Ashes Qualified as a Source of Selected Critical Elements (REY, Co, Ga, V)
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Results of the Granulometric Analysis
3.2. Chemical Composition
3.2.1. Basic Components
3.2.2. Critical Elements
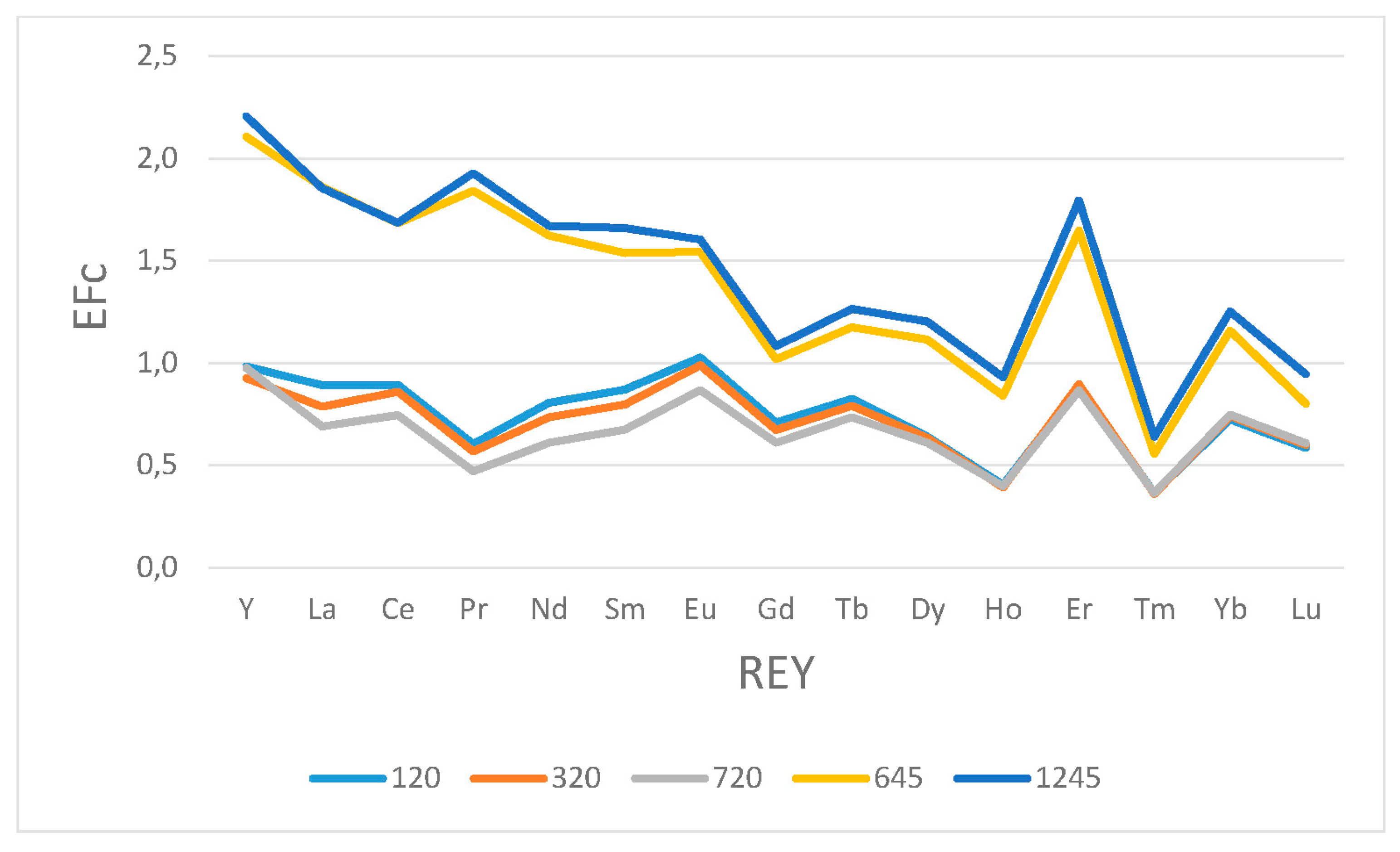
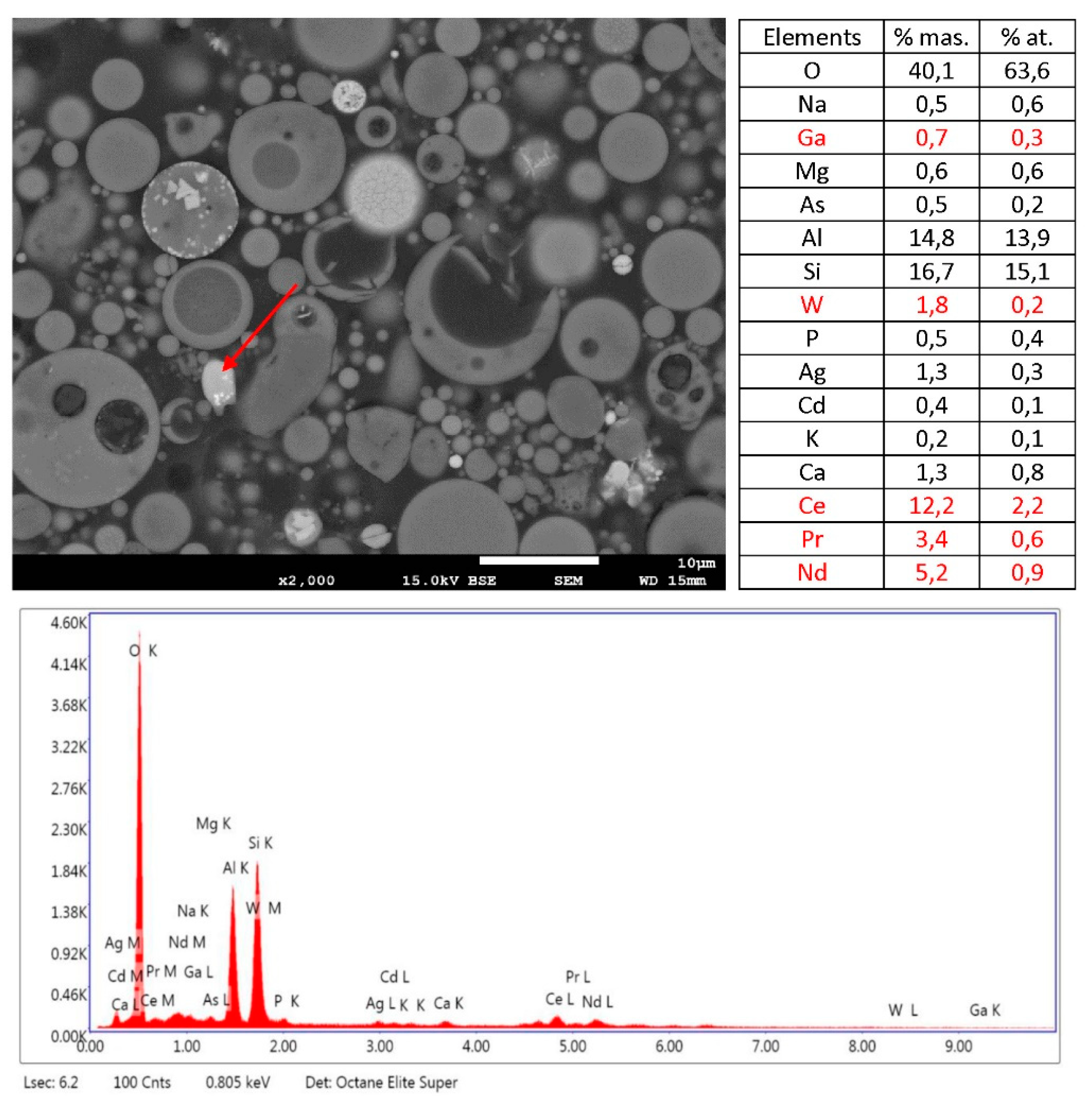
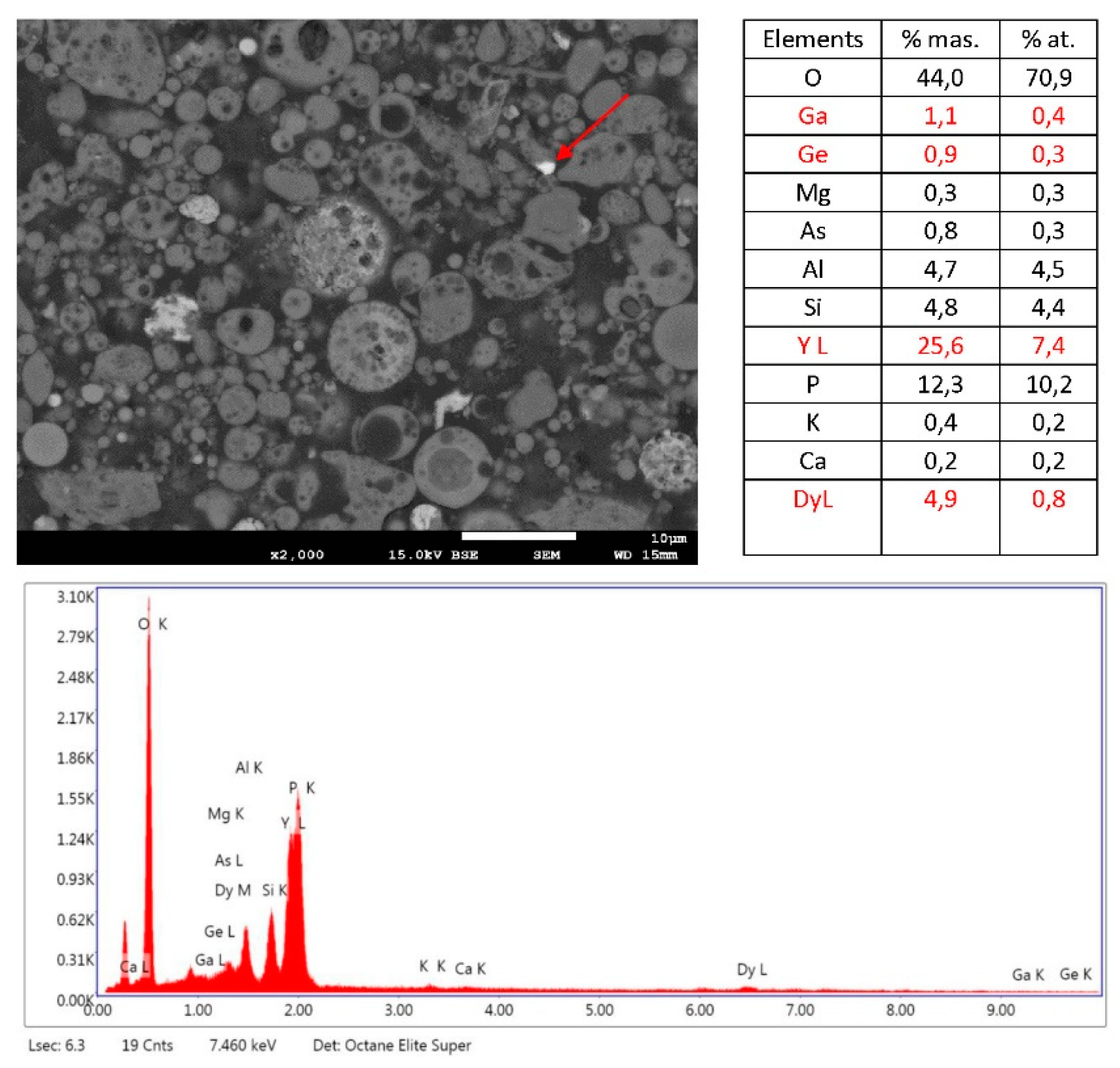
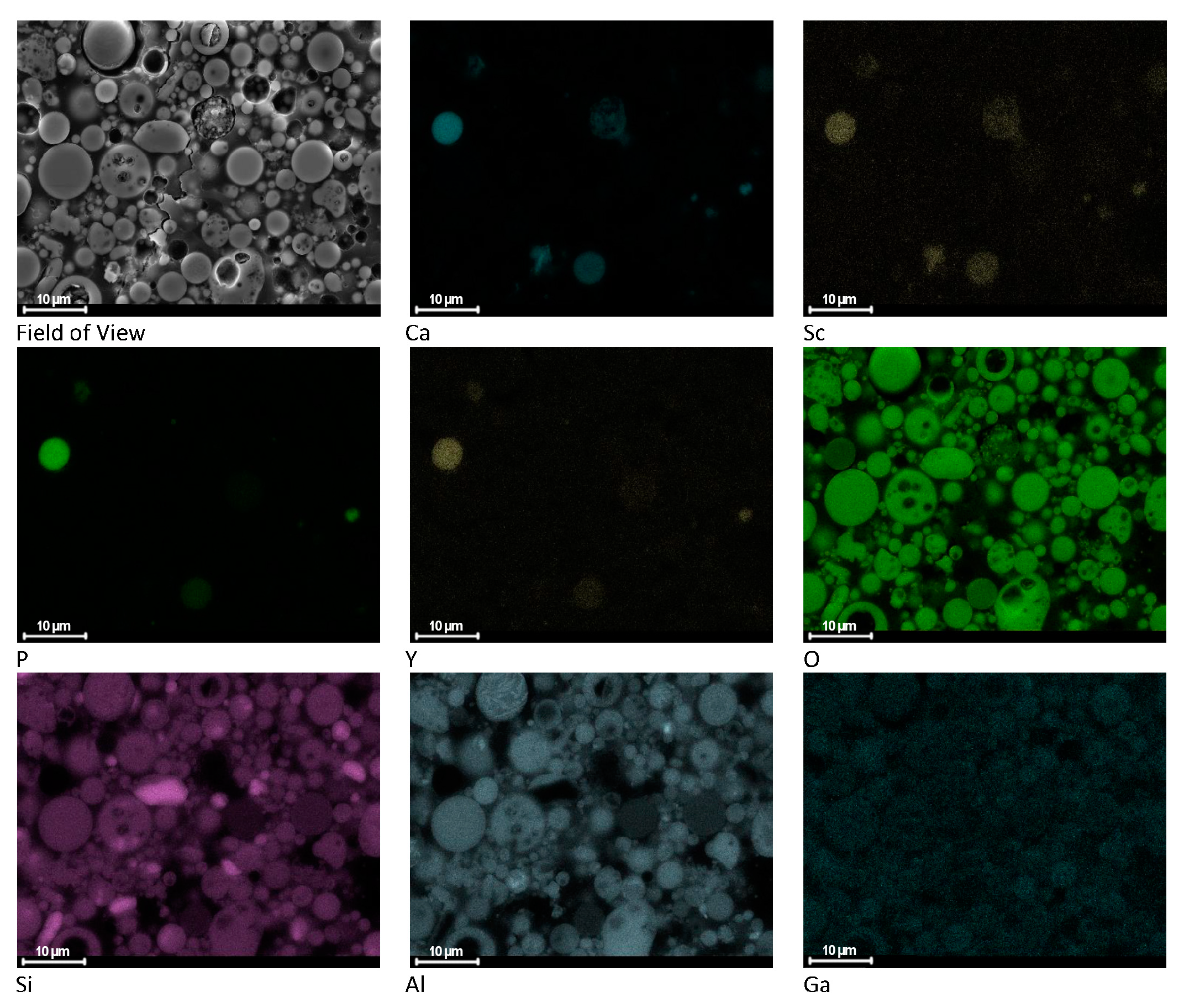
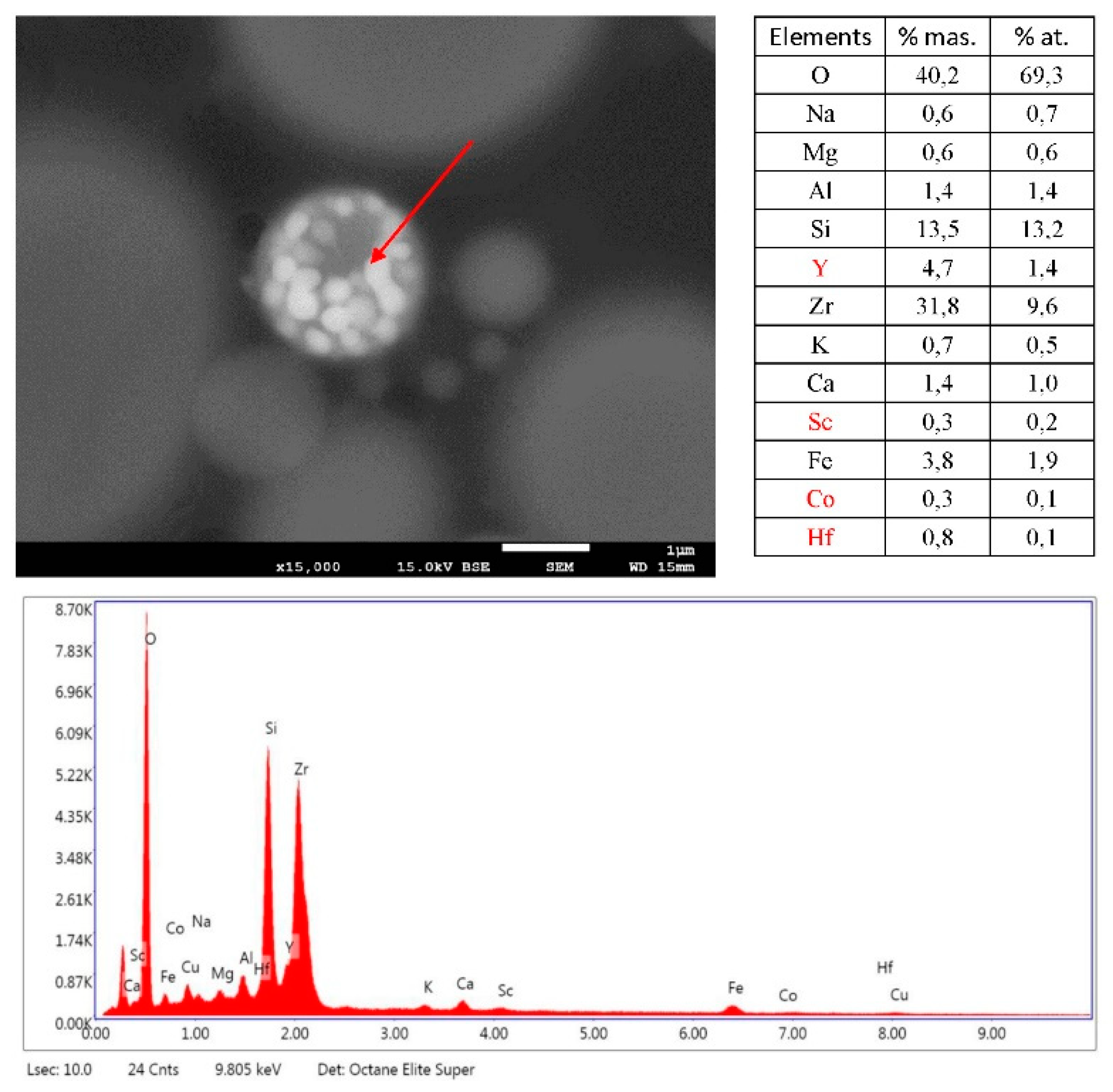
3.3. SEM/EDS
4. Conclusions
- The content of critical elements in ashes from Polish power plants is similar to that of world averages, and in the case of class C ashes, it is exceeded for most REY. It can therefore be assumed that lignite ashes should be given priority in further research on the recovery of rare earth elements from them;
