Review on the Usage of Small-Chain Hydrocarbons (C2—C4) as Aid Gases for Improving the Efficiency of Hydrate-Based Technologies
Abstract
:1. Introduction
2. Main Application of Gas Hydrates
3. Reasons behind the Low Diffusion of Hydrate-Based Technologies
4. Strategies for Improving the Process Efficiency
5. Mixtures Containing Small HCs in Gas Hydrates Production
6. Phase Equilibrium Boundary Conditions for Gas Mixtures Containing Low Concentrations of C2—C4 HCs
7. Practical Applications of Small-Chain HCs in Hydrate-Based Processes
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Rossi, F.; Gambelli, A.M. Thermodynamic and kinetic phase equilibrium of single-guest hydrate and formation data of hydrate in presence of chemical additives: A review. Fluid Phase Equilibr. 2021, 536, 112958. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Thermodynamic and kinetic characterization of methane hydrate nucleation, growth and dissociation processes, according to the Labile Cluster Theory. Chem. Eng. J. 2021, 425, 130706. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Yin, S.; He, G.; Li, J.; Wu, Q. Research progress of the kinetics on natural gas hydrate replacement by CO2-containing mixed gas: A review. J. Nat. Gas Sci. Eng. 2022, 108, 104837. [Google Scholar] [CrossRef]
- Demirbas, A. Methane from gas hydrates in the Black Sea. Energy Sources Part A Recovery Util. Environ. Eff. 2009, 32, 165–171. [Google Scholar] [CrossRef]
- He, J.; Li, X.; Chen, Z.; Li, Q.; Zhang, Y.; Wang, Y.; Xia, Z.; You, C. Combined styles of depressurization and electrical heating for methane hydrate production. Appl. Energy 2021, 282, 266–273. [Google Scholar] [CrossRef]
- Li, Y.; Gambelli, A.M.; Rossi, F. Experimental study on the effect of SDS and micron copper particles mixture on carbon dioxide hydrates formation. Energies 2022, 15, 6540. [Google Scholar] [CrossRef]
- Xu, C.G.; Zhang, W.; Yan, K.F.; Cai, J.; Chen, Z.Y.; Li, X.S. Research on micro mechanism and influence of hydrate-based methane-carbon dioxide replacement for realizing simultaneous clean energy exploitation and carbon emission reduction. Chem. Eng. Sci. 2022, 248, 117266. [Google Scholar] [CrossRef]
- Wang, P.; Teng, Y.; Zhu, J.; Bao, W.; Han, S.; Li, Y.; Zhao, Y.; Xie, H. Review on the synergistic effect between metal-organic frameworks and gas hydrates for CH4 storage and CO2 separation applications. Renew. Sust. Energ. Rev. 2022, 167, 112807. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Filipponi, M.; Rossi, F. How methane release may affect carbon dioxide storage during replacement processes in natural gas hydrate reservoirs. J. Pet. Sci. Eng. 2021, 205, 108895. [Google Scholar] [CrossRef]
- Li, X.S.; Xu, C.G.; Zhang, Y.; Ruan, X.K.; Li, G.; Wang, Y. Investigation into gas production from natural gas hydrate: A review. Appl. Energy 2016, 172, 286–322. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, S.; Wang, Y.; Lang, X.; Li, G.; Liu, F.; Li, M. High storage capacity and high formation rate of carbon dioxide hydrates via super-hydrophobic fluorinated graphenes. Energy 2022, 264, 126405. [Google Scholar] [CrossRef]
- Wu, Y.; Tang, T.; Shi, L.; He, Y. Rapid hydrate-based methane storage promoted by bilayer surfactant-coated Fe3O4 nanoparticles under a magnetic field. Fuel 2021, 303, 121248. [Google Scholar] [CrossRef]
- Mahant, B.; Kushwaha, O.S.; Kumar, R. Synthesis of Cocos nucifera derived surfactant and its application in growth kinetics of methane gas hydrates for energy storage and transportation. Energy Conv. Manag. 2022, 269, 116044. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Castellani, B.; Nicolini, A.; Rossi, F. Gas hydrate formation as a strategy for CH4/CO2 separation: Experimental study on gaseous mixtures produced via Sabatier reaction. J. Nat. Gas Sci. Eng. 2019, 71, 102985. [Google Scholar] [CrossRef]
- Falahieh, M.M.; Bonyadi, M.; Lashanizadegan, A. Seawater desalination by coupling cyclopentane hydrate formation and capacitive deionization processes in the presence of Fe3O4 nanoparticles and magnetic field. Desalination 2022, 543, 116120. [Google Scholar] [CrossRef]
- Goel, N. In situ methane hydrate dissociation with carbon dioxide sequestration: Current knowledge and issues. J. Pet. Sci. Eng. 2006, 51, 169–184. [Google Scholar] [CrossRef]
- Xuan, K.; Yi, W.; Li, X.S.; Zhang, Y.; Chen, Y.Z. Influence of heat conduction and heat convection on hydrate dissociation by depressurization in a pilot-scale hydrate simulator. Appl. Energy 2019, 251, 113405. [Google Scholar]
