Analysis of a Phase Change Material-Based Condenser of a Low-Scale Refrigeration System
Abstract
1. Introduction
2. Numerical Modeling
2.1. Numerical Modeling of the PCM
- (i)
- Homogeneous and isotropic materials;
- (ii)
- Phase change occurs within a fixed temperature range;
- (iii)
- The PCM initial temperature is uniform;
- (iv)
- Expansion and compression during a phase change are negligible;
- (v)
- At the liquid state, the PCM is a Newtonian fluid, and the flow is laminar;
- (vi)
- Incompressible flow (density is assumed constant and equal to the arithmetic mean of the liquid density and solid density).
2.1.1. Mass Conservation and Momentum Conservation Equations
2.1.2. Energy Conservation Equation
2.2. Numerical Modeling of the Refrigerant Fluid
2.2.1. Mass Conservation Equation
2.2.2. Momentum Conservation Equation
2.2.3. Dispersed Phase Transport Equation
2.2.4. Energy Conservation Equation
2.3. Boundary Conditions
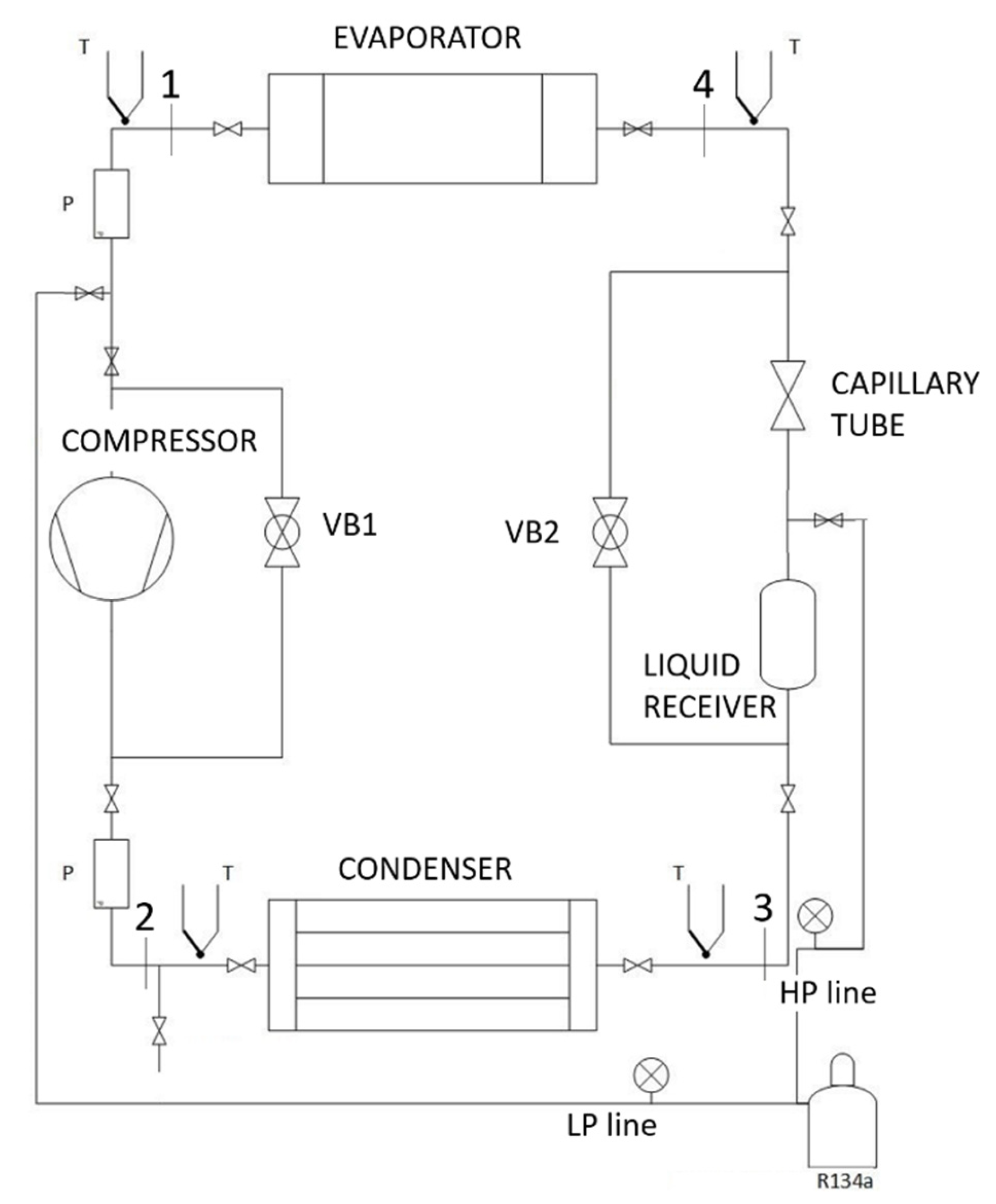
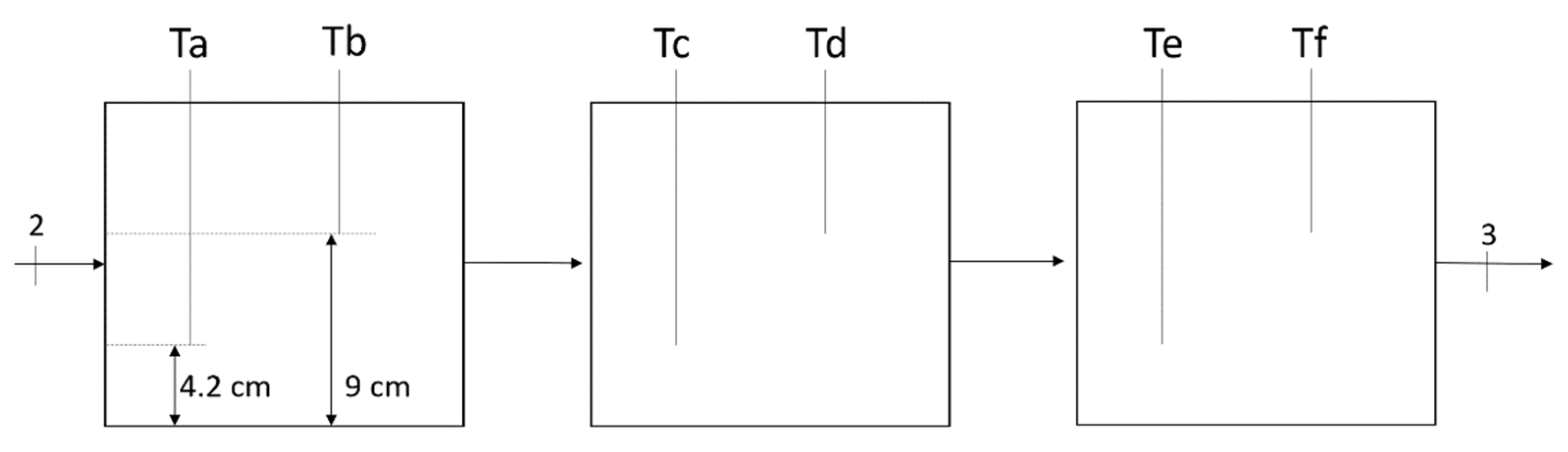
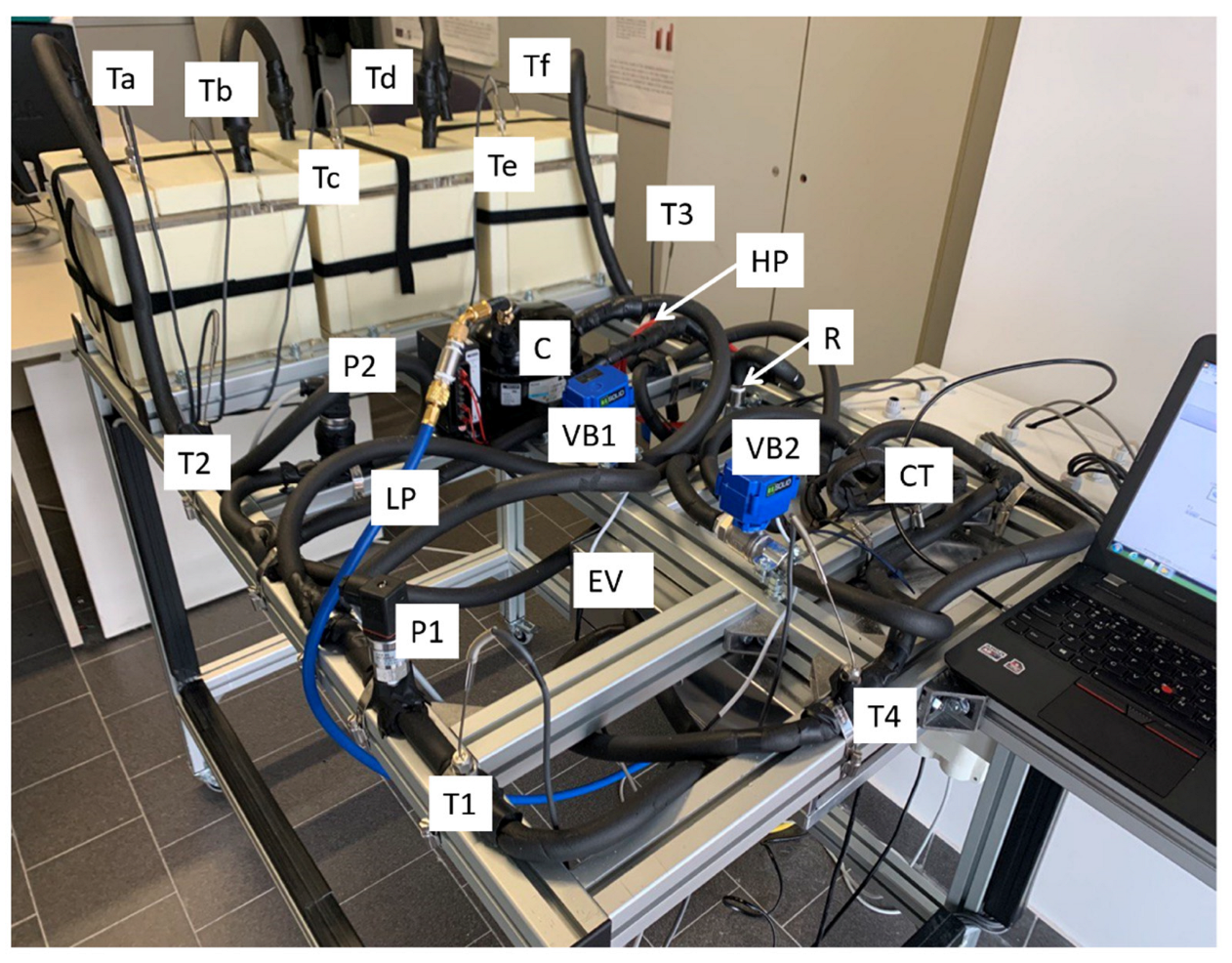
3. Experimental Setup
3.1. System Description
3.2. Components of the Experimental Apparatus
3.3. Measuring and Control Equipment