- The content of REY and that of other critical elements in hard coal ashes increases with decreasing particle size. Despite the increase in the concentration of REY in progressively finer grain classes, the value of their world average, in the class below 20 µm, was not exceeded. High enrichment factors relative to Clarke (1.5; 1.9; 2.5) were noted only for three elements not belonging to the group of lanthanides: Co, Ga, and V. Taking into account the economic aspect, in order to recover these elements, it is important that the share of fine grains in the ashes should be high;
- In lignite ashes, high coefficients of enrichment relative to Clarke (2.2; 1.62; 1.87) were obtained in the class below 45 µm for Y, Nd, and Co. As demonstrated in previous studies, better enrichment effects for these elements are obtained by magnetic separation;
- Both the studies of the chemical composition of FA and microscopic observations combined with EDS microanalyses demonstrated a relationship between the concentration of rare earth elements and the presence of aluminosilicates. A strong positive correlation was reported between REY and the Al2O3 content, with r = 0.94. Therefore, it can be assumed that the extraction of REY from the aluminosilicate glass fraction will allow for the recovery of a significant part of these elements present in the ashes;
- The management of FA brings added value both for the environment and the economy. On the one hand, ashes do not have to be stored, so they do not affect the environment. On the other hand, they can be a source of valuable critical raw materials, which are often expensive to produce. Therefore, it is necessary to continue research on the content of critical elements in coal and its combustion products in order to identify the most promising materials for extraction.