- Nair, V.C.; Prasad, S.K.; Kumar, R.; Sangway, J.S. Energy recovery from simulated clayey gas hydrate reservoir using depressurization by constant rate gas release, thermal stimulation and their combination. Appl. Energy 2018, 225, 755–768. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.C.; Li, X.S.; Zhang, Y. Experimental investigation of optimization of well spacing for gas recovery from methane hydrate reservoir in sandy sediment by heat stimulation. Appl. Energy 2017, 207, 562–572. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Natural gas hydrates: Comparison between two different applications of thermal stimulation for performing CO2 replacement. Energy 2019, 172, 423–434. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Castellani, B.; Nicolini, A.; Rossi, F. Water salinity as potential aid for improving the carbon dioxide replacement process effectiveness in natural gas hydrate reservoirs. Processes 2020, 8, 1298. [Google Scholar] [CrossRef]
- Semenov, A.P.; Mendgaziev, R.I.; Stoporev, A.S.; Istomin, V.A.; Sergeeva, D.V.; Ogienko, A.G.; Vinokurov, V.A. The pursuit of a more powerful thermodynamic hydrate inhibitor than methanol. Dimethyl sulfoxide as a case study. Chem. Eng. J. 2021, 423, 130227. [Google Scholar] [CrossRef]
- Gambelli, A.M. Analyses on CH4 and CO2 hydrate formation to define the optimal pressure for CO2 injection to maximize the replacement efficiency into natural gas hydrate in presence of a silica-based natural porous medium, via depressurization techniques. Chem. Eng. Process. 2021, 167, 108512. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Stornelli, G.; Di Schino, A.; Rossi, F. Methane and carbon dioxide hydrates properties in presence of Inconel 718 particles: Analyses on its potential application in gas separation processes to perform efficiency improvement. J. Environ. Chem. Eng. 2021, 9, 106571. [Google Scholar] [CrossRef]
- Feng, J.C.; Li, G.; Li, X.S.; Li, B.; Chen, Z.Y. Evolution of hydrate dissociation by warm brine stimulation combined depressurization in the South China Sea. Energies 2013, 6, 5402–5425. [Google Scholar] [CrossRef]
- Feng, J.C.; Li, X.S.; Li, G.; Li, B.; Chen, Z.Y.; Wang, Y. Numerical investigation of hydrate dissociation performance in the South China Sea with different horizontal well configurations. Energies 2014, 7, 4813–4834. [Google Scholar] [CrossRef]
- Kumar, A.; Vedula, S.S.; Kumar, R.; Linga, P. Hydrate phase equilibrium data of mixed methane-tetrahydrofuran hydrates in saline water. J. Chem. Thermodyn. 2018, 117, 2–8. [Google Scholar] [CrossRef]
- Cao, X.; Wang, H.; Yang, K.; Wu, S.; Chen, Q.; Bian, J. Hydrate-based CO2 sequestration technology: Feasibilities, mechanisms, influencing factors, and applications. J. Pet. Sci. Eng. 2022, 219, 111121. [Google Scholar] [CrossRef]
- Aminu, M.D.; Nabavi, S.; Rochelle, C.; Manovìc, V. A review of developments in carbon dioxide storage. Appl. Energy 2017, 208, 1389–1419. [Google Scholar] [CrossRef]
- Teng, Y.H.; Zhang, D.X. Long-term viability of carbon sequestration in deep-sea sediments. Sci. Adv. 2018, 4, eaao6588. [Google Scholar] [CrossRef] [PubMed]
- Rochelle, C.A.; Camps, A.P.; Long, D.; Milodowski, A.; Bateman, K.; Gunn, D.; Jackson, P.; Lovell, M.A.; Rees, J. Can CO2 hydrate assist in the underground storage of carbon dioxide? Geol. Soc. London Spec. Publ. 2015, 319, 171–183. [Google Scholar] [CrossRef]
- House, K.Z.; Schrag, D.P.; Harvey, C.F. Permanent carbon dioxide sequestration in deep-sea sediments. Proc. Natl. Acad. Sci. USA 2006, 103, 12291. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cao, Q.; Xu, D.; Luo, S.; Guo, R. Improved methane storage capacity of methane hydrate promoted by vesicles from carboxylate surfactants and quaternary ammonium. J. Nat. Gas Sci. Eng. 2021, 93, 103990. [Google Scholar] [CrossRef]
- Kumar, S.; Kwon, H.T.; Choi, K.H.; Lim, W.; Cho, J.H.; Tak, K.; Moon, L. LNG: An eco-friendly cryogenic fuel for sustainable development. Appl. Energy 2011, 88, 4264–4273. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Wong, A.J.H.; Babu, P.; Kumar, R.; Kulprathipanja, S.; Rangsunvigit, P.; Linga, P. Rapid methane hydrate formation to develop a cost effective large scale energy storage system. Chem. Eng. J. 2016, 290, 161–173. [Google Scholar] [CrossRef]
- Wang, F.; Guo, G.; Liu, G.Q.; Luo, S.J.; Guo, R.B. Effects of surfactants micelles and surfactant-coated nanospheres on methane hydrate growth pattern. Chem. Eng. Sci. 2016, 144, 108–115. [Google Scholar] [CrossRef]
- Hua, T.Q.; Ahluwalia, R.K.; Peng, J.K.; Kromer, M.; Lasher, S.; McKenney, K.; Law, K.; Sinha, J. Technical assessment compressed hydrogen storage tank systems for automotive applications. Int. J. Hydrogen Energy 2011, 36, 3037–3049. [Google Scholar] [CrossRef]
