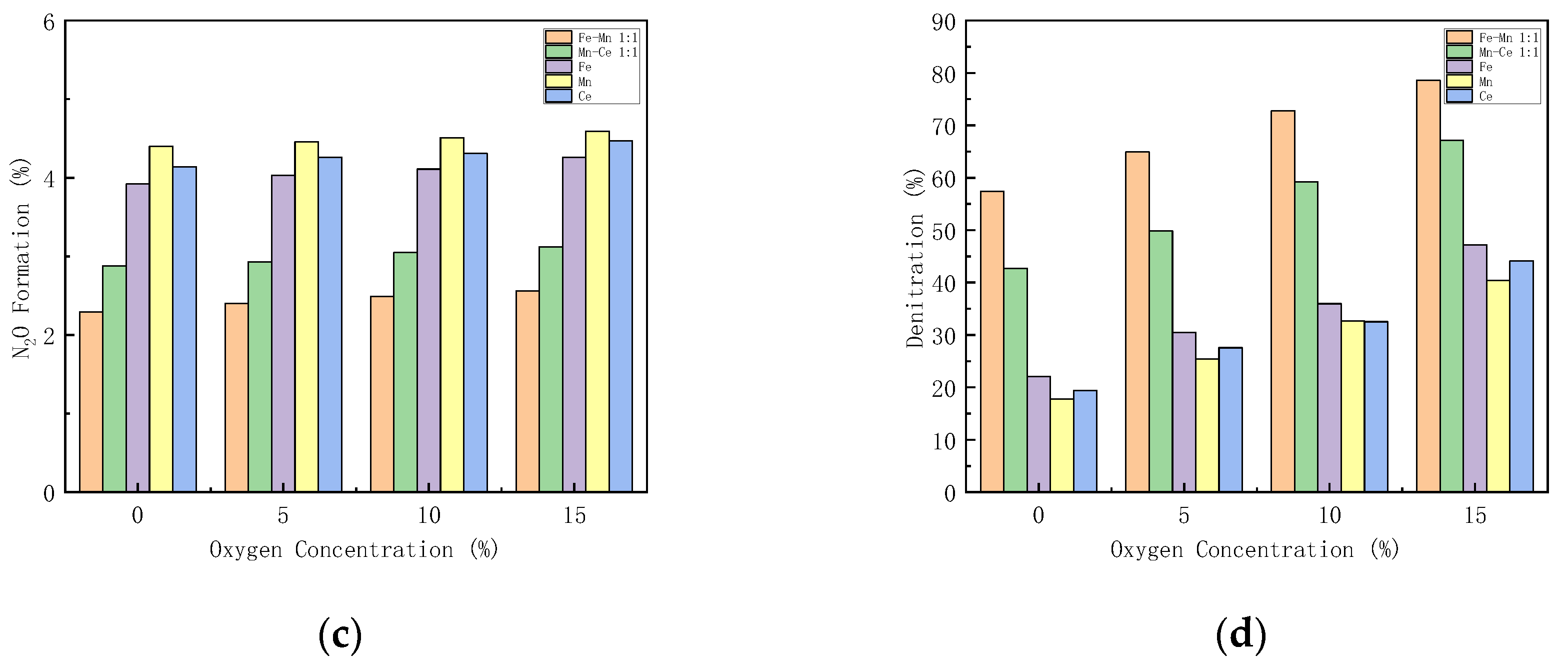Abstract
Gas turbines produce a large amount of NOx and CO due to high temperatures and insufficient combustion. Through the selective catalytic reduction of NO with CO (CO-SCR) in a gas turbine, the activities of the Mn-Fe-Ce/FA catalyst using fly ash (FA) as a carrier under different atmospheres were studied. The catalysts prepared by calcining different active materials under different atmospheres were used to analyze their denitrification abilities and resistance to water vapor. The denitrification performance of the catalyst prepared under reducing atmosphere is about 30 percent higher than that of the catalyst prepared under air atmosphere, and the decarburization performance is about 40 percent higher. In the presence of oxygen, the denitrification rate and decarburization rate of the 1:1 ratio of the Mn-Ce catalyst reach 67.16% and 59.57%, respectively. In an oxygen-containing atmosphere, the catalyst prepared by replacing Ce with Fe shows better denitrification and decarburization performances, which are 78.56% and 78.39%, respectively. When the flue gas space velocity is 4000 h−1 and the carbon-nitrogen ratio is 1.6, the catalyst shows better performance. After the water vapor is introduced, the denitrification and decarbonization rates of the catalyst decrease by about 10% and 9%, respectively. After ceasing water vapor, it rebounds by about 8%, and the activity could not be fully restored. However, the catalyst still shows strong water resistance in general.
1. Introduction
Nowadays, the control of nitrogen oxides has become an indispensable part of environmental management [1]. How to reduce nitrogen oxide emissions as effectively as possible under the condition of complete combustion has become one of the focuses of research [2]. The current common denitrification technologies include liquid adsorption [3], dry gas adsorption [4], biological enzymatic degradation treatment [5], non-selective catalytic reduction (SNCR) [6], selective catalytic reduction (SCR) [7], and so on [8]. NH3-SCR and CO(NH2)2-SCR are the typical SCR methods commonly used in industry, in which redox reaction is taken place between ammonia gases and nitrogen oxides [9]. Liu et al. [10] prepared the Me/SAPO-34 (Me = Mn, Ni, Co) catalyst for NH3-SCR reaction using the impregnation method. The Mn/SAPO-34 catalyst exhibited excellent SCR performance at the temperatures of 150 to 300 °C, and at low temperatures (100 to 300 °C) the N2 selectivity more than 90% is maintained.
However, NH3-SCR has many limitations, such as the addition of ammonia, the high requirements on gas pipe materials, the secondary pollution caused by ammonia leakage, etc [11]. Therefore, it is a better choice to replace ammonia with CO which exists in the exhaust gas produced by combustion reaction [12], which is more economical and environmentally friendly [13,14]. Scholars Tauster and Murrell [15,16] proposed the research area of SCR of NO by CO in the experiment, and have found that precious metals such as Ir-Pt-Pd have better denitrification performance than normal metals [17]. In addition, the reaction rate of CO-SCR is greatly improved in the presence of precious metals in an oxygen atmosphere [18]. However, noble metal catalysts are expensive and susceptible to corrosion by halogens and heavy metals [19]. Although the catalytic activity of transition metals at lower temperatures is lower than that of precious metals, the transition metals such as Cu [20], Ce [21], and Mn [22] also have strong catalytic activity at higher temperatures. Kacimi et al. [23]. prepared a Cu/AlPO4 catalyst with good low-temperature reducibility using Cu (II) ion complex exchange method, and the catalyst can convert 85% of CO into CO2. Zhang et al. [24] found in their study that the complex active component Fe-Ba had a good compound effect on the experimental support ZSM-5, and the loading of active substance could improve the catalyst activity. Moreover, there are interactions between various active components in the composite catalyst, which is beneficial to improve the activity of the catalyst. The denitration efficiency of the complex catalyst in CO atmosphere is improved compared with that of the single metal [25]. MnOx-Fe3O4 nanomaterials fabricated by Zhang et al. [26] employ an innovative scheme of pyrolytically manganese-doped Fe-based metal-organic frameworks in an inert atmosphere, and exhibit extraordinary CO-reduction in NO (CO-SCR) performance.
The catalyst support is one of the components of the catalyst, which is the skeleton of the active component of the catalyst. The carrier can support the active component, disperse the active component, and increase the strength of the catalyst. The carrier generally has no catalytic activity and usually has a large specific surface area and adsorption. Common SCR carriers include TiO2, Al2O3, molecular sieve, activated carbon, and so on. The 20Mn-10Sm/TiO2 catalyst prepared by Liu et al. [27] by ultrasonic impregnation showed good denitration ability. Wang et al. [28] synthesized MnOx-CeO2-Al2O3 catalysts by spray drying (SD), and the NOx conversion rate could also reach 55% and 83% at 50 °C and 100 °C, respectively, and even reach 93% at 150 °C. Zhu et al. [29] synthesized Ni/ETS-10 using an organosilicon surfactant template; good catalytic activity is maintained after many tests. Zhang et al. [30] used the copper-BTC which was prepared by the hydrothermal method as the precursor and obtained the carbon-based catalyst as the low-temperature denitration catalyst, which has excellent catalytic activity. Fly ash (FA for short) is a solid waste discharged and collected in the process of burning coal in thermal power plants [31]. In recent years, the output of fly ash has increased year by year, but the utilization rate of fly ash is seriously insufficient, resulting in most fly ash placed in the open air, causing waste of land resources, polluting the land, and even affecting human health [32]. Fly ash contains a large amount of Al2O3, SiO2, Fe2O3, and other substances, which has a large specific surface area and good adsorption. Compared with the more common catalyst supports, such as activated carbon, fly ash have better heat resistance and low cost, which can effectively reduce industrial costs. Zhang et al. [33] found that with fly ash as the carrier, when Mn:Ce was 1:1, the denitration rate could maintain at 60%.
The paper mainly studies the CO-SCR catalyst for the simultaneous removal of CO and NOx in the flue gas of gas turbine. The experiment was carried out in view of active component and carrier, antioxidant performance, and other problems in the practical use of catalysts. By changing the calcination atmosphere and flue gas conditions, the catalytic performance of the catalyst under different conditions was investigated. By utilizing the cheap and easy-to-obtain characteristics of fly ash as the support of the catalyst, the CO and NO in the exhaust gas are jointly removed through the CO-SCR reaction, to achieve the purpose of treating waste with waste and reducing industrial costs.
2. Experimental
2.1. Catalyst Preparation
In this experiment, fly ash (FA) from China Shenhua (Xinjiang) Coal to Oil Chemical Co., Ltd. was used as the carrier. Ash analysis is shown in Table 1. Cerium nitrate (Ce(NO3)3·6H2O), manganese nitrate (Mn(NO3)2), and iron nitrate (Fe(NO3)3) were taken as active precursors. The active material and the carrier were mixed according to a certain ratio by constant volume impregnation method, stirred, ground, and calcined to prepare a powdered catalyst.

Table 1.
Ash content analysis of fly ash.
The specific process is as follows:
- (1)
- Treatment of carrier: fly ash (FA) was washed with deionized water, dried, and prepared for later experiment.
- (2)
- Preparation of active precursor: metals were weighted and mixed in a certain proportion, then they were dissolved in an appropriate amount of deionized water, fully stirred, and mixed to ensure complete dissolution.
- (3)
- The previously static fly ash was weighed to the required amount, then placed into a beaker, mixed with the prepared active precursor. After that, the sample was placed in a magnetic stirrer for 3 h and standing for another 12 h.
- (4)
- After standing, the mixture was dried in an oven at 80 °C until the moisture had completely evaporated.
- (5)
- The catalyst was dried at 350 °C and 500 °C in a muff furnace for 2 h, then calcined in a reducing atmosphere (2% CO, N2 as equilibrium gas) at 500 °C for 3 h, and then cooled to room temperature.
2.2. Experimental Schemes
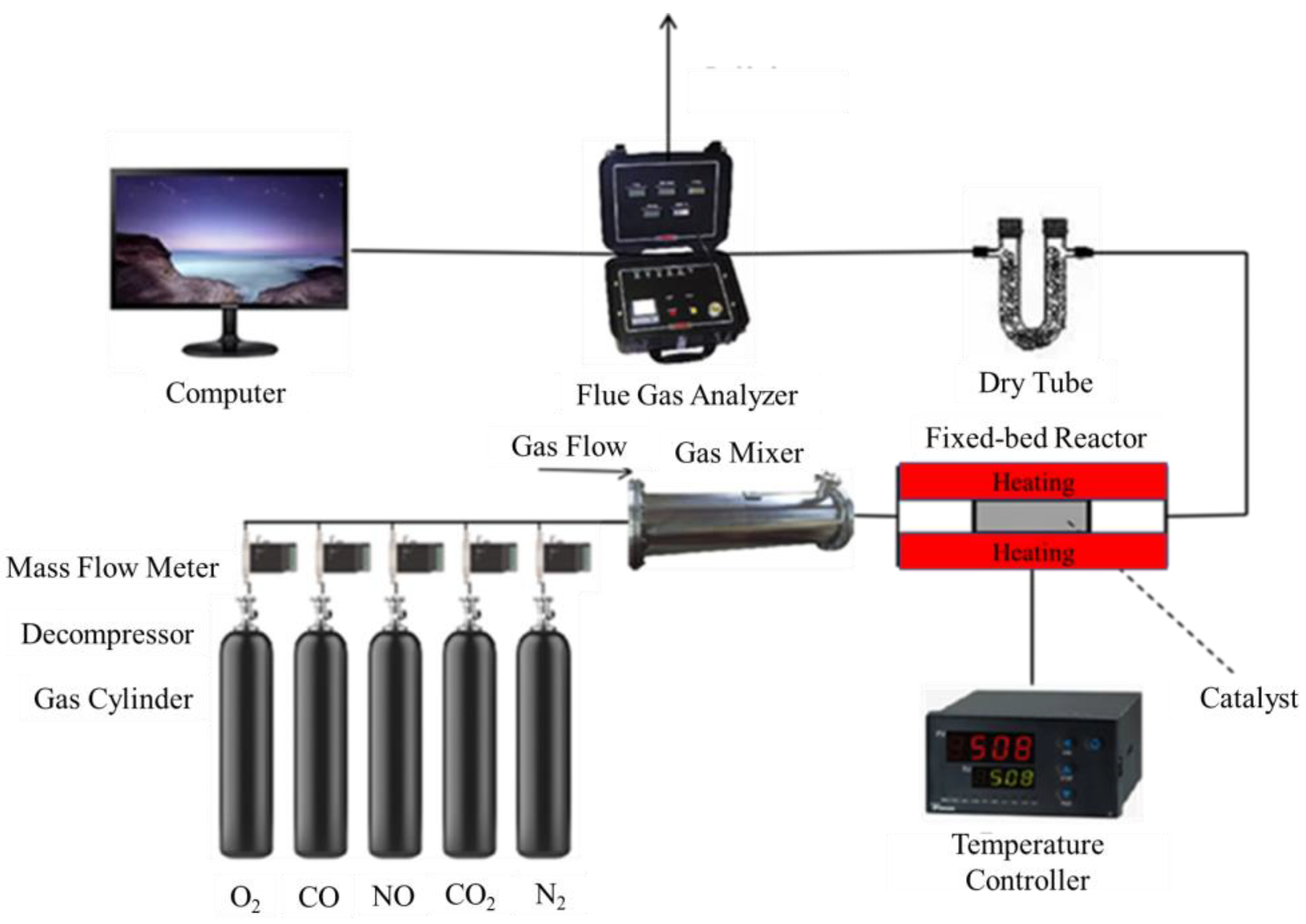
The experiment was set up as shown in Figure 1. The catalyst was placed in a 15 mm diameter ceramic tube of the tubular furnace. All the gas components required by the experiment were passed through the mass flow meter from the gas cylinder, then entered the fixed-bed reactor for the catalytic reaction after fully mixing. The content of each component of the outlet flue gas was measured by a flue gas analyzer before discharging.