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FA | Fly ashes |
| REE | Rare earth elements |
| REY | Rare earth elements and yttrium |
| LREY | Light group (La, Ce, Pr, Nd, Sm) |
| MREY | Medium group (Eu, Gd, Tb, Dy, and Y) |
| HREY | Heavy group (Ho, Er, Yb, Lu) |
| EDS | Energy Dispersive Spectroscopy |
| SEM | Scanning Electron Microscope |
| LOI | Loss on ignition |
| EF | The enrichment factor |
| EFc | The enrichment factor of the component compared to the Clark |
References
- Mokrzycki, E.; Uliasz-Bocheńczyk, A. Alternative fuels for the cement industry. Appl. Energy 2003, 74, 95–100. [Google Scholar] [CrossRef]
- Bielecka, A.; Kulczycka, J. Coal Combustion Products Management toward a Circular Economy—A Case Study of the Coal Power Plant Sector in Poland. Energies 2020, 13, 3603. [Google Scholar] [CrossRef]
- Strzałkowska, E. Fly ash—A valuable material for the circular economy. Gospod. Surowcami Miner. Miner. Resour. Manag. 2021, 37, 49–62. [Google Scholar] [CrossRef]
- Willner, J.; Fornalczyk, A.; Saternus, M.; Franke, D.; Suponik, T.; Turek, M.; Dydo, P.; Jakóbik-Kolon, A.; Kluczka, J.; Mitko, K.; et al. Gospodarka Obiegu Zamkniętego. Circular Economy. In Ochrona Klimatu i Środowiska, Nowoczesna Energetyka: Praca Zbiorowa; Werle, W.S., Ferdyn-Grygierek, J., Szczygieł, M., Eds.; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2021; pp. 71–102. (In Polish) [Google Scholar]
- Uliasz-Bocheńczyk, A.; Mokrzycki, E. The use of waste in cement production in Poland—The move towards sustainable development. Gospod. Surowcami Miner.–Miner. Resour. Manag. 2022, 38, 67–81. [Google Scholar] [CrossRef]
- Odzijewicz, J.I.; Wołejko, E.; Wydro, U.; Wasil, M.; Jabłońska-Trypuć, A. Utilization of Ashes from Biomass Combustion. Energies 2022, 15, 9653. [Google Scholar] [CrossRef]
- Wrona, J.; Żukowski, W.; Bradło, D.; Czupryński, P. Recovery of Cenospheres and Fine Fraction from Coal Fly Ash by a Novel Dry Separation Method. Energies 2020, 13, 3576. [Google Scholar] [CrossRef]
- Grünhäuser Soares, E.; Castro-Gomes, J.; Sitarz, M.; Zdeb, T.; Hager, I. The Immobilisation of Heavy Metals from Sewag Sludge Ash in CO2-Cured Mortars. Sustainability 2021, 13, 12893. [Google Scholar] [CrossRef]
- Giergiczny, Z. Popiół Lotny w Składzie Cementu i Betonu; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2013. [Google Scholar]
- Zhang, X.; Han, J. The effect of ultra-fine admixture on the rheological property of cement paste. Cem. Concr. Res. 2000, 30, 827–830. [Google Scholar] [CrossRef]
- Jones, M.R.; McCarthy, A.; Booth, A.P.P.G. Characteristics of the ultrafine component of fly ash. Fuel 2006, 85, 2250–2259. [Google Scholar] [CrossRef]
- Supit, S.W.M.; Shaikh, F.U.A.; Sarker, P.K. Effect of ultrafine fly ash on mechanical properties of high volume fly ash mortar. Constr. Build. Mater. 2014, 51, 278–286. [Google Scholar] [CrossRef]
- Krishnaraj, L.; Ravichandran, P.T. Characterisation of ultra-fine fly ash as sustainable cementitious material for masonry construction. Ain Shams. Eng. J. 2021, 12, 259–269. [Google Scholar] [CrossRef]
- Haustein, E.; Kuryłowicz-Cudowska, A. Effect of Particle Size of Fly Ash Microspheres (FAMs) on the Selected Properties of Concrete. Minerals 2022, 12, 847. [Google Scholar] [CrossRef]
- Haustein, E.; Kuryłowicz-Cudowska, A. The Effect of Fly Ash Microspheres on the Pore Structure of Concrete. Minerals 2020, 10, 58. [Google Scholar] [CrossRef] [Green Version]
- Hycnar, J. Metody wydzielania koncentratów metali z popiołów elektrownianych. Fizykochem. Probl. Miner. 1987, 19, 243–257. (In Polish) [Google Scholar]
- Lin, R.; Howard, B.H.; Roth, E.A.; Bank, T.L.; Granite, E.J.; Soong, Y. Enrichment of rare earth elements from coal and coal by-products by physical separations. Fuel 2017, 200, 506–520. [Google Scholar] [CrossRef] [Green Version]
- Bielowicz, B.; Botor, D.; Misiak, J.; Wagner, M. Critical Elements in Fly Ash from the Combustion of Bituminous Coal in Major Polish Power Plants. In Proceedings of the E3S Web of Conferences, Krakow, Poland, 20–22 November 2017; Volume 35. [Google Scholar] [CrossRef]
- Fu, B.; Hower, J.C.; Zhang, W.; Luo, G.; Hu, H.; Yao, H. A review of rare earth elements and yttrium in coal ash: Content, modes of occurrences, combustion behavior, and extraction methods. Prog. Energy Combust. Sci. 2022, 88, 100954. [Google Scholar] [CrossRef]
- Zhang, W.; Noble, A.; Yang, X.; Honaker, R. A comprehensive review of rare earth elements recovery from coal-related materials. Minerals 2020, 10, 451. [Google Scholar] [CrossRef]
- Fei, X.; Shenjun, Q.; Shenyong, L.; Jinxi, W.; De’e, Q.; Qingfeng, L.; Jingkai, X. Distribution, occurrence mode, and extraction potential of critical elements in coal ashes of the Chongqing Power Plant. J. Clean. Prod. 2022, 342, 130910. [Google Scholar] [CrossRef]
- Rybak, A.; Rybak, A. Characteristics of Some Selected Methods of Rare Earth Elements Recovery from Coal Fly Ashes. Metals 2021, 11, 142. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. A new approach for the classification of coal fly ashes based on their origin, composition, properties, and behaviour. Fuel 2007, 86, 1490–1512. [Google Scholar] [CrossRef]
- Germani, M.S.; Zoller, W.H. Vapor-phase concentrations of arsenic, selenium, bromine, iodine, and mercury in the stack of a coal-fired power plant. Environ. Sci. Technol. 1988, 22, 1079–1085. [Google Scholar] [CrossRef]
- Seredin, V.V. Rare earth element-bearing coals from the Russian Far East deposits. Int. J. Coal Geol. 1996, 30, 101–129. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. Mineralogy of combustion wastes from coal-fired power stations. Fuel Process. Technol. 1996, 47, 261–280. [Google Scholar] [CrossRef]
