CoOx-Fe3O4/N-rGO Oxygen Reduction Catalyst for Anion-Exchange Membrane Fuel Cells
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Catalyst Synthesis
2.3. Instrumentation
2.4. Electrochemical Characterization
2.5. Anion-Exchange Membrane Fuel Cell Testing
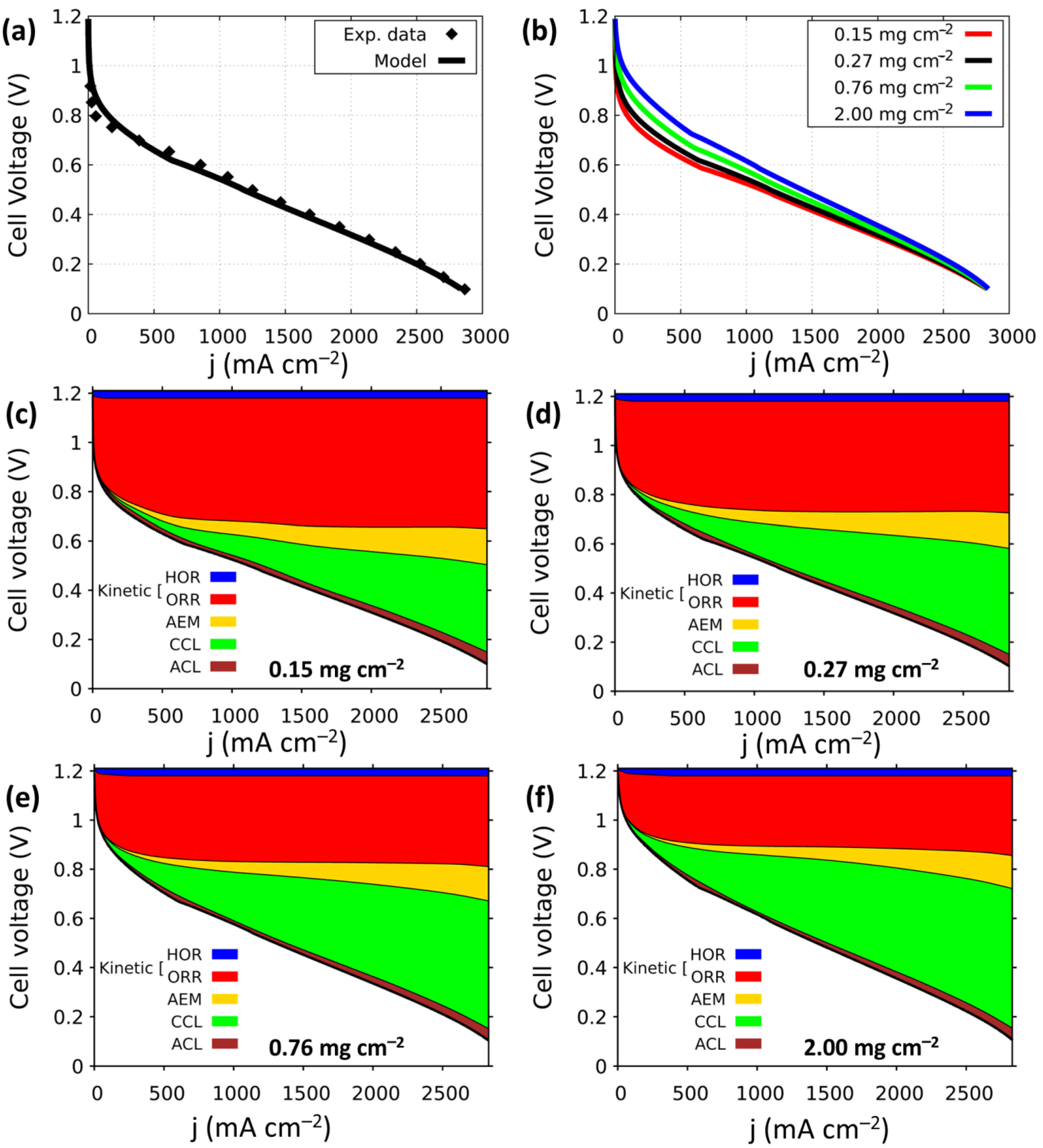
2.6. Anion-Exchange Membrane Fuel Cell Modeling
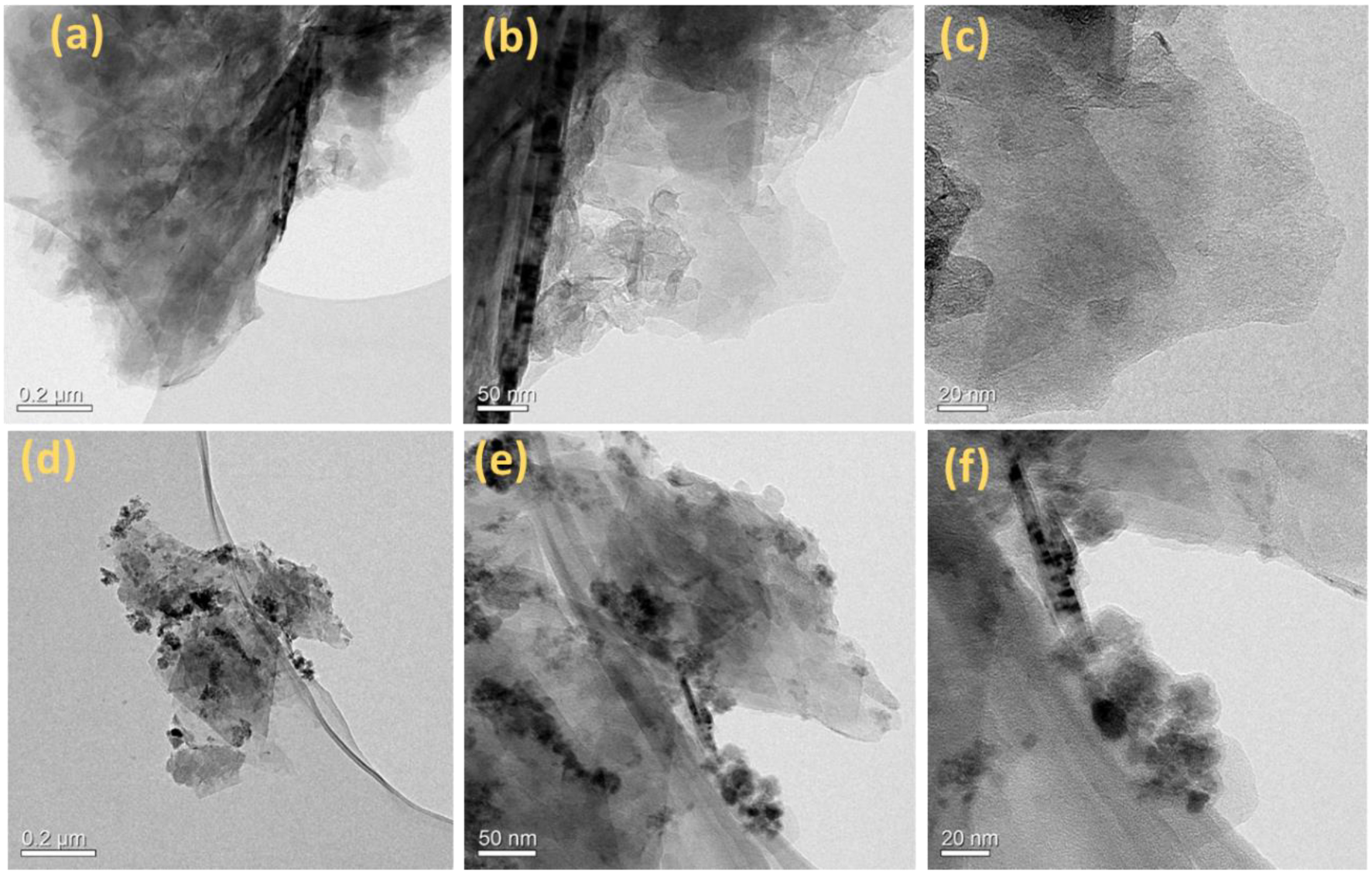
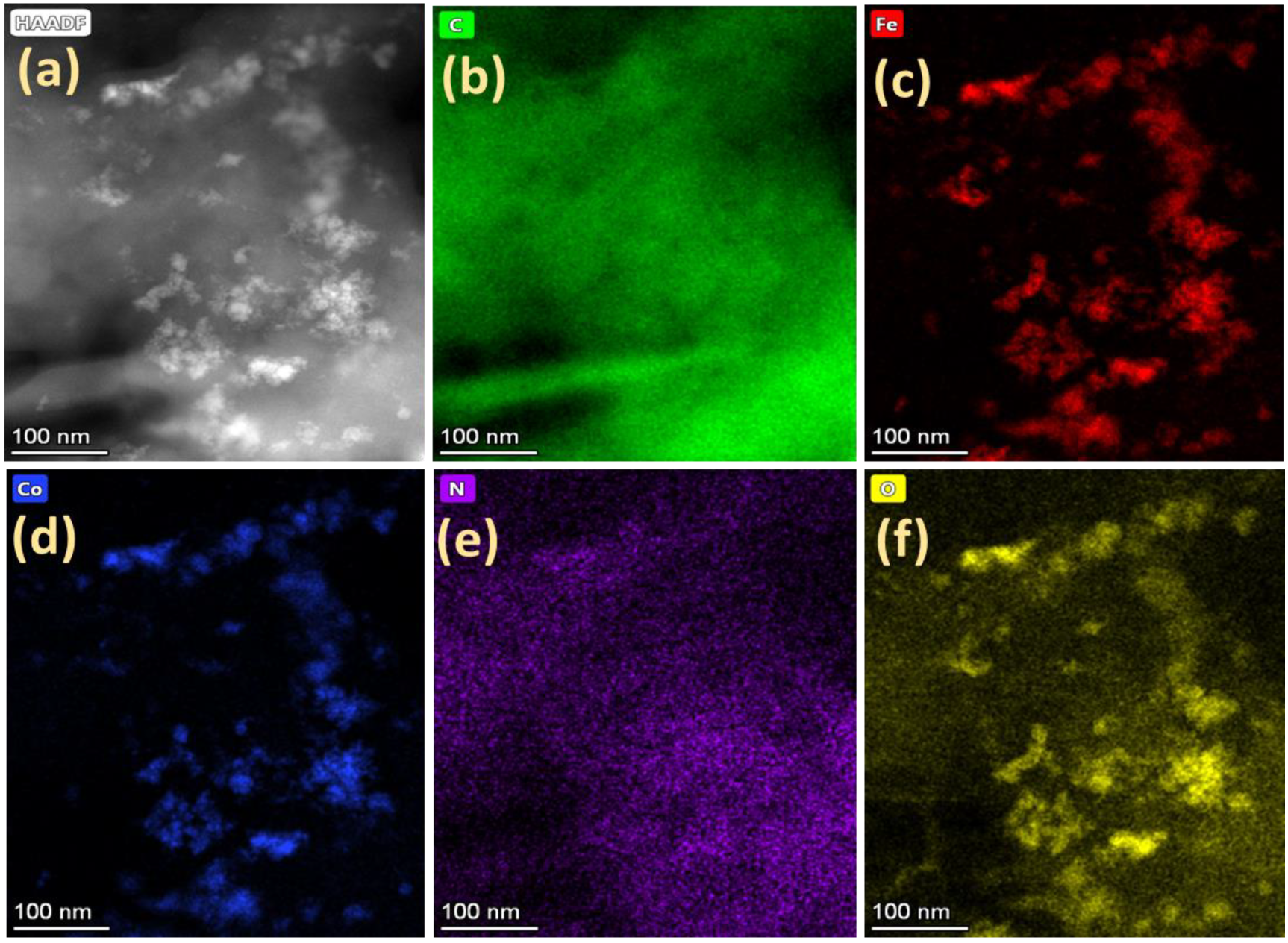
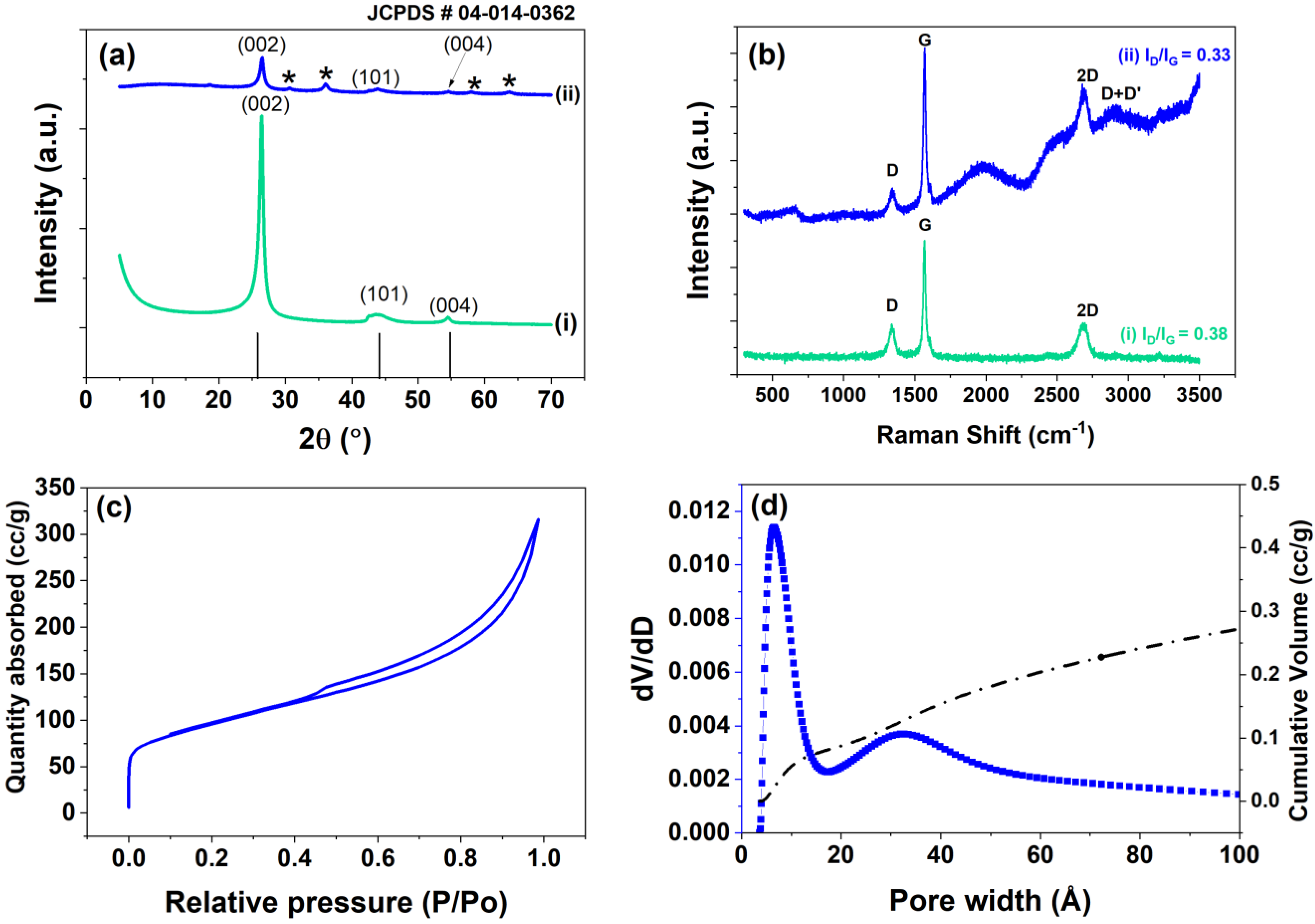
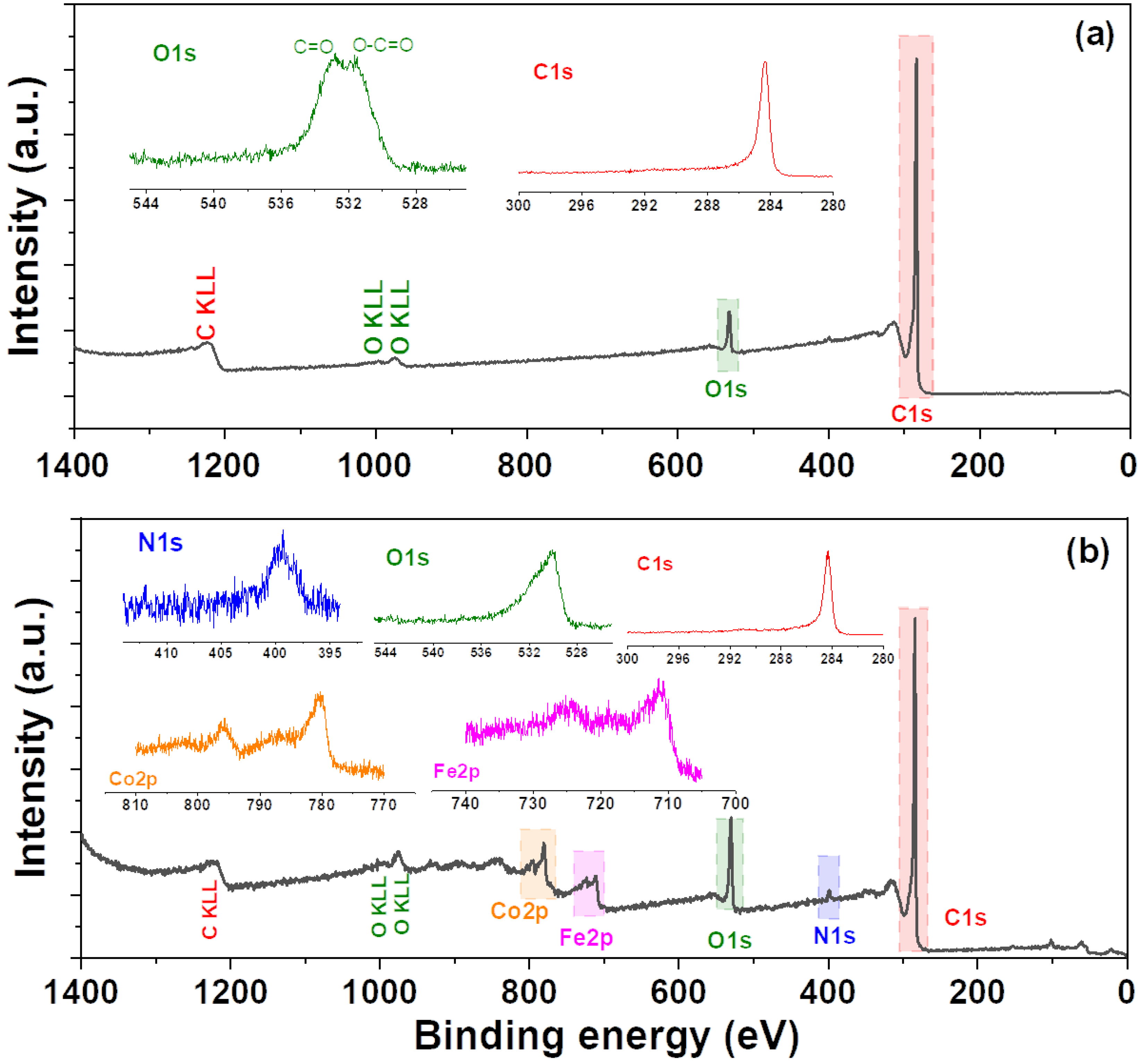
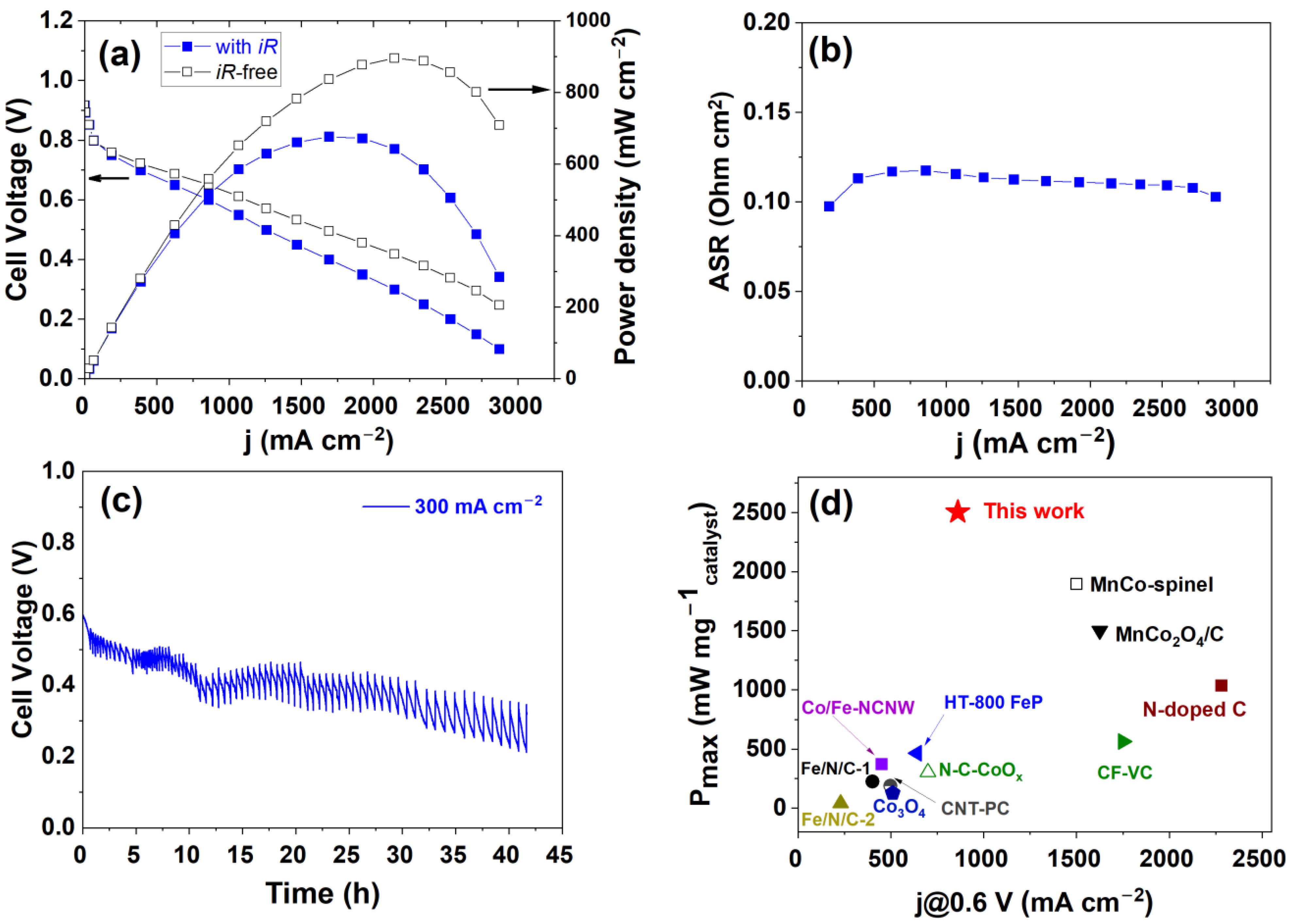
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dekel, D.R. Review of cell performance in anion exchange membrane fuel cells. J. Power Sources 2018, 375, 158–169. [Google Scholar] [CrossRef]
- Gottesfeld, S.; Dekel, D.R.; Page, M.; Bae, C.; Yan, Y.; Zelenay, P.; Kim, Y.S. Anion exchange membrane fuel cells: Current status and remaining challenges. J. Power Sources 2018, 375, 170–184. [Google Scholar] [CrossRef]
- Mustain, W.E.; Chatenet, M.; Page, M.; Kim, Y.S. Durability challenges of anion exchange membrane fuel cells. Energy Environ. Sci. 2020, 13, 2805–2838. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Mukerjee, S. Alkaline Anion-Exchange Membrane Fuel Cells: Challenges in Electrocatalysis and Interfacial Charge Transfer. Chem. Rev. 2019, 119, 11945–11979. [Google Scholar] [CrossRef]
- Willdorf-Cohen, S.; Kaushansky, A.; Dekel, D.R.; Diesendruck, C.E. Hydroxide Chemoselectivity Changes with Water Microsolvation. J. Phys. Chem. Lett. 2022, 13, 10216–10221. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xie, N.; Xue, J.; Li, M.; Zheng, C.; Zhang, J.; Qin, Y.; Yin, Y.; Dekel, D.R.; Guiver, M.D. Magnetic-field-oriented mixed-valence-stabilized ferrocenium anion-exchange membranes for fuel cells. Nat. Energy 2022, 7, 329–339. [Google Scholar] [CrossRef]
- Mandal, M.; Huang, G.; Hassan, N.U.; Peng, X.; Gu, T.; Brooks-Starks, A.H.; Bahar, B.; Mustain, W.E.; Kohl, P.A. The Importance of Water Transport in High Conductivity and High-Power Alkaline Fuel Cells. J. Electrochem. Soc. 2020, 167, 054501. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Setzler, B.P.; Rojas-Carbonell, S.; Ben Yehuda, C.; Amel, A.; Page, M.; Wang, L.; Hu, K.; Shi, L.; et al. Poly(aryl piperidinium) membranes and ionomers for hydroxide exchange membrane fuel cells. Nat. Energy 2019, 4, 392–398. [Google Scholar] [CrossRef]
- Wang, L.; Peng, X.; Mustain, W.E.; Varcoe, J.R. Radiation-grafted anion-exchange membranes: The switch from low- to high-density polyethylene leads to remarkably enhanced fuel cell performance. Energy Environ. Sci. 2019, 12, 1575–1579. [Google Scholar] [CrossRef]
- Fan, J.; Willdorf-Cohen, S.; Schibli, E.M.; Paula, Z.; Li, W.; Skalski, T.J.G.; Sergeenko, A.T.; Hohenadel, A.; Frisken, B.J.; Magliocca, E.; et al. Poly(bis-arylimidazoliums) possessing high hydroxide ion exchange capacity and high alkaline stability. Nat. Commun. 2019, 10, 2306. [Google Scholar] [CrossRef]
