Cellulases: From Lignocellulosic Biomass to Improved Production
Abstract
:1. Introduction
2. Lignocellulosic Biomass
2.1. Lignocellulosic Biomass Sources
2.2. Lignocellulose Composition
2.2.1. Cellulose
2.2.2. Hemicellulose
2.2.3. Lignin
2.3. Lignocellulose Recalcitrance
3. Cellulases—The Cellulose Degrading Enzymes
4. Production of Microbial Cellulases
4.1. Factors Affecting Cellulase Production
4.1.1. Solid State Fermentation vs. Submerged Fermentation
4.1.2. Nutrients
4.1.3. Temperature
4.1.4. pH
4.1.5. Incubation Time
4.1.6. The Content of Moisture
4.2. Approaches for Cost-Effective Cellulase and Bioethanol Production
4.2.1. Strain Improvement and Genetic Engineering
4.2.2. Waste Substrates as Medium Constituents for the Synthesis of Enzymes
4.2.3. Integrated Cellulase Production
4.2.4. Co-Culture System
4.2.5. Improvement of Bioreactor Design
4.2.6. Cellulase Immobilization
4.3. Cellulase Market Outlook
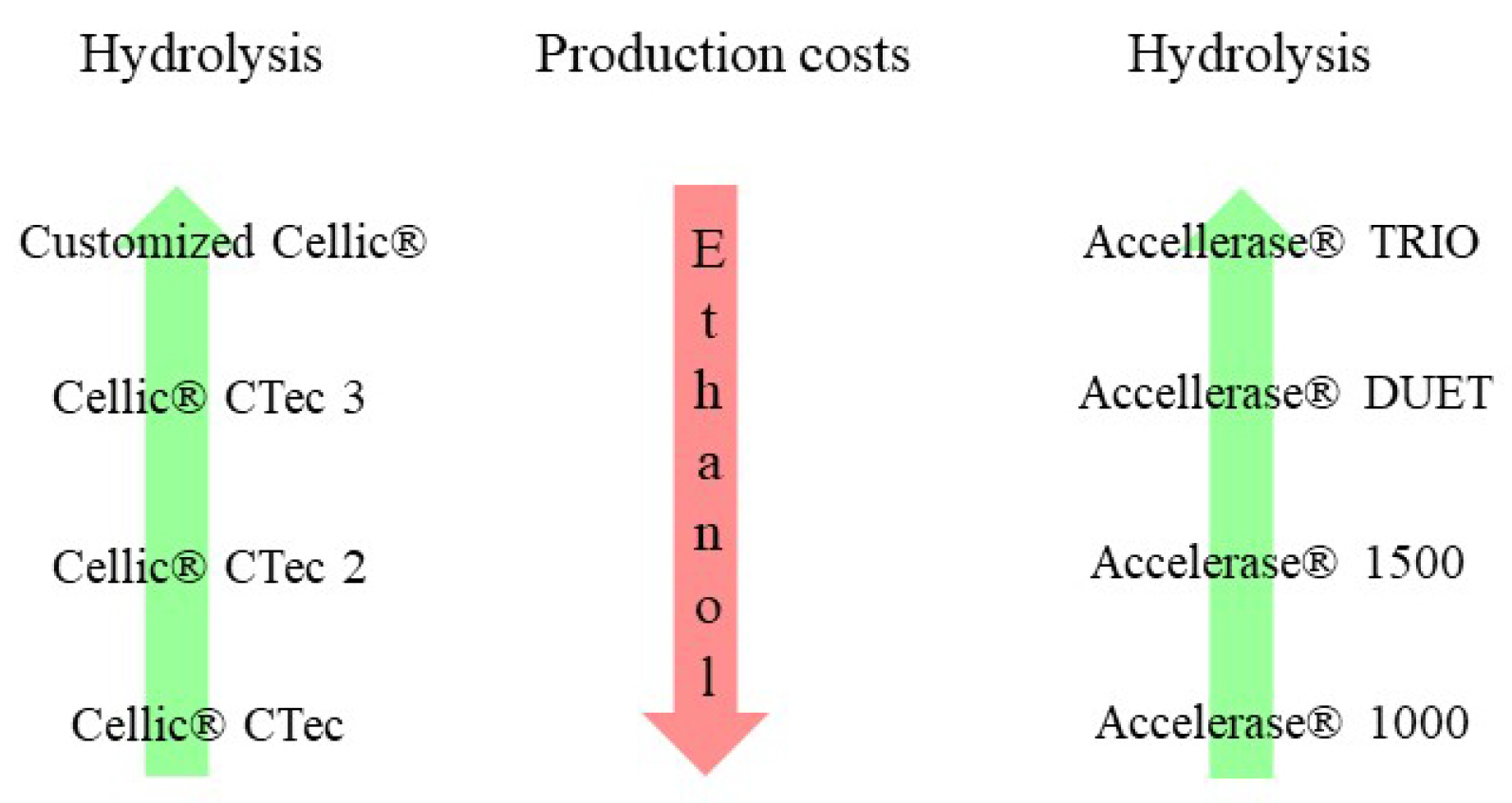
4.4. Commercial Cellulase for Bioethanol Production
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandra, M.R.G.S.; Madakka, M. Comparative biochemistry and kinetics of microbial lignocellulolytic enzymes. In Recent Developments in Applied Microbiology and Biochemistry; Buddolla, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 147–159. [Google Scholar]
- Sjulander, N.; Kikas, T. Two-Step Pretreatment of Lignocellulosic Biomass for High-Sugar Recovery from the Structural Plant Polymers Cellulose and Hemicellulose. Energies 2022, 15, 8898. [Google Scholar] [CrossRef]
- Chandel, A.K.; Albarelli, J.Q.; Santos, D.T.; Chundawat, S.P.; Puri, M.; Meireles, M.A.A. Comparative analysis of key technologies for cellulosic ethanol production from Brazilian sugarcane bagasse at a commercial scale. Biofuels Bioprod. Biorefining 2019, 13, 994–1014. [Google Scholar] [CrossRef]
- El Hage, M.; Rajha, H.N.; Maache-Rezzoug, Z.; Koubaa, M.; Louka, N. Intensification of Bioethanol Production from Different Lignocellulosic Biomasses, Induced by Various Pretreatment Methods: An Updated Review. Energies 2022, 15, 6912. [Google Scholar] [CrossRef]
- Ejaz, U.; Sohail, M.; Ghanemi, A. Cellulases: From bioactivity to a variety of industrial applications. Biomimetics 2021, 6, 44. [Google Scholar] [CrossRef]
- Kuhad, R.C.; Gupta, R.; Singh, A. Microbial cellulases and their industrial applications. Enzyme Res. 2011, 2011, 280696. [Google Scholar] [CrossRef]
- Rastogi, M.; Shrivastava, S. Recent advances in second generation bioethanol production: An insight to pretreatment, saccharification and fermentation processes. Renew. Sustain. Energy Rev. 2017, 80, 330–340. [Google Scholar] [CrossRef]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: Concepts and recent developments. 3 Biotech 2015, 5, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Abdolali, A.; Guo, W.; Ngo, H.; Chen, S.; Nguyen, N.; Tung, K. Typical lignocellulosic wastes and by-products for biosorption process in water and wastewater treatment: A critical review. Bioresour. Technol. 2014, 160, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, B.; Abdullahi, I.I. Bioethanol production from lignocellulosic waste-a review. Biosci. Biotechnol. Res. Asia 2016, 13, 1153–1161. [Google Scholar] [CrossRef]
- Mujtaba, M.; Fraceto, L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; de Medeiros, G.A.; Santo Pereira, A.D.E.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic biomass from agricultural waste to the circular economy: A review with focus on biofuels, biocomposites and bioplastics. J. Clean Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Gupta, A.; Verma, J.P. Sustainable bio-ethanol production from agro-residues: A review. Renew. Sustain. Energy Rev. 2015, 41, 550–567. [Google Scholar] [CrossRef]
- Jatoi, A.S.; Abbasi, S.A.; Hashmi, Z.; Shah, A.K.; Alam, M.S.; Bhatti, Z.A.; Maitlo, G.; Hussain, S.; Khandro, G.A.; Usto, M.A.; et al. Recent trends and future perspectives of lignocellulose biomass for biofuel production: A comprehensive review. Biomass Convers. Biorefin. 2021, 1–13. [Google Scholar] [CrossRef]
- Abo, B.O.; Gao, M.; Wang, Y.; Wu, C.; Ma, H.; Wang, Q. Lignocellulosic biomass for bioethanol: An overview on pretreatment, hydrolysis and fermentation processes. Rev. Environ. Health 2019, 34, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Adewuyi, A. Underutilized lignocellulosic waste as sources of feedstock for biofuel production in developing countries. Front. Energy Res. 2022, 10, 142. [Google Scholar] [CrossRef]
