Experimental Development of Calcium Looping Carbon Capture Processes: An Overview of Opportunities and Challenges
Abstract
:1. Introduction
2. Methods
2.1. Database Formation
- It should not be classified as a conference, abstract, or be under consolidation;
- It must be focused on calcium oxide (CaO) and limestone (CaCO), regardless of whether it has undergone a procedure to enhance its properties or not;
- The research scope must be within experimental carbon capture using fluidized beds, which should be bubbling or circulating;
- The primary focus of the paper must be the development, specific details, phenomena, and limitations of the technique used for CaL carbon capture using fluidized bed reactors;
- In order to validate experiments and analyses, CaO and CaCO conversion processes must be observed in at least one complete cycle.
2.2. Data Analysis
2.3. Co-Citation Analysis Methodology
3. Results and Discussion
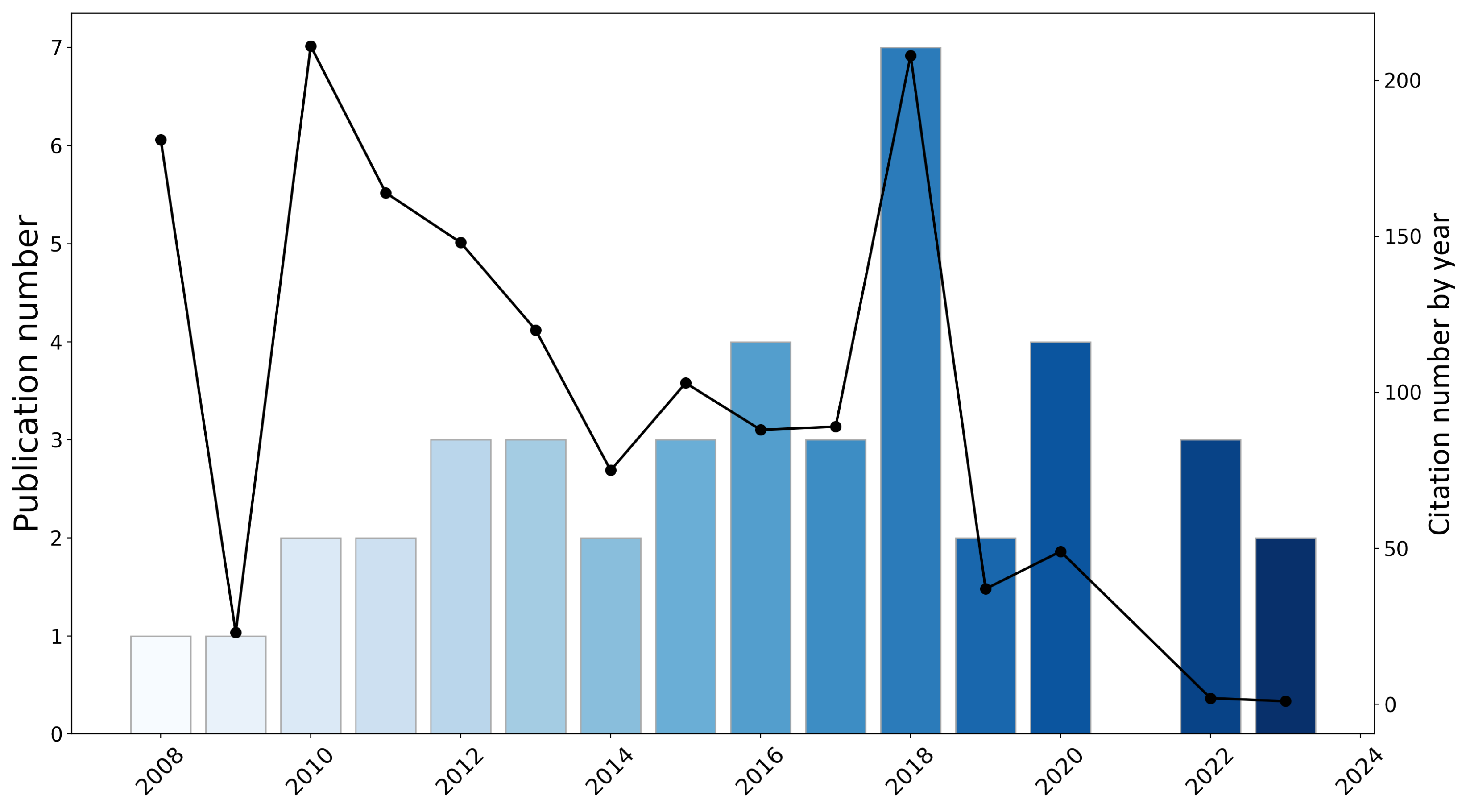
3.1. Analyses of Publication and Citation Tendencies
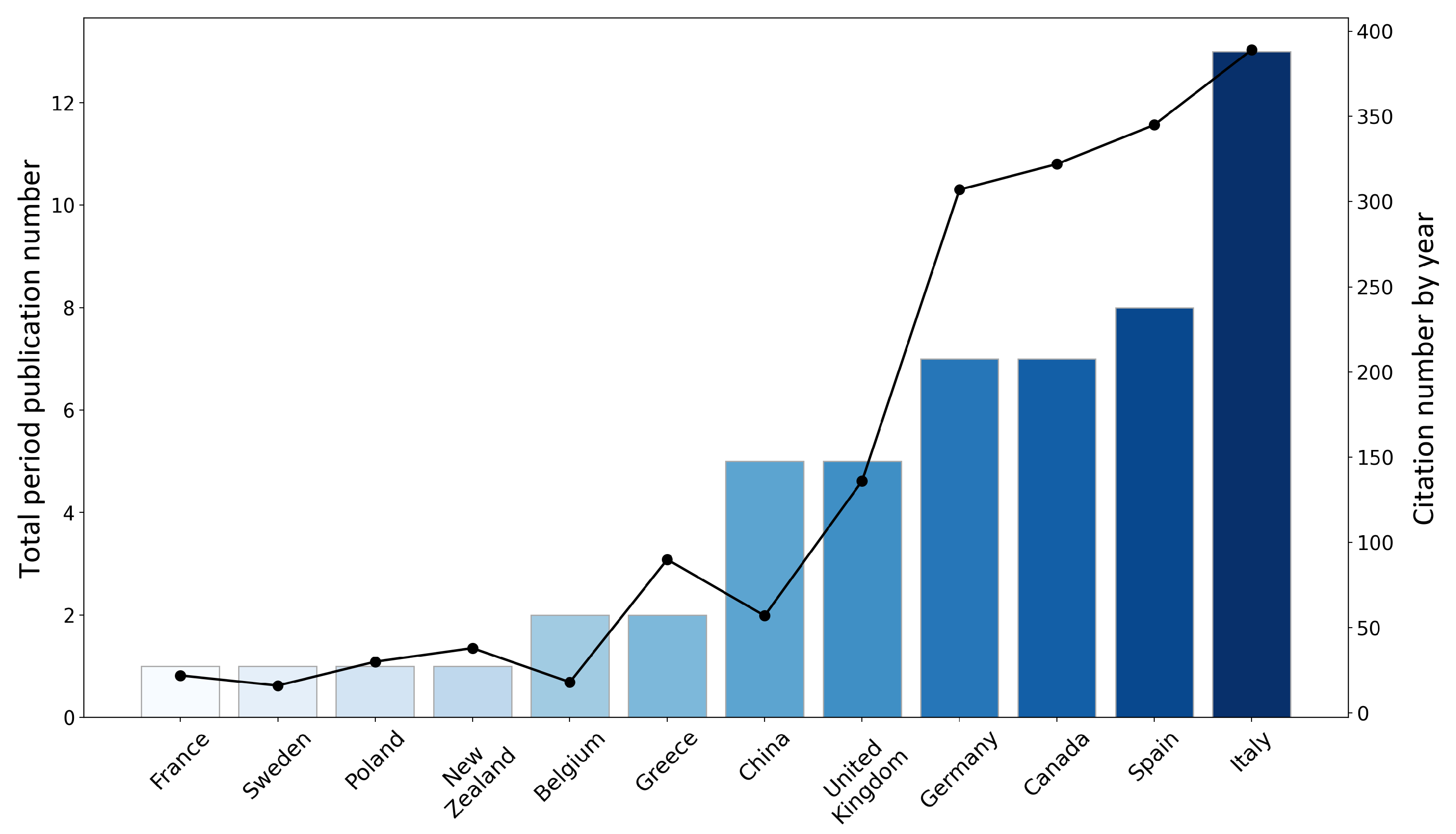
3.1.1. Total Publication by Nation
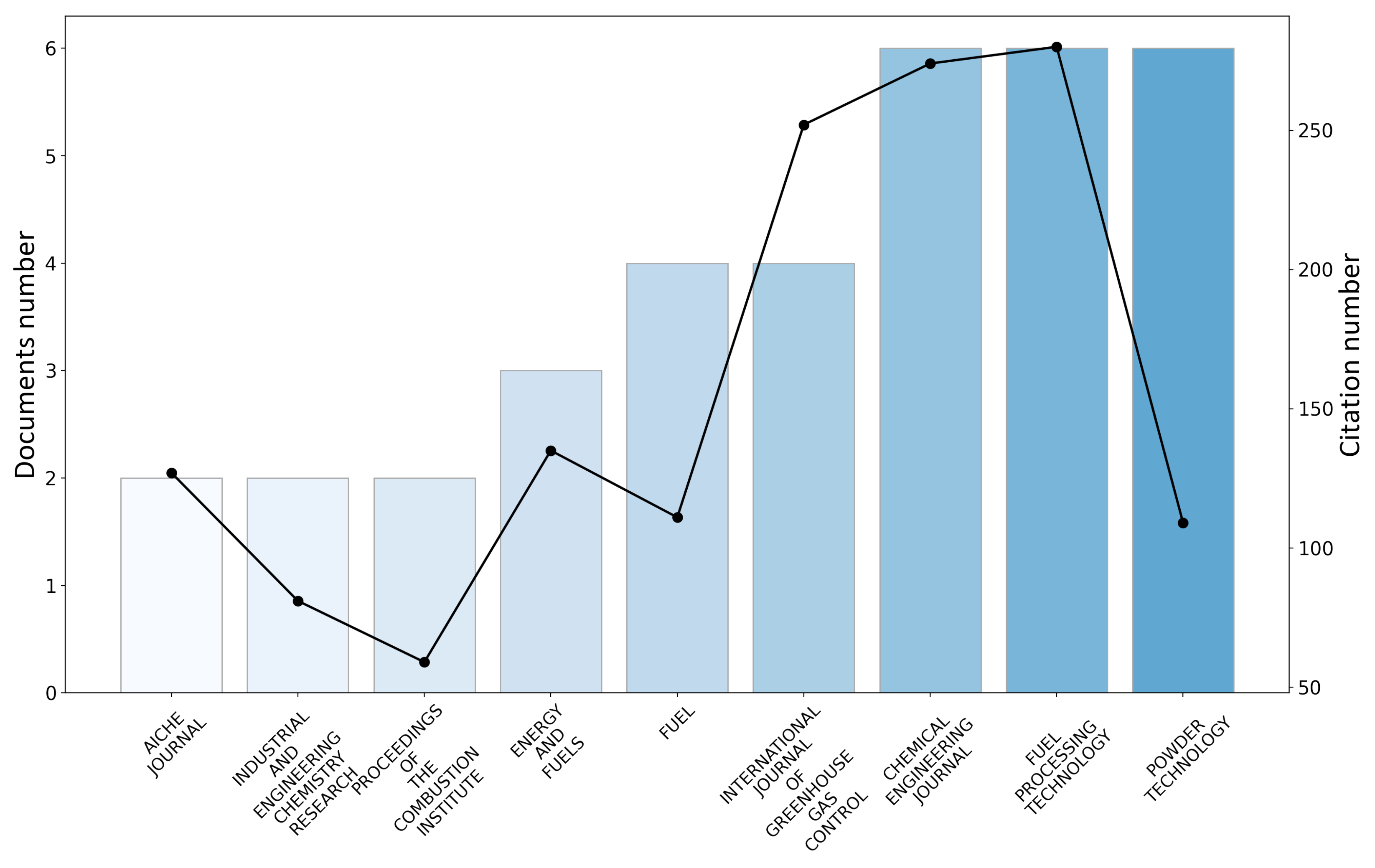
3.1.2. Journal Analysis
3.1.3. Keyword Analysis on Citation
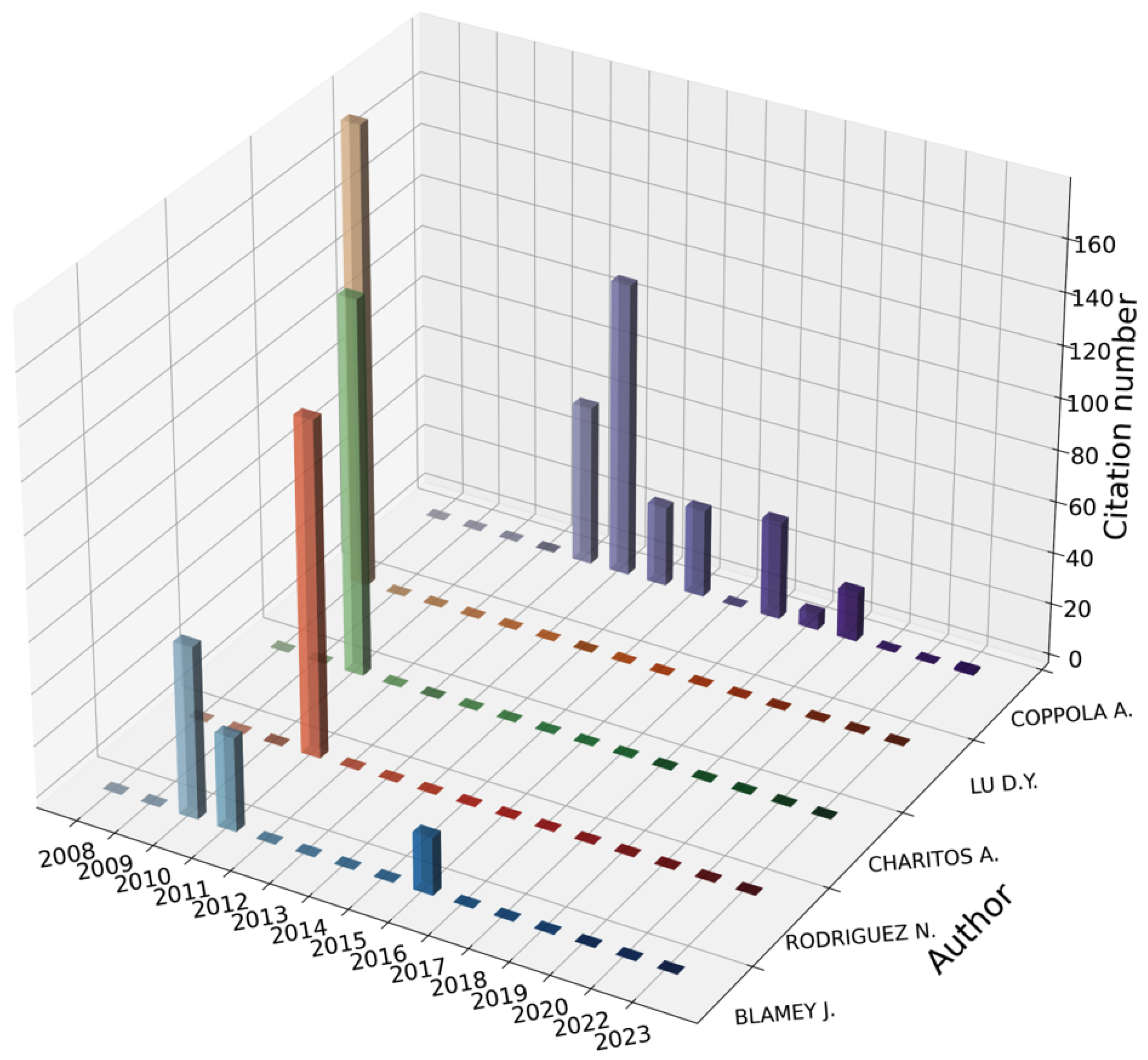
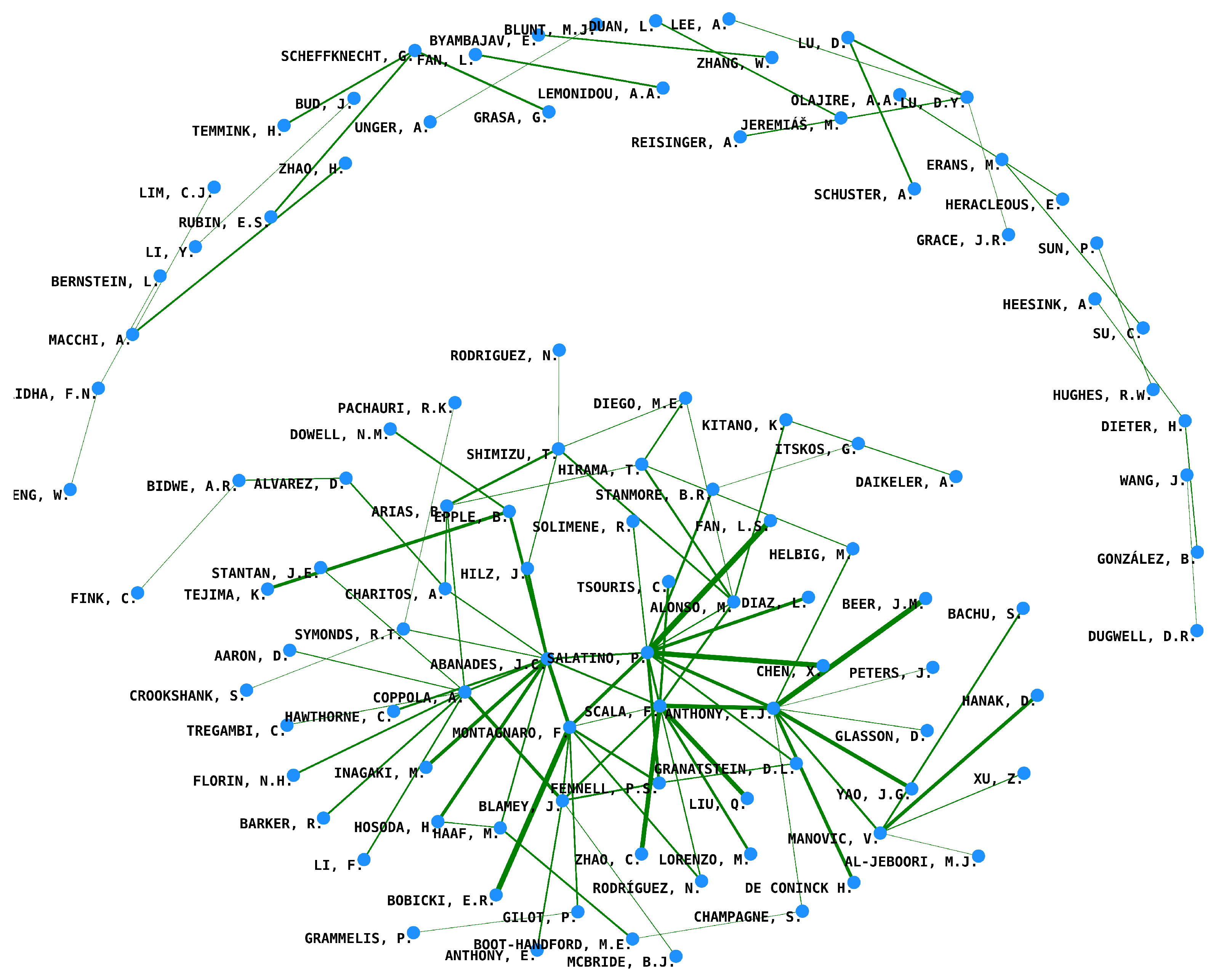
3.1.4. Co-Citation Analysis
3.2. Comparative Analysis of Experimental Data
3.2.1. Experimental Setup
3.2.2. Sorbents
3.2.3. Capture Efficiency
3.2.4. Scientific Gaps Development
| Reference (year) | Sorbent: Type/Pretreatment/ Origin/Particle Size | Fluidized Bed: Type/Dual or Single/Heating Method/Height × Diameter | Process: Calciner × Carbonator Atmosphere/ Calciner × Carbonator Temperature/Investigated Velocity/Wattage/Capture Efficiency | Gaps |
|---|---|---|---|---|
| Coppola et al. [33] | Limestone / no / Italy, Poland, Germany / 600–400 m | Bubbling / Dual / Resistance / n.i. × 0.04 m | Calc 10%CO-air, carb 10%CON / 950 × 650 °C / = 0.5 m/s / n.i / 85–15% | (1) Materials, pre-processed in dual interconnected fluidized beds, underwent impact fragmentation following a chipping/splitting pattern as a function of an increase in impact velocity. |
| Coppola et al. [73] | Limestone / hydration / Italy / 600–400 m | Bubbling / Dual / Resistance / n.i. × 0.04 m | Calc 10%CO-Air carb 10%CO-0-10%HO-N / 850 × 700–600 °C / = 0.5 m/s / n.i / 0.23–0.03 g/g | (1) Optimal carbonation temperature of 650 °C is suggested for this limestone once it both satisfies CO uptake of N number of carbonation stages and slight tendency to attrition/fragmentation. |
| Chai et al. [74] | Limestone and doped CaO / no / China / 180–125 m | Bubbling / Single / Resistance / 0.9 × 0.032 m | CO-CO-NO-O-N balance carb, 20%O-CO calc / 950 × 650 °C / = 0.018 m/s / n.i / n.i | (1) the Ce ((CHCO)CH and limestone mixture) doping is more advantageous for N formation and CO adsorption than doping on the CaO surface. |
| Li et al. [75] | Limestone and pellets / no / Canada / 850–600 m | n.i / Single / Resistance / n.i × 0.0254 m | 17%CO-N / 850 × 675 °C / n.i / n.i / 0.5–0.1 g/g | (1) Synthetic sorbents showed an uptake capacity of 0.4–0.45 g/g after 5 cycles in the reactor system, which was much greater than the uptake capacity of Cadomin limestone (0.15 g/g after 5 cycles). (2) MgO-promoted sorbent indicated a higher uptake capacity than CaZrO-promoted sorbent due to the higher mass ratio of CaO in the sorbent. (3) SEM images indicated formation of cracks in post-cycling samples as a result of expansion and contraction of sorbent grains going through carbonation and calcination. |
| Hashemi et al. (2022) [76] | Limestone / no / China / 1250–150 m | Bubbling / Single / Resistance / n.i × 0.028 m | 17%CO-N / 920–750 × 650 °C / 3x / n.i / 0.5–0.1 g/g | (1) The effect of particle size on limestone calcination kinetics presents three-zone characteristics of the reaction controlling mechanism. In Zone I, particle size is <80 m, which has little effect on consumption rate, and calcination is mainly controlled by the chemical reaction. In Zone III, particle size is >450 m; the calcination process is mainly controlled by gas diffusion, and the effect of particle size is significant. In Zone II, particle size ranges between 80 and 450 m and calcination is controlled by both gas diffusion and the chemical reaction. |
| González et al. [77] | Limestone / hydration / Canada, United Kingdom, Spain / 710–500 m | n.i / Single / Resistance / 0.5 × 0.021 m | 15%CO-0-10%steam-N / 900 × 700–650 °C / 8x / n.i / n.i | (1) Calculation results reveal that it is likely that other researchers investigating seawater and doping of limestone in CaL processes reported reductions in reactivity while overdoping their sorbents. |
| Haaf et al. [13] | Limestone / no / Germany / 500–25 m | Circulating / Dual / Calciner (Fueled by Natural Gas) / 8.6 m carb and 11 m calc × 0.59 m carb and 0.4 m calc | Flue gas 9.5%CO / 920–750 °C × 690–610 °C / = 3.5–2.5 m/s × = 5.5–4.5 m/s / 1 MW/ 92–42% | (1) Attention should be placed upon the hydrodynamics of coupled fluidized bed systems with regard to coarse inert ash fractions. (2) Further works are required to assess the influence of solid recovered fuel during the solid phase and gaseous emission generation by the calciner. |
| Dong et al. [47] | Lab grade CaO / Hydration / n.i / 850–430 m | Bubbling / Single / Resistance / 0.6 m × 0.06 m | Carb = 20%CO-0–40%steam-N, calc = 10–40%steam-N/ 900 × 650 °C / n.i / n.i/ dry = 15–20.5%, hydrated = 15–21.7% | (1) The effect of steam during calcination was influenced by steam concentration, while carbonation conversion efficiency increased by adding 10 vol.% steam after 10 CaL cycles, a concentration of 20 and 40 vol.% led to a negative impact on sorbent reactivity due to sintering acceleration leading to smaller surface area and larger pore size. |