- Pham, T.H.; Olsson, J.S.; Jannasch, P. N-Spirocyclic Quaternary Ammonium Ionenes for Anion-Exchange Membranes. J. Am. Chem. Soc. 2017, 139, 2888–2891. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves Biancolli, A.L.; Herranz, D.; Wang, L.; Stehlíková, G.; Bance-Soualhi, R.; Ponce-González, J.; Ocón, P.; Ticianelli, E.A.; Whelligan, D.K.; Varcoe, J.R.; et al. ETFE-based anion-exchange membrane ionomer powders for alkaline membrane fuel cells: A first performance comparison of head-group chemistry. J. Mater. Chem. A 2018, 6, 24330–24341. [Google Scholar] [CrossRef]
- Aggarwal, K.; Gjineci, N.; Kaushansky, A.; Bsoul, S.; Douglin, J.C.; Li, S.; Salam, I.; Aharonovich, S.; Varcoe, J.R.; Dekel, D.R.; et al. Isoindolinium Groups as Stable Anion Conductors for Anion-Exchange Membrane Fuel Cells and Electrolyzers. ACS Mater. Au 2022, 2, 367–373. [Google Scholar] [CrossRef]
- Yassin, K.; Douglin, J.C.; Rasin, I.G.; Santori, P.G.; Eriksson, B.; Bibent, N.; Jaouen, F.; Brandon, S.; Dekel, D.R. The effect of membrane thickness on AEMFC Performance: An integrated theoretical and experimental study. Energy Convers. Manag. 2022, 270, 116203. [Google Scholar] [CrossRef]
- Omasta, T.J.; Park, A.M.; LaManna, J.M.; Zhang, Y.; Peng, X.; Wang, L.; Jacobson, D.L.; Varcoe, J.R.; Hussey, D.S.; Pivovar, B.S.; et al. Beyond catalysis and membranes: Visualizing and solving the challenge of electrode water accumulation and flooding in AEMFCs. Energy Environ. Sci. 2018, 11, 551–558. [Google Scholar] [CrossRef]
- Zhang, B.; Hua, Y.; Gao, Z. Strategies to optimize water management in anion exchange membrane fuel cells. J. Power Sources 2022, 525, 231141. [Google Scholar] [CrossRef]
- Xue, Y.; Shi, L.; Liu, X.; Fang, J.; Wang, X.; Setzler, B.P.; Zhu, W.; Yan, Y.; Zhuang, Z. A highly-active, stable and low-cost platinum-free anode catalyst based on RuNi for hydroxide exchange membrane fuel cells. Nat. Commun. 2020, 11, 5651. [Google Scholar] [CrossRef]
- Wang, R.; Li, D.; Maurya, S.; Kim, Y.S.; Wu, Y.; Liu, Y.; Strmcnik, D.; Markovic, N.M.; Stamenkovic, V.R. Ultrafine Pt cluster and RuO2 heterojunction anode catalysts designed for ultra-low Pt-loading anion exchange membrane fuel cells. Nanoscale Horiz. 2020, 5, 316–324. [Google Scholar] [CrossRef]
- Miller, H.A.; Bellini, M.; Dekel, D.R.; Vizza, F. Recent developments in Pd-CeO2 nano-composite electrocatalysts for anodic reactions in anion exchange membrane fuel cells. Electrochem. Commun. 2022, 135, 107219. [Google Scholar] [CrossRef]
- Kumar, Y.; Kibena-Põldsepp, E.; Kozlova, J.; Rähn, M.; Treshchalov, A.; Kikas, A.; Kisand, V.; Aruväli, J.; Tamm, A.; Douglin, J.C.; et al. Bifunctional Oxygen Electrocatalysis on Mixed Metal Phthalocyanine-Modified Carbon Nanotubes Prepared via Pyrolysis. ACS Appl. Mater. Interfaces 2021, 13, 41507–41516. [Google Scholar] [CrossRef]
