Bioprocessing of Waste for Renewable Chemicals and Fuels to Promote Bioeconomy
Abstract
1. Introduction
2. Feedstock and Optimization Strategies
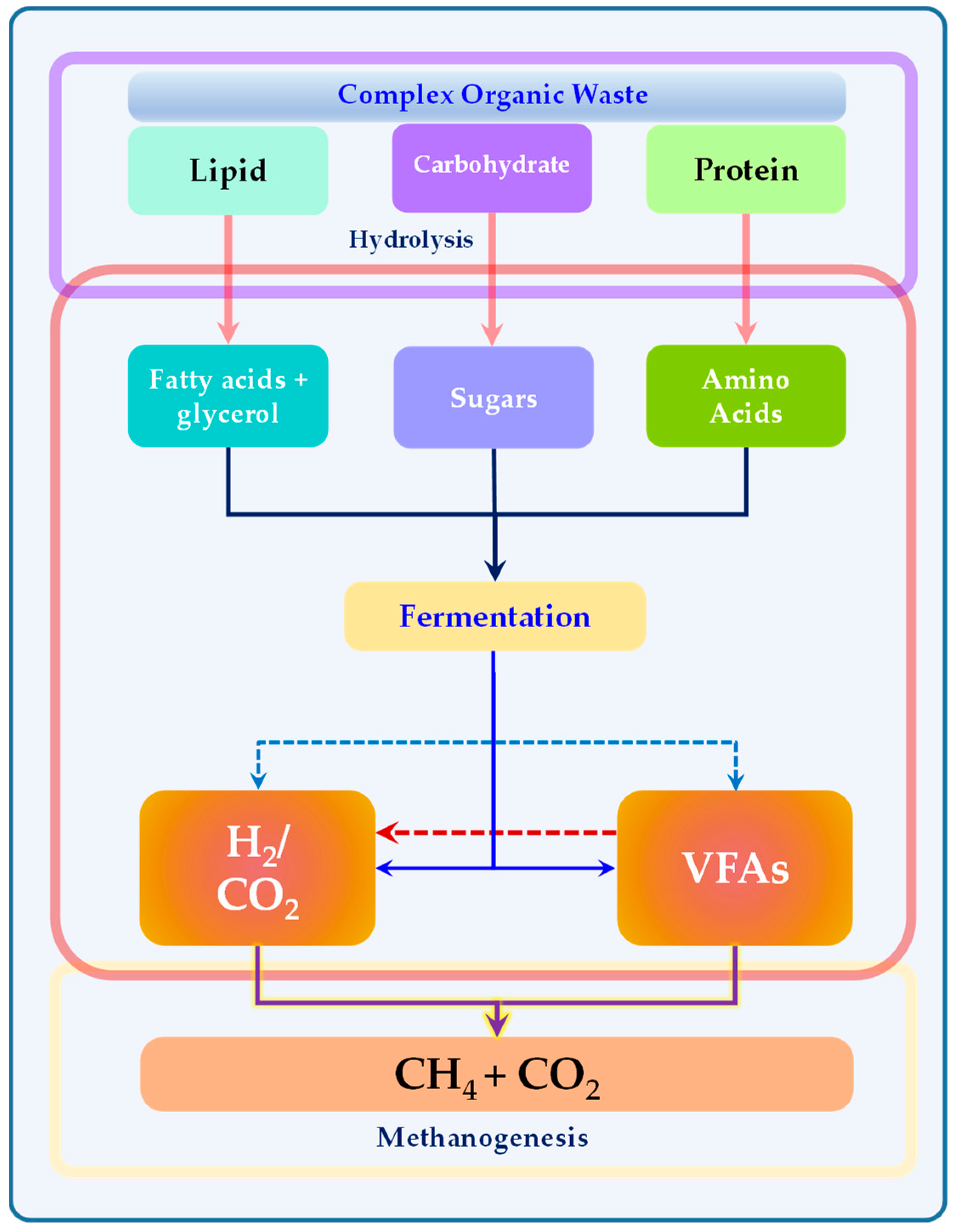
2.1. Organic Waste
2.2. Optimization Strategies
3. Anaerobic Fermentation or Dark Fermentation
3.1. Biohydrogen
3.2. Biomethane
3.3. Bio-LPG (Bio-Propane)
4. Photo Fermentation
4.1. Photosynthetic Bacteria
4.2. Microalgae
5. Renewable Chemicals
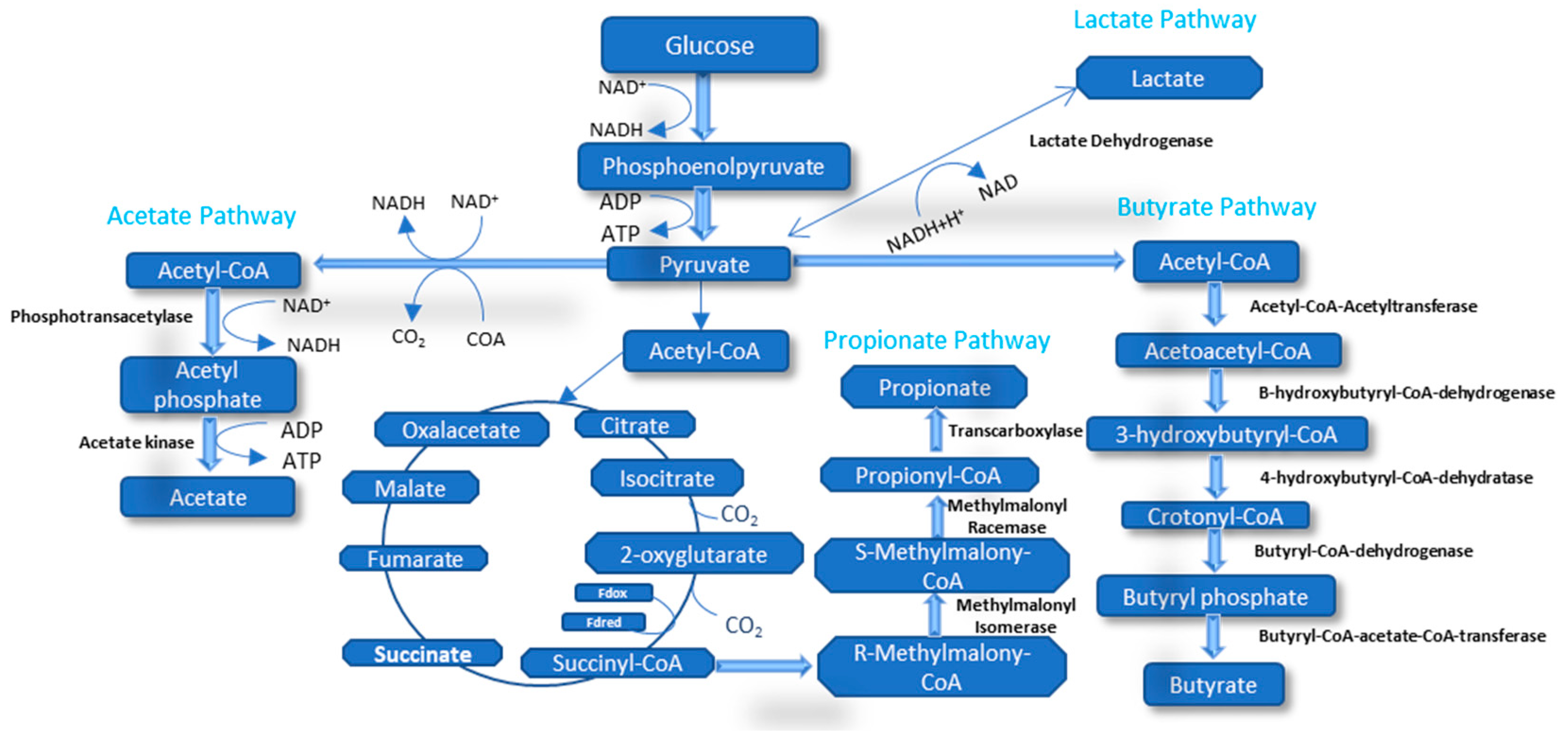
5.1. Volatile Fatty Acids
5.1.1. Acetic Acid
| Culture | Substrate/Fermentation Conditions | Production (g/L) | References |
|---|---|---|---|
| Acetobacter aceti | Cheese whey, 30 L Integrated Fermenter, pH 2–11 | 96.9 | [86] |
| Clostridium acetium | Mixed gas (163 mL Glass Serum bottle), pH 7.08–7.27 | 1.3 | [87] |
| Clostridium lentocellum SG6 | Paddy straw, 120 mL serum vials, pH 7.2 | 30.9 | [88] |
| Moorella thermoacetica | Sugarcane straw hydrolysate, 1.3 L Flask, pH 6.8 | 17.2 | [84] |
| Saccharomyces cerevisiae + Acetobacter pasteurianus | Glucose, 10 L Fed batch | 66.0 | [89] |
| Streptococcus lactis and Clostridium formicoaceticum | Whey lactose, 5 L Fermenter, pH 6.4 | 30 | [90] |
| Acetobacterium woodii | Corn Stover, 3 L sterilized fermenter Bioaugmented with A. woodii, pH 6.5 | 30.8 | [91] |
| Kluyveromyces fragilis | Whey, 500 mL Shake flask, pH 8.5 | 25.85 | [92] |
| Acetobacterium BR-446 | Carbon dioxide (CO2), BR-446 batch cultivation, pH 7.3 | 51 | [93] |
5.1.2. Propionic Acid
| Culture | Substrate/Fermentation Conditions | Concentration (g/L) | References |
|---|---|---|---|