| Diego and Arias [15] | Limestone / no / n.i / 130–80 m | Circulating / Dual / Calciner (n.i) / 15 m × 0.65 m carb and 0.75 m calc | 14%CO carbonator / n.i × 670–660 °C / = 5.5–2.0 m/s and = 5.9–2.6 m/s / 1.7 MW/ 100–50% | (1) Modifications to the make-up flow might be needed in a few cases to counterbalance the hydrodynamic changes occurring in flexible CaL systems to ensure high CO capture efficiencies. |
| Hilz et al. [12] | Limestone / no / n.i/ n.i | Circulating / Dual / Calciner / 8.6 m carb and 11 m calc × 0.6 m carb and 0.4 m calc | Carb = 13%CO × n.i / 900 × 700–630 °C / = 2.2 m/s and = 5.5–5.0 m/s / 1 MW / 88–70% | (1) Conservative parameters, such as specific carbonator inventory > 700 kg/m, make-up ratio >0.15 mole Ca/mole CO, and looping rate of 10–15 mole Ca /mole CO were identified as the basis to scale up the process. |
| Coppola et al. [46] | Limestone / Hydration/ Germany / 600–400 m | Bubbling / Dual / Resistance / 1 × 0.04 m | Calc = 70%CO-air and carb = 15%CO-Air, 15%CO-10%HO-Air / 940 × 650 °C / = 0.5 m/s / n.i/ dry = 0.05–0.20 hydrated = 0.07–0.20 gCO/g Initial sorbent | (1) Steam exerts a beneficial effect on CO uptake, which can be even great enough to counterbalance the detrimental effect of SO when the concentration of the latter is small. (2) With regard to sorbent mechanical properties, attrition is very limited under all operating conditions. The presence of HO and/or SO leads to a slight increase in sorbent fragmentation which, however, is not likely to significantly affect the operation of a CaL process. |
| Su et al. [78] | Limestone, cement, spent bleaching clay / no / China / 600–250 m | Bubbling / Single / Resistance / 1.1 × 0.025 m | Carb = 15–20%CO-N calc = 80%CO-N / 920 × 650 °C / = 0.05 m/s / n.i/ 10–44 g CO/100 g calcined sorbent | (1) The prepared pellets demonstrated lower elutriation rates, as they had greater sphericity than limestone particles. (2) Doped limestone-based pellets showed superior cyclic CO capture capacity and attrition resistance. |
| Alonso et al. [42] | Limestone / no / n.i / 600–0 m | Circulating / Dual / Resistance / 6.3 m carb, 6.1 m calc × 0.1 m | Air and coal fumes / 780 °C × n.i/ n.i/ 30 kW / n.i | (1) Neither the attrition index defined by Gwyn nor that proposed by Forsythe and Hertwig are able to account for the changes observed in power spectral density (PSD) curves sufficiently well. However, the total particle generated index and the maximum particle size generated during attrition, which require a re-construction of PSDs of elutriated and non-elutriated solids, provided a more accurate description of attrition phenomena for this methodology, in addition to distinguishing different attrition mechanisms and ranking the materials |
| Arias et al. [43] | n.i/ no / n.i / n.i | Circulating / Dual / calciner (Fueled by coal) / 15 m × 0.65 carb m and 0.75 calc m | Carb = 12–14%CO and calc = 70%CO / 950–830 × 715–620 °C / = 4.5–2.5 m/s and = 5.0–3.5 m/s / 1.7 MW / 95–40% | (1) Results confirm that it is possible to operate the calciner of a CaL system under oxygen-rich conditions due to the endothermic nature of a calcination reaction and the large flow of solids circulating within the carbonator. The axial temperature profiles measured along the calciner during these tests showed that no hot spots are found as long as there is sufficient circulation and bed inventory of solids in the calciner. |
| Coppola et al. [71] | Limestone / no / Germany / 600–400 m | Bubbling / Dual / Resistance / 1 m × 0.04 m | Carb = 70%CO-air, Calc = 15%CO-air / 940 × 650 °C / = 0.5 m/s / n.i / 0.02–0.22 g co2/ g sorbent | (1) The larger thermal shocks experienced by the sorbent in single bed system tests appear to be detrimental in terms of CO capture and attrition tendency. (2) The thermal history has non-trivial effects on sorbent fragmentation and is largely associated with the tendency of limestone sintering. (3) Sintering strengthens particle structure, which hampers secondary fragmentation on the one hand but intensifies the effects of thermal shock (primary fragmentation) on the other. |
| Antzara et al. [18] | CaO-based, limestone / hydration / Greece / 500–355 m | Bubbling / Single / Resistance / n.i × 0.018 m | Dry carb = 10CO-N-3.2O-N, hydr carb = 10%CO-20%HO-3.2%O-N dry calc = N hydr calc = 20%HO-N / 920–800 × 680–650 °C / = 3.8–2.5 m/s / n.i / hydr = 88–14% | (1) The addition of steam led to higher conversion rates, especially during pre-breakthrough, due to decreased CO diffusion resistance through the formed layer of CaCO. (2) In addition, steam significantly enhanced sorbent stability, leading to <16% deactivation after 20 consecutive cycles. (3) When the sorbent was tested in tests at slightly different temperatures for carbonation (680 °C) and calcination (750 °C), it exhibited similar carbonation conversion but had a higher deactivation. (4) Compared to a natural limestone that was used as reference material, the final capacity of CaO/CaZrO was almost 5.6 times greater. |
| Hilz et al. [44] | Limestone / no / Germany / Mean 175 m | Circulating / Dual / Calciner (Fueled by hard coal and Lignite) / 8.6 m carb 11 m calc × 0.6 m carb 0.4 m calc | n.i / 935–840 × 711–642 °C / n.i / 1 MW / 85–45% | (1) Demonstration of a successful semi-industrial pilot testing of the CaL process on a scale of 1 MWth under realistic operating conditions. |