- Zhu, W.; Pei, Y.; Douglin, J.C.; Zhang, J.; Zhao, H.; Xue, J.; Wang, Q.; Li, R.; Qin, Y.; Yin, Y.; et al. Multi-scale study on bifunctional Co/Fe–N–C cathode catalyst layers with high active site density for the oxygen reduction reaction. Appl. Catal. B Environ. 2021, 299, 120656. [Google Scholar] [CrossRef]
- Douglin, J.C.; Singh, R.K.; Haj-Bsoul, S.; Li, S.; Biemolt, J.; Yan, N.; Varcoe, J.R.; Rothenberg, G.; Dekel, D.R. A high-temperature anion-exchange membrane fuel cell with a critical raw material-free cathode. Chem. Eng. J. Adv. 2021, 8, 100153. [Google Scholar] [CrossRef]
- Zion, N.; Douglin, J.C.; Cullen, D.A.; Zelenay, P.; Dekel, D.R.; Elbaz, L. Porphyrin Aerogel Catalysts for Oxygen Reduction Reaction in Anion-Exchange Membrane Fuel Cells. Adv. Funct. Mater. 2021, 31, 2100963. [Google Scholar] [CrossRef]
- Lilloja, J.; Kibena-Põldsepp, E.; Sarapuu, A.; Douglin, J.C.; Käärik, M.; Kozlova, J.; Paiste, P.; Kikas, A.; Aruväli, J.; Leis, J.; et al. Transition-Metal- and Nitrogen-Doped Carbide-Derived Carbon/Carbon Nanotube Composites as Cathode Catalysts for Anion-Exchange Membrane Fuel Cells. ACS Catal. 2021, 11, 1920–1931. [Google Scholar] [CrossRef]
- Li, Q.; Peng, H.; Wang, Y.; Xiao, L.; Lu, J.; Zhuang, L. The Comparability of Pt to Pt-Ru in Catalyzing the Hydrogen Oxidation Reaction for Alkaline Polymer Electrolyte Fuel Cells Operated at 80 °C. Angew. Chem. Int. Ed. 2019, 58, 1442–1446. [Google Scholar] [CrossRef]
- Cong, Y.; Yi, B.; Song, Y. Hydrogen oxidation reaction in alkaline media: From mechanism to recent electrocatalysts. Nano Energy 2018, 44, 288–303. [Google Scholar] [CrossRef]
- Davydova, E.S.; Mukerjee, S.; Jaouen, F.; Dekel, D.R. Electrocatalysts for Hydrogen Oxidation Reaction in Alkaline Electrolytes. ACS Catal. 2018, 8, 6665–6690. [Google Scholar] [CrossRef]
- Zhuang, Z.; Giles, S.A.; Zheng, J.; Jenness, G.R.; Caratzoulas, S.; Vlachos, D.G.; Yan, Y. Nickel supported on nitrogen-doped carbon nanotubes as hydrogen oxidation reaction catalyst in alkaline electrolyte. Nat. Commun. 2016, 7, 10141. [Google Scholar] [CrossRef]
- Speck, F.D.; Ali, F.S.M.; Paul, M.T.Y.; Singh, R.K.; Böhm, T.; Hofer, A.; Kasian, O.; Thiele, S.; Bachmann, J.; Dekel, D.R.; et al. On the Improved Hydrogen Oxidation Reaction Activity and Stability of Buried Metal-Oxide Electrocatalyst Interfaces. Chem. Mater. 2020, 32, 7716–7724. [Google Scholar] [CrossRef]
- Miller, H.A.; Lavacchi, A.; Vizza, F.; Marelli, M.; Di Benedetto, F.; D’Acapito, F.; Paska, Y.; Page, M.; Dekel, D.R. A Pd/C-CeO2 Anode Catalyst for High-Performance Platinum-Free Anion Exchange Membrane Fuel Cells. Angew. Chem. Int. Ed. 2016, 55, 6004–6007. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, Z.; Jiang, J.; Wang, J.; Song, X.; He, Q.; Ding, W.; Wei, Z. Lattice-confined Ru clusters with high CO tolerance and activity for the hydrogen oxidation reaction. Nat. Catal. 2020, 3, 454–462. [Google Scholar] [CrossRef]