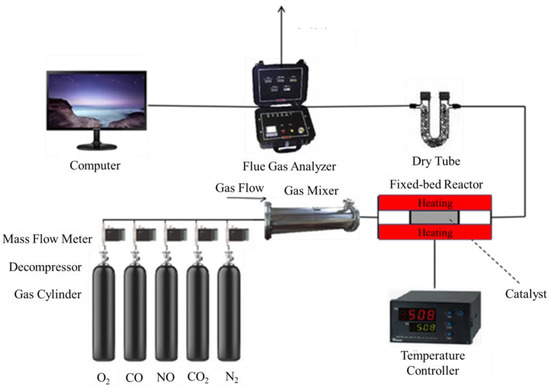
Figure 1.
Experimental system.
According to the actual flue gas composition, N2, O2, CO, NO, and CO2 were supplied by the gas cylinder mixtures. Water vapor was injected into the mixer. The concentration of different compounds in the mixture was controlled by a mass flow meter, and the SCR reaction temperature was adjusted by a temperature controller. The drying tube is to remove the water vapor at the water resistance experiment of the catalyst. Before the experiment starts, it was necessary to check the safety issues of each equipment. The tubular furnace was preheated in advance to check whether the electrical equipment had a short circuit. At the same time, some toxic gases such as CO and NO were used in the experiment, so that it was necessary to maintain good circulation of indoor gas and outside air to ensure the safety of the experiment.
The specific experimental steps are as follows: firstly, after passing the gas for 5 min, turn on the tube furnace and set the temperature at the reaction temperature of the catalyst experiment (150–550 °C). The catalyst was put into the high temperatures area in the middle of the tube from the gas discharge end of the tube furnace to ensure the reliability of the reaction temperature. The data were collected by the flue gas analyzer and recorded in the computer. Each experiment was repeated three times, and the average value was used as the final results.
In the experiment, to simulate the actual gas turbine exhaust conditions (>550 °C), and to select a suitable denitrification location, it is convenient for the arrangement of waste heat recovery. The removal efficiencies of flue gas pollutants including CO and NOx were calculated at the temperatures of 150 °C, 200 °C, 250 °C, 300 °C, 350 °C, 400 °C, 450 °C, 500 °C, and 550 °C, and at the space velocity of 2000 h−1, 3000 h−1, 4000 h−1. 5000 h−1, and 6000 h−1.
The instruments used in the analysis of flue gas were J2KNPRO portable flue gas analyzer from Germany ECOM and 7710 FM made by Signal from UK. The gas components of CO, NO, NO2, N2O, and O2 were monitored and recorded in real time. NO2 and N2O were generated in CO-SCR side reaction.
2.3. Catalyst Characterization
Table 2 lists the characteristic values of specific surface area and pore volume of the catalyst supported by fly ash. It can be seen that the specific surface area of fly ash is the smallest, and the load of active components can effectively increase the specific surface area and pore volume of fly ash carrier. In addition, different metals have different effects on the specific surface area of the catalysts, and the Ce-supported catalyst exhibits the largest specific surface area among the catalysts.

Table 2.
Specific surface area and pore volume of several catalysts.
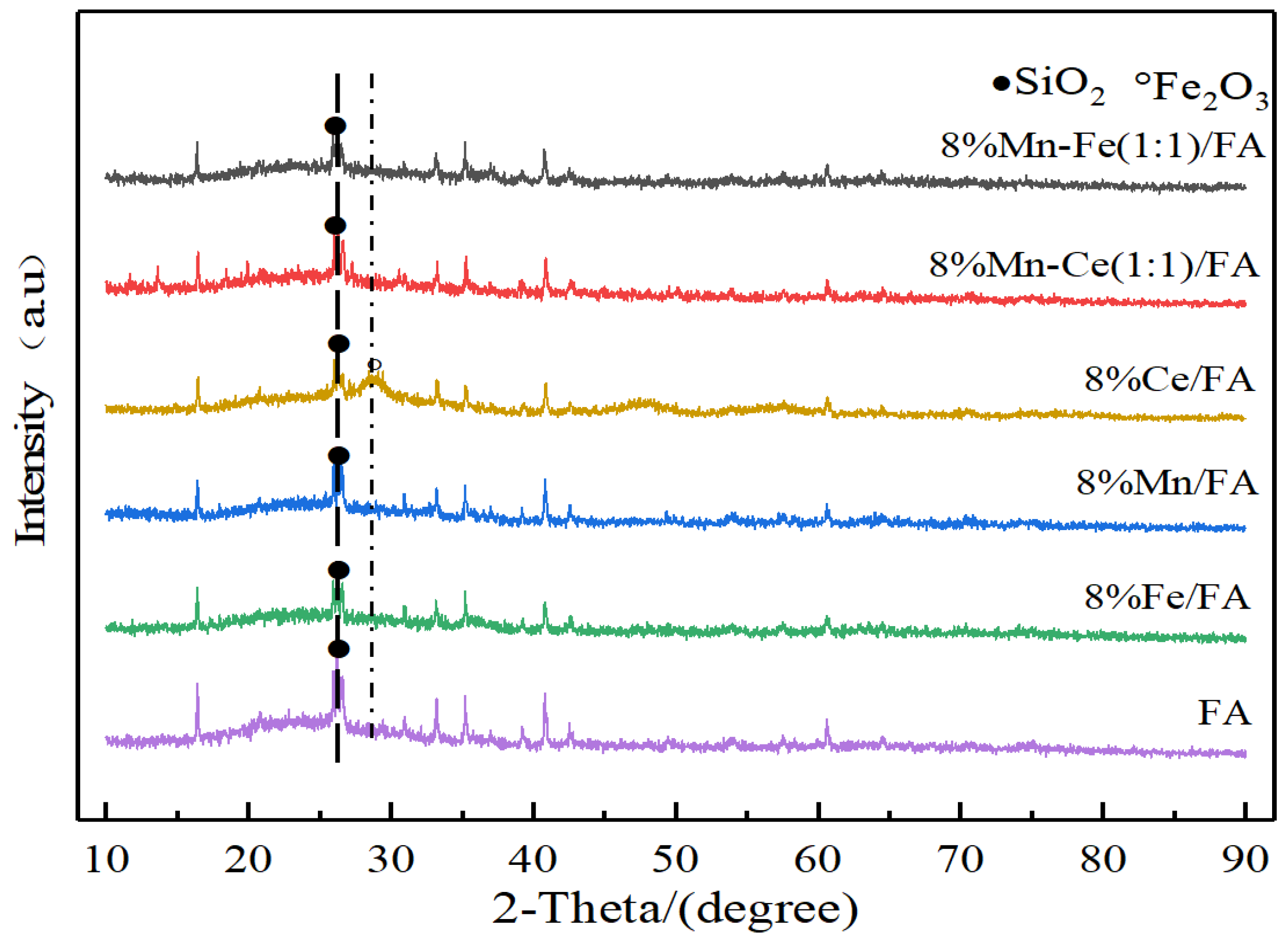
Figure 2 is the XRD characterization analysis of the catalyst supported by fly ash. All the catalysts show obvious diffraction peaks of SiO2 at 27.7°, which indicates that the preparation process of the catalyst and the loading process of active components have not changed the crystal structure of fly ash material, and there are no obvious diffraction peaks of other metal oxides (Al2O3, etc.) in the fly ash in the catalysts, indicating that the content of such substances in the fly ash used in the experiment is low. Moreover, by comparing the catalyst with fly ash alone, it can be found that the addition of active components does not change the crystal structure of the original catalyst support.
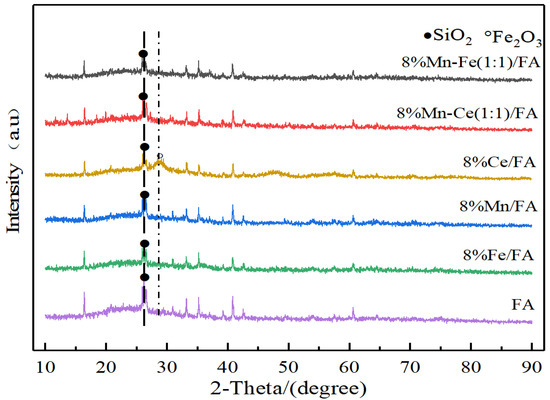
Figure 2.
XRD characterization of catalysts with fly ash as carrier.
No obvious diffraction peaks of active components are found in all five catalysts, which indicates that the active components are highly dispersed on the surface of the catalysts after the loading process. In the experimental catalytic reaction, the active components are supported on the surface of the catalysts in the form of a monolayer to participate in a series of reactions.
The activity of catalyst depends on the phase state and existence form of active component. The dispersion of the active substance on the surface of the catalyst is conducive to the formation of amorphous oxides, which can effectively improve the catalytic activity of the catalyst. The Fe2O3 material peak is observed in the 8% Ce/FA catalyst, and the fly ash material contains a small amount of Fe, which shows that Ce can promote the dioxide in the fly ash after loading. The aggregation of Fe2O3 forms a small amount of Fe2O3 crystal structure. However, no obvious peak of Fe2O3 is observed in the Mn-Ce 1:1 catalyst. Comparing to the previous results, it can be inferred that the promotion of the crystal structure of Fe2O3 requires Ce reaching a certain amount of loading.
2.4. Catalyst Activity Evaluation
(i) Definition of CO conversion rate:
(ii) Definition of NO conversion rate:
(iii) Definition of NO2 generation rate:
(iv) Definition of N2O generation rate:
(v) Definition rate:
where COin is the inlet value of CO; COout represents the outlet value of CO; NOin stands for the inlet value of NO; NOout is the outlet value of NO; NO2out represents the outlet value of NO2; and N2Oout refers to the outlet value of N2O.
3. Results
3.1. Effect of Calcination Atmospheres on Catalyst Performance
In order to study the impact of the calcinating atmosphere on the activity performance of the catalyst, experiments are running under anoxic conditions taking the catalyst Mn-Ce 1:1 as the experimental catalyst. During the activation process of catalyst preparation, air and reducing atmosphere (2% CO, nitrogen balanced gas) were selected as experimental variables. The gas components are 1000 ppm CO, 500 ppm NO, and nitrogen as a balanced gas.
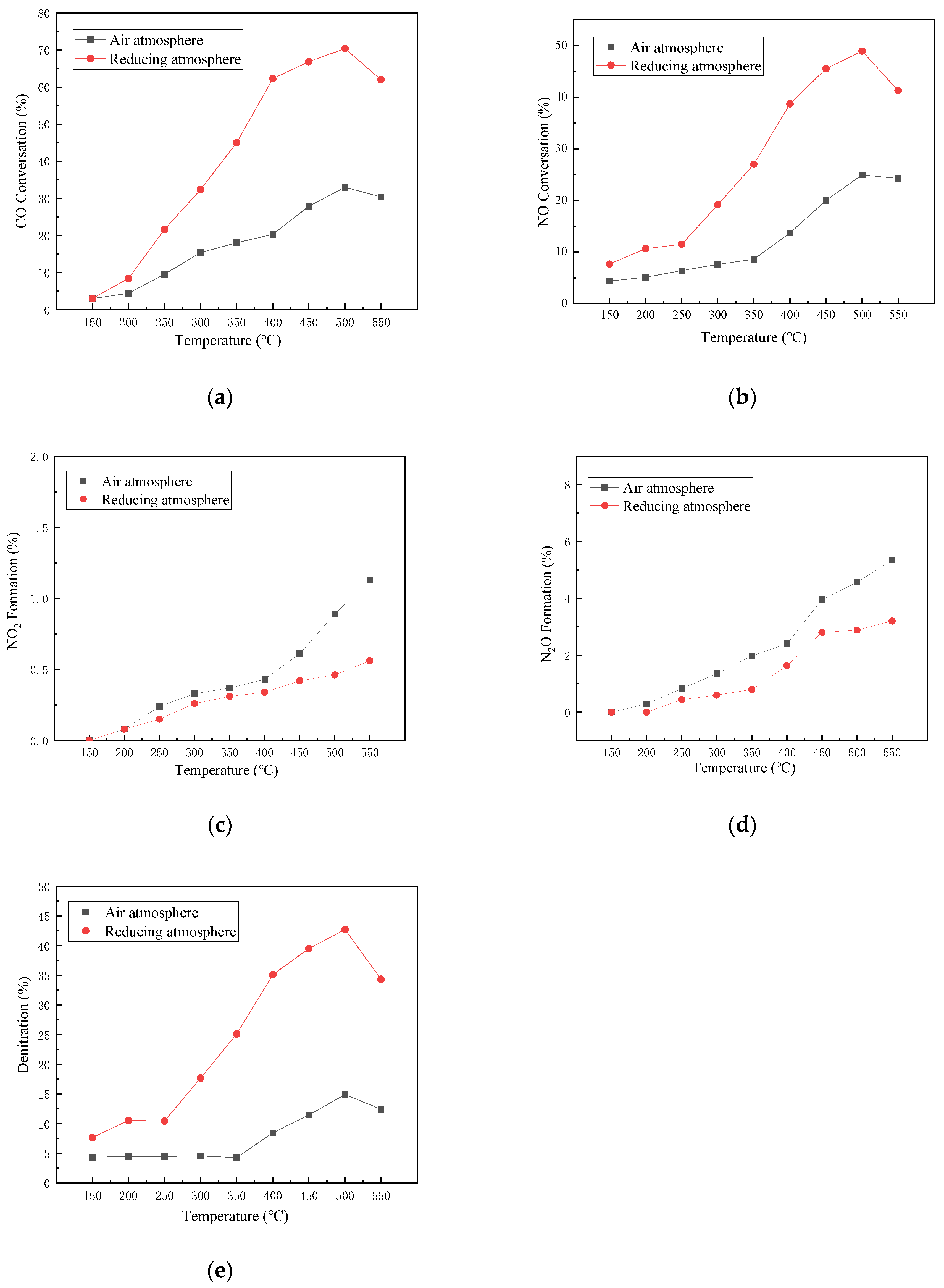
According to the conversion rate and generation rate of various pollutants in reducing atmosphere and air atmosphere shown in Figure 3, it can be inferred that the activity of the catalyst calcined in reducing atmosphere is generally higher than that in air atmosphere. In the experimental temperature range of 150 to 550 °C, the maximum CO conversion rate (Figure 3a), NO conversion rate (Figure 3b), and denitrification rate (Figure 3e) of the catalyst under reducing atmosphere calcination are 70.35%, 48.94%, and 42.72%, respectively. While those of the catalyst under air atmosphere calcination are 32.99%, 24.94%, and 14.91%, respectively. This is mainly because the calcination in reducing atmosphere makes the active ingredients more evenly distributed on the carrier, reducing the metal active material to a lower valence state, so that the metal activation ability is improved, and more active metal sites appear in the carrier. The capacity of the catalyst to adsorb and transform CO and NO is promoted [34,35].