| Tregambi et al. [41] | Limestone / no / Italy / 590–420 m | n.i / Single / Solar-infrared / 0.1 m × 0.102 m | Carb = 15%CO and calc = 70%CO / 950–940 × 650 °C / 2x/ 3.2 kW/ 0.025–0.085 g CO / g sobernt | (1) It is inferred that local and occasional higher peak temperatures experienced by sorbent particles in solar CaL result in more extensive thermal sintering and loss of CO capture capacity. |
| Hilz et al. [68] | Limestone / no / Germany / mean 175 m | Circulating / Dual / Calciner (Fueled by hard coal and lignite) / 8.6 m carb 11 m calc × 0.6 m carb 0.4 m calc | Carbonator 11–16%CO / 940–850 × 700–639 / = 2.5–2.3 m/s, = 5.5–4.5 m/s / 1 MW / 95–45% | (1) An accumulation of inert material shows the dependency of ash and sulphur fractions on fuel combusted in the calciner. (2) High-grade fuel reduces the inactive sorbent share in comparable operation conditions from around 20 to 8 wt.%. |
| Coppola et al. [72] | Limestone / hydration / Germany / 600–400 lm | Bubbling / Single / Resistance / 0.95 m × 0.040 m | Calc = 70%CO-30%air, carb = 15%CO-85%air hydr calc = 10%HO-70%CO-air hydr carb = 10%HO-15%CO-air / 940 × 650 / = 2.5–2 m/s / n.i/ dry = 0.17–0.05 and hydrated = 0.19–0.07 g CO/g sorbent | (1) Synergistic effects were observed when steam was added, both during calcination and carbonation, thus resulting in a very pronounced increase of sorbent CO capture capacity compared to a no-steam case. (2) A characterization of the fragmentation propensity of samples in light of morphological features assessed via microscopy showed that exposure to steam during calcination induces a more resistant external particle structure. |
| Coppola et al. [79] | Limestone / no / Germany/ 600–400 m | Bubbling / Dual / Resistance / 1 m × 0.04 m | 100–15%COair / 850 × 650 °C / = 0.06 m/s / n.i / 0.05–0.20 g CO / g sorbent | (1) CO capture capacity results exhibited a typical decay trend with respect to the number of cycles, as expected in CaL processes. Interestingly enough, a comparison of these results with those previously obtained using the same limestone and under similar operating conditions for single bed apparatuses revealed that capture capacity values were higher than those for single bed reactors. This finding seems to emphasize a non-negligible role of sorbent thermal history in Cal CO capture performance. |
| Peng et al. [80] | / no / China / 300–200 m | Bubbling / Single / Resistance / 0.892 m × 0.026 m | Carb = 10%CO-N, calc = 100%N / 900 × 700 °C / = 5.0–3.0 m/s / n.i / 0.45–0.82 mol CO / mol CaO | (1) The results of ESEM prove its high sintering resistance to maintain abundant available surface area and stable textural structure for CO capture reactions, which evidences the advantage of novel SATS method. In addition to addressing the loss-in-capacity of CaO-based CO sorbents, attrition resistance is another important parameter worth being considered, especially in fluidized bed environments.(2) Pore size distribution and mechanical durability results show that the CaO/TiO–AlO sorbent exhibits high mechanical strength, long-lasting anti-attrition performance, and favorable long-life stability. Finally, a raw material analysis shows that the CaO/TiO–AlO sorbent has a competitive advantage for further continuous long-term large-scale industrial operations. |
| Ridha et al. [81] | Cadomin and Limestone Pellet / no / Canada, Spain / 600–212 m | Mixed / Dual / Resistance and calciner (natural gas carbonator and calcined biomass wood pellets) / 5.1 m calc, 3 m carb × 0.1 m | 80%CO / 900 × 650 °C / = 0.8–0.5 m/s, = 2.6–2.0 m/s / 0.1 mW / 90–80% | (1) Batch and continuous injection of pellets into the system revealed that the injection method exerted an insignificant effect on the attrition of pellets. The similarity of particle size distribution patterns of pellets is indicative of similar attrition tendencies, and it appears to be a characteristic of these pellets, regardless of limestone type and injection method. (2) It was found that the pelletization of CaO-based sorbents using limestone and 10% cement results in a marginal improvement in the mechanical strength of resultant pellets. Therefore, the pelletization of sorbent for CaL CO capture is considered inadequate for sorbent attrition reduction. |
| Symonds et al. [64] | Cadomin and Limestone Pellet / no / Canada, Spain / 600–250 m | Mixed / Dual / Resistance and Calciner (natural gas carbonator and biomass pellets calciner) / 5.1 m calc, 3 m carb × 0.1 m | 80%CO / = 0.8–0.5 m/s, = 2.6–2.0 m/s / 0.1 mW / 100–82% | (1) In accordance with published works on CO capture performance of synthetic/modified CaO-based sorbents at bench-scale, this work aims to highlight the fact that such sorbents could have had a more significant and enhanced performance when tested in a pilot-scale system. This suggests that the assessment of such relatively expensive sorbents should be performed under pilot-scale testing conditions to evaluate their performance with respect to their production costs. |
| Blamey et al. [16] | Limestone and cement pellets / hydration / n.i / 710–500 m | Bubbling / Single / Resistance / n.i × 0.021 m | 15%CO-N and 10%HO13.5%CON / 900 × 700–600 °C / = 7.2–5.8 m/s, = 9.8–7.9 m/s / n.i/ 0.32–0.08 g CO / g calcined sorbent | (1) Bubbling bed reactor tests were carried out using up to 20 CO capture cycles on limestone-based pellets produced using calcium aluminate as the binder. These tests show that pellets exhibit a similar or slightly more enhanced behavior in terms of attrition resistance to its parent limestone, in addition to showing superior CO capture performance altogether. |
