Phase Field Study of Cr-Oxide Growth Kinetics in the Crofer 22 APU Alloy Supported by Wagner’s Theory
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chehrmonavari, H.; Kakaee, A.; Hosseini, S.E.; Desideri, U.; Tsatsaronis, G.; Floerchinger, G.; Braun, R.; Paykani, A. Hybridizing solid oxide fuel cells with internal combustion engines for power and propulsion systems: A review. Renew. Sustain. Energy Rev. 2023, 171, 112982. [Google Scholar] [CrossRef]
- Zarabi Golkhatmi, S.; Asghar, M.I.; Lund, P.D. A review on solid oxide fuel cell durability: Latest progress, mechanisms, and study tools. Renew. Sustain. Energy Rev. 2022, 161, 112339. [Google Scholar] [CrossRef]
- Xu, Q.; Guo, Z.; Xia, L.; He, Q.; Li, Z.; Temitope Bello, I.; Zheng, K.; Ni, M. A comprehensive review of solid oxide fuel cells operating on various promising alternative fuels. Energy Convers. Manag. 2022, 253, 115175. [Google Scholar] [CrossRef]
- Bilal Hanif, M.; Motola, M.; qayyum, S.; Rauf, S.; khalid, A.; Li, C.J.; Li, C.X. Recent advancements, doping strategies and the future perspective of perovskite-based solid oxide fuel cells for energy conversion. Chem. Eng. J. 2022, 428, 132603. [Google Scholar] [CrossRef]
- Hu, Y.Z.; Yao, S.W.; Li, C.X.; Li, C.J.; Zhang, S.L. Influence of pre-reduction on microstructure homogeneity and electrical properties of APS Mn1.5Co1.5O4 coatings for SOFC interconnects. Int. J. Hydrogen Energy 2017, 42, 27241–27253. [Google Scholar] [CrossRef]
- Han, M.; Peng, S.; Wang, Z.; Yang, Z.; Chen, X. Properties of Fe–Cr based alloys as interconnects in a solid oxide fuel cell. J. Power Sources 2007, 164, 278–283. [Google Scholar] [CrossRef]
- Ebrahimifar, H.; Zandrahimi, M. Oxidation and electrical behavior of AISI 430 coated with cobalt spinels for SOFC interconnect applications. Surf. Coat. Technol. 2011, 206, 75–81. [Google Scholar] [CrossRef]
- Thublaor, T.; Chandra-ambhorn, S. High temperature oxidation and chromium volatilisation of AISI 430 stainless steel coated by Mn-Co and Mn-Co-Cu oxides for SOFC interconnect application. Corros. Sci. 2020, 174, 108802. [Google Scholar] [CrossRef]
- Fujita, K.; Ogasawara, K.; Matsuzaki, Y.; Sakurai, T. Prevention of SOFC cathode degradation in contact with Cr-containing alloy. J. Power Sources 2004, 131, 261–269. [Google Scholar] [CrossRef]
