Recent Advances in New-Generation Electrolytes for Sodium-Ion Batteries
Abstract
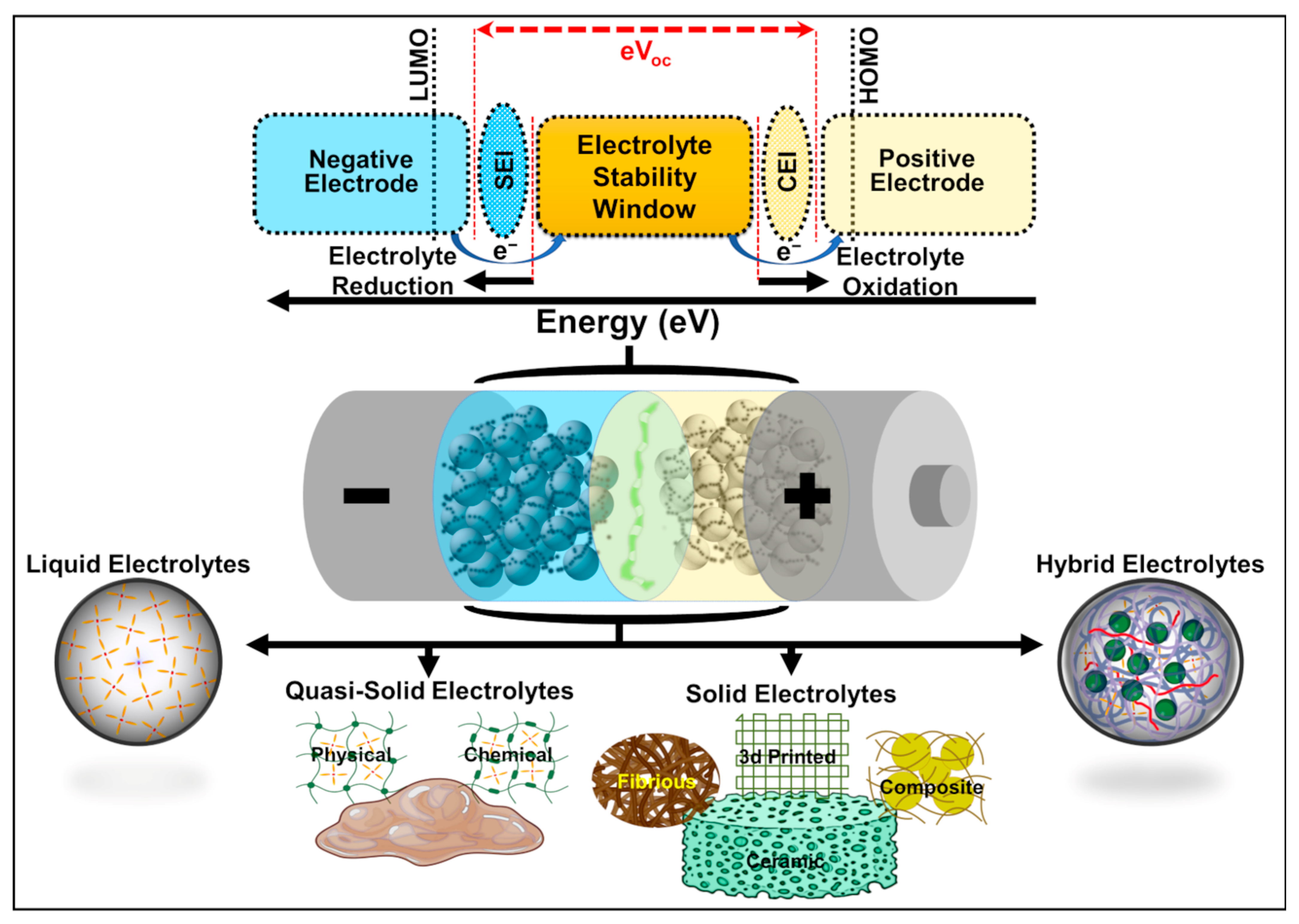
:1. Introduction
2. Liquid Electrolytes
2.1. Non-Aqueous Electrolytes
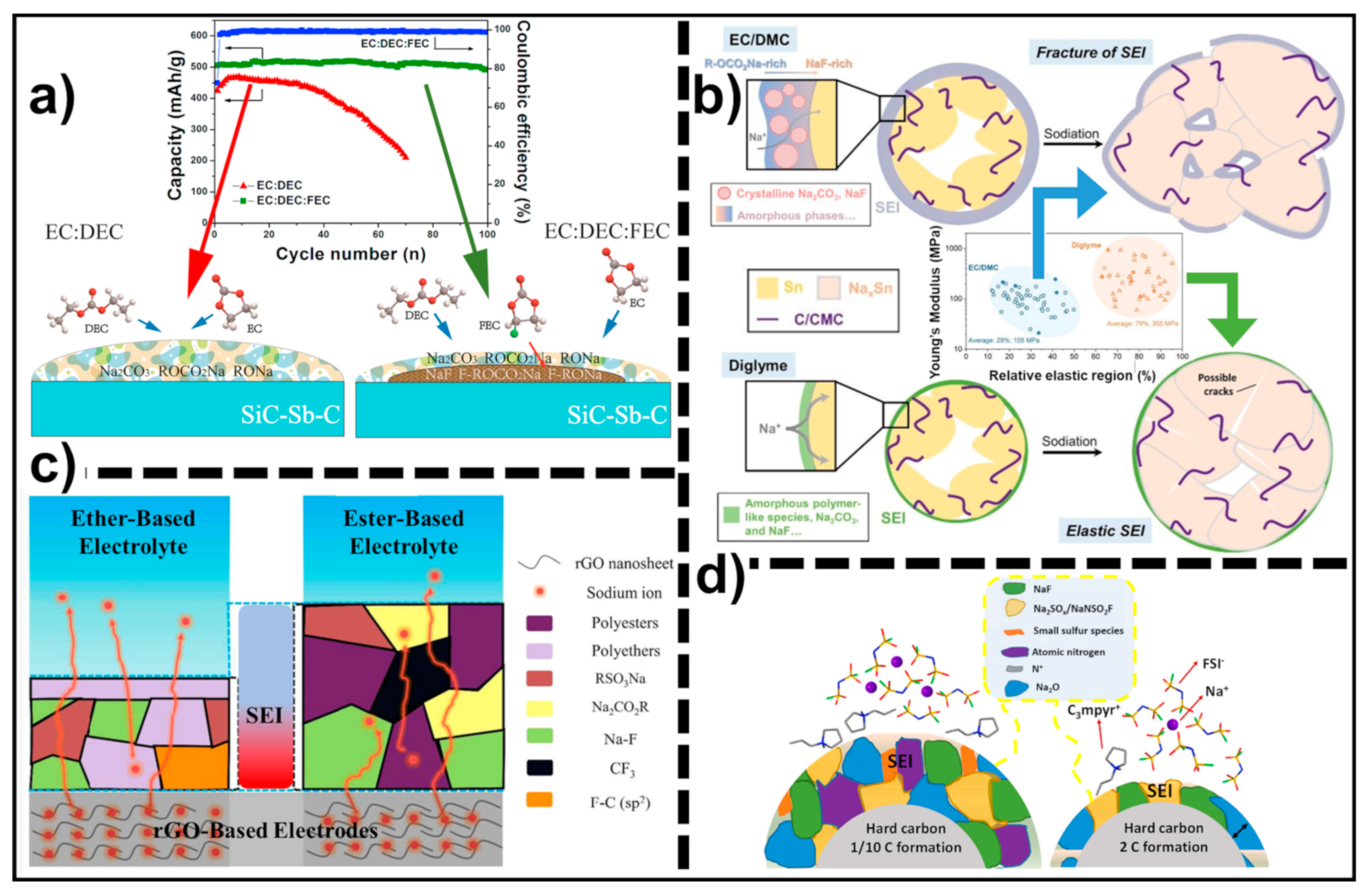
2.1.1. Ester-Based Electrolytes
2.1.2. Ether-Based Electrolytes
2.2. Ionic Liquids
2.3. Aqueous Electrolytes
3. Quasi-Solid Electrolytes
4. Solid Electrolytes
4.1. Inorganic Solid Electrolytes
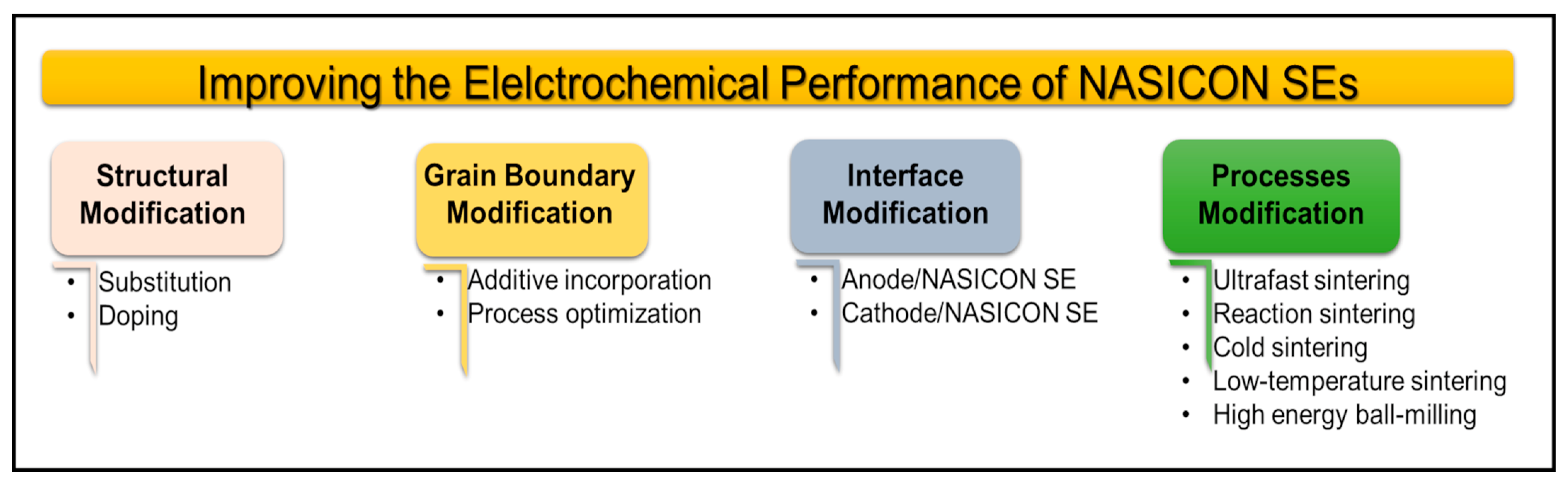
4.1.1. Structural Modification
4.1.2. Grain Boundary Modification
4.1.3. Modification of Interface Properties
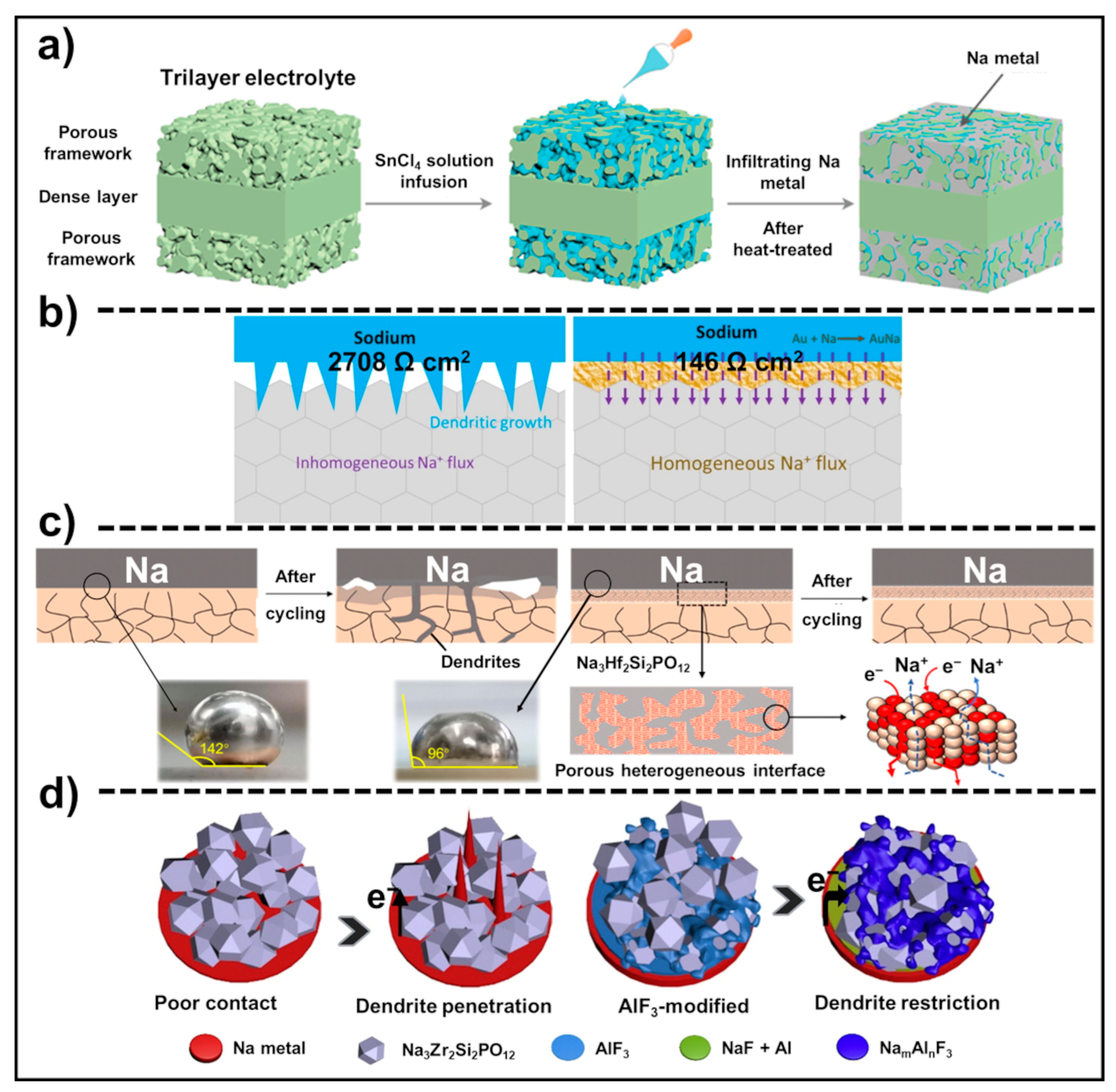
4.1.4. Process Modification
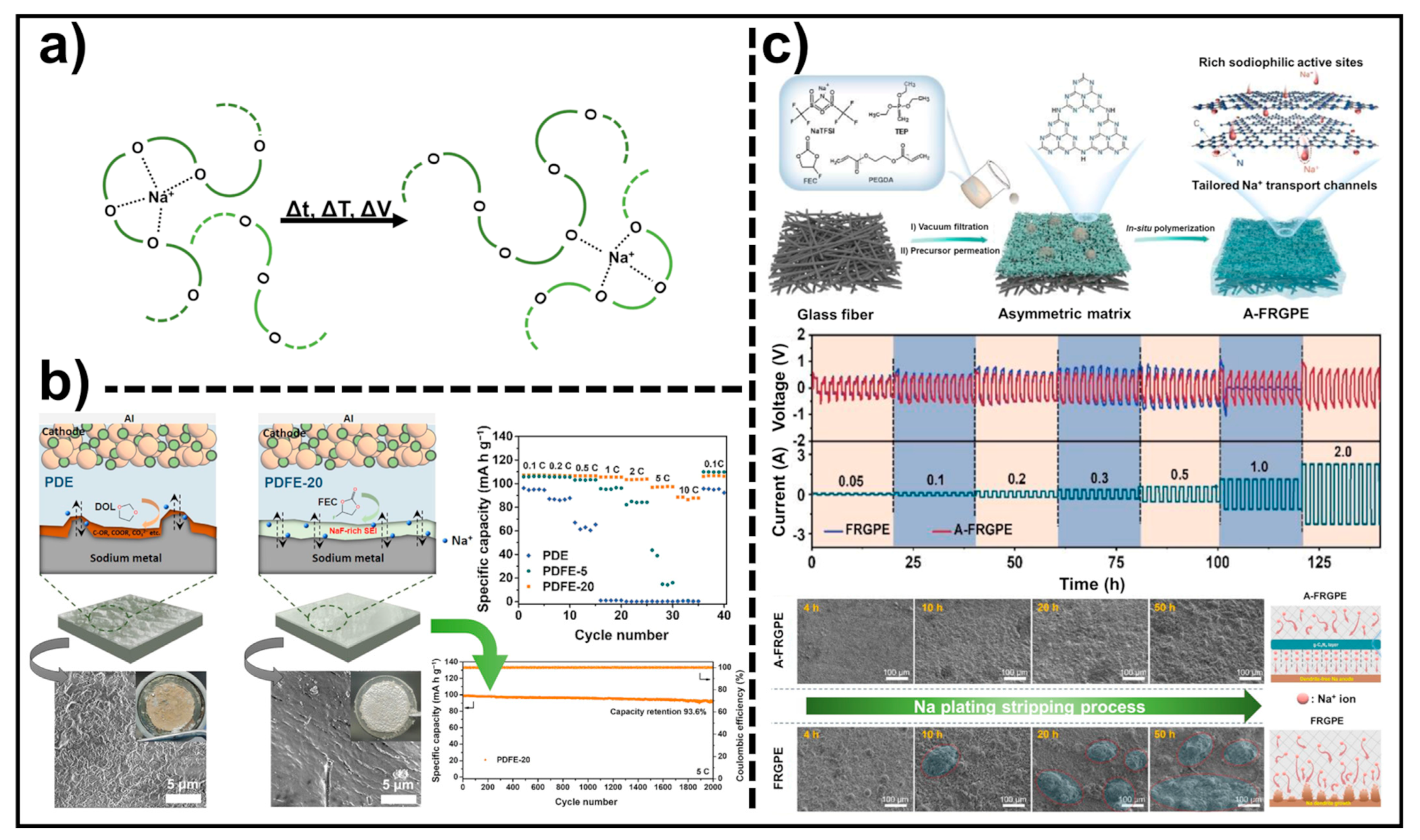
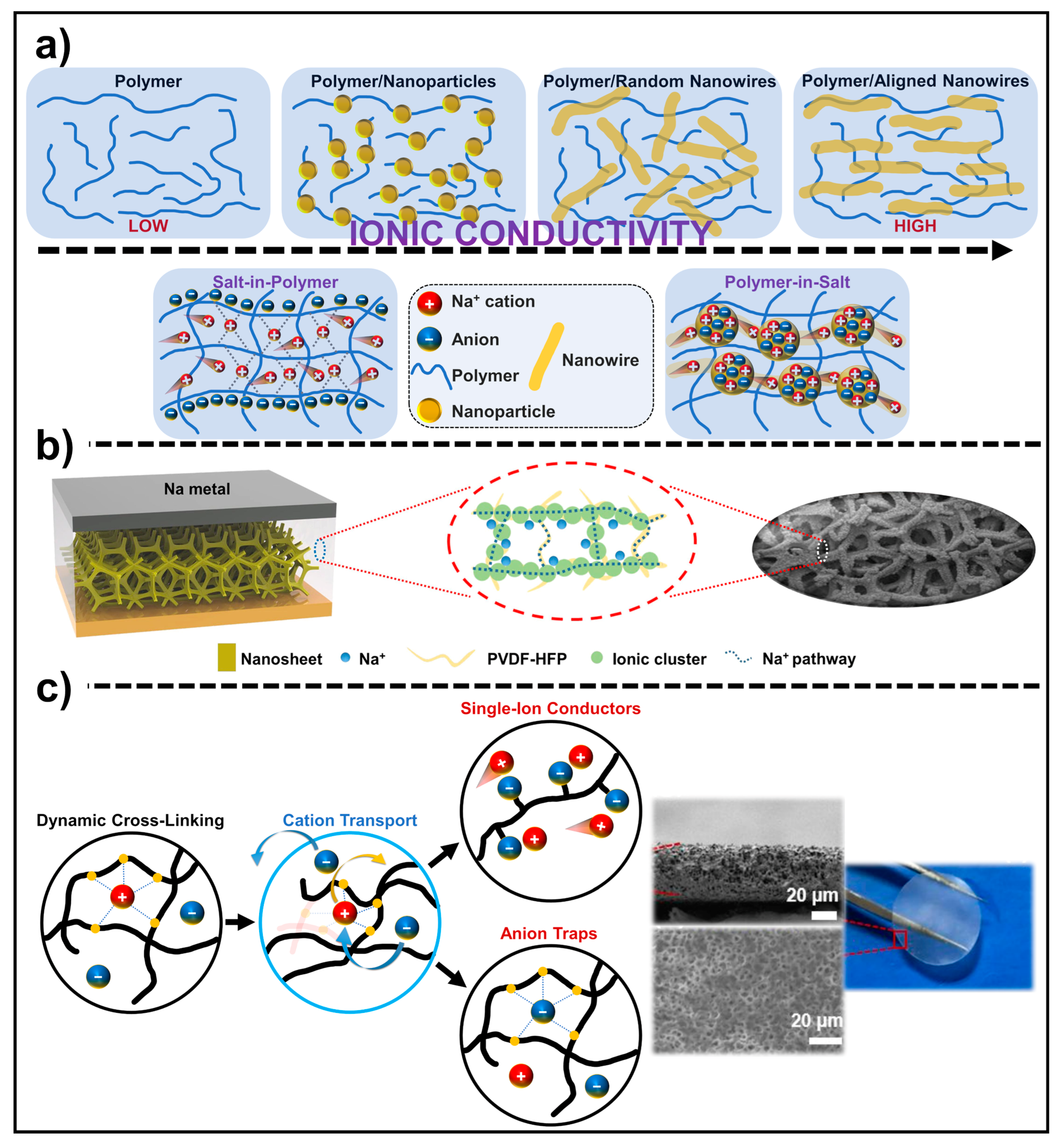
4.2. Polymer Solid Electrolytes
5. Hybrid Electrolytes
5.1. Binary Hybrid Electrolytes
5.1.1. Hybrid Liquid Electrodes
Aqueous/Non-Aqueous Hybrid Liquid Electrolytes
Ionic Liquid/Non-Aqueous Hybrid Liquid Electrolytes
Binary Salt Containing Hybrid Liquid Electrolytes
Binary Solvent Containing Hybrid Liquid Electrolytes
Non-Contact Binary Electrolyte Containing Hybrid Liquid Electrolytes
5.1.2. Hybrid Quasi-Solid Electrolytes
Ionic Liquid/Inorganic Hybrid Quasi-Solid Electrolytes
Ionic Liquid/Polymer Hybrid Quasi-Solid Electrolytes
5.1.3. Hybrid Solid Electrodes
Inorganic/Polymer Hybrid Solid Electrolytes
5.2. Ternary Hybrid Electrolytes
5.2.1. Ionic Liquid/Polymer/Inorganic Hybrid Electrolytes
5.2.2. Ionic Liquid/Polymer/Organic Ionic Plastic Crystal Hybrid Electrolytes
5.2.3. Polymer Gel/Polymer/Inorganic Hybrid Electrolytes
6. Impact of New Generation Electrolytes on SIBs
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Palomares, V.; Casas-Cabanas, M.; Castillo-Martínez, E.; Han, M.H.; Rojo, T. Update on Na-Based Battery Materials. A Growing Research Path. Energy Environ. Sci. 2013, 6, 2312–2337. [Google Scholar] [CrossRef]
- Hong, S.Y.; Kim, Y.; Park, Y.; Choi, A.; Choi, N.S.; Lee, K.T. Charge Carriers in Rechargeable Batteries: Na Ions vs. Li Ions. Energy Environ. Sci. 2013, 6, 2067–2081. [Google Scholar] [CrossRef]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-González, J.; Rojo, T. Na-Ion Batteries, Recent Advances and Present Challenges to Become Low Cost Energy Storage Systems. Energy Environ. Sci. 2012, 5, 5884–5901. [Google Scholar] [CrossRef]
- Pan, H.; Hu, Y.S.; Chen, L. Room-Temperature Stationary Sodium-Ion Batteries for Large-Scale Electric Energy Storage. Energy Environ. Sci. 2013, 6, 2338–2360. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef]
- Peters, J.; Buchholz, D.; Passerini, S.; Weil, M. Life Cycle Assessment of Sodium-Ion Batteries. Energy Environ. Sci. 2016, 9, 1744–1751. [Google Scholar] [CrossRef] [Green Version]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Che, H.; Chen, S.; Xie, Y.; Wang, H.; Amine, K.; Liao, X.Z.; Ma, Z.F. Electrolyte Design Strategies and Research Progress for Room-Temperature Sodium-Ion Batteries. Energy Environ. Sci. 2017, 10, 1075–1101. [Google Scholar] [CrossRef]
- Vignarooban, K.; Kushagra, R.; Elango, A.; Badami, P.; Mellander, B.E.; Xu, X.; Tucker, T.G.; Nam, C.; Kannan, A.M. Current Trends and Future Challenges of Electrolytes for Sodium-Ion Batteries. Int. J. Hydrog. Energy 2016, 41, 2829–2846. [Google Scholar] [CrossRef]
- Ponrouch, A.; Monti, D.; Boschin, A.; Steen, B.; Johansson, P.; Palacín, M.R. Non-aqueous electrolytes for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 22–42. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Yi, Q.; Wang, X.; Camacho, R.A.P.; Kungl, H.; Eichel, R.; Lu, L.; Zhang, H. Ion Conduction in Composite Polymer Electrolytes—Potential Electrolytes for Sodium-Ion Batteries. ChemSusChem 2023, e202202152. [Google Scholar] [CrossRef] [PubMed]
- Nurohmah, A.R.; Nisa, S.S.; Stulasti, K.N.R.; Yudha, C.S.; Suci, W.G.; Aliwarga, K.; Widiyandari, H.; Purwanto, A. Sodium-Ion Battery from Sea Salt: A Review. Mater. Renew. Sustain. Energy 2022, 11, 71–89. [Google Scholar] [CrossRef]
- Maurya, D.K.; Dhanusuraman, R.; Guo, Z.; Angaiah, S. Composite Polymer Electrolytes: Progress, Challenges, and Future Outlook for Sodium-Ion Batteries. Adv. Compos. Hybrid Mater. 2022, 5, 2651–2674. [Google Scholar] [CrossRef]
- Li, P.; Hu, N.; Wang, J.; Wang, S.; Deng, W. Recent Progress and Perspective: Na Ion Batteries Used at Low Temperatures. Nanomaterials 2022, 12, 3529. [Google Scholar] [CrossRef]
- Peljo, P.; Girault, H.H. Electrochemical Potential Window of Battery Electrolytes: The HOMO–LUMO Misconception. Energy Environ. Sci. 2018, 11, 2306–2309. [Google Scholar] [CrossRef]
- Gering, K.L. Prediction of Electrolyte Conductivity: Results from a Generalized Molecular Model Based on Ion Solvation and a Chemical Physics Framework. Electrochim. Acta 2017, 225, 175–189. [Google Scholar] [CrossRef] [Green Version]
- Hayamizu, K.; Chiba, Y.; Haishi, T. Dynamic Ionic Radius of Alkali Metal Ions in Aqueous Solution: A Pulsed-Field Gradient NMR Study. RSC Adv. 2021, 11, 20252–20257. [Google Scholar] [CrossRef]
- Ye, L.; Feng, Z. Polymer Electrolytes as Solid Solvents and Their Applications. In Polymer Electrolytes: Fundamentals and Applications; Woodhead Publishing Limited: Cambridge, UK, 2010. [Google Scholar]
- Wang, Y.; Song, S.; Xu, C.; Hu, N.; Molenda, J.; Lu, L. Development of Solid-State Electrolytes for Sodium-Ion Battery–A Short Review. Nano Mater. Sci. 2019, 1, 91–100. [Google Scholar] [CrossRef]
- Okamoto, Y.; Tsuzuki, S.; Tatara, R.; Ueno, K.; Dokko, K.; Watanabe, M. High Transference Number of Na Ion in Liquid-State Sulfolane Solvates of Sodium Bis(Fluorosulfonyl)Amide. J. Phys. Chem. C 2020, 124, 4459–4469. [Google Scholar] [CrossRef]
- Watanabe, Y.; Ugata, Y.; Ueno, K.; Watanabe, M.; Dokko, K. Does Li-Ion Transport Occur Rapidly in Localized High-Concentration Electrolytes? Phys. Chem. Chem. Phys. 2023, 25, 3092–3099. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Yin, Q.; Huang, Y.; Sun, H.; Chen, Y.; Zhang, R.; Yu, Q.; Gu, L.; Duan, J.; Luo, W. Reducing Interfacial Resistance by Na-SiO2 Composite Anode for NASICON-Based Solid-State Sodium Battery. ACS Mater. Lett. 2020, 2, 127–132. [Google Scholar] [CrossRef]