| Coppola et al. [82] | Limestone / hydration / Germany / 600–400 m | Bubbling / Single / Resistance / n.i × 0.04 m | Calc = 70%CO-air, carb = 15%CO-Air, Hydration = 50%HON / 940 × 650 °C / = 2.5–2.0 m/s / n.i / spent = 0.10–0.48 and hydrated = 0.11–0.51 mol/mol | (1) The effectiveness of sorbent hydration using steam as a means to reactivate CO uptake potential of limestone for applications has been demonstrated. Steam hydration followed by dehydration of reactivated sorbent in a hot fluidized bed reactor leads to increased porosity and, hence, an improved rate and length of CO uptake. (2) Furthermore, attrition and fragmentation propensity of reactivated sorbent is increased. (3) For the aforementinoed limestone, optimal trade-off between sorbent reactivity/uptake and mechanical strength is achieved after 60 min of hydration of spent sorbent, but it is expected that such a result cannot be generalized, since it is critically dependent upon sorbent texture. A comparison between water and steam hydration of spent sorbents as means of reactivation indicated that steam hydration is more favorable. (4) Albeit liquid water hydration gives rise to greater water uptake, prolonged soaking of liquid water makes the reactivated sorbent more susceptible to attrition and fragmentation. |
| Duelli et al. [63] | Limestone / hydration / Germany / 375 m | Mixed / Dual / Resistance / 12.4–0.5 × 0.15–0.071 m | Calc = 0–75%CO0–35%HO-N, carb = 10–16CO0–10HO N / 930–880 × 680–600 / = 5.5–4.5 m/s = 2.0–0.3 m/s / 10 kW / dry = 60–20% hydr = 95–40% | (1) Combustion may lead to faster calcination due to better heat transfer; however, local high temperatures may cause pronounced sintering and consequent further decay of activity as recorded herein, and there might be sorbent hardening. |
| Duelli et al. [62] | Limestone / no / Germany / n.i | Bubbling / Dual / Resistance / 12.4 m × 0.114 m | Calc = 0–65%CO-N, carb = 14%CO-N / 920–905 × 630/ n.i / 10 kW / 18–9% mol CaCO/mol Ca | (1) Full sorbent regeneration could not be achieved primarily due to the bubbling fluidization regime of the bed and the quality of heat provided by electric heaters through the walls to the bed. These limitations impose solid residence times of up to 8 min for a calcination of solids instead of seconds, as it is in TGA investigations as well as industrial applications such as clinker production. This is not the case for process scale-up where these limitations are not found due to a fast fluidization regime and combustion atmosphere; thus, the data presented herein are suggested to be treated qualitatively, not quantitatively. |
| Coppola et al. [83] | Limestone / hydration / Italy / 600–400 m | Bubbling / Single / Resistance / n.i × 0.04 m | 70%CO-Air / 940 × 650 °C / = 0.7 m/s / n.i / hydr = 0.03–0.35 gg−1, dry = 0.03 gg−1 | (1) Optimal hydration time depends on pore size. |
| Alonso et al. [61] | Limestone / no / Spain, Germany / 348–87 m | Circulating / Dual / Calciner (biomass pellets) / 12 m × 0.4 m | n.i / 950–800 × 720–630 °C / = 2.8–0.8 m/s / 300 kW / 85–5% | (1) Large discrepancies were detected in experiments conducted with large particles, probably due to segregation effects of denser and coarser particles in the reactor bed inventory. |
| Coppola et al. [67] | Dolomite, Limestone / no / Italy, Germany, Greece, Poland / 600–400 m | Bubbling / Single / Resistance / 0.95 m × 0.04 m | Calc = 70%CON, carb = 15%CON / 940 × 650 °C / = 0.7–0.6 m/s / n.i / 0.02–0.14 g CO / g Ca | (1) TG and FB devices are characterized by quite different fluid dynamic and mass transfer conditions, which result in different sample heating and reaction rates. However, as far as CO capture capacity is concerned, the results suggest that the type of reactor has a lower influence than the calcination environment on sorbent performance. Factors enhancing sintering (e.g., high temperature and CO concentration) severely impact the sorbent CO capture capacity. On the other hand, factors slowing down sintering (e.g., the presence of MgO, as in dolomite) improve sorbent performance. |
| Coppola et al. [70] | Dolomite, Limestone / no / Italy, Germany, Greece, Poland / 600–400 m | Bubbling / Single / Resistance / 0.95 m × 0.04 m | Calc = 70%CO30%N, Carb = 15%CO85%N / 940 × 650 °C / = 0.7–0.6 m/s / n.i / 80–18% mol/mol | (1) The CO capture capacity of the dolomite was always greater than that of limestone and remained relatively high along cycles, despite the lower calcium content of the sorbent. |
| Coppola et al. [53] | Limestone / no / Italy, Germany, Greece, Poland / 500–30 m | Bubbling / Single / Resistance / 0.95 m × 0.04 m | Calc = 70%CO30%N, carb = 15%CO85%N / 940 × 650 °C / = 0.7–0.6 m/s / n.i / capture capacity = 0.02–0.21 g CO /g CaCO | (1) The measured fine elutriation rate was only moderately large during the first cycle, but it reduced along the cycles, since a combined chemical thermal treatment significantly hardened the particle structure. (2) A high SO concentration had a detrimental effect on the CO capture capacity of all limestones, while a low SO concentration had a more limited effect. |
| Coppola et al. [30] | Limestone / no / Italy / 600–400 m | Bubbling / Single / Resistance / 1 m × 0.04 m | Calc = 20CON, Carb = 16CON / 850 × 700 °C / = 0.7–0.6 m/s / 4.4 kW / 60–10% | (1) Results showed that the presence of SO in the flue gas significantly decreased the sorbent CO capacity, quite possibly due to the formation of an impervious CaSO layer at the periphery of particles. (2) Even a small quantity of SO is sufficient to significantly hinder the sorbent CO capture capacity. (3) An analysis of particle size distribution of bed material over repeated calcination/carbonation cycles indicated that particle fragmentation was very limited in all investigated conditions. (4) Results obtained using 1800 ppm SO showed divergences from other conditions with regard to: cumulative particle size distribution of fragments, cumulative elutriated material over five repeated cycles, and degree of SO uptake over repeated cycles. |
