Recent Advances in Lignin-Based Biofuel Production
Abstract
1. Introduction
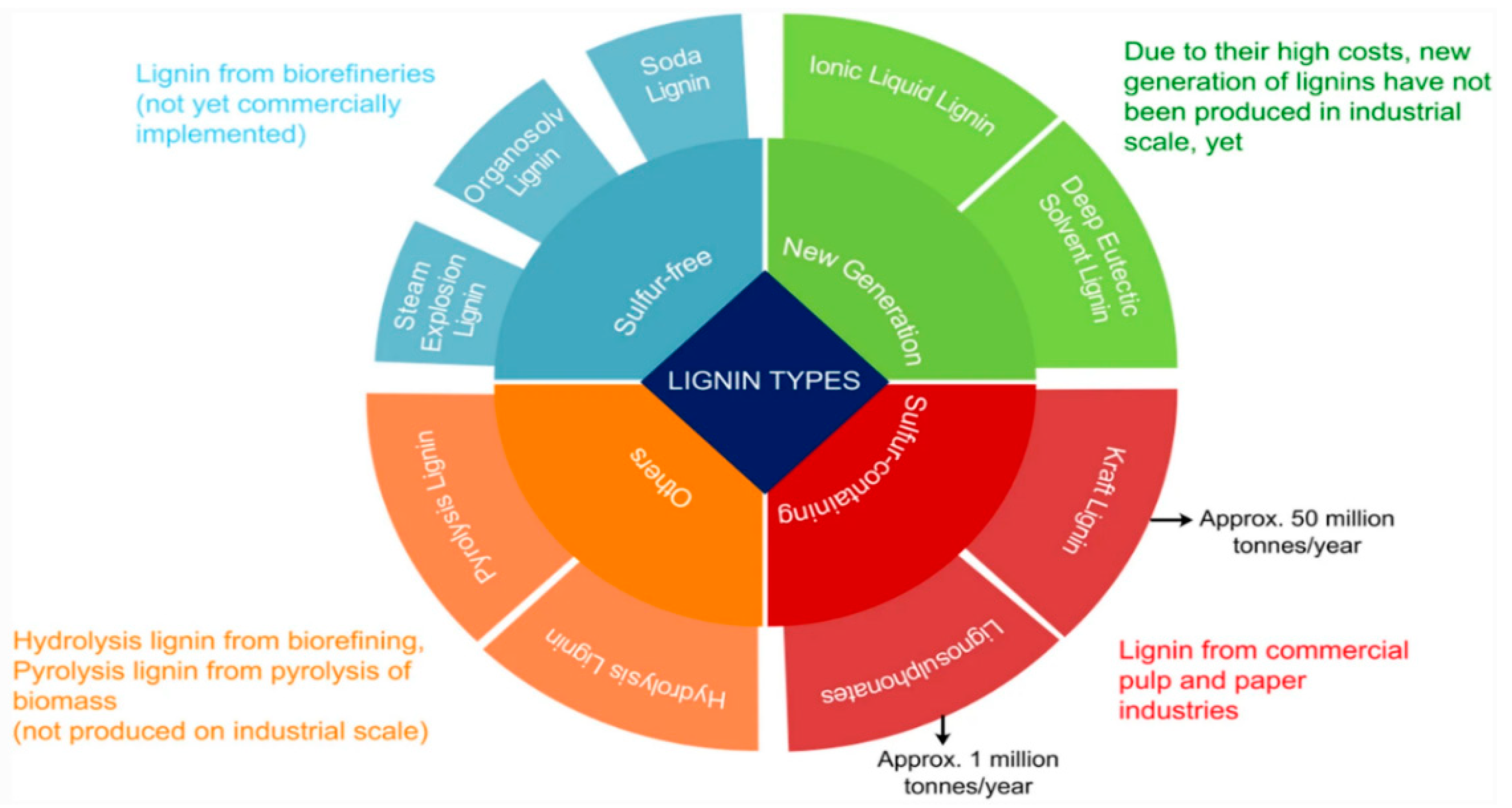
2. Lignin and Its Derivatives
3. Modification and Pretreatment of Lignin for Improved Biofuel Conversion
Different Pretreatment Strategies of Lignin
4. Lignin-Based Biofuels via Thermochemical Routes
4.1. Hydrothermal Carbonization
4.2. Pyrolysis
4.3. Biomass Liquefaction
4.4. Gasification
5. Lignin-Based Biofuels via Catalytic Routes
5.1. Hydrodeoxygenation (HDO)
5.2. Zeolite Creaking
5.3. Hydrogenation
6. Environmental and Cost Impact of Lignin Biofuels
7. Conclusions and Future Prospects
- Abundance: Lignin is a waste product of the paper and pulp industry, and is produced in large quantities. This makes it a potentially cheap and readily available feedstock for biofuel production.
- Renewability: Lignin is a renewable resource, as it can be derived from plants that can be grown and harvested repeatedly.