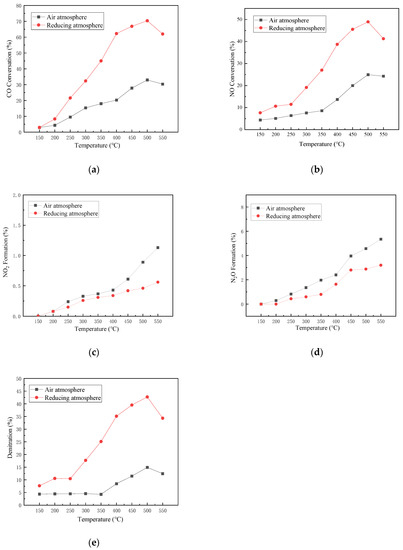
Figure 3.
Influence of different calcination atmospheres on catalyst activity. (a) CO Conversion. (b) NO Conversion. (c) NO2 Formation. (d) N2O Formation. (e) Denitration Rate.
For the reaction by-products of NO2 (Figure 3c) and N2O (Figure 3d), the generation rates of the two reaction by-products in air-calcined catalysts are 0.57% and 2.15% higher than those of the catalyst by-products in the reducing atmosphere, respectively. The catalyst activity in the air atmosphere is poor, and the denitration rate is low. Nitrogen selectivity is also relatively low. It may be that the evenly dispersed active substances and the active site of the low valence state can make the decomposition and reduction reaction route of the catalyst, which is more inclined to produce nitrogen and inhibit the reaction generation of N2O and NO2.
In order to show the difference in catalyst activity more clearly, the apparent activation energy (Ea) and rate constant (k) of each catalyst were calculated. For the CO-SCR reaction, the concentration of CO in the experiment is relatively large, which can be approximately regarded as the calculation of the first-order reaction process, the CO reaction order is zero, and the NO reaction order is one. The formula is as follows [36]:
In the formulas, k is the rate constant, mol/(g·s); V represents the gas molar flow rate, mol/s; W stands for the catalyst mass, g; c is the NO conversion rate, %; Ea refers to the activation energy, kJ/mol; R represents the gas molar constant, 8.314 J/(mol·K); T stands for the reaction temperature, K; and A is the pre-exponential factor, mol/(g·s).
After calculation, the activation energy of the catalyst activated under air atmosphere is 16.83 kJ/mol, and the activation energy of the catalyst activated under reducing atmosphere is 14.63 kJ/mol. The catalyst activated under reducing atmosphere shows lower apparent activation energy.
In summary, the catalyst prepared in the reducing atmosphere shows a better catalytic performance than that prepared in the air atmosphere. Therefore, the catalysts used in subsequent experiments are activated by the reducing atmosphere.
3.2. Effect of Mn-Ce Ratios on the Catalyst Activity under Anoxic Condition
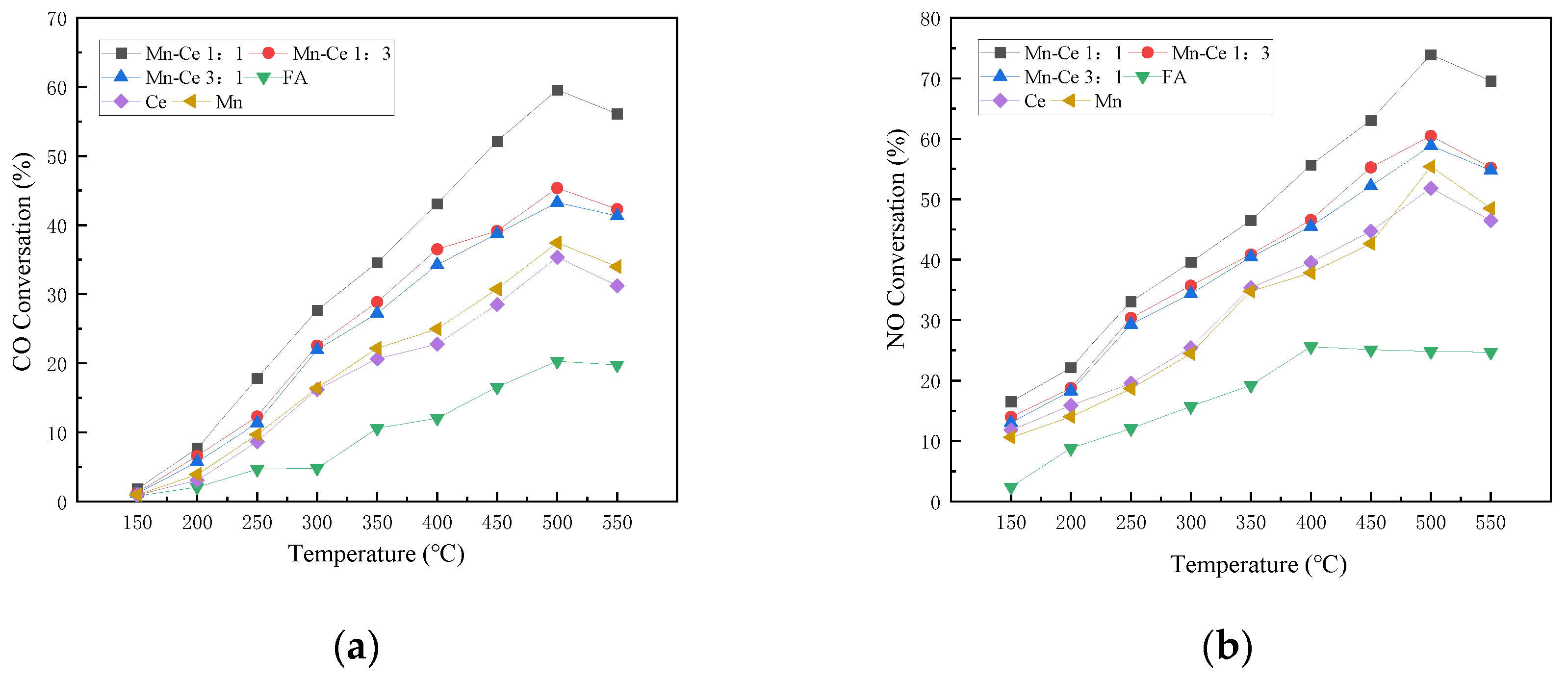
Under anoxic flue gas conditions, the gas components are 1000 ppm CO, 500 ppm NO, and N2 as the balance. The catalytic activities of catalysts with Mn-Ce molar ratios of 1:1, 1:3, 3:1, Mn, and Ce were studied.
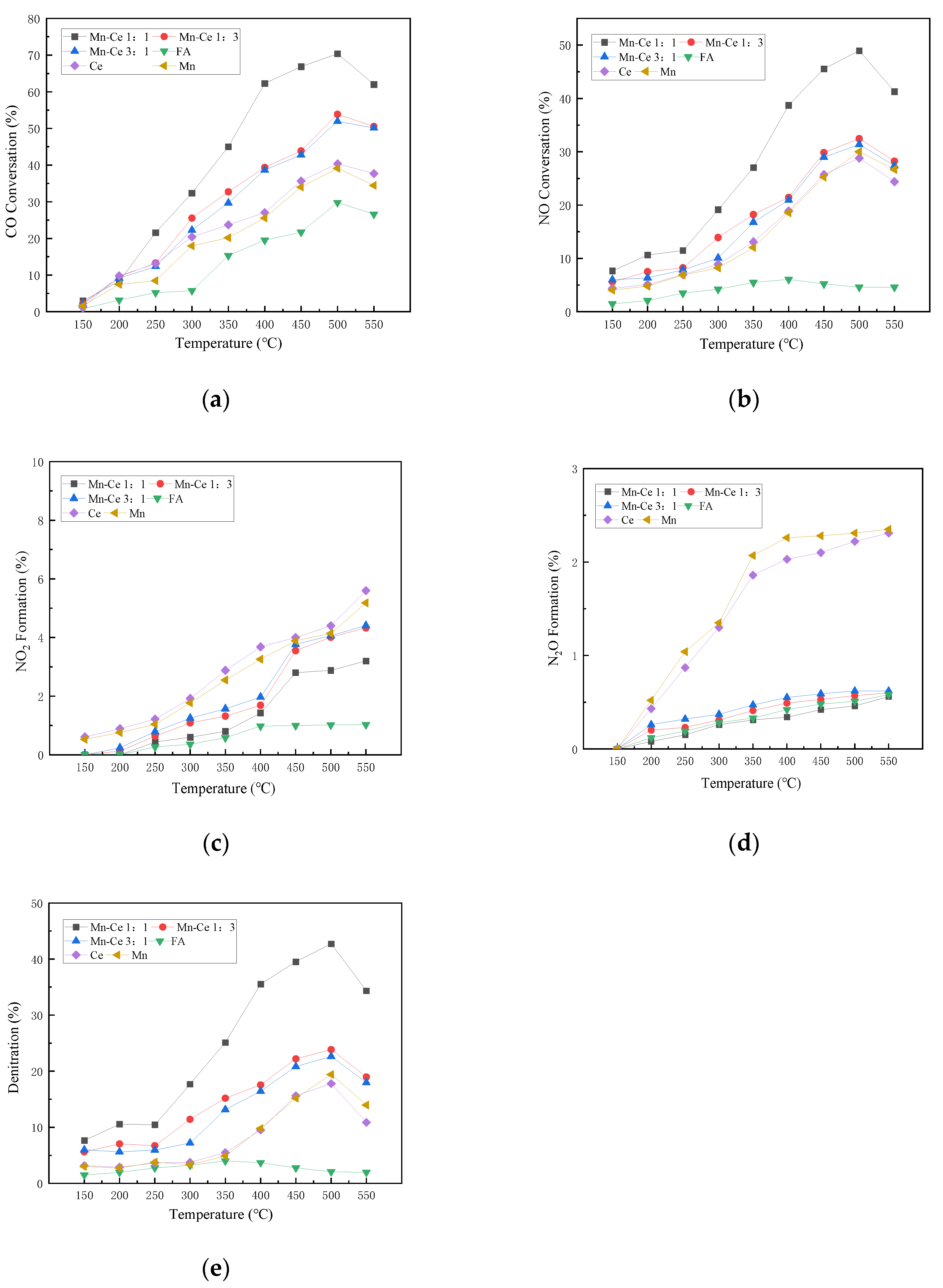
Figure 4 shows the CO and NO removals of flue gas by catalysts with different manganese-cerium ratios under the condition without oxygen. It can be clearly seen that the conversion rates of CO and NO generally increase with the increase in temperature. Among them, CO peaks at 500 °C, and then the conversion rate decreases with increasing temperature. The active components with Mn-Ce molar ratios of 1:1 and 1:3 exhibit relatively excellent CO conversion. Correspondingly, the CO removal performance of the single active component Mn or Ce is generally inferior to that of the composite component, with a maximum difference of about 30%. Ce exhibits better CO removal ability than Mn in an anoxic environment. In addition, when different elements are combined, there will be a synergistic effect between the metal active components, which will promote the adsorption and decomposition of CO by the catalyst, and improve the CO conversion.
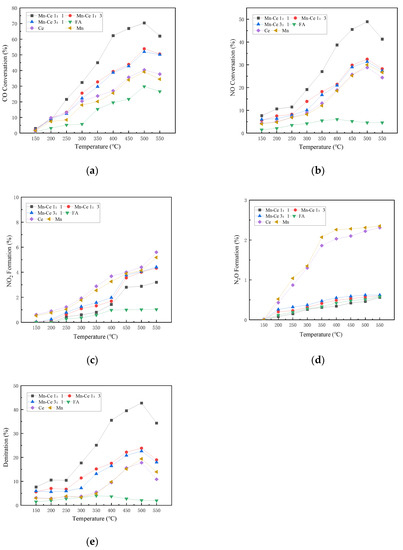
Figure 4.
Influence of different Mn-Ce ratios on catalyst activity under anoxic condition. (a) CO Conversion. (b) NO Conversion. (c) NO2 Formation. (d) N2O Formation. (e) Denitration Rate.
The NO conversion curve is similar to the CO conversion curve. They increase with temperature, peak at 500 °C, and then decrease with temperature. The composite catalyst shows a better NO removal rate than the single catalyst. Ce shows higher NO conversion rate than Mn at low temperature without oxygen, but Mn shows better conversion ability above 500 °C, which shows that the Mn catalyst has better heat resistance.
