Techno-Economic Assessment of High-Safety and Cost-Effective Syngas Produced by O2-Enriched Air Gasification with 40–70% O2 Purity
Abstract
:1. Introduction
2. Process Configuration
- (1)
- O2-enriched air gasification (OAG)
- (2)
- Vacuum Pressure Swing Adsorption (VPSA)
- (3)
- Producer Gas Cleaning (PGC)
- (4)
- Methanation Synthesis
3. Assessment Methodology
3.1. Requirements on Syngas
3.2. Exergy Analysis
3.3. Economic Assessment
3.4. Sensitivity Analysis
4. Results and Discussion
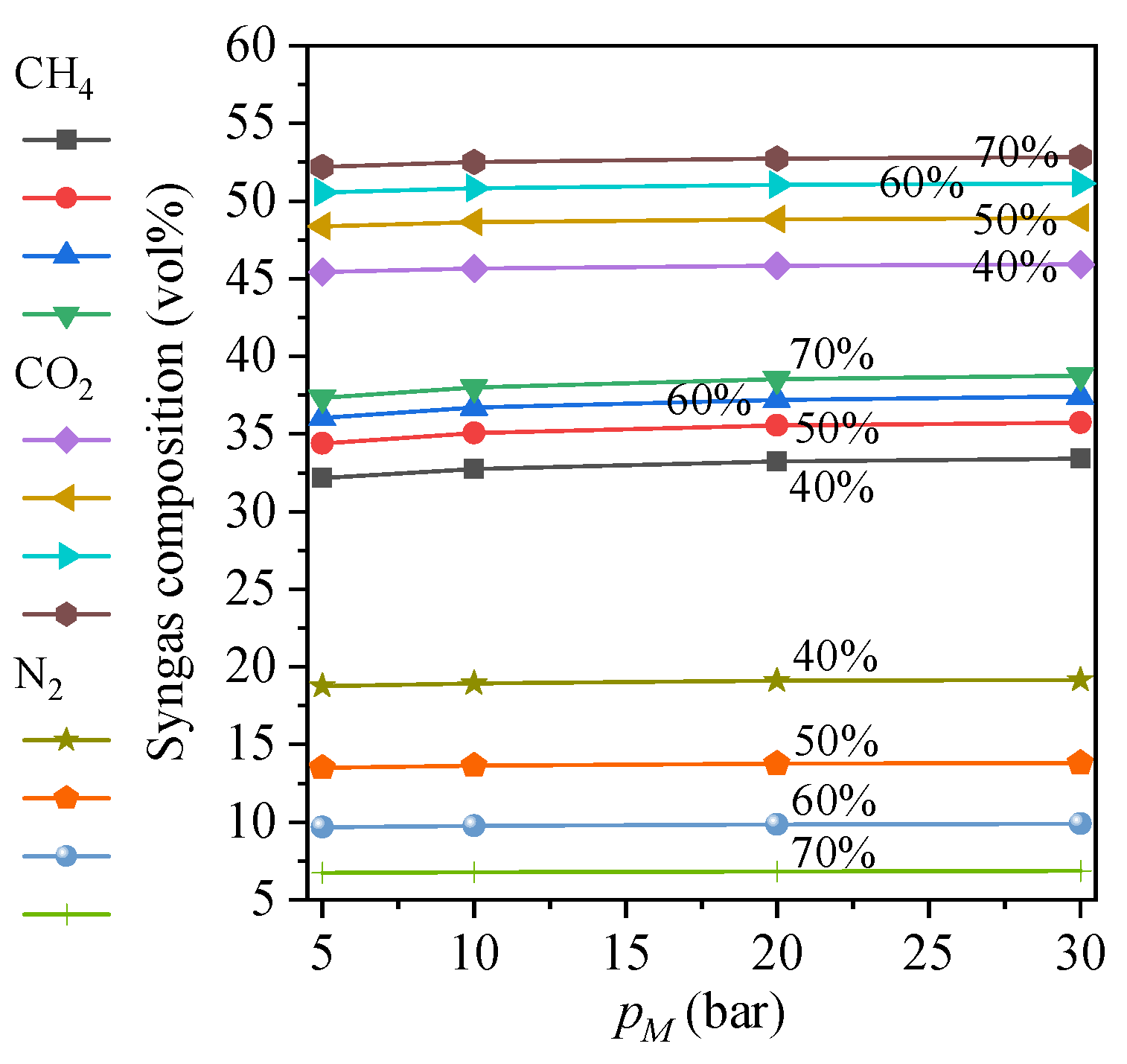
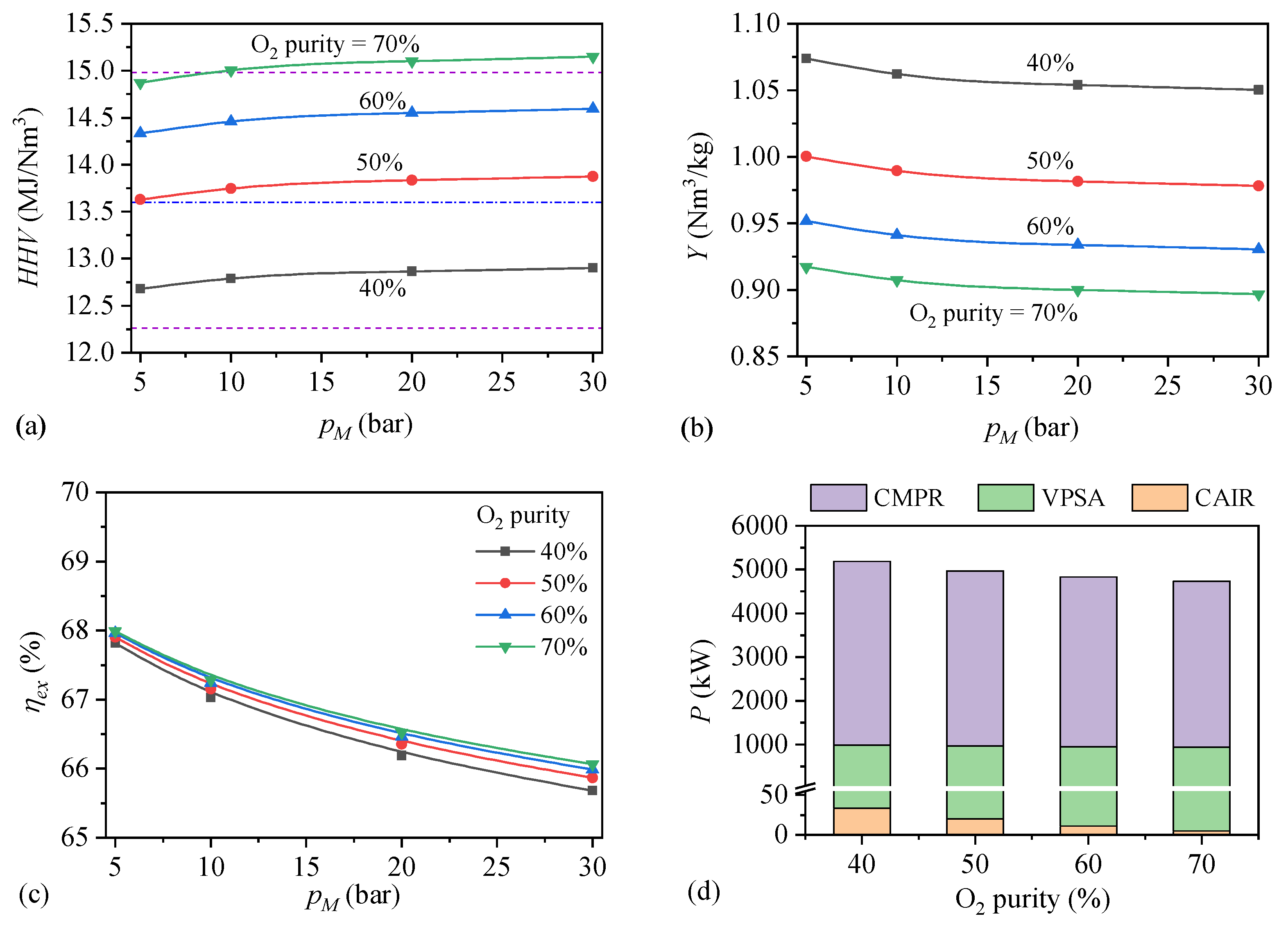
4.1. Technical Performances
- (1)
- Effects of O2 purity and methanation pressure
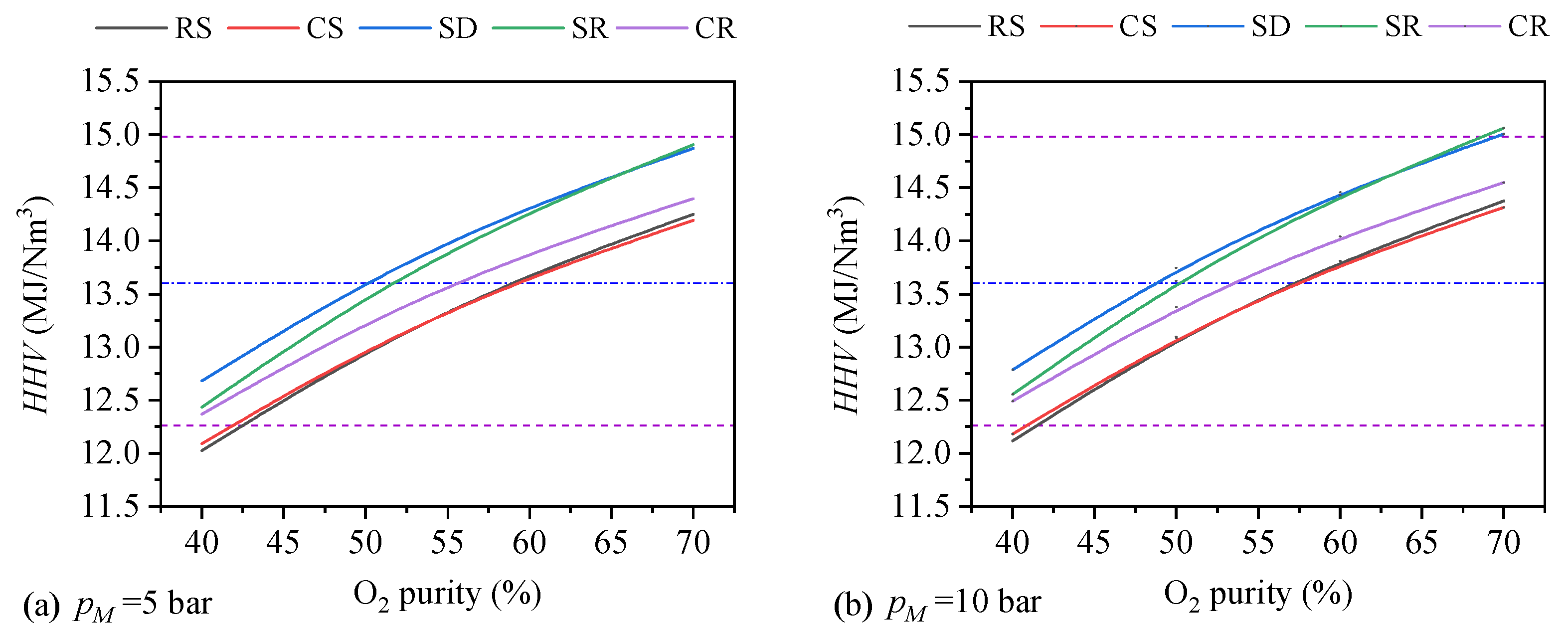
- (2)
- Syngas properties of different feedstock
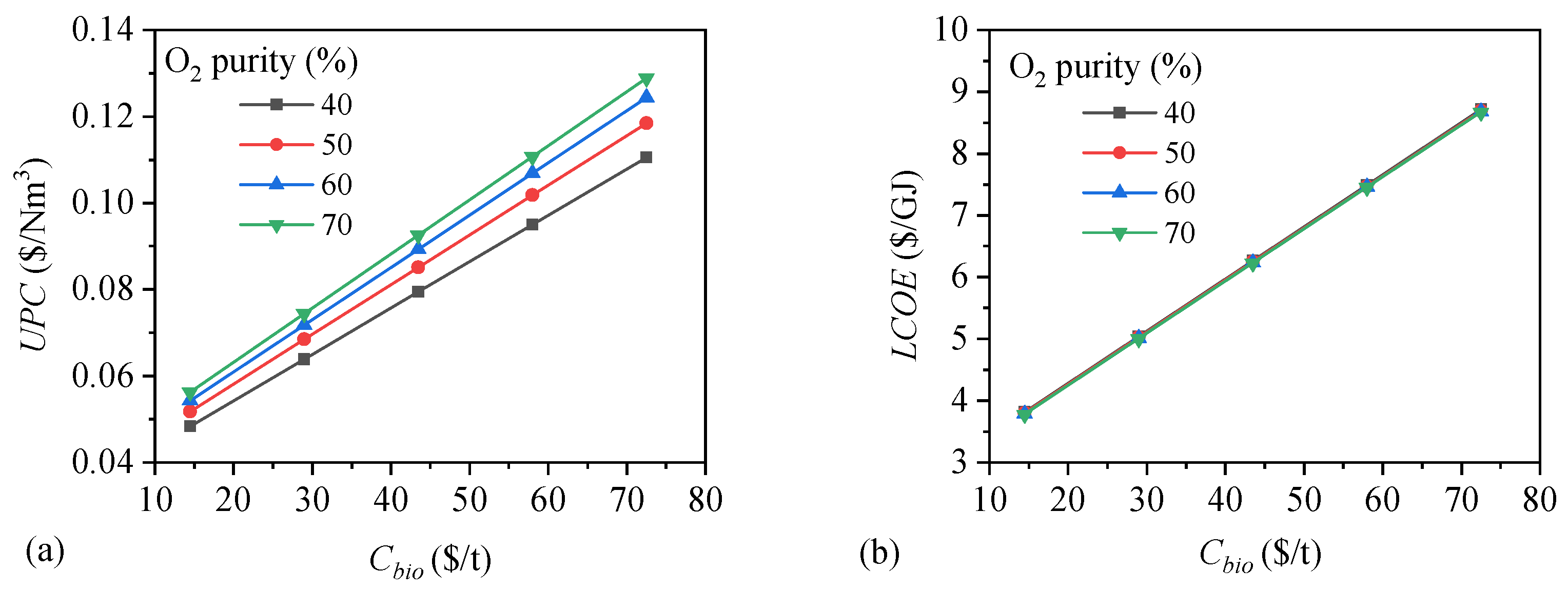
4.2. Economic Performances
- (1)
- Feedstock Cost
- (2)
- Plant Scale
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bailera, M.; Lisbona, P.; Romeo, L.M.; Espatolero, S. Power to Gas projects review: Lab, pilot and demo plants for storing renewable energy and CO2. Renew. Sustain. Energy Rev. 2017, 69, 292–312. [Google Scholar] [CrossRef]