| Acharya et al. [69] | Limestone / n.i / n.i / 325–135 m | Mixed / Dual / Resistance / n.i × 0.101 m | Calc = Steam+Air Carb = 22–25%CO-N / 900 × 600–550 °C / n.i / 100–10% (air) and 100–25% (steam) | (1) The CO absorbing capacity of CaO decreases progressively as it goes through alternating cycles of carbonation and calcination. Such a reduction is due to several factors, including sintering and pore pluggage. (2) The calcination of CaCO in the presence of different media shows that the degree of calcination of CaCO in the presence of HO is similar to that of N. (3) Kinetic rates of calcination are much higher when measured using HO and N than when measured using CO. (4) Sintering leading to sorbent agglomeration cannot be avoided during calcination at higher temperatures, even while using steam. |
| Symonds et al. [66] | Limestone / hydration / Canada, Poland / 612–512 m | Circulating / Single / Resistance + Calciner (Pure fiber hardwood blend) / 5 m × 0.1 m | Calc = 1%CO7.5%O 35%CO or 1%6%O 35%CO and Carb = 8%CO 19.3%O 72.7%N or 8%CO 15.7O 17%HO 59.3%N / 920–860 × 690–610 / n.i / 49–11% | (1) Results suggest that CO capture cycles in TGA experiments should encompass as many factors present during FBC operation as possible (i.e., higher calcination temperatures/CO concentrations, and the presence of steam and other gases such as SO) so as to avoid rather misleading results and conclusions regarding sorbent performance. |
| Rodríguez et al. [52] | Limestone / no / n.i / <350 m | Circulating / Dual / Resistance / calc = 6 m, carb = 6.5 m × 0.1 m | Calc = 5–3%O7–22%CO, Carb = 15–2%CO 17%O / 850–800 × 722–568 °C / n.i / 30 kW/ 92–30% | (1) When there are enough active CaO particles inside the reactor, the low-carrying capacity of highly cycled CaO particles can be counterbalanced by sufficiently large inventories of solids in the carbonator, which can increase the carbonation conversion achieved by CaO to values close to the maximum capture capacity of the material by increasing the average residence time of particles in the reactor. |
| Blamey et al. [60] | Limestone / hydration / Canada / 710–500 m | Bubbling / Single / Resistance / 0.33 m × 0.026 m | 15%CON / 950–850 × 700 °C / = 8 / n.i / 70–10% fresh, 35–0% hydr after 13 cycles | (1) Severe temperatures in calcination cause particles to become friable and increase elutriation process. (2) Results indicated that reactivation would be unsuitable for a bubbling fluidized bed CO capture process, though it may be suitable for a moving or fixed bed reactor. |
| Blamey et al. [84] | Limestone / hydration / United Kingdom, Canada, Spain / 710–500 m | Bubbling / Single / Resistance / 0.33 m × 0.026 m | 15%CON / 1000–840 × 700 °C / = 7 / n.i / 78–2 g/100 g and 58–18 g/100 g hydrated | (1) Significant expansion and break-up of particles was observed in the hydration for particles cycled at higher temperatures, and it is proposed that this is due to a significant densification of particles. |
| Charitos et al. [50] | Limestone / no / Germany / n.i | Bubbling / Dual / Resistance and calciner (Natural gas) / 12.4 m × 0.071 m calc 0.114 m carb | 15%CO / n.i × 700–630 °C / n.i / 10 kW/ 97–90% | (1) The degree of sorbent particle sintering increased along carbonation–calcination cycles and resulted in smaller values of average CO capture capacity, reduction in small pore volume (<200 nm), and a decrease in particle surface area. |
| Hughes et al. [65] | Limestone / no / Poland, Canada / n.i | Mixed / Dual / Resistance and Calciner (Wood pellets) / 5 m × 0.1 m | Calc = 46%NO Carb = 8%COAir or 8%CO17%OAir / 910–860 × 600 °C / n.i / 75 kW /n.i | (1) Sorbent capacity was significantly lower than that expected based on previous thermogravimetric analyses. It is believed to be at least partially captured due to the formation of a thin, low-porosity shell around the sorbent enhanced by the deposition of ash from the solid fuel under oxygen-fired conditions. |
| Lu et al. [49] | Limestone / no / Canada / 800–400 m | Circulating / Dual / Resistance and Calciner (biomass or coal) / 5 m × 0.1 m | 15%COair / 950–850 × 720–580 °C / = 0.8–0.4 m/s / 75 kW / 85–70% | (1) An examination of sorbent surface characteristics suggests that a number of complicated processes are occurring on the particle surface as a consequence of the number of reaction cycles. Issues of sorbent loss through attrition, impact of sulphation, and process optimization require further investigation. |
3.3. Data Analysis Limitations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shukla, P.; Skea, R.J.; Khourdajie, A.A.; van Diemen, R.; McCollum, D.; Pathak, M.; Some, S.; Vyas, P.R.F.; Fradera, R.; Belkacemi, M.; et al. Mitigation of Climate Change Summary for Policymakers (SPM). 2022. Available online: https://www.ipcc.ch/report/ar6/wg3/downloads/report/IPCC_AR6_WGIII_SPM.pdf (accessed on 26 July 2022).
- Pathak, M.; Slade, R.; Shukla, P.; Skea, J.; Pichs-Madruga, R.; Ürge Vorsatz, D. Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. 2022. Available online: https://www.ipcc.ch/report/ar6/wg3/downloads/report/IPCC_AR6_WGIII_TechnicalSummary.pdf (accessed on 26 July 2022).
- Wilberforce, T.; Olabi, A.; Sayed, E.T.; Elsaid, K.; Abdelkareem, M.A. Progress in carbon capture technologies. Sci. Total Environ. 2021, 761, 143203. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Deng, Y.; Dewil, R.; Baeyens, J.; Fan, X. Post-combustion carbon capture. Renew. Sustain. Energy Rev. 2021, 138, 110490. [Google Scholar] [CrossRef]
