Study on the Effect of Dedicated Microelement Mixture (DMM) on the Kick-Off Phase of the Digester and Stabilization of the Methane Fermentation Process
Abstract
1. Introduction
2. Study Methodology
3. Study Results and Their Interpretation
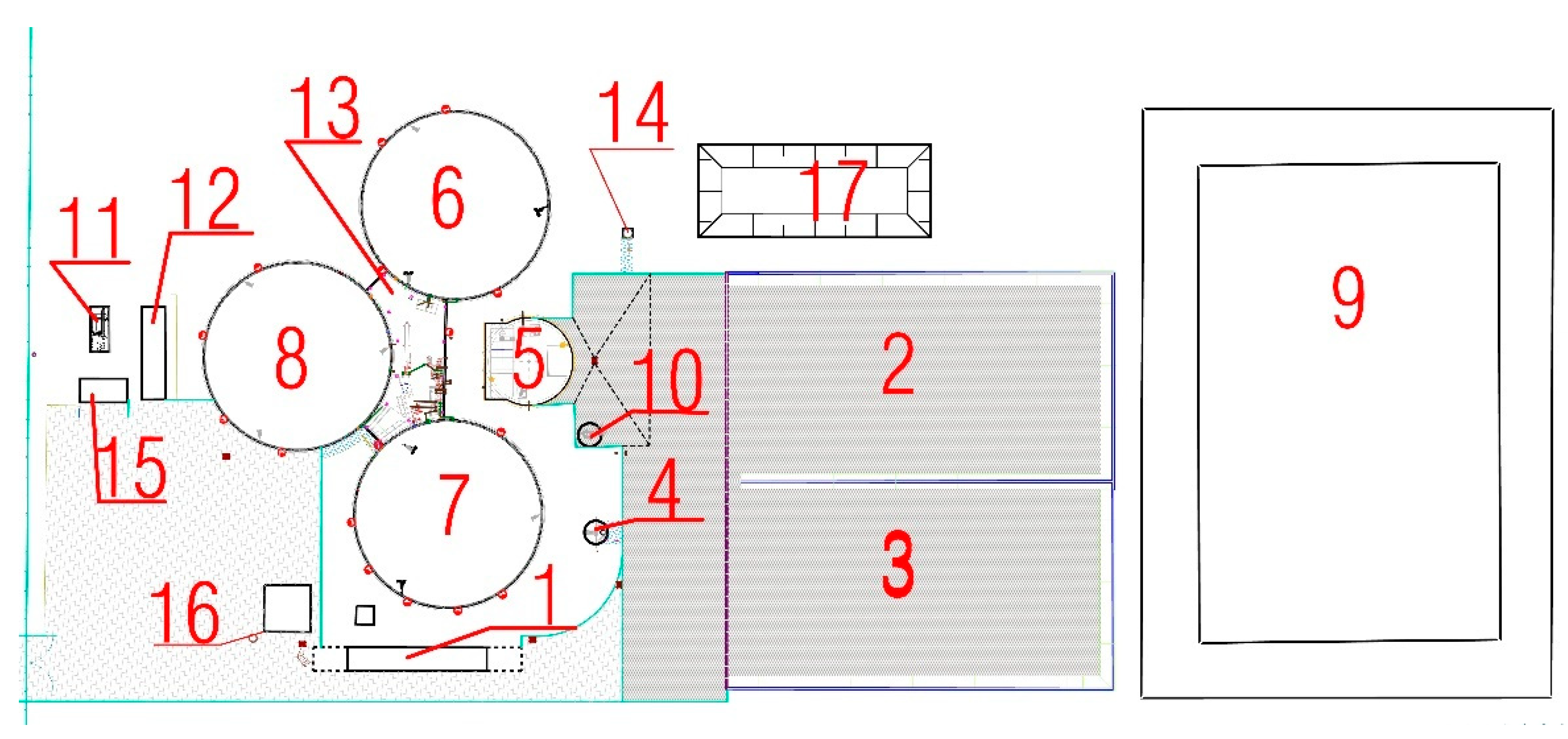
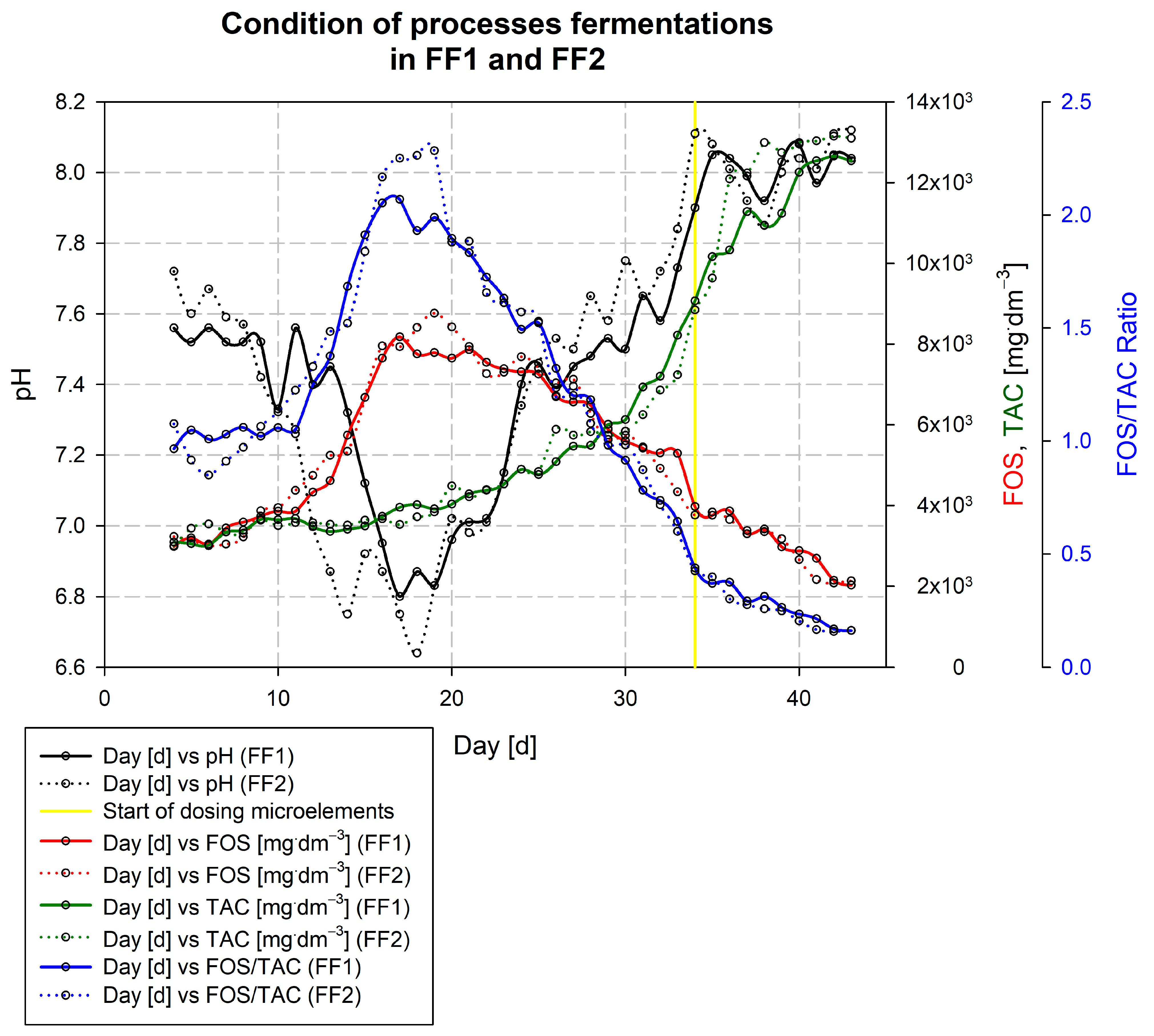
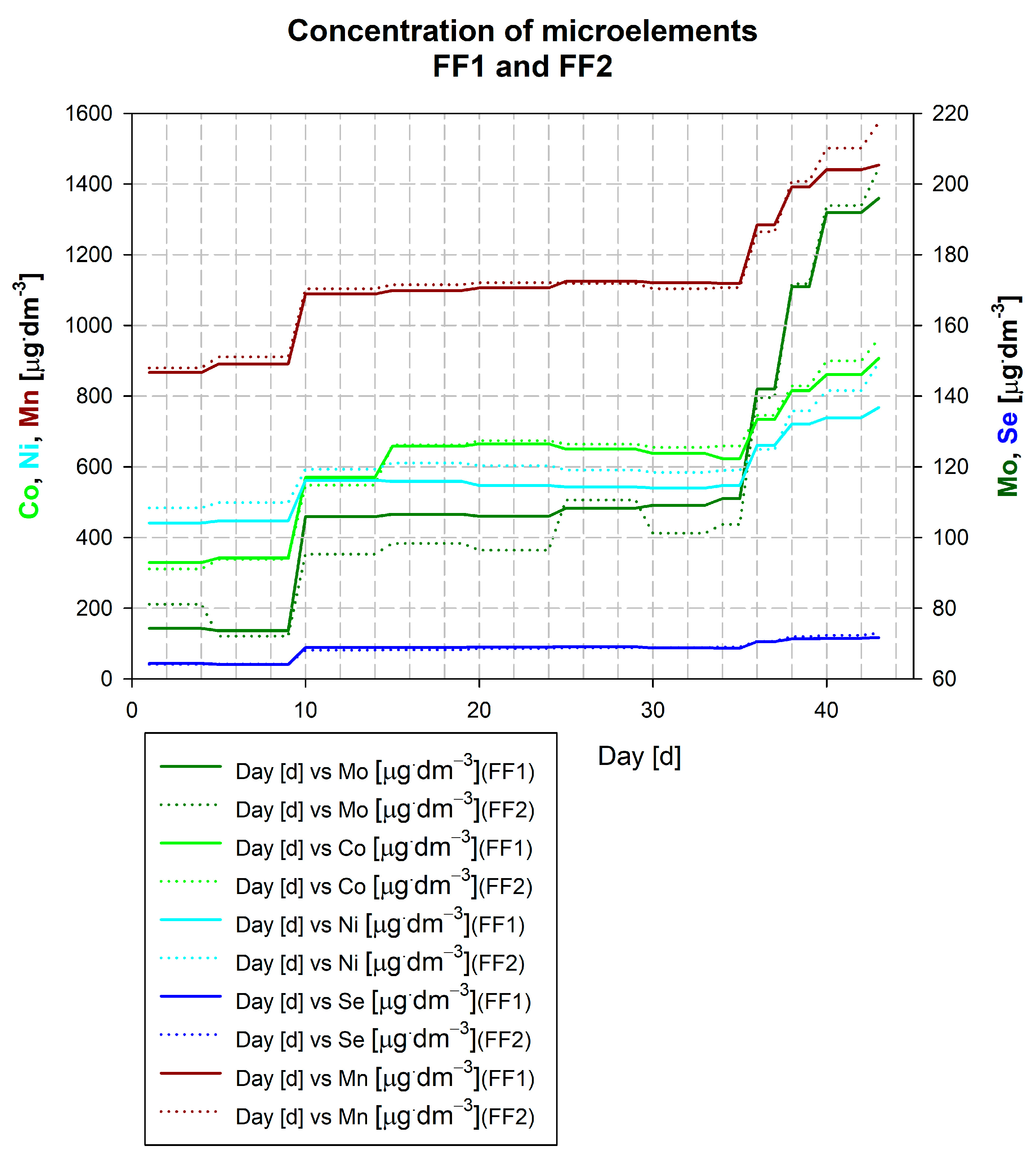
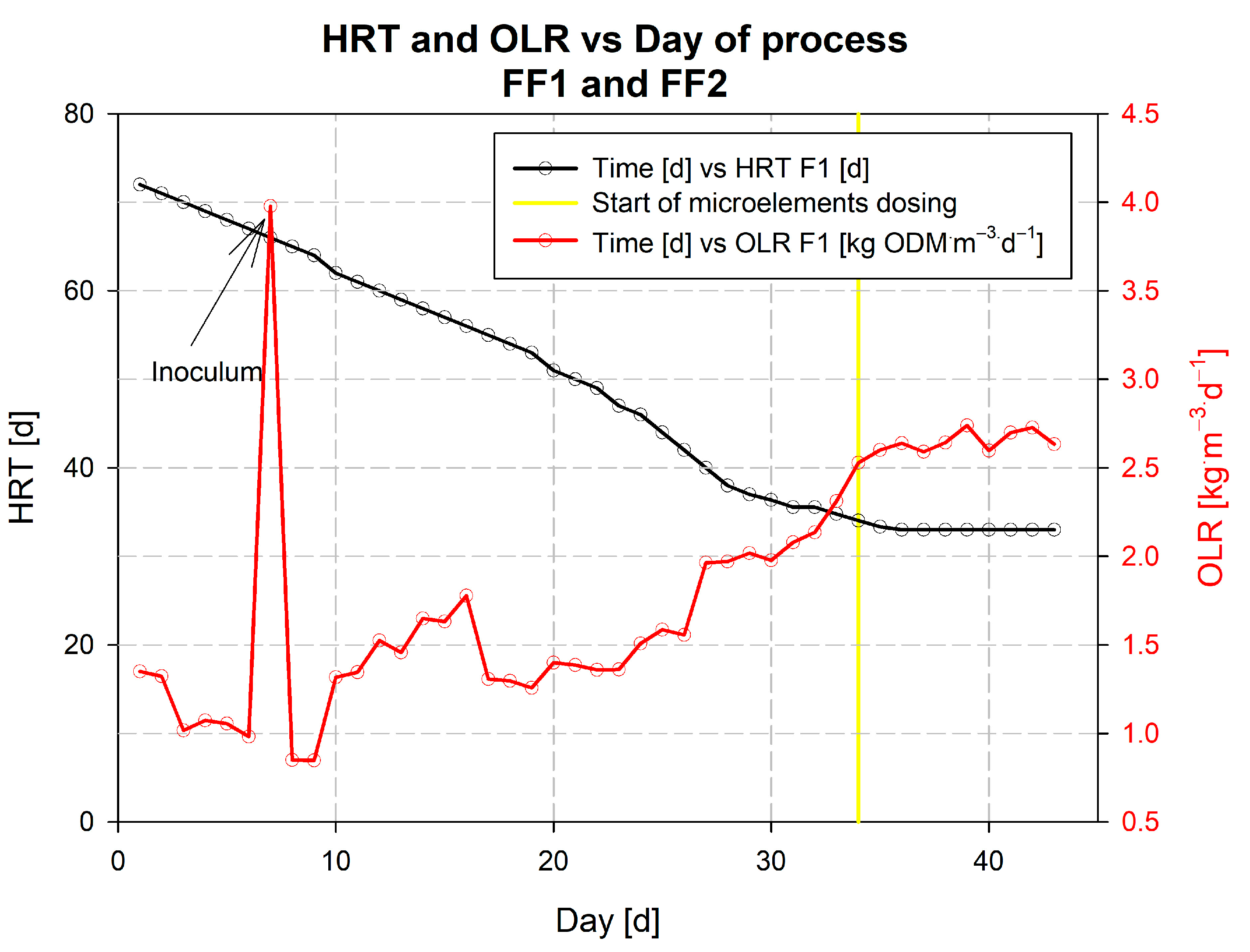