- Deng, T.; Ji, X.; Zou, L.; Chiekezi, O.; Cao, L.; Fan, X.; Adebisi, T.R.; Chang, H.J.; Wang, H.; Li, B.; et al. Interfacial-Engineering-Enabled Practical Low-Temperature Sodium Metal Battery. Nat. Nanotechnol. 2022, 17, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Venkatram, S.; Kim, C.; Batra, R.; Chandrasekaran, A.; Ramprasad, R. Electrochemical Stability Window of Polymeric Electrolytes. Chem. Mater. 2019, 31, 4598–4604. [Google Scholar] [CrossRef]
- Takenaka, N.; Bouibes, A.; Yamada, Y.; Nagaoka, M.; Yamada, A. Frontiers in Theoretical Analysis of Solid Electrolyte Interphase Formation Mechanism. Adv. Mater. 2021, 33, 2100574. [Google Scholar] [CrossRef] [PubMed]
- Kulova, T.L.; Skundin, A.M. Electrode/Electrolyte Interphases of Sodium-Ion Batteries. Energy 2022, 15, 8615. [Google Scholar] [CrossRef]
- Song, J.; Xiao, B.; Lin, Y.; Xu, K.; Li, X. Interphases in Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1703082. [Google Scholar] [CrossRef]
- Zhang, J.; Gai, J.; Song, K.; Chen, W. Advances in Electrode/Electrolyte Interphase for Sodium-Ion Batteries from Half Cells to Full Cells. Cell Rep. Phys. Sci. 2022, 3, 100868. [Google Scholar] [CrossRef]
- Yao, P.; Yu, H.; Ding, Z.; Liu, Y.; Lu, J.; Lavorgna, M.; Wu, J.; Liu, X. Review on Polymer-Based Composite Electrolytes for Lithium Batteries. Front. Chem. 2019, 7, 522. [Google Scholar] [CrossRef] [Green Version]
- Hou, W.; Guo, X.; Shen, X.; Amine, K.; Yu, H.; Lu, J. Solid Electrolytes and Interfaces in All-Solid-State Sodium Batteries: Progress and Perspective. Nano Energy 2018, 52, 279–291. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Li, Z.; Fu, J.-L.; Guo, X. Sodium-Ion Conducting Polymer Electrolytes. Rare Met. 2023, 42, 1–16. [Google Scholar] [CrossRef]
- Li, Y.; Wu, F.; Li, Y.; Liu, M.; Feng, X.; Bai, Y.; Wu, C. Ether-Based Electrolytes for Sodium Ion Batteries. Chem. Soc. Rev. 2022, 51, 4484–4536. [Google Scholar] [CrossRef]
- Suharto, Y.; Lee, Y.; Yu, J.S.; Choi, W.; Kim, K.J. Microporous Ceramic Coated Separators with Superior Wettability for Enhancing the Electrochemical Performance of Sodium-Ion Batteries. J. Power Sources 2018, 376, 184–190. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.; Park, Y.-U.; Kim, J.; Hwang, I.; Kang, K. Sodium Storage Behavior in Natural Graphite Using Ether-Based Electrolyte Systems. Adv. Funct. Mater. 2015, 25, 534–541. [Google Scholar] [CrossRef]
- Lei, S.; Zeng, Z.; Liu, M.; Zhang, H.; Cheng, S.; Xie, J. Balanced Solvation/de-Solvation of Electrolyte Facilitates Li-Ion Intercalation for Fast Charging and Low-Temperature Li-Ion Batteries. Nano Energy 2022, 98, 107265. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, L.; Li, L.; Xie, M.; Wu, F.; Chen, R. Electrolytes and Electrolyte/Electrode Interfaces in Sodium-Ion Batteries: From Scientific Research to Practical Application. Adv. Mater. 2019, 31, 1808393. [Google Scholar] [CrossRef] [PubMed]
- Eshetu, G.G.; Elia, G.A.; Armand, M.; Forsyth, M.; Komaba, S.; Rojo, T.; Passerini, S. Electrolytes and Interphases in Sodium-Based Rechargeable Batteries: Recent Advances and Perspectives. Adv. Energy Mater. 2020, 10, 2000093. [Google Scholar] [CrossRef] [Green Version]
- Eshetu, G.G.; Grugeon, S.; Kim, H.; Jeong, S.; Wu, L.; Gachot, G.; Laruelle, S.; Armand, M.; Passerini, S. Comprehensive Insights into the Reactivity of Electrolytes Based on Sodium Ions. ChemSusChem 2016, 9, 462–471. [Google Scholar] [CrossRef]
- Meng, J.; Jia, G.; Yang, H.; Wang, M. Recent Advances for SEI of Hard Carbon Anode in Sodium-Ion Batteries: A Mini Review. Front. Chem. 2022, 10, 1–7. [Google Scholar] [CrossRef]
- Kamath, G.; Cutler, R.W.; Deshmukh, S.A.; Shakourian-Fard, M.; Parrish, R.; Huether, J.; Butt, D.P.; Xiong, H.; Sankaranarayanan, S.K.R.S. In Silico Based Rank-Order Determination and Experiments on Nonaqueous Electrolytes for Sodium Ion Battery Applications. J. Phys. Chem. C 2014, 118, 13406–13416. [Google Scholar] [CrossRef]
- Komaba, S.; Murata, W.; Ishikawa, T.; Yabuuchi, N.; Ozeki, T.; Nakayama, T.; Ogata, A.; Gotoh, K.; Fujiwara, K. Electrochemical Na Insertion and Solid Electrolyte Interphase for Hard-Carbon Electrodes and Application to Na-Ion Batteries. Adv. Funct. Mater. 2011, 21, 3859–3867. [Google Scholar] [CrossRef]
- Lu, H.; Wu, L.; Xiao, L.; Ai, X.; Yang, H.; Cao, Y. Investigation of the Effect of Fluoroethylene Carbonate Additive on Electrochemical Performance of Sb-Based Anode for Sodium-Ion Batteries. Electrochim. Acta 2016, 190, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Guo, X.; Du, X.; Lin, X.; Huang, J.Q.; Tan, H.; Zhu, Y.; Zhang, B. Nanostructures of Solid Electrolyte Interphases and Their Consequences for Microsized Sn Anodes in Sodium Ion Batteries. Energy Environ. Sci. 2019, 12, 1550–1557. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.W.; Lv, W.; Zhang, S.; Liang, Q.; Zheng, D.; Kang, F.; Yang, Q.H. Achieving Superb Sodium Storage Performance on Carbon Anodes through an Ether-Derived Solid Electrolyte Interphase. Energy Environ. Sci. 2017, 10, 370–376. [Google Scholar] [CrossRef]
- Sun, J.; Gunathilaka, I.E.; O’Dell, L.A.; Howlett, P.C.; Forsyth, M. High-Rate Formation Protocol Enables a High Ionic Conductivity SEI for Sodium-Ion Batteries. J. Power Sources 2023, 554, 232298. [Google Scholar] [CrossRef]
- Dugas, R.; Ponrouch, A.; Gachot, G.; David, R.; Palacin, M.R.; Tarascon, J.M. Na Reactivity toward Carbonate-Based Electrolytes: The Effect of FEC as Additive. J. Electrochem Soc. 2016, 163, A2333. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.; Li, T.; Qiu, Y.; Jiang, M.; Zheng, Q.; Li, X. Weak Coulomb Interaction between Anions and Na+ during Solvation Enabling Desirable Solid Electrolyte Interphase and Superior Kinetics for HC-Based Sodium Ion Batteries. Chem. Eng. J. 2023, 453, 139932. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Martinez-Ibañez, M.; Sánchez-Diez, E.; Gracia, I.; Li, C.; Rodriguez-Martinez, L.M.; Rojo, T.; Zhang, H.; Armand, M. Electrolyte Additives for Room-Temperature, Sodium-Based, Rechargeable Batteries. Chem. Asian J. 2018, 13, 2770–2780. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Diemant, T.; Hekmatfar, M.; Grugeon, S.; Behm, R.J.; Laruelle, S.; Armand, M.; Passerini, S. Impact of the Electrolyte Salt Anion on the Solid Electrolyte Interphase Formation in Sodium Ion Batteries. Nano Energy 2019, 55, 327–340. [Google Scholar] [CrossRef]