- Pardemann, R.; Meyer, B. Pre-Combustion Carbon Capture. In Handbook of Clean Energy Systems; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1–28. [Google Scholar]
- Sreedhar, I.; Nahar, T.; Venugopal, A.; Srinivas, B. Carbon capture by absorption-Path covered and ahead. Renew. Sustain. Energy Rev. 2017, 76, 1080–1107. [Google Scholar] [CrossRef]
- Saxena, A.; Liyanage, W.; Masud, J.; Kapila, S.; Nath, M. Selective electroreduction of CO2 to carbon-rich products with a simple binary copper selenide electrocatalyst. J. Mater. Chem. A 2021, 9, 7150–7161. [Google Scholar] [CrossRef]
- Saxena, A.; Singh, H.; Nath, M. Cobalt telluride electrocatalyst for selective electroreduction of CO2 to value-added chemicals. Mater. Renew. Sustain. Energy 2022, 11, 115–129. [Google Scholar] [CrossRef]
- Saxena, A.; Liyanage, W.P.; Kapila, S.; Nath, M. Nickel selenide as an efficient electrocatalyst for selective reduction of carbon dioxide to carbon-rich products. Catal. Sci. Technol. 2022, 12, 4727–4739. [Google Scholar] [CrossRef]
- Saxena, A.; Kapila, S.; Medvedeva, J.E.; Nath, M. Copper Cobalt Selenide as a Bifunctional Electrocatalyst for the Selective Reduction of CO2 to Carbon-Rich Products and Alcohol Oxidation. ACS Appl. Mater. Interfaces 2023, 15, 14433–14446. [Google Scholar] [CrossRef]
- Khalilpour, R.; Mumford, K.; Zhai, H.; Abbas, A.; Stevens, G.; Rubin, E.S. Membrane-based carbon capture from flue gas: A review. J. Clean. Prod. 2015, 103, 286–300. [Google Scholar] [CrossRef]
- Hilz, J.; Haaf, M.; Helbig, M.; Lindqvist, N.; Ströhle, J.; Epple, B. Scale-up of the carbonate looping process to a 20 MWth pilot plant based on long-term pilot tests. Int. J. Greenh. Gas Control 2019, 88, 332–341. [Google Scholar] [CrossRef]
- Haaf, M.; Hilz, J.; Peters, J.; Unger, A.; Ströhle, J.; Epple, B. Operation of a 1 MWth calcium looping pilot plant firing waste-derived fuels in the calciner. Powder Technol. 2020, 372, 267–274. [Google Scholar] [CrossRef]
- Haaf, M.; Anantharaman, R.; Roussanaly, S.; Ströhle, J.; Epple, B. CO2 capture from waste-to-energy plants: Techno-economic assessment of novel integration concepts of calcium looping technology. Resour. Conserv. Recycl. 2020, 162, 104973. [Google Scholar] [CrossRef]
- Diego, M.; Arias, B. Impact of load changes on the carbonator reactor of a 1.7 MWth calcium looping pilot plant. Fuel Process. Technol. 2020, 200, 106307. [Google Scholar] [CrossRef]
- Blamey, J.; Al-Jeboori, M.J.; Manovic, V.; Fennell, P.S.; Anthony, E.J. CO2 capture by calcium aluminate pellets in a small fluidized bed. Fuel Process. Technol. 2016, 142, 100–106. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Duan, L.; Ma, X.; Wang, Z.; Lu, C. Attrition behavior of calcium-based waste during CO2 capture cycles using calcium looping in a fluidized bed reactor. Chem. Eng. Res. Des. 2016, 109, 806–815. [Google Scholar] [CrossRef]
- Antzara, A.N.; Arregi, A.; Heracleous, E.; Lemonidou, A.A. In-depth evaluation of a ZrO2 promoted CaO-based CO2 sorbent in fluidized bed reactor tests. Chem. Eng. J. 2018, 333, 697–711. [Google Scholar] [CrossRef]
- Soltani, S.M.; Lahiri, A.; Bahzad, H.; Clough, P.; Gorbounov, M.; Yan, Y. Sorption-enhanced steam methane reforming for combined CO2 capture and hydrogen production: A state-of-the-art review. Carbon Capture Sci. Technol. 2021, 1, 100003. [Google Scholar] [CrossRef]
- Shao, B.; Hu, G.; Alkebsi, K.A.; Ye, G.; Lin, X.; Du, W.; Hu, J.; Wang, M.; Liu, H.; Qian, F. Heterojunction-redox catalysts of FexCoyMg10CaO for high-temperature CO2 capture and in situ conversion in the context of green manufacturing. Energy Environ. Sci. 2021, 14, 2291–2301. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, C.; Lu, B.; Luo, C.; Wu, F.; Li, X.; Zhang, L. Study on the effect of NaBr modification on CaO-based sorbent for CO2 capture and SO2 capture. Carbon Capture Sci. Technol. 2021, 1, 100015. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, B.; Luo, C.; Chen, J.; Zhang, Z.; Zhang, L. Sorption enhanced steam reforming of ethanol over Ni-based catalyst coupling with high-performance CaO pellets. Chem. Eng. J. 2021, 406, 126903. [Google Scholar] [CrossRef]
- Chen, J.; Duan, L.; Sun, Z. Review on the development of sorbents for calcium looping. Energy Fuels 2020, 34, 7806–7836. [Google Scholar] [CrossRef]
- Erans, M.; Manovic, V.; Anthony, E.J. Calcium looping sorbents for CO2 capture. Appl. Energy 2016, 180, 722–742. [Google Scholar] [CrossRef]
- Han, R.; Xing, S.; Wu, X.; Pang, C.; Lu, S.; Su, Y.; Liu, Q.; Song, C.; Gao, J. Relevant influence of alkali carbonate doping on the thermochemical energy storage of Ca-based natural minerals during CaO/CaCO3 cycles. Renew. Energy 2022, 181, 267–277. [Google Scholar] [CrossRef]
- Grasa, G.S.; Abanades, J.C. CO2 capture capacity of CaO in long series of carbonation/calcination cycles. Ind. Eng. Chem. Res. 2006, 45, 8846–8851. [Google Scholar] [CrossRef]
- Fennell, P.S.; Pacciani, R.; Dennis, J.S.; Davidson, J.F.; Hayhurst, A.N. The effects of repeated cycles of calcination and carbonation on a variety of different limestones, as measured in a hot fluidized bed of sand. Energy Fuels 2007, 21, 2072–2081. [Google Scholar] [CrossRef]
- Stanmore, B.R.; Gilot, P. calcination and carbonation of limestone during thermal cycling for CO2 sequestration. Fuel Process. Technol. 2005, 86, 1707–1743. [Google Scholar] [CrossRef]
- Anthony, E.; Granatstein, D. Sulfation phenomena in fluidized bed combustion systems. Prog. Energy Combust. Sci. 2001, 27, 215–236. [Google Scholar] [CrossRef]
- Coppola, A.; Montagnaro, F.; Salatino, P.; Scala, F. Fluidized bed calcium looping: The effect of SO2 on sorbent attrition and CO2 capture capacity. Chem. Eng. J. 2012, 207, 445–449. [Google Scholar] [CrossRef]