3.4. Setup of Experimental Tests Initial Conditions
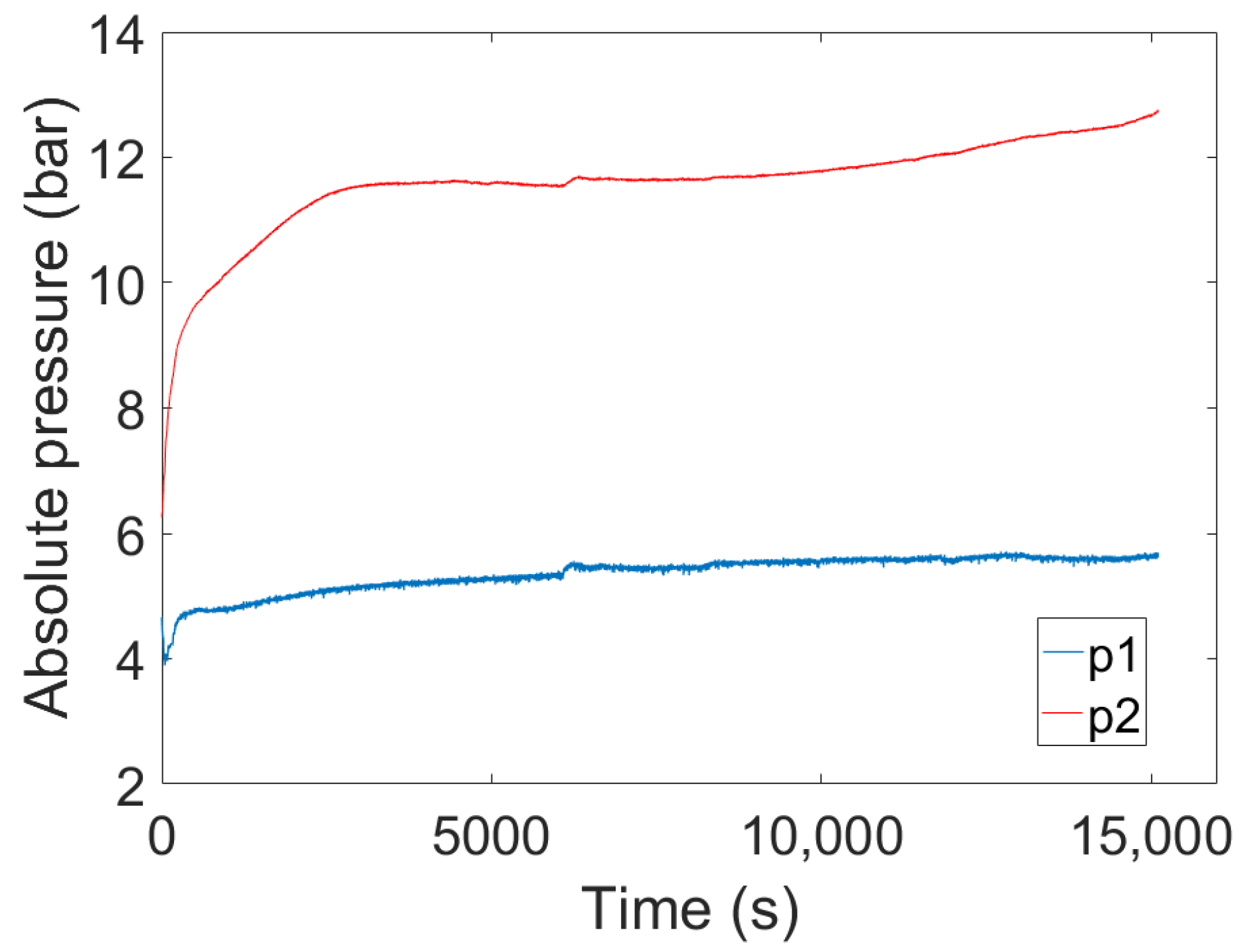
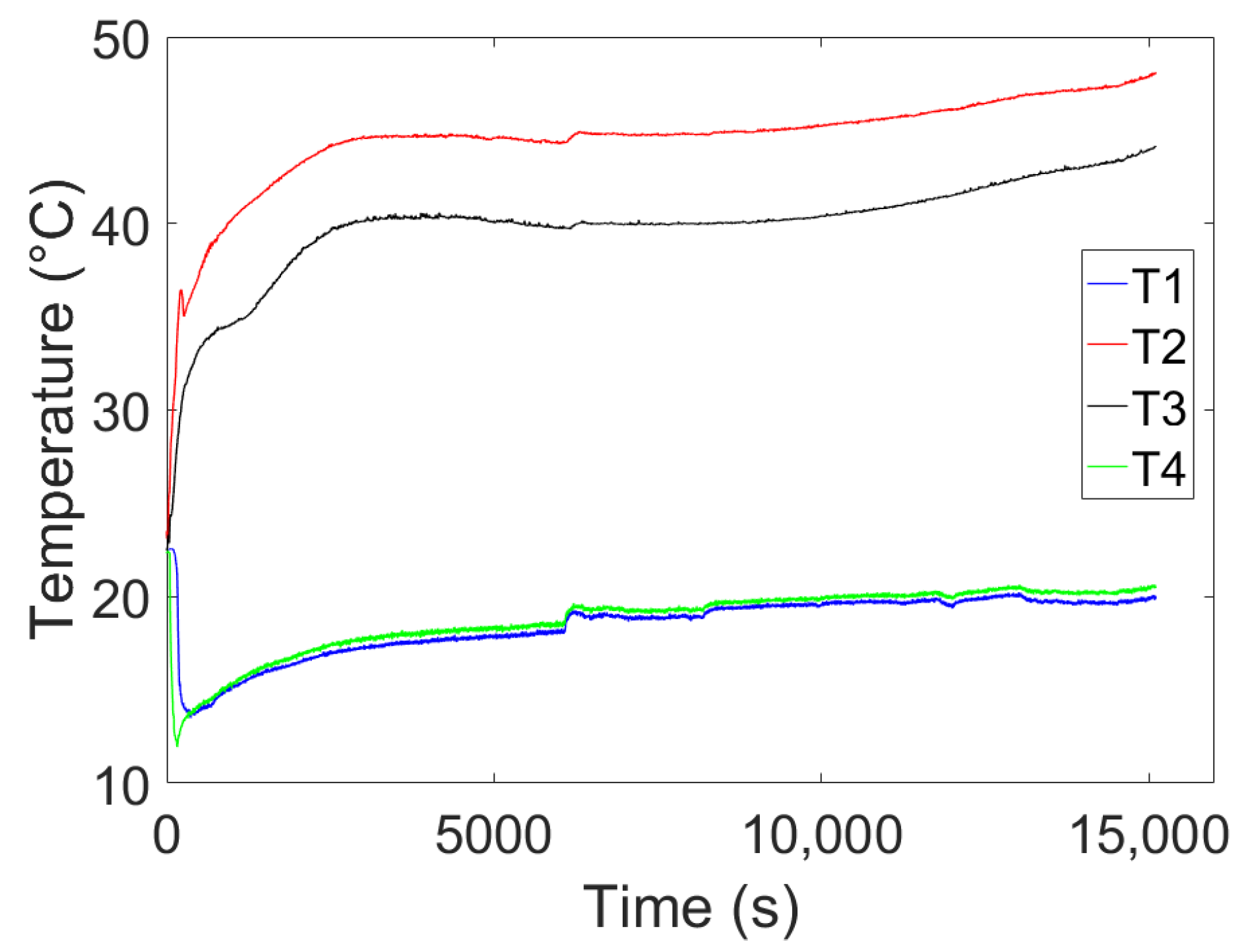
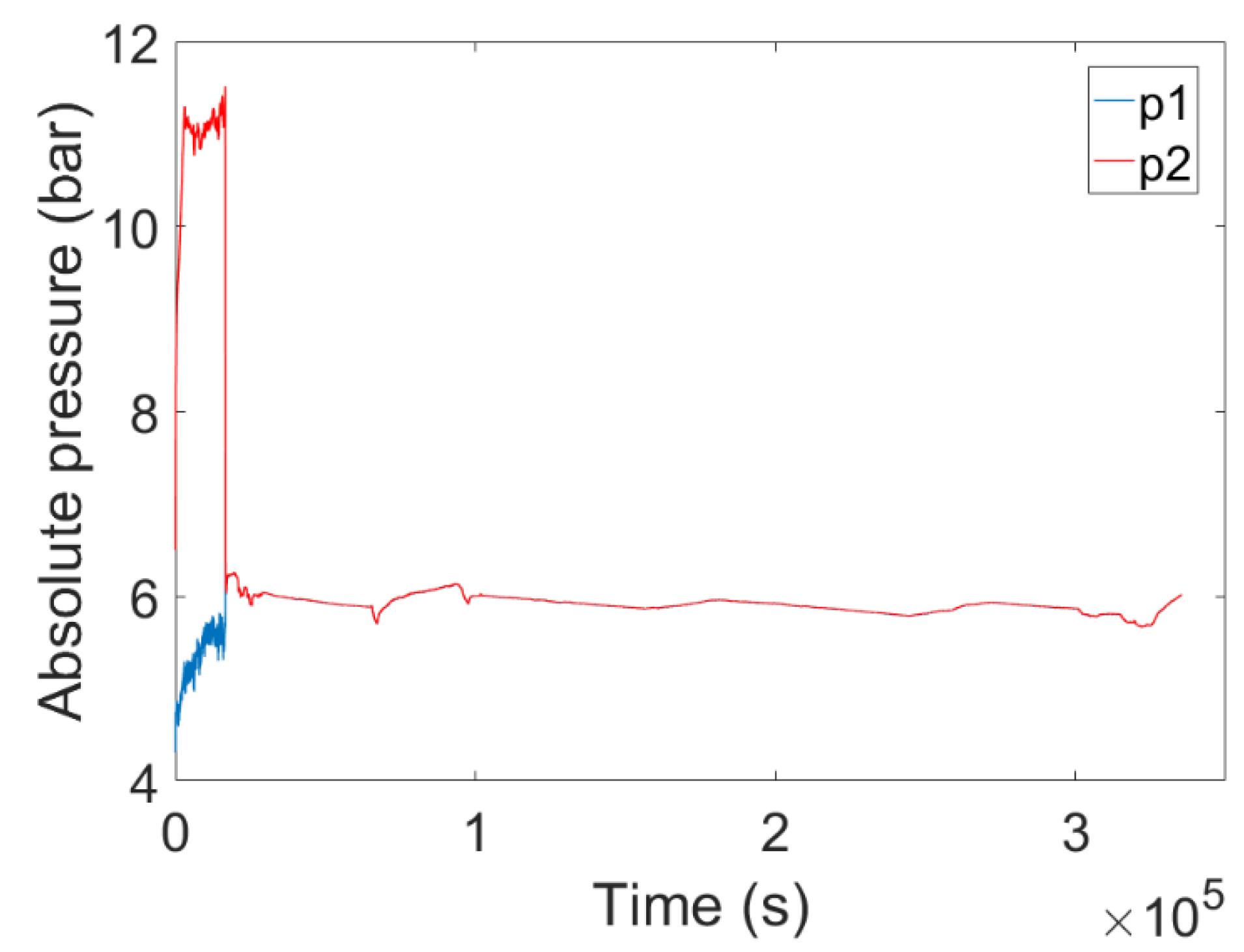
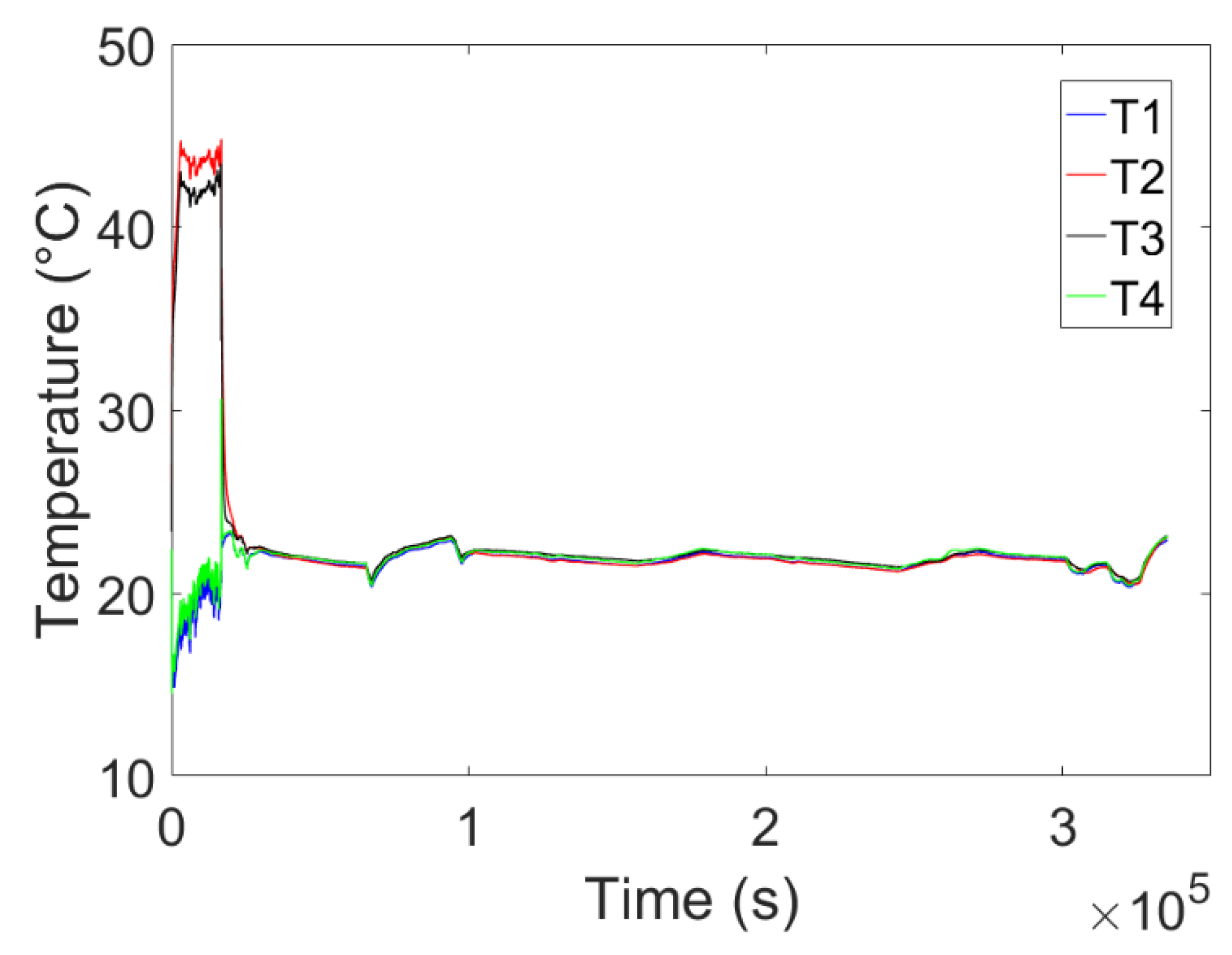
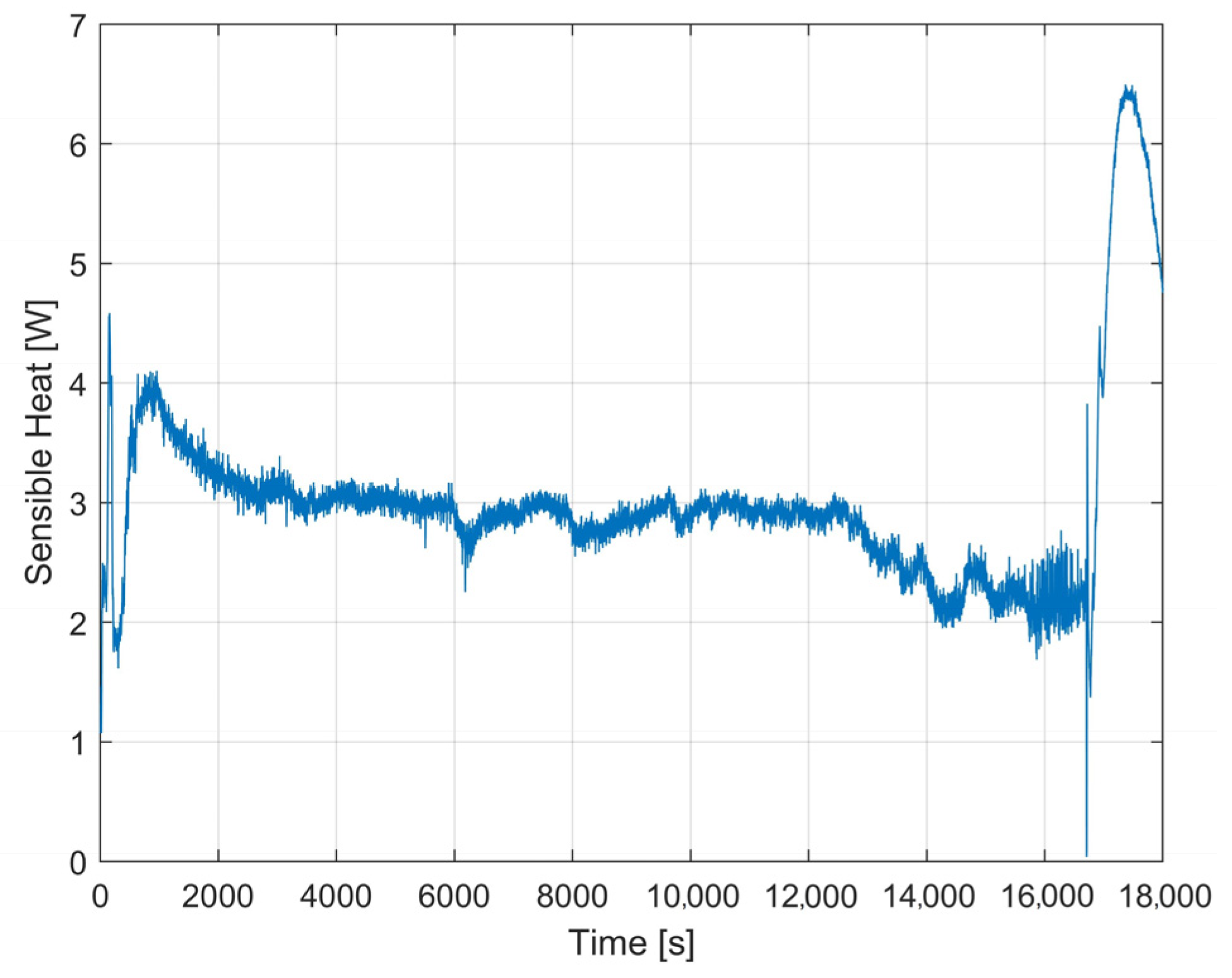
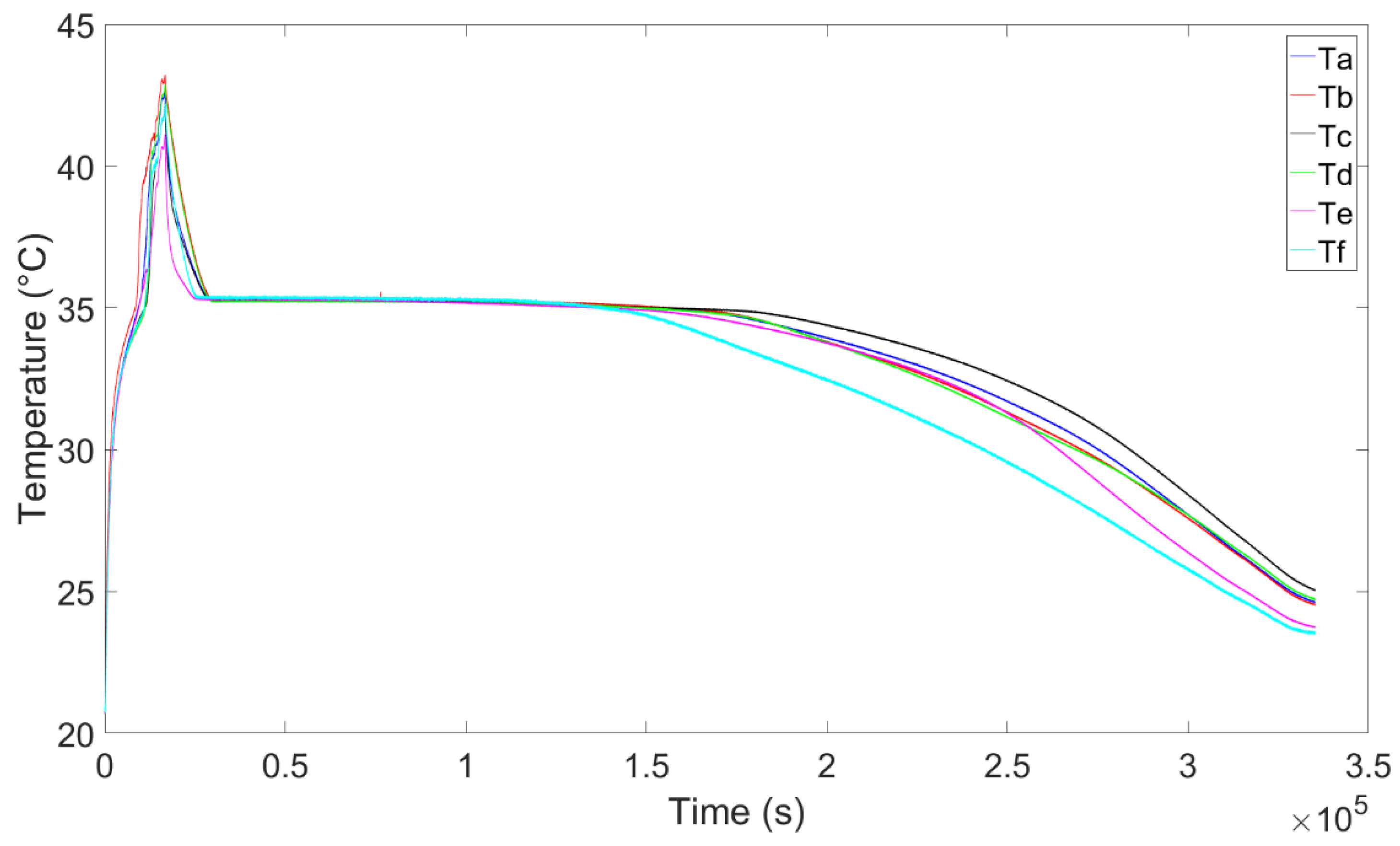
4. Experimental Tests Results
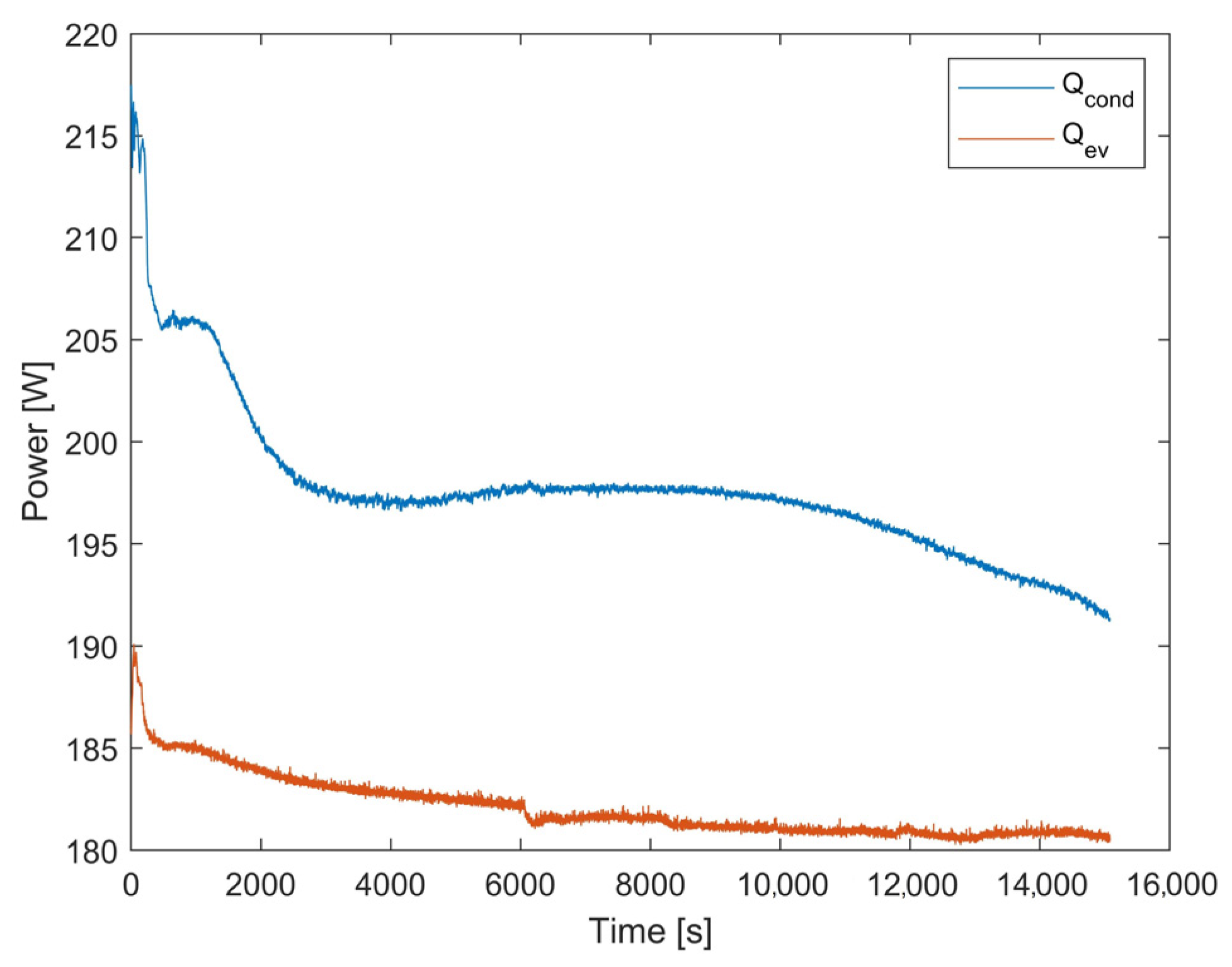
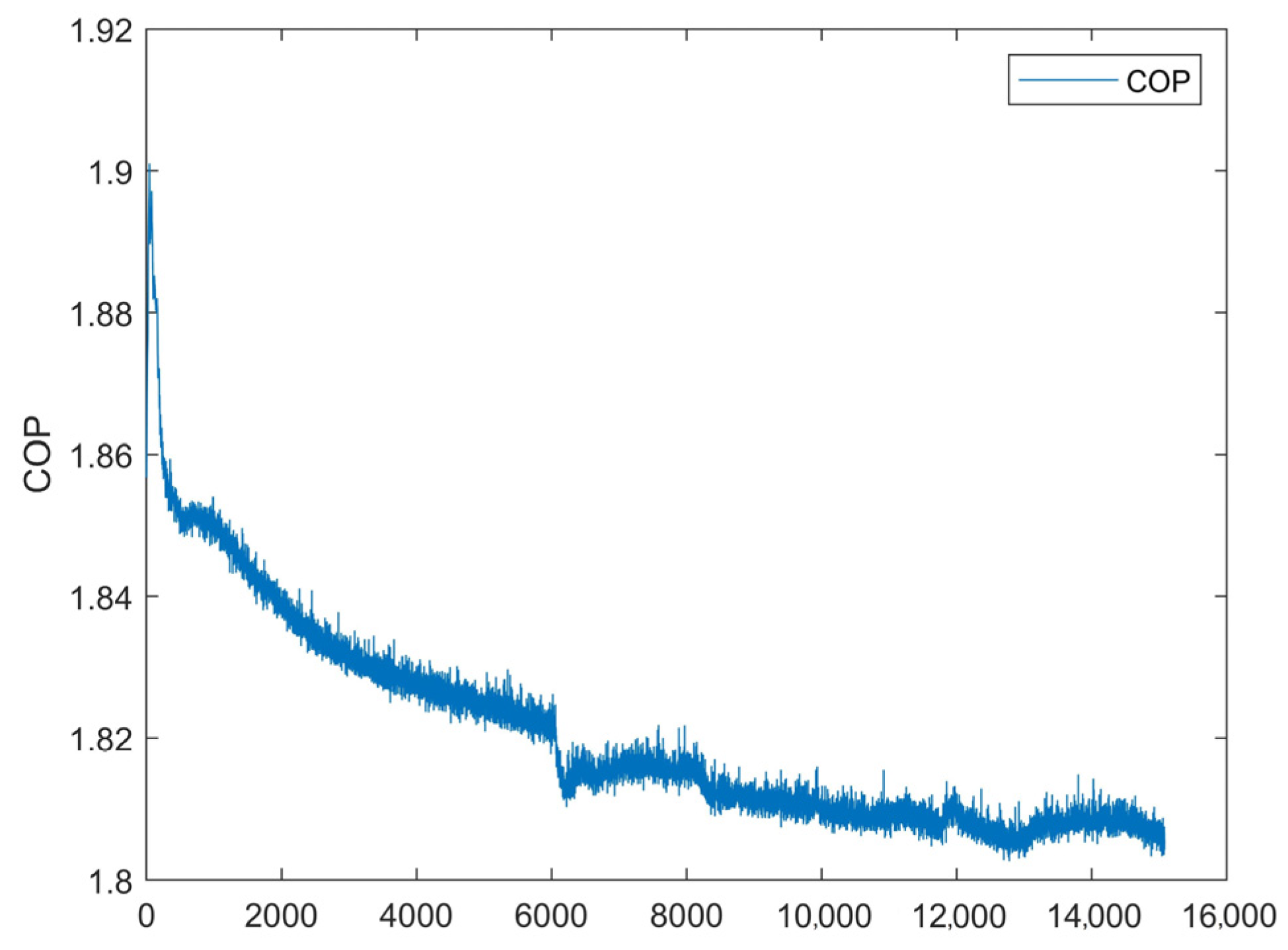
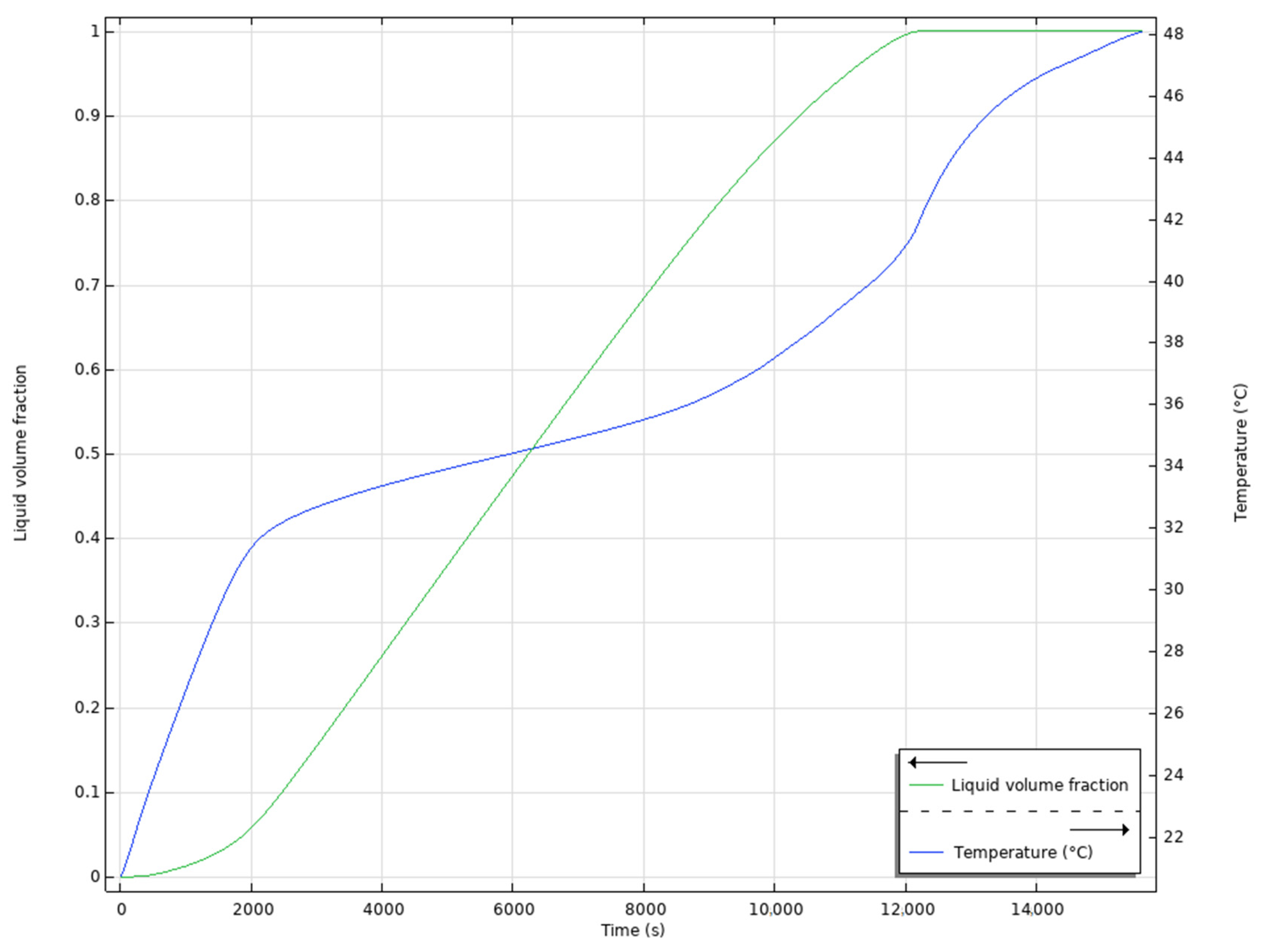
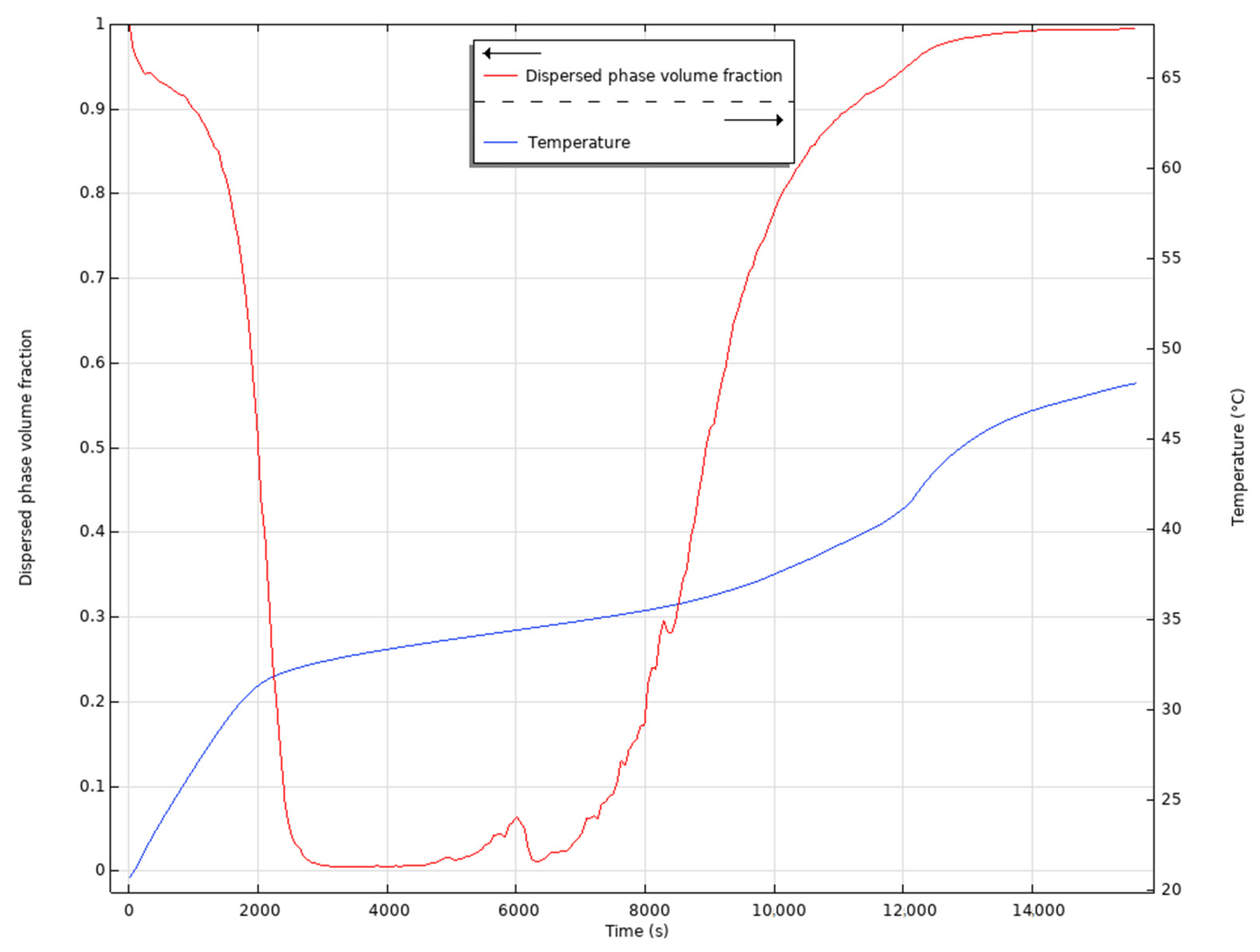
4.1. Results of the First Test
4.2. Results of the Second Test
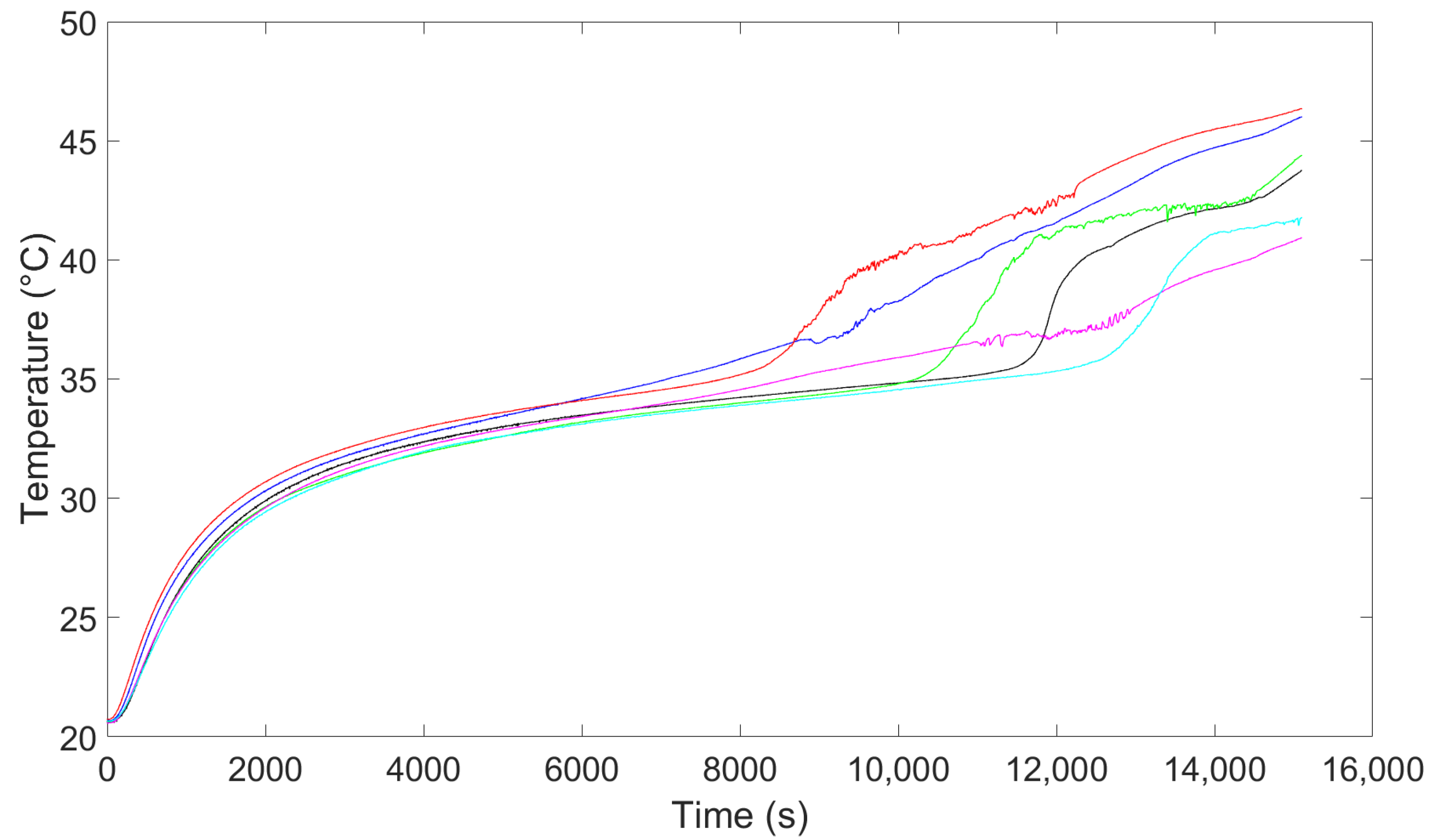
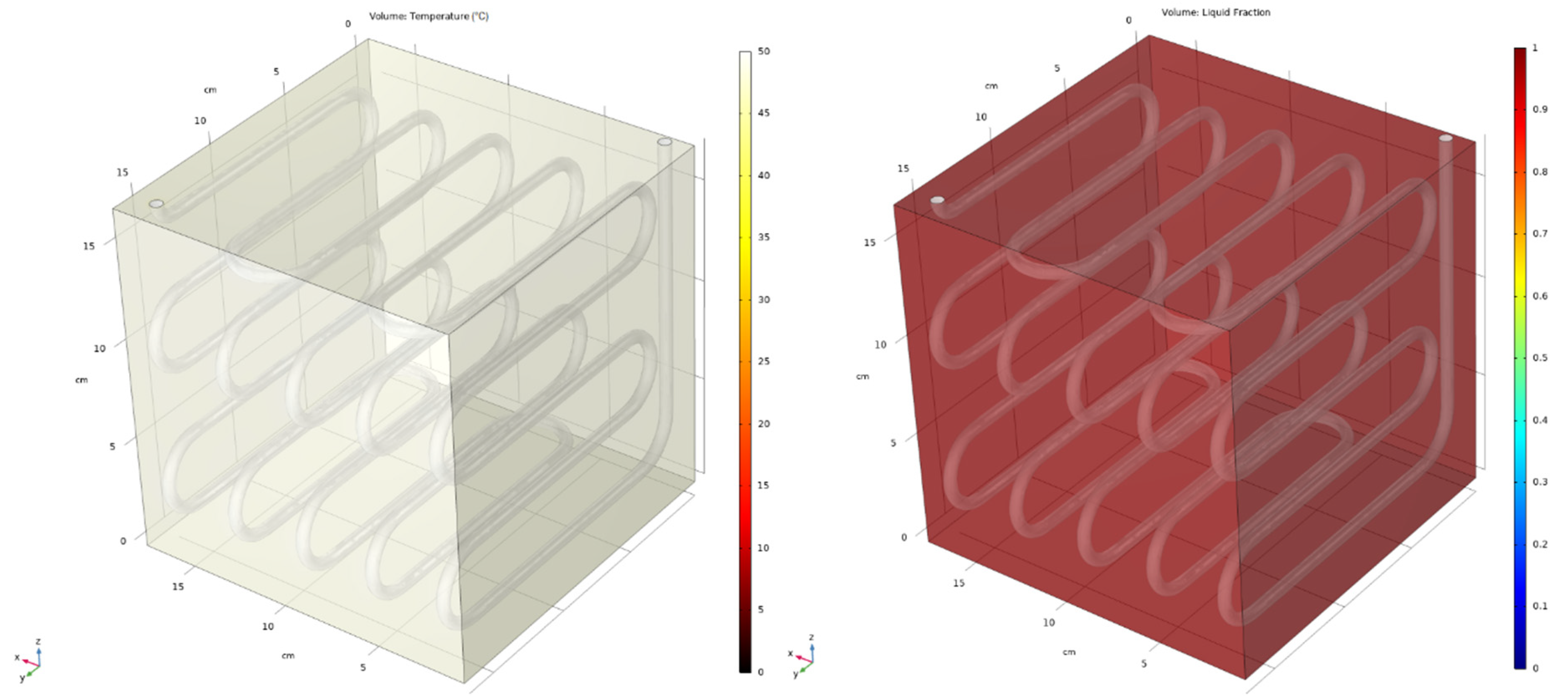
5. Simulation Results
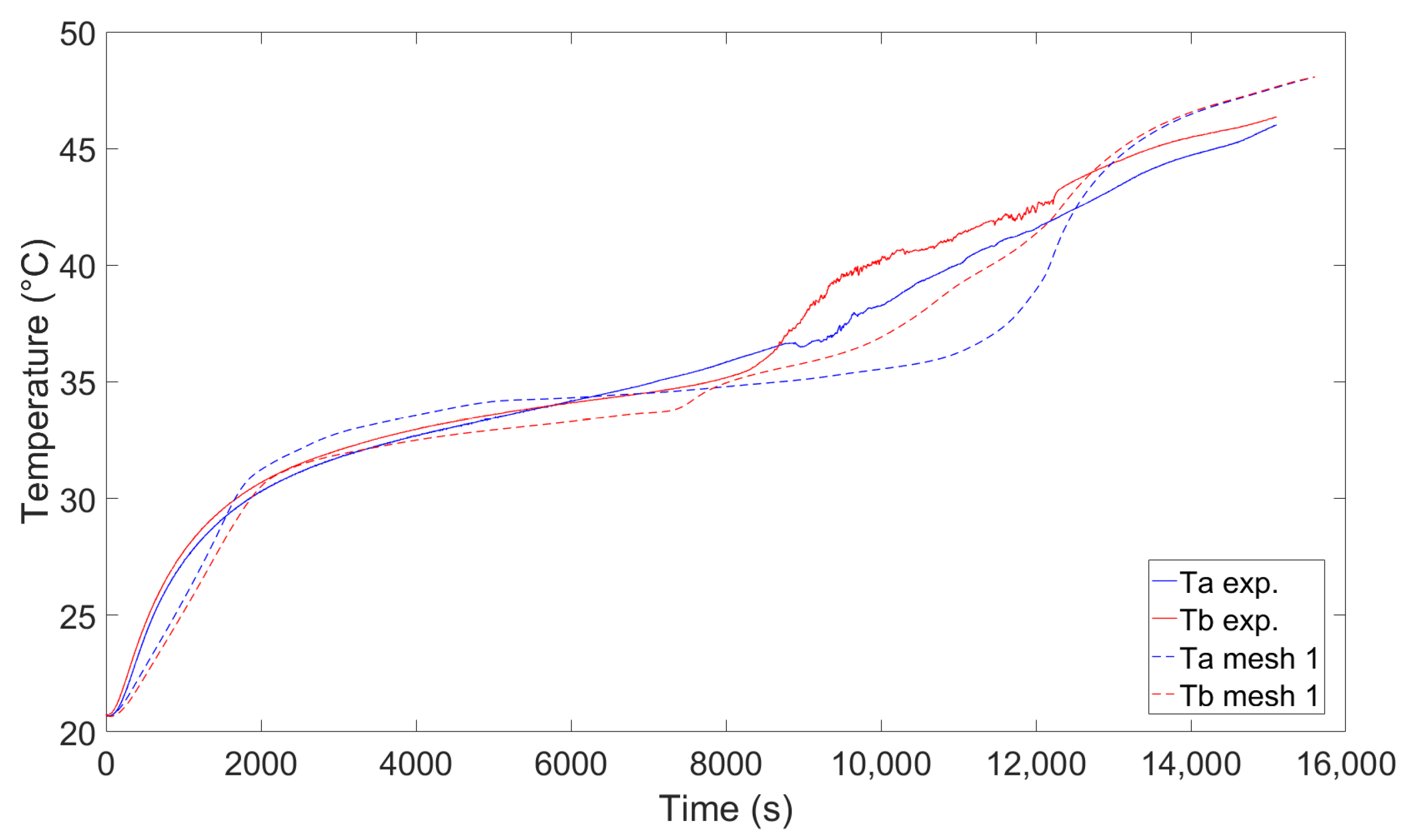
5.1. Simulation Model Implementation and Validation
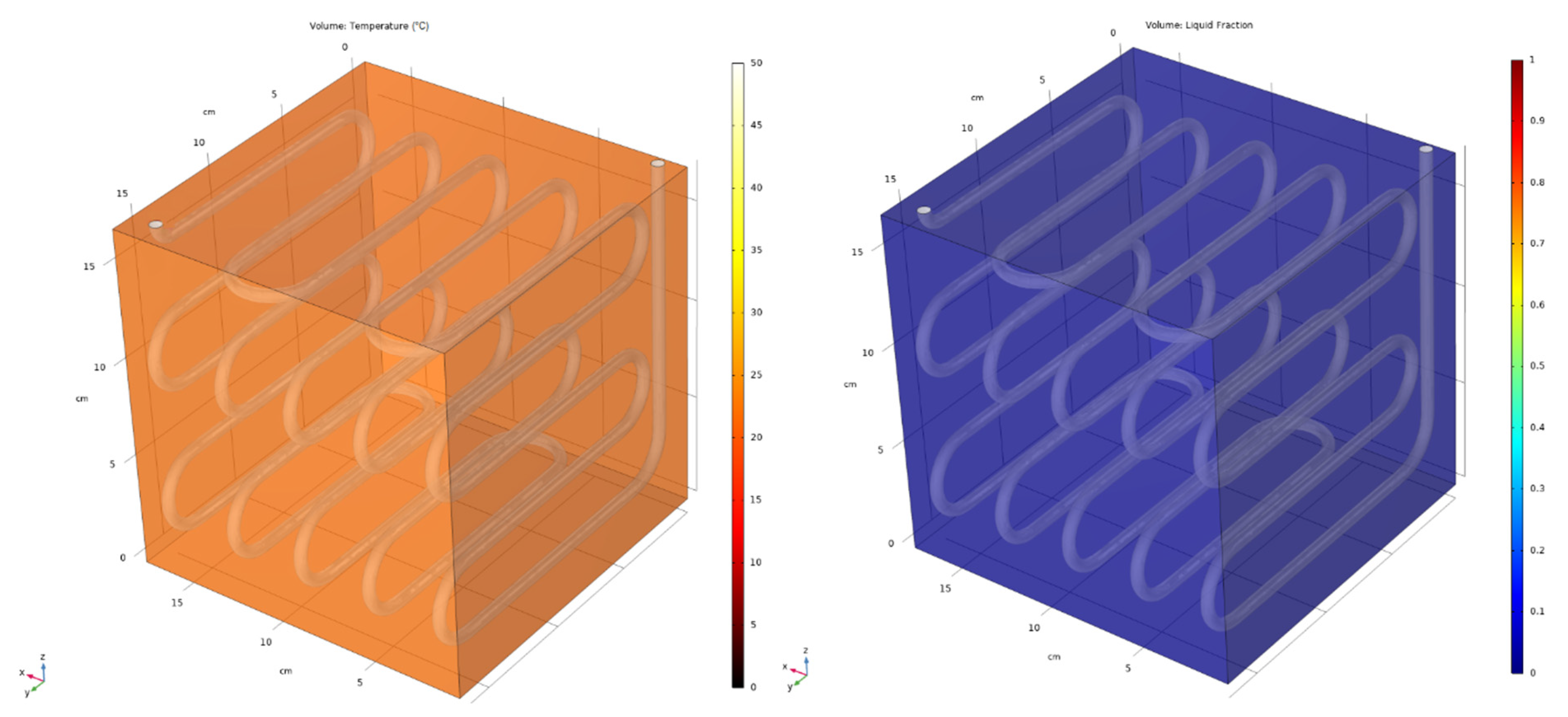
5.2. Averaged and 3D Results
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Veselý, M.; Zeiler, W. Personalized Conditioning and Its Impact on Thermal Comfort and Energy Performance—A Review. Renew. Sustain. Energy Rev. 2014, 34, 401–408. [Google Scholar] [CrossRef]
- Hoyt, T.; Arens, E.; Zhang, H. Extending air temperature setpoints: Simulated energy savings and design considerations for new and retrofit buildings. Build. Environ. 2015, 88, 89–96. [Google Scholar] [CrossRef]