| Propionibacterium acidipropionici (ATCC 4965) | Lactate, glycerol and sugarcane molasses, 1 L Glass Flask, Batch Fermenter, pH 6.87 | 15.1 | [100] |
| Propionibacterium acidipropionici (CGMCC 1.223) | Glycerol, 7 L Fed-Batch Fermenter, pH 7.0 | 44.6 | [101] |
| Propionibacterium acidipropionici (CGMCC 1.223) | Hemicellulose hydrolysate, 2 L Batch fermenter, pH 6.8 | 18.0 | [102] |
| Propionibacterium acidipropionici (ATCC 4875) | Cheese whey, 6 L Continuous Fermentation, pH 6.5 | 19.7 | [103] |
| Propionibacterium freudenreichii CCTCC M207015 | Glucose, 7.5 L Multi-point fibrous-bed (MFB) bioreactor, pH 6.9 | 67.1 | [104] |
| Propionibacterium freudenreichii spp. shermanii | Glycerol, 1.2 L Batch Fermenter, pH 7.0 | 9.0 | [105] |
| Acetobacterium ruminis | Corn stover, 3 L sterilized fermenter, Bioaugmentation with A. ruminis, pH 6.5 | 30.8 | [91] |
| Propionibacterium zeae (CCT 5329) | Sugarcane molasses, Submerged Fermentation, pH 7.0 | 6.83 | [106] |
| Propionibacterium jensenii | Lactate, 1 L Submerged Fermentation, pH 6.83 | 16.31 | [107] |
| Propionibacterium acidipropionici | Flour hydrolysate, 2.5 L Fed-Batch Fermentation, pH 6.0 | 30 | [108] |
5.1.3. Butyric Acid
| Culture | Substrate and Fermentation Conditions | Concentration (g/L) | References |
|---|---|---|---|
| Clostridium butyricum S21 | Sucrose, 500 mL Pertractive fed-batch fermentation, pH 5.2 | 20.0 | [116] |
| Clostridium butyricum ZJUCB | Glucose, 5 L Fed-batch fermentation, pH 6.5 | 16.7 | [109] |
| Clostridium thermobutyricum JW171K | Glucose, 500 mL Rotary fermenter, pH 7.1 | 18.4 | [117] |
| Clostridium tyrobutyricum | Sugarcane Bagasse Hydrolysate, 5 L Batch fermentation, pH 5.0 | 20.9 | [118] |