- Scala, F.; Salatino, P. Limestone fragmentation and attrition during fluidized bed oxyfiring. Fuel 2010, 89, 827–832. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Coppola, A.; Massa, F.; Montagnaro, F.; Scala, F. Analysis of the behaviour of limestone sorbents for sorption-enhanced gasification in dual interconnected fluidised bed reactor. Fuel 2023, 340, 127594. [Google Scholar] [CrossRef]
- Kafshgari, L.A.; Ghorbani, M.; Azizi, A.; Agarwal, S.; Gupta, V.K. Modeling and optimization of Direct Red 16 adsorption from aqueous solutions using nanocomposite of MnFe2O4/MWCNTs: RSM-CCRD model. J. Mol. Liq. 2017, 233, 370–377. [Google Scholar] [CrossRef]
- Galina, N.R.; Arce, G.L.; Maroto-Valer, M.; Ávila, I. Experimental Study on Mineral Dissolution and Carbonation Efficiency Applied to pH-Swing Mineral Carbonation for Improved CO2 Sequestration. Energies 2023, 16, 2449. [Google Scholar] [CrossRef]
- Mortari, D.A.; Avila, I.; Crnkovic, P.M. Response Surface Methodology Applied to the Evaluation of the SO2 Sorption Process in Two Brazilian Limestones. Energy Fuels 2013, 27, 2890–2898. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.; Shahidi, F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005, 93, 47–56. [Google Scholar] [CrossRef]
- Khodaei, B.; Sobati, M.A.; Shahhosseini, S. Optimization of ultrasound-assisted oxidative desulfurization of high sulfur kerosene using response surface methodology (RSM). Clean Technol. Environ. Policy 2016, 18, 2677–2689. [Google Scholar] [CrossRef]
- Pashaei, H.; Mashhadimoslem, H.; Ghaemi, A. Modeling and optimization of CO2 mass transfer flux into Pz-KOH-CO2 system using RSM and ANN. Sci. Rep. 2023, 13, 4011. [Google Scholar] [CrossRef]
- Werther, J. Fluidized-bed reactors. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Tregambi, C.; Salatino, P.; Solimene, R.; Montagnaro, F. An experimental characterization of Calcium Looping integrated with concentrated solar power. Chem. Eng. J. 2018, 331, 794–802. [Google Scholar] [CrossRef]
- Alonso, M.; Arias, B.; Fernández, J.R.; Bughin, O.; Abanades, C. Measuring attrition properties of calcium looping materials in a 30 kW pilot plant. Powder Technol. 2018, 336, 273–281. [Google Scholar] [CrossRef]
- Arias, B.; Diego, M.; Méndez, A.; Alonso, M.; Abanades, J. Calcium looping performance under extreme oxy-fuel combustion conditions in the calciner. Fuel 2018, 222, 711–717. [Google Scholar] [CrossRef]
- Hilz, J.; Helbig, M.; Haaf, M.; Daikeler, A.; Ströhle, J.; Epple, B. Investigation of the fuel influence on the carbonate looping process in 1 MWth scale. Fuel Process. Technol. 2018, 169, 170–177. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Y.; Li, B.; Wang, Z.; Wang, T.; Lei, W. Calcium looping heat storage performance and mechanical property of CaO-based pellets under fluidization. Chin. J. Chem. Eng. 2021, 36, 170–180. [Google Scholar] [CrossRef]
- Coppola, A.; Esposito, A.; Montagnaro, F.; Iuliano, M.; Scala, F.; Salatino, P. The combined effect of H2O and SO2 on CO2 uptake and sorbent attrition during fluidised bed calcium looping. Proc. Combust. Inst. 2019, 37, 4379–4387. [Google Scholar] [CrossRef]
- Dong, J.; Tang, Y.; Nzihou, A.; Weiss-Hortala, E. Effect of steam addition during carbonation, calcination or hydration on re-activation of CaO sorbent for CO2 capture. J. CO2 Util. 2020, 39, 101167. [Google Scholar] [CrossRef]
- Rong, N.; Wang, J.; Han, L.; Wu, Y.; Mu, Z.; Wan, X.; Wang, G. Effect of steam addition during calcination on CO2 capture performance and strength of bio-templated Ca-based pellets. J. CO2 Util. 2022, 63, 102127. [Google Scholar] [CrossRef]
- Lu, D.Y.; Hughes, R.W.; Anthony, E.J. Ca-based sorbent looping combustion for CO2 capture in pilot-scale dual fluidized beds. Fuel Process. Technol. 2008, 89, 1386–1395. [Google Scholar] [CrossRef]
- Charitos, A.; Hawthorne, C.; Bidwe, A.; Sivalingam, S.; Schuster, A.; Spliethoff, H.; Scheffknecht, G. Parametric investigation of the calcium looping process for CO2 capture in a 10 kWth dual fluidized bed. Int. J. Greenh. Gas Control. 2010, 4, 776–784. [Google Scholar] [CrossRef]
- Blamey, J.; Paterson, N.P.; Dugwell, D.R.; Fennell, P.S. Mechanism of particle breakage during reactivation of CaO-based sorbents for CO2 capture. Energy Fuels 2010, 24, 4605–4616. [Google Scholar] [CrossRef]
- Rodríguez, N.; Alonso, M.; Abanades, J. Experimental investigation of a circulating fluidized-bed reactor to capture CO2 with CaO. AIChE J. 2011, 57, 1356–1366. [Google Scholar] [CrossRef]
- Coppola, A.; Scala, F.; Salatino, P.; Montagnaro, F. Fluidized bed calcium looping cycles for CO2 capture under oxy-firing calcination conditions: Part 1. Assessment of six limestones. Chem. Eng. J. 2013, 231, 537–543. [Google Scholar] [CrossRef]
- Chen, L.; Dai, W.; Wang, C.; Wang, W.; Anthony, E.J. The combined effect of SO2 and H2O on CO2 capture performance by calcium looping. J. CO2 Util. 2021, 54, 101798. [Google Scholar] [CrossRef]
- Cotton, A.; Patchigolla, K.; Oakey, J. Hydrodynamic characteristics of a pilot-scale cold model of a CO2 capture fluidised bed reactor. Powder Technol. 2013, 235, 1060–1069. [Google Scholar] [CrossRef]
- Homsy, S.L.; Moreno, J.; Dikhtiarenko, A.; Gascon, J.; Dibble, R.W. Calcium Looping: On the Positive Influence of SO2 and the Negative Influence of H2O on CO2 Capture by Metamorphosed Limestone-Derived Sorbents. ACS Omega 2020, 5, 32318–32333. [Google Scholar] [CrossRef] [PubMed]
- SOCRATCES. Solar Calcium-Looping Integration for Thermo-Chemical Energy Storage. 2018. Available online: https://cordis.europa.eu/project/id/727348 (accessed on 26 July 2022).