- Pagliaro, M.V.; Wen, C.; Sa, B.; Liu, B.; Bellini, M.; Bartoli, F.; Sahoo, S.; Singh, R.K.; Alpay, S.P.; Miller, H.A.; et al. Improving Alkaline Hydrogen Oxidation Activity of Palladium through Interactions with Transition-Metal Oxides. ACS Catal. 2022, 12, 10894–10904. [Google Scholar] [CrossRef]
- Li, J.-C.; Maurya, S.; Kim, Y.S.; Li, T.; Wang, L.; Shi, Q.; Liu, D.; Feng, S.; Lin, Y.; Shao, M. Stabilizing Single-Atom Iron Electrocatalysts for Oxygen Reduction via Ceria Confining and Trapping. ACS Catal. 2020, 10, 2452–2458. [Google Scholar] [CrossRef]
- Mohan, R.; Modak, A.; Schechter, A. NH3-Plasma pre-treated carbon supported active iron–nitrogen catalyst for oxygen reduction in acid and alkaline electrolytes. Catal. Sci. Technol. 2020, 10, 1675–1687. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef]
- Firouzjaie, H.A.; Mustain, W.E. Catalytic Advantages, Challenges, and Priorities in Alkaline Membrane Fuel Cells. ACS Catal. 2020, 10, 225–234. [Google Scholar] [CrossRef]
- Kisand, K.; Sarapuu, A.; Douglin, J.C.; Kikas, A.; Treshchalov, A.; Käärik, M.; Piirsoo, H.-M.; Paiste, P.; Aruväli, J.; Leis, J.; et al. Templated Nitrogen-, Iron-, and Cobalt-Doped Mesoporous Nanocarbon Derived from an Alkylresorcinol Mixture for Anion-Exchange Membrane Fuel Cell Application. ACS Catal. 2022, 12, 14050–14061. [Google Scholar] [CrossRef]
- Ren, H.; Wang, Y.; Yang, Y.; Tang, X.; Peng, Y.; Peng, H.; Xiao, L.; Lu, J.; Abruña, H.D.; Zhuang, L. Fe/N/C Nanotubes with Atomic Fe Sites: A Highly Active Cathode Catalyst for Alkaline Polymer Electrolyte Fuel Cells. ACS Catal. 2017, 7, 6485–6492. [Google Scholar] [CrossRef]
- Sa, Y.J.; Seo, D.-J.; Woo, J.; Lim, J.T.; Cheon, J.Y.; Yang, S.Y.; Lee, J.M.; Kang, D.; Shin, T.J.; Shin, H.S.; et al. A General Approach to Preferential Formation of Active Fe–Nx Sites in Fe–N/C Electrocatalysts for Efficient Oxygen Reduction Reaction. J. Am. Chem. Soc. 2016, 138, 15046–15056. [Google Scholar] [CrossRef]
- Hossen, M.; Artyushkova, K.; Atanassov, P.; Serov, A. Synthesis and characterization of high performing Fe-N-C catalyst for oxygen reduction reaction (ORR) in Alkaline Exchange Membrane Fuel Cells. J. Power Sources 2018, 375, 214–221. [Google Scholar] [CrossRef]
- Sun, Y.; Silvioli, L.; Sahraie, N.R.; Ju, W.; Li, J.; Zitolo, A.; Li, S.; Bagger, A.; Arnarson, L.; Wang, X.; et al. Activity–Selectivity Trends in the Electrochemical Production of Hydrogen Peroxide over Single-Site Metal–Nitrogen–Carbon Catalysts. J. Am. Chem. Soc. 2019, 141, 12372–12381. [Google Scholar] [CrossRef] [PubMed]
- Santori, P.G.; Speck, F.D.; Li, J.; Zitolo, A.; Jia, Q.; Mukerjee, S.; Cherevko, S.; Jaouen, F. Effect of pyrolysis atmosphere and electrolyte pH on the oxygen reduction activity, stability and spectroscopic signature of FeNx moieties in Fe-NC catalysts. J. Electrochem. Soc. 2019, 166, F3311–F3320. [Google Scholar] [CrossRef]