- Sun, Z.; Fu, W.; Liu, M.Z.; Lu, P.; Zhao, E.; Magasinski, A.; Liu, M.; Luo, S.; McDaniel, J.; Yushin, G. A Nanoconfined Iron(Iii) Fluoride Cathode in a NaDFOB Electrolyte: Towards High-Performance Sodium-Ion Batteries. J. Mater. Chem. A Mater. 2020, 8, 4091–4098. [Google Scholar] [CrossRef]
- Tao, W.; Chen, J.; Xu, C.; Liu, S.; Fakudze, S.; Wang, J.; Wang, C. Nanostructured MoS2 with Interlayer Controllably Regulated by Ionic Liquids/Cellulose for High-Capacity and Durable Sodium Storage Properties. Small 2023, 2207397. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, Z.; Vanaphuti, P.; Yang, X.; Mei, L.; Zhu, X.; Liu, S.; Wang, Y. Stable Fast-Charging Sodium-Ion Batteries Achieved by a Carbomethoxy-Modified Disodium Organic Material. Cell Rep. Phys. Sci. 2023, 4, 101240. [Google Scholar] [CrossRef]
- Yang, R.; Sun, L.; Liu, W.; Zhang, Y.; Cui, Y.; Du, Y.; Liu, S.; Wang, H.; Huang, M. Bio-Derived 3D TiO 2 Hollow Spheres with a Mesocrystal Nanostructure to Achieve Improved Electrochemical Performance of Na-Ion Batteries in Ether-Based Electrolytes. J. Mater. Chem. A Mater. 2019, 7, 3399–3407. [Google Scholar] [CrossRef]
- Nacimiento, F.; Cabello, M.; Ortiz, G.F.; Alcántara, R.; Lavela, P.; Tirado, J.L. Morphological Adaptability of Graphitic Carbon Nanofibers to Enhance Sodium Insertion in a Diglyme-Based Electrolyte. Dalton Trans. 2019, 48, 5417–5424. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, Y.; Jie, Y.; Lang, S.; Song, J.; Lei, Z.; Wang, S.; Ren, X.; Wang, D.; Li, X.; et al. Stable Sodium Metal Batteries via Manipulation of Electrolyte Solvation Structure. Small Methods 2020, 4, 1900856. [Google Scholar] [CrossRef]
- Zhang, K.; Yoon, G.; Zhang, J.; Park, M.; Yang, J.; Kang, K.; Kang, Y.-M. Pseudocapacitive Behavior and Ultrafast Kinetics from Solvated Ion Cointercalation into MoS2 for Its Alkali Ion Storage. ACS Appl. Energy Mater. 2019, 2, 3726–3735. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.; Yoon, G.; Kim, H.; Park, K.-Y.; Park, M.-S.; Yoon, W.-S.; Kang, K. Sodium Intercalation Chemistry in Graphite. Energy Environ. Sci. 2015, 8, 2963–2969. [Google Scholar] [CrossRef]
- Song, K.; Wang, X.; Xie, Z.; Zhao, Z.; Fang, Z.; Zhang, Z.; Luo, J.; Yan, P.; Peng, Z.; Chen, W. Ultrathin CuF2-Rich Solid-Electrolyte Interphase Induced by Cation-Tailored Double Electrical Layer toward Durable Sodium Storage. Angew. Chem. Int. Ed. 2023, 62, e202216450. [Google Scholar] [CrossRef]
- Yu, X.; Lu, T.; Li, X.; Qi, J.; Yuan, L.; Man, Z.; Zhuo, H. Realizing Outstanding Electrochemical Performance with Na3V2(PO4)2F3 Modified with an Ionic Liquid for Sodium-Ion Batteries. RSC Adv. 2022, 12, 14007–14017. [Google Scholar] [CrossRef]
- Yu, X.; Lu, T.; Li, X.; Qi, J.; Yuan, L.; Man, Z.; Zhuo, H. Ionic Liquid–Acrylic Acid Copolymer Derived Nitrogen–Boron Codoped Carbon-Covered Na3V2(PO4)2F3 as Cathode Material of High-Performance Sodium-Ion Batteries. Langmuir 2022, 38, 7815–7824. [Google Scholar] [CrossRef]
- Xu, X.; Si, L.; Zhou, X.; Tu, F.; Zhu, X.; Bao, J. Chemical bonding between antimony and ionic liquid derived nitrogen-doped carbon for sodium-ion battery. J. Power Sources 2017, 349, 37–44. [Google Scholar] [CrossRef]
- Maça, R.R.; Etacheri, V. Effect of Vinylene Carbonate Electrolyte Additive on the Surface Chemistry and Pseudocapacitive Sodium-Ion Storage of TiO2 Nanosheet Anodes. Batteries 2021, 7, 1. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, T.; Fang, T.; Gao, Y.; Gao, S.; Wang, W.; Liao, L. A Novel MoS2@C Framework Architecture Composites with Three-Dimensional Cross-Linked Porous Carbon Supporting MoS2 Nanosheets for Sodium Storage. J. Alloys Compd. 2020, 818, 152821. [Google Scholar] [CrossRef]
- Dahbi, M.; Fukunishi, M.; Horiba, T.; Yabuuchi, N.; Yasuno, S.; Komaba, S. High Performance Red Phosphorus Electrode in Ionic Liquid-Based Electrolyte for Na-Ion Batteries. J. Power Sources 2017, 363, 404–412. [Google Scholar] [CrossRef]
- Santamaría, C.; Morales, E.; del Rio, C.; Herradón, B.; Amarilla, J.M. Studies on Sodium-Ion Batteries: Searching for the Proper Combination of the Cathode Material, the Electrolyte and the Working Voltage. The Role of Magnesium Substitution in Layered Manganese-Rich Oxides, and Pyrrolidinium Ionic Liquid. Electrochim. Acta 2023, 439, 141654. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, B.; Boebinger, M.G.; Magasinski, A.; Jhulki, S.; Zhang, Y.; Fu, W.; McDowell, M.T.; Yushin, G. Stability of FeF3-Based Sodium-Ion Batteries in Nonflammable Ionic Liquid Electrolytes at Room and Elevated Temperatures. ACS Appl. Mater. Interfaces 2022, 14, 33447–33456. [Google Scholar] [CrossRef]
- Reber, D.; Grissa, R.; Becker, M.; Kühnel, R.S.; Battaglia, C. Anion Selection Criteria for Water-in-Salt Electrolytes. Adv Energy Mater. 2021, 11, 2002913. [Google Scholar] [CrossRef]
- Bi, H.; Luo, Y.; Zhao, C.; Ma, L.; Huang, H. Graphene Oxide Suspension-Based Electrolyte Promotes the Cycling Performance of Aqueous Sodium-Ion Batteries through the Interaction between Metal Ions, Free Water Molecules and Functional Groups. J. Power Sources 2023, 555, 232380. [Google Scholar] [CrossRef]
- Yang, W.; Wang, X.; Lu, S.; Gao, Y.; Gao, T.; Guo, T.; Xie, Q.; Ruan, Y. Bimetallic Synergies Help the Application of Sodium Vanadyl Phosphate in Aqueous Sodium-Ion Batteries Possible. ChemSusChem 2023, e202202257. [Google Scholar] [CrossRef]
- Nakamoto, K.; Sakamoto, R.; Nishimura, Y.; Xia, J.; Ito, M.; Okada, S. A Trifluoroacetate-Based Concentrated Electrolyte for Symmetrical Aqueous Sodium-Ion Battery with NASICON-Type Na2VTi(PO4)3 Electrodes. Electrochemistry 2021, 89, 415–419. [Google Scholar] [CrossRef]
- Sun, J.; O’Dell, L.A.; Armand, M.; Howlett, P.C.; Forsyth, M. Anion-Derived Solid-Electrolyte Interphase Enables Long Life Na-Ion Batteries Using Superconcentrated Ionic Liquid Electrolytes. ACS Energy Lett. 2021, 6, 2481–2490. [Google Scholar] [CrossRef]
- Fiates, J.; Ratochinski, R.H.; Lourenço, T.C.; da Silva, J.L.F.; Dias, L.G. Fluoroalkoxyaluminate-Based Ionic Liquids as Electrolytes for Sodium-Ion Batteries. J. Mol. Liq. 2023, 369, 120919. [Google Scholar] [CrossRef]
- Yang, M.; Luo, J.; Guo, X.; Chen, J.; Cao, Y.; Chen, W. Aqueous Rechargeable Sodium-Ion Batteries: From Liquid to Hydrogel. Batteries 2022, 8, 180. [Google Scholar] [CrossRef]