- Pensupa, N.; Jin, M.; Kokolski, M.; Archer, D.B.; Du, C. A solid state fungal fermentation-based strategy for the hydrolysis of wheat straw. Bioresour. Technol. 2013, 149, 261–267. [Google Scholar] [CrossRef]
- Shao, H.; Zhao, H.; Xie, J.; Qi, J.; Shupe, T.F. Agricultural and forest residues towards renewable chemicals and materials using microwave liquefaction. Int. J. Polym. Sci. 2019, 2019, 7231263. [Google Scholar] [CrossRef]
- Barakat, A.; de Vries, H.; Rouau, X. Dry fractionation process as an important step in current and future lignocellulose biorefineries: A review. Bioresour. Technol. 2013, 134, 362–373. [Google Scholar] [CrossRef]
- Taylor, N.G. Cellulose biosynthesis and deposition in higher plants. New Phytol. 2008, 178, 239–252. [Google Scholar] [CrossRef]
- Zoghlami, A.; Paës, G. Lignocellulosic biomass: Understanding recalcitrance and predicting hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef]
- Adapa, P.K.; Karunakaran, C.; Tabil, L.G.; Schoenau, G.J. Potential applications of infrared and Raman spectromicroscopy for agricultural biomass. Agric. Eng. Int. CIGR J. 2009, 11, 1–25. [Google Scholar]
- Thomas, L.H.; Forsyth, V.T.; Šturcová, A.; Kennedy, C.J.; May, R.P.; Altaner, C.M.; Apperley, D.C.; Wess, T.J.; Jarvis, M.C. Structure of cellulose microfibrils in primary cell walls from collenchyma. Plant Physiol. 2013, 161, 465–476. [Google Scholar] [CrossRef]
- Weidener, D.; Dama, M.; Dietrich, S.K.; Ohrem, B.; Pauly, M.; Leitner, W.; Domínguez de María, P.; Grande, P.M.; Klose, H. Multiscale analysis of lignocellulose recalcitrance towards OrganoCat pretreatment and fractionation. Biotechnol. Biofuels 2020, 13, 155. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Mishra, P.K.; Gupta, V.K. Bioprocessing for Biofuel Production: Strategies to Improve Process Parameters, 1st ed.; Neha, S., Manish, S., Mishra, P.K., Vijai, K.G., Eds.; Springer: Singapore, 2021; pp. 1–231. [Google Scholar]
- Gupta, M.N.; Bisaria, V.S. Stable cellulolytic enzymes and their application in hydrolysis of lignocellulosic biomass. Biotechnol. J. 2018, 13, 1700633. [Google Scholar]
- Mihajlovski, K.R.; Milić, M.D. The Role of Plant Cell Wall Degrading Enzymes in Biorefinery Development. In Lignocellulose Bioconversion through White Biotechnology, 1st ed.; Chandel, A.K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 99–135. [Google Scholar]
- Mihajlovski, K.R.; Carević, M.B.; Dević, M.L.; Šiler-Marinković, S.; Rajilić-Stojanović, M.D.; Dimitrijević-Branković, S. Lignocellulosic waste material as substrate for Avicelase production by a new strain of Paenibacillus chitinolyticus CKS1. Int. Biodeterior. Biodegrad. 2015, 104, 426–434. [Google Scholar] [CrossRef]
- De Souza, T.S.; Kawaguti, H.Y. Cellulases, hemicellulases, and pectinases: Applications in the food and beverage industry. Food Bioprocess Technol. 2021, 14, 1446–1477. [Google Scholar] [CrossRef]
- Naraian, R.; Gautam, R.L. Penicillium enzymes for the saccharification of lignocellulosic feedstocks. In New and Future Developments in Microbial Biotechnology and Bioengineering, 1st ed.; Gupta, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 121–136. [Google Scholar]
- Dimarogona, M.; Topakas, E.; Christakopoulos, P. Cellulose degradation by oxidative enzymes. Comput. Struct. Biotechnol. J. 2012, 2, e201209015. [Google Scholar] [CrossRef]
- Quinlan, R.J.; Sweeney, M.D.; Lo Leggio, L.; Otten, H.; Poulsen, J.-C.N.; Johansen, K.S.; Krogh, K.B.; Jørgensen, C.I.; Tovborg, M.; Anthonsen, A.; et al. Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. Proc. Natl. Acad. Sci. USA 2011, 108, 15079–15084. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chandra, R. Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon 2020, 6, e03170. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Anwar, Z.; Zafar, M.; Ali, A.; Arif, M. Production and characterization of commercial cellulase produced through Aspergillus niger IMMIS1 after screening fungal species. Pak. J. Bot. 2018, 50, 1563–1570. [Google Scholar]
- Bhati, N.; Sharma, A.K. Cost-effective cellulase production, improvement strategies, and future challenges. J. Food Process Eng. 2021, 44, e13623. [Google Scholar] [CrossRef]