- Carbon neutrality: Lignin is composed of carbon, hydrogen, and oxygen, and when it is burned as a biofuel, it releases the same amount of carbon dioxide as was absorbed by the plants during their growth. This means that the carbon emissions of lignin-derived biofuels are considered to be carbon neutral.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, H.; Yadav, V.; Zhong, M.; Bilal, M.; Taherzadeh, M.J.; Iqbal, H.M. Bioengineered microbial platforms for biomass-derived biofuel production—A review. Chemosphere 2022, 288, 132528. [Google Scholar] [CrossRef]
- Li, P.; Fu, X.; Zhang, L.; Li, S. CRISPR/Cas-based screening of a gene activation library in Saccharomyces cerevisiae identifies a crucial role of OLE 1 in thermotolerance. Microb. Biotechnol. 2019, 12, 1154–1163. [Google Scholar] [CrossRef]
- Sarker, T.R.; Nanda, S.; Dalai, A.K.; Meda, V. A review of torrefaction technology for upgrading lignocellulosic biomass to solid biofuels. Bioenergy Res. 2021, 14, 645–669. [Google Scholar] [CrossRef]
- Xia, J.; Yu, Y.; Chen, H.; Zhou, J.; Tan, Z.; He, S.; Zhu, X.; Shi, H.; Liu, P.; Bilal, M.; et al. Improved lignocellulose degradation efficiency by fusion of β-glucosidase, exoglucanase, and carbohydrate-binding module from Caldicellulosiruptor saccharolyticus. BioResources 2019, 14, 6767–6780. [Google Scholar] [CrossRef]
- Amusa, A.A.; Ahmad, A.L.; Adewole, J.K. Mechanism and compatibility of pretreated lignocellulosic biomass and polymeric mixed matrix membranes: A review. Membranes 2020, 10, 370. [Google Scholar] [CrossRef] [PubMed]
- Halmann, M.; Steinfeld, A. Fuel saving, carbon dioxide emission avoidance, and syngas production by tri-reforming of flue gases from coal-and gas-fired power stations, and by the carbothermic reduction of iron oxide. Energy 2006, 31, 3171–3185. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H. Recent advancements in the life cycle analysis of lignocellulosic biomass. Curr. Sustain. Renew. Energy Rep. 2020, 7, 100–107. [Google Scholar] [CrossRef]
- Zhu, J.; Yan, C.; Zhang, X.; Yang, C.; Jiang, M.; Zhang, X. A sustainable platform of lignin: From bioresources to materials and their applications in rechargeable batteries and supercapacitors. Prog. Energy Combust. Sci. 2020, 76, 100788. [Google Scholar] [CrossRef]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crops Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Huang, C.; Jiang, X.; Shen, X.; Hu, J.; Tang, W.; Wu, X.; Ragauskas, A.; Jameel, H.; Meng, X.; Yong, Q. Lignin-enzyme interaction: A roadblock for efficient enzymatic hydrolysis of lignocellulosics. Renew. Sustain. Energy Rev. 2022, 154, 111822. [Google Scholar] [CrossRef]
- Avilov, O. Management of consumption according to the special role of need for safety. Ekon. Manaz. Spektrum 2021, 15, 75–83. [Google Scholar] [CrossRef]
- Novak, A.; Bennett, D.; Kliestik, T. Product decision-making information systems, real-time sensor networks, and artificial intelligence-driven big data analytics in sustainable Industry 4.0. Econ. Manag. Financ. Mark. 2021, 16, 62–72. [Google Scholar]
- Ahmad, F.B.; Kalam, M.A.; Zhang, Z.; Masjuki, H.H. Sustainable production of furan-based oxygenated fuel additives from pentose-rich biomass residues. Energy Convers. Manag. 2022, 14, 100222. [Google Scholar] [CrossRef]
- Gundekari, S.; Karmee, S.K. Catalytic Hydropyrolysis of Lignin for the Preparation of Cyclic Hydrocarbon-Based Biofuels. Catalysts 2022, 12, 1651. [Google Scholar] [CrossRef]
- RajeshKumar, B.; Saravanan, S.; NiranjanKumar, R.; Nishanth, B.; Rana, D.; Nagendran, A. Effect of lignin-derived cyclohexanol on combustion, performance and emissions of a direct-injection agricultural diesel engine under naturally aspirated and exhaust gas recirculation (EGR) modes. Fuel 2016, 181, 630–642. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Liu, R.; Wang, S.; Chen, L.; Li, K. Hydrodeoxygenation of Lignin-Derived Phenolic Monomers and Dimers to Alkane Fuels over Bi functional Zeolite-Supported Metal Catalysts. ACS Sustain. Chem. Eng. 2014, 2, 683–691. [Google Scholar] [CrossRef]
- Roman-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of dimethyl furan for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Herreros, J.M.; Jones, A.; Sukjit, E.; Tsolakis, A. Blending lignin-derived oxygenate in enhanced multi-component diesel fuel for improved emissions. Appl. Energy 2014, 116, 58–65. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin structure and its engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef]
- Fang, Z.; Smith, R.L., Jr. Production of Biofuels and Chemicals from Lignin; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Zeng, Y.; Zhao, S.; Yang, S.; Ding, S.Y. Lignin plays a negative role in the biochemical process for producing lignocellulosic biofuels. Curr. Opin. Biotechnol. 2014, 27, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Arancon, R.A.D.; Labidi, J.; Luque, R. Lignin depolymerisation strategies: Towards valuable chemicals and fuels. Chem. Soc. Rev. 2014, 43, 7485–7500. [Google Scholar] [CrossRef]
- Ma, R.; Xu, Y.; Zhang, X. Catalytic oxidation of biorefinery lignin to value-added chemicals to support sustainable biofuel production. ChemSusChem 2015, 8, 24–51. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, Z.; Feng, M.; Cort, J.R.; Gieleciak, R.; Heyne, J.; Yang, B. Lignin-based jet fuel and its blending effect with conventional jet fuel. Fuel 2022, 321, 124040. [Google Scholar] [CrossRef]
- Ruan, H.; Qin, Y.; Heyne, J.; Gieleciak, R.; Feng, M.; Yang, B. Chemical compositions and properties of lignin-based jet fuel range hydrocarbons. Fuel 2019, 256, 115947. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Buffi, M.; Palmisano, P.; Redaelli, S. Lignin-based advanced biofuels: A novel route towards aviation fuels. Chem. Eng. Trans. 2016, 50, 109–114. [Google Scholar]