3.1. Description of the Kick-Off of the Falknowo Biogas Plant
3.2. Description of the Kick-Off of the Kupin Biogas Plant
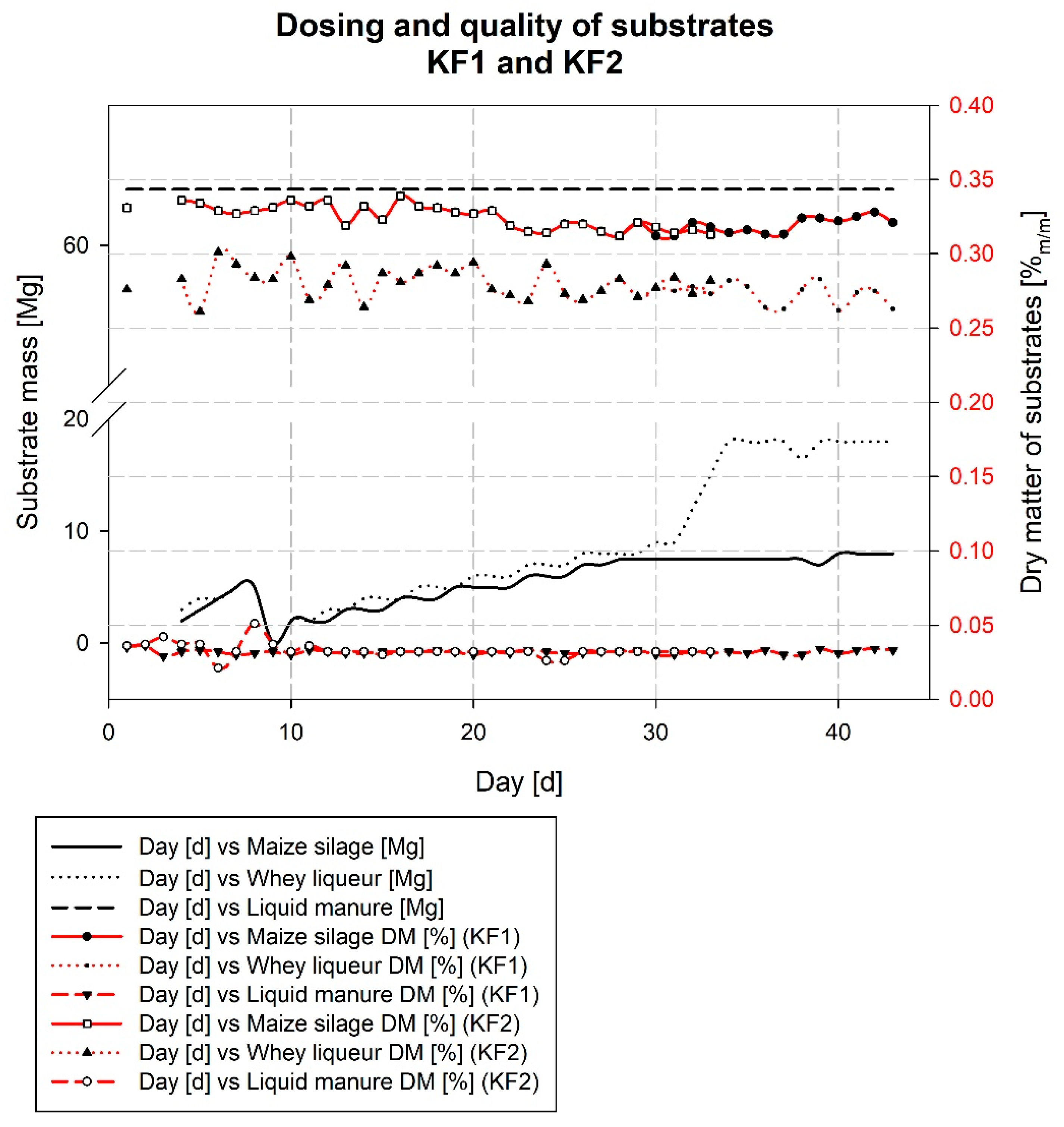
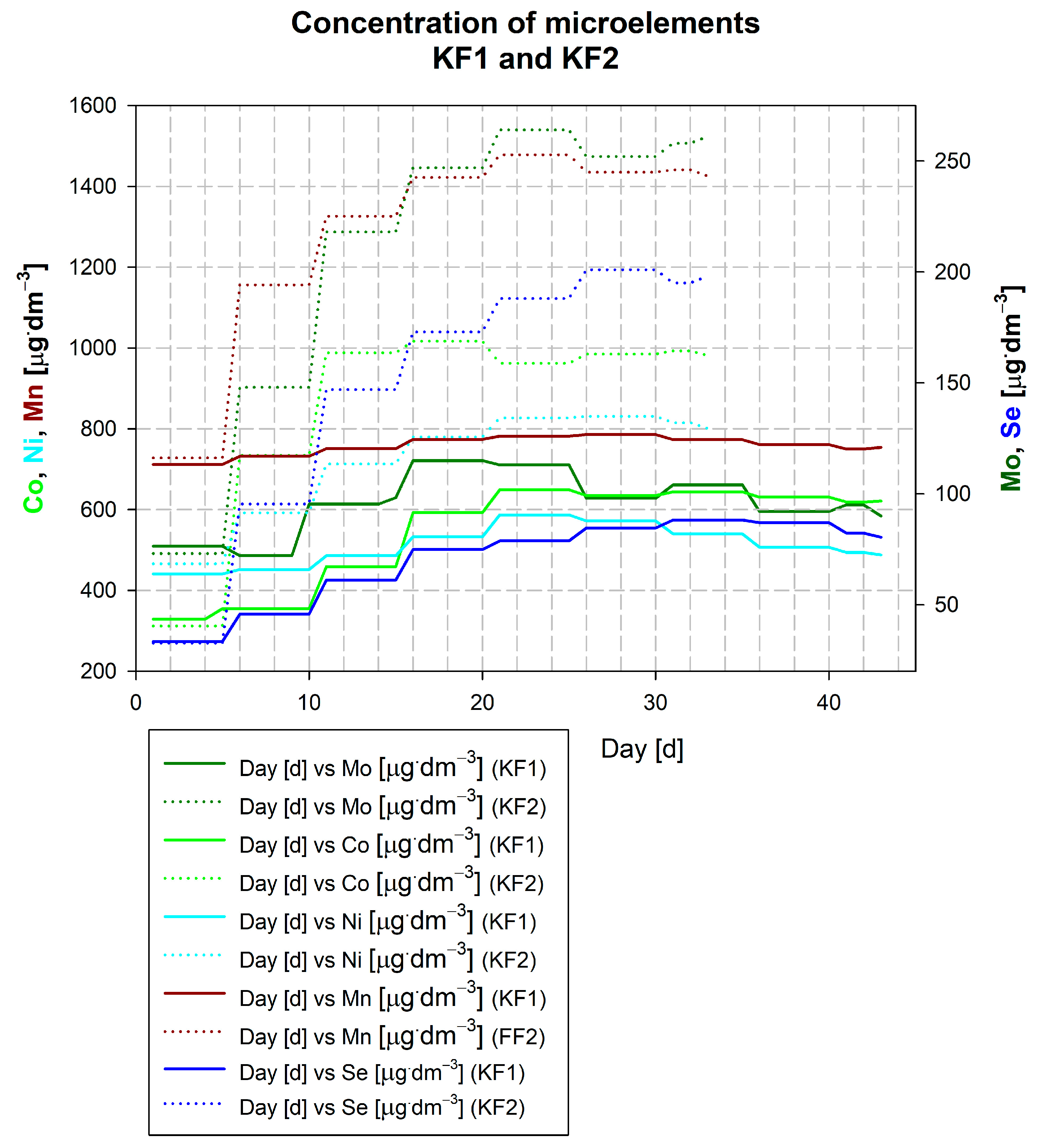