- Prussi, M.; Padella, M.; Conton, M.; Postma, E.D.; Lonza, L. Review of technologies for biomethane production and assessment of Eu transport share in 2030. J. Clean. Prod. 2019, 222, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Xiao, J.; Yu, Y.; Shen, L. A techno-economic assessment of SNG production from agriculture residuals in China. Energy Sources Part B Econ. Plan. Policy 2016, 11, 465–471. [Google Scholar] [CrossRef]
- Haro, P.; Johnsson, F.; Thunman, H. Improved syngas processing for enhanced Bio-SNG production: A techno-economic assessment. Energy 2016, 101, 380–389. [Google Scholar] [CrossRef]
- Cui, X.; Song, G.; Yao, A.; Wang, H.; Wang, L.; Xiao, J. Technical and Economic Assessments of a novel biomass-to-synthetic natural gas (SNG) process integrating O2-enriched air gasification. Process Saf. Environ. Prot. 2021, 156, 417–428. [Google Scholar] [CrossRef]
- Song, G.; Wang, L.; Yao, A.; Cui, X.; Xiao, J. Technical and Economic Assessment of a High-Quality Syngas Production Process Integrating Oxygen Gasification and Water Electrolysis: The Chinese Case. ACS Omega 2021, 6, 27851–27864. [Google Scholar] [CrossRef]
- Wang, Z.; He, T.; Qin, J.; Wu, J.; Li, J.; Zi, Z.; Liu, G.; Wu, J.; Sun, L. Gasification of biomass with oxygen-enriched air in a pilot scale two-stage gasifier. Fuel 2015, 150, 386–393. [Google Scholar] [CrossRef]
- Nam, H.; Maglinao, A.L.; Capareda, S.C.; Rodriguez-Alejandro, D.A. Enriched-air fluidized bed gasification using bench and pilot scale reactors of dairy manure with sand bedding based on response surface methods. Energy 2016, 95, 187–199. [Google Scholar] [CrossRef]
- Gu, H.; Tang, Y.; Yao, J.; Chen, F. Study on biomass gasification under various operating conditions. J. Energy Inst. 2019, 92, 1329–1336. [Google Scholar] [CrossRef]
- Song, G.; Xiao, J.; Yan, C.; Gu, H.; Zhao, H. Quality of gaseous biofuels: Statistical assessment and guidance on production technologies. Renew. Sustain. Energy Rev. 2022, 169, 112959. [Google Scholar] [CrossRef]
- Feng, F.; Song, G.; Shen, L.; Xiao, J. Environmental benefits analysis based on life cycle assessment of rice straw-based synthetic natural gas in China. Energy 2017, 139, 341–349. [Google Scholar] [CrossRef]
- Ying, T.; Cao, H. Progress of biomass gasification technology. Chinese J. Bioprocess Eng. 2017, 15, 57–65. (In Chinese) [Google Scholar] [CrossRef]
- Ye, R.; Liu, J.; Li, X.; Zheng, L.; Li, M.; Wang, G.; Zhang, D. Experimental study on biomass of bubbling testbed with steam-oxygen enriched air gasification of directional. Acta Energiae Solaris Sin. 2016, 37, 1636–1642. [Google Scholar]
- Air-Liquide Standard Plants Fully-Packaged Modular Solutions. Available online: https://www.engineering-airliquide.com/sites/activity_eandc/files/2017/04/12/air-liquide-brochure-standard-plants-170418.pdf (accessed on 15 February 2021).