The production rates of N2O and NO2 are low, with less than 3% and 6%, respectively, but the general formation rates increase with the increase in temperature. High temperature may promote the reaction of O(ads) and NO(ads), and lead to the detachment of SCR reaction N2 route [37].
The denitrification performance curves of each catalyst are generally similar to that of the NO conversion rate, indicating that the N2 selectivity of the catalyst is better, and most of the NO is converted to nitrogen. Mn showed better catalytic performance than Ce at medium and high temperatures. The Mn-Ce 1:1 catalyst shows better denitration performance, and the denitration efficiency reaches 42.72% at 500 °C. From the experimental results, it can be inferred that the catalyst loaded with active metals can significantly promote the forward progress of the CO-SCR reaction, and can effectively promote the reaction route towards nitrogen during the reaction. After the composite metal is loaded, the synergistic effect between metals reduces the electron transfer energy, which is beneficial to nitrogen generation.
3.3. Effect of Mn-Ce Ratios on the Catalyst Activity with Oxygen
In order to study the oxygenated flue gas conditions of gas turbines and explore the activity of catalysts in the atmosphere with oxygen, the activity of catalysts with different manganese-cerium ratios was studied with the gas composition of 1000 ppm CO, 500 ppm NO, 15% O2, 5% CO2, and N2 as the balance.
It can be found that, when there is no active component in the catalyst, the CO conversion rate of FA alone in the reaction is low, which is almost the same as the situation under the anoxic condition as shown in Figure 5a. When the catalyst has active components in the reaction, the CO conversion of the catalyst generally increases as the temperature increases. The catalyst with a molar ratio of Mn-Ce of 1:1 shows better activity for CO conversion, and reaches a peak of 59.57% at 500 °C. The two catalysts with Mn-Ce ratios of 1:3 and 3:1 have slightly lower CO conversion than the Mn-Ce 1:1. Compared with anoxic conditions, the CO conversion rate drops slightly in the same temperature range. The reason may be the presence of CO2 in the reaction gas. The increase in concentration of CO2 inhibits the conversion process of CO to CO2, thereby reducing the removal efficiency of CO.
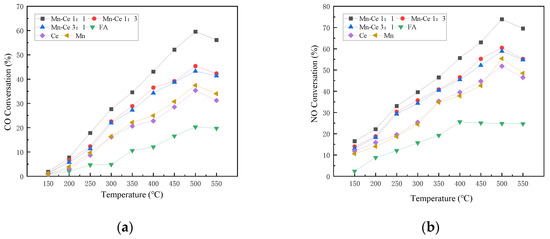
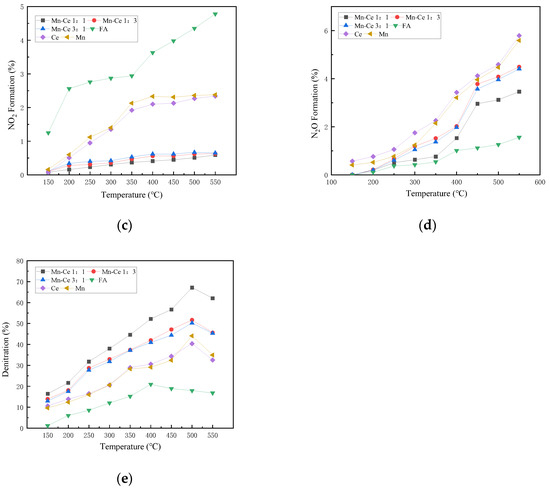
Figure 5.
Influence of different Mn-Ce ratios on catalyst activity. (a) CO Conversion. (b) NO Conversion. (c) NO2 Formation. (d) N2O Formation. (e) Denitration Rate.
In the atmosphere with oxygen, the NO conversion rate is lower when the catalyst has no active components. Over 300 °C, the NO conversion rate rises to 25%, and then keeps around 25% as the temperature rises. The conversion effect of the active component on NO has been significantly improved. The Mn-Ce 1:1 catalyst shows better NO conversion activity. The NO conversion of the catalyst first rises with the temperature increasing, reaches a peak value of 73.9% at 500 °C, and then decreases slightly. The results show that an appropriate ratio of active loading can improve the catalyst performance. Compared with anoxic conditions, the NO conversion rate is significantly improved in the atmosphere with oxygen. The reason may be that oxygen can promote the progress of the catalytic reaction, increase the activity of surface metal, improve the adsorption of NO by the catalyst, and promote the reaction between NO and CO. Some scholars believe that the presence of O2 in the flue gas has a positive effect on the CO-SCR reaction [38]. The dissociated O(ads) will combine with adsorbed NO on the surface of the catalyst to form adsorbed NO2, and then SCR reactions will occur fast. The NO2 participates in the SCR reaction to transform the reaction route into fast SCR reactions to promote the conversion of nitrogen oxides. During the reaction, NO2 undergoes a condensation and disproportionation reaction in the SCR reaction to generate nitrate on the reaction surface, which then reacts with NO to generate nitrite, and then decomposes to form N2. The series of reaction formulas are as follows:
where * stands for active site. Part of NO is converted into NO2 with the participation of oxygen, which improves the conversion rate of NO. When the catalyst has no active component, the amount of NO2 produced increases with the increase in temperature, and the overall trend is consistent with the NO conversion rate of FA, indicating that most of the NO in the reaction is converted to NO2. When the catalyst carries active component, the production rate of NO2 increases slightly with the increase in temperature, but it is significantly lower than that of FA alone. Then, when the temperature reaches the range of 250 to 400 °C, NO2 will participates in fast SCR reactions so as to reduce the formation. At the same time, the formation and desorption of NO2 are carried out at high temperatures (>400 °C), which causes the fluctuation of the NO2 formation rate. The amount of N2O production in the atmosphere with oxygen generally rises with the increase in temperature, and there is little difference among catalysts with different active ratios, with a small increase at over 400 °C.
The trend of the overall denitration efficiency of the catalyst is consistent with that of the NO conversion rate and the value is not much different, indicating that the N2 selectivity of each catalyst is relatively high, and the most NO is converted into nitrogen. The Mn-Ce1:1/FA catalyst shows more excellent denitration performance. The denitration rate is higher and the denitration temperature range is wider. The denitration efficiency of the composite active catalyst is generally higher than that of a single metal, and the denitration temperature range of the composite catalyst is wider than that of a single metal. The denitration temperature range of the Ce/FA catalyst is less than that of the Mn/FA catalyst, which indicates that the two elements have different catalytic activity temperatures for denitration, and the combination of the two elements can effectively broaden the temperature range and improve the overall denitrification performance of the catalyst. Compared with the Mn alone as the active component in the composite catalyst, the introduction of Ce can effectively enhance and broaden the denitration temperature range of the Mn-based catalyst. The synergistic effect of the composite metal can still be used in the presence of oxygen. It can improve the activity of the catalyst. Although the introduction of oxygen can increase the NO conversion rate of the catalyst, at the same time, compared with anoxic condition, the generation of NO2 also increases. The synergistic effect of the composite metal active material can promote the reaction path to the direction of the nitrogen route, which can relatively suppress the generation of NO2, increase the nitrogen selectivity of the catalyst, and improve the denitration efficiency of the catalyst.
From the overall results of the experiment, the Mn-Ce 1:1 catalyst exhibits good CO conversion activity and NO conversion activity in this temperature range under oxygenated and anoxic conditions. At the same time, the NO2 production is small, and the nitrogen selectivity is relatively high, therefore it is a catalyst with high activity.
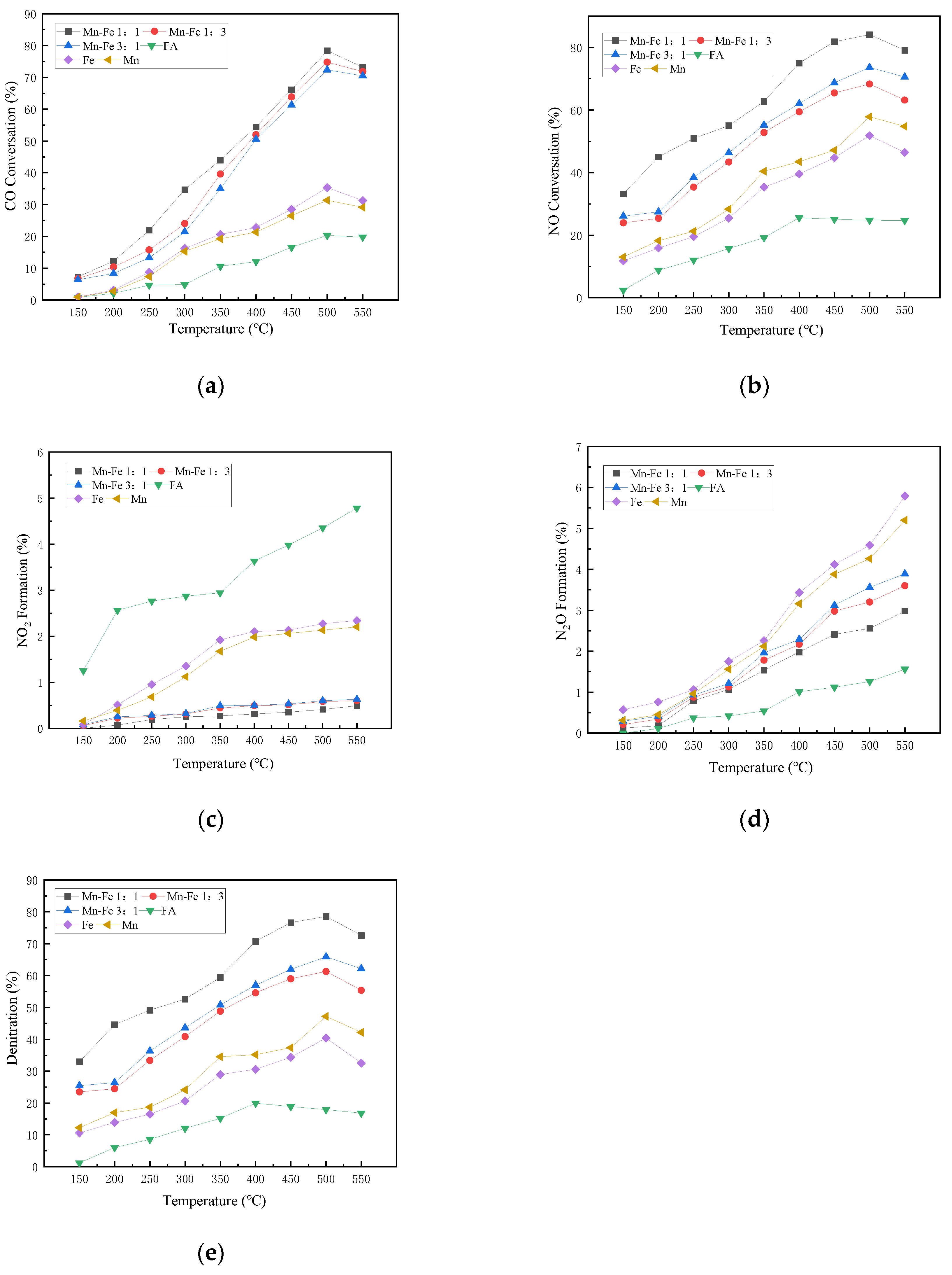
3.4. Effect of Mn-Fe Ratios on the Activity of CO-SCR Catalyst Supported by FA
In the experiment, Mn with superior performance is retained, and Fe with better antioxidant performance is used to replace Ce as the active component to explore the influence of different proportions of metal active components on the activity of the catalyst.
The operation conditions are 1000 ppm CO, 500 ppm NO, 15% O2, 5% CO2, N2 as the balance, and the airspeed of 4000 h−1. The molar ratios of Mn-Fe are 1:1, 1:3, 3:1, with Fe and Mn metals as active controls and FA as blank control.
As shown in Figure 6, the CO conversion efficiency of the Fe-Mn catalyst increases with the increase in temperature, peaking at 500 °C. The overall efficiency of the complex active substance is slightly higher than that of the single metal substance. Compared with the condition without the active component, the activity of the catalyst with the active component increases significantly. In the condition without the active substance, the CO reaction is slightly clear at over 300 °C as the temperature rises, but the overall removal efficiency is not high. Under the condition with active components, the conversion rate of CO increases rapidly from 300 °C to a peak at 500 °C and then declines slowly.
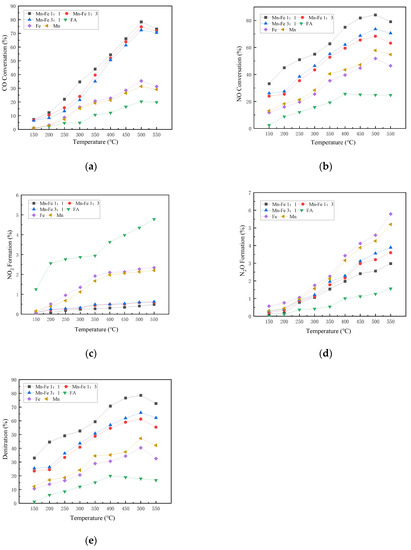
Figure 6.
Influence of different Mn-Fe ratios on catalyst activity. (a) CO Conversion. (b) NO Conversion. (c) NO2 Formation. (d) N2O Formation. (e) Denitration Rate.
We can see that the NO conversion rate of each catalyst rises with the increase in temperature below 500 °C. Among them, the efficiency of the blank catalyst at 500 °C is about 25%, and the Mn-Fe molar ratios of 1:1, 3:1, and 1:3 reach 84.09%, 73.61%, and 68.29% at 500 °C, respectively. The catalyst has shown obvious enhancement effect in NO reaction. The performance of Fe metal alone is better than that of Mn metal. Fe as an auxiliary element can provide better oxygen resistance and improve the catalytic activity of the catalyst under the condition of high concentration of oxygen. However, the more Fe is not the better conversion rate. The NO conversion performance of the Mn-Fe 1:1 catalyst is better than that of the Mn-Fe 1:3 catalyst, which indicates that the synergistic effect of the two metal active compounds shows more superior performance at an appropriate ratio [39].