- Peng, X.; Omasta, T.J.; Magliocca, E.; Wang, L.; Varcoe, J.R.; Mustain, W.E. Nitrogen-doped Carbon–CoOx Nanohybrids: A Precious Metal Free Cathode that Exceeds 1.0 W cm−2 Peak Power and 100 h Life in Anion-Exchange Membrane Fuel Cells. Angew. Chem. Int. Ed. 2019, 58, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Yang, Y.; Feng, X.; DiSalvo, F.J.; Abruña, H.D. A Strategy for Increasing the Efficiency of the Oxygen Reduction Reaction in Mn-Doped Cobalt Ferrites. J. Am. Chem. Soc. 2019, 141, 4412–4421. [Google Scholar] [CrossRef]
- Jaouen, F.; Proietti, E.; Lefèvre, M.; Chenitz, R.; Dodelet, J.-P.; Wu, G.; Chung, H.T.; Johnston, C.M.; Zelenay, P. Recent advances in non-precious metal catalysis for oxygen-reduction reaction in polymer electrolyte fuel cells. Energy Environ. Sci. 2011, 4, 114–130. [Google Scholar] [CrossRef]
- Zhou, Y.; Neyerlin, K.; Olson, T.S.; Pylypenko, S.; Bult, J.; Dinh, H.N.; Gennett, T.; Shao, Z.; O’Hayre, R. Enhancement of Pt and Pt-alloy fuel cell catalyst activity and durability via nitrogen-modified carbon supports. Energy Environ. Sci. 2010, 3, 1437–1446. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, H.; Xiong, Y.; Li, Q.; Lu, J.; Xiao, L.; DiSalvo, F.J.; Zhuang, L.; Abruña, H.D. High-Loading Composition-Tolerant Co–Mn Spinel Oxides with Performance beyond 1 W/cm2 in Alkaline Polymer Electrolyte Fuel Cells. ACS Energy Lett. 2019, 4, 1251–1257. [Google Scholar] [CrossRef]
- Truong, V.M.; Tolchard, J.R.; Svendby, J.; Manikandan, M.; Miller, H.A.; Sunde, S.; Yang, H.; Dekel, D.R.; Barnett, A.O. Platinum and Platinum Group Metal-Free Catalysts for Anion Exchange Membrane Fuel Cells. Energies 2020, 13, 582. [Google Scholar] [CrossRef]
- Chung, H.T.; Cullen, D.A.; Higgins, D.; Sneed, B.T.; Holby, E.F.; More, K.L.; Zelenay, P. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst. Science 2017, 357, 479–484. [Google Scholar] [CrossRef]
- Bashyam, R.; Zelenay, P. A class of non-precious metal composite catalysts for fuel cells. Nature 2006, 443, 63–66. [Google Scholar] [CrossRef]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef]
- Wang, L.; Brink, J.J.; Liu, Y.; Herring, A.M.; Ponce-González, J.; Whelligan, D.K.; Varcoe, J.R. Non-fluorinated pre-irradiation-grafted (peroxidated) LDPE-based anion-exchange membranes with high performance and stability. Energy Environ. Sci. 2017, 10, 2154–2167. [Google Scholar] [CrossRef]
- Kar, T.; Devivaraprasad, R.; Singh, R.K.; Bera, B.; Neergat, M. Reduction of graphene oxide—A comprehensive electrochemical investigation in alkaline and acidic electrolytes. RSC Adv. 2014, 4, 57781–57790. [Google Scholar] [CrossRef]