- Beijing-Peking-University- Pioneer-Technology-Co., -Ltd Vacuum Pressure Swing Adsorption Oxygen Plant (VPSA-O2). Available online: https://www.vpsatech.com/VPSA-Oxygen.html#local (accessed on 15 February 2021).
- Liu, X.; Dou, Q.; Min, W.; Chen, T.; Xue, Z. Pressure swing adsorption for oxygen generation and its application in EAF steelmaking. Steelmaking 2020, 36, 6–10. [Google Scholar]
- Vilela, C.M.; Boymans, E.; Vreugdenhil, B. Co-production of aromatics in biomass and waste gasification. Processes 2021, 9, 463. [Google Scholar] [CrossRef]
- Zwart, R.W.R.; Boerrigter, H.; Deurwaarder, E.P.; van der Meijden, C.M.; Paasen, S.V.B. Production of Synthetic Natural Gas (SNG) from Biomass. Available online: https://www.biosng.com/fileadmin/biosng/user/documents/reports/e06018.pdf (accessed on 19 September 2021).
- Seemann, M.C.; Schildhauer, T.J.; Biollaz, S.M.A. Fluidized bed methanation of wood-derived producer gas for the production of synthetic natural gas. Ind. Eng. Chem. Res. 2010, 49, 7034–7038. [Google Scholar] [CrossRef]
- Gassner, M.; Maréchal, F. Thermo-economic process model for thermochemical production of Synthetic Natural Gas (SNG) from lignocellulosic biomass. Biomass Bioenergy 2009, 33, 1587–1604. [Google Scholar] [CrossRef] [Green Version]
- GB/T 13611-2018; Classification and Basic Characteristics of City Gas. Technical-Standard; Standards Press of China: Beijing, China, 2018.
- Szargut, J. Exergy Method: Technical and Ecological Applications; WIT Press: Southampton, UK, 2005. [Google Scholar]
- Song, G.; Xiao, J.; Zhao, H.; Shen, L. A unified correlation for estimating specific chemical exergy of solid and liquid fuels. Energy 2012, 40, 164–173. [Google Scholar] [CrossRef]
- Watson, J.; Zhang, Y.; Si, B.; Chen, W.T.; de Souza, R. Gasification of biowaste: A critical review and outlooks. Renew. Sustain. Energy Rev. 2018, 83, 1–17. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Q.; Du, J.; Chen, J. Oxygen-enriched air gasification of biomass materials for high-quality syngas production. Energy Convers. Manag. 2019, 199, 111628. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, L.; Sheng, X.; Liu, M.; Xu, Y.; Tang, Y.; Wang, Q.; Ma, Q.; Zuo, J. Life Cycle Cost of Electricity Production: A Comparative Study of Coal-Fired, Biomass, and Wind Power in China. Energies 2021, 14, 3463. [Google Scholar] [CrossRef]


| Composition | RS | CS | SD | SR | CR |
|---|---|---|---|---|---|
| Proximate analysis ar | |||||
| Moisture | 9.10 | 9.36 | 7.89 | 8.10 | 5.40 |
| Fixed Carbon | 16.75 | 16.75 | 14.77 | 76.40 | 75.80 |
| Volatile Matter | 63.68 | 70.74 | 75.78 | 12.20 | 12.30 |
| Ash | 10.46 | 3.15 | 1.56 | 3.30 | 6.50 |
| Ultimate analysis daf | |||||
| Carbon | 43.97 | 44.85 | 44.24 | 50.19 | 42.10 |