- Sethi, S.; Datta, A.; Gupta, B.L.; Gupta, S. Optimization of cellulase production from bacteria isolated from soil. Int. Sch. Res. Notices 2013, 2013, 985685. [Google Scholar] [CrossRef] [PubMed]
- Khianngam, S.; Pootaeng-On, Y.; Techakriengkrai, T.; Tanasupawat, S. Screening and identification of cellulase producing bacteria isolated from oil palm meal. J. Appl. Pharm. Sci. 2014, 4, 090–096. [Google Scholar]
- Paudel, Y.P.; Qin, W. Characterization of novel cellulase-producing bacteria isolated from rotting wood samples. Appl. Biochem. Biotechnol. 2015, 177, 1186–1198. [Google Scholar] [CrossRef]
- Shaikh, N.M.; Patel, A.; Mehta, S.; Patel, N. Isolation and Screening of Cellulolytic Bacteria Inhabiting Different Environment and Optimization of Cellulase Production. Univers. J. Environ. Res. Technol. 2013, 3, 39–49. [Google Scholar]
- Islam, F.; Roy, N. Screening, purification and characterization of cellulase from cellulase producing bacteria in molasses. BMC Res. Notes 2018, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- Mawadza, C.; Hatti-Kaul, R.; Zvauya, R.; Mattiasson, B. Purification and characterization of cellulases produced by two Bacillus strains. J. Biotechnol. 2000, 83, 177–187. [Google Scholar] [CrossRef]
- Liang, Y.-L.; Zhang, Z.; Wu, M.; Wu, Y.; Feng, J.-X. Isolation, screening, and identification of cellulolytic bacteria from natural reserves in the subtropical region of China and optimization of cellulase production by Paenibacillus terrae ME27-1. BioMed Res. Int. 2014, 2014, 512497. [Google Scholar] [CrossRef]
- López-Contreras, A.M.; Gabor, K.; Martens, A.A.; Renckens, B.A.; Claassen, P.A.; Van Der Oost, J.; De Vos, W.M. Substrate-induced production and secretion of cellulases by Clostridium acetobutylicum. Appl. Environ. Microbiol. 2004, 70, 5238–5243. [Google Scholar] [CrossRef]
- de Lima, A.L.G.; do Nascimento, R.P.; da Silva Bon, E.P.; Coelho, R.R.R. Streptomyces drozdowiczii cellulase production using agro-industrial by-products and its potential use in the detergent and textile industries. Enzym. Microb. Technol. 2005, 37, 272–277. [Google Scholar] [CrossRef]
- Mihajlovski, K.; Buntić, A.; Milić, M.; Rajilić-Stojanović, M.; Dimitrijević-Branković, S. From agricultural waste to biofuel: Enzymatic potential of a bacterial isolate Streptomyces fulvissimus CKS7 for bioethanol production. Waste Biomass Valorization 2021, 12, 165–174. [Google Scholar] [CrossRef]
- Irfan, M.; Safdar, A.; Syed, Q.; Nadeem, M. Isolation and screening of cellulolytic bacteria from soil and optimization of cellulase production and activity. Turk. J. Biochem. 2012, 37, 287–293. [Google Scholar] [CrossRef]
- Yin, L.-J.; Huang, P.-S.; Lin, H.-H. Isolation of cellulase-producing bacteria and characterization of the cellulase from the isolated bacterium Cellulomonas sp. YJ5. J. Agric. Food Chem. 2010, 58, 9833–9837. [Google Scholar] [CrossRef]
- Soares, F.L.; Melo, I.S.; Dias, A.C.F.; Andreote, F.D. Cellulolytic bacteria from soils in harsh environments. World J. Microbiol. Biotechnol. 2012, 28, 2195–2203. [Google Scholar] [CrossRef]
- Ahmed, A.; Bibi, A. Fungal cellulase; production and applications: Minireview. Int. J. Health Life Sci. 2018, 4, 19–36. [Google Scholar] [CrossRef]
- Sethi, S.; Gupta, S. Optimization of cultural parameters for cellulase enzyme production from fungi. BioLife 2014, 2, 989–996. [Google Scholar]
- Hu, Y.; Du, C.; Pensupa, N.; Lin, C.S.K. Optimisation of fungal cellulase production from textile waste using experimental design. Process Saf. Environ. Prot. 2018, 118, 133–142. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Brar, S.K.; Verma, M. Potential of apple pomace as a solid substrate for fungal cellulase and hemicellulase bioproduction through solid-state fermentation. Ind. Crops Prod. 2012, 38, 6–13. [Google Scholar] [CrossRef]
- Ja’afaru, M.I. Screening of fungi isolated from environmental samples for xylanase and cellulase production. Int. Sch. Res. Notices 2013, 2013, 283423. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, M.; Valkonen, M.J.; Westerholm-Parvinen, A.; Aro, N.; Arvas, M.; Vitikainen, M.; Penttilä, M.; Saloheimo, M.; Pakula, T.M. Screening of candidate regulators for cellulase and hemicellulase production in Trichoderma reesei and identification of a factor essential for cellulase production. Biotechnol. Biofuels 2014, 7, 14. [Google Scholar] [CrossRef]