- Pires, M.; Querol, X. Characterization of Candiota (South Brazil) coal and combustion by-product. Int. J. Coal Geol. 2004, 60, 57–72. [Google Scholar] [CrossRef]
- Mazur, J.; Konieczyński, J. Distribution of Trace Elements in Granulometric Fractions of Fly-Ash Emitted from Power Stations; Monografia Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2004. (In Polish) [Google Scholar]
- Vassilev, S.V.; Eskenazy, G.M.; Vassileva, C.G. Behaviour of elements and minerals during preparation and combustion of the Pernik coal, Bulgaria. Fuel Process. Technol. 2001, 72, 103–129. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Karayigit, A.I.; Bulut, Y.; Alastuey, A.; Querol, X. Phase–mineral and chemical composition of composite samples from feed coals, bottom ashes and fly ashes at the Soma power station, Turkey. Int. J. Coal Geol. 2005, 61, 35–63. [Google Scholar] [CrossRef]
- Smolka-Danielowska, D. Rare earth elements in fly ashes created during the coal burning process in certain coal-fired power plants operating in Poland–Upper Silesian Industrial Region. J. Environ. Radioact. 2010, 101, 965–968. [Google Scholar] [CrossRef]
- Zajusz Zubek, E. Evaluation of Forms in which Occur Selected Trace Elements in Suspended Dust (PM10) and Respirable Fraction (PM2.5) in the Surroundings of Coal-Fired Power Plants and Coking Plants in the Non-Heating Season; Monografia, Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2016. (In Polish) [Google Scholar]
- Parzentny, H.R.; Róg, L. Distribution and Mode of Occurrence of Co, Ni, Cu, Zn, As, Ag, Cd, Sb, Pb in the Feed Coal, Fly Ash, Slag, in the Topsoil and in the Roots of Trees and Undergrowth Downwind of Three Power Stations in Poland. Minerals 2021, 11, 133. [Google Scholar] [CrossRef]
- Chlebnikovas, A.; Paliulis, D.; Kilikevičius, A.; Selech, J.; Matijošius, J.; Kilikevičienė, K.; Vainorius, D. Possibilities and Generated Emissions of Using Wood and Lignin Biofuel for Heat Production. Energies 2021, 14, 8471. [Google Scholar] [CrossRef]
- Hower, J.C.; Dai, S.; Seredin, V.V.; Zhao, L.; Kostova, I.J.; Silva, L.F.O.; Mardon, S.M.; Gurdal, G. A Note on the Occurrence of Yttrium and Rare Earth Elements in Coal Combustion Products. Coal Combust. Gasif. Prod. 2013, 5, 39–47. [Google Scholar]
- Wu, L.; Ma, L.; Huang, G.; Li, J.; Xu, H. Distribution and Speciation of Rare Earth Elements in Coal Fly Ash from the Qianxi Power Plant, Guizhou Province, Southwest China. Minerals 2022, 12, 1089. [Google Scholar] [CrossRef]
- Seredin, V.V.; Dai, S. Coal deposits as potential alternative sources for lanthanides and yttrium. Int. J. Coal Geol. 2012, 94, 67–93. [Google Scholar] [CrossRef]
- Dai, S.; Arbuzov, S.I.; Chekryzhov, I.Y.; French, D.; Feole, I.; Folkedahl, B.C.; Graham, I.T.; Hower, J.C.; Nechaev, V.P.; Wagner, N.J.; et al. Metalliferous Coals of Cretaceous Age: A Review. Minerals 2022, 12, 1154. [Google Scholar] [CrossRef]
- Hower, J.C.; Groppo, J.G.; Joshi, P.; Dai, S.; Moecher, D.P.; Johnston, M.N. Location of Cerium in Coal Combustion Fly Ashes: Implications for Recovery of Lanthanides. Coal Combust. Gasif. Prod. 2013, 5, 73–78. [Google Scholar]
- Blissett, R.S.; Smalley, N.; Rowson, N.A. An investigation into six coal fly ashes from the United Kingdom and Poland to evaluate rare earth element content. Fuel 2014, 119, 236–239. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Zhou Ch Tang, M.; Cao, S.; Liu Ch Zhang, N.; Wen, M.; Luo, Y.; Hu, T.; Ji, W. Study on the modes of occurrence of rare earth elements in coal fly ash by statistics and a sequential chemical extraction procedure. Fuel 2019, 237, 555–565. [Google Scholar] [CrossRef]
- Li Ch Zhou Ch Li, W.; Zhu, W.; Shi, J.; Liu, G. Enrichment of critical elements from coal fly ash by the combination of physical separations. Fuel 2023, 336, 127156. [Google Scholar] [CrossRef]
- Dai, S.; Zhao, L.; Hower, J.C.; Johnston, M.N.; Song, W.; Wang, P.; Zhang, S. Petrology, mineralogy, and chemistry of size-fractioned fly ash from the Jungar power plant, Inner Mongolia, China, with emphasis on the distribution of rare earth elements. Energy Fuel 2014, 28, 1502–1514. [Google Scholar] [CrossRef]
- Martinez-Tarazona, M.; Spears, D. The fate of trace elements and bulk minerals in pulverized coal combustion in a power station. Fuel Process. Technol. 1996, 47, 79–92. [Google Scholar] [CrossRef]
- Clarke, L.B.; Sloss, L.L. Trace Elements-Emissions from Coal Combustion and Gasification; IEA Coal Research: London, UK, 1992. [Google Scholar]
- Hower, J.C.; Groppo, J.G.; Hopps, S.D.; Morgan, T.D.; Hsu-Kim, H.; Taggart, R.K. Coal Feed-Dependent Variation in Fly Ash Chemistry in a Single Pulverized-Combustion Unit. Minerals 2022, 12, 1071. [Google Scholar] [CrossRef]
- Lanzerstorfer, C. Fly ash from coal combustion: Dependence of the concentration of various elements on the particle size. Fuel 2018, 228, 263–271. [Google Scholar] [CrossRef]
- Sakulpitakphon, T.; Hower, J.C.; Trimble, A.S.; Schram, W.H.; Thomas, G.A. Mercury capture by fly ash: Study of the combustion of a high-mercury coal at a utility boiler. Energy Fuels 2000, 14, 727–733. [Google Scholar] [CrossRef]
- Vilakazi, A.Q.; Ndlovu, S.; Chipise, L.; Shemi, A. The Recycling of Coal Fly Ash: A Review on Sustainable Developments and Economic Considerations. Sustainability 2022, 14, 1958. [Google Scholar] [CrossRef]
- Europa.eu. Available online: http://EUR-Lex-52020DC0474-EN-EUR-Lex (accessed on 3 September 2020).
