2016, 95, 64–77. [Google Scholar] [CrossRef]
- Banja, M.; Sikkema, R.; Jégard, M.; Motola, V.; Dallemand, J.-F. Biomass for energy in the EU—The support framework. Energy Policy 2019, 131, 215–228. [Google Scholar] [CrossRef]
- Singh, R.; Shukla, A. A review on methods of flue gas cleaning from combustion of biomass. Renew. Sustain. Energy Rev. 2014, 29, 854–864. [Google Scholar] [CrossRef]
- Höfer, I.; Kaltschmitt, M.; Beckendorff, A. Emissions from solid biofuel combustion, pollutant formation and control options. In Encyclopedia of Sustainability Science and Technology Meyers; Meyers, R.A., Ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Sofia, D.; Gioiella, F.; Lotrecchiano, N.; Giuliano, A. Mitigation strategies for reducing air pollution. Environ. Sci. Pollut. Res. Int. 2020, 27, 19226–19235. [Google Scholar] [CrossRef]
- Obernberger, I.; Brunner, T.; Mandl, C.; Kerschbaum, M.; Svetlik, T. Strategies and technologies towards zero emission biomass combustion by primary measures. Energy Procedia 2017, 120, 681–688. [Google Scholar] [CrossRef]
- Lim, M.T.; Phan, A.; Roddy, D.; Harvey, A. Technologies for measurement and mitigation of particulate emissions from domestic combustion of biomass: A review. Renew. Sustain. Energy Rev. 2015, 49, 574–584. [Google Scholar] [CrossRef]
- Lamberg, H.; Sippula, O.; Tissari, J.; Jokiniemi, J. Effects of Air Staging and Load on Fine-Particle and Gaseous Emissions from a Small-Scale Pellet Boiler. Energy Fuels 2011, 25, 4952–4960. [Google Scholar] [CrossRef]
- Gehrig, M.; Jaeger, D.; Pelz, S.K.; Weissinger, A.; Groll, A.; Thorwarth, H.; Haslinger, W. Influence of firebed temperature on inorganic particle emissions in a residential wood pellet boiler. Atmos. Environ. 2016, 136, 61–67. [Google Scholar] [CrossRef]
- Gollmer, C.; Höfer, I.; Kaltschmitt, M. Additives as a fuel-oriented measure to mitigate inorganic particulate matter (PM) emissions during small-scale combustion of solid biofuels. Biomass Convers. Biorefin. 2019, 9, 3–20. [Google Scholar] [CrossRef]
- Míguez, J.L.; Porteiro, J.; Behrendt, F.; Blanco, D.; Patiño, D.; Dieguez-Alonso, A. Review of the use of additives to mitigate operational problems associated with the combustion of biomass with high content in ash-forming species. Renew. Sustain. Energy Rev. 2021, 141, 110502. [Google Scholar] [CrossRef]
- Wang, L.; Hustad, J.E.; Skreiberg, Ø.; Skjevrak, G.; Grønli, M. A Critical Review on Additives to Reduce Ash Related Operation Problems in Biomass Combustion Applications. Energy Procedia 2012, 20, 20–29. [Google Scholar] [CrossRef]
- Pollex, A.; Zeng, T.; Khalsa, J.; Erler, U.; Schmersahl, R.; Schön, C.; Kuptz, D.; Lenz, V.; Nelles, M. Content of potassium and other aerosol forming elements in commercially available wood pellet batches. Fuel 2018, 232, 384–394. [Google Scholar] [CrossRef]
- Sippula, O.; Hytönen, K.; Tissari, J.; Raunemaa, T.; Jokiniemi, J. Effect of Wood Fuel on the Emissions from a Top-Feed Pellet Stove. Energy Fuels 2007, 21, 1151–1160. [Google Scholar] [CrossRef]
- Lamberg, H.; Tissari, J.; Jokiniemi, J.; Sippula, O. Fine Particle and Gaseous Emissions from a Small-Scale Boiler Fueled by Pellets of Various Raw Materials. Energy Fuels 2013, 27, 7044–7053. [Google Scholar] [CrossRef]
- van Lith, S.C.; Jensen, P.A.; Frandsen, F.J.; Glarborg, P. Release to the Gas Phase of Inorganic Elements during Wood Combustion. Part 2: Influence of Fuel Composition. Energy Fuels 2008, 22, 1598–1609. [Google Scholar] [CrossRef]