- Bhattacharya, A.S.; Bhattacharya, A.; Pletschke, B.I. Synergism of fungal and bacterial cellulases and hemicellulases: A novel perspective for enhanced bio-ethanol production. Biotechnol. Lett. 2015, 37, 1117–1129. [Google Scholar] [CrossRef]
- Pandey, S.; Srivastava, M.; Shahid, M.; Kumar, V.; Singh, A.; Trivedi, S.; Srivastava, Y. Trichoderma species cellulases produced by solid state fermentation. J. Data Min. Genom. Proteom. 2015, 6, 170. [Google Scholar]
- Liu, J.; Yang, J. Cellulase production by Trichoderma koningii AS3. 4262 in solid-state fermentation using lignocellulosic waste from the vinegar industry. Food Technol. Biotechnol. 2007, 45, 420–425. [Google Scholar]
- Singhania, R.R.; Ruiz, H.A.; Awasthi, M.K.; Dong, C.-D.; Chen, C.-W.; Patel, A.K. Challenges in cellulase bioprocess for biofuel applications. Renew. Sustain. Energy Rev. 2021, 151, 111622. [Google Scholar] [CrossRef]
- Bentil, J.A.; Thygesen, A.; Mensah, M.; Lange, L.; Meyer, A.S. Cellulase production by white-rot basidiomycetous fungi: Solid-state versus submerged cultivation. Appl. Microbiol. Biotechnol. 2018, 102, 5827–5839. [Google Scholar] [CrossRef]
- Sirohi, R.; Singh, A.; Tarafdar, A.; Shahi, N.C.; Verma, A.K.; Kushwaha, A. Cellulase production from pre-treated pea hulls using Trichoderma reesei under submerged fermentation. Waste Biomass Valorization 2019, 10, 2651–2659. [Google Scholar] [CrossRef]
- Infanzón-Rodríguez, M.; Ragazzo-Sánchez, J.; Del Moral, S.; Calderón-Santoyo, M.; Gutiérrez-Rivera, B.; Aguilar-Uscanga, M. Optimization of cellulase production by Aspergillus niger ITV 02 from sweet Sorghum bagasse in submerged culture using a Box–Behnken design. Sugar Tech 2020, 22, 266–273. [Google Scholar] [CrossRef]
- Ghazanfar, M.; Irfan, M.; Shakir, H.A.; Khan, M.; Nadeem, M.; Ahmad, I. Cellulase production optimization by Bacillus aerius through response surface methodology in submerged fermentation. Cellul. Chem. Technol. 2022, 56, 321–330. [Google Scholar] [CrossRef]
- Irfan, M.; Mushtaq, Q.; Tabssum, F.; Shakir, H.A.; Qazi, J.I. Carboxymethyl cellulase production optimization from newly isolated thermophilic Bacillus subtilis K-18 for saccharification using response surface methodology. AMB Express 2017, 7, 29. [Google Scholar] [CrossRef]
- Mihajlovski, K.; Pecarski, D.; Rajilić-Stojanović, M.; Dimitrijević-Branković, S. Valorization of corn stover and molasses for enzyme synthesis, lignocellulosic hydrolysis and bioethanol production by Hymenobacter sp. CKS3. Environ. Technol. Innov. 2021, 23, 101627. [Google Scholar] [CrossRef]
- Bezerra, C.O.; Carneiro, L.L.; Carvalho, E.A.; das Chagas, T.P.; de Carvalho, L.R.; Uetanabaro, A.P.T.; da Silva, G.P.; da Silva, E.G.P.; da Costa, A.M. Artificial intelligence as a combinatorial optimization strategy for cellulase production by Trichoderma stromaticum AM7 using peach-palm waste under solid-state fermentation. BioEnergy Res. 2021, 14, 1161–1170. [Google Scholar] [CrossRef]
- Julia, B.M.; Belén, A.M.; Georgina, B.; Beatriz, F. Potential use of soybean hulls and waste paper as supports in SSF for cellulase production by Aspergillus niger. Biocatal. Agric. Biotechnol. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Buntić, A.; Milić, M.; Stajković-Srbinović, O.S.; Rasulić, N.V.; Delić, D.I.; Mihajlovski, K. Cellulase production by Sinorhizobium meliloti strain 224 using waste tobacco as substrate: Utilization of waste tobacco for cellulase production. Int. J. Environ. Sci. Technol. 2019, 16, 5881–5890. [Google Scholar] [CrossRef]
- Su, L.-H.; Zhao, S.; Jiang, S.-X.; Liao, X.-Z.; Duan, C.-J.; Feng, J.-X. Cellulase with high β-glucosidase activity by Penicillium oxalicum under solid state fermentation and its use in hydrolysis of cassava residue. World J. Microbiol. Biotechnol. 2017, 33, 37. [Google Scholar] [CrossRef]
- Sharma, M.; Kumar Bajaj, B. Optimization of bioprocess variables for production of a thermostable and wide range pH stable carboxymethyl cellulase from Bacillus subtilis MS 54 under solid state fermentation. Environ. Prog. Sustain. Energy 2017, 36, 1123–1130. [Google Scholar] [CrossRef]