- Sethia, G.; Sayari, A. Activated carbon with optimum pore size distribution for hydrogen storage. Carbon 2016, 99, 289–294. [Google Scholar] [CrossRef]
- Farha, O.K.; Hupp, J.T. Rational design, synthesis, purification, and activation of metal-organic framework materials. Acc. Chem. Res. 2010, 43, 1166–1175. [Google Scholar] [CrossRef]
- Li, G.; Kobayashi, H.; Taylor, J.M.; Ikeda, R.; Kubota, Y.; Kato, K.; Takata, M.; Yamamoto, T.; Toh, S.; Matsumura, S.; et al. Hydrogen storage in Pd nanocrystals covered with a metal organic framework. Nat. Mater. 2014, 13, 802–806. [Google Scholar] [CrossRef]
- Tong, L.; Xiao, J.; Bénard, P.; Chanine, R. Thermal management of metal hydride hydrogen storage reservoir using phase change materials. Int. J. Hydrogen Energy 2019, 44, 21055–21066. [Google Scholar] [CrossRef]
- Yanxing, Z.; Maoqiong, G.; Yuan, Z.; Xueqiang, D.; Jun, S. Thermodynamics analysis of hydrogen storage based on compressed gaseous hydrogen, liquid hydrogen and cryo-compressed hydrogen. Int. J. Hydrogen Energy 2019, 22, 16833–16840. [Google Scholar] [CrossRef]
- Di Profio, P.; Arca, S.; Rossi, F.; Filipponi, M. Reverse micelles enhance the formation of clathrate hydrates of hydrogen. J. Colloid Interf. Sci. 2018, 516, 224–231. [Google Scholar] [CrossRef]
- Jorgensen, S.W. Hydrogen storage tanks for vehicles: Recent progress and current status. Curr. Opin. Solid State Mater. Sci. 2011, 15, 39–43. [Google Scholar] [CrossRef]
- Dagdougui, H.; Sacile, R.; Bersani, C.; Ouammi, A. Chapter 4–Hydrogen storage and distribution: Implementation scenarios. Hydrog. Infrastruct. Energy Appl. 2018, 4, 37–52. [Google Scholar]
- Stamatakis, E.; Zoulias, E.; Tzamalis, G.; Massina, Z.; Analytis, V.; Christodoulou, C.; Stubos, A. Metal hydride hydrogen compressors: Current developments and early markets. Renew. Energy 2018, 127, 850–862. [Google Scholar] [CrossRef]
- Zohuri, B. Cryogenics and liquid hydrogen storage. In Hydrogen Energy; Springer: Cham, Switzerland, 2019; pp. 121–139. [Google Scholar]
- Dyadin, Y.A.; Larionov, E.G.; Manakov, A.Y.; Zhurko, F.V.; Aladko, E.Y.; Mikina, T.V.; Komarov, V.Y. Clathrate hydrates of hydrogen and neon. Mendeleev Commun. R. Soc. Chem. 1999, 9, 209–210. [Google Scholar] [CrossRef]
- Smirnov, G.S.; Stegailov, V.V. Toward determination of the new hydrogen hydrate clathrate structures. J. Phys. Chem. Lett. 2013, 4, 3560–3564. [Google Scholar] [CrossRef]
- Du, J.; Wang, L.; Liang, D.; Li, D. Phase equilibria and dissociation enthalpies of hydrogen semi-clathrate hydrate with tetrabutyl ammonium nitrate. J. Chem. Eng. Data 2021, 57, 603–609. [Google Scholar] [CrossRef]
- Hashimoto, S.; Sugahara, T.; Moritoki, M.; Sato, H.; Ohgaki, K. Thermodynamic stability of hydrogen + tetra-n-butyl ammonium bromide mixed gas hydrate in nonstoichiometric aqueous solutions. Chem. Eng. Sci. 2008, 63, 1092–1097. [Google Scholar] [CrossRef]
- Wang, P.; Li, K.; Yang, J.; Zhu, J.; Zhao, Y.; Teng, Y. Experimental and theoretical study on dissociation thermodynamics and kinetics of hydrogen-propane hydrate. Chem. Eng. J. 2021, 426, 131279. [Google Scholar] [CrossRef]
- Zhang, Y.; Bhattacharjee, G.; Kumar, R.; Linga, P. Solidified hydrogen storage (Solid-HyStore) via clathrate hydrates. Chem. Eng. J. 2022, 431, 133702. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F.; Cotana, F. Gas hydrates as high-efficiency storage system: Perspectives and potentialities. Energies 2022, 15, 8728. [Google Scholar] [CrossRef]
- Sinehbaghizadeh, S.; Saptoro, A.; Mohammadi, A.H. CO2 hydrate properties and applications: A state of the art. Prog. Energy Combust. Sci. 2022, 93, 101026. [Google Scholar] [CrossRef]
- Sahu, P.; Krishnaswamy, S.; Ponnani, K.; Pande, N.K. A thermodynamic approach to selection of suitable hydrate formers for seawater desalination. Desalination 2018, 436, 144–151. [Google Scholar] [CrossRef]
- Tromp, R.H.; Neilson, G.W.; Soper, A.K. Water structure in concentrated lithium chloride solutions. J. Chem. Phys. 1992, 96, 8460–8469. [Google Scholar] [CrossRef]
- Karamoddin, M.; Varaminian, F. Water desalination using R141b gas hydrate formation. Desalin. Water Treat. 2014, 52, 2450–2456. [Google Scholar] [CrossRef]
- Nam Park, K.; Hong, S.Y.; Lee, J.W.; Kang, K.C.; Lee, Y.C.; Ha, M.G.; Lee, J.D. A new apparatus for seawater desalination by gas hydrate process and removal characteristics of dissolved minerals (Na+, Mg2+, Ca2+, K+, B3+). Desalination 2011, 274, 91–96. [Google Scholar] [CrossRef]
- Gaikward, N.; Nakka, R.; Khavala, V.; Bhadani, A.; Mamane, H.; Kumar, R. Gas hydrate-based process for desalination of heavy metal ions from an aqueous solution: Kinetics and rate of recovery. ACS Es&T Water 2021, 1, 134–144. [Google Scholar]