- Singh, R.K.; Davydova, E.S.; Douglin, J.; Godoy, A.O.; Tan, H.; Bellini, M.; Allen, B.J.; Jankovic, J.; Miller, H.A.; Alba-Rubio, A.C.; et al. Synthesis of CeOx-Decorated Pd/C Catalysts by Controlled Surface Reactions for Hydrogen Oxidation in Anion Exchange Membrane Fuel Cells. Adv. Funct. Mater. 2020, 30, 2002087. [Google Scholar] [CrossRef]
- Hamo, E.R.; Singh, R.K.; Douglin, J.C.; Chen, S.; Hassine, M.B.; Carbo-Argibay, E.; Lu, S.; Wang, H.; Ferreira, P.J.; Rosen, B.A.; et al. Carbide-Supported PtRu Catalysts for Hydrogen Oxidation Reaction in Alkaline Electrolyte. ACS Catal. 2021, 11, 932–947. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef]
- Paulus, U.A.; Schmidt, T.J.; Gasteiger, H.A.; Behm, R.J. Oxygen reduction on a high-surface area Pt/Vulcan carbon catalyst: A thin-film rotating ring-disk electrode study. J. Electroanal. Chem. 2001, 495, 134–145. [Google Scholar] [CrossRef]
- Douglin, J.C.; Singh, R.K.; Hamo, E.R.; Hassine, M.B.; Ferreira, P.J.; Rosen, B.A.; Miller, H.A.; Rothenberg, G.; Dekel, D.R. Performance optimization of PGM and PGM-free catalysts in anion-exchange membrane fuel cells. J. Solid State Electrochem. 2022, 26, 2049–2057. [Google Scholar] [CrossRef]
- Praats, R.; Käärik, M.; Kikas, A.; Kisand, V.; Aruväli, J.; Paiste, P.; Merisalu, M.; Leis, J.; Sammelselg, V.; Zagal, J.H.; et al. Tammeveski, Electrocatalytic oxygen reduction reaction on iron phthalocyanine-modified carbide-derived carbon/carbon nanotube composite electrocatalysts. Electrochim. Acta 2020, 334, 135575. [Google Scholar] [CrossRef]
- Wierzbicki, S.; Douglin, J.C.; Singh, R.K.; Dekel, D.R.; Kruczała, K. Operando EPR Study of Radical Formation in Anion-Exchange Membrane Fuel Cells. ACS Catal. 2023, 13, 2744–2750. [Google Scholar] [CrossRef]
- Hassan, N.U.; Mandal, M.; Huang, G.; Firouzjaie, H.A.; Kohl, P.A.; Mustain, W.E. Achieving High-Performance and 2000 h Stability in Anion Exchange Membrane Fuel Cells by Manipulating Ionomer Properties and Electrode Optimization. Adv. Energy Mater. 2020, 10, 2001986. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Panels, J.E.; Yan, S.G. Dependence of PEM fuel cell performance on catalyst loading. J. Power Sources 2004, 127, 162–171. [Google Scholar] [CrossRef]
- Omasta, T.J.; Peng, X.; Miller, H.A.; Vizza, F.; Wang, L.; Varcoe, J.R.; Dekel, D.R.; Mustain, W.E. Beyond 1.0 W cm−2Performance without Platinum: The Beginning of a New Era in Anion Exchange Membrane Fuel Cells. J. Electrochem. Soc. 2018, 165, J3039–J3044. [Google Scholar] [CrossRef]
- Dekel, D.R.; Rasin, I.G.; Page, M.; Brandon, S. Steady state and transient simulation of anion exchange membrane fuel cells. J. Power Sources 2018, 375, 191–204. [Google Scholar] [CrossRef]
- Dekel, D.R.; Rasin, I.G.; Brandon, S. Predicting performance stability of anion exchange membrane fuel cells. J. Power Sources 2019, 420, 118–123. [Google Scholar] [CrossRef]
- Yassin, K.; Rasin, I.G.; Brandon, S.; Dekel, D.R. Elucidating the role of anion-exchange ionomer conductivity within the cathode catalytic layer of anion-exchange membrane fuel cells. J. Power Sources 2022, 524, 231083. [Google Scholar] [CrossRef]