- Chen, Y.; Liu, J.; Zeng, Q.; Liang, Z.; Ye, X.; Lv, Y.; Liu, M. Preparation of Eucommia ulmoides lignin-based high-performance biochar containing sulfonic group: Synergistic pyrolysis mechanism and tetracycline hydrochloride adsorption. Bioresour. Technol. 2021, 329, 124856. [Google Scholar] [CrossRef]
- Gul, E.; Alrawashdeh, K.A.B.; Masek, O.; Skreiberg, Ø.; Corona, A.; Zampilli, M.; Wang, L.; Samaras, P.; Yang, Q.; Fantozzi, F. Production and use of biochar from lignin and lignin-rich residues (such as digestate and olive stones) for wastewater treatment. J. Anal. Appl. Pyrolysis 2021, 158, 105263. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, M.; Li, Q.; Liu, Z.; Hao, N.; Meng, X.; Li, J.; Lin, F.; Fang, L.; Yuan, J.S.; et al. Phototunable Lignin Plastics to Enable Recyclability. ChemSusChem 2021, 14, 4260–4269. [Google Scholar] [CrossRef]
- Huang, J.; Liu, W.; Qiu, X. High performance thermoplastic elastomers with biomass lignin as plastic phase. ACS Sustain. Chem. Eng. 2019, 7, 6550–6560. [Google Scholar] [CrossRef]
- Poovaiah, C.R.; Nageswara-Rao, M.; Soneji, J.R.; Baxter, H.L.; Stewart, C.N., Jr. Altered lignin biosynthesis using biotechnology to improve lignocellulosic biofuel feedstocks. Plant Biotechnol. J. 2014, 12, 1163–1173. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef] [PubMed]
- Nigam, P.S.; Singh, A. Production of liquid biofuels from renewable resources. Prog. Energy Combust. Sci. 2011, 37, 52–68. [Google Scholar] [CrossRef]
- Borges, C.S.; Akhavan-Safar, A.; Marques, E.A.; Carbas, R.J.; Ueffing, C.; Weißgraeber, P.; da Silva, L.F. Effect of water ingress on the mechanical and chemical properties of polybutylene terephthalate reinforced with glass fibers. Materials 2021, 14, 1261. [Google Scholar] [CrossRef]
- Zor, M.; Mengeloğlu, F.; Aydemir, D.; Şen, F.; Kocatürk, E.; Candan, Z.; Ozcelik, O. Wood Plastic Composites (WPCs): Applications of Nanomaterials. In Emerging Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2023; pp. 97–133. [Google Scholar]
- Bruijnincx, P.C.; Rinaldi, R.; Weckhuysen, B.M. Unlocking the potential of a sleeping giant: Lignins as sustainable raw materials for renewable fuels, chemicals and materials. Green Chem. 2015, 17, 4860–4861. [Google Scholar] [CrossRef]
- Torres, L.A.Z.; Woiciechowski, A.L.; de Andrade Tanobe, V.O.; Karp, S.G.; Lorenci, L.C.G.; Faulds, C.; Soccol, C.R. Lignin as a potential source of high-added value compounds: A review. J. Clean. Prod. 2020, 263, 121499. [Google Scholar] [CrossRef]
- Ghaffar, S.H.; Fan, M. Structural analysis for lignin characteristics in biomass straw. Biomass Bioenergy 2013, 57, 264–279. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Feldman, D. Lignin nanocomposites. J. Macromol. Sci. A 2016, 53, 382–387. [Google Scholar] [CrossRef]
- Liu, C.-J. Deciphering the enigma of lignification: Precursor transport, oxidation, and the topochemistry of lignin assembly. Mol. Plant 2012, 5, 304–317. [Google Scholar] [CrossRef]
- Grabber, J.H. How do lignin composition, structure, and cross-linking affect degradability? A review of cell wall model studies. Crop. Sci. 2005, 45, 820–831. [Google Scholar] [CrossRef]
- Rosillo-Calle, F.; de Groot, P.; Hemstock, S.L.; Woods, J. Non-Woody Biomass and Secondary Fuels. In The Biomass Assessment Handbook; Routledge: Abingdon-on-Thames, UK, 2012; p. 110. [Google Scholar]
- Doherty, W.O.S.; Mousavioun, P.; Fellows, C.M. Value-adding to cellulosic ethanol: Lignin polymers. Ind. Crops. Prod. 2011, 33, 259–276. [Google Scholar] [CrossRef]
- Norgren, M.; Edlund, H. Lignin: Recent advances and emerging applications. Curr. Opin. Colloid Interface Sci. 2014, 19, 409–416. [Google Scholar] [CrossRef]
- Stojanovska, E.; Pampal, E.S.; Kilic, A.; Quddus, M.; Candan, Z. Developing and characterization of lignin-based fibrous nanocarbon electrodes for energy storage devices. Compos. B Eng. 2019, 158, 239–248. [Google Scholar] [CrossRef]
- Stojanovska, E.; Kurtulus, M.; Abdelgawad, A.; Candan, Z.; Kilic, A. Developing lignin-based bio-nanofibers by centrifugal spinning technique. Int. J. Biol. Macromol. 2018, 113, 98–105. [Google Scholar] [CrossRef]
- Sharma, V.; Tsai, M.L.; Nargotra, P.; Chen, C.W.; Sun, P.P.; Singhania, R.R.; Dong, C.D. Journey of lignin from a roadblock to bridge for lignocellulose biorefineries: A comprehensive review. Sci. Total Environ. 2023, 861, 160560. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Recent advances in green hydrogels from lignin: A review. Int. J. Biol. Macromol. 2015, 72, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Pan, X.; Kadla, J.F.; Ehara, K.; Gilkes, N.; Saddler, J.N. Organosolv ethanol lignin from hybrid poplar as a radical scavenger: Relationship between lignin structure, extraction conditions, and antioxidant activity. J. Agric. Food Chem. 2006, 54, 5806–5813. [Google Scholar] [CrossRef]
- Cruz, J.M.; Domínguez, J.M.; Domínguez, H.; Parajó, J.C. Antioxidant and antimicrobial effects of extracts from hydrolysates of lignocellulosic materials. J. Agric. Food Chem. 2001, 49, 2459–2464. [Google Scholar] [CrossRef]
- Toh, K.; Nakano, S.; Yokoyama, H.; Ebe, K.; Gotoh, K.; Noda, H. Anti-deterioration effect of lignin as an ultraviolet absorbent in polypropylene and polyethylene. Polym. J. 2005, 37, 633. [Google Scholar] [CrossRef]
- Réti, C.; Casetta, M.; Duquesne, S.; Bourbigot, S.; Delobel, R. Flammability properties of intumescent PLA including starch and lignin. Polym. Adv. Technol. 2008, 19, 628–635. [Google Scholar] [CrossRef]
- Wang, J.; Deng, Y.; Qian, Y.; Qiu, X.; Ren, Y.; Yang, D. Reduction of lignin color via one-step UV irradiation. Green Chem. 2016, 18, 695–699. [Google Scholar] [CrossRef]
