Hydrogen Production by Catalytic Supercritical Water Gasification of Black Liquor-Based Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
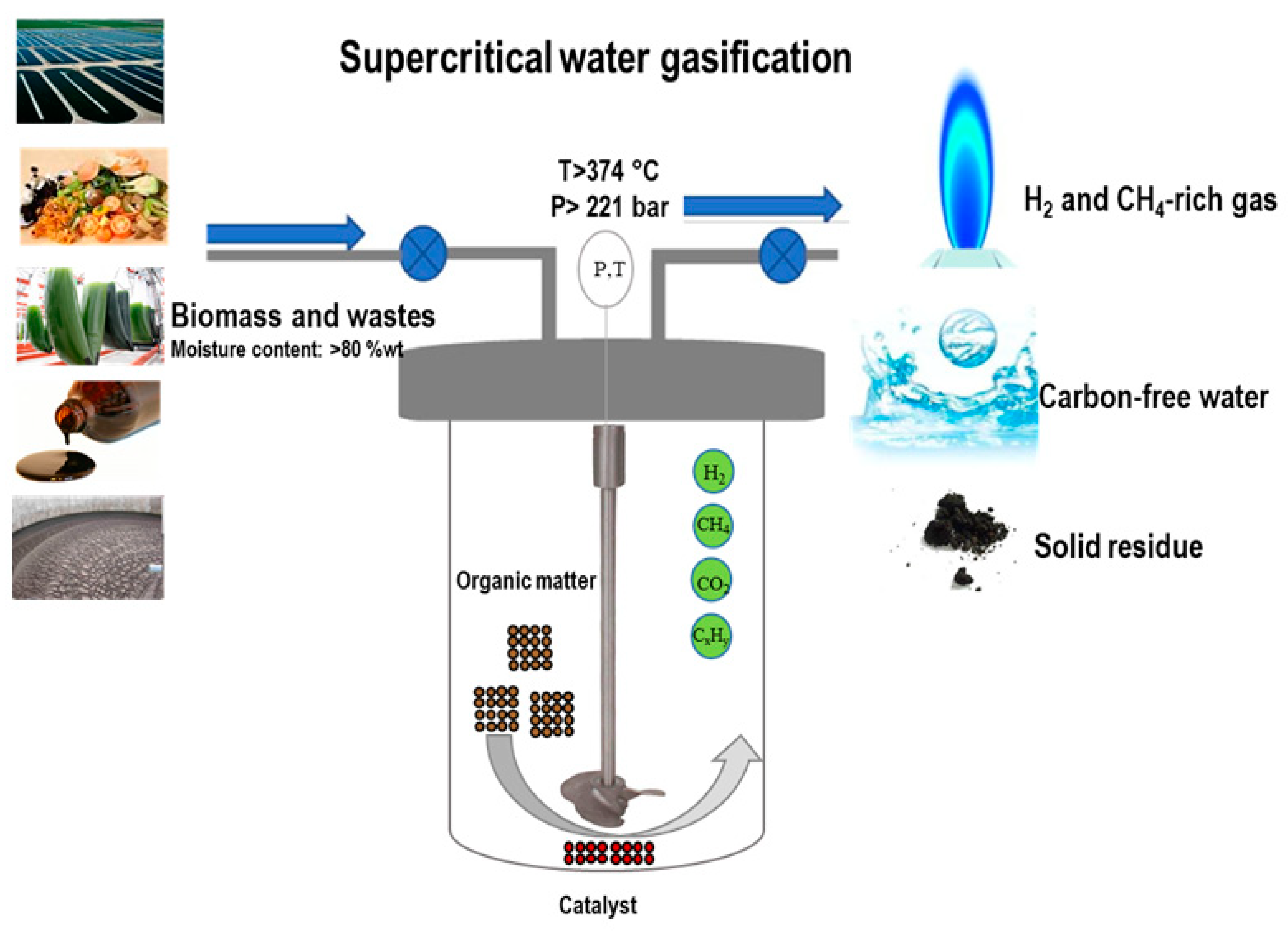
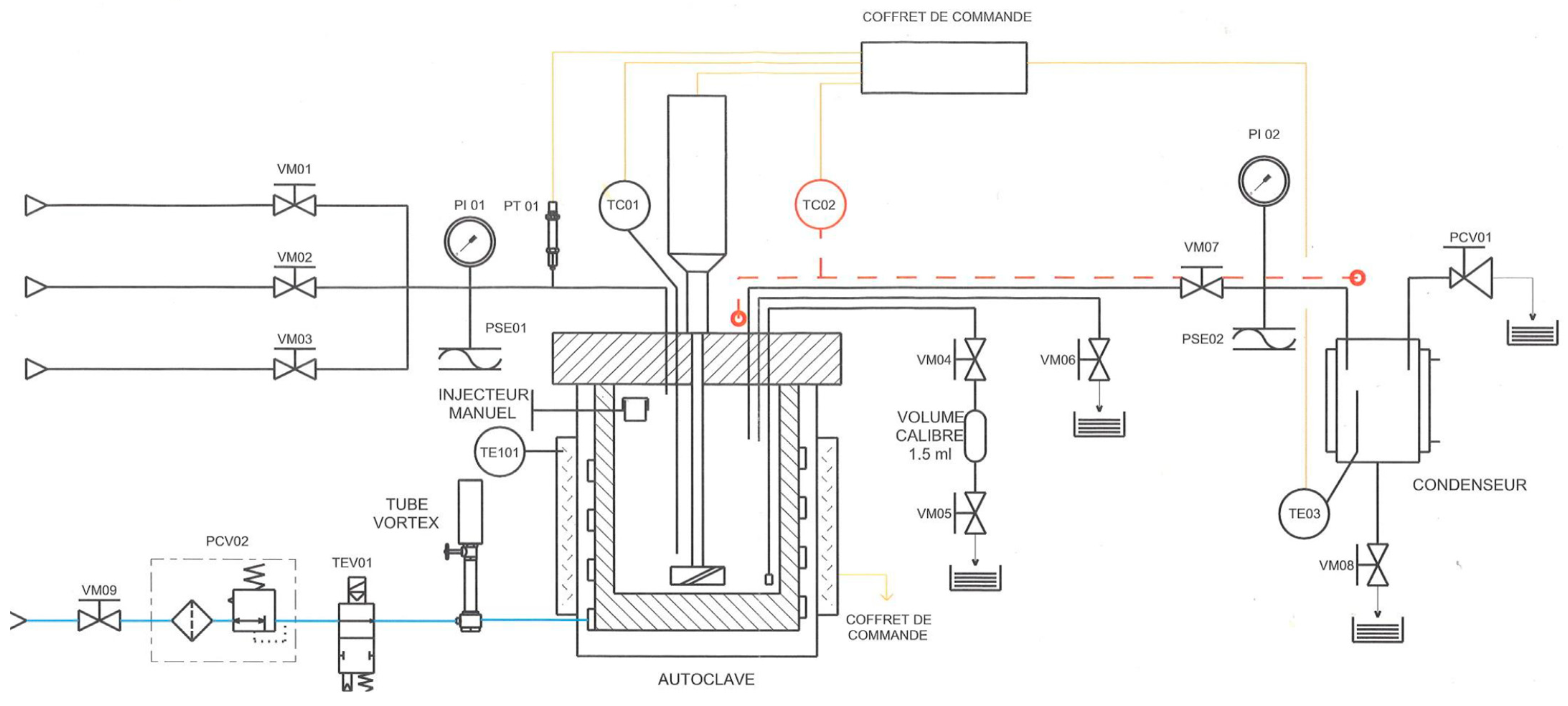
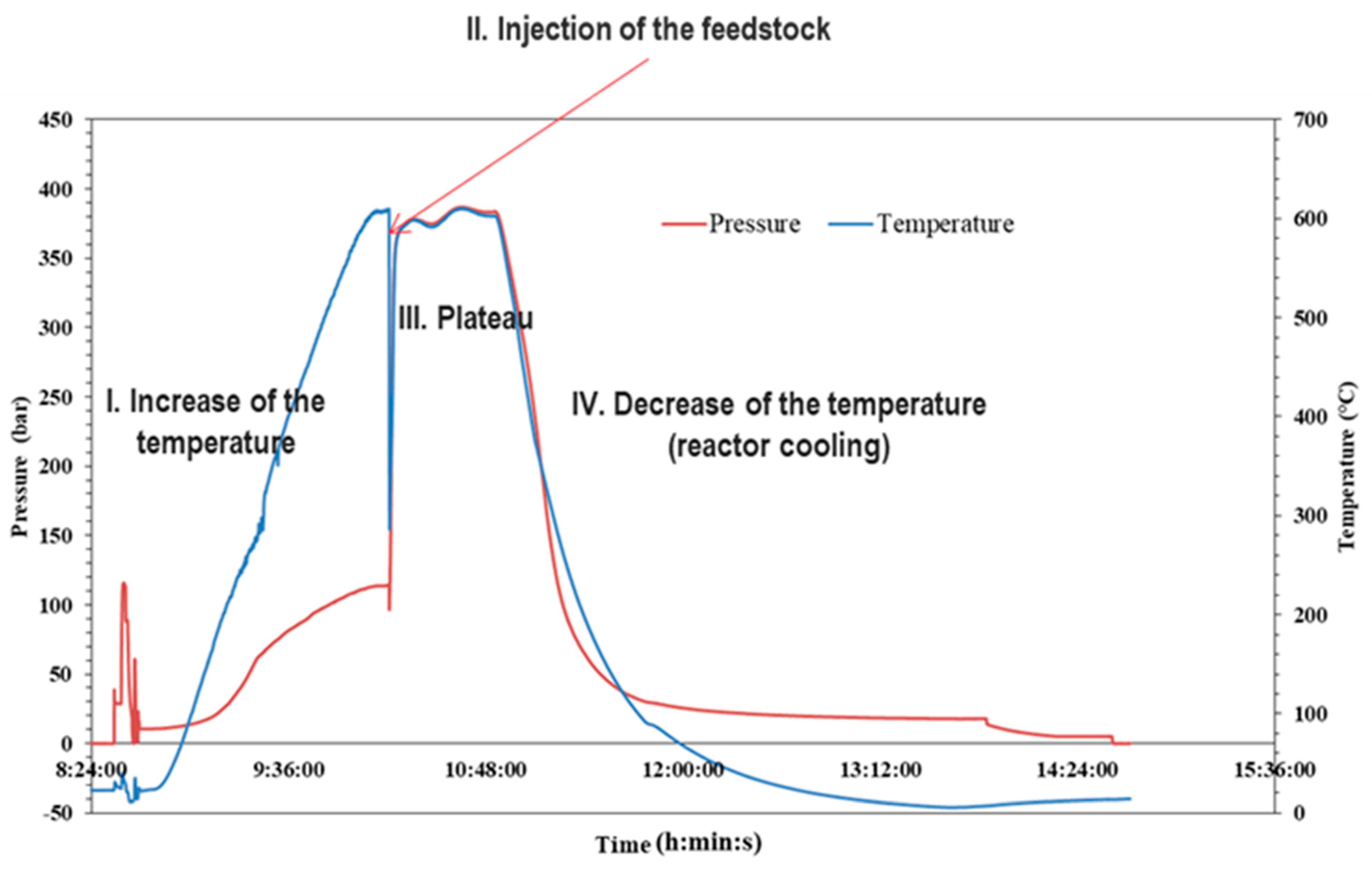
2.3. Supercritical Water Gasification Process
3. Results and Discussion
3.1. Characterization of the Feedstock
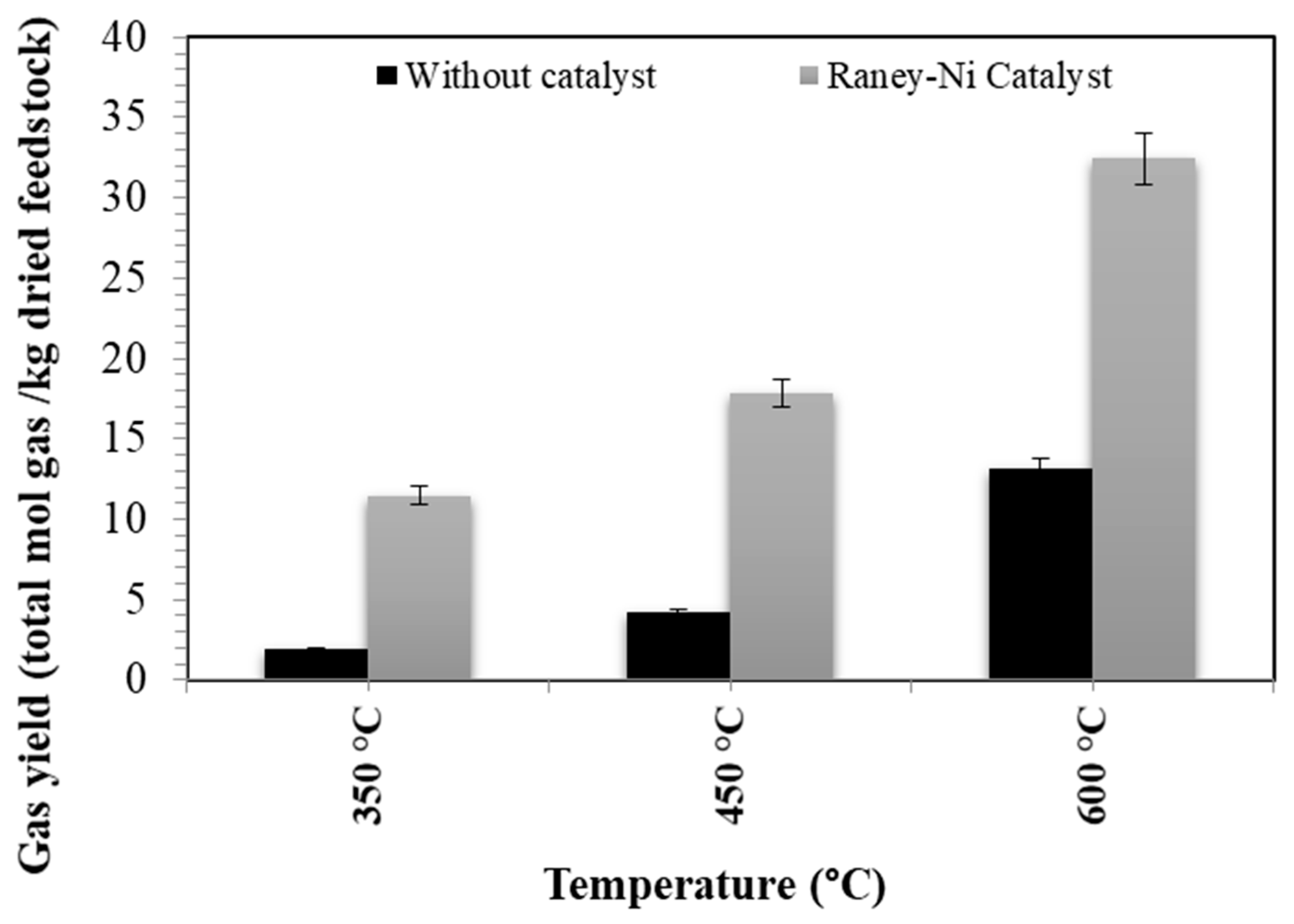