| Acetobacterium woodii | Corn Stover, 3 L sterilized fermenter Bioaugmentation with A. woodii, pH 6.5 | 49.31 | [91] |
| Clostridium tyrobutyricum | Cane Molasses, 5 L Fed-batch/Immobilized. Fibrous bed bioreactor, pH 6.0 | 55.52 | [119] |
| Clostridium thermobutyricum | Corn Stalk, Immobilized continuous reactor, pH 6.0 | 15.82 | [120] |
| Clostridium tyrobutyricum | Jerusalem artichoke, 5 L Fed-batch/Immobilized fibrous-bed bioreactor, pH 6.0 | 27.5 | [121] |
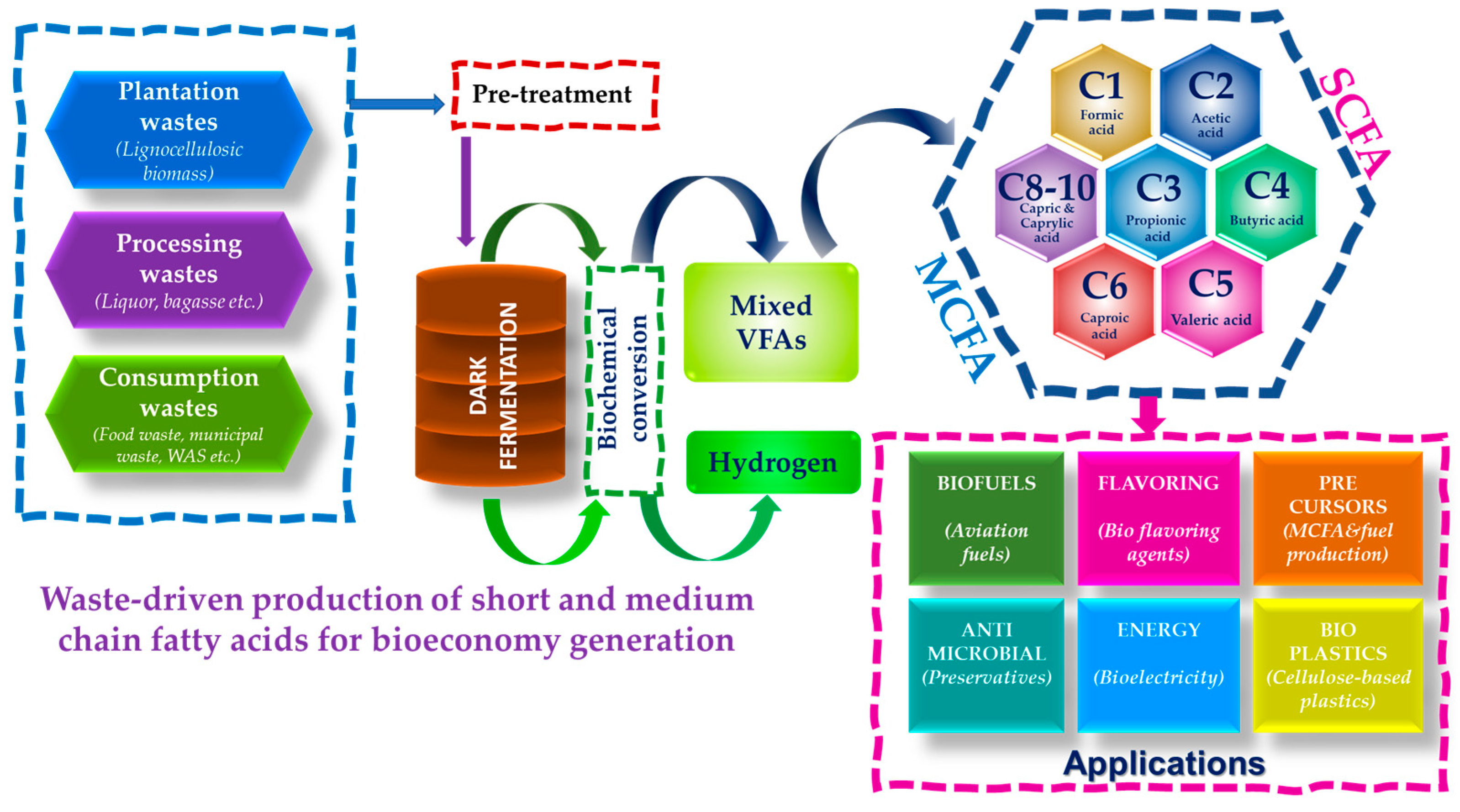
5.2. Medium Chain Fatty Acids
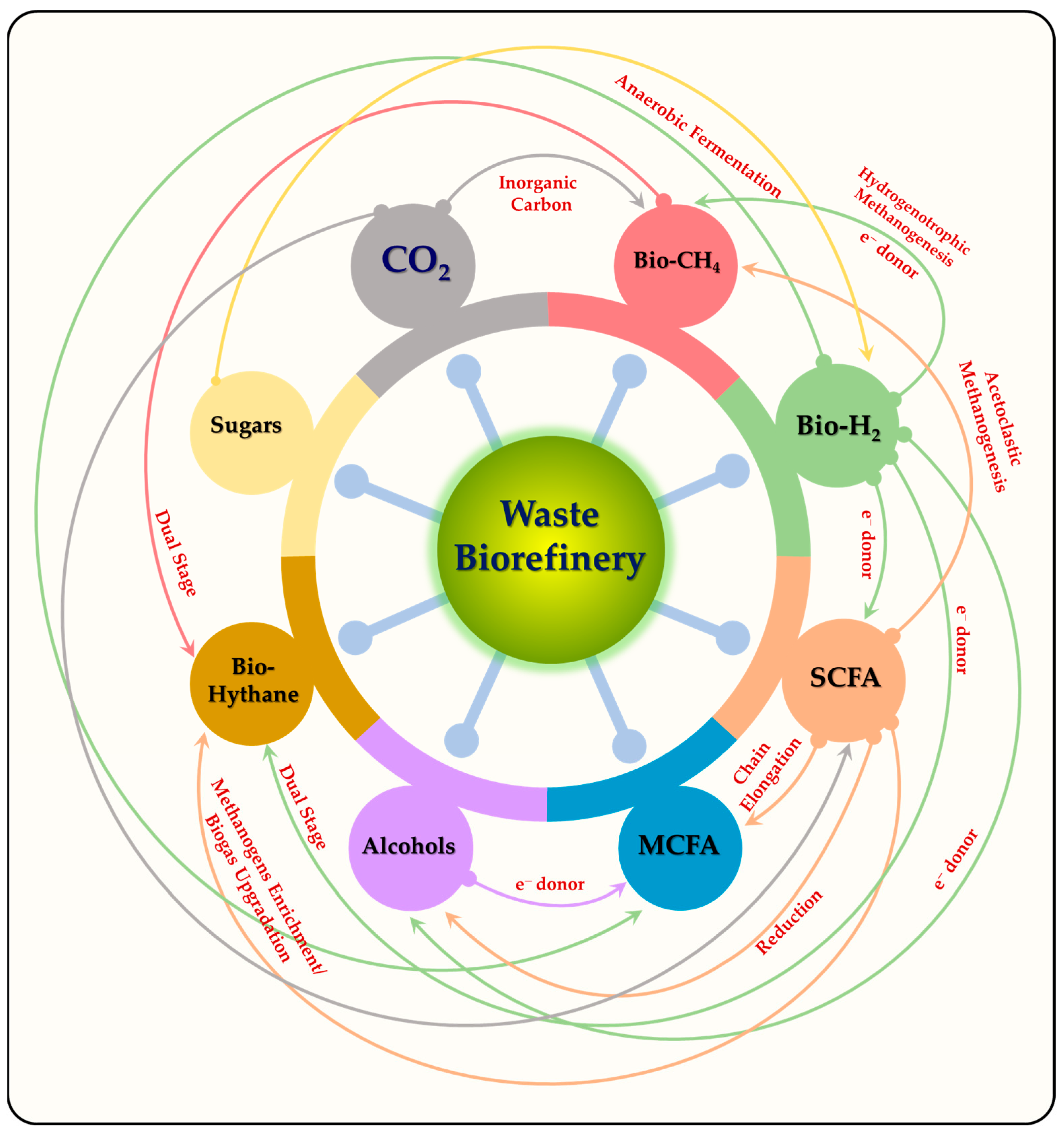
6. Biorefinery Approach for Biofuels and Renewable Chemicals
7. Road Map for Waste Derived Bioeconomy Promotion
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Leong, H.Y.; Chang, C.-K.; Khoo, K.S.; Chew, K.W.; Chia, S.R.; Lim, J.W.; Chang, J.-S.; Show, P.L. Waste Biorefinery towards a Sustainable Circular Bioeconomy: A Solution to Global Issues. Biotechnol. Biofuels 2021, 14, 87. [Google Scholar] [CrossRef]
- Bhatt, A.H.; Ren, Z.; Tao, L. Value Proposition of Untapped Wet Wastes: Carboxylic Acid Production through Anaerobic Digestion. iScience 2020, 23, 101221. [Google Scholar] [CrossRef] [PubMed]
- Venkata Mohan, S.; Nikhil, G.N.N.; Chiranjeevi, P.; Nagendranatha Reddy, C.; Rohit, M.V.V.; Kumar, A.N.N.; Sarkar, O.; Mohan, S.V.; Nikhil, G.N.N.; Chiranjeevi, P.; et al. Waste Biorefinery Models towards Sustainable Circular Bioeconomy: Critical Review and Future Perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Mallouppas, G.; Yfantis, E.A.; Ioannou, C.; Paradeisiotis, A.; Ktoris, A. Application of Biogas and Biomethane as Maritime Fuels: A Review of Research, Technology Development, Innovation Proposals, and Market Potentials. Energies 2023, 16, 2066. [Google Scholar] [CrossRef]