- Kühnel, R.S.; Reber, D.; Battaglia, C. A High-Voltage Aqueous Electrolyte for Sodium-Ion Batteries. ACS Energy Lett. 2017, 2, 2005–2006. [Google Scholar] [CrossRef]
- Ge, J.; Fan, L.; Rao, A.M.; Zhou, J.; Lu, B. Surface-Substituted Prussian Blue Analogue Cathode for Sustainable Potassium-Ion Batteries. Nat. Sustain. 2022, 5, 225–234. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, Y.; Xie, Z.; Xie, H.; Chen, W.; Lu, B. Tailored ZnF2/ZnS-Rich Interphase for Reversible Aqueous Zn Batteries. Nano Res. 2023. [Google Scholar] [CrossRef]
- Guo, J.Z.; Yang, A.B.; Gu, Z.Y.; Wu, X.L.; Pang, W.L.; Ning, Q.L.; Li, W.H.; Zhang, J.P.; Su, Z.M. Quasi-Solid-State Sodium-Ion Full Battery with High-Power/Energy Densities. ACS Appl. Mater. Interfaces 2018, 10, 17903–17910. [Google Scholar] [CrossRef] [PubMed]
- Janakiraman, S.; Surendran, A.; Ghosh, S.; Anandhan, S.; Venimadhav, A. Electroactive Poly(Vinylidene Fluoride) Fluoride Separator for Sodium Ion Battery with High Coulombic Efficiency. Solid State Ion. 2016, 292, 130–135. [Google Scholar] [CrossRef]
- DeBlock, R.H.; Lai, C.-H.; Butts, D.M.; Dunn, B.S. Sodium-Ion Conducting Pseudosolid Electrolyte for Energy-Dense, Sodium-Metal Batteries. J. Power Sources 2023, 554, 232305. [Google Scholar] [CrossRef]
- Zhang, Y.; Bakenov, Z.; Tan, T.; Huang, J. Polyacrylonitrile-Nanofiber-Based Gel Polymer Electrolyte for Novel Aqueous Sodium-Ion Battery Based on a Na4Mn9O18 Cathode and Zn Metal Anode. Polymers 2018, 10, 853. [Google Scholar] [CrossRef] [Green Version]
- Rani, M.A.A.; Hwang, J.; Matsumoto, K.; Hagiwara, R. Poly(Vinyl Chloride) Ionic Liquid Polymer Electrolyte Based on Bis(Fluorosulfonyl)Amide for Sodium Secondary Batteries. J. Electrochem Soc. 2017, 164, H5031. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Che, H.; Feng, F.; Liao, J.; Wang, H.; Yin, Y.; Ma, Z.F. Poly(Vinylene Carbonate)-Based Composite Polymer Electrolyte with Enhanced Interfacial Stability to Realize High-Performance Room-Temperature Solid-State Sodium Batteries. ACS Appl. Mater. Interfaces 2019, 11, 43056–43065. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Z.; Tang, Y.; Chen, J.; Mao, Z.; Wang, D. PVDF-HFP/PMMA/TPU-Based Gel Polymer Electrolytes Composed of Conductive Na3Zr2Si2PO12 Filler for Application in Sodium Ions Batteries. Solid State Ion. 2021, 359, 115532. [Google Scholar] [CrossRef]
- Gebert, F.; Knott, J.; Gorkin, R.; Chou, S.L.; Dou, S.X. Polymer Electrolytes for Sodium-Ion Batteries. Energy Storage Mater. 2021, 36, 10–30. [Google Scholar] [CrossRef]
- Ma, J.; Feng, X.; Wu, Y.; Wang, Y.; Liu, P.; Shang, K.; Jiang, H.; Hou, X.; Mitlin, D.; Xiang, H. Stable Sodium Anodes for Sodium Metal Batteries (SMBs) Enabled by in-Situ Formed Quasi Solid-State Polymer Electrolyte. J. Energy Chem. 2023, 77, 290–299. [Google Scholar] [CrossRef]
- Yang, M.; Feng, F.; Shi, Z.; Guo, J.; Wang, R.; Xu, Z.; Liu, Z.; Cai, T.; Wang, Z.; Wang, C.; et al. Facile Design of Asymmetric Flame-Retardant Gel Polymer Electrolyte with Excellent Interfacial Stability for Sodium Metal Batteries. Energy Storage Mater. 2023, 56, 611–620. [Google Scholar] [CrossRef]
- Colò, F.; Bella, F.; Nair, J.R.; Gerbaldi, C. Light-Cured Polymer Electrolytes for Safe, Low-Cost and Sustainable Sodium-Ion Batteries. J. Power Sources 2017, 365, 293–302. [Google Scholar] [CrossRef]
- Parveen, S.; Sehrawat, P.; Hashmi, S.A. Triglyme-Based Solvate Ionic Liquid Gelled in a Polymer: A Novel Electrolyte Composition for Sodium Ion Battery. Mater. Today Commun. 2022, 31, 103392. [Google Scholar] [CrossRef]
- Zhao, C.d.; Guo, J.Z.; Gu, Z.Y.; Wang, X.T.; Zhao, X.X.; Li, W.H.; Yu, H.Y.; Wu, X.L. Flexible Quasi-Solid-State Sodium-Ion Full Battery with Ultralong Cycle Life, High Energy Density and High-Rate Capability. Nano Res. 2022, 15, 925–932. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Y.; Fu, L.; Wu, Y. A Porous Gel-Type Composite Membrane Reinforced by Nonwoven: Promising Polymer Electrolyte with High Performance for Sodium Ion Batteries. Electrochim. Acta 2017, 224, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Zhang, X.; Shi, W.; Liang, S.; Cao, H.; Fu, Y.; Wang, H.; Zhu, Y. A P(VDF-HFP) and Nonwoven-Fabric Based Composite as High-Performance Gel Polymer Electrolyte for Fast-Charging Sodium Metal Batteries. Polymers 2023, 269, 125751. [Google Scholar] [CrossRef]
- Lei, D.; He, Y.B.; Huang, H.; Yuan, Y.; Zhong, G.; Zhao, Q.; Hao, X.; Zhang, D.; Lai, C.; Zhang, S.; et al. Cross-Linked Beta Alumina Nanowires with Compact Gel Polymer Electrolyte Coating for Ultra-Stable Sodium Metal Battery. Nat. Commun. 2019, 10, 4244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Liu, H.; Meng, X.; Liu, A.; Chen, Y.; Ma, T. A Cross-Linked Tin Oxide/Polymer Composite Gel Electrolyte with Adjustable Porosity for Enhanced Sodium Ion Batteries. Chem. Eng. J. 2022, 431, 133922. [Google Scholar] [CrossRef]
- Saroja, A.P.V.K.; Kumar, A.; Moharana, B.C.; Kamaraj, M.; Ramaprabhu, S. Design of Porous Calcium Phosphate Based Gel Polymer Electrolyte for Quasi-Solid State Sodium Ion Battery. J. Electroanal. Chem. 2020, 859, 113864. [Google Scholar] [CrossRef]
- Lai, H.; Lu, Y.; Zha, W.; Hu, Y.; Zhang, Y.; Wu, X.; Wen, Z. In Situ Generated Composite Gel Polymer Electrolyte with Crosslinking Structure for Dendrite-Free and High-Performance Sodium Metal Batteries. Energy Storage Mater. 2023, 54, 478–487. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, K.; Liu, Y.; Ye, L.; Gao, Y.; Lin, W.; Xu, H.; Wang, X.; Bai, Y.; Wu, C. Flame-Retardant Gel Polymer Electrolyte and Interface for Quasi-Solid-State Sodium Ion Batteries. Chem. Eng. J. 2020, 401, 126065. [Google Scholar] [CrossRef]
- Zheng, J.; Li, W.; Zhang, J.; Sun, Y.; Chen, W. Effects of Flexible Group Length of Phosphonate Monomers on the Performance of Gel Polymer Electrolytes for Sodium-Ion Batteries with Ultralong Cycling Life. ACS Sustain. Chem. Eng. 2022, 10, 7158–7168. [Google Scholar] [CrossRef]
- Sehrawat, P.; Parveen, S.; Hashmi, S.A. High-Performance Sodium Ion Conducting Gel Polymer Electrolyte Based on a Biodegradable Polymer Polycaprolactone. Energy Storage 2022, e375. [Google Scholar] [CrossRef]