- Vishtal, A.G.; Kraslawski, A. Challenges in industrial applications of technical lignins. BioResources 2011, 6, 3547–3568. [Google Scholar] [CrossRef]
- Chakar, F.S.; Ragauskas, A.J. Review of current and future softwood kraft lignin process chemistry. Ind. Crop. Prod. 2004, 20, 131–141. [Google Scholar] [CrossRef]
- Matsushita, Y.; Yasuda, S. Preparation and evaluation of lignosulfonates as a dispersant for gypsum paste from acid hydrolysis lignin. Bioresour. Technol. 2005, 96, 465–470. [Google Scholar] [CrossRef]
- Mousavioun, P.; Doherty, W.O. Chemical and thermal properties of fractionated bagasse soda lignin. Ind. Crop. Prod. 2010, 31, 52–58. [Google Scholar] [CrossRef]
- Glasser, W.G. About making lignin great again—Some lessons from the past. Front. Chem. 2019, 7, 565. [Google Scholar] [CrossRef]
- Wang, K.; Bauer, S.; Sun, R.-C. Structural transformation of miscanthus × giganteus lignin fractionated under mild formosolv, basic organosolv, and cellulolytic enzyme conditions. J. Agric. Food Chem. 2012, 60, 144–152. [Google Scholar] [CrossRef]
- Watkins, D.; Nuruddin, M.; Hosur, M.; Tcherbi-Narteh, A.; Jeelani, S. Extraction and characterization of lignin from different biomass resources. J. Mater. Res. Technol. 2015, 4, 26–32. [Google Scholar] [CrossRef]
- Samuel, R.; Pu, Y.; Raman, B.; Ragauskas, A.J. Structural characterization and comparison of switchgrass ball-milled lignin before and after dilute acid pretreatment. Appl. Biochem. Biotechnol. 2010, 162, 62–74. [Google Scholar] [CrossRef]
- Xf, T.; Fang, Z.; Guo, F. Impact and prospective of fungal pre-treatment of lignocellulosic biomass for enzymatic hydrolysis. Biofuels Bioprod. Biorefin. 2012, 6, 335–350. [Google Scholar]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef]
- Mankar, A.R.; Modak, A.; Pant, K.K. Recent advances in the valorization of lignin: A key focus on pretreatment, characterization, and catalytic depolymerization strategies for future biorefineries. Adv. Sustain. Syst. 2022, 6, 2100299. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Huang, D.; Li, R.; Xu, P.; Li, T.; Deng, R.; Chen, S.; Zhang, Q. The cornerstone of realizing lignin value-addition: Exploiting the native structure and properties of lignin by extraction methods. J. Chem. Eng. 2020, 402, 126237. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Narron, R.H.; Kim, H.; Chang, H.M.; Jameel, H.; Park, S. Biomass pretreatments capable of enabling lignin valorization in a biorefinery process. Curr. Opin. Biotechnol. 2016, 38, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Lam, S.S.; Wu, Y.; Ge, S.; Wu, J.; Cai, L.; Huang, Z.; Le, Q.V.; Sonne, C.; Xia, C. Enzymatic conversion of pretreated lignocellulosic biomass: A review on influence of structural changes of lignin. Bioresour. Technol. 2021, 324, 124631. [Google Scholar] [CrossRef]
- Carvalho, D.M.; Colodette, J.L. Comparative study of acid hydrolysis of lignin and polysaccharides in biomasses. Bioresour. Technol. 2017, 12, 6907–6923. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, S.J.; Zhong, C.; Li, B.Z.; Yuan, Y.J. Alkali-based pretreatment-facilitated lignin valorization: A review. Ind. Eng. Chem. Res. 2020, 59, 16923–16938. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, J.; Wang, G.; Chen, G. Enzymatic hydrolysis of lignin by ligninolytic enzymes and analysis of the hydrolyzed lignin products. Bioresour. Technol. 2020, 304, 122975. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Jiang, B.; Chen, H.; Wu, W.; Wu, S.; Jin, Y.; Xiao, H. Recent advances in understanding the effects of lignin structural characteristics on enzymatic hydrolysis. Biotechnol. Biofuels 2021, 14, 205. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef]
- Rehman, M.S.U.; Kim, I.; Chisti, Y.; Han, J.I. Use of ultrasound in the production of bioethanol from lignocellulosic biomass. Energy Educ. Sci. Technol. Part A Energy Sci. Res. 2013, 30, 1391–1410. [Google Scholar]
- Luo, J.; Fang, Z.; Smith Jr, R.L. Ultrasound-enhanced conversion of biomass to biofuels. Prog. Energy Combust. Sci. 2014, 41, 56–93. [Google Scholar] [CrossRef]
- Bundhoo, Z.M.; Mohee, R. Ultrasound-assisted biological conversion of biomass and waste materials to biofuels: A review. Ultrason. Sonochem. 2018, 40, 298–313. [Google Scholar] [CrossRef]
- Madadi, M.; Abbas, A. Lignin degradation by fungal pretreatment: A review. J. Plant. Pathol. Microbiol. 2017, 8, 398–404. [Google Scholar]
- Zhang, X.S.; Yang, G.X.; Jiang, H.; Liu, W.J.; Ding, H.S. Mass production of chemicals from biomass-derived oil by directly atmospheric distillation coupled with co-pyrolysis. Sci. Rep. 2013, 3, 1120. [Google Scholar] [CrossRef]
- Dodds, D.R.; Gross, R.A. Chemicals from biomass. Science 2007, 318, 1250–1251. [Google Scholar] [CrossRef]
- Vispute, T.P.; Zhang, H.; Sanna, A.; Xiao, R.; Huber, G.W. Renewable chemical commodity feedstocks from integrated catalytic processing of pyrolysis oils. Science 2010, 330, 1222–1227. [Google Scholar] [CrossRef]
- Kageyama, H.; Osada, S.; Nakata, H.; Kubota, M.; Matsuda, H. Effect of coexisting inorganic chlorides on lead volatilization from CaO–SiO2–Al2O3 molten slag under municipal solid waste gasification and melting conditions. Fuel 2013, 103, 94–100. [Google Scholar] [CrossRef]
- Arena, U. Process and technological aspects of municipal solid waste gasification. A review. J. Waste Manag. 2012, 32, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Champagne, P.; Xu, C.C. Bio-crude production from secondary pulp/paper-mill sludge and waste newspaper via co-liquefaction in hot-compressed water. Energy 2011, 36, 2142–2150. [Google Scholar] [CrossRef]