- Shi, F.; Li, Y.; Zhang, Q.; Wang, H. Synthesis of Fe3O4/C/TiO2 Magnetic Photocatalyst via Vapor Phase Hydrolysis. Int. J. Photoenergy 2012, 2012, 365401. [Google Scholar] [CrossRef]
- Iqbal, M.W.; Singh, A.K.; Iqbal, M.Z.; Eom, J. Raman fingerprint of doping due to metal adsorbates on graphene. J. Phys. Condens. Matter. 2012, 24, 335301. [Google Scholar] [CrossRef]
- Xie, L.; Ling, X.; Fang, Y.; Zhang, J.; Liu, Z. Graphene as a Substrate To Suppress Fluorescence in Resonance Raman Spectroscopy. J. Am. Chem. Soc. 2009, 131, 9890–9891. [Google Scholar] [CrossRef]
- Kondo, T.; Guo, D.; Shikano, T.; Suzuki, T.; Sakurai, M.; Okada, S.; Nakamura, J. Observation of Landau levels on nitrogen-doped flat graphite surfaces without external magnetic fields. Sci. Rep. 2015, 5, 16412. [Google Scholar] [CrossRef]
- Zhong, G.; Xu, S.; Liu, L.; Zheng, C.Z.; Dou, J.; Wang, F.; Fu, X.; Liao, W.; Wang, H. Effect of experimental operations on the limiting current density of oxygen reduction reaction evaluated by rotating-disk electrode. ChemElectroChem 2020, 7, 1107–1114. [Google Scholar] [CrossRef]
- Chung, H.T.; Won, J.H.; Zelenay, P. Active and stable carbon nanotube/nanoparticle composite electrocatalyst for oxygen reduction. Nat. Commun. 2013, 4, 1922. [Google Scholar] [CrossRef]
- Neergat, M.; Gunasekar, V.; Singh, R.K. Oxygen reduction reaction and peroxide generation on Ir, Rh, and their selenides—A comparison with Pt and RuSe. J. Electrochem. Soc. 2011, 158, B1060. [Google Scholar] [CrossRef]
- Ramaswamy, N.; Mukerjee, S. Fundamental Mechanistic Understanding of Electrocatalysis of Oxygen Reduction on Pt and Non-Pt Surfaces: Acid versus Alkaline Media. Adv. Phys. Chem. 2012, 2012, 491604. [Google Scholar] [CrossRef]
- Pylypenko, S.; Queen, A.; Neyerlin, K.C.; Olson, T.; Dameron, A.; O’Neill, K.; Ginley, D.; Gorman, B.; Kocha, S.; Dinh, H.N.; et al. The Role of Nitrogen Doping on Durability in the Pt-Ru/HOPG System. ECS Trans. 2010, 33, 351–357. [Google Scholar] [CrossRef]
- Adabi, H.; Santori, P.G.; Shakouri, A.; Peng, X.; Yassin, K.; Rasin, I.G.; Brandon, S.; Dekel, D.R.; Hassan, N.U.; Sougrati, M.-T.; et al. Understanding how single-atom site density drives the performance and durability of PGM-free Fe–N–C cathodes in anion exchange membrane fuel cells. Mater. Today Adv. 2021, 12, 100179. [Google Scholar] [CrossRef]
- Adabi, H.; Shakouri, A.; Hassan, N.U.; Varcoe, J.R.; Zulevi, B.; Serov, A.; Regalbuto, J.R.; Mustain, W.E. High-performing commercial Fe–N–C cathode electrocatalyst for anion-exchange membrane fuel cells. Nat. Energy 2021, 6, 834–843. [Google Scholar] [CrossRef]