- Manjunath, N.; Santhy, K.; Rajasekaran, B. Thermal expansion of Crofer 22 APU steel used for SOFC interconnect using in-situ high temperature X-ray diffraction. In Materials Today: Proceedings; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Topcu, A.; Öztürk, B.; Cora, Ö.N. Performance evaluation of machined and powder metallurgically fabricated Crofer®22 APU interconnects for SOFC applications. Int. J. Hydrog. Energy 2022, 47, 3437–3448. [Google Scholar] [CrossRef]
- Yin, X.; Beez, A.; Menzler, N.H.; Spatschek, R. Combined experimental and ab initio based determination of the thermal expansion of La0.5Sr0.5Co0.25Fe0.75O3. J. Am. Ceram. Soc. 2018, 101, 3086. [Google Scholar] [CrossRef]
- Beez, A.; Yin, X.; Menzler, N.H.; Spatschek, R.; Bram, M. Insight into the Reaction Mechanism of (La0.58Sr0.40)(Co0.20Fe0.80)O3-δ Cathode with Volatile Chromium Species at High Current Density in a Solid Oxide Fuel Cell Stack. J. Electrochem. Soc. 2017, 164, F3028. [Google Scholar] [CrossRef]
- Fang, Q.; Menzler, N.H.; Blum, L. Degradation Analysis of Long-Term Solid Oxide Fuel Cell Stacks with Respect to Chromium Poisoning in La0.58Sr0.4Co0.2Fe0.8O3-δ and La0.6Sr0.4CoO3-δ Cathodes. J. Electrochem. Soc. 2021, 168, 104505. [Google Scholar] [CrossRef]
- Sachitanand, R.; Sattari, M.; Svensson, J.E.; Froitzheim, J. Evaluation of the oxidation and Cr evaporation properties of selected FeCr alloys used as SOFC interconnects. Int. J. Hydrogen Energy 2013, 38, 15328–15334. [Google Scholar] [CrossRef]
- Magdefrau, N.J.; Chen, L.; Sun, E.Y.; Aindow, M. Effects of alloy heat treatment on oxidation kinetics and scale morphology for Crofer 22 APU. J. Power Sources 2013, 241, 756–767. [Google Scholar] [CrossRef]
- Falk-Windisch, H.; Svensson, J.E.; Froitzheim, J. The effect of temperature on chromium vaporization and oxide scale growth on interconnect steels for Solid Oxide Fuel Cells. J. Power Sources 2015, 287, 25–35. [Google Scholar] [CrossRef]
- Wagner, C. Reaktionstypen bei der Oxydation von Legierungen. Z. Für Elektrochem. Berichte Bunsenges. Für Phys. Chem. 1959, 63, 772–782. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High Temperature Oxidation of Metals, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar] [CrossRef]