- Gehrig, M.; Wöhler, M.; Pelz, S.; Steinbrink, J.; Thorwarth, H. Kaolin as additive in wood pellet combustion with several mixtures of spruce and short-rotation-coppice willow and its influence on emissions and ashes. Fuel 2019, 235, 610–616. [Google Scholar] [CrossRef]
- Van Lith, S.C.; Alonso-Ramírez, V.; Jensen, P.A.; Frandsen, F.J.; Glarborg, P. Release to the Gas Phase of Inorganic Elements during Wood Combustion. Part 1: Development and Evaluation of Quantification Methods. Energy Fuels 2006, 20, 964–978. [Google Scholar] [CrossRef]
- Boman, C.; Nordin, A.; Boström, D.; Öhman, M. Characterization of Inorganic Particulate Matter from Residential Combustion of Pelletized Biomass Fuels. Energy Fuels 2004, 18, 338–348. [Google Scholar] [CrossRef]
- Knudsen, J.N.; Jensen, P.A.; Dam-Johansen, K. Transformation and Release to the Gas Phase of Cl, K, and S during Combustion of Annual Biomass. Energy Fuels 2004, 18, 1385–1399. [Google Scholar] [CrossRef]
- Wang, G.; Jensen, P.A.; Wu, H.; Frandsen, F.J.; Sander, B.; Glarborg, P. Potassium Capture by Kaolin, Part 1: KOH. Energy Fuels 2018, 32, 1851–1862. [Google Scholar] [CrossRef]
- Wang, G.; Jensen, P.A.; Wu, H.; Frandsen, F.J.; Sander, B.; Glarborg, P. Potassium Capture by Kaolin, Part 2: K2CO3 KCl, and K2SO4. Energy Fuels 2018, 32, 3566–3578. [Google Scholar] [CrossRef]
- Johansson, L.S.; Tullin, C.; Leckner, B.; Sjövall, P. Particle emissions from biomass combustion in small combustors. Biomass Bioenergy 2003, 25, 435–446. [Google Scholar] [CrossRef]
- Gollmer, C.; Höfer, I.; Harms, D.; Kaltschmitt, M. Potential additives for small-scale wood chip combustion—Laboratory-scale estimation of the possible inorganic particulate matter reduction potential. Fuel 2019, 254, 115695. [Google Scholar] [CrossRef]
- Steenari, B.-M.; Lundberg, A.; Pettersson, H.; Wilewska-Bien, M.; Andersson, D. Investigation of Ash Sintering during Combustion of Agricultural Residues and the Effect of Additives. Energy Fuels 2009, 23, 5655–5662. [Google Scholar] [CrossRef]
- Steenari, B.-M.; Lindqvist, O. High-temperature reactions of straw ash and the anti-sintering additives Kaolin and Dolomite. Biomass Bioenergy 1998, 14, 67–76. [Google Scholar] [CrossRef]
- Huelsmann, T.; Mack, R.; Kaltschmitt, M.; Hartmann, H. Influence of kaolinite on the PM emissions from small-scale combustion. Biomass Convers. Biorefinery 2019, 9, 55–70. [Google Scholar] [CrossRef]
- Khalil, R.A.; Todorovic, D.; Skreiberg, O.; Becidan, M.; Backman, R.; Goile, F.; Skreiberg, A.; Sørum, L. The effect of kaolin on the combustion of demolition wood under well-controlled conditions. Waste Manag. Res. 2012, 30, 672–680. [Google Scholar] [CrossRef]
- Öhman, M.; Boström, D.; Nordin, A.; Hedman, H. Effect of Kaolin and Limestone Addition on Slag Formation during Combustion of Wood Fuels. Energy Fuels 2004, 18, 1370–1376. [Google Scholar] [CrossRef]
- Mack, R.; Kuptz, D.; Schön, C.; Hartmann, H. Combustion behavior and slagging tendencies of kaolin additivated agricultural pellets and of wood-straw pellet blends in a small-scale boiler. Biomass Bioenergy 2019, 125, 50–62. [Google Scholar] [CrossRef]
- Höfer, I.; Huelsmann, T.; Kaltschmitt, M. Influence of Ca- and Al-additives on the pollutant emissions from blends of wood and straw in small-scale combustion. Biomass Bioenergy 2021, 150, 106135. [Google Scholar] [CrossRef]