- Arens, E.; Humphreys, M.A.; de Dear, R.; Zhang, H. Are “class A” temperature requirements realistic or desirable? Build. Environ. 2010, 45, 4–10. [Google Scholar] [CrossRef]
- Zhu, S.; Dalgo, D.; Srebric, J.; Kato, S. Cooling efficiency of a spot-type personalized air-conditioner. Build. Environ. 2017, 121, 35–48. [Google Scholar] [CrossRef]
- Sharma, S.D.; Sagara, K. Latent heat storage materials and systems: A review. Int. J. Green Energy 2005, 2, 1–56. [Google Scholar] [CrossRef]
- Nkwetta, D.N.; Haghighat, F. Thermal energy storage with phase change material—A state-of-the art review. Sustain. Cities Soc. 2014, 10, 87–100. [Google Scholar] [CrossRef]
- Beyne, W.; T’Jollyn, I.; Lecompte, S.; Cabeza, L.F.; De Paepe, M. De Paepe Standardised methods for the determination of key performance indicators for thermal energy storage heat exchangers. Renew. Sustain. Energy Rev. 2023, 176, 113139. [Google Scholar] [CrossRef]
- Medrano, M.; Yilmaz, M.O.; Nogués, M.; Martorell, I.; Roca, J.; Cabeza, L.F. Experimental evaluation of commercial heat exchangers for use as PCM thermal storage systems. Appl. Energy 2009, 86, 2047–2055. [Google Scholar] [CrossRef]