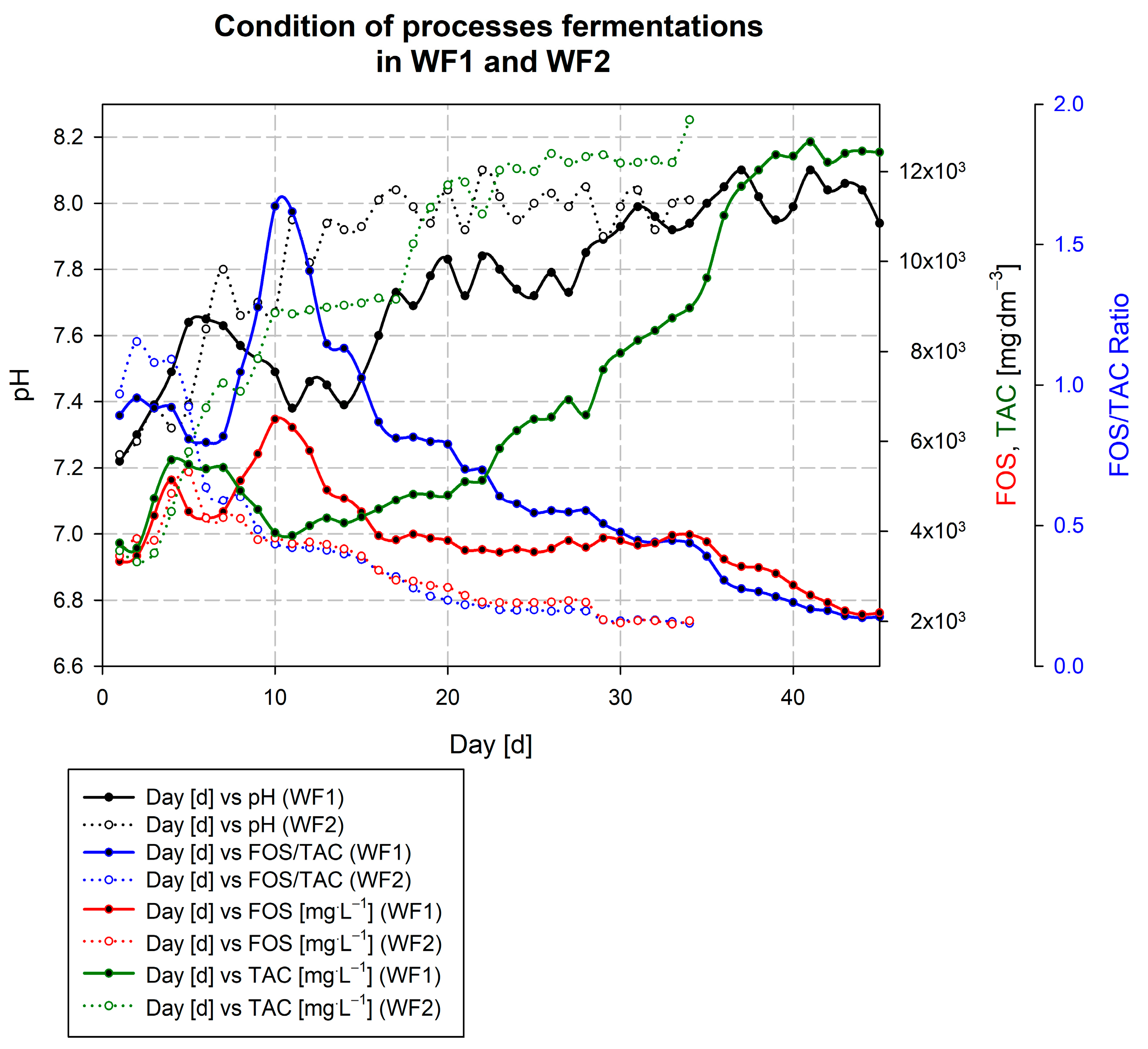
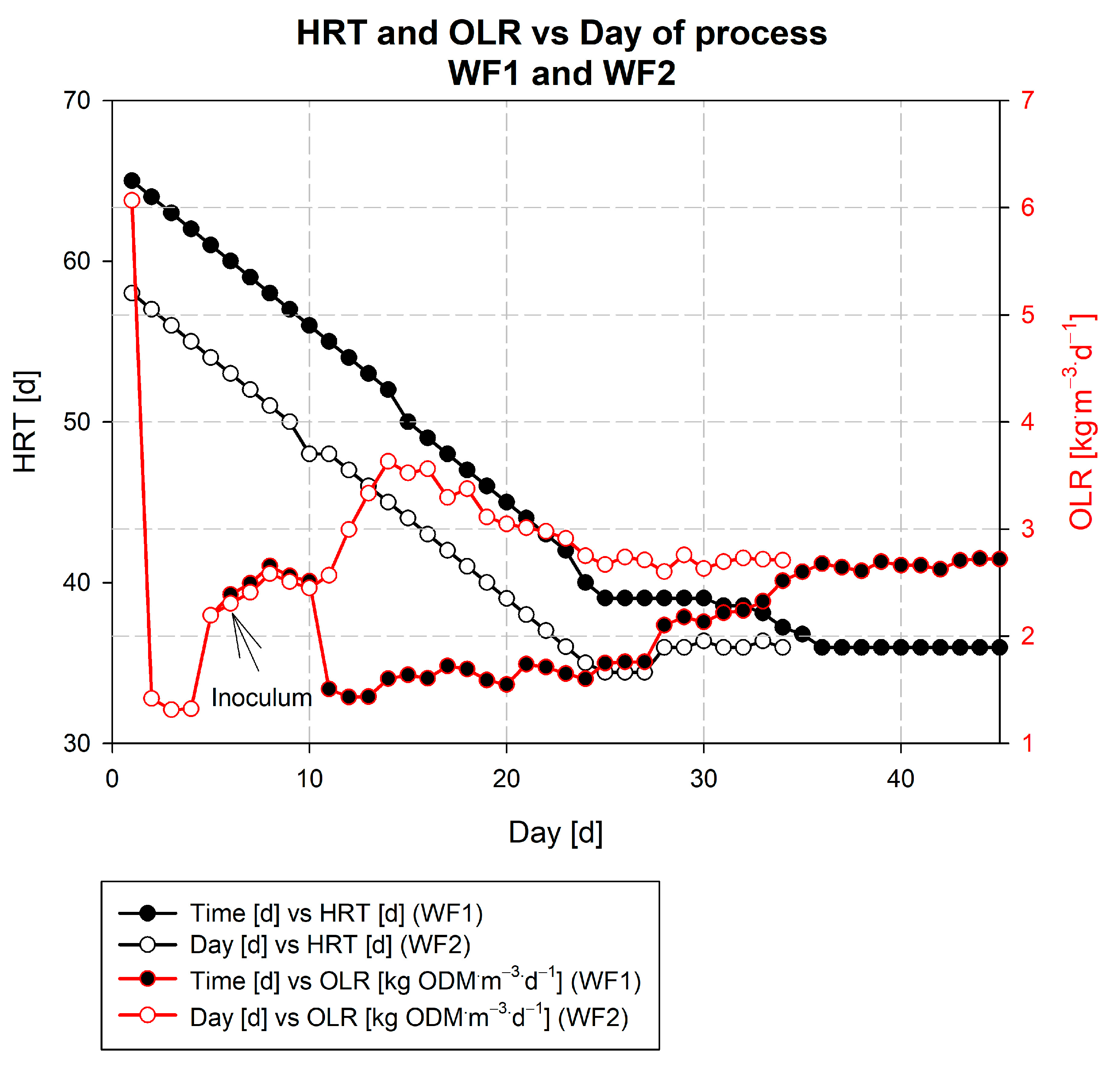
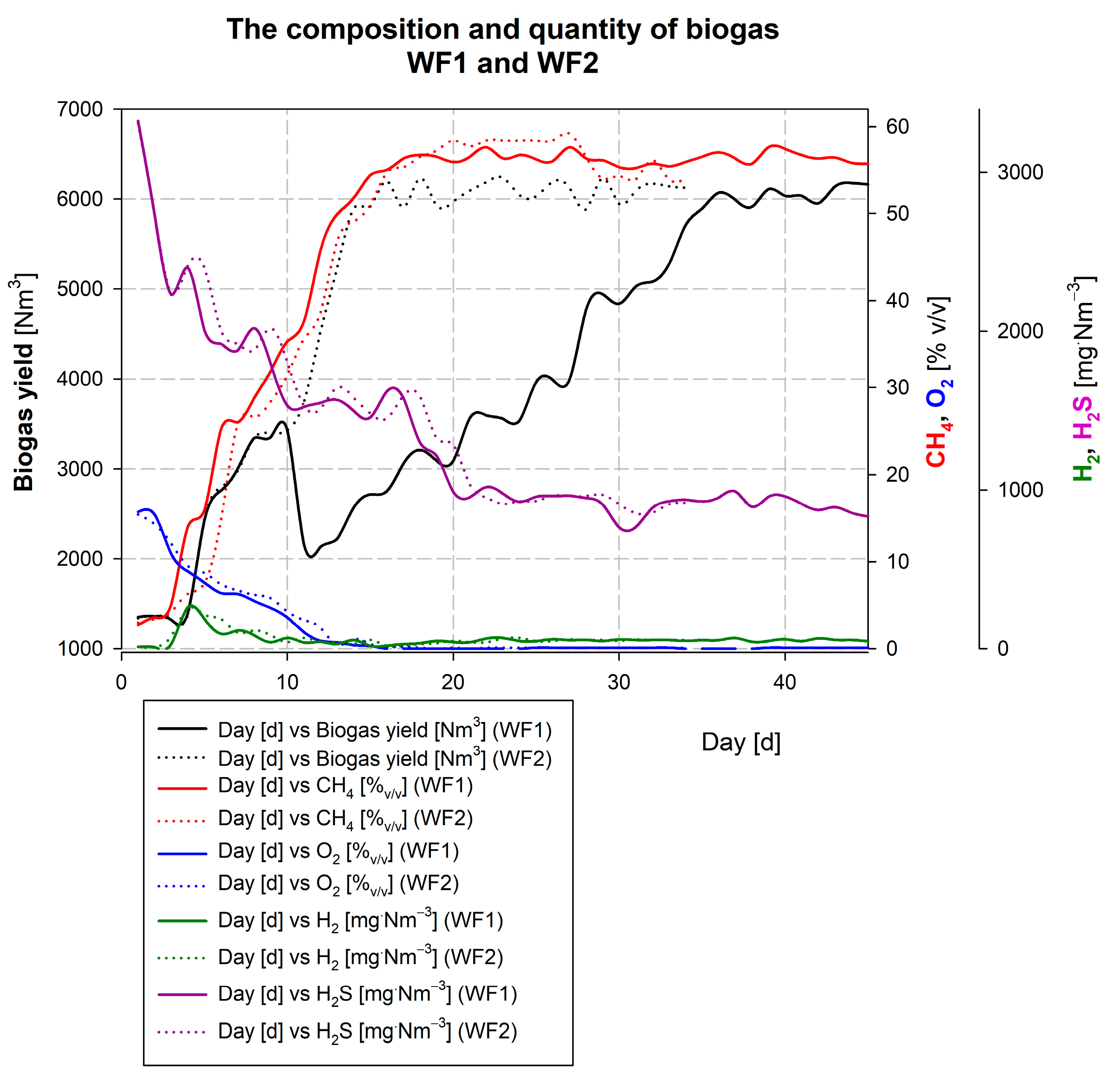
3.3. Description of the Wicie Biogas Plant Kick-Off
4. Conclusions
- –
- The rapid elimination of microelement deficiencies;
- –
- The activation of methanogenic microorganisms in the fermenter; and
- –
- The stabilization of the microbial metabolic process.