With no active substance involved in the reaction, the NO2 production increases with the rise in temperature, and begins to slow down at over 450 °C, and starts to fluctuate with increasing temperature. As the active substance participates in the reaction, NO2 increases with the increase in temperature, and Mn-Fe 1:1 is lower than that of blank control. The catalyst can reduce and inhibit the generation of NO2 below 450 °C. The catalyst with Mn-Fe 1:1 shows the lowest NO2 production, and peaks 0.49% at 550 °C.
The generation of N2O increases with the increase in temperature. At over 400 °C, the formation rate of N2O is accelerate. The yield of by-products corresponding to the reaction will increase as the increase in temperature. After 400 °C, the amount of N2O generated by the catalyst will increase. In the high temperature range, the side reaction rate accelerates, resulting in the increase in by-product production. The study of Lu et al. [40] shows that the mechanism route of NO2 generation in low temperature area is mainly L-H route. When the temperature is higher than 250 °C, the reaction mechanism is mainly converted to E-R route, and N2 is generated through decomposition [41].
The addition of Fe can effectively increase the activity of the catalyst under high oxygen conditions. The denitration rate of the catalyst with Mn-Fe ratio of 1:1 reaches 78.56% at 500 °C, and the catalyst also has good N2 selectivity by comparing the NO conversion rate, NO2 generation rate, and N2O generation rate. Compared with other catalysts, Mn-Fe 1:1 is better. The denitration rate of each catalyst decreases at over 500 °C. It may be that, when the temperature is higher than the appropriate reaction temperature, the original internal structure of the catalyst is destroyed, leading to the decline of specific surface and the reduction in activity [42].
On the whole, Fe replaces Ce as the active component, which improves the catalyst activity. The antioxidation activity of Fe in the denitration reaction can increase the NO conversion rate and the denitration rate. Mn-Fe catalysts exhibit higher activity than Fe catalysts, indicating that Fe and Mn metals also have a synergistic effect in the SCR reaction [43]. Appropriate metal ratio can improve the overall activity of the catalyst more effectively
3.5. Effect of Reaction Flue Gas Condition on Catalyst Performance
3.5.1. Effect of Space Velocity on Catalyst Performance
Volumetric space velocity is an important parameter in catalytic reactions. It is the processing amount of the gas flowing per unit time through the unit volume of the catalyst, which reflects the degree of residence of the gas in the catalyst, and the unit is h−1.
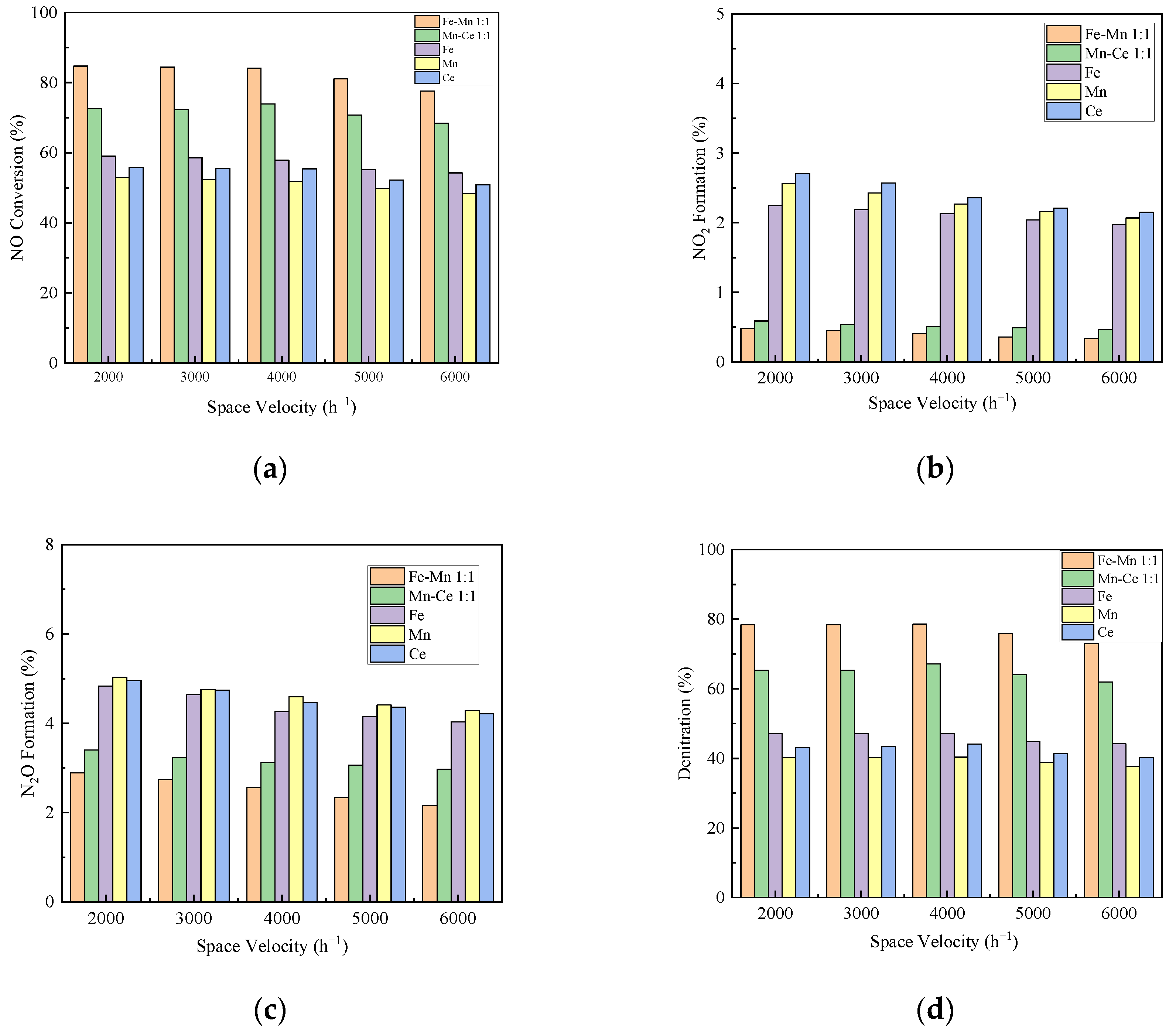
In the atmosphere with oxygen, the denitrification activity of the catalysts with FA as the carrier supporting Mn-Ce 1:1, Mn-Fe 1:1, Mn, Fe, and Ce was studied. The reaction temperature was the optimum temperature of 500 °C, and the space velocity of the experimental catalyst was changed by controlling the gas flow rate.
Figure 7 displays the influence of the space velocity on the denitration performance of the catalyst. It can be seen from Figure 7b,c that the conversion rate of NO2 and N2O of each catalyst decreases with the increase in space velocity. In the range of experimental space velocity, the decreases of NO2 and N2O are within the range of 1%, and 2%, respectively. At 4000 h−1, the NO conversion rates of Mn-Fe 1:1/FA, Mn-Ce 1:1/FA, Fe/FA, Mn/FA, and Ce/FA were 84.09%, 73.91%, 57.83%, 51.82%, and 55.4%, as shown in Figure 7a, respectively. Under the condition of space velocity below 4000 h−1, the conversion rate of NO increases slightly. When the space velocity is greater than 4000 h−1, the conversion rate of NO decreases significantly, and the decrease value is about 7% compared to that of 6000 h−1. The overall denitration performance of the catalyst also shows the same trend as the NO conversion rate. At 4000 h−1, the denitrification rates of Mn-Fe 1:1/FA, Mn-Ce 1:1/FA, Fe/FA, Mn/FA, and Ce/FA are 78.56%, 67.16%, 47.18%, 40.37%, and 44.1%, respectively. This is mainly because when the space velocity value is low, the reaction gas can stay on the catalyst for a long time, which can fully diffuse the gas to the active surface of the catalyst, reduce the interference of external diffusion, and increase the rate of reactions, thereby improving the catalytic efficiency. The increase in space velocity helps the gas diffusion rate inside the catalyst, but will shorten the contact time between the reactant gas and the catalyst. It will lead to the reaction insufficient, and even the reactant gas may flow out of the reaction zone without contacting the catalyst [44]. At the same time, it should be noted that the lower the space velocity is not better. When the space velocity is lower than the appropriate value, the gas will stay in the reaction area for a long time, reflecting the reverse reaction and reducing the catalyst denitration rate [45]. In general, the change in space velocity has a little effect on the denitration efficiency of each component catalyst. This shows that the catalyst prepared in this experiment has good anti-scour ability and adaptability to the flow rate of gas.
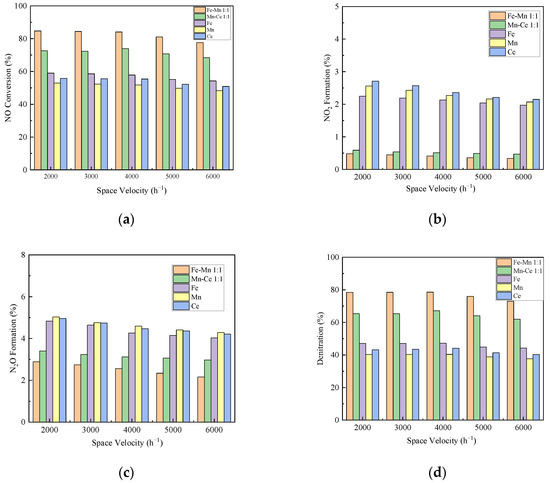
Figure 7.
Influence of space velocity on catalytic efficiency. (a) NO Conversion. (b) NO2 Formation. (c) N2O Formation. (d) Denitration Rate.
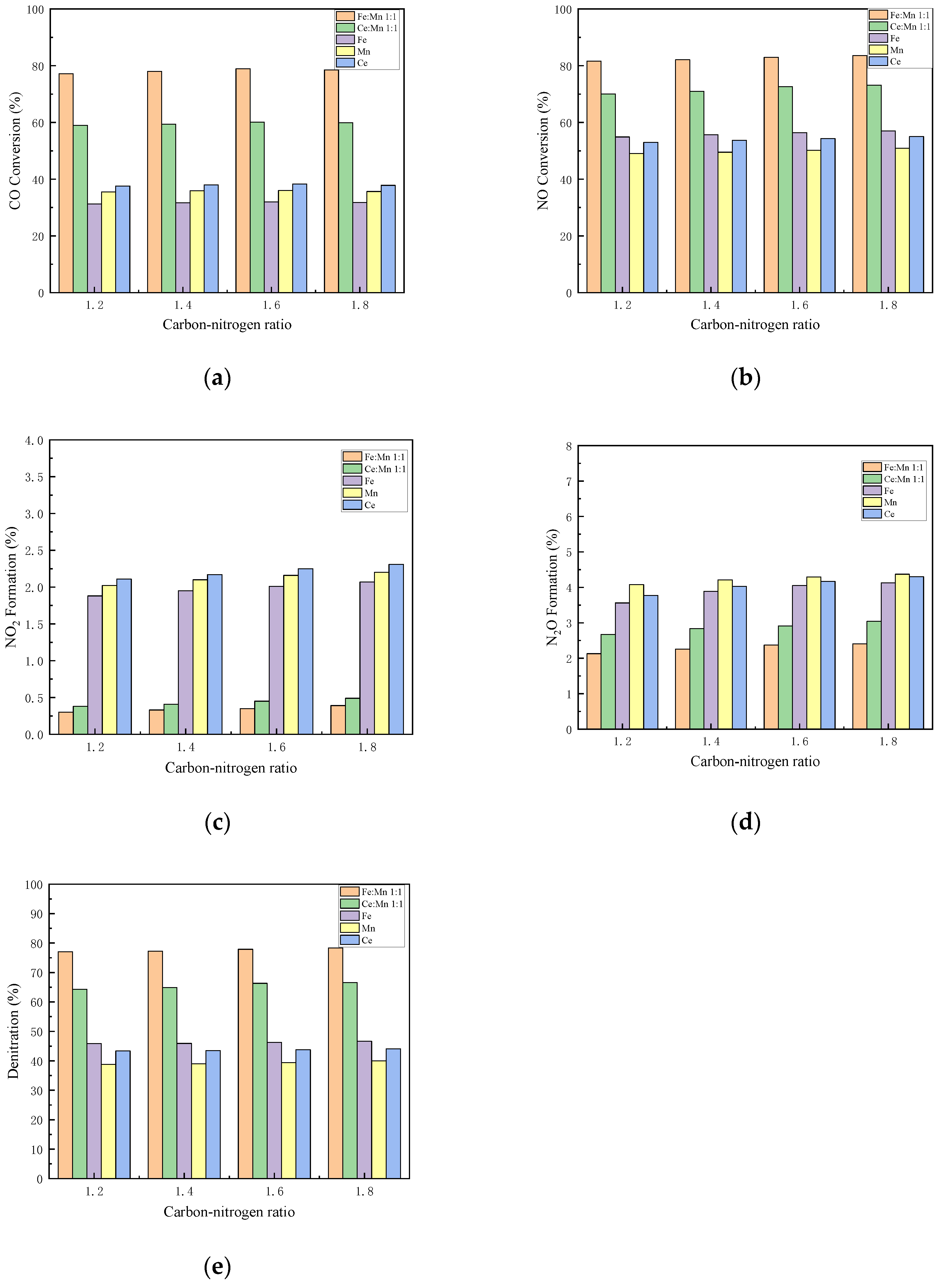
3.5.2. Effect of C/N Ratio on Catalyst Performance
The activity curves of different catalysts under different C/N ratios are shown in Figure 8. This experiment is based on the condition with oxygen and 500 ppm NO. By changing the CO concentration of flue gas, at C/N ratios of 1.2, 1.4, 1.6, and 1.8, the denitration performances of Mn-Fe 1:1/FA, Mn-Ce 1:1/FA, Fe/FA, Mn/FA, and Ce/FA catalysts at 500 °C were studied, respectively.