- Sarkar, O.; Venkata Mohan, S. Deciphering Acidogenic Process towards Biohydrogen, Biohythane, and Short Chain Fatty Acids Production: Multi-Output Optimization Strategy. Biofuel Res. J. 2016, 3, 458–469. [Google Scholar] [CrossRef]
- Dahiya, S.; Kumar, A.N.N.; Shanthi Sravan, J.; Chatterjee, S.; Sarkar, O.; Mohan, S.V.V. Food Waste Biorefinery: Sustainable Strategy for Circular Bioeconomy. Bioresour. Technol. 2018, 248, 2–12. [Google Scholar] [CrossRef]
- Lee, S.Y.; Sankaran, R.; Chew, K.W.; Tan, C.H.; Krishnamoorthy, R.; Chu, D.-T.; Show, P.-L. Waste to Bioenergy: A Review on the Recent Conversion Technologies. BMC Energy 2019, 1, 4. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F. Food Waste as a Valuable Resource for the Production of Chemicals, Materials and Fuels. Current Situation and Global Perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Micoli, L.; Di Rauso Simeone, G.; Turco, M.; Toscano, G.; Rao, M.A. Anaerobic Digestion of Olive Mill Wastewater in the Presence of Biochar. Energies 2023, 16, 3259. [Google Scholar] [CrossRef]
- Molino, A.; Nanna, F.; Ding, Y.; Bikson, B.; Braccio, G. Biomethane Production by Anaerobic Digestion of Organic Waste. Fuel 2013, 103, 1003–1009. [Google Scholar] [CrossRef]