- Brüggemann, C.; Brügger, E.; Dörr, I.; Hansen, H.; Krapf, G.; Krämer, G.; Kuptz, D.; Langer, S.; Stanev, A.; Schmoeckel, G.; et al. Hackschnitzelheizungen 2018—Was Muss Beachtet Werden? Fachagentur Nachwachsende Rohstoffe e.V.: Gülzow-Prüzen, Germany, 2018. [Google Scholar]
- Davidsson, K.O.; Steenari, B.-M.; Eskilsson, D. Kaolin Addition during Biomass Combustion in a 35 MW Circulating Fluidized-Bed Boiler. Energy Fuels 2007, 21, 1959–1966. [Google Scholar] [CrossRef]
- Broström, M.; Kassman, H.; Helgesson, A.; Berg, M.; Andersson, C.; Backman, R.; Nordin, A. Sulfation of corrosive alkali chlorides by ammonium sulfate in a biomass fired CFB boiler. Fuel Process. Technol. 2007, 88, 1171–1177. [Google Scholar] [CrossRef]
- Geisen, B.; Givers, F.; Kuptz, D.; Peetz, D.; Schmidt-Baum, T.; Schön, C.; Schreiber, K.; Schulmeyer, F.; Thudium, T.; Zelinski, V.; et al. Handbuch zum Qualitätsmanagement von Holzhackschnitzeln; Fachagentur Nachwachsende Rohstoffe e.V.: Gülzow-Prüzen, Germany, 2017. [Google Scholar]
- Gollmer, C.; Höfer, I.; Kaltschmitt, M. Laboratory-scale additive content assessment for aluminum-silicate-based wood chip additivation. Renew. Energy 2021, 164, 1471–1484. [Google Scholar] [CrossRef]
- Diedrich, H.; Stahl, A. NCHS-Elementaranalyse. M02.001. 02; Technische Universität Hamburg, Zentrallabor Chemische Analytik: Hamburg, Germany, 2021. [Google Scholar]
- Fütterer, C.; Stahl, A. Elementbestimmung mit ICP-OEM02.015. 03; Technische Universität Hamburg, Zentrallabor Chemische Analytik: Hamburg, Germany, 2021. [Google Scholar]
- Cöllen, H.; Frerichs, H.; Stahl, A. Elementbestimmung per ICP-MM02.013. 01; Technische Universität Hamburg, Zentrallabor Chemische Analytik: Hamburg, Germany, 2021. [Google Scholar]
- DIN EN ISO 17225-1; Biogene Festbrennstoffe—Brennstoffspezifikationen und -Klassen—Teil 1: Allgemeine Anforderungen. Beuth Verlag GmbH: Berlin, Germany, 2014.
- DIN EN ISO 17225-4; Biogene Festbrennstoffe—Brennstoffspezifikationen und -Klassen—Teil 4: Klassifizierung von Holzhackschnitzeln. Beuth Verlag GmbH: Berlin, Germany, 2014.
- Nordin, A. Chemical elemental characteristics of biomass fuels. Biomass Bioenergy 1994, 6, 339–347. [Google Scholar] [CrossRef]
- Gebrüder Dorfner GmbH & Co. Kaolin- und Kristallquarzsand-Werke KG. Sicherheitsdatenblatt—Kaolin Gemahlen; Gebrüder Dorfner GmbH & Co. Kaolin- und Kristallquarzsand-Werke KG: Hirschau, Germany, 2018. [Google Scholar]
- Gebrüder Dorfner GmbH & Co. Kaolin- und Kristallquarzsand-Werke KG. Sicherheitsdatenblatt—Kaolin Granuliert; Gebrüder Dorfner GmbH & Co. Kaolin- und Kristallquarzsand-Werke KG: Hirschau, Germany, 2018. [Google Scholar]
- Sigma-Aldrich Chemie GmbH. Sicherheitsdatenblatt Kaolinit; Sigma-Aldrich Chemie GmbH: Munich, Germany, 2021. [Google Scholar]
- Heizomat—Gerätebau + Energiesysteme GmbH: Technische Daten RHK-AK 30, Gunzenhausen. 2021. Available online: https://www.heizomat.de/images/downloads/rhk-30.pdf (accessed on 2 March 2023).
- DIN EN 15259; Luftbeschaffenheit—Messung von Emissionen aus Stationären Quellen—Anforderungen an Messstrecken und Messplätze und an die Messaufgabe, den Messplan und den Messbericht. Beuth Verlag GmbH: Berlin, Germany, 2008.
- DIN EN 13284-1; Emissionen aus Stationären Quellen—Ermittlung der Staubmassenkonzentration bei geringen Staubkonzentrationen—Teil 1: Manuelles gravimetrisches Verfahren. Beuth Verlag GmbH: Berlin, Germany, 2018.