- Rapp, R. The transition from internal to external oxidation and the formation of interruption bands in silver-indium alloys. Acta Metall. 1961, 9, 730–741. [Google Scholar] [CrossRef]
- Zhao, W.; Kang, Y.; Orozco, J.M.A.; Gleeson, B. Quantitative approach for determining the critical volume fraction for the transition from internal to external oxidation. Oxid. Met. 2015, 83, 187–201. [Google Scholar] [CrossRef]
- Wang, K.; Wei, M.; Zhang, L.; Du, Y. Morphologies of Primary Silicon in Hypereutectic Al-Si Alloys: Phase-Field Simulation Supported by Key Experiments. Metall. Mater. Trans. A 2016, 47, 1510–1516. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, L. Quantitative phase-field simulation of the entire solidification process in one hypereutectic Al–Si alloy considering the effect of latent heat. Prog. Nat. Sci. Mater. Int. 2021, 31, 428–433. [Google Scholar] [CrossRef]
- Van der Waals, J.D. The thermodynamic theory of capillarity under the hypothesis of a continuous variation of density. J. Stat. Phys. 1979, 20, 200–244. [Google Scholar] [CrossRef]
- Ginzburg, V.L.; Ginzburg, V.L.; Landau, L. On the Theory of Superconductivity; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Cahn, J.W.; Hilliard, J.E. Free energy of a nonuniform system. I. Interfacial free energy. J. Chem. Phys. 1958, 28, 258–267. [Google Scholar] [CrossRef]
- Hohenberg, P.C.; Halperin, B.I. Theory of dynamic critical phenomena. Rev. Mod. Phys. 1977, 49, 435. [Google Scholar] [CrossRef]
- Karma, A.; Rappel, W.J. Quantitative phase-field modeling of dendritic growth in two and three dimensions. Phys. Rev. E 1998, 57, 4323. [Google Scholar] [CrossRef]
- Karma, A. Phase-field model of eutectic growth. Phys. Rev. E 1994, 49, 2245. [Google Scholar] [CrossRef]
- Nestler, B.; Wheeler, A. A multi-phase-field model of eutectic and peritectic alloys: Numerical simulation of growth structures. Phys. D Nonlinear Phenom. 2000, 138, 114–133. [Google Scholar] [CrossRef]
- Chen, L.Q. Phase-field models for microstructure evolution. Annu. Rev. Mater. Res. 2002, 32, 113–140. [Google Scholar] [CrossRef]