- Raj, V.A.A.; Velraj, R. Heat transfer and pressure drop studies on a PCM-heat exchanger module for free cooling applications. Int. J. Therm. Sci. 2011, 50, 1573–1582. [Google Scholar]
- Bianco, N.; Fragnito, A.; Iasiello, M.; Mauro, G.M.; Mongibello, L. Multi-objective optimization of a phase change material-based shell-and-tube heat exchanger for cold thermal energy storage: Experiments and numerical modeling. Appl. Therm. Eng. 2022, 215, 119047. [Google Scholar] [CrossRef]
- Fragnito, A.; Bianco, N.; Iasiello, M.; Mauro, G.M.; Mongibello, L. Experimental and numerical analysis of a phase change material-based shell-and-tube heat exchanger for cold thermal energy storage. J. Energy Storage 2022, 56, 105975. [Google Scholar] [CrossRef]
- Hosseini, M.J.; Rahimi, M.; Bahrampoury, R. Experimental and computational evolution of a shell and tube heat exchanger as a PCM thermal storage system. Int. Commun. Heat Mass Transf. 2014, 50, 128–136. [Google Scholar] [CrossRef]
- Yu, M.; Sun, X.; Su, W.; Li, D.; Shen, J.; Zhang, X.; Jiang, L. Investigation on the Melting Performance of a Phase Change Material Based on a Shell-and-Tube Thermal Energy Storage Unit with a Rectangular Fin Configuration. Energies 2022, 15, 8200. [Google Scholar] [CrossRef]