- Strzałkowska, E. Morphology, chemical and mineralogical composition of magnetic fraction of coal fly ash. Int. J. Coal Geol. 2021, 240, 103746. [Google Scholar] [CrossRef]
- Strzałkowska, E. Rare earth elements and other critical elements in the magnetic fraction of fly ash from several Polish power plants. Int. J. Coal Geol. 2022, 258, 104015. [Google Scholar] [CrossRef]
- Ketris, M.P.; Yudovich, Y.E. Estimations of Clarkes for Carbonaceous biolithes: World averages for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Idzikowski, A. O występowaniu niektórych mikroelementów w węglach kamiennych warstw rudzkich i siodłowych na Górnym Śląsku. Arch. Mineral. 1959, 23, 2. (In Polish) [Google Scholar]
- Bielowicz, B. Ash Characteristics and Selected Critical Elements (Ga, Sc, V) in Coal and Ash in Polish Deposits. Resources 2020, 9, 115. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Z.; Guo, J.; Xu, Z. Distribution of heavy metals and release mechanism for respirable fine particles incineration ashes from lignite. Resour. Conserv. Recycl. 2021, 166, 105282. [Google Scholar] [CrossRef]
- Taggart, R.K.; Hower, J.C.; Dwyer, G.S.; Hsu-Kim, H. Trends in the rare-earth element content of U.S.-based coal combustion fly ashes. Environ. Sci. Technol. 2016, 50, 5919–5926. [Google Scholar] [CrossRef]
- Querol, X.; Fernandez Turiel, J.; Lopez Soler, A. The behaviour of mineral matter during combustion of Spanish subbituminous and brown coals. Mineral. Mag. 1994, 58, 119–133. [Google Scholar] [CrossRef]
- Polański, A.; Smulikowski, K. Geochemia; Wydawnictwa Geologiczne: Warszawa, Poland, 1969. (In Polish) [Google Scholar]
- Valentim, B.; Białecka, B.; Gonçalves, P.A.; Guedes, A.; Guimarães, R.; Cruceru, M.; Całus-Moszko, J.; Popescu, L.G.; Predeanu, G.; Santos, A.C. Undifferentiated inorganics’ in coal fly ash and bottom ash: Calcispheres, magnesiacalcispheres, and magnesiaspheres. Minerals 2018, 8, 140. [Google Scholar] [CrossRef] [Green Version]
- Dai, S.; Zhao, L.; Peng, S.; Chou, C.L.; Wang, X.; Zhang, Y.; Li, D.; Sun, Y. Abundances and distribution of minerals and elements in high-alumina coal fly ash from the Jungar Power Plant, Inner Mongolia, China. Int. J. Coal Geol. 2010, 81, 320–332. [Google Scholar] [CrossRef]
- Kolker, A.; Scott, C.; Hower, J.C.; Vazquez, J.A.; Lopano, C.L.; Dai, S. Distribution of rare earth elements in coal combustion fly ash, determined by SHRIMP-RG ion microprobe. Int. J. Coal Geol. 2017, 184, 1–10. [Google Scholar] [CrossRef]
- Thompson, R.L.; Bank, T.; Montross, S.; Roth, E.; Howard, B.; Verba, C.; Granite, E. Analysis of rare earth elements in coal fly ash using laser ablation inductively coupled plasma mass spectrometry and scanning electron microscopy. Spectrochim. Acta Part B At. Spectrosc. 2018, 143, 1–11. [Google Scholar] [CrossRef]
- Franus, W.; Wiatros-Motyka, M.M.; Wdowin, M. Coal fly ash as a resource for rare earth elements. Environ. Sci. Pollut. Res. 2015, 22, 9464–9474. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Dai, S.; Zou, J.; French, D.; Graham, I.T. Rare earth elements and yttrium in coal ash from the Luzhou power plant in Sichuan, Southwest China: Concentration, characterization and optimized extraction. Int. J. Coal Geol. 2019, 203, 1–14. [Google Scholar] [CrossRef]
- Hower, J.C.; Groppo, J.G.; Joshi, P.; Preda, D.V.; Gamliel, D.P.; Mohler, D.T.; Wiseman, J.D.; Hopps, S.D.; Morgan, T.D.; Beers, T.; et al. Distribution of Lanthanides, Yttrium, and Scandium in the Pilot-Scale Beneficiation of Fly Ashes Derived from Eastern Kentucky Coals. Minerals 2020, 10, 105. [Google Scholar] [CrossRef] [Green Version]
- Kabata-Pendias, A.; Pendias, H. Biogeochemia Pierwiastków Śladowych; Wydawnictwo Naukowe PWN: Warszawa, Poland, 1999. (In Polish) [Google Scholar]
- Querol, X.; Fernández-Turiel, J.; López-Soler, A. Trace elements in coal and their behaviour during combustion in a large power station. Fuel 1995, 74, 331–343. [Google Scholar] [CrossRef]