- VDI 2066 Blatt 1; Messen von Partikeln—Staubmessung in Strömenden Gasen—Gravimetrische Bestimmung der Staubbeladung. Beuth Verlag GmbH: Berlin, Germany, 2019.
- DIN 22022-1; Feste Brennstoffe—Bestimmung der Gehalte an Spurenelementen—Teil 1: Allgemeine Regeln, Probenahme und Probenvorbereitung—Vorbereitung der Analysenprobe für die Bestimmung (Aufschlussverfahren). Beuth Verlag GmbH: Berlin, Germany, 2014.
- DIN 22022-3; Feste Brennstoffe—Bestimmung der Gehalte an Spurenelementen—Teil 3: AAS-Flammentechnik. Beuth Verlag GmbH: Berlin, Germany, 2001.
- DIN 38406 Teil 13; Deutsche Einheitsverfahren zur Wasser-, Abwasser- und Schlammuntersuchung—Kationen (Gruppe E)—Bestimmung von Kalium mittels Atomabsorptionsspektrometrie (AAS) in der Luft-Acetylen-Flamme (E 13). Beuth Verlag GmbH: Berlin, Germany, 1992.
- DIN 38406 Teil 14; Deutsche Einheitsverfahren zur Wasser-, Abwasser- und Schlammuntersuchung—Kationen (Gruppe E)—Bestimmung von Natrium mittels Atomabsorptionsspektrometrie (AAS) in der Luft-Acetylen-Flamme (E14). Beuth Verlag GmbH: Berlin, Germany, 1992.
- DIN EN ISO 7980; Wasserbeschaffenheit—Bestimmung von Calcium und Magnesium—Verfahren mittels Absorptionsspektrometrie. Beuth Verlag GmbH: Berlin, Germany, 2000.
- DIN EN ISO 10304-1; Wasserbeschaffenheit—Bestimmung von gelösten Anionen mittels Flüssigkeits-Ionenchromatographie—Teil 1: Bestimmung von Bromid, Chlorid, Fluorid, Nitrat, Nitrit, Phosphat und Sulfat. Beuth Verlag GmbH: Berlin, Germany, 2009.
- Tritscher, T.; Koched, A.; Han, H.-S.; Filimundi, E.; Johnson, T.; Elzey, S.; Avenido, A.; Kykal, C.; Bischof, O.F. Multi-Instrument Manager Tool for Data Acquisition and Merging of Optical and Electrical Mobility Size Distributions. J. Phys. Conf. Ser. 2014, 617, 012013. [Google Scholar] [CrossRef]
- DIN EN ISO 16967; Biogene Festbrennstoffe—Bestimmung von Hauptelementen—Al, Ca, Fe, Mg, P, K, Si, Na und Ti. Beuth Verlag GmbH: Berlin, Germany, 2015.
- DIN EN ISO 16968; Biogene Festbrennstoffe—Bestimmung von Spurenelementen. Beuth Verlag GmbH: Berlin, Germany, 2015.
- DIN EN 13925-1; Zerstörungsfreie Prüfung—Röntgendiffraktometrie von Polykristallinen und Amorphen Materialien—Teil 1: Allgemeine Grundlagen. Beuth Verlag GmbH: Berlin, Germany, 2003.
- DIN EN 13925-2; Zerstörungsfreie Prüfung—Röntgendiffraktometrie von Polykristallinen und Amorphen Materialien—Teil 2: Verfahrensabläufe. Beuth Verlag GmbH: Berlin, Germany, 2003.
