The petrochemical industry is based on raw materials from the energy industry, mainly naphtha, natural gas, liquified natural gas, and coal. Naphtha is the main raw material for the petrochemical industries of China, Japan, and Europe, occupying 50% of the global cracking raw materials [
1]. Specifically, in South America, naphtha is the main raw material in the petrochemical chain for Brazil in (92%), followed by natural gas (8%). Based in capacity and production, Brazilian companies represent an interest of study compared with other samples in South America and the world. The Petrobras company is practically the only naphtha and natural gas producer in Brazil, meeting part of the national demand with its production and imports in the region [
2].
The origins of naphtha describes multiple properties in relation to molecule structure and chemical characteristics, especially the relevant composition of aromatics and olefins which define the origins. In this way, different methodologies and sets in petrochemical refineries were improved with the knowledge and characterization of naphtha in the specific PIONA components presented. This raw material undergoes a cracking process, which results in basic petrochemicals such as olefins (ethylene and propylene) and aromatics (gasoline, benzene, toluene, and xylenes). These chemicals can be sold to third parties or, to create value, they can be polymerized in a process where they become thermoplastic resins. The resins are sold to processing industries, which convert them into a final product for sale on the market [
3].
This work presents an experimental study applied in 1849 different samples of naphtha obtained in Latin America and their comparison with imported naphtha to quantify properties. The novelty results present the evaluated properties and their relationships with the quality of raw material and subproducts obtained to improve the knowledge of naphtha applications and processes for obtention.
1.1. Naphtha
Naphtha is produced by several methods, which include (1) fractionation of straight run, cracked, reforming distillates, and even crude petroleum; (2) solvent extraction; (3) hydrogenation of cracked distillates; (4) polymerization of unsaturated compounds (olefins); and (5) alkylation, direct distillation, and/or catalytic reforming processes [
3,
4].
The typical composition of naphtha is constituted of 15–30% crude oil by weight and boils at 30–200 °C. This complex mixture consists of hydrocarbon molecules with 4–12 carbon atoms, mainly including paraffins (P), iso-paraffins (I), olefins (O), naphthene (N), and aromatics (A) (PIONA). Some compounds contain sulfur, nitrogen, and oxygen as heteroatoms, while metallic derivatives (e.g., vanadium, nickel, and silicon) can also exist [
5]. Besides the complexity of crude oil composition, the naphtha fraction properties vary according to the reservoir location. Since there is variability in the organic matter (aquatic plants and animals) and decomposition variables (layer deposits, climatic conditions (pressure, low oxygenation, and bacteria)), the crude oil derivatives also have different compositions [
6,
7].
The composition variability of naphtha is related to its origin and locality, directly impacting its conversion processes, as shown in
Table 1 [
8,
9].
In
Table 1, it is possible to see that there is a significant difference between naphtha from different origins. Brazilian naphtha had a higher amount of sulfur and paraffin compared with Norway, but compared to Argentina, it had a lower amount of sulfur and a smaller number of aromatics, whereas paraffins are in the same range for PIONA.
Naphtha available on the market is characterized by some properties defined at the time of contract. Among these properties, density, distillation curve, PIONA composition, and sulfur content are the main evaluated properties [
1].
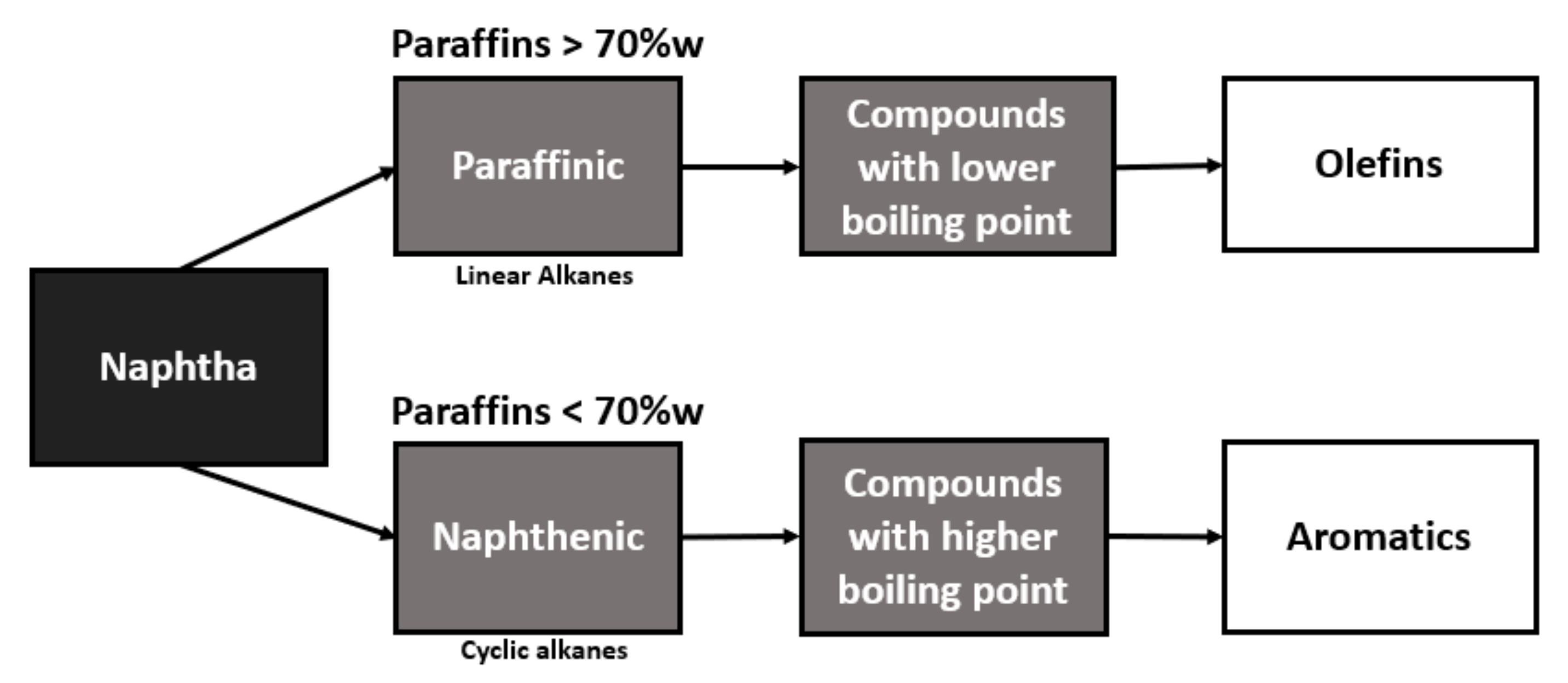
The naphtha destination in the petrochemical industry depends on the content of paraffinic hydrocarbons, which is why the precision and detailing of naphtha composition is essential for the optimization of the process since the ethylene yield in the pyrolysis furnace is based on the quality of naphtha that will be used as feedstock [