- Hwang, I.H.; Aoyama, H.; Matsuto, T.; Nakagishi, T.; Matsuo, T. Recovery of solid fuel from municipal solid waste by hydrothermal treatment using subcritical water. J. Waste Manag. 2012, 32, 410–416. [Google Scholar] [CrossRef]
- Luo, S.; Xiao, B.; Hu, Z.; Liu, S. Effect of particle size on pyrolysis of single-component municipal solid waste in fixed bed reactor. Int. J. Hydrog. Energy 2010, 35, 93–97. [Google Scholar] [CrossRef]
- Ateş, F.; Miskolczi, N.; Borsodi, N. Comparision of real waste (MSW and MPW) pyrolysis in batch reactor over different catalysts. Part I: Product yields, gas and pyrolysis oil properties. Bioresour. Technol. 2013, 133, 443–454. [Google Scholar] [CrossRef]
- Bhaskar, T.; Matsui, T.; Kaneko, J.; Uddin, M.A.; Muto, A.; Sakata, Y. Novel calcium based sorbent (Ca-C) for the dehalogenation (Br, Cl) process during halogenated mixed plastic (PP/PE/PS/PVC and HIPS-Br) pyrolysis. Green Chem. 2002, 4, 372–375. [Google Scholar] [CrossRef]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Thermochemical conversion of lignin to functional materials: A review and future directions. Green Chem. 2015, 17, 4888–4907. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Al-Hinai, A.; Al-Muhtaseb, A.A.H.; Rooney, D.W. Conversion of biomass to biofuels and life cycle assessment: A review. Environ. Chem. Lett. 2021, 19, 4075–4118. [Google Scholar] [CrossRef]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Froling, M.; Antal, J.M.J.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub-and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Barbier, J.; Charon, N.; Dupassieux, N.; Loppinet-Serani, A.; Mahé, L.; Ponthus, J.; Courtiade, M.; Ducrozet, A.; Quoineaud, A.-A.; Cansell, F. Hydrothermal conversion of lignin compounds. A detailed study of fragmentation and condensation reaction pathways. Biomass Bioenergy 2012, 46, 479–491. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Classified separation of lignin hydrothermal liquefied products. Ind. Eng. Chem. Res. 2011, 50, 11288–11296. [Google Scholar] [CrossRef]
- Ehara, K.; Saka, S.; Kawamoto, H. Characterization of the lignin-derived products from wood as treated in supercritical water. J. Wood Sci. 2002, 48, 320–325. [Google Scholar] [CrossRef]
- Wang, H.J.; Zhao, Y.; Wang, C.; Fu, Y.; Guo, Q. Theoretical study on the pyrolysis process of lignin dimer model compounds. Acta Chim. Sin. 2009, 67, 893–900. [Google Scholar]
- Singh, R.; Prakash, A.; Balagurumurthy, B.; Bhaskar, T. Recent Advances in Thermo-Chemical Conversion of Biomass; Pandey, A., Bhaskar, T., Stöcker, M., Sukumaran, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 269–291. [Google Scholar]
- Czernik, S.; Bridgwater, A.V. Overview of applications of biomass fast pyrolysis oil. Energy Fuels 2004, 18, 590–659. [Google Scholar] [CrossRef]
- Fu, J.; Bai, L.; Chi, M.; Xu, X.; Chen, Z.; Yu, K. Study on the evolution pattern of the chemical structure of Fenton pretreated lignin during hydrothermal carbonization. J. Environ. Chem. Eng. 2022, 10, 107184. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Park, K.Y. Upgrading the characteristics of biochar from cellulose, lignin, and xylan for solid biofuel production from biomass by hydrothermal carbonization. J. Ind. Eng. Chem. 2016, 42, 95–100. [Google Scholar] [CrossRef]
- Xiao, L.P.; Shi, Z.J.; Xu, F.; Sun, R.C. Hydrothermal carbonization of lignocellulosic biomass. Bioresour. Technol. 2012, 118, 619–623. [Google Scholar] [CrossRef]
- Cagnon, B.; Py, X.; Guillot, A.; Stoeckli, F.; Chambat, G. Contributions of hemicellulose, cellulose and lignin to the mass and the porous properties of chars and steam activated carbons from various lignocellulosic precursors. Bioresour. Technol. 2009, 100, 292–298. [Google Scholar] [CrossRef]
- Broch, A.; Jena, U.; Hoekman, S.K.; Langford, J. Analysis of solid and aqueous phase products from hydrothermal carbonization of whole and lipid-extracted algae. Energies 2013, 7, 62–79. [Google Scholar] [CrossRef]
- Zhou, S.; Pecha, B.; van Kuppevelt, M.; McDonald, A.G.; Garcia-Perez, M. Slow and fast pyrolysis of Douglas-fir lignin: Importance of liquid-intermediate formation on the distribution of products. Biomass Bioenergy 2014, 66, 398–409. [Google Scholar] [CrossRef]
- Poveda-Giraldo, J.A.; Solarte-Toro, J.C.; Alzate, C.A.C. The potential use of lignin as a platform product in biorefineries: A review. Renew. Sustain. Energy Rev. 2021, 138, 110688. [Google Scholar] [CrossRef]
- Babu, B.V. Biomass pyrolysis: A state-of-the-art review. Biofuels Bioprod. Bior. 2008, 2, 393–414. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Peacocke, G.V.C. Fast pyrolysis processes for biomass. Renew. Sust. Energ. Rev. 2000, 4, 1–73. [Google Scholar] [CrossRef]
- Xiangyu, L.; Lu, S.; Yujue, W.; Yanqing, Y.; Chengwen, W.; Li, X.; Wang, Z. Catalytic fast pyrolysis of Kraft lignin with HZSM-5 zeolite for producing aromatic hydrocarbons, Frontiers Environ. Sci. Eng. 2012, 6, 295–303. [Google Scholar]
- Jegers, H.E.; Klein, M.T. Primary and secondary lignin pyrolysis reaction pathways. Ind. Eng. Chem. Process Des. Dev. 1985, 24, 173–183. [Google Scholar] [CrossRef]
- Wright, M.M.; Daugaard, D.E.; Satrio, J.A.; Brown, R.C. Techno-Economic Analysis of Biomass Fast Pyrolysis to Transportation Fuels. Fuel 2010, 89, S2–S10. [Google Scholar] [CrossRef]
- Kawamoto, H. Lignin pyrolysis reactions. J. Wood Sci. 2017, 6, 117–132. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Benali, M.; Périn-Levasseur, Z.; Savulescu, L.; Kouisni, L.; Jemaa, N.; Kudra, T.; Paleologou, M. Implementation of lignin-based biorefinery into a Canadian softwood kraft pulp mill: Optimal resources integration and economic viability assessment. Biomass Bioenergy 2014, 67, 473–482. [Google Scholar] [CrossRef]