| Sample Number | Fly Ash | Sample Number | Grain Class below 45 μm | Sample Number | Grain Class below 20 μm |
|---|---|---|---|---|---|
| 1 | from hard coal | 145 | ash from hard coal | 120 | ash from hard coal |
| 3 | 345 | 320 | |||
| 7 | 745 | 720 | |||
| 11 | 1145 | - | |||
| - | 945 | 920 | |||
| 6 | from lignite | 645 | ash from lignite | 620 | ash from lignite |
| 12 | 1245 | - |
| Sample | Particle Size Distribution | |||
|---|---|---|---|---|
| 1 | X10.3 = 9.99 µm | X50.3 = 68.14 µm | X90.3 = 288.44 µm | X99.3 = 371.76 µm |
| 3 | X10.3 = 8.91 µm | X50.3 = 38.66 µm | X90.3 = 163.74 µm | X99.3 = 220.36 µm |
| 7 | X10.3 = 7.73 µm | X50.3 = 16.84 µm | X90.3 = 60.94 µm | X99.3 = 128.02 µm |
| 11 | X10.3 = 8.54 µm | X50.3 = 35.37 µm | X90.3 = 138.25 µm | X99.3 = 228.39 µm |
| 6 | X10.3 = 18.95 µm | X50.3 = 77.01 µm | X90.3 = 283.10 µm | X99.3 = 371.63 µm |
| 12 | X10.3 = 10.51 µm | X50.3 = 31.53 µm | X90.3 = 100.82 µm | X99.3 = 313.25 µm |
| Sample | Grain Class below 45 µm | Grain Class below 20 µm |
|---|---|---|
| 1 | 40 | 25 |
| 3 | 53 | 36 |
| 7 | 82 | 60 |
| 11 | 55 | 36 |
| 6 | 31 | 11 |
| 12 | 64 | 32 |
| Sample | Chemical Components (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | TiO2 | P2O5 | MnO | Cr2O3 | LOI | Total | |
| 1 | 50.03 | 25.37 | 6.58 | 2.5 | 2.95 | 0.95 | 2.93 | 1.05 | 0.53 | 0.07 | 0.021 | 6.6 | 99.58 |
| 145 | 50.04 | 25.76 | 6.9 | 2.71 | 3.21 | 1.32 | 3.32 | 1.09 | 0.56 | 0.07 | 0.023 | 4.5 | 99.50 |
| 120 | 49.74 | 27.27 | 6.67 | 2.47 | 3.19 | 1.34 | 3.17 | 1.23 | 0.94 | 0.07 | 0.026 | 3.3 | 99.42 |
| 3 | 42.05 | 21.2 | 4.34 | 1.84 | 3.04 | 0.6 | 2.3 | 0.92 | 0.43 | 0.05 | 0.020 | 22.8 | 99.59 |
| 345 | 45.32 | 24.19 | 5.1 | 2.27 | 4.04 | 0.78 | 2.59 | 1.11 | 0.57 | 0.06 | 0.025 | 13.5 | 99.56 |
| 320 | 47.54 | 27.2 | 4.92 | 2.02 | 3.61 | 0.96 | 2.91 | 1.32 | 0.59 | 0.06 | 0.026 | 8.4 | 99.56 |
| 7 | 51.64 | 20.69 | 11.27 | 1.69 | 4.39 | 2.17 | 1.99 | 0.92 | 0.22 | 0.05 | 0.026 | 4.3 | 99.36 |
| 745 | 51.32 | 20.86 | 11.54 | 1.77 | 4.63 | 2.37 | 2.01 | 0.99 | 0.24 | 0.05 | 0.028 | 3.6 | 99.41 |
| 720 | 50.01 | 21.54 | 12.67 | 2.01 | 4.65 | 2.86 | 2.14 | 1.08 | 0.22 | 0.06 | 0.031 | 1.9 | 99.17 |
| 6 | 33.02 | 28.98 | 8 | 0.99 | 22.48 | 0.07 | 0.12 | 0.7 | 0.57 | 0.06 | 0.025 | 4.7 | 99.72 |
| 645 | 22.76 | 24.68 | 12.11 | 1.09 | 28.32 | 0.08 | 0.05 | 0.55 | 0.52 | 0.07 | 0.012 | 2.5 | 92.74 |
| 620 | 17.46 | 19.84 | 20.94 | 1.03 | 29.49 | 0.09 | 0.06 | 0.46 | 0.41 | 0.08 | 0.016 | 2.1 | 91.98 |
| 945 | 51.23 | 21.1 | 10.52 | 2.23 | 4.74 | 2.03 | 2.13 | 0.98 | 0.21 | 0.07 | 0.025 | 4.1 | 99.37 |
| 920 | 49.28 | 23.18 | 10.42 | 2.48 | 4.86 | 2.55 | 2.29 | 1.1 | 0.32 | 0.07 | 0.032 | 2.7 | 99.28 |
| 11 | 50.37 | 26.28 | 5.44 | 2.6 | 3.54 | 1.08 | 2.84 | 1.09 | 0.46 | 0.07 | 0.023 | 5.8 | 99.59 |
| 1145 | 50.77 | 26.76 | 4.89 | 2.83 | 4.12 | 1.29 | 2.86 | 1.2 | 0.56 | 0.08 | 0.026 | 4.1 | 99.49 |
| 12 | 31.68 | 25.39 | 9.19 | 1.27 | 27.03 | 0.06 | 0.14 | 0.91 | 0.44 | 0.06 | 0.028 | 3.4 | 99.60 |
| 1245 | 28.41 | 24.82 | 11.67 | 1.34 | 29.75 | 0.09 | 0.13 | 0.83 | 0.44 | 0.07 | 0.027 | 2 | 99.58 |
| Sample | Element (ppm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sc | Be | Co | Ga | Hf | Nb | Sr | Ta | V | W | Sb | Bi | |
| 1 | 28 | 7 | 39.3 | 33.8 | 5.8 | 21.5 | 621 | 1.6 | 241 | 4.7 | 4 | 0.9 |
| 145 | 29 | 8 | 48.9 | 47.4 | 5 | 21.1 | 649.3 | 1.5 | 295 | 5.5 | 6.3 | 1.2 |
| 120 | 32 | 11 | 56.1 | 69.6 | 5.9 | 24.3 | 830.9 | 1.5 | 353 | 7.7 | 9.8 | 1.7 |
| 3 | 24 | 8 | 30.5 | 30.1 | 4.3 | 17.2 | 476.7 | 1.2 | 196 | 4.8 | 3 | 0.8 |
| 345 | 29 | 5 | 39.5 | 37.1 | 5.2 | 20.5 | 543.5 | 1.7 | 252 | 5.5 | 5.5 | 0.5 |
| 320 | 32 | 12 | 52.7 | 48.8 | 4.4 | 23.2 | 677 | 1.9 | 330 | 6.3 | 6.7 | 1.3 |
| 7 | 29 | 4 | 28.4 | 25.7 | 6.3 | 17.4 | 815 | 1.1 | 344 | 3.5 | 5.9 | 0.5 |
| 745 | 31 | 7 | 27.8 | 29.3 | 6.2 | 16.7 | 845.5 | 1.1 | 361 | 3.9 | 6.4 | 0.6 |
| 720 | 31 | 8 | 42.4 | 44 | 7 | 21.5 | 1228.6 | 1.2 | 422 | 8.4 | 8.1 | 0.7 |
| 6 | 24 | 3 | 30.1 | 27.3 | 4 | 13.7 | 458.1 | 0.9 | 192 | 1.7 | 0.1 | 0.6 |