- Sandt, R.; Le Bouar, Y.; Spatschek, R. Quantum annealing for microstructure equilibration with long-range elastic interactions. Sci. Rep. 2023, 13, 6036. [Google Scholar] [CrossRef]
- Borden, M.J.; Verhoosel, C.V.; Scott, M.A.; Hughes, T.J.; Landis, C.M. A phase-field description of dynamic brittle fracture. Comput. Methods Appl. Mech. Eng. 2012, 217, 77–95. [Google Scholar] [CrossRef]
- Mellenthin, J.; Karma, A.; Plapp, M. Phase-field crystal study of grain-boundary premelting. Phys. Rev. B 2008, 78, 184110. [Google Scholar] [CrossRef]
- Miehe, C.; Dal, H.; Schänzel, L.M.; Raina, A. A phase-field model for chemo-mechanical induced fracture in lithium-ion battery electrode particles. Int. J. Numer. Methods Eng. 2016, 106, 683–711. [Google Scholar] [CrossRef]
- Jin, Y.M. Domain microstructure evolution in magnetic shape memory alloys: Phase-field model and simulation. Acta Mater. 2009, 57, 2488–2495. [Google Scholar] [CrossRef]
- Wang, K.; Boussinot, G.; Hüter, C.; Brener, E.A.; Spatschek, R. Modeling of dendritic growth using a quantitative nondiagonal phase field model. Phys. Rev. Mater. 2020, 4, 033802. [Google Scholar] [CrossRef]
- Wang, K.; Boussinot, G.; Brener, E.A.; Spatschek, R. Quantitative nondiagonal phase field modeling of eutectic and eutectoid transformations. Phys. Rev. B 2021, 103, 184111. [Google Scholar] [CrossRef]
- Wang, K.; Wei, M.; Zhang, L. Anomalous Halo Formation in an Arc-Melted ScNi-Sc2Ni Off-Eutectic Binary Alloy. Materials 2016, 9, 584. [Google Scholar] [CrossRef]
- Asle Zaeem, M.; El Kadiri, H. An elastic phase field model for thermal oxidation of metals: Application to zirconia. Comput. Mater. Sci. 2014, 89, 122–129. [Google Scholar] [CrossRef]
- Sherman, Q.C.; Voorhees, P.W. Phase-field model of oxidation: Equilibrium. Phys. Rev. E 2017, 95, 032801. [Google Scholar] [CrossRef]
- Kim, K.; Sherman, Q.C.; Aagesen, L.K.; Voorhees, P.W. Phase-field model of oxidation: Kinetics. Phys. Rev. E 2020, 101, 022802. [Google Scholar] [CrossRef]
- Wang, R.; Ji, Y.; Cheng, T.; Xue, F.; Chen, L.Q.; Wen, Y.H. A Phase-Field Study on Internal to External Oxidation Transition in High-Temperature Structural Alloys. JOM 2022, 74, 1435–1443. [Google Scholar] [CrossRef]