- –
- Reaching full power took place over a span of 20 days;
- –
- An average of 52% less substrate was used until full power in biogas production was achieved; and
- –
- 27% less fuel oil required to power mobile boiler plants warming the digesters was used.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CMP-S1 | Name of the formulation developed by authors. |
| DM | Dry matter (%). |
| DMM | Dedicated mixture of microelements. |
| ODM | Organic dry matter (% DM). |
| HRT | Hydraulic retention time (d). |
| IC50 | The half-maximal inhibitory concentrations |
| FOS | Value corresponds to the volatile fatty acids content (De: Flüchtigen Organischen Säuren) (mg·dm−1). |
| TAC | Estimation of the buffer capacity of the sample (De: gesam Ten Anorganischen Carbonat) (mg·dm−1). |
| OLR | Organic loading rate (kg ODM·m−3·d−1). |
| FF1 | Falknowo fermenter 01. |
| FF2 | Falknowo fermenter 02. |
| WF1 | Wicie fermenter 01. |
| WF2 | Wicie fermenter 02. |
| KF1 | Kornin fermenter 01. |
| KF2 | Kornin fermenter 02. |
References
- Janke, L.; Weinrich, S.; Leite, A.F.; Schüch, A.; Nikolausz, M.; Nelles, M.; Stinner, W. Optimization of semi-continuous anaerobic digestion of sugarcane straw co-digested with filter cake: Effects of macronutrients supplementation on conversion kinetics. Bioresour. Technol. 2017, 245, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Zagklis, D.; Kornaros, M.; Sun, J. Cobalt oxide nanoparticles as a new strategy for enhancing methane production from anaerobic digestion of noxious aquatic weeds. Bioresour. Technol. 2023, 368, 128308. [Google Scholar] [CrossRef] [PubMed]
- Sicchieri, I.M.; de Quadros, T.C.F.; Bortoloti, M.A.; Fernandes, F.; Kuroda, E.K. Selection, composition, and validation of standard inoculum for anaerobic digestion assays. Biomass Bioenergy 2022, 164, 106558. [Google Scholar] [CrossRef]
- Simioni, T.; Agustini, C.B.; Dettmer, A.; Gutterres, M. Nutrient balance for anaerobic co-digestion of tannery wastes: Energy efficiency, waste treatment and cost-saving. Bioresour. Technol. 2020, 308, 123255. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.C.; Lu, Q.; de Toledo, R.A.; Gu, J.-D.; Shim, H. Improved anaerobic co-digestion of food waste and domestic wastewater by copper supplementation—Microbial community change and enhanced effluent quality. Sci. Total Environ. 2019, 670, 337–344. [Google Scholar] [CrossRef]
- Samoraj, M.; Mironiuk, M.; Izydorczyk, G.; Witek-Krowiak, A.; Szopa, D.; Moustakas, K.; Chojnacka, K. The challenges and perspectives for anaerobic digestion of animal waste and fertilizer application of the digestate. Chemosphere 2022, 295, 133799. [Google Scholar] [CrossRef]
- Chan, P.C.; Toledo, R.A.d.; Iu, H.I.; Shim, H. Co-digestion of food waste and domestic wastewater—Effect of copper supplementation on biogas production. Energy Procedia 2018, 153, 237–241. [Google Scholar] [CrossRef]
- Ganchev, G.T.U.; Mikhajlova, M.; Dzhurbinev, D. The new norms for dry matter and protein in feeding of lactatinf ewes. Anim. Sci. 2004, 41, 22–25. [Google Scholar]
- Pinto-Ibieta, F.; Serrano, A.; Jeison, D.; Borja, R.; Fermoso, F.G. Effect of cobalt supplementation and fractionation on the biological response in the biomethanization of Olive Mill Solid Waste. Bioresour. Technol. 2016, 211, 58–64. [Google Scholar] [CrossRef]
- Molaey, R.; Bayrakdar, A.; Sürmeli, R.Ö.; Çalli, B. Anaerobic digestion of chicken manure: Mitigating process inhibition at high ammonia concentrations by selenium supplementation. Biomass Bioenergy 2018, 108, 439–446. [Google Scholar] [CrossRef]
- Juntupally, S.; Begum, S.; Anupoju, G.R. Impact of additives (macronutrient and nanoparticles of micronutrients) on the anaerobic digestion of food waste: Focus on feeding strategy for improved performance. Biomass Bioenergy 2023, 172, 106751. [Google Scholar] [CrossRef]
- Schönheit, P.; Moll, J.; Thauer, R.K. Nickel, cobalt, and molybdenum requirement for growth of Methanobacterium thermoautotrophicum. Arch. Microbiol. 1979, 123, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Oleszkiewicz, J.A.; Sharma, V.K. Stimulation and inhibition of anaerobic processes by heavy metals—A review. Biol. Wastes 1990, 31, 45–67. [Google Scholar] [CrossRef]
- Friedmann, H.C.; Klein, A.; Thauer, R.K. Structure and function of the nickel porphinoid, coenzyme F430, and of its enzyme, methyl coenzyme M reductase. FEMS Microbiol. Lett. 1990, 87, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Langenhoff, A.A.M.; Brouwers-Ceiler, D.L.; Engelberting, J.H.L.; Quist, J.J.; Wolkenfelt, J.G.P.N.; Zehnder, A.J.B.; Schraa, G. Microbial reduction of manganese coupled to toluene oxidation. FEMS Microbiol. Ecol. 1997, 22, 119–127. [Google Scholar] [CrossRef]
- Evranos, B.; Demirel, B. The impact of Ni, Co and Mo supplementation on methane yield from anaerobic mono-digestion of maize silage. Environ. Technol. 2015, 36, 1556–1562. [Google Scholar] [CrossRef]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Banks, C.J.; Zhang, Y.; Jiang, Y.; Heaven, S. Trace element requirements for stable food waste digestion at elevated ammonia concentrations. Bioresour. Technol. 2012, 104, 127–135. [Google Scholar] [CrossRef]
- Kletzin, A.; Adams, M.W.W. Tungsten in biological systems. FEMS Microbiol. Rev. 1996, 18, 5–63. [Google Scholar] [CrossRef]
- Thanh, P.M.; Ketheesan, B.; Yan, Z.; Stuckey, D. Trace metal speciation and bioavailability in anaerobic digestion: A review. Biotechnol. Adv. 2016, 34, 122–136. [Google Scholar] [CrossRef]