- Windt, M.; Meier, D.; Marsman, J.H.; Heeres, H.J.; de Koning, S. Micro-pyrolysis of technical lignins in a new modular rig and product analysis by GC-MS/FID and GC x GC-TOFMS/FID. J. Anal. Appl. Pyrolysis 2009, 85, 38–46. [Google Scholar] [CrossRef]
- Pandey, M.P.; Kim, C.S. Lignin Depolymerization and Conversion: A Review of Thermochemical Methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Rahimi, Z.; Anand, A.; Gautam, S. An overview on thermochemical conversion and potential evaluation of biofuels derived from agricultural wastes. Energy Nexus 2022, 7, 100125. [Google Scholar] [CrossRef]
- Robinson, K.K. Reaction engineering of direct coal liquefaction. Energies 2009, 2, 976–10006. [Google Scholar] [CrossRef]
- Elliott, D.C.; Biller, P.; Ross, A.B.; Schmidt, A.J.; Jones, S.B. Hydrothermal liquefaction of biomass: Developments from batch to continuous process. Bioresour. Technol. 2015, 178, 147–156. [Google Scholar] [CrossRef]
- Xiu, S.; Shahbazi, A. Bio-oil production and upgrading research: A review. Renew. Sustain. Energy Rev. 2012, 16, 4406–4414. [Google Scholar] [CrossRef]
- Bensaid, S.; Conti, R.; Fino, D. Direct liquefaction of ligno-cellulosic residues for liquid fuel production. Fuel 2012, 94, 324–332. [Google Scholar] [CrossRef]
- Abdelaziz, O.Y.; Li, K.; Tunå, P.; Hulteberg, C.P. Continuous catalytic depolymerisation and conversion of industrial kraft lignin into low-molecular-weight aromatics. Biomass Convers. Biorefin. 2018, 8, 455–470. [Google Scholar] [CrossRef]
- Abdelaziz, O.Y.; Hulteberg, C.P. Lignin Depolymerization under Continuous-Flow Conditions: Highlights of Recent Developments. ChemSusChem 2020, 13, 4382–4384. [Google Scholar] [CrossRef]
- Rana, M.; Islam, M.N.; Agarwal, A.; Taki, G.; Park, S.J.; Dong, S.; Jo, Y.-T.; Park, J.H. Production of phenol-rich monomers from kraft lignin hydrothermolysates in basic-subcritical water over MoO3/SBA-15 catalyst. Energy Fuels 2018, 32, 11564–11575. [Google Scholar] [CrossRef]
- Feng, L.; Li, X.; Wang, Z.; Liu, B. Catalytic hydrothermal liquefaction of lignin for production of aromatic hydrocarbon over metal supported mesoporous catalyst. Bioresour. Technol. 2021, 323, 124569. [Google Scholar] [CrossRef]
- Lawoko, M.; Samec, J.S. Kraft lignin valorization: Biofuels and thermoset materials in focus. Curr. Opin. Green Sustain. Chem. 2022, 40, 100738. [Google Scholar] [CrossRef]
- Durak, H. Bio-oil production from biomass via supercritical fluid extraction. AIP Conf. Proc. 2016, 1726, 020099. [Google Scholar]
- Akalın, M.K.; Tekin, K.; Karagöz, S. Supercritical fluid extraction of biofuels from biomass. Environ. Chem. Lett. 2017, 15, 29–41. [Google Scholar] [CrossRef]
- Durak, H. Bio-oil production from Glycyrrhiza glabra through supercritical fluid extraction. J. Supercrit. Fluids 2014, 95, 373–386. [Google Scholar] [CrossRef]
- Ibarra-Gonzalez, P.; Rong, B.G. A review of the current state of biofuels production from lignocellulosic biomass using thermochemical conversion routes. Chin. J. Chem. Eng. 2019, 27, 1523–1535. [Google Scholar] [CrossRef]
- Mu, W.; Ben, H.; Ragauskas, A.; Deng, Y. Lignin pyrolysis components and upgrading—Technology review. Bioenergy Res. 2013, 6, 1183–1204. [Google Scholar] [CrossRef]
- Roberts, V.M.; Stein, V.; Reiner, T.; Lemonidou, A.; Li, X.; Lercher, J.A. Towards Quantitative Catalytic Lignin Depolymerization. Chem. Eur. J. 2011, 17, 5939–5948. [Google Scholar] [CrossRef]
- Osada, M.; Sato, T.; Watanabe, M.; Shirai, M.; Arai, K. Catalytic gasification of wood biomass in subcritical and supercritical water. Combust. Sci. Technol. 2006, 178, 537–552. [Google Scholar] [CrossRef]
- Kijima, M.; Hirukawa, T.; Hanawa, F.; Hata, T. Thermal conversion of alkaline lignin and its structured derivatives to porous carbonized materials. Bioresour. Technol. 2011, 102, 6279–6285. [Google Scholar] [CrossRef]
- Sturgeon, M.R.; O’Brien, M.H.; Ciesielski, P.N.; Katahira, R.; Kruger, J.S.; Chmely, S.C.; Hamlin, J.; Kelsey, L.; Hunsinger, G.B.; Beckham, G.T.; et al. Lignin depolymerisation by nickel supported layered-double hydroxide catalysts. Green Chem. 2014, 16, 824–835. [Google Scholar] [CrossRef]
- Wang, X.; Rinaldi, R. Solvent effects on the hydrogenolysis of diphenyl ether with Raney nickel and their implications for the conversion of lignin. ChemSusChem 2012, 5, 1455–1466. [Google Scholar] [CrossRef]
- Azadi, P.; Inderwildi, O.R.; Farnood, R.; King, D.A. Liquid fuels, hydrogen and chemicals from lignin: A critical review. Renew. Sustain. Energy Rev. 2013, 21, 506–523. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, Y.; Liu, S.; Zhou, N.; Chen, P.; Cheng, Y.; Addy, M.; Lu, Q.; Omar, M.M.; Liu, Y. Bio-oil from fast pyrolysis of lignin: Effects of process and upgrading parameters. Bioresour. Technol. 2017, 241, 1118–1126. [Google Scholar] [CrossRef]
- Saidi, M.; Samimi, F.; Karimipourfard, D.; Nimmanwudipong, T.; Gates, B.C.; Rahimpour, M.R. Upgrading of lignin-derived bio-oils by catalytic hydrodeoxygenation. Energy Environ. Sci. 2014, 7, 103–129. [Google Scholar] [CrossRef]
- McCarty, T.; Sesmero, J. Uncertainty, irreversibility, and investment in second-generation biofuels. BioEnergy Res. 2015, 8, 675–687. [Google Scholar] [CrossRef]
- Diao, X.; Ji, N. Rational design of MoS2-based catalysts toward lignin hydrodeoxygenation: Interplay of structure, catalysis, and stability. J. Energy Chem. 2022, 77, 601–631. [Google Scholar] [CrossRef]
- Elliott, D.C. Historical developments in hydroprocessing bio-oils. Energy Fuels 2007, 21, 1792–1815. [Google Scholar] [CrossRef]
- Furimsky, E. Catalytic hydrodeoxygenation. Appl. Catal. A Gen. 2000, 199, 147–190. [Google Scholar] [CrossRef]