- Jain, L.; Kurmi, A.K.; Agrawal, D. Conclusive selection of optimal parameters for cellulase production by Talaromyces verruculosus IIPC 324 under SSF via saccharification of acid-pretreated sugarcane bagasse. Biofuels 2018, 12, 61–69. [Google Scholar] [CrossRef]
- Ilić, N.; Davidović, S.; Milić, M.; Rajilić-Stojanović, M.; Pecarski, D.; Ivančić-Šantek, M.; Mihajlovski, K.; Dimitrijević-Branković, S. Valorization of lignocellulosic wastes for extracellular enzyme production by novel Basidiomycetes: Screening, hydrolysis, and bioethanol production. Biomass Convers. Biorefin. 2022, 1–12. [Google Scholar] [CrossRef]
- Yoon, L.W.; Ang, T.N.; Ngoh, G.C.; Chua, A.S.M. Fungal solid-state fermentation and various methods of enhancement in cellulase production. Biomass Bioenergy 2014, 67, 319–338. [Google Scholar] [CrossRef]
- Prasanna, H.; Ramanjaneyulu, G.; Reddy, B. Optimization of cellulase production by Penicillium sp. 3 Biotech 2016, 6, 162. [Google Scholar] [CrossRef]
- Imran, M.; Anwar, Z.; Irshad, M.; Asad, M.J.; Ashfaq, H. Cellulase production from species of fungi and bacteria from agricultural wastes and its utilization in industry: A review. Adv. Enzym. Res. 2016, 4, 44–55. [Google Scholar] [CrossRef]
- Sarkar, N.; Aikat, K. Aspergillus fumigatus NITDGPKA3 provides for increased cellulase production. Int. J. Chem. Eng. 2014, 2014, 959845. [Google Scholar] [CrossRef]
- Prasetyo, J.; Sumita, S.; Okuda, N.; Park, E.Y. Response of cellulase activity in pH-controlled cultures of the filamentous fungus Acremonium cellulolyticus. Appl. Biochem. Biotechnol. 2010, 162, 52–61. [Google Scholar] [CrossRef] [PubMed]
- El-Nahrawy, S.; Metwally, M.; El-Kodoos, A.; Rizk, Y.; Belal, E.-S.B.; Shabana, S.A.; El-Refai, I.M. Optimization of culture conditions for production of cellulase by Aspergillus tubingensis KY615746 using rice straw waste. Environ. Biodivers. Soil Secur. 2017, 1, 177–189. [Google Scholar]
- El-Hadi, A.A.; El-Nour, S.A.; Hammad, A.; Kamel, Z.; Anwar, M. Optimization of cultural and nutritional conditions for carboxymethylcellulase production by Aspergillus hortai. J. Radiat. Res. Appl. Sci. 2014, 7, 23–28. [Google Scholar] [CrossRef]
- Teixeira da Silva, V.D.C.; de Souza Coto, A.L.; de Carvalho Souza, R.; Bertoldi Sanchez Neves, M.; Gomes, E.; Bonilla-Rodriguez, G.O. Effect of pH, temperature, and chemicals on the endoglucanases and β-glucosidases from the thermophilic fungus Myceliophthora heterothallica F. 2.1. 4. Obtained by solid-state and submerged cultivation. Biochem. Res. Int. 2016, 2016, 9781216. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Mishra, P.; Gupta, V.K.; Molina, G.; Rodriguez-Couto, S.; Manikanta, A.; Ramteke, P. Applications of fungal cellulases in biofuel production: Advances and limitations. Renew. Sustain. Energy Rev. 2018, 82, 2379–2386. [Google Scholar] [CrossRef]
- Singh, A.; Bajar, S.; Devi, A.; Pant, D. An overview on the recent developments in fungal cellulase production and their industrial applications. Bioresour. Technol. Rep. 2021, 14, 100652. [Google Scholar] [CrossRef]
- Payne, C.M.; Knott, B.C.; Mayes, H.B.; Hansson, H.; Himmel, M.E.; Sandgren, M.; Stahlberg, J.; Beckham, G.T. Fungal cellulases. Chem. Rev. 2015, 115, 1308–1448. [Google Scholar] [CrossRef]
- Amore, A.; Giacobbe, S.; Faraco, V. Regulation of cellulase and hemicellulase gene expression in fungi. Curr. Genom. 2013, 14, 230–249. [Google Scholar] [CrossRef]
- Shen, L.; Yan, A.; Wang, Y.; Wang, Y.; Liu, H.; Zhong, Y. Tailoring the expression of Xyr1 leads to efficient production of lignocellulolytic enzymes in Trichoderma reesei for improved saccharification of corncob residues. Biotechnol. Biofuels Bioprod. 2022, 15, 142. [Google Scholar] [CrossRef]
- Gielkens, M.M.; Dekkers, E.; Visser, J.; de Graaff, L.H. Two cellobiohydrolase-encoding genes from Aspergillus niger require D-xylose and the xylanolytic transcriptional activator XlnR for their expression. Appl. Environ. Microbiol. 1999, 65, 4340–4345. [Google Scholar] [CrossRef] [PubMed]
- Shida, Y.; Furukawa, T.; Ogasawara, W. Deciphering the molecular mechanisms behind cellulase production in Trichoderma reesei, the hyper-cellulolytic filamentous fungus. Biosci. Biotechnol. Biochem. 2016, 80, 1712–1729. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Tsai, M.L.; Nargotra, P.; Chen, C.W.; Kuo, C.H.; Sun, P.P.; Dong, C.D. Agro-industrial food waste as a low-cost substrate for sustainable production of industrial enzymes: A Critical Review. Catalysts 2022, 12, 1373. [Google Scholar] [CrossRef]