- Peng, X.; Kashyap, V.; Ng, B.; Kurungot, S.; Wang, L.; Varcoe, J.R.; Mustain, W.E. High-performing pgm-free aemfc cathodes from carbon-supported cobalt ferrite nanoparticles. Catalysts 2019, 9, 264. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Jia, S.; Wang, X.; Lyu, K.; Peng, Y.; Zheng, H.; Wei, X.; Ren, H.; Xiao, L.; et al. Synergistic Mn-Co catalyst outperforms Pt on high-rate oxygen reduction for alkaline polymer electrolyte fuel cells. Nat. Commun. 2019, 10, 1506. [Google Scholar] [CrossRef]
- Yassin, K.; Rasin, I.G.; Brandon, S.; Dekel, D.R. Quantifying the critical effect of water diffusivity in anion exchange membranes for fuel cell applications. J. Memb. Sci. 2020, 608, 118206. [Google Scholar] [CrossRef]

| Elements | GO (at %) | CoOx-Fe3O4/N-rGO (at %) |
|---|---|---|
| C | 93.34 | 81.83 |
| O | 6.03 | 11.24 |
| N | 0.63 | 1.70 |
| Fe | - | 2.62 |
| Co | - | 2.61 |
| Cathode Catalyst | Cathode Catalyst Loading (mg cm−2) | Power Density per Catalyst Mass (mW mg−1) | References |
|---|---|---|---|
| CoOx-Fe3O4/N-rGO | 0.27 | 2504 | This work |
| Fe-N-C cathode | 1.0 | 2005 | [77] |
| MnCo2O4/C | 0.80 | 1500 | [47] |
| MnCo-spinel | 0.58 | 1897 | [79] |
| N-doped C | 1.10 | 1036 | [22] |
| CF-VC | 2.40 | 563 | [78] |
| HT800-FeP | 1.25 | 464 | [23] |
| Ce/Fe-NCNW | 1.00 | 372 | [33] |
| N-C-CoOx | 2.40 | 304 | [43] |
| Fe/N/C-1 | 2.00 | 225 | [38] |
| CNT/PC | 2.00 | 90 | [39] |
| Co3O4 | 3.00 | 129 | [48] |
| Fe/N/C-2 | 3.50 | 40 | [40] |
| Voltage Loss (mV) @ 500 mA cm−2 | |||||
|---|---|---|---|---|---|
| Loading (mg cm−2) | HOR | ORR | AEM | CCL | ACL |
| 0.15 | 30.5 | 470.1 | 28.4 | 30.5 | 19.3 |
| 0.27 | 30.5 | 416.2 | 26.4 | 54.8 | 22.3 |
| 0.76 | 30.5 | 332.0 | 23.4 | 99.5 | 20.3 |
| 2.00 | 31.5 | 272.1 | 18.3 | 111.7 | 20.3 |
| Voltage Loss (mV) @ 1000 mA cm−2 | |||||
| Loading (mg cm−2) | HOR | ORR | AEM | CCL | ACL |
| 0.15 | 30.5 | 498.5 | 53.8 | 84.3 | 18.3 |
| 0.27 | 31.5 | 442.6 | 50.8 | 128.9 | 14.2 |
| 0.76 | 30.5 | 348.2 | 43.7 | 195.9 | 14.2 |
| 2.00 | 30.5 | 286.3 | 35.5 | 230.5 | 12.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, R.K.; Douglin, J.C.; Jiang, L.; Yassin, K.; Brandon, S.; Dekel, D.R. CoOx-Fe3O4/N-rGO Oxygen Reduction Catalyst for Anion-Exchange Membrane Fuel Cells. Energies 2023, 16, 3425. https://doi.org/10.3390/en16083425
Singh RK, Douglin JC, Jiang L, Yassin K, Brandon S, Dekel DR. CoOx-Fe3O4/N-rGO Oxygen Reduction Catalyst for Anion-Exchange Membrane Fuel Cells. Energies. 2023; 16(8):3425. https://doi.org/10.3390/en16083425
Chicago/Turabian StyleSingh, Ramesh K., John C. Douglin, Lanjie Jiang, Karam Yassin, Simon Brandon, and Dario R. Dekel. 2023. "CoOx-Fe3O4/N-rGO Oxygen Reduction Catalyst for Anion-Exchange Membrane Fuel Cells" Energies 16, no. 8: 3425. https://doi.org/10.3390/en16083425
APA StyleSingh, R. K., Douglin, J. C., Jiang, L., Yassin, K., Brandon, S., & Dekel, D. R. (2023). CoOx-Fe3O4/N-rGO Oxygen Reduction Catalyst for Anion-Exchange Membrane Fuel Cells. Energies, 16(8), 3425. https://doi.org/10.3390/en16083425