- Cai, Y.; Zhao, X.; Zhao, Y.; Wang, H.; Yuan, X.; Zhu, W.; Cui, Z.; Wang, X. Optimization of Fe2+ supplement in anaerobic digestion accounting for the Fe-bioavailability. Bioresour. Technol. 2018, 250, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Neumann, L.; Scherer, P. Impact of bioaugmentation by compost on the performance and ecology of an anaerobic digester fed with energy crops. Bioresour. Technol. 2011, 102, 2931–2935. [Google Scholar] [CrossRef]
- Worm, P.; Fermoso, F.G.; Stams, A.J.M.; Lens, P.N.L.; Plugge, C.M. Transcription of fdh and hyd in Syntrophobacter spp. and Methanospirillum spp. as a diagnostic tool for monitoring anaerobic sludge deprived of molybdenum, tungsten and selenium. Environ. Microbiol. 2011, 13, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, J.; Duan, L.; Song, Y.; Hu, X.; Wei, J. Enhancing the production of butyric acid from sludge fermentation with an emphasis on zinc, cobalt, cuprum, ferrum and manganese. Environ. Earth Sci. 2015, 73, 5057–5066. [Google Scholar] [CrossRef]
- Hendawy, A.O.; Sugimura, S.; Sato, K.; Mansour, M.M.; Abd El-Aziz, A.H.; Samir, H.; Islam, M.A.; Bostami, A.B.M.R.; Mandour, A.S.; Elfadadny, A.; et al. Effects of Selenium Supplementation on Rumen Microbiota, Rumen Fermentation, and Apparent Nutrient Digestibility of Ruminant Animals: A Review. Fermentation 2022, 8, 4. [Google Scholar] [CrossRef]
- Takashima, M.; Speece, R.E.; Parkin, G.F. Mineral requirements for methane fermentation. Crit. Rev. Environ. Control 1990, 19, 465–479. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, X.; Zhang, Y.; Zhang, Y.; Sun, Y.; Zhang, L. Organic matter conversion and contributors to bioavailability in biogas slurry treated by biochar/persulfate during the oxidation pond process. J. Clean. Prod. 2022, 355, 131770. [Google Scholar] [CrossRef]
- Marchetti, R.; Vasmara, C.; Orsi, A. Inoculum production from pig slurry for potential use in agricultural biogas plants. Sustain. Energy Technol. Assess. 2022, 52, 102310. [Google Scholar] [CrossRef]
- Stadtlander, T.; Bandy, J.; Rosskothen, D.; Pietsch, C.; Tschudi, F.; Sigrist, M.; Seitz, A.; Leiber, F. Dilution rates of cattle slurry affect ammonia uptake and protein production of duckweed grown in recirculating systems. J. Clean. Prod. 2022, 357, 131916. [Google Scholar] [CrossRef]
- Pedersen, I.F.; Rubæk, G.H.; Nyord, T.; Sørensen, P. Data on initial leaf P concentrations and final dry matter yields of silage maize in response to row-injected cattle slurry. Data Brief 2020, 30, 105570. [Google Scholar] [CrossRef]
- Kowalczyk-Juśko, A.; Pochwatka, P.; Zaborowicz, M.; Czekała, W.; Mazurkiewicz, J.; Mazur, A.; Janczak, D.; Marczuk, A.; Dach, J. Energy value estimation of silages for substrate in biogas plants using an artificial neural network. Energy 2020, 202, 117729. [Google Scholar] [CrossRef]
- Jelen, P. 10—Whey-based functional beverages. In Functional and Speciality Beverage Technology; Paquin, P., Ed.; Woodhead Publishing: Sawston, UK, 2009; pp. 259–280. [Google Scholar]
- Jelen, P. WHEY PROCESSING|Utilization and Products. In Encyclopedia of Dairy Sciences (Second Edition); Fuquay, J.W., Ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 731–737. [Google Scholar]
- Reguengo, L.M.; Salgaço, M.K.; Sivieri, K.; Maróstica Júnior, M.R. Agro-industrial by-products: Valuable sources of bioactive compounds. Food Res. Int. 2022, 152, 110871. [Google Scholar] [CrossRef] [PubMed]
- Pobeheim, H.; Munk, B.; Johansson, J.; Guebitz, G.M. Influence of trace elements on methane formation from a synthetic model substrate for maize silage. Bioresour. Technol. 2010, 101, 836–839. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Ye, Z.; Yang, C. Effect of manganese oxide-modified biochar addition on methane production and heavy metal speciation during the anaerobic digestion of sewage sludge. J. Environ. Sci. 2019, 76, 267–277. [Google Scholar] [CrossRef]
- Ferry, J.G. Enzymology of one-carbon metabolism in methanogenic pathways. FEMS Microbiol. Rev. 1999, 23, 13–38. [Google Scholar] [CrossRef]
- Cai, Y.; Zheng, Z.; Zhao, Y.; Zhang, Y.; Guo, S.; Cui, Z.; Wang, X. Effects of molybdenum, selenium and manganese supplementation on the performance of anaerobic digestion and the characteristics of bacterial community in acidogenic stage. Bioresour. Technol. 2018, 266, 166–175. [Google Scholar] [CrossRef]
- Chen, J.L.; Steele, T.W.J.; Stuckey, D.C. Stimulation and Inhibition of Anaerobic Digestion by Nickel and Cobalt: A Rapid Assessment Using the Resazurin Reduction Assay. Environ. Sci. Technol. 2016, 50, 11154–11163. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Hua, B.; Gao, L.; Hu, Y.; Yuan, X.; Cui, Z.; Zhu, W.; Wang, X. Effects of adding trace elements on rice straw anaerobic mono-digestion: Focus on changes in microbial communities using high-throughput sequencing. Bioresour. Technol. 2017, 239, 454–463. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. Trace element requirements of agricultural biogas digesters during biological conversion of renewable biomass to methane. Biomass Bioenergy 2011, 35, 992–998. [Google Scholar] [CrossRef]
- Ortner, M.; Rameder, M.; Rachbauer, L.; Bochmann, G.; Fuchs, W. Bioavailability of essential trace elements and their impact on anaerobic digestion of slaughterhouse waste. Biochem. Eng. J. 2015, 99, 107–113. [Google Scholar] [CrossRef]
- Milán, Z.; Montalvo, S.; Ruiz-Tagle, N.; Urrutia, H.; Chamy, R.; Sánchez, E.; Borja, R. Influence of heavy metal supplementation on specific methanogenic activity and microbial communities detected in batch anaerobic digesters. J. Environ. Sci. Health Part A 2010, 45, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Logan, M.; Tan, L.C.; Lens, P.N.L. Anaerobic co-digestion of dissolved air floatation slurry and selenium rich wastewater for simultaneous methane production and selenium bioremediation. Int. Biodeterior. Biodegrad. 2022, 172, 105425. [Google Scholar] [CrossRef]