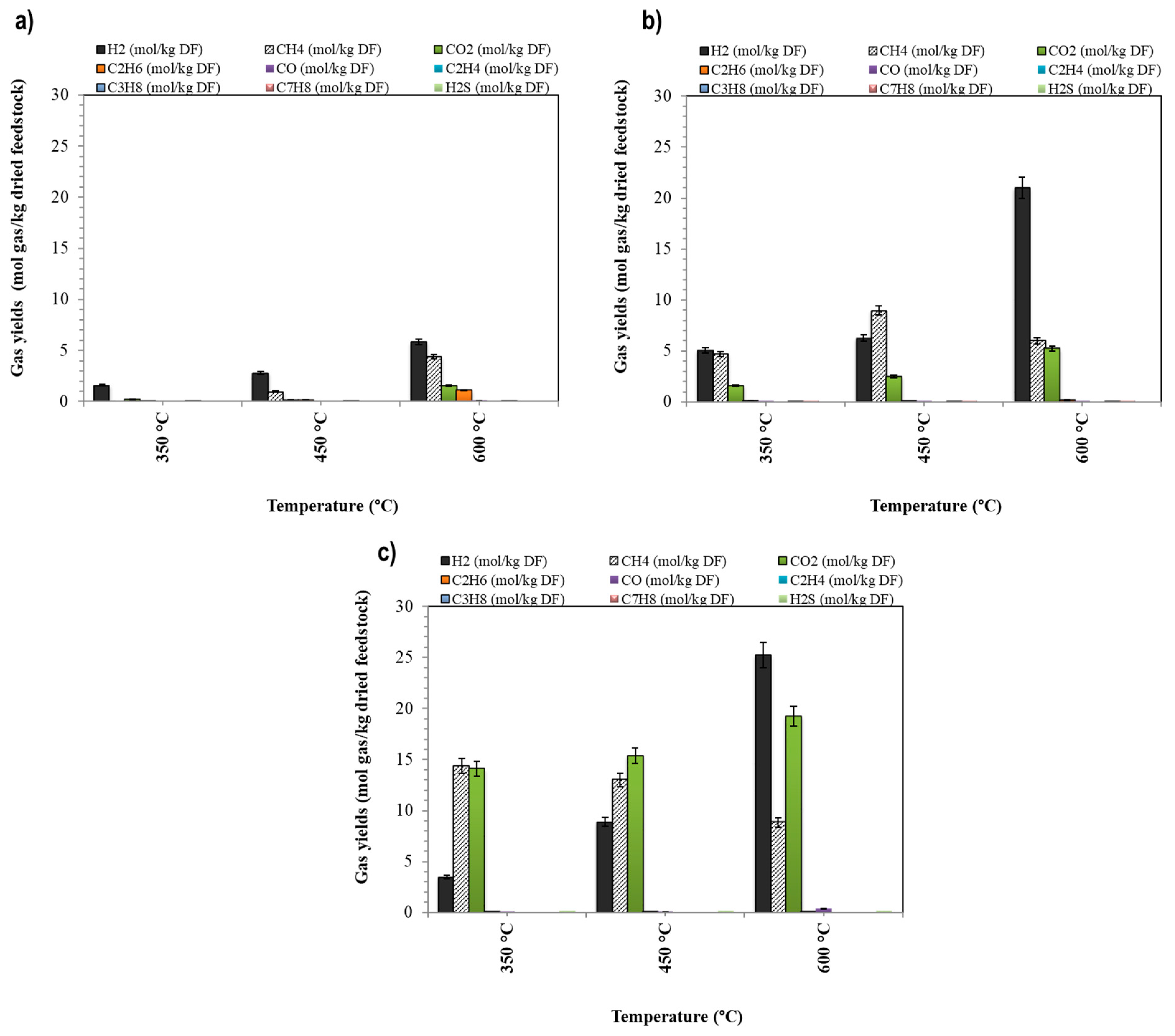
3.2. Effect of the Temperature on the Conversion Yield
| Feedstock | Operating Conditions | Catalyst | H2 yield | Reference |
|---|---|---|---|---|
| Kraft lignin | 651 °C, 25 MPa | Without catalyst | 1.60 mol H2/kg lignin | Kang et al. [19] |
| Paper waste sludge | 450 °C, 60 min | Ni/Al2O3-SiO2 | 5.8 mol H2/kg | Louw et al. [20] |