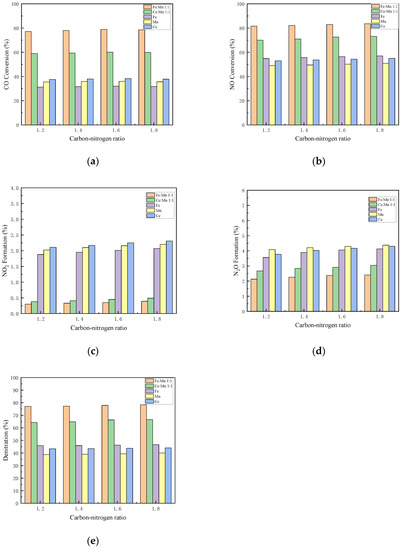
Figure 8.
Influence of C/N ratio on the performance of each catalyst. (a) CO Conversion. (b) NO Conversion. (c) NO2 Formation. (d) N2O Formation. (e) Denitration Rate.
When the C/N ratio is less than 1.6, the CO conversion rate rises as the C/N ratio increases. At the C/N ratio of 1.6, the CO conversion rates of Mn-Fe 1:1/FA, Mn-Ce 1:1, Fe/FA, Mn/FA, and Ce/FA catalysts peak 78.96%, 60.13%, 31.95%, 36.01%, and 38.25%, respectively. However, when the C/N ratio is greater than 1.6, the CO conversion rate decreases, which may be because the part of CO directly flows through the catalyst without participating in the reaction, leading to the overall CO conversion rate declining, and the excess of CO emission.
The conversion rates of NO increase with the increase in C/N ratio. The NO conversion rates of Mn-Fe 1:1/FA, Mn-Ce 1:1, Fe/FA, Mn/FA, and Ce/FA catalysts are, respectively, 83.56%, 73.15%, 57.01%, 50.94%, and 55% at the peaks. However, when the ratio of C/N exceeds 1.6, the increase in conversion rate is not obvious, indicating that the NO reaction is close to full reaction when the C/N ratio exceeds 1.6. The overall trend of NO2 production is almost the same as that of NO. The increase in NO2 production increases at over 1.6, but the increase in NO2 production does not exceed 0.5%. The trend of N2O increases with the increase in C/N ratio, just like the trend of NO2, but the increase is also not obvious. As the C/N ratio exceeds 1.6, the N2 selectivity of the catalyst reaction decreases, and the reaction tends to generate N2O and NO2, and the denitrification rate of the reaction shows the maximum value when C/N ratio is 1.6.
According to the reaction mechanism [46], in the CO-SCR reaction, CO is first adsorbed by the catalyst, and then decomposed on the surface of the catalyst or in the pores of the catalyst at an adsorbed state, and then undergoes a redox reaction with the adsorbed NO. After the reaction produces CO2 and N2, the gas is dissociated and released. The increase in the concentration of CO helps the forward reaction of CO in the adsorption and dissociation process, and the increase in the amount of CO adsorbed and dissociated on the catalyst increases the collision probability of adsorbed ions and accelerates the reaction of adsorbed NO, which in turn promotes the overall progress of the NO reaction in the reaction toward the forward reaction. However, when the amount of CO is too much, the amount of CO that can be over-adsorbed by the catalyst itself, but the amount of CO that can be reacted with NO are limited. When the concentration of CO exceeds the maximum required for the reaction, the excess CO will not be adsorbed. After that, it is directly released without participating in the reaction, resulting in a decrease in the conversion rate of CO. Moreover, the excessively adsorbed CO will occupy a large number of adsorption sites, affecting the adsorption of NO, resulting in a decrease in nitrogen selectivity.
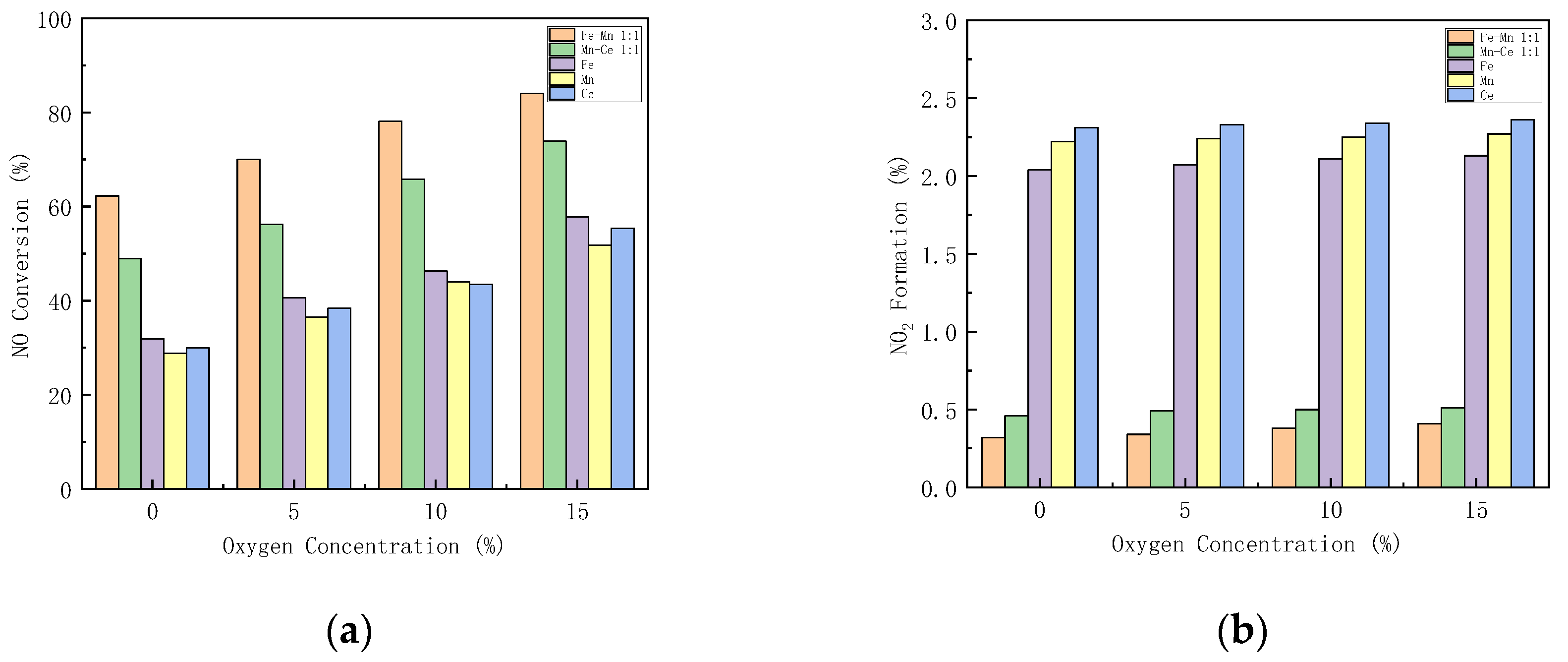
3.5.3. Effect of Oxygen Concentration on Catalyst Performance
The excess air coefficient in the gas turbine combustion chamber is large, and there is still about 15% oxygen in the flue gas. Figure 9 shows how different oxygen concentration influences the performance of the catalysts. The performance of Mn-Fe 1:1/FA, Mn-Ce 1:1/FA, Fe/FA, Mn/FA, and Ce/FA catalysts at different oxygen concentrations is studied.
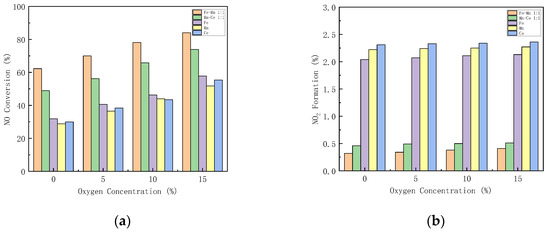
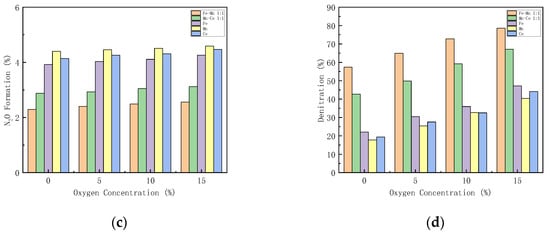
Figure 9.
Influence of different oxygen concentrations on CO-SCR catalyst. (a) NO Conversion. (b) NO2 Formation. (c) N2O Formation. (d) Denitration Rate.
With the increase in oxygen concentration, the NOx conversion rate increases. When the oxygen concentration is lower than 5%, the NOx conversion rate increases rapidly. When the oxygen concentration is higher than 5%, the growth rate decreases (Figure 9a). This reflects that oxygen can play a catalytic role in SCR. Under the condition of 15% oxygen concentration, the NO conversion rates of Mn-Fe 1:1/FA, Mn-Ce 1:1/FA, Fe/FA, Mn/FA, and Ce/FA were 84.09%, 73.91%, 57.83%, 51.82%, and 55.4%, respectively. NO2 formation rises with the increase in oxygen concentration. The introduction of oxygen promotes the production of NO2. The production of N2O increases with the increase in oxygen concentration, but the overall range is within 2%. The denitration rate of the catalyst increases with the increase in oxygen concentration. The denitration rate of the catalyst increases significantly after the oxygen concentration is 5%, and the trend is similar to the NO conversion rate, but the increase in oxygen concentration will decrease the N2 selectivity in the SCR reaction.
Studies [47] have shown that the reaction of oxygen on the catalyst first requires being diffused and adsorbed on the surface of the catalyst, and then fixation and adsorption on the active sites of the catalyst improve to form adsorbed oxygen molecules, which participate in the catalytic reaction at an adsorbed state. The introduction of oxygen, on the one hand, reacts with NO as a gas state, or reacts with NO on the surface of the catalyst in an adsorbed state to generate NO2, which promotes the reaction of part of NO to generate NO2 reacting with CO, so that the SCR reaction path is transformed into a fast SCR reaction path, increasing the reaction path, the reaction rate, and the activity of the catalyst. However, on the other hand, the oxygen in the reaction will compete with the other reaction gases for active sites, resulting in the reduction in the active sites of CO and NO, which hinders the progress of the SCR reaction. Too high oxygen concentration will increase the conversion path of NO, generate more NO2 and N2O, reduce nitrogen selectivity, and lead to catalyst degradation [48]. Therefore, the oxygen concentration in the catalytic system needs to be maintained in an appropriate range to help maintain the activity of the catalyst.