- Liu, D.; Du, G.; Qi, Y.; Niu, Y.; Bao, S.; Xu, M. Bacterial Cellulose Network Based Gel Polymer Electrolyte for Quasi-Solid-State Sodium-Ion Battery. J. Mater. Sci. Mater. Electron. 2022, 33, 15313–15322. [Google Scholar] [CrossRef]
- Mittal, N.; Tien, S.; Lizundia, E.; Niederberger, M. Hierarchical Nanocellulose-Based Gel Polymer Electrolytes for Stable Na Electrodeposition in Sodium Ion Batteries. Small 2022, 18, 2107183. [Google Scholar] [CrossRef]
- Chen, J.; Luo, C.; Huang, Y.; Liu, J.; Li, C.; Zhao, Z.; Xu, X.; Zheng, H.; Tang, Z.; Li, X.; et al. Hydroxypropyl Methyl Cellulose-Based Gel Polymer Electrolyte Provides a Fast Migration Channel for Sodium-Ion Batteries. J. Mater. Sci. 2022, 57, 4311–4322. [Google Scholar] [CrossRef]
- Cai, S.; Meng, W.; Tian, H.; Luo, T.; Wang, L.; Li, M.; Luo, J.; Liu, S. Artificial Porous Heterogeneous Interface for All-Solid-State Sodium Ion Battery. J. Colloid Interface Sci. 2023, 632, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Tietz, F. Solid-State Electrolyte Materials for Sodium Batteries: Towards Practical Applications. ChemElectroChem 2020, 7, 2691–2713. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, L.; Qi, X.; Lu, Y.; Wu, F.; Zhao, J.; Yu, Y.; Hu, Y.S.; Chen, L. Solid-State Sodium Batteries. Adv. Energy Mater. 2018, 8, 1703012. [Google Scholar] [CrossRef]
- Delmas, C. Sodium and Sodium-Ion Batteries: 50 Years of Research. Adv. Energy Mater. 2018, 8, 1703137. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Hong, H.Y.P.; Kafalas, J.A. Fast Na+-Ion Transport in Skeleton Structures. Mater. Res. Bull. 1976, 11, 203–220. [Google Scholar] [CrossRef]
- Li, C.; Li, R.; Liu, K.; Si, R.; Zhang, Z.; Hu, Y.-S. NaSICON: A Promising Solid Electrolyte for Solid-State Sodium Batteries. Interdiscip. Mater. 2022, 1, 396–416. [Google Scholar] [CrossRef]
- Samiee, M.; Radhakrishnan, B.; Rice, Z.; Deng, Z.; Meng, Y.S.; Ong, S.P.; Luo, J. Divalent-Doped Na3Zr2Si2PO12 Natrium Superionic Conductor: Improving the Ionic Conductivity via Simultaneously Optimizing the Phase and Chemistry of the Primary and Secondary Phases. J. Power Sources 2017, 347, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zou, Z.; Kaup, K.; Xiao, R.; Shi, S.; Avdeev, M.; Hu, Y.S.; Wang, D.; He, B.; Li, H.; et al. Correlated Migration Invokes Higher Na+-Ion Conductivity in NaSICON-Type Solid Electrolytes. Adv. Energy Mater. 2019, 9, 1902373. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; You, Y.; Vinu, A.; Mai, L. NASICONs-Type Solid-State Electrolytes: The History, Physicochemical Properties, and Challenges. Interdiscip. Mater. 2023, 2, 91–110. [Google Scholar] [CrossRef]
- Singh, K.; Chakraborty, A.; Thirupathi, R.; Omar, S. Recent Advances in NASICON-Type Oxide Electrolytes for Solid-State Sodium-Ion Rechargeable Batteries. Ionics 2022, 28, 5289–5319. [Google Scholar] [CrossRef]
- Yang, Z.; Tang, B.; Xie, Z.; Zhou, Z. NASICON-Type Na3Zr2Si2PO12 Solid-State Electrolytes for Sodium Batteries. ChemElectroChem 2021, 8, 1035–1047. [Google Scholar] [CrossRef]
- Pal, S.K.; Saha, R.; Kumar, G.V.; Omar, S. Designing High Ionic Conducting NASICON-Type Na3Zr2Si2PO12 Solid-Electrolytes for Na-Ion Batteries. J. Phys. Chem. C 2020, 124, 9161–9169. [Google Scholar] [CrossRef]
- Miao, R.J.; Cao, X.G.; Wang, W.G.; Zhang, H.Y. Influence of Bi2O3 Additive on the Electrochemical Performance of Na3.1Y0.1Zr1.9Si2PO12 Inorganic Solid Electrolyte. Ceram Int. 2021, 47, 17455–17462. [Google Scholar] [CrossRef]
- Jiang, P.; Du, G.; Shi, Y.; She, F.; Guo, P.; Qian, G.; Lu, X.; Xie, F.; Lu, X. Ultrafast Sintering of Na3Zr2Si2PO12 Solid Electrolyte for Long Lifespan Solid-State Sodium Ion Batteries. Chem. Eng. J. 2023, 451, 138771. [Google Scholar] [CrossRef]
- Shindrov, A.A. Increasing Sinterability and Ionic Conductivity of Na3Zr2Si2PO12 Ceramics by High Energy Ball-Milling. Solid State Ion. 2023, 391, 116139. [Google Scholar] [CrossRef]
- Noguchi, Y.; Kobayashi, E.; Plashnitsa, L.S.; Okada, S.; Yamaki, J.I. Fabrication and Performances of All Solid-State Symmetric Sodium Battery Based on NASICON-Related Compounds. Electrochim. Acta 2013, 101, 59–65. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Q.; Lu, Y.; Shao, Y.; Qi, Y.; Qi, X.; Zhong, G.; Yang, Y.; Chen, L.; Hu, Y.S. Modification of NASICON Electrolyte and Its Application in Real Na-Ion Cells. Engineering 2022, 8, 170–180. [Google Scholar] [CrossRef]
- Lu, Y.; Alonso, J.A.; Yi, Q.; Lu, L.; Wang, Z.L.; Sun, C. A High-Performance Monolithic Solid-State Sodium Battery with Ca2+ Doped Na3Zr2Si2PO12 Electrolyte. Adv. Energy Mater. 2019, 9, 1901205. [Google Scholar] [CrossRef]
- Jolley, A.G.; Cohn, G.; Hitz, G.T.; Wachsman, E.D. Improving the Ionic Conductivity of NASICON through Aliovalent Cation Substitution of Na3Zr2Si2PO12. Ionics 2015, 21, 3031–3038. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Mao, Z.; Wang, D. Effective Resistance to Dendrite Growth of NASICON Solid Electrolyte with Lower Electronic Conductivity. Chem. Eng. J. 2022, 427, 130899. [Google Scholar] [CrossRef]
- Chen, D.; Luo, F.; Zhou, W.; Zhu, D. Influence of Nb5+, Ti4+, Y3+ and Zn2+ Doped Na3Zr2Si2PO12 Solid Electrolyte on Its Conductivity. J. Alloys Compd. 2018, 757, 348–355. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Peng, J.; Zhou, X.; Liang, D.; Zhao, L.; Su, J.; Zhang, B.; Li, S.; Zhang, N.; et al. A Niobium-Substituted Sodium Superionic Conductor with Conductivity Higher than 5.5 MS Cm−1 Prepared by Solution-Assisted Solid-State Reaction Method. J. Power Sources 2022, 518, 230765. [Google Scholar] [CrossRef]
- Li, D.; Sun, C.; Wang, C.; Li, J.; Wang, Z.; Jin, H. Regulating Na/NASCION Electrolyte Interface Chemistry for Stable Solid-State Na Metal Batteries at Room Temperature. Energy Storage Mater. 2023, 54, 403–409. [Google Scholar] [CrossRef]
- Yang, J.; Liu, G.; Avdeev, M.; Wan, H.; Han, F.; Shen, L.; Zou, Z.; Shi, S.; Hu, Y.S.; Wang, C.; et al. Ultrastable All-Solid-State Sodium Rechargeable Batteries. ACS Energy Lett. 2020, 5, 2835–2841. [Google Scholar] [CrossRef]
- Luo, J.; Zhao, G.; Qiang, W.; Huang, B. Synthesis of Na Ion-Electron Mixed Conductor Na3Zr2Si2PO12 by Doping with Transition Metal Elements (Co, Fe, Ni). J. Am. Ceram. Soc. 2022, 105, 3428–3437. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Shi, J.; Chu, Y.S.; Yu, X.; Xu, K.; Ge, M.; Yan, H.; Li, W.; Gu, L.; et al. A Self-Forming Composite Electrolyte for Solid-State Sodium Battery with Ultralong Cycle Life. Adv. Energy Mater. 2017, 7, 1601196. [Google Scholar] [CrossRef]