- CLEANKER. CLEAN clinKER Production by Calcium Looping Process. 2017. Available online: https://cordis.europa.eu/project/id/764816 (accessed on 26 July 2022).
- Natural Resources Canada. Available online: https://www.nrcan.gc.ca/our-natural-resources/energy-sources-distribution/carbon-capture-utilization-and-storage/4275 (accessed on 27 July 2022).
- Blamey, J.; Paterson, N.P.; Dugwell, D.R.; Stevenson, P.; Fennell, P.S. Reactivation of a CaO-based sorbent for CO2 capture from stationary sources. Proc. Combust. Inst. 2011, 33, 2673–2681. [Google Scholar] [CrossRef]
- Alonso, M.; Diego, M.; Pérez, C.; Chamberlain, J.; Abanades, J. Biomass combustion with in situ CO2 capture by CaO in a 300 kWth circulating fluidized bed facility. Int. J. Greenh. Gas Control 2014, 29, 142–152. [Google Scholar] [CrossRef]
- Duelli, G.; Bidwe, A.R.; Papandreou, I.; Dieter, H.; Scheffknecht, G. Characterization of the oxy-fired regenerator at a 10 kWth dual fluidized bed calcium looping facility. Appl. Therm. Eng. 2015, 74, 54–60. [Google Scholar] [CrossRef]
- Duelli, G.; Charitos, A.; Diego, M.E.; Stavroulakis, E.; Dieter, H.; Scheffknecht, G. Investigations at a 10 kWth calcium looping dual fluidized bed facility: Limestone calcination and CO2 capture under high CO2 and water vapor atmosphere. Int. J. Greenh. Gas Control 2015, 33, 103–112. [Google Scholar] [CrossRef]
- Symonds, R.T.; Champagne, S.; Ridha, F.N.; Lu, D.Y. CO2 capture performance of CaO-based pellets in a 0.1 mWth pilot-scale calcium looping system. Powder Technol. 2016, 290, 124–131. [Google Scholar] [CrossRef]
- Hughes, R.W.; Macchi, A.; Lu, D.Y.; Anthony, E.J. Changes in Limestone Sorbent Morphology during CaO-CaCO3 Looping at Pilot Scale. Chem. Eng. Technol. 2009, 32, 425–434. [Google Scholar] [CrossRef]
- Symonds, R.T.; Lu, D.Y.; Manovic, V.; Anthony, E.J. Pilot-scale study of CO2 capture by CaO-based sorbents in the presence of steam and SO2. Ind. Eng. Chem. Res. 2012, 51, 7177–7184. [Google Scholar] [CrossRef]
- Coppola, A.; Scala, F.; Itskos, G.; Grammelis, P.; Pawlak-Kruczek, H.; Antiohos, S.K.; Salatino, P.; Montagnaro, F. Performance of natural sorbents during calcium looping cycles: A comparison between fluidized bed and thermo-gravimetric tests. Energy Fuels 2013, 27, 6048–6054. [Google Scholar] [CrossRef]
- Hilz, J.; Helbig, M.; Haaf, M.; Daikeler, A.; Ströhle, J.; Epple, B. Long-term pilot testing of the carbonate looping process in 1 MWth scale. Fuel 2017, 210, 892–899. [Google Scholar] [CrossRef]
- Acharya, B.; Dutta, A.; Basu, P. Circulating-fluidized-bed-based calcium-looping gasifier: Experimental studies on the calcination–carbonation cycle. Ind. Eng. Chem. Res. 2012, 51, 8652–8660. [Google Scholar] [CrossRef]
- Coppola, A.; Scala, F.; Salatino, P.; Montagnaro, F. Fluidized bed calcium looping cycles for CO2 capture under oxy-firing calcination conditions: Part 2. Assessment of dolomite vs. limestone. Chem. Eng. J. 2013, 231, 544–549. [Google Scholar] [CrossRef]
- Coppola, A.; Scala, F.; Salatino, P. Characterization of calcium looping sorbents with a novel twin bed reactor. Fuel Process. Technol. 2018, 172, 49–54. [Google Scholar] [CrossRef]
- Coppola, A.; Gais, E.; Mancino, G.; Montagnaro, F.; Scala, F.; Salatino, P. Effect of steam on the performance of Ca-based sorbents in calcium looping processes. Powder Technol. 2017, 316, 578–584. [Google Scholar] [CrossRef]
- Coppola, A.; Sattari, A.; Montagnaro, F.; Scala, F.; Salatino, P. Performance of limestone-based sorbent for sorption-enhanced gasification in dual interconnected fluidized bed reactors. AIChE J. 2023, 69, e17588. [Google Scholar] [CrossRef]
- Chai, S.; Li, Y.; Zhang, W.; He, Z. Simultaneous NO/CO2 removal performance using Ce-doped CaO in calcium looping process: Experimental and DFT studies. J. Environ. Chem. Eng. 2022, 10, 108236. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Li, Z. Limestone Calcination Kinetics in Microfluidized Bed Thermogravimetric Analysis (MFB-TGA) for Calcium Looping. Catalysts 2022, 12, 1661. [Google Scholar] [CrossRef]
- Hashemi, S.M.; Zhou, R.; Mahinpey, N. Evaluation of MgO- and CaZrO3-promoted CaO-based pellets produced via solution combustion synthesis. Chem. Eng. J. 2022, 450, 138274. [Google Scholar] [CrossRef]
- González, B.; Kokot-Blamey, J.; Fennell, P. Enhancement of CaO-based sorbent for CO2 capture through doping with seawater. Greenh. Gases Sci. Technol. 2020, 10, 878–883. [Google Scholar] [CrossRef]
- Su, C.; Duan, L.; Anthony, E.J. CO2 capture and attrition performance of competitive eco-friendly calcium-based pellets in fluidized bed. Greenh. Gases Sci. Technol. 2018, 8, 1124–1133. [Google Scholar] [CrossRef]
- Coppola, A.; Scala, F.; Gargiulo, L.; Salatino, P. A twin-bed test reactor for characterization of calcium looping sorbents. Powder Technol. 2017, 316, 585–591. [Google Scholar] [CrossRef]
- Peng, W.; Xu, Z.; Zhao, H. Batch fluidized bed test of SATS-derived CaO/TiO2–Al2O3 sorbent for calcium looping. Fuel 2016, 170, 226–234. [Google Scholar] [CrossRef]
- Ridha, F.N.; Lu, D.Y.; Symonds, R.T.; Champagne, S. Attrition of CaO-based pellets in a 0.1 mWth dual fluidized bed pilot plant for post-combustion CO2 capture. Powder Technol. 2016, 291, 60–65. [Google Scholar] [CrossRef]
- Coppola, A.; Palladino, L.; Montagnaro, F.; Scala, F.; Salatino, P. Reactivation by steam hydration of sorbents for fluidized-bed calcium looping. Energy Fuels 2015, 29, 4436–4446. [Google Scholar] [CrossRef]
- Coppola, A.; Salatino, P.; Montagnaro, F.; Scala, F. Hydration-induced reactivation of spent sorbents for fluidized bed calcium looping (double looping). Fuel Process. Technol. 2014, 120, 71–78. [Google Scholar] [CrossRef]
- Blamey, J.; Anthony, E.; Wang, J.; Fennell, P. The use of the calcium looping cycle for post-combustion CO2 capture. Prog. Energy Combust. Sci. 2010, 36, 260–279. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toledo, R.C.; Arce, G.L.A.F.; Carvalho, J.A., Jr.; Ávila, I. Experimental Development of Calcium Looping Carbon Capture Processes: An Overview of Opportunities and Challenges. Energies 2023, 16, 3623. https://doi.org/10.3390/en16093623
Toledo RC, Arce GLAF, Carvalho JA Jr., Ávila I. Experimental Development of Calcium Looping Carbon Capture Processes: An Overview of Opportunities and Challenges. Energies. 2023; 16(9):3623. https://doi.org/10.3390/en16093623
Chicago/Turabian StyleToledo, Rubens C., Gretta L. A. F. Arce, João A. Carvalho, Jr., and Ivonete Ávila. 2023. "Experimental Development of Calcium Looping Carbon Capture Processes: An Overview of Opportunities and Challenges" Energies 16, no. 9: 3623. https://doi.org/10.3390/en16093623
APA StyleToledo, R. C., Arce, G. L. A. F., Carvalho, J. A., Jr., & Ávila, I. (2023). Experimental Development of Calcium Looping Carbon Capture Processes: An Overview of Opportunities and Challenges. Energies, 16(9), 3623. https://doi.org/10.3390/en16093623