- Siqueira, J.G.W.; Rodrigues, C.; de Souza Vandenberghe, L.P.; Woiciechowski, A.L.; Soccol, C.R. Current advances in on-site cellulase production and application on lignocellulosic biomass conversion to biofuels: A review. Biomass Bioenergy 2020, 132, 105419. [Google Scholar] [CrossRef]
- Martins, D.A.B.; do Prado, H.F.A.; Leite, R.S.R.; Ferreira, H.; de Souza, M.M.; Moretti, R.D.S.; Gomes, E. Agroindustrial wastes as substrates for microbial enzymes production and source of sugar for bioethanol production. In Integrated Waste Management-Volume II; IntechOpen: London, UK, 2011. [Google Scholar]
- Bhardwaj, N.; Kumar, B.; Agrawal, K.; Verma, P. Current perspective on production and applications of microbial cellulases: A review. Bioresour. Bioprocess. 2021, 8, 95. [Google Scholar] [CrossRef]
- Andriani, D.; Sunwoo, C.; Ryu, H.-W.; Prasetya, B.; Park, D.-H. Immobilization of cellulase from newly isolated strain Bacillus subtilis TD6 using calcium alginate as a support material. Bioprocess Biosyst. Eng. 2012, 35, 29–33. [Google Scholar] [CrossRef]
- Buntić, A.V.; Pavlović, M.D.; Antonović, D.G.; Šiler-Marinković, S.S.; Dimitrijević-Branković, S.I. Utilization of spent coffee grounds for isolation and stabilization of Paenibacillus chitinolyticus CKS1 cellulase by immobilization. Heliyon 2016, 2, e00146. [Google Scholar] [CrossRef]
- Podrepšek, G.H.; Primožič, M.; Knez, Ž.; Habulin, M. Immobilization of cellulase for industrial production. Chem. Eng. 2012, 27, 235–240. [Google Scholar]
- Zhang, D.; Hegab, H.E.; Lvov, Y.; Dale Snow, L.; Palmer, J. Immobilization of cellulase on a silica gel substrate modified using a 3-APTES self-assembled monolayer. SpringerPlus 2016, 5, 48. [Google Scholar] [CrossRef]
- Coherent Market Insights. Available online: https://www.coherentmarketinsights.com/market-insight/cellulase-market-2146 (accessed on 7 March 2023).
- Transparency Market Research. Cellulase Market. Available online: https://www.transparencymarketresearch.com/cellulase-market.html (accessed on 5 December 2022).
- MarketResearch. Global Cellulase (CAS 9012-54-8) Market Trends, Applications, Analysis, Growth, and Forecast to 2028. Available online: https://marketresearch.biz/report/cellulase-cas-9012-54-8-market (accessed on 7 March 2023).
- Available online: https://www.futuremarketinsights.com/reports/cellulase-market (accessed on 7 March 2023).
- MarketWatch. Cellulase Market Size and Forecast till 2028. Available online: https://www.marketwatch.com/press-release/cellulase-market-size-and-forecast-till-2028-2023-03-16 (accessed on 7 March 2023).
- Market.US. Global Cellulase Market by Type (EG, CBH, and BG), by Application (Animal Feed, Textile Industry, Food & Beverages, and Biofuels), by Region and Key Companies—Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2019–2028. Available online: https://market.us/report/cellulase-market/#major-market-players (accessed on 7 March 2023).
- Ranganathan, S.; Mahesh, S.; Suresh, S.; Nagarajan, A.; Sen, T.Z.; Yennamalli, R. Experimental and computational studies of cellulases as bioethanol enzymes. Bioengineered 2022, 13, 14028–14046. [Google Scholar] [CrossRef]
- Rajeswari, S.; Baskaran, D.; Saravanan, P.; Rajasimman, M.; Rajamohan, N.; Vasseghian, Y. Production of ethanol from biomass—Recent research, scientometric review and future perspectives. Fuel 2022, 317, 123448. [Google Scholar] [CrossRef]
- Novozymes. Bioenergy Your Partner for Better Plant Performance. Available online: https://www.novozymes.com/en/advance-your-business/cn/bioenergy-cn/lignocellulosic-hydrolysis (accessed on 25 November 2022).
- Novozymes. Biomass Conversion on the Cusp of Commercialization. Available online: https://documents.pub/document/biomass-conversion-on-the-cusp-of-commercialization-on-the-cusp-of-commercialization.html?page=1 (accessed on 30 November 2022).
- Sørensen, A.; Lübeck, P.S.; Lübeck, M.; Teller, P.J.; Ahring, B.K. β-Glucosidases from a new Aspergillus species can substitute commercial β-glucosidases for saccharification of lignocellulosic biomass. Can. J. Microbiol. 2011, 57, 638–650. [Google Scholar] [CrossRef] [PubMed]
- Biofuel. Available online: https://biofuel.org.uk/Novozymes.html (accessed on 25 November 2022).