- Ruan, Y.; Song, S.; Liu, J.; Liu, P.; Cheng, B.; Song, X.; Battaglia, V. Improved Structural Stability and Ionic Conductivity of Na3Zr2Si2PO12 Solid Electrolyte by Rare Earth Metal Substitutions. Ceram. Int. 2017, 43, 7810–7815. [Google Scholar] [CrossRef]
- Sun, F.; Xiang, Y.; Sun, Q.; Zhong, G.; Banis, M.N.; Liu, Y.; Li, R.; Fu, R.; Zheng, M.; Sham, T.K.; et al. Origin of High Ionic Conductivity of Sc-Doped Sodium-Rich NASICON Solid-State Electrolytes. Adv. Funct. Mater. 2021, 31, 2102129. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, C.; Li, L.; Liu, X.; Zhang, X.; Gao, G.; Wang, Y.; Li, G. Sc-Doping in Na3Zr2Si2PO12 Electrolytes Enables Preeminent Performance of Solid-State Sodium Batteries in a Wide Temperature Range. Energy Storage Mater. 2023, 54, 135–145. [Google Scholar] [CrossRef]
- Thirupathi, R.; Omar, S. A Strategic Co-Doping Approach Using Sc3+ and Ce4+ toward Enhanced Conductivity in NASICON-Type Na3Zr2Si2PO12. J. Phys. Chem. C 2021, 125, 27723–27735. [Google Scholar] [CrossRef]
- Ran, L.; Baktash, A.; Li, M.; Yin, Y.; Demir, B.; Lin, T.; Li, M.; Rana, M.; Gentle, I.; Wang, L.; et al. Sc, Ge Co-Doping NASICON Boosts Solid-State Sodium Ion Batteries’ Performance. Energy Storage Mater. 2021, 40, 282–291. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Dai, Y.; Jin, H. Homogeneous Na+ Transfer Dynamic at Na/Na3Zr2Si2PO12 Interface for All Solid-State Sodium Metal Batteries. Nano Energy 2021, 88, 106293. [Google Scholar] [CrossRef]
- Noi, K.; Suzuki, K.; Tanibata, N.; Hayashi, A.; Tatsumisago, M. Liquid-Phase Sintering of Highly Na+ Ion Conducting Na3Zr2Si2PO12 Ceramics Using Na3BO3 Additive. J. Am. Ceram. Soc. 2018, 101, 1255–1265. [Google Scholar] [CrossRef]
- He, S.; Xu, Y.; Ma, X.; Chen, Y.; Lin, J.; Wang, C. Mg2+/F− Synergy to Enhance the Ionic Conductivity of Na3Zr2Si2PO12 Solid Electrolyte for Solid-State Sodium Batteries. ChemElectroChem 2020, 7, 2087–2094. [Google Scholar] [CrossRef]
- Cao, X.G.; Zhang, X.H.; Tao, T.; Zhang, H.Y. Effects of Antimony Tin Oxide (ATO) Additive on the Properties of Na3Zr2Si2PO12 Ceramic Electrolytes. Ceram. Int. 2020, 46, 8405–8412. [Google Scholar] [CrossRef]
- Wang, C.; Sun, C.; Sun, Z.; Wang, B.; Song, T.; Zhao, Y.; Li, J.; Jin, H. Optimizing the Na Metal/Solid Electrolyte Interface through a Grain Boundary Design. J. Mater. Chem. A Mater. 2022, 10, 5280–5286. [Google Scholar] [CrossRef]
- Zhou, P.; Sun, K.; Ji, S.; Zhao, Z.; Fu, Y.; Xia, J.; Wu, S.; Zhu, Y.; Hui, K.N.; Li, H.-F. MgF2 as an Effective Additive for Improving Ionic Conductivity of Ceramic Solid Electrolytes. Mater. Today Energy 2023, 32, 101248. [Google Scholar] [CrossRef]
- Oh, J.A.S.; He, L.; Plewa, A.; Morita, M.; Zhao, Y.; Sakamoto, T.; Song, X.; Zhai, W.; Zeng, K.; Lu, L. Composite NASICON (Na3Zr2Si2PO12) Solid-State Electrolyte with Enhanced Na+ Ionic Conductivity: Effect of Liquid Phase Sintering. ACS Appl. Mater. Interfaces 2019, 11, 40125–40133. [Google Scholar] [CrossRef]
- Lou, S.; Zhang, F.; Fu, C.; Chen, M.; Ma, Y.; Yin, G.; Wang, J. Interface Issues and Challenges in All-Solid-State Batteries: Lithium, Sodium, and Beyond. Adv. Mater. 2021, 33, 2000721. [Google Scholar] [CrossRef]
- Yang, J.; Wan, H.L.; Zhang, Z.H.; Liu, G.Z.; Xu, X.X.; Hu, Y.S.; Yao, X.Y. NASICON-Structured Na3.1Zr1.95Mg0.05Si2PO12 Solid Electrolyte for Solid-State Sodium Batteries. Rare Met. 2018, 37, 480–487. [Google Scholar] [CrossRef]
- Sampathkumar, R.; Echeverría, M.; Zhang, Y.; Armand, M.; Galceran, M. Interface Stability between Na3Zr2Si2PO12 Solid Electrolyte and Sodium Metal Anode for Quasi-Solid-State Sodium Battery. Batteries 2023, 9, 8. [Google Scholar] [CrossRef]
- Kehne, P.; Guhl, C.; Ma, Q.; Tietz, F.; Alff, L.; Hausbrand, R.; Komissinskiy, P. Sc-Substituted Nasicon Solid Electrolyte for an All-Solid-State NaxCoO2/Nasicon/Na Sodium Model Battery with Stable Electrochemical Performance. J. Power Sources 2019, 409, 86–93. [Google Scholar] [CrossRef]
- Uchida, Y.; Hasegawa, G.; Shima, K.; Inada, M.; Enomoto, N.; Akamatsu, H.; Hayashi, K. Insights into Sodium Ion Transfer at the Na/NASICON Interface Improved by Uniaxial Compression. ACS Appl. Energy Mater. 2019, 2, 2913–2920. [Google Scholar] [CrossRef]
- Miao, X.; Di, H.; Ge, X.; Zhao, D.; Wang, P.; Wang, R.; Wang, C.; Yin, L. AlF3-Modified Anode-Electrolyte Interface for Effective Na Dendrites Restriction in NASICON-Based Solid-State Electrolyte. Energy Storage Mater. 2020, 30, 170–178. [Google Scholar] [CrossRef]
- Jung, S.W.; Wang, J.E.; Kim, D.G.; Ma, H.J.; Kim, D.K.; Kim, D.J. Rare-Earth Element Substitution of Na1+xZr2SixP3–XO12 (x = 2) Solid Electrolyte: Implications for All-Solid-State Na Ion Batteries. ACS Appl. Nano Mater. 2022, 5, 13894–13902. [Google Scholar] [CrossRef]
- Grady, Z.M.; Tsuji, K.; Ndayishimiye, A.; Hwan-Seo, J.; Randall, C.A. Densification of a Solid-State NASICON Sodium-Ion Electrolyte below 400 °c by Cold Sintering with a Fused Hydroxide Solvent. ACS Appl. Energy Mater. 2020, 3, 4356–4366. [Google Scholar] [CrossRef]
- Okubo, K.; Wang, H.; Hayashi, K.; Inada, M.; Enomoto, N.; Hasegawa, G.; Osawa, T.; Takamura, H. A Dense NASICON Sheet Prepared by Tape-Casting and Low Temperature Sintering. Electrochim. Acta 2018, 278, 176–181. [Google Scholar] [CrossRef]
- Suzuki, K.; Noi, K.; Hayashi, A.; Tatsumisago, M. Low Temperature Sintering of Na1 + XZr2SixP3 − XO12 by the Addition of Na3BO3. Scr. Mater. 2018, 145, 67–70. [Google Scholar] [CrossRef]
- Gadjourova, Z.; Andreev, Y.G.; Tunstall, D.P.; Bruce, P.G. Ionic Conductivity in Crystalline Polymer Electrolytes. Nature 2001, 412, 520–523. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhou, B.; Wang, J.; Zuo, C.; He, D.; Xie, X.; Xue, Z. PEO-Based Electrolytes Blended with Star Polymers with Precisely Imprinted Polymeric Pseudo-Crown Ether Cavities for Alkali Metal Ion Batteries. J. Memb. Sci. 2019, 576, 182–189. [Google Scholar] [CrossRef]
- Boschin, A.; Johansson, P. Characterization of NaX (X: TFSI, FSI)—PEO Based Solid Polymer Electrolytes for Sodium Batteries. Electrochim. Acta 2015, 175, 124–133. [Google Scholar] [CrossRef]