- Rahimi, M.; Ranjbar, A.A.; Ganji, D.D.; Sedighi, K.; Hosseini, M.J.; Bahrampoury, R. Analysis of geometrical and operational parameters of PCM in a fin and tube heat exchanger. Int. Commun. Heat Mass Transf. 2014, 53, 109–115. [Google Scholar] [CrossRef]
- Lv, L.; Zou, Y.; Huang, S.; Wang, X.; Shao, R.; Xue, X.; Rong, Y.; Zhou, H. Experimental study on a pilot-scale medium-temperature latent heat storage system with various fins. Renew. Energy 2023, 205, 499–508. [Google Scholar] [CrossRef]
- Stathopoulos, N.; El Mankibi, M.; Issoglio, R.; Michel, P.; Haghighat, F. Air–PCM heat exchanger for peak load management: Experimental and simulation. Sol. Energy 2016, 132, 453–466. [Google Scholar] [CrossRef]
- Elarem, R.; Mellouli, S.; Abhilash, E.; Jemni, A. Performance analysis of a household refrigerator integrating a PCM heat exchanger. Appl. Therm. Eng. 2017, 125, 1320–1333. [Google Scholar] [CrossRef]
- Ben Nasrallah, S.; Ben Khedher, N. Three-dimensional simulation of a porous thermal energy storage system using solid-liquid phase change material. J. Porous Media 2011, 2011, 777–790. [Google Scholar] [CrossRef]