- Sun, F.F.; Zhao, X.; Hong, J.; Tang, Y.; Wang, L.; Sun, H.; Li, X.; Hu, J. Industrially relevant hydrolyzability and fermentability of sugarcane bagasse improved effectively by glycerol organosolv pretreatment. Biotechnol. Biofuels 2016, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.; Smart, K.A.; James, S.; Cook, D.J. Maximising high solid loading enzymatic saccharification yield from acid-catalysed hydrothermally-pretreated brewers spent grain. Biofuel Res. J. 2016, 3, 417–429. [Google Scholar] [CrossRef]
- Novozymes. Fuel Ethanol Application Sheet. Available online: http://www.shinshu-u.ac.jp/faculty/engineering/chair/chem010/manual/Ctec2.pdf (accessed on 28 November 2022).
- McBrayer, B.; Shaghasi, T.; Vlasenko, E. Compositions for Saccharification of Cellulosic Material. U.S. Patent US9587262B2, 16 October 2015. [Google Scholar]
- Triwahyuni, E.; Sudiyani, Y.; Abimanyu, H. The effect of substrate loading on simultaneous saccharification and fermentation process for bioethanol production from oil palm empty fruit bunches. Energy Procedia 2015, 68, 138–146. [Google Scholar] [CrossRef]
- Zhao, X.; Moates, G.; Elliston, A.; Wilson, D.; Coleman, M.; Waldron, K. Simultaneous saccharification and fermentation of steam exploded duckweed: Improvement of the ethanol yield by increasing yeast titre. Bioresour. Technol. 2015, 194, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Niyomvong, N. Ethanol production from cassava stem using Saccharomyces cerevisiae TISTR 5339 through simultaneous saccharification and fermentation. Agric. Nat. Resour. 2019, 53, 667–673. [Google Scholar]
- Novozymes. Our Media Relations Team and Latest News. Available online: https://www.novozymes.com/en/news/news-archive/2012/02/advanced-biofuels-becoming-reality-with-novozymes-new-enzyme-technology) (accessed on 1 December 2022).
- Greencarcongress. New Novozymes Enzymes for Cellulosic Ethanol Enable Production Cost Below US$2 per Gallon. Available online: https://www.greencarcongress.com/2010/02/cellicctec2-20100216.html (accessed on 1 December 2022).
- Novozymes. Cellic® CTec3 HS. Available online: https://biosolutions.novozymes.com/en/bioenergy/products/biomass-conversion/cellic-ctec3-hs (accessed on 2 December 2022).
- Yumpu. Available online: https://www.yumpu.com/en/document/read/15813202/improvements-in-enzymes-for-cellulosic-ethanol/13 (accessed on 2 December 2022).
- Sun, F.F.; Hong, J.; Hu, J.; Saddler, J.N.; Fang, X.; Zhang, Z.; Shen, S. Accessory enzymes influence cellulase hydrolysis of the model substrate and the realistic lignocellulosic biomass. Enzym. Microb. Technol. 2015, 79, 42–48. [Google Scholar] [CrossRef]
- Shinshu University. Available online: http://www.shinshu-u.ac.jp/faculty/engineering/chair/chem010/manual/accellerase1500_Dupont.pdf (accessed on 2 December 2022).
- Marcos, M.; Garcia-Cubero, M.T.; González-Benito, G.; Coca, M.; Bolado, S.; Lucas, S. Optimization of the enzymatic hydrolysis conditions of steam-exploded wheat straw for maximum glucose and xylose recovery. J. Chem. Technol. Biotechnol. 2013, 88, 237–246. [Google Scholar] [CrossRef]
- Gutierrez, C.; Mitchinson, C.; Huang, T.T.; Diner, B.A.; Fagan, P.J.; Hitz, W.D. Methods for Improving the Efficiency of Simultaneous Saccharification and Fermentation Reactions. U.S. Patent US10138499B2, 27 November 2018. [Google Scholar]
- De Silva, W.I.N.; Fagan, P.J.; Gallagher, F.G.; Huang, Z.-Z.; Liu, G.G.; Perelman, A.; Millan, L.F.R.; Shpilsky, A.; Slanac, D.A.; Walsh, M. High Force and High Stress Destructuring of Cellulosic Biomass. U.S. Patent US10227623B2, 18 November 2014. [Google Scholar]
- Genencor. Available online: http://www.genencor.com/fileadmin/user_upload/genencor/documents/accellerase_duet_product_info_sheet.pdf (accessed on 2 December 2022).