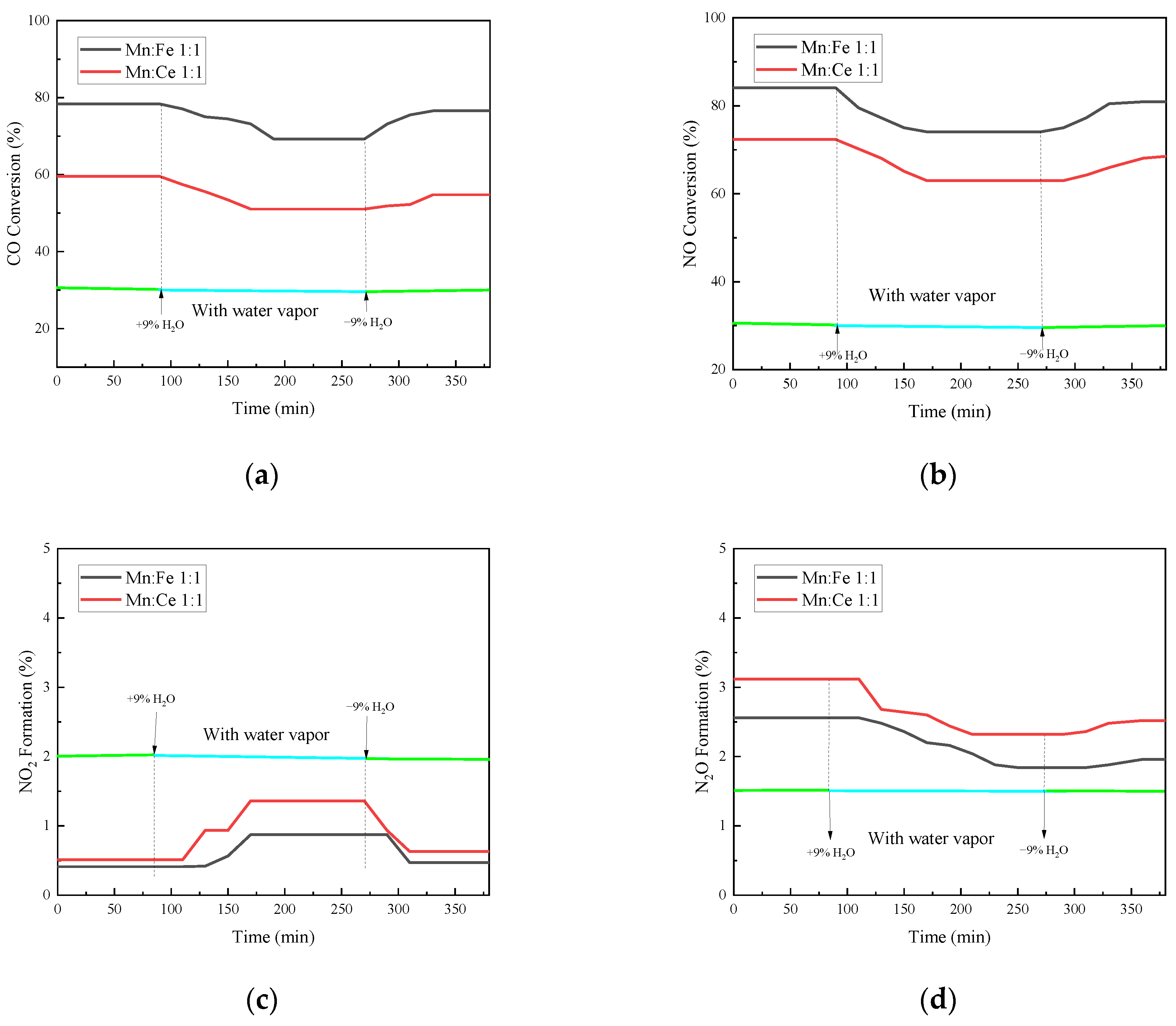
3.5.4. Study on Water Resistance of Catalyst
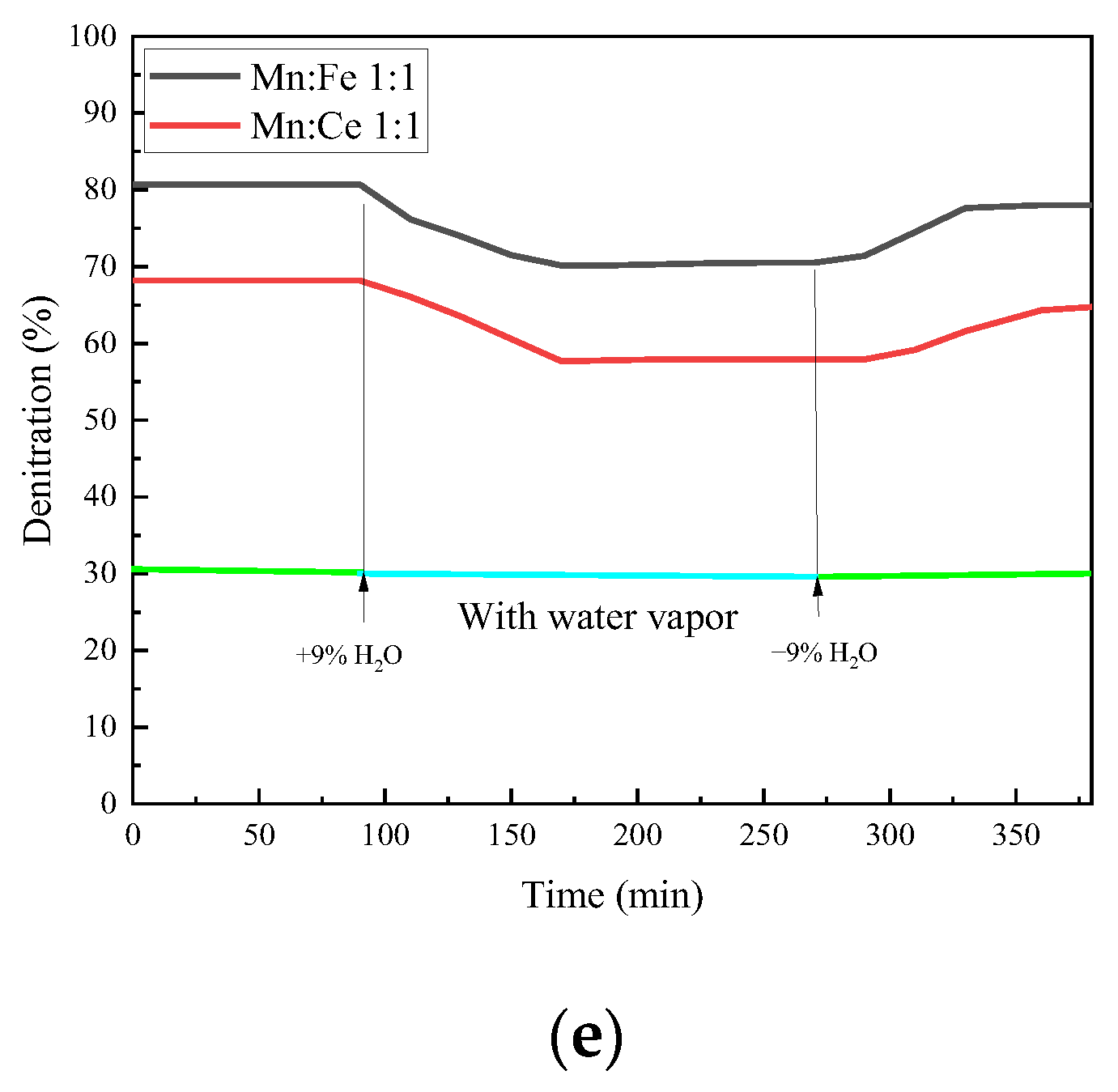
In order to simulate the actual operating atmosphere of a gas turbine, water vapor needs to be added to the atmosphere in the experiment to test the water resistance of the catalyst. The gas turbine used in the industry is mainly F-class gas turbine flue gas, and the water vapor concentration is 5–10%. Therefore, 9% water vapor concentration is selected as the concentration of water resistance experiment in this study. The other conditions were the same as the oxygenated conditions, 1000 ppm CO, 500 ppm NO, 15% O2, and N2 as the balance. Mn-Ce 1:1 and Mn-Fe 1:1 are selected as catalysts. Water resistance test is carried out for the experimental catalyst. At the 80th minute, 9% water vapor is injected, and the water vapor is stopped at 270th minute.
As shown in Figure 10, the CO conversion rate has a trend of decline after a period of reaction after steam inlet, with a decrease of 9.0% compared with that before steam inlet. After the water vapor is stopped, the conversion rate rises to some extent, but it is still lower than that without the water vapor. The inhibition and passivation effect of vapor on the CO conversion rate of the catalyst is irreversible within a certain range. The NO conversion efficiency of the two groups of catalysts decreases with the passage of water vapor, and the decrease rate reaches 10%. The effect of water vapor on the adsorption and removal of NO is more obvious than that of CO. It is possible that the competition of water vapor on the active site of NO is greater than that of CO. The N2O production and NO conversion of the catalyst show a similar trend after the injection of water vapor, both of which decrease. From the series of reactions in the previous section, the generation of N2O is related to the adsorption and decomposition of NO, which is the previous step of N2O generation. Therefore, the changes of N2O and NO show a consistent trend. However, the effect of water vapor on the amount of N2O formation is relatively limited because the total amount of N2O formation is small. The reaction of NO2 generation is also affected to a small extent under water vapor, but the overall effect is not significant. The injection of water vapor at 500 °C will slightly increase the production of NO2. The introduction of water vapor can increase the formation of NO2 in the reaction and promote the conversion of NO to NO2. When the water vapor is stopped, the production of NO2 slowly returns to the amount before the water vapor is injected. This indicates that the formation of NO2 by water vapor is relatively reversible.
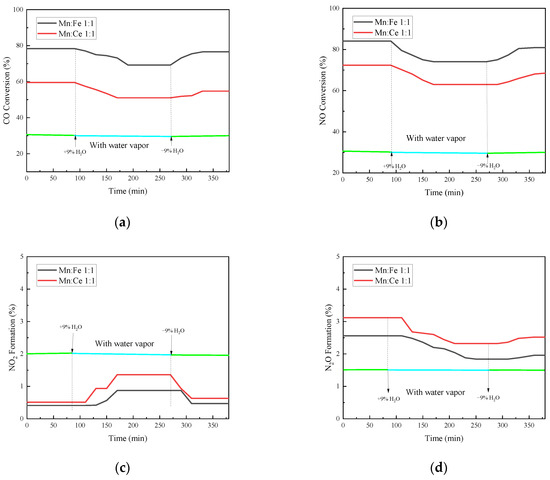
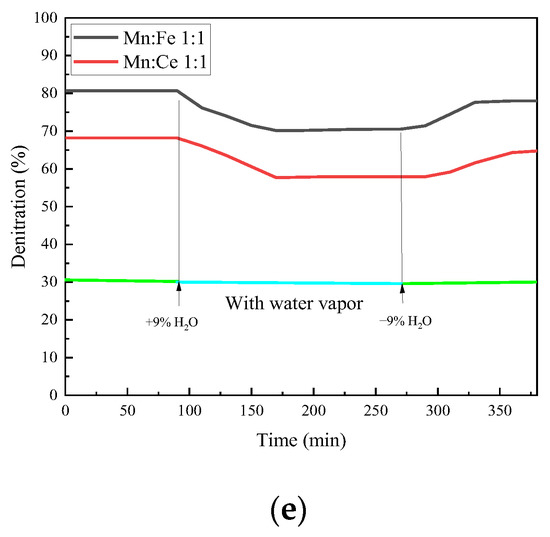
Figure 10.
Water resistance test of catalyst supported by fly ash. (a) CO Conversion. (b) NO Conversion. (c) NO2 Formation. (d) N2O Formation. (e) Denitration Rate.
The overall denitration efficiency of the catalysts decreases with the addition of water vapor, and even if water vapor is stopped, the denitration rate of the two groups of catalysts fails to return to before injection. The hindrance of water vapor to the two groups of catalysts has different degree of irreversibility. At the same time, it can be noted that the addition of water vapor not only reduces the NO conversion rate, but also increases the generation of NO2 in a small range, which reduces the N2 selectivity of the catalyst. However, in general, the denitrification and decarburization performance of each catalyst did not change much before and after water vapor injection. The catalyst in this experiment has a certain water resistance.
The water vapor inhibits the catalyst mainly from two aspects [49,50]. On the one hand, water vapor will cover the surface of the catalyst to form a water vapor film, especially the metal active site, hinder the contact between the catalyst and the reaction gas, directly reduce the effective catalytic area, and reduce the activity of the catalyst. However, in this case, the structure of the catalyst will not be affected, and water vapor does not participate in the catalytic reaction. After stopping water vapor injection and heating the catalyst for a period of time, the water vapor film on the catalyst surface will release off with the gas, and the metal active sites can be restored. On the other hand, the water that occupies the surface of the catalyst will hydrolyze on the surface of the catalyst to generate hydroxyl groups, occupy the active sites on the catalyst surface, and compete with the other gases for active metal sites, resulting in the reduction in the active sites and the catalytic activity. It will not be eliminated with the stop of water vapor injection, which is an irreversible effect. Therefore, in the catalytic system, the water vapor will affect the denitration efficiency of the catalyst. In the application of the catalytic system, the removal of water vapor should be placed before the catalytic system [51].
4. Conclusions
This research is mainly based on the background of gas turbine, and the relevant performance of the CO-SCR catalyst has been experimentally explored. The main conclusions are as follows:
(i) The activity of the catalyst is affected by different calcination atmospheres. The calcination of the catalyst in a reducing atmosphere helps to reduce the valence of the active metal on the catalyst surface, thereby increasing the activity of the catalyst. Among the Mn-Ce/FA catalysts with different ratios, the catalyst with the best denitration is the Mn-Ce 1:1/FA catalyst.
(ii) The denitration performance of the Fe/FA catalyst is better than that of Mn/FA and Ce/FA in the atmosphere with oxygen. The replacement of Ce by Fe as a load can improve the performance of the catalyst. Among them, the overall performance of the Mn-Fe 1:1 catalyst is the best.
(iii) The conversion rate of NO and the generation rate of NO2 and N2O decrease with the increase in the space velocity, and the reduction rate of each space velocity has a little difference. The main factor affecting space velocity resistance may be the material structure of the carrier itself.
(iv) When the C/N ratio is less than 1.6, the CO conversion rate increases with the rise in the C/N ratio. When the C/N ratio is greater than 1.6, the CO conversion rate decreases instead. This shows that when the concentration of CO in the reaction is slightly higher than that of NO, it will promote the SCR reaction, but the excess CO will escape and affect the environment.
(v) The NO conversion rate increases significantly with the addition of oxygen, but NO2 generation also increases. The trend of denitration rate is almost the same as that of NO conversion rate, but the introduction of oxygen reduces the N2 selectivity of the catalyst.
(vi) The catalyst activity decreases with the addition of water vapor. After the water vapor injection is stopped, the denitration efficiency of the two groups of catalysts could not be restored to the state before injection, which shows that the obstacle of water vapor to the catalyst is irreversible. In general, the activity of the catalyst decreases slightly, so it has a certain degree of water resistance.
(vii) Since the experiment is carried out under the conditions of simulated flue gas composition, it may be different from the actual gas turbine flue gas conditions, and there may be influences such as unburned tiny particles. Furthermore, research on catalyst molding is needed to facilitate practical industrial applications.