- Karamoddin, M.; Varaminian, F. Water purification by freezing and gas hydrate processes, and removal of dissolved minerals (Na+, K+, Mg2+, Ca2+). J. Mol. Liq. 2016, 223, 1021–1031. [Google Scholar] [CrossRef]
- Montazeri, S.M.; Kolliopoulos, G. Hydrate based desalination for sustainable water treatment: A review. Desalination 2022, 537, 115855. [Google Scholar] [CrossRef]
- Lu, H.; Matsumoto, R.; Tsuji, Y.; Oda, H. Anion plays a more important role than cation in affecting gas hydrate stability in electrolyte solution?—A recognition from experimental results. Fluid Phase Equilibr. 2001, 178, 225–232. [Google Scholar] [CrossRef]
- Wu, J.-J.; Kang, M.-Q.; Hu, F.-L.; Yan, Y.-H.; Liu, C.-Z.; Chen, J.; Liang, Z.-K.; Zeng, Y.-S.; Jiang, J.-H.; Deng, B. Comparing hydrate-based method with freezing/thawing method for chromium hydroxide sulfate removal close to the melting point of ice. Sep. Purif. Technol. 2021, 266, 118523. [Google Scholar] [CrossRef]
- Neves, M.F.; Trombin, V.G.; Marques, V.N.; Martinez, L.F. Global orange juice market: A 16-year summary and opportunities for creating value. Trop. Plant Pathol. 2020, 45, 166–174. [Google Scholar] [CrossRef]
- Adnan, A.; Mushtaq, M.; Islam, T. Fruit juice concentrates. Fruit Juices 2018, 5, 217–240. [Google Scholar]
- Claben, T.; Seidl, P.; Loekman, S.; Gatternig, B.; Rauch, C.; Delgado, A. Revie on the food technological potentials for gas hydrate technology. Curr. Opin. Food Sci. 2019, 29, 48–55. [Google Scholar]
- Srivastava, S.; Hitzmann, B.; Zettel, V. A future road map for carbon dioxide (CO2) gas hydrate as an emerging technology in food research. Food Bioprocesses Technol. 2021, 14, 1758–1762. [Google Scholar] [CrossRef]
- Lv, Y.; Xia, X.; Wang, F.; Wu, X.; Cheng, C.; Zhang, L.; Yang, L.; Zhao, J.; Song, Y. Clathrate hydrate for phase change cold storage: Simulation advances and potential applications. J. Energy Storage 2022, 55, 105835. [Google Scholar] [CrossRef]
- Li, Y.; Kumar, N.; Hirschey, J.; Akamo, D.O.; Li, K.; Tugba, T.; Goswami, M.; Orlando, R.; LaClair, T.J.; Graham, S.; et al. Stable salt hydrate-based thermal energy storage materials. Compos. B Eng. 2022, 233, 109621. [Google Scholar] [CrossRef]
- Chang, Y.; Sun, Z. Synthesis and thermal properties of n-tetradecane phase change microcapsules for cold storage technology. Renew. Sust. Energ. Rev. 2020, 117, 104959. [Google Scholar]
- Cheng, C.; Wang, F.; Tian, Y.; Wu, X.; Zheng, J.; Zhang, J.; Li, L.; Yang, P.; Zhao, J. Review and prospects of hydrate cold storage technology. Renew. Sust. Energ. Rev. 2020, 117, 109492. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, M.; Wang, T.; Yang, L.; Zhang, X.; Zhao, J.; Song, Y. An in-situ MRI method for quantifying temperature changes during crystal hydrate growth in porous medium. Int. J. Therm. Sci. 2022, 31, 1542–1550. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, H.; Dai, S.; Kuang, Y.; Yang, L.; Wang, J.; Zhao, J.; Song, Y. Effects of depressurization on gas production and water performance from excess-gas and excess-water methane hydrate accumulations. Chem. Eng. J. 2022, 431, 133223. [Google Scholar] [CrossRef]
- Li, G.; Li, X.S.; Li, B.; Wang, Y. Methane hydrate dissociation using inverted five-spot water flooding method in cubic hydrate simulator. Energy 2014, 64, 298–306. [Google Scholar] [CrossRef]
- Collett, T.S.; Ginsburg, G.D. Gas hydrates in the Messoyakha gas field of the west Siberian basin–a re-examination of the geologic evidence. Int. J. Offshore Polar Eng. 1997, 1, 96–103. [Google Scholar]
- Lu, X.; Wang, L.; Zhang, X.; Wang, S.; Yao, H.; Li, Q. Instability of seabed and pipes induced by NGH dissociation. In Proceedings of the Twentieth International Offshore and Polar Engineering Conference, Beijing, China, 20–25 June 2010. [Google Scholar]
- Sultan, N.; Choconat, P.; Foucher, J.P.; Mienert, J. Effect of gas hydrates melting on seafloor slope instability. Mar. Geol. 2004, 213, 379–401. [Google Scholar] [CrossRef]
- Xu, W.; Germanovich, L.N. Excess pore pressure resulting from methane hydrate dissociation in marine sediments: A theoretical approach. J. Geophys. Res. Solar Earth 2006, 111, 1978–2012. [Google Scholar] [CrossRef]
- Miyazaki, K.; Masui, A.; Yamamoto, Y.; Ogata, Y.; Aoki, K.; Yamaguchi, T. Investigation of deformation mechanism for methane hydrate sediment based upon mechanical properties in unloading and reloading process under triaxial compression. In Proceedings of the Eighth ISOPE Ocean Mining Symposium, Chennai, India, 20–24 September 2009. [Google Scholar]
- Circone, S.; Stern, L.A.; Kirby, S.H.; Durham, W.B.; Chakoumakos. CO2 hydrate: Synthesis, composition, structure, dissociation behavior, and a comparison to structure I CH4 hydrate. J. Phys. Chem. B 2003, 107, 5529. [Google Scholar] [CrossRef]