- Buchauer, K. A comparison of two simple titration procedures to determine volatile fatty acids in influents to waste-water and sludge treatment processes. Water SA 1998, 24, 49–56. [Google Scholar]
- Haas, D.d.; Adam, N. Use of a simple titration procedure to determine H2CO3* alkalinity and volatile fatty acids for process control in waste-water treatment. Water SA 1995, 21, 307–318. [Google Scholar]
- Saravanan, A.; Senthil Kumar, P.; Rangasamy, G.; Hariharan, R.; Hemavathy, R.V.; Deepika, P.D.; Anand, K.; Karthika, S. Strategies for enhancing the efficacy of anaerobic digestion of food industry wastewater: An insight into bioreactor types, challenges, and future scope. Chemosphere 2023, 310, 136856. [Google Scholar] [CrossRef]
- Calbry-Muzyka, A.; Madi, H.; Rüsch-Pfund, F.; Gandiglio, M.; Biollaz, S. Biogas composition from agricultural sources and organic fraction of municipal solid waste. Renew. Energy 2022, 181, 1000–1007. [Google Scholar] [CrossRef]
- Capa, A.; García, R.; Chen, D.; Rubiera, F.; Pevida, C.; Gil, M.V. On the effect of biogas composition on the H2 production by sorption enhanced steam reforming (SESR). Renew. Energy 2020, 160, 575–583. [Google Scholar] [CrossRef]
- Fuksa, P.; Hakl, J.; Míchal, P.; Hrevušová, Z.; Šantrůček, J.; Tlustoš, P. Effect of silage maize plant density and plant parts on biogas production and composition. Biomass Bioenergy 2020, 142, 105770. [Google Scholar] [CrossRef]
- Romero-Güiza, M.S.; Vila, J.; Mata-Alvarez, J.; Chimenos, J.M.; Astals, S. The role of additives on anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2016, 58, 1486–1499. [Google Scholar] [CrossRef]
- Gustavsson, J.; Shakeri Yekta, S.; Sundberg, C.; Karlsson, A.; Ejlertsson, J.; Skyllberg, U.; Svensson, B.H. Bioavailability of cobalt and nickel during anaerobic digestion of sulfur-rich stillage for biogas formation. Appl. Energy 2013, 112, 473–477. [Google Scholar] [CrossRef]
- Cao, W.; Wang, M.; Liu, M.; Zhang, Z.; Sun, Z.; Miao, Y.; Sun, C.; Hu, C. The chemical and dynamic distribution characteristics of iron, cobalt and nickel in three different anaerobic digestates: Effect of pH and trace elements dosage. Bioresour. Technol. 2018, 269, 363–374. [Google Scholar] [CrossRef] [PubMed]
- De Silva, J.A.; Braga, A.F.M.; Fermoso, F.G.; Zaiat, M.; Silva, G.H.R. Evaluation of the influence of trace metals on methane production from domestic sewage, using the Plackett-Burman experimental design. J. Environ. Manag. 2021, 294, 113002. [Google Scholar] [CrossRef] [PubMed]
- Pornmai, K.; Itsadanont, S.; Lertpattanapong, M.; Seneesrisakul, K.; Jiraprasertwong, A.; Leethochawalit, M.; Sekiguchi, H.; Chavadej, S. Enhancement of methanogenic activity by micronutrient control: Micronutrient availability in relation to sulfur transport. J. Environ. Sci. 2023, 127, 738–752. [Google Scholar] [CrossRef] [PubMed]
- Trisakti, B.; Batubara, F.; Daimon, H. The Minimum Requirements for Nickel and Cobalt as Trace Metals in Thermophilic Biogas Fermentation of Palm Oil Mill Effluent. Orient. J. Chem. 2018, 34, 1278. [Google Scholar] [CrossRef]
- Speece, R.E.; Parkin, G.F.; Gallagher, D. Nickel stimulation of anaerobic digestion. Water Res. 1983, 17, 677–683. [Google Scholar] [CrossRef]
- Šafarič, L.; Yekta, S.S.; Svensson, B.H.; Schnürer, A.; Bastviken, D.; Björn, A. Effect of Cobalt, Nickel, and Selenium/Tungsten Deficiency on Mesophilic Anaerobic Digestion of Chemically Defined Soluble Organic Compounds. Microorganisms 2020, 8, 598. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Yi, X.; Yang, F.; Wang, D.; Han, H. Dissimilatory manganese reduction facilitates synergistic cooperation of hydrolysis, acidogenesis, acetogenesis and methanogenesis via promoting microbial interaction during anaerobic digestion of waste activated sludge. Environ. Res. 2023, 218, 114992. [Google Scholar] [CrossRef]
- Liu, G.; Liu, L.; Huo, Y.; Dai, Z.; Zhang, L.; Wang, Q. Enhanced two-phase anaerobic digestion of waste activated sludge by combined free nitrous acid and manganese dioxide. J. Clean. Prod. 2022, 379, 134777. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, N.; Huang, D.; Yuan, T.; Wu, H.; Xu, Q. Enhancement of methane production from anaerobic digestion using different manganese species. Biomass Convers. Biorefin. 2021. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łazarski, S.; Butarewicz, A.; Cichosz, M.; Kiełkowska, U. Study on the Effect of Dedicated Microelement Mixture (DMM) on the Kick-Off Phase of the Digester and Stabilization of the Methane Fermentation Process. Energies 2023, 16, 3763. https://doi.org/10.3390/en16093763
Łazarski S, Butarewicz A, Cichosz M, Kiełkowska U. Study on the Effect of Dedicated Microelement Mixture (DMM) on the Kick-Off Phase of the Digester and Stabilization of the Methane Fermentation Process. Energies. 2023; 16(9):3763. https://doi.org/10.3390/en16093763
Chicago/Turabian StyleŁazarski, Sławomir, Andrzej Butarewicz, Marcin Cichosz, and Urszula Kiełkowska. 2023. "Study on the Effect of Dedicated Microelement Mixture (DMM) on the Kick-Off Phase of the Digester and Stabilization of the Methane Fermentation Process" Energies 16, no. 9: 3763. https://doi.org/10.3390/en16093763
APA StyleŁazarski, S., Butarewicz, A., Cichosz, M., & Kiełkowska, U. (2023). Study on the Effect of Dedicated Microelement Mixture (DMM) on the Kick-Off Phase of the Digester and Stabilization of the Methane Fermentation Process. Energies, 16(9), 3763. https://doi.org/10.3390/en16093763