Author Contributions
Conceptualization, X.G.; methodology, X.G., Z.X., R.Z., Y.L. and Z.L.; validation, Z.X., R.Z., J.D. and Y.L.; formal analysis, Z.X., R.Z. and Z.L.; investigation, Z.X., R.Z. and J.D.; resources, Z.X., Y.L. and Z.L.; data curation, Z.X. and R.Z.; writing—original draft preparation, Z.X. and R.Z.; writing—review and editing, Y.L., Z.L. and X.G.; visualization, Z.X., R.Z. and J.D.; supervision, X.G.; project administration, J.D. and X.G.; funding acquisition, X.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Tianjin Science and Technology Plan Project (18YFCZZC00080).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Damma, D.; Ettireddy, P.R.; Reddy, B.M.; Smirniotis, P.G. A review of low temperature NH3-SCR for removal of NOx. Catalysts 2019, 9, 349. [Google Scholar] [CrossRef]
- Guo, K.; Ji, J.; Song, W.; Sun, J.; Tang, C.; Dong, L. Conquering ammonium bisulfate poison over low-temperature NH3-SCR catalysts: A critical review. Appl. Catal. B Environ. 2021, 297, 120388. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Wang, Y.; Yin, Y.; Pan, J.; Zhang, J.; Wang, Q. Simultaneous absorption of SO2 and NO from flue gas using ultrasound/Fe2+/heat coactivated persulfate system. J. Hazard. Mater. 2018, 342, 326–334. [Google Scholar] [CrossRef]
- Liu, F.; Cai, M.; Liu, X.; Zhu, T.; Zou, Y. O3 oxidation combined with semi-dry method for simultaneous desulfurization and denitrification of sintering/pelletizing flue gas. J. Environ. Sci. 2021, 104, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Huan, C.; Yan, Z.; Sun, J.; Liu, Y.; Zeng, Y.; Qin, W.; Cheng, Y.; Tian, X.; Tan, Z.; Lyu, Q. Nitrogen removal characteristics of efficient heterotrophic nitrification-aerobic denitrification bacterium and application in biological deodorization. Bioresour. Technol. 2022, 363, 128007. [Google Scholar] [CrossRef] [PubMed]
- Van Roekel, C.A.; Montgomery, D.T.; Singh, J.; Olsen, D.B. Analysis of Non-Selective Catalyst Reduction Performance with Dedicated Exhaust Gas Recirculation. Adv. Chem. Eng. Sci. 2022, 12, 114–129. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Tian, J.; Zhong, Y.; Zou, Z.; Dong, R.; Gao, S.; Xu, W.; Tan, D. The effects of Mn-based catalysts on the selective catalytic reduction of NOx with NH3 at low temperature: A review. Fuel Process. Technol. 2022, 230, 107213. [Google Scholar] [CrossRef]
- Gou, X.; Zhang, R.; Xu, G.; Zhao, D. Comparative study of low temperature denitration performance of Mn-Ce/ACFA-TiO2 catalysts under oxy-fuel and air-fuel combustion flue gases. Energy Rep. 2020, 6, 1545–1552. [Google Scholar] [CrossRef]
- Kubota, H.; Toyao, T.; Maeno, Z.; Inomata, Y.; Murayama, T.; Nakazawa, N.; Inagaki, S.; Kubota, Y.; Shimizu, K. Analogous mechanistic features of NH3-SCR over vanadium oxide and copper zeolite catalysts. ACS Catal. 2021, 11, 11180–11192. [Google Scholar] [CrossRef]
- Liu, X.; Sui, Z.; Chen, H.; Chen, Y.; Liu, H.; Jiang, P.; Shen, Z.; Linghu, W.; Wu, X. Structures and catalytic performances of Me/SAPO-34 (Me = Mn, Ni, Co) catalysts for low-temperature SCR of NOx by ammonia. J. Environ. Sci. 2021, 104, 137–149. [Google Scholar] [CrossRef]
- Tian, J.; Li, Y.; Zhou, X.; Yao, Y.; Wang, D.; Dan, J.; Dai, B.; Wang, Q.; Yu, F. Overwhelming low ammonia escape and low temperature denitration efficiency via MnOx-decorated two-dimensional MgAl layered double oxides. Chin. J. Chem. Eng. 2020, 28, 1925–1934. [Google Scholar] [CrossRef]
- Fal Desai, M.; Kerkar, R.; Salker, A. Detoxification of NO and CO gases over effectively substituted Pd and Rh in cupric oxide catalysts. Int. J. Environ. Sci. Technol. 2019, 16, 1541–1550. [Google Scholar] [CrossRef]
- Ji, Y.; Chen, X.; Liu, S.; Song, S.; Xu, W.; Jiang, R.; Chen, W.; Li, H.; Zhu, T.; Li, Z. Tailoring the Electronic Structure of Single Ag Atoms in Ag/WO3 for Efficient NO Reduction by CO in the Presence of O2. ACS Catal. 2023, 13, 1230–1239. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Liu, Y.; Lian, D.; Chen, M.; Ji, Y.; Xing, L.; Wu, K.; Liu, S. Recent Advances of Cu-Based Catalysts for NO Reduction by CO under O2-Containing Conditions. Catalysts 2022, 12, 1402. [Google Scholar] [CrossRef]
- Murrell, L.; Tauster, S.; Anderson, D. Laser Raman Characterization of Surface Phase Precious Metal Oxides Formed on CeO2, in Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1991; Volume 71, pp. 275–289. [Google Scholar]
- Ogura, M.; Kawamura, A.; Matsukata, M.; Kikuchi, E. Catalytic activity of Ir for NO-CO reaction in the presence of SO2 and excess oxygen. Chem. Lett. 2000, 29, 146–147. [Google Scholar] [CrossRef]
- Zhao, R.; Wei, X.; Chu, B.; Chen, K.; Qin, Q.; Liu, H.; Zhou, Y.; Li, B.; Dong, L. Multi-phase coexisting metal oxide derived by MOFs for the CO-SCR reaction at low temperature and in situ DRIFTS study on reaction mechanism. Appl. Surf. Sci. 2022, 580, 1269–1275. [Google Scholar] [CrossRef]
- Oton, L.F.; Oliveira, A.C.; de Araujo, J.C.; Araujo, R.S.; de Sousa, F.F.; Saraiva, G.D.; Lang, R.; Otubo, L.; da Silva Duarte, G.C.; Campos, A. Selective catalytic reduction of NOx by CO (CO-SCR) over metal-supported nanoparticles dispersed on porous alumina. Adv. Powder Technol. 2020, 31, 464–476. [Google Scholar] [CrossRef]
- Delpeuch, A.B.; Maillard, F.; Chatenet, M.; Soudant, P.; Cremers, C. Ethanol oxidation reaction (EOR) investigation on Pt/C, Rh/C, and Pt-based bi-and tri-metallic electrocatalysts: A DEMS and in situ FTIR study. Appl. Catal. B Environ. 2016, 181, 672–680. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Kang, M.; Chen, Z.; Gao, S.; Hao, H. Insights into high CO-SCR performance of CuCoAlO catalysts derived from LDH/MOFs composites and study of H2O/SO2 and alkali metal resistance. Chem. Eng. J. 2021, 426, 131873. [Google Scholar] [CrossRef]
- Liu, H.; Liang, Q.; Liu, J.; Liu, X.; Li, D.; Xie, S.; Jin, L.; Dong, L.; Li, B.; Yao, Y. Promotional mechanism of activity via three-dimensional ordered macroporous Cu-doped Ce–Fe mixed oxides for the CO-SCR reaction. Environ. Sci. Nano 2020, 7, 3136–3154. [Google Scholar] [CrossRef]
- Yan, X.; Liu, J.; Yang, Y.; Wang, Z.; Zheng, Y. A catalytic reaction scheme for NO reduction by CO over Mn-terminated LaMnO3 perovskite: A DFT study. Fuel Process. Technol. 2021, 216, 106798. [Google Scholar] [CrossRef]
- Kacimi, M.; Ziyad, M.; Liotta, L.F. Cu on amorphous AlPO4: Preparation, characterization and catalytic activity in NO reduction by CO in presence of oxygen. Catal. Today 2015, 241, 151–158. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, C.; Cheng, X.; Wang, Z. Performance of Fe-Ba/ZSM-5 catalysts in NO+ O2 adsorption and NO+ CO reduction. Int. J. Hydrogen Energy 2017, 42, 7077–7088. [Google Scholar] [CrossRef]
- Liu, T.; Qian, J.; Yao, Y.; Shi, Z.; Han, L.; Liang, C.; Li, B.; Dong, L.; Fan, M.; Zhang, L. Research on SCR of NO with CO over the Cu0. 1La0. 1Ce0. 8O mixed-oxide catalysts: Effect of the grinding. Mol. Catal. 2017, 430, 43–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Chen, Z.; Li, X. Promotional effect for SCR of NO with CO over MnO -doped Fe3O4 nanoparticles derived from metal-organic frameworks. Chin. J. Chem. Eng. 2022, 46, 113–125. [Google Scholar] [CrossRef]
- Liu, L.; Xu, K.; Su, S.; He, L.; Qing, M.; Chi, H.; Liu, T.; Hu, S.; Wang, Y.; Xiang, J. Efficient Sm modified Mn/TiO2 catalysts for selective catalytic reduction of NO with NH3 at low temperature. Appl. Catal. A Gen. 2020, 592, 117413. [Google Scholar] [CrossRef]
- Wang, C.; Yu, F.; Zhu, M.; Tang, C.; Zhang, K.; Zhao, D.; Dong, L.; Dai, B. Highly selective catalytic reduction of NOx by MnOx–CeO2–Al2O3 catalysts prepared by self-propagating high-temperature synthesis. J. Environ. Sci. 2019, 75, 124–135. [Google Scholar] [CrossRef]
- Zhu, C.; Shen, R.; Fang, Z.; Zhang, L.; Wu, D.; Wu, M.; Tang, T.; Fu, W.; Chen, Q. Basic mesoporous zeolite ETS-10 supported Ni catalyst with bi-functional properties for efficiently catalyzing arenes fluorination. Catal. Commun. 2018, 117, 63–68. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, L.; Qin, Y.; Chen, B. Structure and denitration performance of carbon-based catalysts prepared from Cu–BTC precursor. Trans. Nonferrous Met. Soc. China 2018, 28, 980–988. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Chen, L.; Ma, B.; Zhang, Y.; Ni, W.; Tsang, D.C. Treatment of municipal solid waste incineration fly ash: State-of-the-art technologies and future perspectives. J. Hazard. Mater. 2021, 411, 125132. [Google Scholar] [CrossRef]
- Zou, D.; Qiu, M.; Chen, X.; Drioli, E.; Fan, Y. One step co-sintering process for low-cost fly ash based ceramic microfiltration membrane in oil-in-water emulsion treatment. Sep. Purif. Technol. 2019, 210, 511–520. [Google Scholar] [CrossRef]
- Lei, Z.; Hao, S.; Yang, J.; Zhang, L.; Fang, B.; Wei, K.; Lingbo, Q.; Jin, S.; Wei, C. Study on denitration and sulfur removal performance of Mn–Ce supported fly ash catalyst. Chemosphere 2021, 270, 128646. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.L.; Young, C.W.; Pan, G.T.; Chang, M.B. Catalytic reduction of NO by CO with Cu-based and Mn-based catalysts. Catal. Today 2020, 348, 15–25. [Google Scholar] [CrossRef]
- Liu, T.; Wei, L.; Yao, Y.; Dong, L.; Li, B. La promoted CuO-MnOx catalysts for optimizing SCR performance of NO with CO. Appl. Surf. Sci. 2021, 546, 148971. [Google Scholar] [CrossRef]
- Zhdanov, V.P.; Kasemo, B. Mechanism and kinetics of the NO-CO reaction on Rh. Surf. Sci. Rep. 1997, 29, 31–90. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Liu, Y.; Xu, H.; Wu, Z. A rational construction of Pt@ Cu-ZSM-5@ CuOx catalyst with detached multi-functional Pt and Cu sites for high-performance acetonitrile decomposition. Chem. Eng. J. 2023, 463, 142343. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Duan, J.; Bi, S. Insights into deNOx processing over Ce-modified Cu-BTC catalysts for the CO-SCR reaction at low temperature by in situ DRIFTS. Sep. Purif. Technol. 2020, 234, 2568. [Google Scholar] [CrossRef]
- Pan, K.; Yu, F.; Liu, Z.; Zhou, X.; Sun, R.; Li, W.; Zhao, H.; Liu, M.; Guo, X.; Dai, B. Enhanced low-temperature CO-SCR denitration performance and mechanism of two-dimensional CuCoAl layered double oxide. J. Environ. Chem. Eng. 2022, 10, 108030. [Google Scholar] [CrossRef]
- Suhong, L.; Fan, W.; Chen, C.; Huang, F.; Li, K. Catalytic oxidation of formaldehyde over CeO2-Co3O4 catalysts. J. Rare Earths 2017, 35, 867–874. [Google Scholar]
- Pan, Y.; Li, N.; Li, K.; Ran, S.; Wu, C.; Zhou, Q.; Liu, J.; Li, S. Enhanced low-temperature behavior of selective catalytic reduction of NOx by CO on Fe-based catalyst with looping oxygen vacancy. Chem. Eng. J. 2023, 461, 141814. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, X.; Wang, Z.; Ma, C.; Qin, Y. Investigation on Fe-Co binary metal oxides supported on activated semi-coke for NO reduction by CO. Appl. Catal. B Environ. 2017, 201, 636–651. [Google Scholar] [CrossRef]
- Han, Y.; Wen, B.; Zhu, M.; Dai, B. Lanthanum incorporated in MCM-41 and its application as a support for a stable Ni-based methanation catalyst. J. Rare Earths 2018, 36, 367–373. [Google Scholar] [CrossRef]
- Guo, M.; Liu, Q.; Liu, C.; Wang, X.; Bi, Y.; Fan, B.; Ma, D.; Liang, X.; Li, Z. Rational design of novel CrZrOx catalysts for efficient low temperature SCR of NOx. Chem. Eng. J. 2021, 413, 127554. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, T.; Guo, Y.; Wang, J.; Wei, J.; Yu, Q. Recent advances in simultaneous removal of SO2 and NOx from exhaust gases: Removal process, mechanism and kinetics. Chem. Eng. J. 2021, 420, 127588. [Google Scholar] [CrossRef]
- Li, J.; Zhu, J.; Fu, S.; Tao, L.; Chu, B.; Qin, Q.; Wang, J.; Li, B.; Dong, L. Insight into copper-cerium catalysts with different Cu valence states for CO-SCR and in-situ DRIFTS study on reaction mechanism. Fuel 2023, 339, 126962. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, F.; Dong, D.; Gui, R.; Li, W.; Sun, R.; Wan, Y.; Dan, J.; Wang, Q.; Dai, B. Transition-metal-doped ceria carried on two-dimensional vermiculite for selective catalytic reduction of NO with CO: Experiments and density functional theory. Appl. Surf. Sci. 2021, 566, 20–23. [Google Scholar] [CrossRef]
- Heck, R.M. Catalytic abatement of nitrogen oxides–stationary applications. Catal. Today 1999, 53, 519–523. [Google Scholar] [CrossRef]
- Niu, C.; Shi, X.; Liu, K.; You, Y.; Wang, S.; He, H. A novel one-pot synthesized CuCe-SAPO-34 catalyst with high NH3-SCR activity and H2O resistance. Catal. Commun. 2016, 81, 20–23. [Google Scholar] [CrossRef]
- Gui, R.; Yan, Q.; Xue, T.; Gao, Y.; Li, Y.; Zhu, T.; Wang, Q. The promoting/inhibiting effect of water vapor on the selective catalytic reduction of NOx. J. Hazard. Mater. 2022, 439, 129665. [Google Scholar] [CrossRef]
- Souza, M.S.; Martins, A.J.; Ribeiro, J.A.S.; Campos, A.; Oliveira, A.C.; Juca, R.F.; Saraiva, G.D.; Torres, M.A.M.; Rodriguez-Castellon, E.; Araujo, R.S. Selective Catalytic Reduction of NOx by CO over Cu (Fe)/SBA-15 Catalysts: Effects of the Metal Loading on the Catalytic Activity. Catalysts 2023, 13, 527. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).