| Paper waste sludge | 450 °C, 60 min | K2CO3 | 7.5 mol H2/kg | Louw et al. [20] |
| Glucose | 750 °C, 28 MPa | Without catalyst | 4.78 mol H2/mol glucose | Lee et al. [21] |
| Xylose | 600 °C, 21 MPa, 60 min | Without catalyst | 15 mol H2/kg | Gokkaya et al. [16] |
| Xylose | 600 °C, 21 MPa, 60 min | K2CO3 | 18 mol H2/kg | Gokkaya et al. [16] |
| Timothy grass | 650 °C, 23–25 MPa, 45 min | Without catalyst | 5.15 mol H2/kg | Nanda et al. [22] |
| Timothy grass | 650 °C, 23–25 MPa, 45 min | 3% w/w KOH | 8.91 mol H2/kg | Nanda et al. [22] |
| Alkaline black liquor from wheat straw | 650 °C, 25 MPa, 12.4 min | Without catalyst | 11.3 mol H2/kg | Cao et al. [23] |
| Coconut shell | 650 °C, 23–25 MPa, 45 min | 2% w/w K2CO3 | 4.78 mol H2/kg | Nanda et al. [24] |
| Dewatered sewage sludge | 400 °C, 10 min | 3.33% w/w Ni and 1.67% w/w NaOH | 4.8 mol H2/kg of organic matter | Gong et al. [15] |
| Sewage sludge | 750 °C, 30 min | Without catalyst | 20.66 mol H2/kg | Chen et al. [17] |
| Sewage sludge | 750 °C, 30 min | 50% w/w K2CO3 25% w/w Raney Ni/Mo2 | 20.03 mol H2/kg | Chen et al. [17] |
| Food waste | 450 °C, 25 MPa, 60 min | 5% w/w NaOH | 12.73 mol H2/kg | Su et al. [18] |