| 645 | 22 | 2 | 48.6 | 25.6 | 4.3 | 12.2 | 587.2 | 1 | 244 | 1.3 | 0.3 | 0.9 |
| 620 | 20 | 2 | 70.4 | 25.1 | 4.3 | 11 | 529.5 | 0.6 | 248 | 3.2 | 0.4 | 1.4 |
| 945 | 26 | 5 | 31 | 27.8 | 6.3 | 17.7 | 874.7 | 1.2 | 315 | 5.1 | 5.9 | 0.5 |
| 920 | 30 | 10 | 43.6 | 47.8 | 6.2 | 21 | 1064 | 1.3 | 410 | 7.3 | 9.7 | 0.9 |
| 11 | 28 | 10 | 37.7 | 35 | 5.6 | 21.3 | 578.4 | 1.7 | 235 | 5.2 | 3.9 | 1.3 |
| 1145 | 30 | 9 | 41.9 | 44.7 | 6.3 | 23.6 | 657.8 | 1.6 | 268 | 6 | 5.7 | 1.4 |
| 12 | 27 | 1 | 42 | 34.5 | 5.9 | 22 | 586.1 | 1.5 | 274 | 2.7 | 5.7 | 1.4 |
| 1245 | 29 | 5 | 46.9 | 32.1 | 5.5 | 18 | 572.9 | 1.2 | 288 | 2.4 | 0.4 | 1.3 |
| CVH | 24 ± 1 | 12 ± 1 | 37 ± 2 | 36 ± 1 | 9 ± 0.3 | 22 ± 1 | 730 ± 50 | 2 ± 0.1 | 170 ± 10 | 7.8 ± 0.6 | 7.5 ± 0.6 | 7.5 ± 0.4 |
| CVB | 23 ± 1 | 6.7 ± 0.5 | 26 ± 1 | 29 ± 1 | 7.5 ± 0.4 | 18 ± 1 | 740 ± 70 | 1.4 ± 0.1 | 140 ± 10 | 6 ± 1.7 | 5 ± 0.4 | 4.3 ± 0.8 |
| Sample | Element [ppm] | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ∑ REY | |
| 1 | 49.7 | 62.9 | 123.5 | 14.59 | 56.2 | 10.92 | 2.33 | 9.82 | 1.47 | 8.69 | 1.72 | 4.82 | 0.72 | 4.55 | 0.71 | 353 |
| 145 | 49.9 | 58 | 109.7 | 13.87 | 53 | 10.86 | 2.36 | 10.16 | 1.49 | 8.78 | 1.72 | 4.99 | 0.71 | 4.64 | 0.72 | 331 |
| 120 | 56 | 67.7 | 125 | 15.69 | 60.4 | 12.16 | 2.67 | 11.33 | 1.73 | 9.6 | 1.94 | 5.65 | 0.8 | 5 | 0.76 | 376 |
| 3 | 42 | 48.9 | 93.5 | 12.37 | 46.7 | 9.5 | 1.98 | 8.63 | 1.3 | 7.69 | 1.52 | 4.38 | 0.63 | 4.04 | 0.59 | 284 |
| 345 | 48.5 | 52.7 | 104.8 | 13.74 | 51.9 | 10.72 | 2.29 | 9.72 | 1.52 | 8.82 | 1.79 | 4.87 | 0.7 | 4.64 | 0.68 | 317 |
| 320 | 52.7 | 59.7 | 120.1 | 14.75 | 55.1 | 11.14 | 2.57 | 10.74 | 1.66 | 9.51 | 1.88 | 5.73 | 0.79 | 5.11 | 0.78 | 352 |
| 7 | 47.1 | 44.6 | 85.8 | 11.04 | 42.8 | 8.57 | 1.76 | 8.12 | 1.3 | 8.03 | 1.74 | 4.91 | 0.68 | 4.61 | 0.69 | 272 |
| 745 | 47.4 | 43.8 | 82.7 | 10.88 | 41.8 | 8.3 | 1.82 | 8.03 | 1.26 | 7.97 | 1.59 | 4.91 | 0.7 | 4.47 | 0.68 | 266 |
| 720 | 55.6 | 52.3 | 104.4 | 12.23 | 45.7 | 9.44 | 2.258 | 9.74 | 1.54 | 9.14 | 1.91 | 5.54 | 0.8 | 5.16 | 0.79 | 317 |
| 6 | 81.8 | 93.2 | 168.9 | 22.43 | 86.3 | 16.06 | 3.3 | 15.14 | 2.12 | 12.16 | 2.38 | 6.87 | 0.91 | 5.56 | 0.81 | 518 |
| 645 | 92.7 | 113.5 | 202.1 | 23.94 | 94.2 | 16.92 | 3.55 | 16.3 | 2.35 | 13.38 | 2.6 | 7.58 | 1 | 6.36 | 0.88 | 597 |
| 620 | 83 | 95.6 | 174.5 | 21.41 | 81 | 15.11 | 3.24 | 14.39 | 2.1 | 12.71 | 2.47 | 6.8 | 0.9 | 5.53 | 0.86 | 520 |
| 945 | 47 | 45.5 | 86.3 | 10.82 | 40.5 | 7.89 | 1.75 | 8.1 | 1.25 | 7.47 | 1.56 | 4.67 | 0.65 | 4.28 | 0.65 | 268 |
| 920 | 52 | 50.4 | 93.9 | 11.76 | 45.1 | 8.72 | 2.04 | 9.08 | 1.4 | 8.32 | 1.83 | 5.13 | 0.76 | 4.76 | 0.77 | 296 |
| 11 | 48.2 | 61.8 | 122.4 | 14.13 | 53.5 | 10.78 | 2.35 | 9.84 | 1.51 | 8.72 | 1.78 | 5.27 | 0.73 | 4.55 | 0.69 | 346 |
| 1145 | 53.2 | 65.1 | 126.9 | 14.89 | 58.6 | 11.33 | 2.4 | 10.22 | 1.63 | 9.21 | 1.89 | 5.42 | 0.81 | 4.98 | 0.75 | 367 |
| 12 | 92.7 | 113.5 | 197 | 24.05 | 90.6 | 17.33 | 3.66 | 16.07 | 2.42 | 13.76 | 2.79 | 7.76 | 1.06 | 6.71 | 1.02 | 590 |
| 1245 | 97.1 | 113.1 | 202.2 | 25.06 | 96.9 | 18.25 | 3.69 | 17.31 | 2.53 | 14.44 | 2.88 | 8.26 | 1.15 | 6.88 | 1.04 | 611 |
| CVH | 57 ± 2 | 76 ± 3 | 140 ± 10 | 26 ± 3 | 75 ± 4 | 14 ± 1 | 2.6 ± 0.1 | 16 ± 1 | 2.1 ±0.1 | 15 ± 1 | 4.8 ± 0.2 | 6.4 ± 0.3 | 2.2 ± 0.1 | 6.9 ± 0.3 | 1.3 ± 0.1 | - |
| CVB | 44 ± 3 | 61 ± 3 | 120 ± 10 | 13 ± 2 | 58 ± 5 | 11 ± 1 | 2.3 ± 0.2 | 16 ± 1 | 2 ± 0.1 | 12 ± 1 | 3.1 ± 0.3 | 4.6 ± 0.2 | 1.8 ± 0.3 | 5.5 ± 0.2 | 1.1 ± 0.1 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strzałkowska, E. Ashes Qualified as a Source of Selected Critical Elements (REY, Co, Ga, V). Energies 2023, 16, 3331. https://doi.org/10.3390/en16083331
Strzałkowska E. Ashes Qualified as a Source of Selected Critical Elements (REY, Co, Ga, V). Energies. 2023; 16(8):3331. https://doi.org/10.3390/en16083331
Chicago/Turabian StyleStrzałkowska, Ewa. 2023. "Ashes Qualified as a Source of Selected Critical Elements (REY, Co, Ga, V)" Energies 16, no. 8: 3331. https://doi.org/10.3390/en16083331