- Devi, C.; Gellanki, J.; Pettersson, H.; Kumar, S. High Sodium Ionic Conductivity in PEO/PVP Solid Polymer Electrolytes with InAs Nanowire Fillers. Sci. Rep. 2021, 11, 20180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Q.; Ren, C.; Luo, F.; Ma, Q.; Hu, Y.-S.; Zhou, Z.; Li, H.; Huang, X.; Chen, L. A Ceramic/Polymer Composite Solid Electrolyte for Sodium Batteries. J. Mater. Chem. A Mater. 2016, 4, 15823–15828. [Google Scholar] [CrossRef]
- Zhao, Y.; Meng, X.; Kang, Q.; Yan, L.; Ye, X.; Zhang, J.; Liu, H.; Han, Q.; Chen, Y.; Ma, T. Integrating a Three-Dimensional Cu2MoS4 Electrode and Solid-State Polymer Electrolyte for Sodium-Ion Batteries. Chem. Eng. J. 2022, 450, 137903. [Google Scholar] [CrossRef]
- Colò, F.; Bella, F.; Nair, J.R.; Destro, M.; Gerbaldi, C. Cellulose-Based Novel Hybrid Polymer Electrolytes for Green and Efficient Na-Ion Batteries. Electrochim. Acta 2015, 174, 185–190. [Google Scholar] [CrossRef]
- Chen, G.; Ye, L.; Zhang, K.; Gao, M.; Lu, H.; Xu, H.; Bai, Y.; Wu, C. Hyperbranched Polyether Boosting Ionic Conductivity of Polymer Electrolytes for All-Solid-State Sodium Ion Batteries. Chem. Eng. J. 2020, 394, 124885. [Google Scholar] [CrossRef]
- Ni’Mah, Y.L.; Cheng, M.Y.; Cheng, J.H.; Rick, J.; Hwang, B.J. Solid-State Polymer Nanocomposite Electrolyte of TiO2/PEO/NaClO4 for Sodium Ion Batteries. J. Power Sources 2015, 278, 375–381. [Google Scholar] [CrossRef]
- Qi, X.; Ma, Q.; Liu, L.; Hu, Y.-S.; Li, H.; Zhou, Z.; Huang, X.; Chen, L. Sodium Bis(Fluorosulfonyl)Imide/Poly(Ethylene Oxide) Polymer Electrolytes for Sodium-Ion Batteries. ChemElectroChem 2016, 3, 1741–1745. [Google Scholar] [CrossRef]
- Zhou, Z.; Tao, Z.; Zhang, L.; Zheng, X.; Xiao, X.; Liu, Z.; Li, X.; Liu, G.; Zhao, P.; Zhang, P. Scalable Manufacturing of Solid Polymer Electrolytes with Superior Room-Temperature Ionic Conductivity. ACS Appl. Mater. Interfaces 2022, 14, 32994–33003. [Google Scholar] [CrossRef]
- Schauser, N.S.; Seshadri, R.; Segalman, R.A. Multivalent Ion Conduction in Solid Polymer Systems. Mol. Syst. Des. Eng. 2019, 4. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Liu, Z.; Cao, Y.; Yang, L.; Jiang, Y.; Lei, Y.; Yuan, T.; Lu, H.; Feng, J. Novel Sodium−poly(Tartaric Acid)Borate-Based Single-Ion Conducting Polymer Electrolyte for Sodium−metal Batteries. ACS Appl. Energy Mater. 2020, 3, 10053–10060. [Google Scholar] [CrossRef]
- Pritam; Arya, A.; Sharma, A.L. Selection of Best Composition of Na+ Ion Conducting PEO-PEI Blend Solid Polymer Electrolyte Based on Structural, Electrical, and Dielectric Spectroscopic Analysis. Ionics 2020, 26, 745–766. [Google Scholar] [CrossRef]
- Makhlooghiazad, F.; Nti, F.; Sun, J.; Mendes, T.C.; Malunavar, S.S.; Pringle, J.M.; Forsyth, M. Composite Electrolytes Based on Electrospun PVDF and Ionic Plastic Crystal Matrices for Na-Metal Battery Applications. J. Phys. Mater. 2021, 4, 034003. [Google Scholar] [CrossRef]
- Kim, J.-K.; Lim, Y.J.; Kim, H.; Cho, G.-B.; Kim, Y. A Hybrid Solid Electrolyte for Flexible Solid-State Sodium Batteries. Energy Environ. Sci. 2015, 8, 3589–3596. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Qin, B.; Han, J.; Passerini, S. Aqueous/Nonaqueous Hybrid Electrolyte for Sodium-Ion Batteries. ACS Energy Lett. 2018, 3, 1769–1770. [Google Scholar] [CrossRef]
- Shen, Y.; Han, X.; Cai, T.; Hu, H.; Li, Y.; Zhao, L.; Hu, H.; Xue, Q.; Zhao, Y.; Zhou, J.; et al. High-Performance Aqueous Sodium-Ion Battery Using a Hybrid Electrolyte with a Wide Electrochemical Stability Window. RSC Adv. 2020, 10, 25496–25499. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Yang, H.; Matsumoto, K.; Hagiwara, R. Benefits of the Mixtures of Ionic Liquid and Organic Electrolytes for Sodium-Ion Batteries. J. Electrochem. Soc. 2021, 168, 030508. [Google Scholar] [CrossRef]
- Monti, D.; Ponrouch, A.; Palacín, M.R.; Johansson, P. Towards Safer Sodium-Ion Batteries via Organic Solvent/Ionic Liquid Based Hybrid Electrolytes. J. Power Sources 2016, 324, 712–721. [Google Scholar] [CrossRef] [Green Version]
- Karlsmo, M.; Bouchal, R.; Johansson, P. High-Performant All-Organic Aqueous Sodium-Ion Batteries Enabled by PTCDA Electrodes and a Hybrid Na/Mg Electrolyte. Angew. Chem. Int. Ed. 2021, 60, 24709–24715. [Google Scholar] [CrossRef]
- Wang, H.; Liu, T.; Du, X.; Wang, J.; Yang, Y.; Qiu, H.; Lu, G.; Li, H.; Chen, Z.; Zhao, J.; et al. Hybrid Electrolytes Enabling In-Situ Interphase Protection and Suppressed Electrode Dissolution for Aqueous Sodium-Ion Batteries. Batter Supercaps 2022, 5, e202200246. [Google Scholar] [CrossRef]
- Senthilkumar, S.T.; Abirami, M.; Kim, J.; Go, W.; Hwang, S.M.; Kim, Y. Sodium-Ion Hybrid Electrolyte Battery for Sustainable Energy Storage Applications. J. Power Sources 2017, 341, 404–410. [Google Scholar] [CrossRef]
- Kim, Y.; Künzel, M.; Steinle, D.; Dong, X.; Kim, G.-T.; Varzi, A.; Passerini, S. Anode-Less Seawater Batteries with a Na-Ion Conducting Solid-Polymer Electrolyte for Power to Metal and Metal to Power Energy Storage. Energy Environ. Sci. 2022, 15, 2610–2618. [Google Scholar] [CrossRef]
- Martínez-Cisneros, C.S.; Pandit, B.; Antonelli, C.; Sanchez, J.Y.; Levenfeld, B.; Varez, A. Development of Sodium Hybrid Quasi-Solid Electrolytes Based on Porous NASICON and Ionic Liquids. J. Eur. Ceram Soc. 2021, 41, 7723–7733. [Google Scholar] [CrossRef]
- Shen, L.; Deng, S.; Jiang, R.; Liu, G.; Yang, J.; Yao, X. Flexible Composite Solid Electrolyte with 80 Wt% Na3.4Zr1.9Zn0.1Si2.2P0.8O12 for Solid-State Sodium Batteries. Energy Storage Mater. 2022, 46, 175–181. [Google Scholar] [CrossRef]
- de la Torre-Gamarra, C.; Appetecchi, G.B.; Ulissi, U.; Varzi, A.; Varez, A.; Passerini, S. Na3Si2Y0.16Zr1.84PO12-Ionic Liquid Hybrid Electrolytes: An Approach for Realizing Solid-State Sodium-Ion Batteries? J. Power Sources 2018, 383, 157–163. [Google Scholar] [CrossRef]
- Johari, N.S.M.; Adnan, S.B.R.S.; Mohamed, N.S.; Ahmad, N. Structural and Electrical Properties of Na2ZnSiO4—Py14TFSI Hybrid Solid Electrolyte. Ceram. Int. 2020, 46, 8039–8046. [Google Scholar] [CrossRef]
- Johari, N.S.M.; Jonderian, A.; Jia, S.; Cozea, V.; Yao, E.; Adnan, S.B.R.S.; Ahmad, N.; McCalla, E. High-Throughput Development of Na2ZnSiO4-Based Hybrid Electrolytes for Sodium-Ion Batteries. J. Power Sources 2022, 541, 231706. [Google Scholar] [CrossRef]