| HTL effluent based on Black liquor | 450 °C, 30 min | Raney Ni | 6.25 mol H2/kg | This work |
| HTL effluent based on Black liquor | 600 °C, 30 min | Raney Ni | 20.98 mol H2/kg | This work |
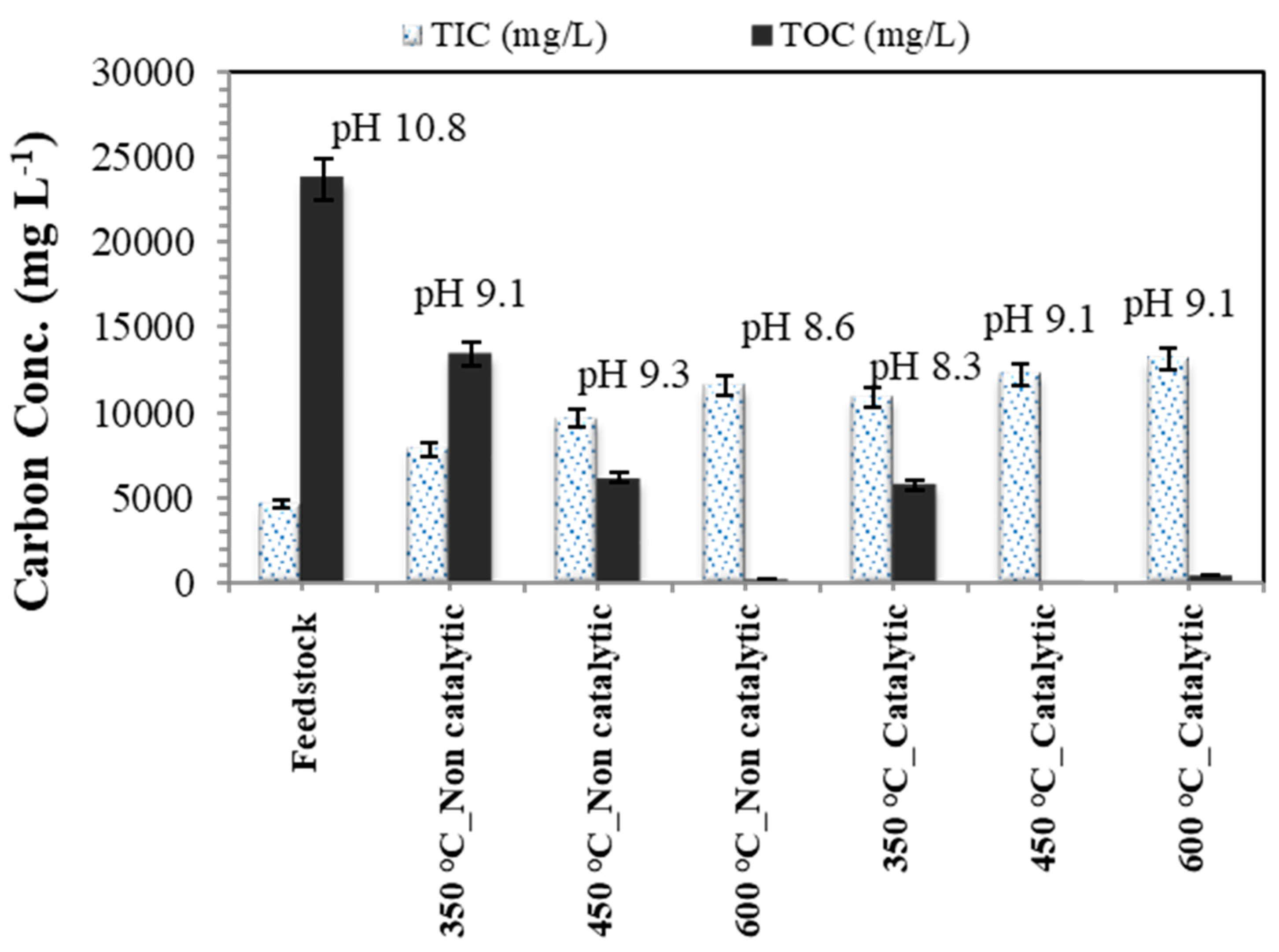
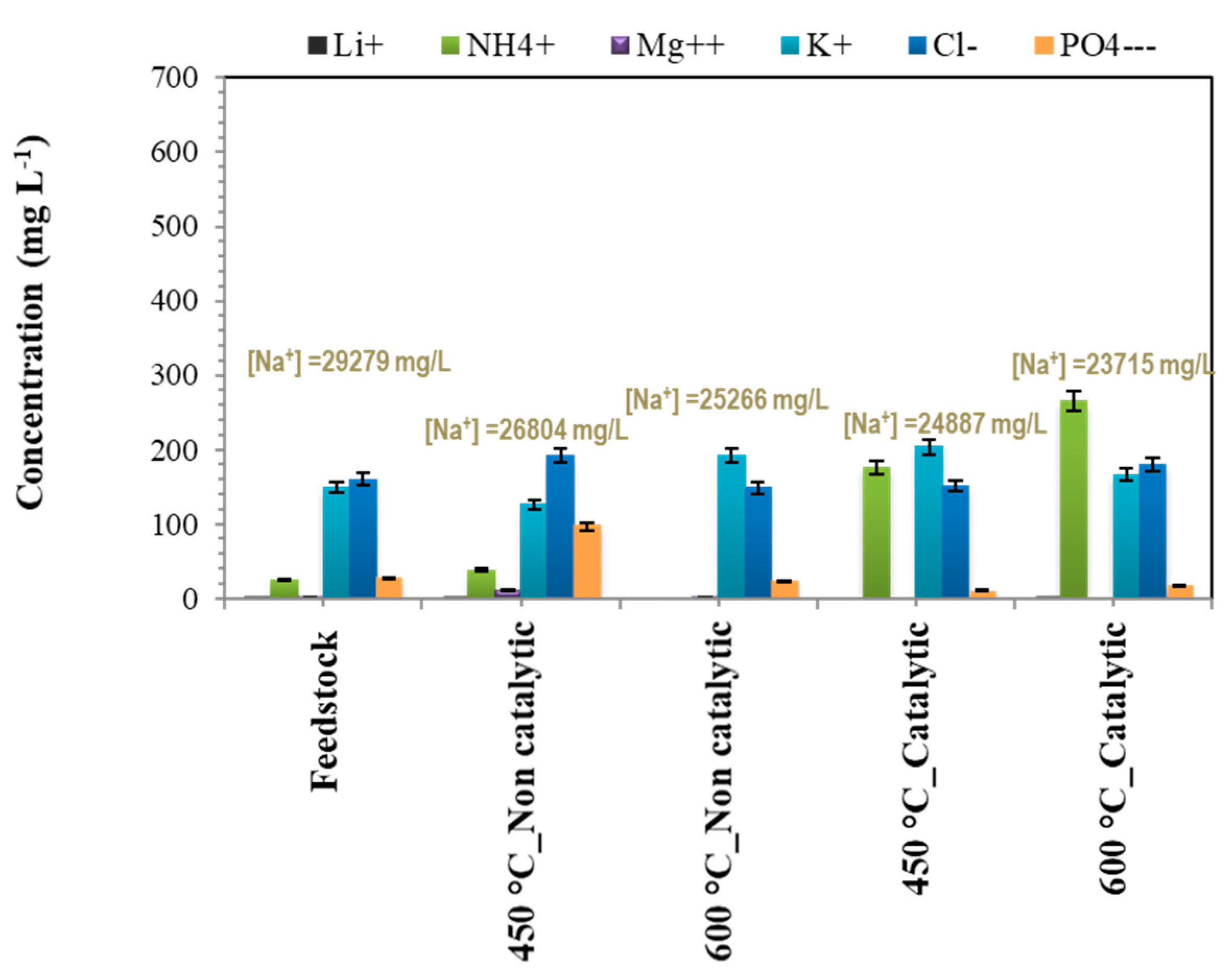
3.3. Aqueous Phase Analyses
4. Conclusions
- The results confirm that an increase in temperature conditions improves the conversion yield of the evaluated waste.