- Qiao, Y.; Cao, T.; Muehlbauer, J.; Hwang, Y.; Radermacher, R. Experimental study of a personal cooling system integrated with phase change material. Appl. Therm. Eng. 2020, 170, 115026. [Google Scholar] [CrossRef]
- Cheng, W.L.; Yuan, X.D. Numerical analysis of a novel household refrigerator with shape-stabilized PCM (phase change material) heat storage condensers. Energy 2013, 59, 265–276. [Google Scholar] [CrossRef]
- Bakhshipour, S.; Valipour, M.S.; Pahamli, Y. Parametric analysis of domestic refrigerators using PCM heat exchanger. Int. J. Refrig. 2017, 83, 1–13. [Google Scholar] [CrossRef]
- Yan, G.; Liu, Y.; Qian, S.; Yu, J. Theoretical study on a vapor compression refrigeration system with cold storage for freezer applications. Appl. Therm. Eng. 2019, 160, 114091. [Google Scholar] [CrossRef]
- Mosleh, H.J.; Kavian, S.; Shafii, M.B. Performance analysis and transient simulation of a vapor compression cooling system integrated with phase change material as thermal energy storage for electric peak load shaving. J. Energy Storage 2021, 35, 102316. [Google Scholar]
- Riffat, J.; Kutlu, C.; Tapia-Brito, E.; Tekpetey, S.; Agyenim, F.B.; Su, Y.; Riffat, S. Development and testing of a PCM enhanced domestic refrigerator with use of miniature DC compressor for weak/off grid locations. Int. J. Green Energy 2021, 19, 118–1131. [Google Scholar] [CrossRef]
- Sun, Z.; Peng, J.; Shi, Y.; Wang, H.; Li, J.; Zhu, S.; Yuan, Y.; Xu, Z.; Feng, Z.; Liu, J. Experimental study of a single-tube multi-fin row of tube evaporator. Appl. Therm. Eng. 2022, 211, 118385. [Google Scholar] [CrossRef]
- Abdolmaleki, L.; Berardi, U. Single and Multi-phase Change Materials Used in Cooling Systems. Appl. Therm. Eng. 2022, 182, 116160. [Google Scholar] [CrossRef]
- Dhumane, R.; Qiao, Y.; Ling, J.; Muehlbauer, J.; Aute, V.; Hwang, Y.; Radermacher, R. Improving system performance of a personal conditioning system integrated with thermal storage. Appl. Therm. Eng. 2019, 147, 40–51. [Google Scholar] [CrossRef]
- Dhumane, R.; Ling, J.; Aute, V.; Radermacher, R. Performance comparison of low GWP refrigerants for a miniature vapor compression system integrated with enhanced phase change material. Appl. Therm. Eng. 2021, 182, 116160. [Google Scholar] [CrossRef]
- Available online: www.rubitherm.eu (accessed on 4 April 2022).