- Obernberger, I.; Brunner, T.; Baernthaler, G. Fine particulate emissions from modern Austrian small-scale biomass combustion plants. In Proceedings of the 15th European Biomass Conference and Exhibition for Research to Market Deployment: For Research to Market Deployment, Berlin, Germany, 7–11 May 2007. [Google Scholar]
- Obaidullah, M.; Bram, S.; Verma, V.K.; de Ruyck, J. A Review on Particle Emissions from Small Scale Biomass Combustion. Int. J. Renew. Energy Res. IJRER 2012, 2, 147–159. [Google Scholar]
- Yang, W.; Zhu, Y.; Cheng, W.; Sang, H.; Xu, H.; Yang, H.; Chen, H. Effect of minerals and binders on particulate matter emission from biomass pellets combustion. Appl. Energy 2018, 215, 106–115. [Google Scholar] [CrossRef]
- Höfer, I.; Gollmer, C.; Kaltschmitt, M. Inorganic PM and K emissions during ashing of solid biofuels and Kaolinite—Data measurement in laboratory scale. Fuel 2021, 296, 120704. [Google Scholar] [CrossRef]
- Wiinikka, H.; Gebart, R.; Boman, C.; Boström, D.; Nordin, A.; Öhman, M. High-temperature aerosol formation in wood pellets flames: Spatially resolved measurements. Combust. Flame 2006, 147, 278–293. [Google Scholar] [CrossRef]
- Zeng, T.; Weller, N.; Pollex, A.; Lenz, V. Blended biomass pellets as fuel for small scale combustion appliances: Influence on gaseous and total particulate matter emissions and applicability of fuel indices. Fuel 2016, 184, 689–700. [Google Scholar] [CrossRef]
- Hueglin, C.; Gaegauf, C.; Künzel, S.; Burtscher, H. Characterization of Wood Combustion Particles: Morphology, Mobility, and Photoelectric Activity. Environ. Sci. Technol. 1997, 31, 3439–3447. [Google Scholar] [CrossRef]
- Leskinen, J.; Tissari, J.; Uski, O.; Virén, A.; Torvela, T.; Kaivosoja, T.; Lamberg, H.; Nuutinen, I.; Kettunen, T.; Joutsensaari, J.; et al. Fine particle emissions in three different combustion conditions of a wood chip-fired appliance—Particulate physico-chemical properties and induced cell death. Atmos. Environ. 2014, 86, 129–139. [Google Scholar] [CrossRef]
- Boström, D.; Boman, C.; Öhman, M. Effect of Fuel Additive Sorbents (Kaolin and Calcite) on Aerosol Particle Emission and Characteristics during Combustion of Pelletized Woody Biomass. In Proceedings of the 16th European Biomass Conference & Exibition, Valencia, Spain, 2–6 June 2008. [Google Scholar]
- Tissari, J.; Lyyränen, J.; Hytönen, K.; Sippula, O.; Tapper, U.; Frey, A.; Saarnio, K.; Pennanen, A.S.; Hillamo, R.; Salonen, R.O.; et al. Fine particle and gaseous emissions from normal and smouldering wood combustion in a conventional masonry heater. Atmos. Environ. 2008, 42, 7862–7873. [Google Scholar] [CrossRef]
- Olanders, B.; Steenari, B.-M. Characterization of ashes from wood and straw. Biomass Bioenergy 1995, 8, 105–115. [Google Scholar] [CrossRef]
| Parameter | Unit | Wood Chips |
|---|---|---|
| Moisture content | wt%a.r. | 6.6 |
| Ash content | wt%d.b. | 0.3 |
| Carbon (C) | wt%d.b. | 49.8 |
| Hydrogen (H) | wt%d.b. | 6.4 |
| Oxygen (O) * | wt%d.b. | 43.6 |
| Nitrogen (N) | wt%d.b. | <0.1 |
| Sulfur (S) | wt%d.b. | <0.2 |
| Potassium (K) | mg/kgd.b. | 475 |
| Sodium (Na) | mg/kgd.b. | 8 |
| Calcium (Ca) | mg/kgd.b. | 713 |
| Magnesium (Mg) | mg/kgd.b. | 171 |
| Silicon (Si) | mg/kgd.b. | <250 |
| Manganese (Mn) | mg/kgd.b. | 28 |
| Phosphorus (P) | mg/kgd.b. | n.d. |
| Aluminum (Al) | mg/kgd.b. | 10 |
| Iron (Fe) | mg/kgd.b. | 10 |