- The best operating conditions for valorizing the aqueous effluent (from the HTL process) were obtained at 600 °C with a Raney nickel catalyst. The gas yield obtained was 32.43 mol gas/kg of dried feedstock (containing 21 mol H2/kg dried feedstock and 6 mol CH4/kg dried feedstock). The higher heating value (HHV) of this gas was 80.2 MJ/kg.
- The utilization of a Raney nickel catalyst enhances the H2/CH4 molar ratio, which is advantageous for producing gas with a higher energy content (HHV) at lower temperatures (450 °C).
- The characterization results of the aqueous effluent after SCWG confirm the decrease of organic carbon concentration because of the conversion yield into gas molecules. The organic carbon concentration of the wastewater decreased notably after SCWG treatment from T > 450 °C with a catalyst.
- The Raney nickel catalyst improves the organic carbon conversion at lower temperatures (i.e., 450 °C), which is the major contribution of this work for decreasing the input of energy to the system and decreasing the process costs (linked to the special alloy materials conceived for operating at higher temperature and pressure conditions).
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Db | dry basis |
| DF | Dried feedstock |
| HHV | Higher heating value |
| LOD | Limit of detection |
| SCWG | Supercritical water gasification |
References
- Dafinov, A.; Font, J.; Garcia-Valls, R. Processing of black liquors by UF/NF ceramic membranes. Desalination 2005, 173, 83–90. [Google Scholar] [CrossRef]
- Bajpai, P. Black Liquor Gasification; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Naqvi, M.; Yan, J.; Dahlquist, E. Black liquor gasification integrated in pulp and paper mills: A critical review. Bioresour. Technol. 2010, 101, 8001–8015. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Conradie, A.V.; Lester, E. Review of supercritical water gasification with lignocellulosic real biomass as the feedstocks: Process parameters, biomass composition, catalyst development, reactor design and its challenges. Chem. Eng. J. 2021, 415, 128837. [Google Scholar] [CrossRef]
- Chen, Y.; Yi, L.; Li, S.; Yin, J.; Jin, H. Catalytic gasification of sewage sludge in near and supercritical water with different catalysts. Chem. Eng. J. 2020, 388, 124292. [Google Scholar] [CrossRef]
- Correa, C.R.; Kruse, A. Supercritical water gasification of biomass for hydrogen production—Review. J. Supercrit. Fluids 2018, 133, 573–590. [Google Scholar] [CrossRef]
- Sınaǧ, A.; Kruse, A.; Rathert, J. Influence of the Heating Rate and the Type of Catalyst on the Formation of Key Intermediates and on the Generation of Gases During Hydropyrolysis of Glucose in Supercritical Water in a Batch Reactor. Ind. Eng. Chem. Res. 2004, 43, 502–508. [Google Scholar] [CrossRef]
- Sheikhdavoodi, M.J.; Almassi, M.; Ebrahimi-Nik, M.; Kruse, A.; Bahrami, H. Gasification of sugarcane bagasse in supercritical water; evaluation of alkali catalysts for maximum hydrogen production. J. Energy Inst. 2015, 88, 450–458. [Google Scholar] [CrossRef]
- Azadi, P.; Afif, E.; Foroughi, H.; Dai, T.; Azadi, F.; Farnood, R. Catalytic reforming of activated sludge model compounds in supercritical water using nickel and ruthenium catalysts. Appl. Catal. B Environ. 2013, 134–135, 265–273. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Hiyoshi, N.; Sato, O.; Bando, K.K.; Osada, M.; Shirai, M. Hydrogen production from woody biomass over supported metal catalysts in supercritical water. Catal. Today 2009, 146, 192–195. [Google Scholar] [CrossRef]
- Huet, M.; Roubaud, A.; Chirat, C.; Lachenal, D. Hydrothermal treatment of black liquor for energy and phenolic platform molecules recovery in a pulp mill. Biomass Bioenergy 2016, 89, 105–112. [Google Scholar] [CrossRef]
- Curmi, H.; Chirat, C.; Roubaud, A.; Peyrot, M.; Haarlemmer, G.; Lachenal, D. Extraction of phenolic compounds from sulfur-free black liquor thanks to hydrothermal treatment before the production of syngas for biofuels. J. Supercrit. Fluids 2022, 181, 105489. [Google Scholar] [CrossRef]
- Yan, M.; Hantoko, D.; Kanchanatip, E.; Zheng, R.; Zhong, Y.; Mubeen, I. Valorization of sewage sludge through catalytic sub- and supercritical water gasification. J. Energy Inst. 2020, 93, 1419–1427. [Google Scholar] [CrossRef]