- Henning, R.W.; Schultz, A.J.; Thieu, V. Neutron diffraction studies of CO2 clathrate hydrate: Formation from deuterated ice. J. Phys. Chem. A 2003, 104, 5066–5071. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Li, Y.; Rossi, F. Influence of different proportion of CO2/N2 binary gas mixture on methane recovery through replacement processes in natural gas hydrate. Chem. Eng. Process. 2022, 175, 108932. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Gambelli, A.M.; Zhao, Z.; Gao, Y.; Rossi, F.; Mei, S. In situ experimental study on the effect of mixed inhibitors on the phase equilibrium of carbon dioxide hydrate. Chem. Eng. Sci. 2022, 248, 117230. [Google Scholar] [CrossRef]
- Li, Y.; Gambelli, A.M.; Rossi, F.; Mei, S. Effect of promoters on CO2 hydrates formation: Thermodynamic assessment and microscale Raman spectroscopy/hydrate crystal morphology characterization analysis. Fluid Phase Equlibr. 2021, 550, 113218. [Google Scholar] [CrossRef]
- Bradshaw, R.W.; Grathouse, J.A.; Cygan, R.T.; Simmons, B.A.; Dedrick, D.E.; Majzoub, E.H. Desalination utilizing clathrate hydrates. (LDRD Final Report). Contract 2008. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Joonaki, E.; Vasheghani Farahani, M.; Takeya, S.; Ruppel, C.; Yang, J.; English, N.J.; Schicks, J.M.; Edlmann, K.; Mehrabian, H.; et al. Gas hydrates in sustainable chemistry. Chem. Soc. 2020, 49, 5225–5309. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.; Kumar, R.; Linga, P. Unusual behavior of propane as co-guest during hydrate formation in silica sand: Potential application to seawater desalination and carbon dioxide capture. Chem. Eng. Sci. 2014, 117, 342–351. [Google Scholar] [CrossRef]
- Fazlali, A.; Kazemi, S.A.; Keshavarz-Moraveji, M.; Mohammadi, A.H. Impact of different surfactants and their mixtures on methane-hydrate formation. Energy Technol. 2013, 1, 471–477. [Google Scholar] [CrossRef]
- Bhattacharjee, G.; Linga, P. Amino acids as kinetic promoters for gas hydrate applications: A mini review. Energy Fuels 2021, 35, 7553–7571. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Laurens, S.; Richon, D. Experimental study of methane hydrate phase equilibrium in the presence of polyethylene glycol-400aqueous solution. J. Chem. Eng. Data 2009, 54, 3118–3120. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Richon, D. Gas hydrate phase equilibrium in the presence of ethylene glycol or methanol aqueous solution. Ind. Eng. Chem. Res. 2010, 49, 8865–8869. [Google Scholar] [CrossRef]
- Broni-Bediako, E.; Amorin, R.; Bavoh, G.B. Gas hydrate formation phase boundary behaviour of synthetic natural gas system of the Keta basin of Ghana. Open Pet. Eng. J. 2017, 10, 64–72. [Google Scholar] [CrossRef]
- Partoon, B.; Wong, N.M.S.; Sabil, K.M.; Nasrifar, K.; Ahmad, M.R. A study on thermodynamics effect of [EMIM]-Cl and [OH-C2MIM]-Cl on methane hydrate equilibrium line. Fluid Phase Equilibr. 2013, 337, 26–31. [Google Scholar] [CrossRef]
- Xiao, C.; Adidharma, H. Dual function inhibitors for methane hydrate. Chem. Eng. Sci. 2009, 64, 1522–1527. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Partoon, B.; Lal, B.; Keong, L.K. Methane hydrate-liquid-vapor-equilibrium phase condition measurements in the presence of natural amino acids. J. Nat. Gas Sci. Eng. 2017, 37, 425–434. [Google Scholar] [CrossRef]
- Badawy, W.A.; Ismail, K.M.; Fathi, A.M. Effect of Ni content on the corrosion behavior of Cu-Ni alloys in neutral chloride solutions. Electrochim. Acta 2005, 50, 3603–3608. [Google Scholar] [CrossRef]
- Madeira, P.P.; Bessa, A.; Alvares-Ribeiro, L.; Aires-Barros, M.R.; Rodrigues, A.E.; Uversky, V.N.; Zaslavsky, B.Y. Amino acid/water interactions study: A new amino acid scale. J. Biomol. Struct. Dyn. 2014, 32, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, B.; Li, K.; Song, Y.; Yang, M. Stability and structure of multiply occupied sII CO2 clathrate hydrates: A possibility for carbon capturing. J. Mol. Liq. 2023, 380, 121746. [Google Scholar] [CrossRef]
- Juan, J.W.; Tang, M.; Chen, L.J.; Lin, S.T.; Chen, P.C.; Chen, Y.P. Measurements for the equilibrium conditions of methane hydrate in the presence of cyclopentanone or 4-hydroxy-4-methyl-2-pentanone additives. Fluid Phase Equilibr. 2015, 386, 162–167. [Google Scholar] [CrossRef]
- Komarov, V.Y.; Rodionova, T.V.; Terekhova, I.S.; Kuratieva, N.V. The cubic superstructure-I of tetrabutylammonium fluoride (C4H9)4NF29.7H2O clathrate hydrate. J. Incl. Phenom. Macrocycl. Chem. 2007, 59, 11–15. [Google Scholar] [CrossRef]