10].
Naphtha with a higher content of paraffin, in its conversion, will present a higher yield in the production of olefins, while naphtha with a higher content of naphthenic will be better used for the production of aromatics, as can be seen in the flowchart in
Figure 1.
1.3. Naphtha Characterization
Several analytical methods have been proposed to determine naphtha composition. Most methods are based on chromatographic techniques, such as gas chromatography, multidimensional chromatography, and gas chromatography coupled with mass spectrometry (GC/MS) and intensified separation [
3,
21].
Gas chromatography (GC) is considered to be the most appropriate method for determining the detailed molecular composition of naphtha fractions [
22]. The sample is injected into the chromatograph, and at the same time, the obtained chromatogram is compared with a reference database called Detailed Hydrocarbon Analysis (DHA), where the peaks are categorized by group type and carbon number.
The ASTM D5134 method, based on gas chromatography with a flame ionization detector (GC/FID), is commonly used to determine PIONA composition by petrochemical industry laboratories [
23]. GC/MS, for example, can only be used for fractions up until 300 °C, because the higher the boiling point, the greater the number of carbons, which exponentially increases the number of isomers [
24]. GC/MS analysis also has limitations in terms of analysis time, components, complexity, and cost, thus, not making it inconvenient for industrial applications. An example of the PIONA analysis result is described in
Table 3 [
22,
25,
26,
27].
Other important parameters in naphtha’s characterization are the distillation curve and density values. Such information combined with PIONA analysis enables estimation of naphtha quality, besides the parameter adjustments for the conversion processes. The petrochemical naphtha specification depends on the place where it is sold, while national naphtha specifications are defined by negotiation between suppliers and users in the same country. In Brazil, the National Petroleum Agency (Agência Nacional de Petróleo—ANP) regulates the performed analysis and the respective method to be used [
25].
Therefore, the main techniques for measuring the most relevant properties to describe the quality of naphtha are described in
Table 4 [
12].
Naphtha quality is superior for the fractions with the absence of some contaminants, such as catalyst poisoning agents (metals such as copper, lead, and iron) as well as environmentally aggressive (chlorides) and corrosive materials (sulfur derivatives) [
28].
This work presents the characterization and comparison between naphtha from different origins to correctly qualify them and correlate their composition according to the crude oil reservoir locality.
Although naphtha is the main raw material of petrochemical industries in the world, little is known about its characterization and its impacts on conversion processes. The large quantities of work on the subject are related to the catalysts involved in their production processes and not to their qualification.
The petrochemical industries have faced the challenge of adapting to each type of naphtha received to guarantee the quality of manufactured products. The naphtha cracking process in a pyrolysis furnace consists of a sequence of endothermic reactions that occur in a serpentine (tubular reactor) heated in the presence of water vapor. The average coil’s useful lifetime is around six years, with cleaning intervention every 45 to 60 days, for naphtha with low contaminant levels (sulfur, paraffins, and aromatics, within the specified temperature range of oven operation). However, due to the lack of knowledge about the quality of received naphtha, these interventions lasted less than 25 days, consequently reducing the coil’s useful lifetime, which considerably increases the cost of petrochemicals, generates waste, and impacts the products’ quality, due to loss of reaction efficiency.
Therefore, prior knowledge of the characteristics of naphtha is extremely important for the rapid parameterization of its conversion processes, reducing losses, and minimizing environmental impacts, combined with an economic approach.