- Acelas, N.Y.; López, D.P.; Brilman, D.W.; Kersten, S.R.; Kootstra, A.M. Supercritical water gasification of sewage sludge: Gas production and phosphorus recovery. Bioresour. Technol. 2014, 174, 167–175. [Google Scholar] [CrossRef]
- Gong, M.; Zhu, W.; Zhang, H.W.; Ma, Q.; Su, Y.; Fan, Y.J. Influence of NaOH and Ni catalysts on hydrogen production from the supercritical water gasification of dewatered sewage sludge. Int. J. Hydrogen Energy 2014, 39, 19947–19954. [Google Scholar] [CrossRef]
- Gökkaya, D.S.; Saglam, M.; Yuksel, M.; Ballice, L. Hydrothermal gasification of xylose: Effects of reaction temperature, pressure, and K2CO3 as a catalyst on product distribution. Biomass Bioenergy 2016, 91, 26–36. [Google Scholar] [CrossRef]
- Chen, Y.; Yi, L.; Wei, W.; Jin, H.; Guo, L. Hydrogen production by sewage sludge gasification in supercritical water with high heating rate batch reactor. Energy 2022, 238, 121740. [Google Scholar] [CrossRef]
- Su, W.; Cai, C.; Liu, P.; Lin, W.; Liang, B.; Zhang, H.; Ma, Z.; Ma, H.; Xing, Y.; Liu, W. Supercritical water gasification of food waste: Effect of parameters on hydrogen production. Int. J. Hydrogen Energy 2020, 45, 14744–14755. [Google Scholar] [CrossRef]
- Kang, K.; Azargohar, R.; Dalai, A.K.; Wang, H. Noncatalytic Gasification of Lignin in Supercritical Water Using a Batch Reactor for Hydrogen Production: An Experimental and Modeling Study. Energy Fuels 2015, 29, 1776–1784. [Google Scholar] [CrossRef]
- Louw, J.; Schwarz, C.E.; Burger, A.J. Catalytic supercritical water gasification of primary paper sludge using a homogeneous and heterogeneous catalyst: Experimental vs thermodynamic equilibrium results. Bioresour. Technol. 2016, 201, 111–120. [Google Scholar] [CrossRef]
- Lee, I.G.; Kim, M.S.; Ihm, S.K. Gasification of Glucose in Supercritical Water. Ind. Eng. Chem. Res. 2002, 41, 1182–1188. [Google Scholar] [CrossRef]
- Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical water gasification of timothy grass as an energy crop in the presence of alkali carbonate and hydroxide catalysts. Biomass Bioenergy 2016, 95, 378–387. [Google Scholar] [CrossRef]
- Cao, C.; Guo, L.; Chen, Y.; Guo, S.; Lu, Y. Hydrogen production from supercritical water gasification of alkaline wheat straw pulping black liquor in continuous flow system. Int. J. Hydrogen Energy 2011, 36, 13528–13535. [Google Scholar] [CrossRef]
- Nanda, S.; Isen, J.; Dalai, A.K.; Kozinski, J.A. Gasification of fruit wastes and agro-food residues in supercritical water. Energy Convers. Manag. 2016, 110, 296–306. [Google Scholar] [CrossRef]

| Parameter | Unit | Feedstock |
|---|---|---|
| pH | 10.8 | |
| Dry matter | % w/w | 9.8 |
| Ash content (815 °C) | % w/w db * | 32 |
| C | % w/w db * | 28.4 |
| H | % w/w db * | 2.52 |
| N | % w/w db * | 0.22 |
| S | % w/w db * | 0.31 |
| Total organic carbon content (TOC) | mg/L | 23,692 |
| Total inorganic carbon content (TIC) | mg/L | 4658 |
| Total carbon content (TC) | mg/L | 28,350 |
| HHV | MJ/kg db * | 7.42 |
| Na+ content | mg/L | 29,279 |
| NH4+ content | mg/L | 24 |
| Mg2+ content | mg/L | 2 |
| K+ content | mg/L | 149 |
| Ca2+ content | mg/L | 5 |
| Cl− content | mg/L | 160 |
| NO3− content | mg/L | <LOD |
| SO42− content | mg/L | 546 |
| PO43− content | mg/L | 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demey, H.; Ratel, G.; Lacaze, B.; Delattre, O.; Haarlemmer, G.; Roubaud, A. Hydrogen Production by Catalytic Supercritical Water Gasification of Black Liquor-Based Wastewater. Energies 2023, 16, 3343. https://doi.org/10.3390/en16083343
Demey H, Ratel G, Lacaze B, Delattre O, Haarlemmer G, Roubaud A. Hydrogen Production by Catalytic Supercritical Water Gasification of Black Liquor-Based Wastewater. Energies. 2023; 16(8):3343. https://doi.org/10.3390/en16083343
Chicago/Turabian StyleDemey, Hary, Gilles Ratel, Bruno Lacaze, Olivier Delattre, Geert Haarlemmer, and Anne Roubaud. 2023. "Hydrogen Production by Catalytic Supercritical Water Gasification of Black Liquor-Based Wastewater" Energies 16, no. 8: 3343. https://doi.org/10.3390/en16083343
APA StyleDemey, H., Ratel, G., Lacaze, B., Delattre, O., Haarlemmer, G., & Roubaud, A. (2023). Hydrogen Production by Catalytic Supercritical Water Gasification of Black Liquor-Based Wastewater. Energies, 16(8), 3343. https://doi.org/10.3390/en16083343