- Sun, Z.G.; Liu, G.C.; Zhou, B.; Xu, L.Z. Phase equilibrium and latent heat of tetra-n-butylammonium chloride semi-clathrate hydrate. J. Chem. Eng. Data 2011, 56, 3416–3418. [Google Scholar] [CrossRef]
- Muromachi, S.; Kida, M.; Takeya, S.; Yamamoto, Y.; Ohmura, R. Characterization of the ionic clathrate hydrate of tetra-n-butylammonium acrylate. Can. J. Chem. 2015, 93, 954–959. [Google Scholar] [CrossRef]
- Fakharian, H.; Ganji, H.; Naderi Far, A.; Kameli, M. Potato starch as methane hydrate promoter. Fuel 2012, 94, 356–360. [Google Scholar] [CrossRef]
- Ricaurte, M.; Dicharry, C.; Broseta, D.; Renaud, X.; Torré, J.P. CO2 removal from a CO2-CH4 gas mixture by clathrate hydrate formation using THF and SDS as water-soluble hydrate promoters. Ind. Eng. Chem. Res. 2013, 52, 899–910. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Stornelli, G.; Di Schino, A.; Rossi, F. Methane and carbon dioxide hydrate formation and dissociation in presence of a pure quartz porous framework impregnated with CuSn12 metallic powder: An experimental report. Materials 2021, 14, 5115. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Gambelli, A.M.; Sharma, D.K.; Castellani, B.; Nicolini, A.; Castaldi, M.J. Experiments on methane hydrates formation in seabed deposits and gas recovery adopting carbon dioxide replacement strategies. Appl. Therm. Eng. 2019, 148, 371–381. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, B.; Chen, Y.; Zhang, S.; Guo, W.; Cai, Y.; Tai, B.; Wang, W. Methane storage in a hydrated form as promoted by leucines for possible application to natural gas transportation and storage. Energy Technol. 2015, 3, 815–819. [Google Scholar] [CrossRef]
- Yan, C.; Li, Y.; Cheng, Y.; Wei, J.; Tian, W.; Li, S.; Wang, Z. Multifield coupling mechanism in formations around wellbore during the exploitation of methane hydrate with CO2 replacement. Energy 2022, 245, 123283. [Google Scholar] [CrossRef]
- Li, X.Y.; Li, X.S.; Wang, Y.; Liu, J.W.; Hu, H.Q. The optimization mechanism for gas hydrate dissociation by depressurization in the sediment with different water saturations and different particle sizes. Energy 2021, 215, 119129. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kawamura, T.; Yamamoto, Y.; Komai, T. Transformation of methane hydrate to carbon dioxide hydrate: In situ Raman spectroscopic observations. In Proceedings of the 15th International Offshore and Polar Engineering Conference, OnePetro, Shanghai, China, 14–19 June 2020. [Google Scholar]
- Xu, C.G.; Cai, J.; Yu, Y.S.; Chen, Z.Y.; Li, X.S. Research on micro-mechanism and efficiency of CH4 exploitation via CH4-CO2 replacement from natural gas hydrates. Fuel 2018, 216, 255–265. [Google Scholar] [CrossRef]
- Tung, Y.T.; Chen, L.J.; Chen, Y.P.; Lin, S.T. In situ methane recovery and carbon dioxide sequestration in methane hydrates: A molecular dynamics simulation study. J. Phys. Chem. B 2011, 115, 15295–15302. [Google Scholar] [CrossRef]
- Baldwin, B.A.; Stevens, J.; Howard, J.J.; Graue, A.; Kvamme, B.; Aspenes, E.; Ersland, G.; Husebo, J.; Zornes, D.R. Using magnetic resonance imaging to monitor CH4 hydrate formation and spontaneous conversion of CH4 hydrate to CO2 hydrate in porous media. Magn. Reson. Imaging 2009, 27, 720–726. [Google Scholar] [CrossRef]
- Li, Y.; Gambelli, A.M.; Chen, J.; Yin, Z.; Rossi, F.; Tronconi, E.; Mei, S. Experimental study on the competition between carbon dioxide hydrate and ice below the freezing point. Chem. Eng. Sci. 2023, 268, 118426. [Google Scholar] [CrossRef]
- Gambelli, A.M. Variations in terms of CO2 capture and CH4 recovery during replacement processes in gas hydrate reservoirs, associated to the “memory effect”. J. Clean. Prod. 2022, 360, 132154. [Google Scholar] [CrossRef]
- Saberi, A.; Alamdari, A.; Shariati, A.; Mohammadi, A.H. Experimental measurement and thermodynamic modelling of equilibrium condition for natural gas hydrate in MEG aqueous solution. Fluid Phase Equilibr. 2018, 459, 110–118. [Google Scholar] [CrossRef]
- Sadeq, D.; Iglauer, S.; Lebedev, M.; Smith, C.; Barifcani, A. Experimental determination of hydrate phase equilibrium for different gas mixtures containing methane, carbon dioxide and nitrogen with motor current measurements. J. Nat. Gas Sci. Eng. 2017, 38, 59–73. [Google Scholar] [CrossRef]
- Semenov, A.P.; Medvedev, V.I.; Guschin, P.A.; Yakushev, V. Effect of heating rate on the accuracy of measuring equilibrium conditions for methane and argon hydrates. Chem. Eng. Sci. 2015, 137, 161–169. [Google Scholar] [CrossRef]
- Seo, Y.T.; Lee, H. Multiple-phase hydrate equilibria of the ternary carbon dioxide, methane, and water mixtures. J. Phys. Chem. B 2001, 105, 10084–10090. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Presciutti, A.; Rossi, F. Review on the characteristics and advantages related to the use of flue-gas as CO2/N2 mixture for gas hydrate production. Fluid Phase Equilibr. 2021, 541, 113077. [Google Scholar] [CrossRef]