| Hydrogen | 5.99 | 5.63 | 6.20 | 6.10 | 6.00 |
| Nitrogen | 1.20 | 0.92 | 0.99 | 0.02 | 3.09 |
| Sulfur | 0.17 | 0.13 | 0.11 | 0.00 | 0.00 |
| Oxygen | 48.67 | 48.47 | 48.46 | 43.69 | 48.80 |
| Item | HHV (MJ/Nm3) | |
|---|---|---|
| Class | 3T | 4T |
| Upper Value | 14.98 | 19.04 |
| Standard Value | 13.62 | 17.31 |
| Lower Value | 12.26 | 15.58 |
| Equipment | Benchmark | PC | EC (Million $) |
|---|---|---|---|
| Two-stage Gasifier | Biomass | 25 t/h | 3.266 |
| Gas Cleaning | Syngas | 30.7 t/h | 2.025 |
| Compressors | Syngas | 30.7 t/h | 0.494 |
| Methanation reactor | Syngas | 30.7 t/h | 0.877 |
| Heat exchangers | Water | 143 t/h | 0.119 |
| ORC | Output | 2.39 MW | 0.510 |
| VPSA | O2 stream | 2510 m3/h | 0.809 |
| Item | O2 Purity | |||
|---|---|---|---|---|
| 40% | 50% | 60% | 70% | |
| Producer gas | ||||
| CH4 (vol.%) | 6.7 | 7.0 | 7.2 | 7.4 |
| H2 (vol.%) | 32.9 | 34.5 | 35.7 | 36.6 |
| CO (vol.%) | 28.6 | 30.0 | 31.1 | 31.8 |
| CO2 (vol.%) | 17.5 | 18.3 | 18.9 | 19.3 |
| N2 (vol.%) | 14.2 | 10.0 | 7.1 | 4.8 |
| UEL (%) | 63.2 | 62.1 | 61.3 | 60.7 |
| LEL (%) | 8.2 | 7.9 | 7.6 | 7.5 |
| Toxicity | P | P | P | P |
| Syngas | ||||
| CH4 (vol.%) | 30.8 | 33.1 | 34.9 | 36.2 |
| H2 (vol.%) | 3.6 | 3.6 | 3.6 | 3.7 |
| CO (vol.%) | 0.02 | 0.02 | 0.03 | 0.03 |
| CO2 (vol.%) | 45.2 | 48.4 | 50.8 | 52.6 |
| N2 (vol.%) | 20.4 | 14.8 | 10.6 | 7.3 |
| UEL (%) | 36.3 | 34.6 | 33.5 | 32.6 |
| LEL (%) | 13.0 | 12.3 | 11.8 | 11.4 |
| Toxicity | E | E | E | E |
| Feedstock | Composition (vol.%) | HHV | Y | ηex | ||
|---|---|---|---|---|---|---|
| CH4 | H2 | CO | (MJ/Nm3) | (Nm3/kgdaf) | (%) | |
| RS | 33.28 | 3.64 | 0.03 | 13.69 | 0.956 | 66.96 |
| CS | 33.24 | 3.57 | 0.03 | 13.67 | 0.964 | 67.10 |
| SD | 34.89 | 3.63 | 0.03 | 14.33 | 0.952 | 67.95 |
| SR | 34.80 | 3.55 | 0.03 | 14.28 | 1.088 | 68.68 |
| CR | 33.80 | 3.61 | 0.03 | 13.89 | 0.922 | 67.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Gu, H.; Qian, J.; Raróg-Pilecka, W.; Wang, Y.; Wu, Q.; Zhao, H. Techno-Economic Assessment of High-Safety and Cost-Effective Syngas Produced by O2-Enriched Air Gasification with 40–70% O2 Purity. Energies 2023, 16, 3414. https://doi.org/10.3390/en16083414
Zhang S, Gu H, Qian J, Raróg-Pilecka W, Wang Y, Wu Q, Zhao H. Techno-Economic Assessment of High-Safety and Cost-Effective Syngas Produced by O2-Enriched Air Gasification with 40–70% O2 Purity. Energies. 2023; 16(8):3414. https://doi.org/10.3390/en16083414
Chicago/Turabian StyleZhang, Siwen, Haiming Gu, Jing Qian, Wioletta Raróg-Pilecka, Yuan Wang, Qijing Wu, and Hao Zhao. 2023. "Techno-Economic Assessment of High-Safety and Cost-Effective Syngas Produced by O2-Enriched Air Gasification with 40–70% O2 Purity" Energies 16, no. 8: 3414. https://doi.org/10.3390/en16083414