- Yun, Y.-M.; Lee, M.-K.; Im, S.-W.; Marone, A.; Trably, E.; Shin, S.-R.; Kim, M.-G.; Cho, S.-K.; Kim, D.-H. Biohydrogen Production from Food Waste: Current Status, Limitations, and Future Perspectives. Bioresour. Technol. 2018, 248, 79–87. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Kampranis, A.; Ntaikou, I.; Lyberatos, G. Enhancement of Liquid and Gaseous Biofuels Production from Agro-Industrial Residues after Thermochemical and Enzymatic Pretreatment. Front. Sustain. Food Syst. 2019, 3, 92. [Google Scholar] [CrossRef]
- Konti, A.; Kekos, D.; Mamma, D. Life Cycle Analysis of the Bioethanol Production from Food Waste—A Review. Energies 2020, 13, 5206. [Google Scholar] [CrossRef]
- Cheng, C.-L.; Che, P.-Y.; Chen, B.-Y.; Lee, W.-J.; Lin, C.-Y.; Chang, J.-S. Biobutanol Production from Agricultural Waste by an Acclimated Mixed Bacterial Microflora. Appl. Energy 2012, 100, 3–9. [Google Scholar] [CrossRef]
- Xing, T.; Yu, S.; Tang, J.; Liu, H.; Zhen, F.; Sun, Y.; Kong, X. Liquid–Liquid Extraction of Volatile Fatty Acids from Anaerobic Acidification Broth Using Ionic Liquids and Cosolvent. Energies 2023, 16, 785. [Google Scholar] [CrossRef]
- Sarkar, O.; Rova, U.; Christakopoulos, P.; Matsakas, L. Green Hydrogen and Platform Chemicals Production from Acidogenic Conversion of Brewery Spent Grains Co-Fermented with Cheese Whey Wastewater: Adding Value to Acidogenic CO2. Sustain. Energy Fuels 2022, 6, 778–790. [Google Scholar] [CrossRef]
- Sarkar, O.; Chatterjee, S.; Mohan, S.V.; da Silva, G.A.; Kulay, L.A. Acidogenic Outlet from Biohydrogen Reactor as Phosphate Solubilizing Agent for Integrated Organic Farming. J. Clean. Prod. 2019, 208, 490–498. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, Y.; Chen, Y.; Liu, M.; Bao, X.; Guo, W. Opportunities and Challenges in Microbial Medium Chain Fatty Acids Production from Waste Biomass. Bioresour. Technol. 2021, 340, 125633. [Google Scholar] [CrossRef]