- Boschin, A.; Johansson, P. Plasticization of NaX-PEO Solid Polymer Electrolytes by Pyr13X Ionic Liquids. Electrochim. Acta 2016, 211, 1006–1015. [Google Scholar] [CrossRef]
- Cheng, M.; Qu, T.; Zi, J.; Yao, Y.; Liang, F.; Ma, W.; Yang, B.; Dai, Y.; Lei, Y. A Hybrid Solid Electrolyte for Solid-State Sodium Ion Batteries with Good Cycle Performance. Nanotechnology 2020, 31, 425401. [Google Scholar] [CrossRef]
- Sirigiri, N.; Chen, F.; Forsyth, C.M.; Yunis, R.; O’Dell, L.; Pringle, J.M.; Forsyth, M. Factors Controlling the Physical Properties of an Organic Ionic Plastic Crystal. Mater. Today Phys. 2022, 22, 100603. [Google Scholar] [CrossRef]
- Yi, Q.; Zhang, W.; Li, S.; Li, X.; Sun, C. Durable Sodium Battery with a Flexible Na3Zr2Si2PO12–PVDF–HFP Composite Electrolyte and Sodium/Carbon Cloth Anode. ACS Appl. Mater. Interfaces 2018, 10, 35039–35046. [Google Scholar] [CrossRef] [PubMed]



| Cathode | Anode | Solvent | Na Salt | Molarity | Additive | Ref. | |
|---|---|---|---|---|---|---|---|
| Non-aqueous Electrolytes | NaNi0.5Mn0.5O2 | HC | PC | NaClO4 | 1 | VC | [41] |
| Na3V2(PO4)3 | HC | EC:DEC | NaPF6 | 1 | FEC | [47] | |
| Na | SiC-Sb-C | EC:DEC | NaClO4 | 1 | FEC | [42] | |
| Na | TiO2 | PC | NaClO4 | 1 | VC | [62] | |
| Na | MoS2/C | EC:DEC | NaClO4 | 1 | FEC | [63] | |
| Na0.67Mn0.75Li0.25O2 | CNT/Na-IL | DME | NaPF6 | 1 | [52] | ||
| Na | rGO | Diglyme | NaOTf | 1 | [44] | ||
| Na | TiO2 | Tetraglyme | NaOTf | 1 | [53] | ||
| Na3V2(PO4)3 | CNF | Diglyme | NaPF6 | 1 | [54] | ||
| Na3V2(PO4)3 | Na | Diglyme | NaBF4 | 0.5 | [55] | ||
| Ionic Liquids | Na | Red P | MPPFSA | NaFSI | 0.25 | [64] | |
| Na0.67Mn0.67Ni0.23Mg0.102 | Na | Pyr1.4FSI | NaFSI | 1 | [65] | ||
| FeF3/C NFs | Na | Pyr1.3FSI | NaFSI | 1 | [66] | ||
| Na | HC | C3mpyrFSI | NaFSI | 0.5 | [45] | ||
| Aqueous | Na2MnFe(CN)6 | NaTi2(PO4)3 | H2O | NaTFSI | 30 | EMImTFSI | [67] |
| PBA | AC | H2O | NaClO4 | 1 | GO | [68] | |
| Na3V1.3Fe0.5W0.2(PO4)3 | NaTi2(PO4)3 | H2O | Na2SO4 | 1 | [69] | ||
| Na2VTi(PO4)3 | Na2VTi(PO4)3 | H2O | CF3COONa | 26 | [70] |
| Electrolyte Content | IC (mS cm−1) | T (°C) | Ref. |
|---|---|---|---|
| PEO/NaClO4/PC | >1 | 25 | [87] |
| PVDF-HFP/NaClO4/triglyme | 2.54 | 25 | [88] |
| PVDF-HFP/NaClO4/PC:FEC | 0.42 | [77] | |
| PVDF-HFP/PP/NaClO4/EC:DMC:EMC | 0.82 | 25 | [90] |
| PVDF-HFP/PP/NaClO4/EC:DEC:FEC | 1.38 | 25 | [91] |
| PVDF-HFP/SnO2/1-(4-cyanophenyl)guanidine/NaClO4/EC:DMC:FEC | 0.232 | 70 | [93] |
| PVDF-HFP/PMMA/TPU-Na3Zr2Si2PO12, NaClO4/EC:DMC | 2.83 | 25 | [83] |
| PVDF-HFP/SiO2/NaBF4/tetraglyme | 0.9 | 25 | [79] |
| PEGMA/glass fiber/triethyl phosphate | 0.91 | 25 | [96] |
| PCL/NaOTf/EC:PC | ~1.3 | 25 | [98] |
| BCGE/NaClO4/EC:DEC:FEC | 0.632 | 20 | [99] |
| CNC/CNF/NaClO4/EC:PC | 2.32 | 25 | [100] |
| HPMC/NaClO4/PC | 3.3 | 25 | [101] |
| Type | Content | IC (mS cm−1) | T (°C) | Ref. |
|---|---|---|---|---|
| Inorganic Solid Electrolyte | Na3.256Mg0.128Zr1.872Si2PO12 | 2.7 | 25 | [108] |
| Na3.2Zr1.9Ca0.1Si2PO12 | 10−3 | 25 | [119] | |
| Na3.3Zr1.85Mg0.15Si2PO12 | 3.54 | 25 | [118] | |
| Na3.4Zr1.9Zn0.1Si2.2P0.8O12 | 5.27 | 25 | [125] | |
| 0.1 Co-Na3Zr2Si2PO12 | 5.03 × 10−3 | 25 | [126] | |
| Na3.3Zr1.7La0.3Si2PO12 | 3.4 | 25 | [127] | |
| Na3.3Zr1.7Eu0.3Si2PO12 | 1.08 | 25 | [121] | |
| Na3Zr1.8Zn0.2Si2PO11.8 | 1.44 | 25 | [122] | |
| Na3.3Zr1.9Nb0.1Si2.4P0.6O12 | 5.51 | 25 | [123] | |
| Na3Zr2Si2PO12 | 0.61 | 25 | [113] | |
| Na3.33Zr1.67Sc0.33Si2PO12 | 0.96 | 25 | [113] | |
| Na3.33Zr1.67Sc0.29Yb0.04Si2PO12 | 1.62 | 25 | [113] | |
| Na3Zr2Si2PO12 | 0.61 | 25 | [131] | |
| Na3.33Sc0.33Zr1.67Si2PO12 | 0.96 | 25 | [131] | |
| 1 mol% Ce4+ +Na3.33Sc0.33Zr1.67Si2PO12 | 2.44 | 25 | [131] | |
| Na3Zr2Si2PO12 | 0.39 | 30 | [132] | |
| Na3.125Zr1.75Sc0.125Ge0.125Si2PO12 | 4.64 | 30 | [132] | |
| 1 wt.% Bi2O3-Na3.1Y0.1Zr1.9Si2PO12 | 1.21 | 25 | [114] | |
| 5 wt.% Na2SiO3-Na3Zr2Si2PO12 | 1.45 | 25 | [139] | |
| 4.8 wt.% Na3BO3-Na3Zr2Si2PO12 | 1 | 25 | [134] | |
| 1 wt.% MgF2-Na3.4Zr2Si2.4P0.6O12 | 2.03 | 25 | [138] | |
| Na3Zr2Si2PO12 | 0.262 | 25 | [115] | |
| La doped Na3Zr2Si2PO12 | 1.017 | 25 | [146] | |
| Na3Zr2Si2PO12 | 2.12 | 25 | [116] | |
| Polymer Solid Electrolyte | NaTFSI/PEO | 4.5 × 10−3 | 20 | [152] |
| NaFSI/PEO | 0.41 | 80 | [159] | |
| TiO2 nanofillers/NaClO4/PEO | 0.262 | 60 | [158] | |
| Na3.4Zr1.8Mg0.2Si2PO12/NaFSI/PEO | 2.4 | 80 | [154] | |
| Na-CMC/NaClO4/PEO | 10−4 | 60 | [156] | |
| PEO:PEI blend/NaPF6 | 5.12 × 10−3 | 40 | [163] | |
| Indium arsenide nanowires/PVP/PEO/NaPF6 | 0.15 | 40 | [153] | |
| Star polymer:PEO blend | 2.37 × 10−2 | 25 | [151] | |
| Hyperbranched copolymer:PVDF-HFP blend/NaClO4 | 0.57 | 30 | [157] | |
| PVDF-HFP/1-(4-cyanophenyl) guanidine/NaPF6 | 1.76 × 10−2 | 30 | [155] |
| Content | IC (mS cm−1) | T (°C) | Ref. |
|---|---|---|---|
| NaClO4, acetonitrile, water | ~67 | 25 | [167] |
| [C3C1pyrr][FSA]/PC/NaFSA/NaClO4 | 8.4 | 25 | [168] |
| Na2SO4/MgSO4/water | 55 | 30 | [170] |
| NaTFSI, adiponitrile, H2O | 3.39 | 25 | [171] |
| Na3Zr1.84Y0.16Si2PO12/NaTFSI/Pyr0408a | 0.9 | 30 | [174] |
| Halloysite clay-derived Na2ZnSiO, ionic liquids | 0.166 | 25 | [178] |
| PEO/NaTFSI/NaFSI/Pyr13TFSI/Pyr13FSI | 10−3 | 70 | [179] |
| Na3Zr2Si2PO12/PVDF-HFP/NaClO4/EC:DMC | 2.25 | 25 | [180] |
| PEO/NaFSI/Pyr14FSI | 1.43 | 20 | [173] |
| Na3.4Zr1.9Zn0.1Si2.2P0.8O12/[Py13] + [NTf2]/PEO/NaClO4 | 0.148 | 25 | [175] |
| [P1i444][FSI] organic ionic plastic crystal, NaFSI/NaTFSI/PVDF | 2.1, 2.5 | 50 | [164,182] |
| NZSPO-PVDF-HFP composite | 2.78 | 30 | [182] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karahan Toprakci, H.A.; Toprakci, O. Recent Advances in New-Generation Electrolytes for Sodium-Ion Batteries. Energies 2023, 16, 3169. https://doi.org/10.3390/en16073169
Karahan Toprakci HA, Toprakci O. Recent Advances in New-Generation Electrolytes for Sodium-Ion Batteries. Energies. 2023; 16(7):3169. https://doi.org/10.3390/en16073169
Chicago/Turabian StyleKarahan Toprakci, Hatice Aylin, and Ozan Toprakci. 2023. "Recent Advances in New-Generation Electrolytes for Sodium-Ion Batteries" Energies 16, no. 7: 3169. https://doi.org/10.3390/en16073169
APA StyleKarahan Toprakci, H. A., & Toprakci, O. (2023). Recent Advances in New-Generation Electrolytes for Sodium-Ion Batteries. Energies, 16(7), 3169. https://doi.org/10.3390/en16073169