- Faden, M.; König-Haagen, A.; Höhlein, S.; Brüggemann, D. An implicit algorithm for melting and settling of phase change material inside macrocapsules. Int. J. Heat Mass Transf. 2018, 117, 757–767. [Google Scholar] [CrossRef]
- Lee, W.H. A Pressure Iteration Scheme for Two-Phase Flow Modeling. In Computational Methods for Two-Phase Flow and Particle Transport; World Scientific Publishing Company: Singapore, 2013; pp. 61–82. [Google Scholar] [CrossRef]
- ASHRAE Handbook; Ashrae Handbook: Refrigeration; ASHRAE (American Society of Heating, Refrigerating and Air-Conditioning Engineers): Peachtree Corners, GA, USA, 2006.









| Melting Area (°C) | 34–36 Main peak 35 |
| Congealing Area (°C) | 36–34 Main peak 35 |
| Heat storage capacity ± 7.5% (J·kg−1) | 240 × 103 |
| Combination of sensible and latent heat in the temperature range 27–42 °C (J·kg−1) | 241.2 |
| Specific heat capacity (J·kg−1·K−1) | 2000 |
| Density solid at 25 °C (kg·m−3) | 880 |
| Density liquid at 40 °C (kg·m−3) | 770 |
| Thermal conductivity (both phases) (W·m−1·K−1) | 0.2 |
| Volume expansion (%) | 12 |
| Flash point (°C) | 177 |
| Max. operation temperature (°C) | 70 |
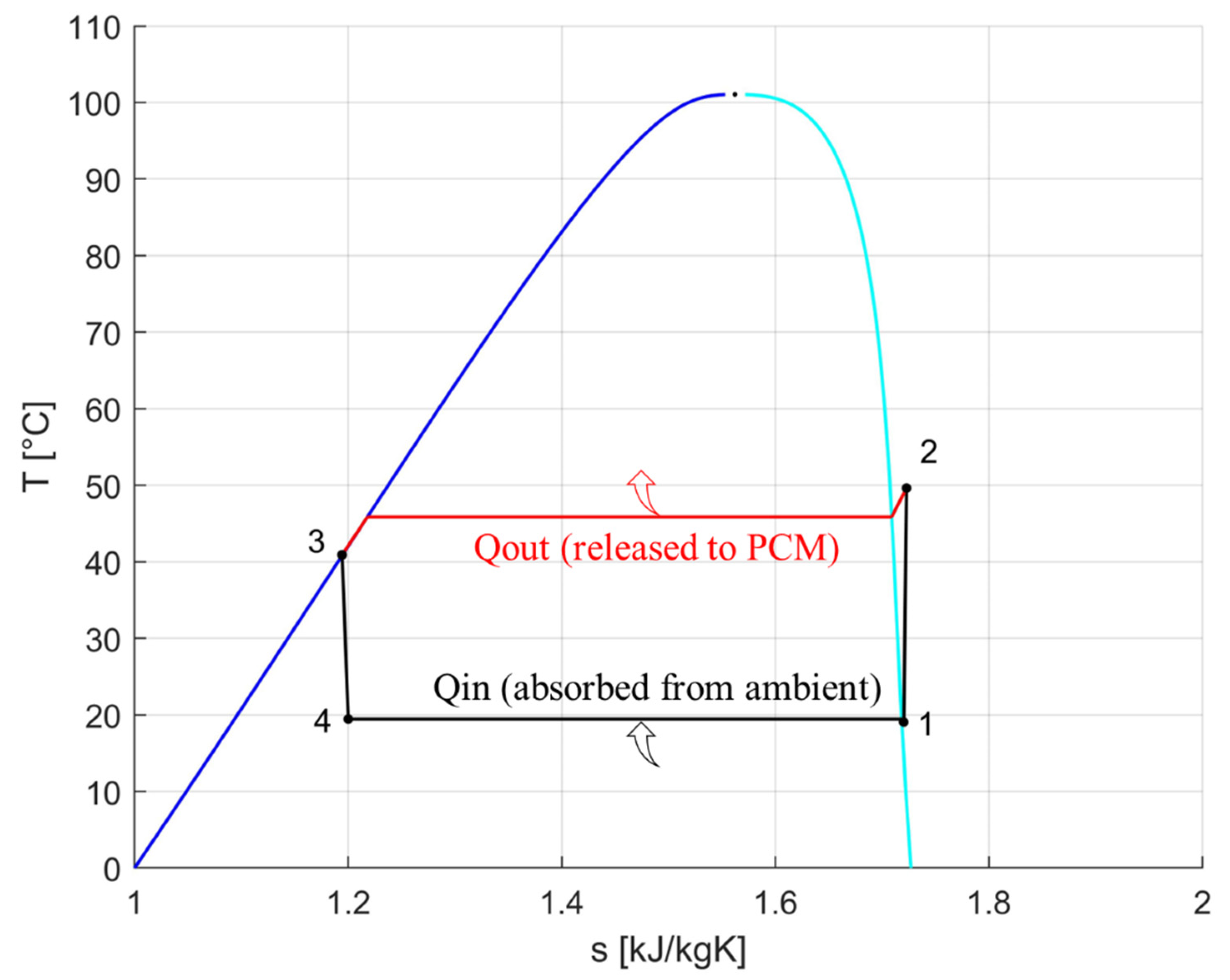
| T [°C] | P [bar] | vs [m3/kg] | h [kJ/kg] | s [kJ/kgK] | x | |
|---|---|---|---|---|---|---|
| 1 (evaporator outlet/compressor inlet) | 11.10 | 4.30 | 0.0477 | 404.928 | 1.722 | 1 |
| 2id. (ideal compressor outlet/condenser inlet) | 53.89 | 13.20 | 0.0155 | 428.144 | 1.722 | \ |
| 2 (real compressor outlet/condenser inlet) | 60.46 | 13.20 | 0.0163 | 435.882 | 1.745 | \ |
| 3 (condenser outlet/receiver inlet) | 50.06 | 13.20 | 0.00091 | 271.721 | 1.238 | 0 |
| 4 (capillary tube outlet/evaporator inlet) | 11.10 | 4.30 | 0.0148 | 271.721 | 1.253 | 0.298 |
| C | Compressor |
| CT | Capillary tube |
| EV | Evaporator |
| LP | Low pressure line |
| HP | High pressure line |
| P1, P2 | Pressure sensors |
| R | Receiver |
| T1-4, Ta-f | Temperature sensors (RTD) |
| VB1, VB2 | Bypass valves |
| T [°C] | P [bar] | vs [m3/kg] | h [kJ/kg] | S [kJ/kgK] | x | |
|---|---|---|---|---|---|---|
| 1 (evaporator outlet/compressor inlet) | 19.05 | 5.47 | 0.037 | 409.46 | 1.720 | ~1 |
| 2 (compressor outlet/condenser inlet) | 49.63 | 11.86 | 0.0174 | 426.32 | 1.723 | / |
| 3 (condenser outlet/receiver inlet) | 40.90 | 11.86 | 0.0008 | 257.72 | 1.194 | ~0 |
| 4 (capillary tube outlet/evaporator inlet) | 19.45 | 5.47 | 0.0073 | 257.72 | 1.200 | 0.176 |
| 2* (real measurement of the point 2) | 46.02 | 11.86 | 0.0169 | 422.05 | 1.709 | ~1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavargna, A.; Mongibello, L.; Iasiello, M.; Bianco, N. Analysis of a Phase Change Material-Based Condenser of a Low-Scale Refrigeration System. Energies 2023, 16, 3798. https://doi.org/10.3390/en16093798
Cavargna A, Mongibello L, Iasiello M, Bianco N. Analysis of a Phase Change Material-Based Condenser of a Low-Scale Refrigeration System. Energies. 2023; 16(9):3798. https://doi.org/10.3390/en16093798
Chicago/Turabian StyleCavargna, Augusto, Luigi Mongibello, Marcello Iasiello, and Nicola Bianco. 2023. "Analysis of a Phase Change Material-Based Condenser of a Low-Scale Refrigeration System" Energies 16, no. 9: 3798. https://doi.org/10.3390/en16093798
APA StyleCavargna, A., Mongibello, L., Iasiello, M., & Bianco, N. (2023). Analysis of a Phase Change Material-Based Condenser of a Low-Scale Refrigeration System. Energies, 16(9), 3798. https://doi.org/10.3390/en16093798