- Paritosh, K.; Yadav, M.; Mathur, S.; Balan, V.; Liao, W.; Pareek, N.; Vivekanand, V. Organic Fraction of Municipal Solid Waste: Overview of Treatment Methodologies to Enhance Anaerobic Biodegradability. Front. Energy Res. 2018, 6, 75. [Google Scholar] [CrossRef]
- Chiranjeevi, P.; Dahiya, S.; Kumar, N. Waste Derived Bioeconomy in India: A Perspective. New Biotechnol. 2018, 40, 60–69. [Google Scholar]
- Pheakdey, D.V.; Quan, N.V.; Xuan, T.D. Economic and Environmental Benefits of Energy Recovery from Municipal Solid Waste in Phnom Penh Municipality, Cambodia. Energies 2023, 16, 3234. [Google Scholar] [CrossRef]
- Mohan, S.V.; Hemalatha, M.; Amulya, K.; Velvizhi, G.; Chiranjeevi, P.; Sarkar, O.; Kumar, A.N.; Krishna, K.V.; Modestra, J.A.; Dahiya, S.; et al. Decentralized Urban Farming Through Keyhole Garden: A Case Study with Circular Economy and Regenerative Perspective. Mater. Circ. Econ. 2020, 2, 12. [Google Scholar] [CrossRef]
- Martin-Rios, C.; Hofmann, A.; Mackenzie, N. Sustainability-Oriented Innovations in Food Waste Management Technology. Sustainability 2020, 13, 210. [Google Scholar] [CrossRef]
- Strazzera, G.; Battista, F.; Garcia, N.H.; Frison, N.; Bolzonella, D. Volatile Fatty Acids Production from Food Wastes for Biorefinery Platforms: A Review. J. Environ. Manag. 2018, 226, 278–288. [Google Scholar] [CrossRef]
- Li, Y.; He, D.; Niu, D.; Zhao, Y. Acetic Acid Production from Food Wastes Using Yeast and Acetic Acid Bacteria Micro-Aerobic Fermentation. Bioprocess Biosyst. Eng. 2015, 38, 863–869. [Google Scholar] [CrossRef]