- Cheung, P.; Fox, B.C.; Lau, M.W.; Selby, J.M.; Shankwitz, G.P.; Thomas, S.M.; Warner, R.E. Gradient Pretreatment of Lignocellulosic Biomass. U.S. Patent US20140273105A1, 5 March 2014. [Google Scholar]
- Nghiem, N.P.; Ellis, C.W., Jr.; Montanti, J. The effects of ethanol on hydrolysis of cellulose and pretreated barley straw by some commercial cellulolytic enzyme products. AIMS Bioeng. 2016, 3, 441–453. [Google Scholar] [CrossRef]
- Imam, T.; Capareda, S. Ultrasonic and high-temperature pretreatment, enzymatic hydrolysis and fermentation of lignocellulosic sweet sorghum to bio-ethanol. Int. J. Ambient Energy 2012, 33, 152–160. [Google Scholar] [CrossRef]
- Salleh, N.S.; Murad, A.M.A. Enzymatic hydrolysis of oil palm empty fruits bunch fiber using Celluclast® and Accellerase® BG for sugar production. AIP Conf. Proc. 2016, 33, 152–160. [Google Scholar]
- Mogensen, K.; Jeppesen, M.; Populsen, N.; Larsen, J. Methods of Processing Lignocellulosic Biomass Using Single-Stage Autohydrolysis and Enzymatic Hydrolysis with c5 Bypass and Post-Hydrolysis. U.S. Patent US 2016/0160253 A1, 9 June 2016. [Google Scholar]
| Microorganism | Process | Substrate | Fermentation Conditions | Cellulase Activity | Reference |
|---|---|---|---|---|---|
| Trichoderma reesei QM9414 | SmF | Pea hulls | 91 h, 30 °C, pH 5 | Filter paper activity 0.372 ± 0.019 U/mL | [60] |
| Aspergillus niger ITV 02 | SmF | Delignified sweet sorghum bagasse (10% w/v), 0.9 g/L urea, 2.4 g/L ammonium sulfate, and 1.5 g/L yeast extrac | 50 h, 30 °C | Endoglucanase activity 0.61 ± 0.025 Endoglucanase specific activity 126.72 ± 1.83 β-glucosidase ativity 0.41 ± 0.006 Specific β-glucosidase ativity 85.0 ± 0.40 | [61] |
| Bacillus aerius MG597041 | SmF | Yeast extract of 0.5 g/L, peptone of 0.5 g/L, FeSO4 of 0.2 g/L, and K2HPO4 of 0.02 g/L | 24 h, pH 5.5, 37°C | Filter paper activity 127.4 IU/mL/min | [62] |
| Bacillus subtilis K-18 | SmF | Potato peel | 50 °C for 24 h of fermentation period; 2% substrate concentration, 2% inoculum size, 1% yeast extract, and pH 5.0, | 3.50 ± 0.11 IU/mL | [63] |
| Hymenobacter sp. CKS3 | SmF | 5.0% corn stover, 2.5% molasses | 4 days | CMCase 1.11 IU/mL Avicelase 0.92 IU/mL | [64] |
| Trichoderma stromaticum AM7 | SSF | Peach-palm waste | 12 days 26 °C | CMCase 120 U/g | [65] |
| Aspergillus niger NRRL3 | SSF | Soybean hulls | 96 h 30 °C | Endoglucanase activity 5914.29 U/L Exoglucanase activity 4551.19 U/L β-glucosidase activity 984.01 U/L | [66] |
| Streptomyces fulvissimus CKS7 | SSF | Rye bran | 6 days 30 °C Solid moisture ratio 1:1 | Endoglucanase (CMCase) 8.62± 0.08; Exoglucanase (Avicelase) 5.98 ± 0.22 | [45] |
| Sinorhizobium meliloti 224 | SSF | Waste tobacco | 2 days 28 °C | Avicelase activity 1.503 U/g Carboxymethyl cellulase activity of 1.615 U/g | [67] |
| Penicillium oxalicum EU2106 | SSF | Cassava residue | 5 days 28 °C | 34.0 ± 2.8 filter-paper units/g dry substrate | [68] |
| Bacillus subtilis MS 54 | SSF | Maize bran | 3 days 37 °C | CMCase 28.84 IU/g) | [69] |
| Talaromyces verruculosus IIPC 324 | SSF | Wheat bran | 4 days 24 °C Moisture content 62.5% | Endoglucanase 250 U/g | [70] |
| Fomes fomentarius TMF2 | SSF | Sunfower meal | 6 days 30 °C Moisture content 62.5% | CMCase 1.49 ± 0.10 U/g Avicelase 1.02 ± 0.07 U/g | [71] |
| Schizophyllum commune TMF3 | SSF | Sunfower meal | 6 days 30 °C Moisture content 62.5% | CMCase 2.51 ± 0.13 U/g Avicelase 1.60 ± 0.09 U/g | [71] |
| Bjerkandera adusta TMF1 | SSF | Brewer’s spent grain | 6 days 30 °C Moisture content 62.5% | CMCase 2.76 ± 0.16 U/g Avicelase 2.76 ± 0.16 U/g | [71] |
| SSF | SmF | |
|---|---|---|
| Strengths | The high productivity Utilization of alternative low-cost substrate Minimal power required Minimal water production No or little foam up creation Displays the native habitat of filamentous fungi, which is why they are better adjusted Generating greater enzyme volumes that can be isolated or used for direct, extraction-free hydrolysis of biomass | Sterilization, heating, and mass transfer are simplified Enhanced process tracking (temperature, pH, and soluble molecules), and automation Simple access to extracellular secreted enzymes Large commercial facilities use SmF for cellulase synthesis since there are advanced bioreactors available, which allow simple mass transfer and easy processing |
| Weaknesses | Heat generation Lack of knowledge of automation Slow aeration and reduced mass transfer Possible high level of mycotoxins | Continuous supply with medium supplements or nutrients is needed Low-end product purity necessitates additional refining |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilić, N.; Milić, M.; Beluhan, S.; Dimitrijević-Branković, S. Cellulases: From Lignocellulosic Biomass to Improved Production. Energies 2023, 16, 3598. https://doi.org/10.3390/en16083598
Ilić N, Milić M, Beluhan S, Dimitrijević-Branković S. Cellulases: From Lignocellulosic Biomass to Improved Production. Energies. 2023; 16(8):3598. https://doi.org/10.3390/en16083598
Chicago/Turabian StyleIlić, Nevena, Marija Milić, Sunčica Beluhan, and Suzana Dimitrijević-Branković. 2023. "Cellulases: From Lignocellulosic Biomass to Improved Production" Energies 16, no. 8: 3598. https://doi.org/10.3390/en16083598
APA StyleIlić, N., Milić, M., Beluhan, S., & Dimitrijević-Branković, S. (2023). Cellulases: From Lignocellulosic Biomass to Improved Production. Energies, 16(8), 3598. https://doi.org/10.3390/en16083598