- Li, X.; Chen, G.; Liu, C.; Ma, W.; Yan, B.; Zhang, J. Hydrodeoxygenation of lignin-derived bio-oil using molecular sieves supported metal catalysts: A critical review. Renew. Sustain. Energy Rev. 2017, 71, 296–308. [Google Scholar] [CrossRef]
- Laskar, D.D.; Yang, B.; Wang, H.; Lee, J. Pathways for biomass-derived lignin to hydrocarbon fuels. Biofuels Bioprod. Biorefin. 2013, 7, 602–626. [Google Scholar] [CrossRef]
- Do, P.T.; Foster, A.J.; Chen, J.; Lobo, R.F. Bimetallic effects in the hydrodeoxygenation of meta-cresol on γ-Al2O3 supported Pt–Ni and Pt–Co catalysts. Green Chem. 2012, 14, 1388–1397. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Wang, S. Hydrodeoxygenation of bio-oil over Pt-based supported catalysts: Importance of mesopores and acidity of the support to compounds with different oxygen contents. RSC Adv. 2013, 3, 12635–12640. [Google Scholar] [CrossRef]
- Ruiz, P.E.; Frederick, B.G.; De Sisto, W.J.; Austin, R.N.; Radovic, L.R.; Leiva, K.; Garcia, R.; Escalona, N.; Wheeler, M.C. Guaiacol hydrodeoxygenation on MoS2 catalysts: Influence of activated carbon supports. Catal. Commun. 2012, 27, 44–48. [Google Scholar] [CrossRef]
- Ghampson, I.T.; Sepúlveda, C.; Garcia, R.; Radovic, L.R.; Fierro, J.G.; DeSisto, W.J.; Escalona, N. Hydrodeoxygenation of guaiacol over carbon-supported molybdenum nitride catalysts: Effects of nitriding methods and support properties. Appl. Catal. A Gen. 2012, 439, 111–124. [Google Scholar] [CrossRef]
- Li, K.; Wang, R.; Chen, J. Hydrodeoxygenation of anisole over silica-supported Ni2P, MoP, and NiMoP catalysts. Energy Fuels 2011, 25, 854–863. [Google Scholar] [CrossRef]
- Olcese, R.N.; Bettahar, M.; Petitjean, D.; Malaman, B.; Giovanella, F.; Dufour, A. Gas-phase hydrodeoxygenation of guaiacol over Fe/SiO2 catalyst. Appl. Catal. B Environ. 2012, 115, 63–73. [Google Scholar] [CrossRef]
- Ambursa, M.M.; Juan, J.C.; Yahaya, Y.; Taufiq-Yap, Y.H.; Lin, Y.C.; Lee, H.V. A review on catalytic hydrodeoxygenation of lignin to transportation fuels by using nickel-based catalysts. Renew. Sustain. Energy Rev. 2021, 138, 110667. [Google Scholar] [CrossRef]
- Jan, O.; Marchand, R.; Anjos, L.C.; Seufitelli, G.V.; Nikolla, E.; Resende, F.L. Hydropyrolysis of lignin using Pd/HZSM-5. Energy Fuels 2015, 29, 1793–1800. [Google Scholar] [CrossRef]
- Wang, H.; Ben, H.; Ruan, H.; Zhang, L.; Pu, Y.; Feng, M.; Ragauskas, A.J.; Yang, B. Effects of lignin structure on hydrodeoxygenation reactivity of pine wood lignin to valuable chemicals. ACS Sustain. Chem. Eng. 2017, 5, 1824–1830. [Google Scholar] [CrossRef]
- Yang, S.; Shi, C.; Shen, Z.; Pan, L.; Huang, Z.; Zhang, X.; Zou, J.J. Conversion of lignin oil and hemicellulose derivative into high-density jet fuel. J. Energy Chem. 2023, 77, 452–460. [Google Scholar] [CrossRef]
- Zhong, Z.; Li, J.; Jian, M.; Shu, R.; Tian, Z.; Wang, C.; Chen, Y.; Shi, N.; Wu, Y. Hydrodeoxygenation of lignin-derived phenolic compounds over Ru/TiO2 catalyst: Effect of TiO2 morphology. Fuel 2023, 333, 126241. [Google Scholar] [CrossRef]
- Dwiatmoko, A.A.; Zhou, L.; Kim, I.; Choi, J.W.; Suh, D.J.; Ha, J.M. Hydrodeoxygenation of lignin-derived monomers and lignocellulose pyrolysis oil on the carbon-supported Ru catalysts. Catal. Today 2016, 265, 192–198. [Google Scholar] [CrossRef]
- Han, Y.; Gholizadeh, M.; Tran, C.C.; Kaliaguine, S.; Li, C.Z.; Olarte, M.; Garcia-Perez, M. Hydrotreatment of pyrolysis bio-oil: A review. Fuel Process. Technol. 2019, 195, 106140. [Google Scholar] [CrossRef]
- Lazaridis, P.A.; Fotopoulos, A.P.; Karakoulia, S.A.; Triantafyllidis, K.S. Catalytic fast pyrolysis of kraft lignin with conventional, mesoporous and nanosized ZSM-5 zeolite for the production of alkyl-phenols and aromatics. Front. Chem. 2018, 6, 295. [Google Scholar] [CrossRef]
- Valle, B.; Palos, R.; Bilbao, J.; Gayubo, A.G. Role of zeolite properties in bio-oil deoxygenation and hydrocarbons production by catalytic cracking. Fuel Process. Technol. 2022, 227, 107130. [Google Scholar] [CrossRef]
- Rezaei, P.S.; Shafaghat, H.; Daud WM, A.W. Production of green aromatics and olefins by catalytic cracking of oxygenate compounds derived from biomass pyrolysis: A review. Appl. Catal. 2014, 469, 490–511. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, Y.; He, T.; Hu, H.; Wu, J. Hydrodeoxygenation of dibenzofuran over noble metal supported on mesoporous zeolite. Catal. Commun. 2011, 12, 1201–1205. [Google Scholar] [CrossRef]
- Akhade, S.A.; Singh, N.; Gutiérrez, O.Y.; Lopez-Ruiz, J.; Wang, H.; Holladay, J.D.; Liu, Y.; Karkamkar, A.; Weber, R.S.; Glezakou, V.A.; et al. Electrocatalytic hydrogenation of biomass-derived organics: A review. Chem. Rev. 2020, 120, 11370–11419. [Google Scholar] [CrossRef]
- Yan, Z.P.; Lin, L.; Liu, S. Synthesis of γ-valerolactone by hydrogenation of biomass-derived levulinic acid over Ru/C catalyst. Energy Fuels 2009, 23, 3853–3858. [Google Scholar] [CrossRef]
- Rinaldi, R. Catalytic Hydrogenation for Biomass Valorization; Royal Society of Chemistry: London, UK, 2015; Volume 13. [Google Scholar]
- Yan, N.; Zhao, C.; Dyson, P.J.; Wang, C.; Liu, L.T.; Kou, Y. Selective degradation of wood lignin over noble-metal catalysts in a two-step process. ChemSusChem 2008, 1, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Petrus, L.; Noordermeer, M.A. Biomass to biofuels, a chemical perspective. Green Chem. 2006, 8, 861–867. [Google Scholar] [CrossRef]
- Shu, R.; Xu, Y.; Chen, P.; Ma, L.; Zhang, Q.; Zhou, L.; Wang, C. Mild hydrogenation of lignin depolymerization products over Ni/SiO2 catalyst. Energy Fuels 2017, 31, 7208–7213. [Google Scholar] [CrossRef]