- Sulewski, P.; Ignaciuk, W.; Szymańska, M.; Wąs, A. Development of the Biomethane Market in Europe. Energies 2023, 16, 2001. [Google Scholar] [CrossRef]
- Rogala, Z.; Stanclik, M.; Łuszkiewicz, D.; Malecha, Z. Perspectives for the Use of Biogas and Biomethane in the Context of the Green Energy Transformation on the Example of an EU Country. Energies 2023, 16, 1911. [Google Scholar] [CrossRef]
- Naresh Kumar, A.; Venkata Mohan, S. Acidogenesis of Waste Activated Sludge-Biohydrogen Production with Simultaneous Short Chain Carboxylic Acids. J. Environ. Chem. Eng. 2018, 6, 2983–2991. [Google Scholar] [CrossRef]
- Patel, A.; Sarkar, O.; Rova, U.; Christakopoulos, P.; Matsakas, L. Valorization of Volatile Fatty Acids Derived from Low-Cost Organic Waste for Lipogenesis in Oleaginous Microorganisms-A Review. Bioresour. Technol. 2021, 321, 124457. [Google Scholar] [CrossRef]
- Amer, M.; Wojcik, E.Z.; Sun, C.; Hoeven, R.; Hughes, J.M.X.; Faulkner, M.; Yunus, I.S.; Tait, S.; Johannissen, L.O.; Hardman, S.J.O. Low Carbon Strategies for Sustainable Bio-Alkane Gas Production and Renewable Energy. Energy Environ. Sci. 2020, 13, 1818–1831. [Google Scholar] [CrossRef]
- Yunus, I.S.; Anfelt, J.; Sporre, E.; Miao, R.; Hudson, E.P.; Jones, P.R. Synthetic Metabolic Pathways for Conversion of CO2 into Secreted Short-to Medium-Chain Hydrocarbons Using Cyanobacteria. Metab. Eng. 2022, 72, 14–23. [Google Scholar] [CrossRef]
- Menon, N.; Pásztor, A.; Menon, B.R.K.; Kallio, P.; Fisher, K.; Akhtar, M.K.; Leys, D.; Jones, P.R.; Scrutton, N.S. A Microbial Platform for Renewable Propane Synthesis Based on a Fermentative Butanol Pathway. Biotechnol. Biofuels 2015, 8, 61. [Google Scholar] [CrossRef]