- Xu, S.; Fan, S.; Yao, H.; Wang, Y.; Lang, X.; Lv, P.; Fang, S. The phase equilibria of multicomponent gas hydrate in methanol/ethylene glycol solution based formation water. J. Chem. Thermodyn. 2017, 104, 212–217. [Google Scholar] [CrossRef]
- Yu, Y.S.; Zhou, S.D.; Li, X.S.; Wang, S.L. Effect of graphite nanoparticles on CO2 hydrate phase equilibrium. Fluid Phase Equilibr. 2016, 414, 23–28. [Google Scholar] [CrossRef]
- Shicai, S.; Yong, Z.; Changling, L.; Yufeng, L. Preliminary study on measurement technology for hydrate phase equilibrium. Fluid Phase Equilibr. 2015, 403, 60–69. [Google Scholar] [CrossRef]
- Pahlavanzadeh, H.; Nouri, S.; Mohammadi, A.H.; Manteghian, M. Experimental measurements and thermodynamic modelling of hydrate dissociation conditions in CO2 + THF + NaCl + water systems. J. Chem. Thermodyn. 2020, 141, 105956. [Google Scholar] [CrossRef]
- Yasuda, K.; Oto, Y.; Shen, R.; Uchida, T.; Ohmura, R. Phase equilibrium condition measurements in nitrogen and air clathrate hydrate forming systems at temperatures below freezing point of water. J. Chem. Thermodyn. 2013, 67, 143–147. [Google Scholar] [CrossRef]
- Belandria, V.; Mohammadi, A.H.; Eslamimanesh, A.; Richon, D.; Sànchez-Mora, M.F.; Galicia-Luna, L.A. Phase equilibrium measurements for semi-clathrate hydrates of the (CO2 + N2 + tetra-n-butylammonium bromide) aqueous solution systems: Part 2. Fluid Phase Equilibr. 2012, 322–323, 102–112. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, J.D.; Lee, H.J.; Lee, E.K.; Kim, Y. Gas hydrate formation method to capture the carbon dioxide for pre-combustion process in IGCC plant. Int. J. Hyd. 2011, 36, 1115–1121. [Google Scholar] [CrossRef]
- Sun, S.C.; Liu, C.L.; Meng, Q.G. Hydrate phase equilibrium of binary guest-mixtures containing CO2 and N2 in various system. J. Chem. Thermodyn. 2015, 84, 1–6. [Google Scholar] [CrossRef]
- Sfaxi, I.B.A.; Durand, I.; Lugo, R.; Mohammadi, A.H.; Richon, D. Hydrate phase equilibria of CO2+N2+aqueous solution of THF, TBAB or TBAF system. Int. J. Greenh. Gas Control 2014, 26, 185–192. [Google Scholar] [CrossRef]
- Schoderbek, D.; Boswell, R. Successful Test of Gas Hydrate Production Test Well Ignik Sikumi on Alaska’s North Slope. Methane hydrate newsletter. US DOE Fire Ice 2011, 11, 1–5. [Google Scholar]
- Park, Y.; Kim, D.Y.; Lee, J.W.; Huh, D.G.; Park, K.P.; Lee, J.; Lee, H. Sequestering carbon dioxide into complex structures of naturally occurring gas hydrates. Proc. Natl. Acad. Sci. USA 2006, 103, 12690–12694. [Google Scholar] [CrossRef]
- Lim, D.; Ro, H.; Seo, Y.; Seo, Y.J.; Lee, J.Y.; Kim, S.J.; Lee, J.; Lee, H. Thermodynamic stability and guest distribution of CH4/N2/CO2 mixed hydrates for methane hydrate production using N2/CO2 injection. J. Chem. Thermodyn. 2017, 106, 16–21. [Google Scholar] [CrossRef]
- Ricaurte, M.; Torré, J.P.; Diaz, J.; Dicharry, C. In situ injection of THF to trigger gas hydrate crystallization: Application to the evaluation of a kinetic hydrate promoter. Chem. Eng. Res. Des. 2014, 92, 1674–1680. [Google Scholar] [CrossRef]
- Torré, J.P.; Ricaurte, M.; Dicharry, C.; Boseta, D. CO2 enclathration in the presence of water-soluble hydrate promoters: Hydrate phase equilibria and kinetic studies in quiescent conditions. Chem. Eng. Sci. 2012, 82, 1–13. [Google Scholar] [CrossRef]
- Ripmeester, J.A.; Tse, J.S.; Ratcliffe, C.I.; Powell, B.M. A new clathrate hydrate structure. Nature 1987, 325, 135–136. [Google Scholar] [CrossRef]
- Sundramoorthy, J.D.; Hammonds, P.; Lal, B.; Philips, G. Gas hydrate equilibrium measurement and observation of gas hydrate dissociation with/without KHI. Procedia Eng. 2016, 148, 870–877. [Google Scholar] [CrossRef]
- Moojer-van den Heuvel, M.M.; Peters, C.J.; Arons, J.S. Gas hydrate phase equilibria for propane in the presence of additive components. Fluid Phase Equilibr. 2002, 193, 245–249. [Google Scholar] [CrossRef]
- Buleiko, V.M.; Grigoriev, B.A.; Mendoza, J. Calorimetric investigation of hydrates of pure isobutane and iso- and normal butane mixtures. Fluid Phase Equilibr. 2018, 462, 14–24. [Google Scholar] [CrossRef]
- Babu, P.; Yang, T.; Veluswamy, H.P.; Kumar, R.; Linga, P. Hydrate phase equilibrium of ternary gas mixture containing carbon dioxide, hydrogen and propane. J. Chem. Thermodyn. 2013, 61, 58–63. [Google Scholar] [CrossRef]
- Sun, Z.G.; Fan, S.S.; Guo, K.H.; Shi, L.; Wang, R.Z. Equilibrium hydrate formation conditions for methylcyclohexane with methane and a ternary gas mixture. Fluid Phase Equilibr. 2002, 198, 293–298. [Google Scholar] [CrossRef]