- Vriamont, C.E.; Chen, T.; Romain, C.; Corbett, P.; Manageracharath, P.; Peet, J.; Conifer, C.M.; Hallett, J.P.; Britovsek, G.J.P. From lignin to chemicals: Hydrogenation of lignin models and mechanistic insights into hydrodeoxygenation via low-temperature C–O bond cleavage. ACS Catal. 2019, 9, 2345–2354. [Google Scholar] [CrossRef]
- Song, Q.; Wang, F.; Cai, J.; Wang, Y.; Zhang, J.; Yu, W.; Xu, J. Lignin depolymerization (LDP) in alcohol over nickel-based catalysts via a fragmentation–hydrogenolysis process. Energy Environ. Sci. 2013, 6, 994–1007. [Google Scholar] [CrossRef]
- Shu, R.; Long, J.; Yuan, Z.; Zhang, Q.; Wang, T.; Wang, C.; Ma, L. Efficient and product-controlled depolymerization of lignin oriented by metal chloride cooperated with Pd/C. Bioresour. Technol. 2015, 179, 84–90. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, Y.; Fan, J.; Chang, J. Selective production of 4-ethylphenolics from lignin via mild hydrogenolysis. Bioresour. Technol. 2012, 118, 648–651. [Google Scholar] [CrossRef]
- Singh, S.K.; Ekhe, J.D. Towards effective lignin conversion: HZSM-5 catalyzed one-pot solvolytic depolymerization/hydrodeoxygenation of lignin into value added compounds. RSC Adv. 2014, 4, 27971–27978. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, B.; Li, C.; Peng, C.; Dai, T.; Xie, H.; Wang, A.; Zhang, T. Tungsten carbide: A remarkably efficient catalyst for the selective cleavage of lignin C− O bonds. ChemSusChem 2016, 9, 3220–3229. [Google Scholar] [CrossRef]
- Barta, K.; Warner, G.R.; Beach, E.S.; Anastas, P.T. Depolymerization of organosolv lignin to aromatic compounds over Cu-doped porous metal oxides. Green Chem. 2014, 16, 191–196. [Google Scholar] [CrossRef]
- Hermansson, F.; Janssen, M.; Svanström, M. Allocation in life cycle assessment of lignin. Int. J. Life Cycle Assess. 2020, 25, 1620–1632. [Google Scholar] [CrossRef]
- Beaucamp, A.; Muddasar, M.; Amiinu, I.S.; Leite, M.M.; Culebras, M.; Latha, K.; Gutierrez, M.C.; Rodriguez-Padron, D.; del Monte, F.; Collins, M.N. Lignin for energy applications–state of the art, life cycle, technoeconomic analysis and future trends. Green Chem. 2022, 24, 8193–8226. [Google Scholar] [CrossRef]
- Kosamia, N.M.; Samavi, M.; Piok, K.; Rakshit, S.K. Perspectives for scale up of biorefineries using biochemical conversion pathways: Technology status, techno-economic, and sustainable approaches. Fuel 2022, 324, 124532. [Google Scholar] [CrossRef]
- Budsberg, E.; Crawford, J.T.; Morgan, H.; Chin, W.S.; Bura, R.; Gustafson, R. Hydrocarbon bio-jet fuel from bioconversion of poplar biomass: Life cycle assessment. Biotechnol. Biofuels 2016, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Dang, Q.; Smith, R.; Brown, R.C.; Wright, M.M. Techno-economic analysis of the stabilization of bio-oil fractions for insertion into petroleum refineries. ACS Sustain. Chem. Eng. 2017, 5, 1528–1537. [Google Scholar] [CrossRef]
- Obydenkova, S.V.; Kouris, P.D.; Hensen, E.J.; Heeres, H.J.; Boot, M.D. Environmental economics of lignin derived transport fuels. Bioresour. Technol. 2017, 243, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; AlNouss, A.; Parthasarathy, P.; Abdelaal, A.H.; Mackey, H.; McKay, G.; Al-Ansari, T. Investigation of biomass components on the slow pyrolysis products yield using Aspen Plus for techno-economic analysis. Biomass Convers. Biorefin. 2020, 12, 669–681. [Google Scholar] [CrossRef]
- Shen, R.; Tao, L.; Yang, B. Techno-economic analysis of jet-fuel production from biorefinery waste lignin. Biofuels Bioprod. Biorefin. 2019, 13, 486–501. [Google Scholar] [CrossRef]
- Kumaniaev, I.; Navare, K.; Mendes, N.C.; Placet, V.; Van Acker, K.; Samec, J.S. Conversion of birch bark to biofuels. Green Chem. 2020, 22, 2255–2263. [Google Scholar] [CrossRef]
- Moretti, C.; Corona, B.; Hoefnagels, R.; Vural-Gürsel, I.; Gosselink, R.; Junginger, M. Review of life cycle assessments of lignin and derived products: Lessons learned. Sci. Total Environ. 2021, 770, 144656. [Google Scholar] [CrossRef] [PubMed]
| Process | Process Requirements | Product | Advantages and Disadvantages |
|---|---|---|---|
| Hydrothermal Carbonization | 180–250 °C several hours | Gas Liquid Hydrochar |
|
| Pyrolysis | 350–700 °C >30 min | Bio-oil Biochar Gas |
|
| Biomass Liquefaction | 300–400 °C 0.2–1.0 h 5–20 MPa | Bio-oil |
|
| Supercritical Fluid Extraction | 250–400 °C | Bio-oil |
|
| Gasification | >700 °C atm pressure | Syngas Fuel gas |
|
| Process | Process Requirements | Advantages and Disadvantages |
|---|---|---|
| Hydrodeoxygenation | 120–400 °C 10–20 MPa hydrogen pressure. |
|
| Zeolite Cracking | 350–500 °C atmospheric pressure |
|
| Hydrogenation | Exposure to hydrogen in a liquid phase at 100 °C to 500 °C with suitable catalyst. |
|
| Catalyst | Temperature | Yield | Reference |
|---|---|---|---|
| Ni/C | 200 °C | 54% | [172] |
| Pd/C with CrCl3 | 280 °C | 78.2–83.2% | [173] |
| Ru/C | 200–250 °C | 76.7% | [174] |
| HZSM-5 with NaOH | 220 °C | 61% | [175] |
| W2C/AC | 260 °C | 12.7% | [176] |
| Porous Cu–Mg–Al oxide | 140–220 °C | 63% | [177] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocaturk, E.; Salan, T.; Ozcelik, O.; Alma, M.H.; Candan, Z. Recent Advances in Lignin-Based Biofuel Production. Energies 2023, 16, 3382. https://doi.org/10.3390/en16083382
Kocaturk E, Salan T, Ozcelik O, Alma MH, Candan Z. Recent Advances in Lignin-Based Biofuel Production. Energies. 2023; 16(8):3382. https://doi.org/10.3390/en16083382
Chicago/Turabian StyleKocaturk, Engin, Tufan Salan, Orhan Ozcelik, Mehmet Hakkı Alma, and Zeki Candan. 2023. "Recent Advances in Lignin-Based Biofuel Production" Energies 16, no. 8: 3382. https://doi.org/10.3390/en16083382
APA StyleKocaturk, E., Salan, T., Ozcelik, O., Alma, M. H., & Candan, Z. (2023). Recent Advances in Lignin-Based Biofuel Production. Energies, 16(8), 3382. https://doi.org/10.3390/en16083382