| Copper (Cu) | mg/kgd.b. | 3 |
| Zinc (Zn) | mg/kgd.b. | 7 |
| Lead (Pb) | mg/kgd.b. | <1 |
| Parameter | Unit | Kaolin I | Kaolin II | Kaolin III | Kaolinite |
|---|---|---|---|---|---|
| SiO2 | wt%a.r. | 46.9 | 44.6 | 50.2 | 46.5 |
| Al2O3 | wt%a.r. | 37.2 | 37.5 | 34.4 | 39.5 |
| H2O | wt%a.r. | 13.3 | 14.3 | 12.0 | 14.0 |
| Fe2O3 | wt%a.r. | 0.9 | 2.0 | 0.5 | - |
| TiO2 | wt%a.r. | 0.4 | 1.1 | 0.4 | - |
| K2O | wt%a.r. | 1.0 | <0.1 | 2.1 | - |
| Na2O | wt%a.r. | 0.1 | 0.2 | 0.2 | - |
| CaO | wt%a.r. | <0.1 | <0.1 | <0.1 | - |
| MgO | wt%a.r. | <0.1 | <0.1 | <0.1 | - |
| P2O5 | wt%a.r. | 0.1 | 0.2 | 0.2 | - |
| Parameter | Unit | Without Additive | Kaolin I | Kaolin II | Kaolin III | Kaolinite | ESP |
|---|---|---|---|---|---|---|---|
| Potassium (K) | wt%d.b. | 33.3 | 17.8 | 9.9 | 4.3 | 5.7 | 38.5 |
| Sodium (Na) | wt%d.b. | 0.5 | 0.7 | 0.5 | 0.5 | 1.2 | 2.5 |
| Calcium (Ca) | wt%d.b. | 4.2 | 6.9 | 6.4 | 6.2 | 7.2 | 7.6 |
| Magnesium (Mg) | wt%d.b. | 0.3 | 0.2 | <0.1 | 0.1 | <0.1 | <0.1 |
| Aluminum (Al) | wt%d.b. | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Iron (Fe) | wt%d.b. | 1.8 | 3.5 | 3.4 | 2.9 | 3.7 | 3.7 |
| Zinc (Zn) | wt%d.b. | 1.0 | 1.6 | 1.8 | 1.5 | 2.0 | 1.8 |
| Sulfate (SO42−) | wt%d.b. | 36.9 | 17.2 | 9.8 | 4.0 | 7.6 | 35.1 |
| Chloride (Cl−) | wt%d.b. | 5.6 | 6.1 | 7.0 | 4.2 | 11.0 | 7.3 |
| Parameter | Unit | Without Additive | Kaolin I | Kaolin II | Kaolin III | Kaolinite | ESP |
|---|---|---|---|---|---|---|---|
| Potassium (K) | wt%d.b. | 7.7 | 7.9 | 6.9 | 6.9 | 6.7 | 13.3 |
| Sodium (Na) | wt%d.b. | 0.4 | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 |
| Calcium (Ca) | wt%d.b. | 18.3 | 11.0 | 10.3 | 9.1 | 9.2 | 23.6 |
| Magnesium (Mg) | wt%d.b. | 3.6 | 2.7 | 2.6 | 2.2 | 2.0 | 5.7 |
| Aluminum (Al) | wt%d.b. | 7.4 | 11.9 | 15.0 | 14.3 | 13.4 | 4.3 |
| Iron (Fe) | wt%d.b. | 2.0 | 1.1 | 1.5 | 1.2 | 0.8 | 0.9 |
| Zinc (Zn) | wt%d.b. | 0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Sulfate (SO42−) | wt%d.b. | 1.6 | 1.1 | 1.5 | 1.2 | 1.2 | 1.4 |
| Chloride (Cl−) | wt%d.b. | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 |
| Parameter | Without Additive | Kaolin I | Kaolin II | Kaolin III | Kaolinite | ESP |
|---|---|---|---|---|---|---|
| K2SO4 | x | x | ||||
| K2CO3 | x | x | ||||
| K2Ca(CO3)2 | x | |||||
| KAlSiO4 | x | x | x | |||
| KAlSi3O8 | x | |||||
| Al2O3 | x | |||||
| SiO2 | x | x | ||||
| CaCO3 | x | x | x | x | x | x |
| MgO | x | x | x | x |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gollmer, C.; Weigel, V.; Kaltschmitt, M. Emission Mitigation by Aluminum-Silicate-Based Fuel Additivation of Wood Chips with Kaolin and Kaolinite. Energies 2023, 16, 3095. https://doi.org/10.3390/en16073095
Gollmer C, Weigel V, Kaltschmitt M. Emission Mitigation by Aluminum-Silicate-Based Fuel Additivation of Wood Chips with Kaolin and Kaolinite. Energies. 2023; 16(7):3095. https://doi.org/10.3390/en16073095
Chicago/Turabian StyleGollmer, Christian, Vanessa Weigel, and Martin Kaltschmitt. 2023. "Emission Mitigation by Aluminum-Silicate-Based Fuel Additivation of Wood Chips with Kaolin and Kaolinite" Energies 16, no. 7: 3095. https://doi.org/10.3390/en16073095
APA StyleGollmer, C., Weigel, V., & Kaltschmitt, M. (2023). Emission Mitigation by Aluminum-Silicate-Based Fuel Additivation of Wood Chips with Kaolin and Kaolinite. Energies, 16(7), 3095. https://doi.org/10.3390/en16073095