- Nh, H.J.; Robinson, D. The role of n-butane in hydrate formation. AlChE J. 1976, 22, 656–661. [Google Scholar]
- McLeod, H.; Campbell, J. Natural gas hydrates at pressures to 10,000 psia. J. Petrol. Technol. 1961, 13, 590–594. [Google Scholar] [CrossRef]
- Smith, C.; Pack, D.; Barifcani, A. Propane, n-butane and i-butane stabilization effects on methane hydrates. J. Chem. Thermodyn. 2017, 115, 293–301. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Experimental characterization of the difference in induction period between CH4 and CO2 hydrates: Motivations and possible consequences on the replacement process. J. Nat. Gas Sci. Eng. 2022, 108, 104848. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Re-definition of the region suitable for CO2/CH4 replacement into hydrates as a function of the thermodynamic difference between CO2 hydrate formation and dissociation. Process Saf. Environ. Prot. 2023, 169, 132–141. [Google Scholar] [CrossRef]
- Yousefi, S.; Bahrami, M.; Hesam Najibi, S. Hydrate phase equilibria of carbon dioxide/propane gas mixture in concentrated aqueous sodium chloride solutions: Experimental data and prediction. J. Mol. Liq. 2023, 376, 121417. [Google Scholar] [CrossRef]
- Wu, B.; Horvat, K.; Mahajan, D.; Chai, X.; Yang, D.; Dai, X. Free-conditioning dewatering of sewage sludge through in situ propane hydrate formation. Water Res. 2018, 145, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Cha, M.; Jang, J.; Lim, S.G.; Oh, C.Y.; Lee, J.W.; Park, J.; Yoon, J.H. Thermodynamic behavior and spectroscopic properties of CO and C3H8 mixed gas hydrates: Implications for hydrate-based gas separation. Chem. Eng. J. 2022, 428, 132076. [Google Scholar] [CrossRef]
- Misawa, T.; Ishikawa, T.; Takeya, S.; Alavi, S.; Ohmura, R. Continuous hydrate-based CO2 separation from H2 + CO2 gas mixture using cyclopentane as co-guest. J. Ind. Eng. Chem. 2023, article in press. [Google Scholar] [CrossRef]
- Du, S.; Han, X.; Cai, W.; Zhu, J.; Ma, X.; Han, S.; Chen, D.; Zhao, Y.; Li, H.; Lu, H.; et al. Formation of the structure-II gas hydrate from low-concentration propane mixed with methane. Chin. J. Chem. Eng. 2022, article in press. [Google Scholar] [CrossRef]

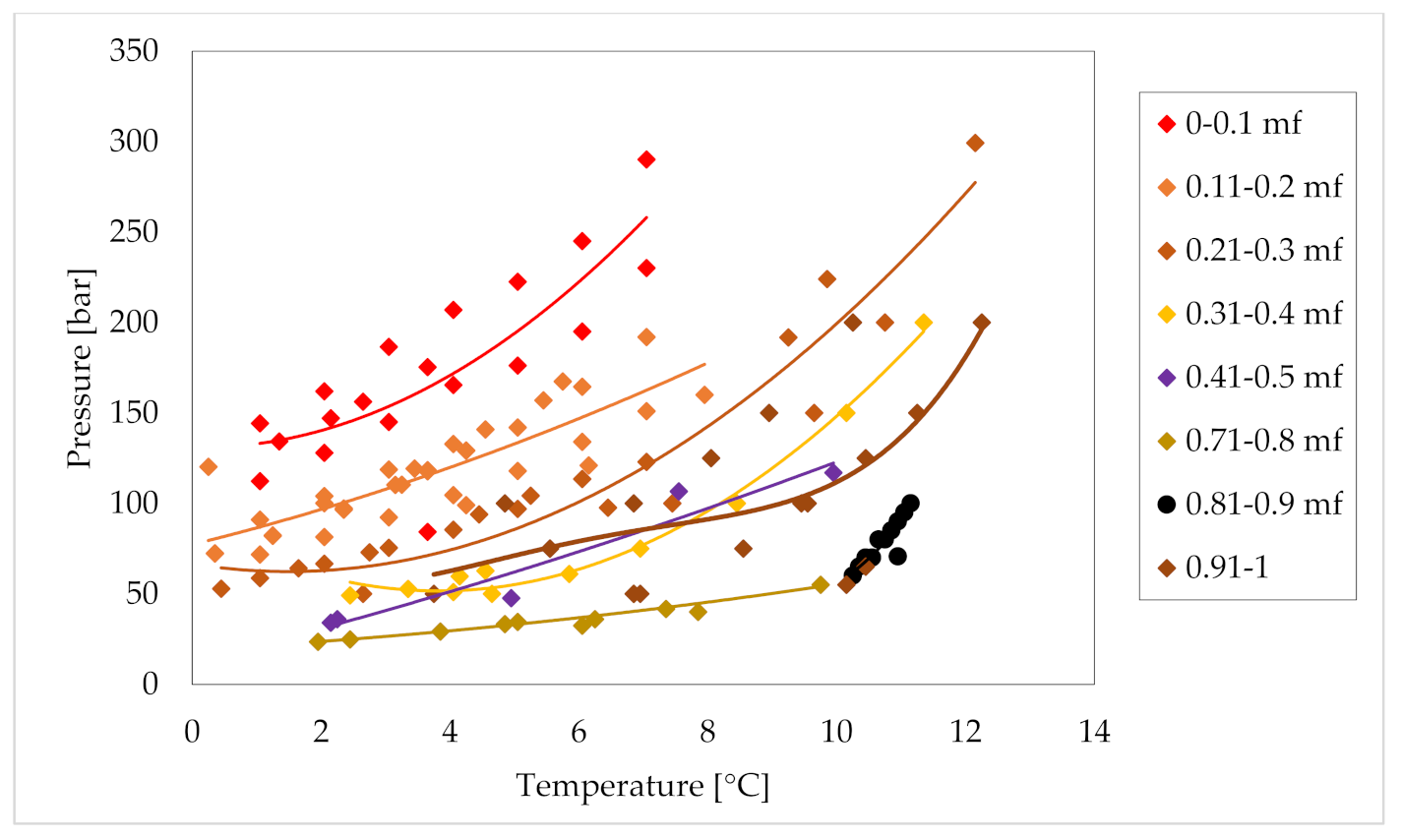

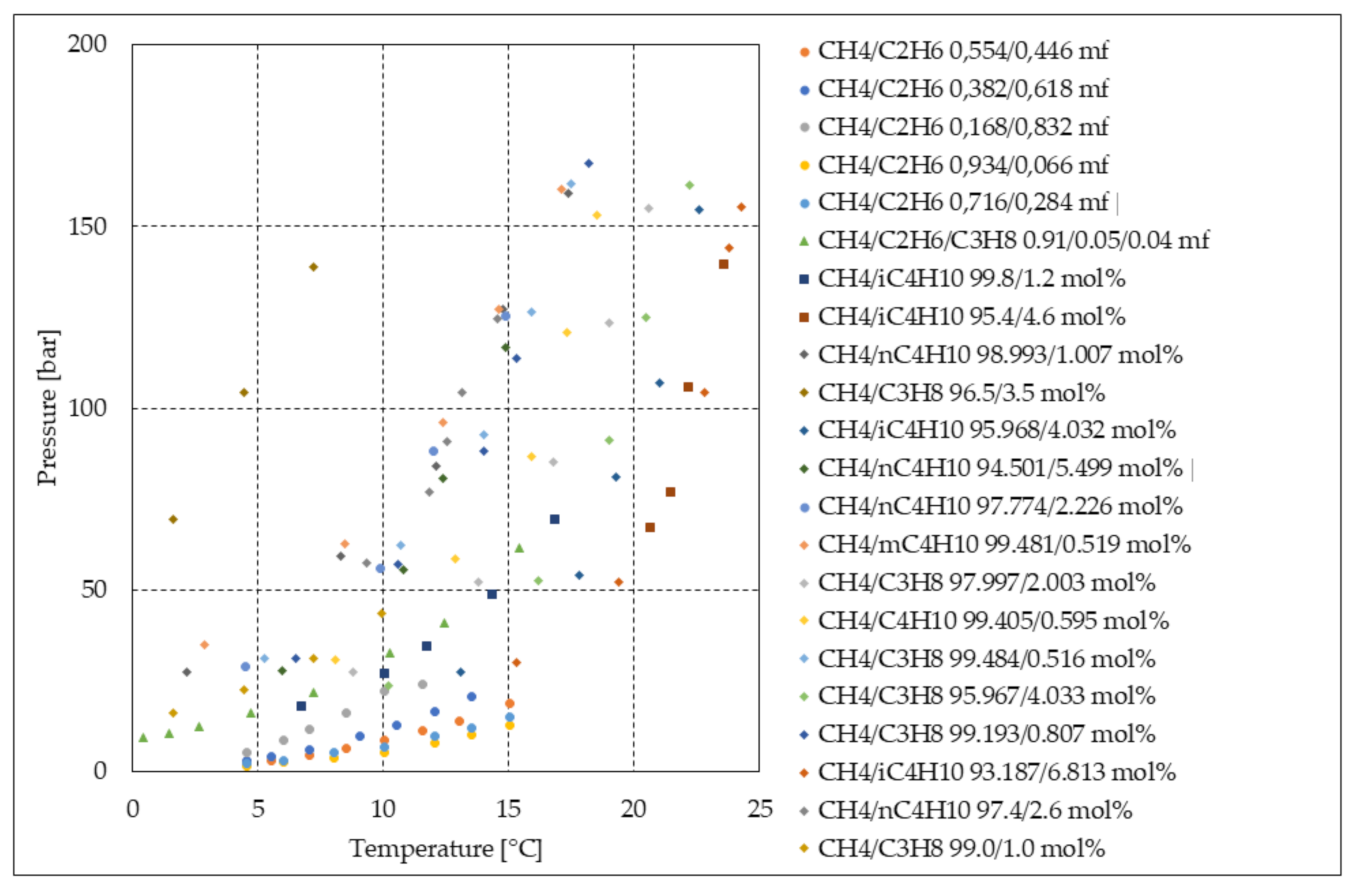
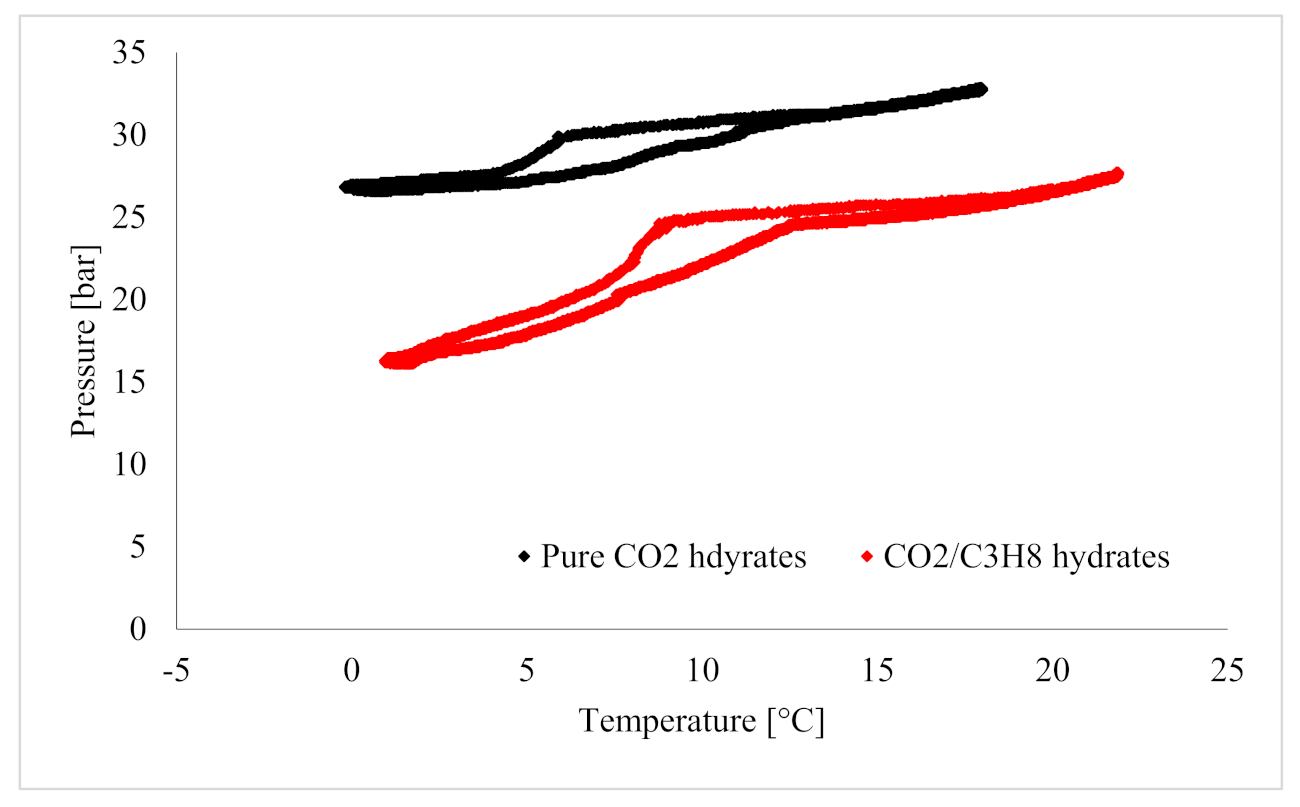
| Application | Advantages | Limitations |
|---|---|---|
| Energy production | Abundancy (1015–1017 m3 CH4) | Risks for the environment: deformations, soil failures, and landslides |
| Potential carbon-neutral energy source | Risks for the atmosphere: gas leaks | |
| Higher distribution of natural reservoirs (if compared with conventional energy sources) | High energy spent/energy recovered ratio | |
| Technical maturity still not reached | ||
| CO2 disposal in deep oceans | Suitable pressures and temperatures | Complexity of the CO2 injection phase |
| Unlimited availability of space | Too elevated costs (without the contemporary production of energy) | |
| Environmentally friendly | ||
| Storage of energy gases | Ease of transportation | Low efficiency for small-size molecules (H2) |
| Energy density similar to that of pressurized vessels | Necessity of using gas mixtures to improve the storage efficiency | |
| Safety for operators | ||
| Safety for the environment | ||
| Desalination, wastewater treatment, and food concentration | High efficiency (up to 98%) | Need for chemical additives |
| Possibility for CO2 reuse prior to final disposal | Recovery of additives from water | |
| Mature technology | Overall costs | |
| Separation of hydrates from residual brine | ||
| Cold storage | Possibility for CO2 reuse prior to final disposal | Technical maturity still not reached |
| High energy density | Elevated plant costs | |
| Promising application in key sectors (nuclear plants) | Need for chemical additives | |
| Use for security systems |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambelli, A.M.; Rossi, F. Review on the Usage of Small-Chain Hydrocarbons (C2—C4) as Aid Gases for Improving the Efficiency of Hydrate-Based Technologies. Energies 2023, 16, 3576. https://doi.org/10.3390/en16083576
Gambelli AM, Rossi F. Review on the Usage of Small-Chain Hydrocarbons (C2—C4) as Aid Gases for Improving the Efficiency of Hydrate-Based Technologies. Energies. 2023; 16(8):3576. https://doi.org/10.3390/en16083576
Chicago/Turabian StyleGambelli, Alberto Maria, and Federico Rossi. 2023. "Review on the Usage of Small-Chain Hydrocarbons (C2—C4) as Aid Gases for Improving the Efficiency of Hydrate-Based Technologies" Energies 16, no. 8: 3576. https://doi.org/10.3390/en16083576