- ACCESS, e.V. MICRESS. Available online: https://micress.rwth-aachen.de (accessed on 2 April 2023).
- Taylor, J.R.; Dinsdale, A.T. A Thermodynamic Assessment of the Cr-Fe-O System. Int. J. Mater. Res. 1993, 84, 335–345. [Google Scholar] [CrossRef]
- Auinger, M.; Naraparaju, R.; Christ, H.J.; Rohwerder, M. Modelling High Temperature Oxidation in Iron–Chromium Systems: Combined Kinetic and Thermodynamic Calculation of the Long-Term Behaviour and Experimental Verification. Oxid. Met. 2011, 76, 247–258. [Google Scholar] [CrossRef]
- Saeidpour, F.; Zandrahimi, M.; Ebrahimifar, H. Evaluation of pulse electroplated cobalt/yttrium oxide composite coating on the Crofer 22 APU stainless steel interconnect. Int. J. Hydrogen Energy 2019, 44, 3157–3169. [Google Scholar] [CrossRef]
- Sun, J.; Stirner, T.; Matthews, A. Structure and surface energy of low-index surfaces of stoichiometric α-Al2O3 and α-Cr2O3. Surf. Coat. Technol. 2006, 201, 4205–4208. [Google Scholar] [CrossRef]
- Nakajima, K.; Apel, M.; Steinbach, I. The role of carbon diffusion in ferrite on the kinetics of cooperative growth of pearlite: A multi-phase field study. Acta Mater. 2006, 54, 3665–3672. [Google Scholar] [CrossRef]
- Krause, D.; Thörnig, P. JURECA: Modular supercomputer at Jülich supercomputing centre. J. Large-Scale Res. Facil. 2018, 4, A132. [Google Scholar] [CrossRef]

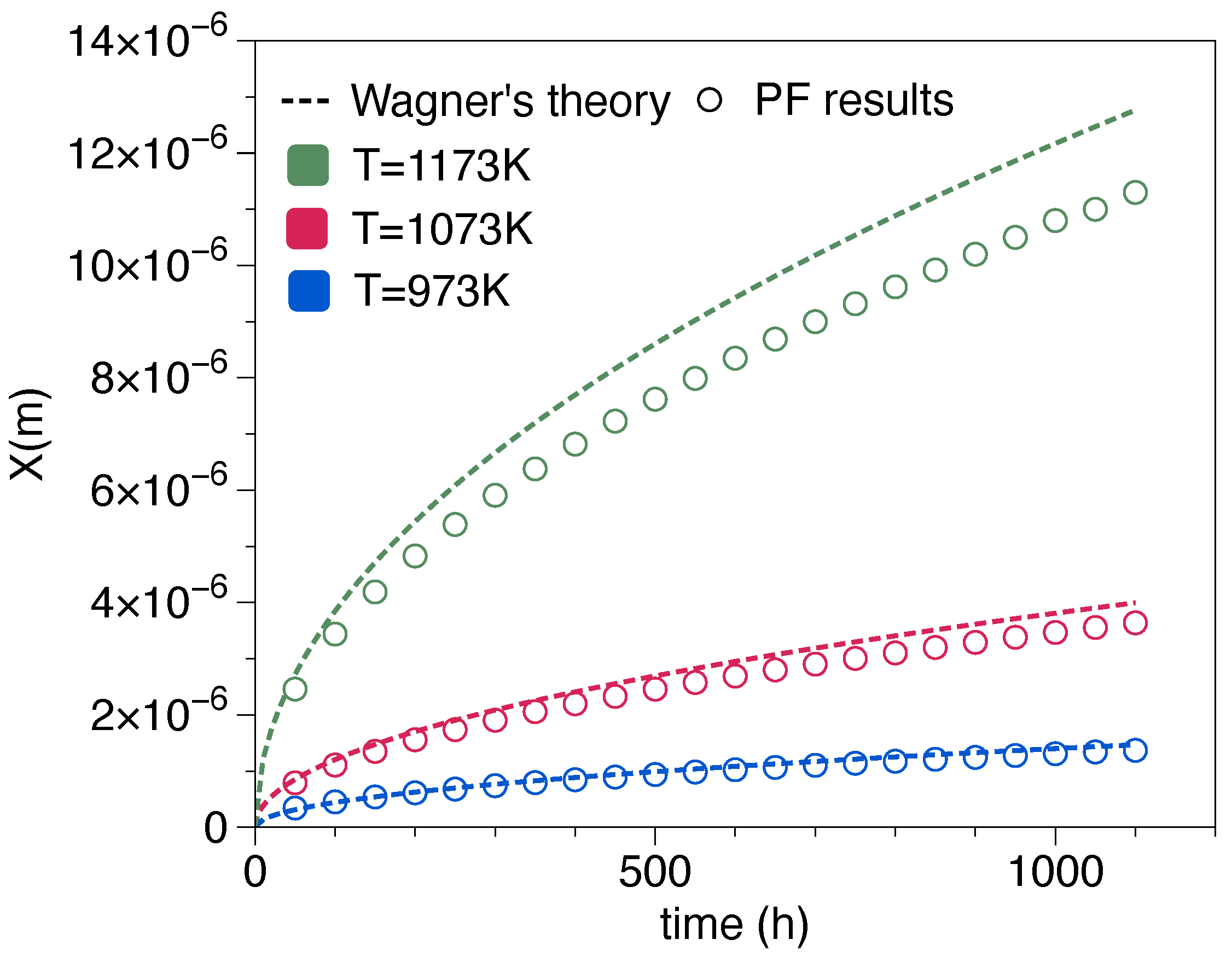

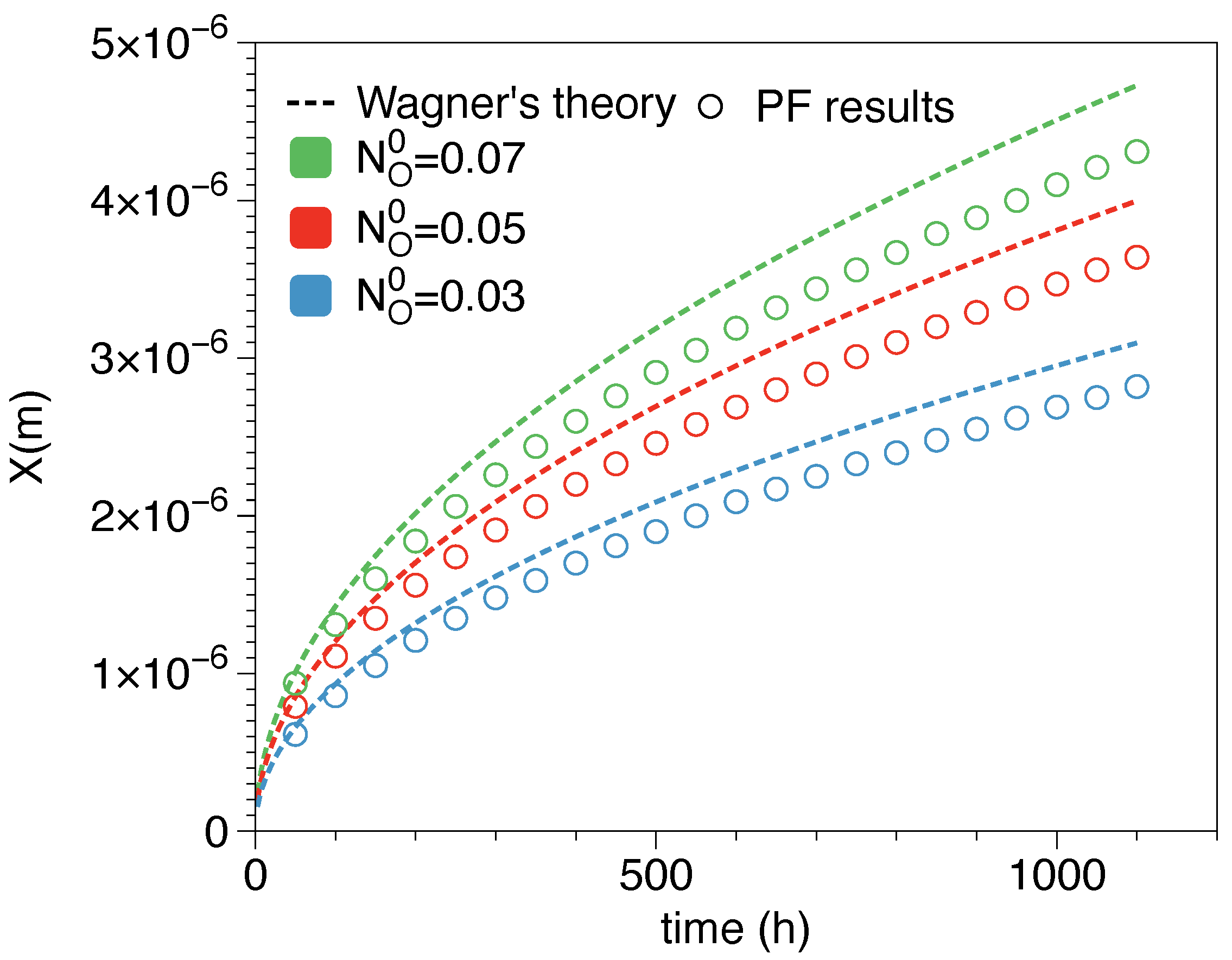

| Element | Fe | Cr | Mn | Ti | La | C | Si | Cu | P | Al | S |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | Bal. | 20–24 | 0.42 | 0.07 | 0.04–0.2 | 0.03 | 0.5 | 0.5 | 0.005 | 0.5 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Spatschek, R. Phase Field Study of Cr-Oxide Growth Kinetics in the Crofer 22 APU Alloy Supported by Wagner’s Theory. Energies 2023, 16, 3574. https://doi.org/10.3390/en16083574
Wang K, Spatschek R. Phase Field Study of Cr-Oxide Growth Kinetics in the Crofer 22 APU Alloy Supported by Wagner’s Theory. Energies. 2023; 16(8):3574. https://doi.org/10.3390/en16083574
Chicago/Turabian StyleWang, Kai, and Robert Spatschek. 2023. "Phase Field Study of Cr-Oxide Growth Kinetics in the Crofer 22 APU Alloy Supported by Wagner’s Theory" Energies 16, no. 8: 3574. https://doi.org/10.3390/en16083574