- Dahiya, S.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Renewable Hydrogen Production by Dark-Fermentation: Current Status, Challenges and Perspectives. Bioresour. Technol. 2021, 321, 124354. [Google Scholar] [CrossRef]
- Kanwal, F.; Torriero, A.A.J. Biohydrogen—A Green Fuel for Sustainable Energy Solutions. Energies 2022, 15, 7783. [Google Scholar] [CrossRef]
- Rabbi, M.F.; Popp, J.; Máté, D.; Kovács, S. Energy Security and Energy Transition to Achieve Carbon Neutrality. Energies 2022, 15, 8126. [Google Scholar] [CrossRef]
- Lee, D.-J.; Show, K.-Y.; Su, A. Dark Fermentation on Biohydrogen Production: Pure Culture. Bioresour. Technol. 2011, 102, 8393–8402. [Google Scholar] [CrossRef]
- Sarkar, O.; Matsakas, L.; Rova, U.; Christakopoulos, P. Ultrasound-Controlled Acidogenic Valorization of Wastewater for Biohydrogen and Volatile Fatty Acids Production: Microbial Community Profiling. iScience 2023, 26, 106519. [Google Scholar] [CrossRef]
- Sarkar, O.; Katakojwala, R.; Mohan, S.V.; Venkata Mohan, S. Low Carbon Hydrogen Production from a Waste-Based Biorefinery System and Environmental Sustainability Assessment. Green Chem. 2021, 23, 561–574. [Google Scholar] [CrossRef]
- Karthikeyan, B.; Gokuladoss, V. Fusion of Vermicompost and Sewage Sludge as Dark Fermentative Biocatalyst for Biohydrogen Production: A Kinetic Study. Energies 2022, 15, 6917. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dębowski, M.; Zieliński, M. Progress and Challenges in Biohydrogen Production. Energies 2022, 15, 5413. [Google Scholar] [CrossRef]
- Sarkar, O.; Kumar, A.N.N.; Dahiya, S.; Krishna, K.V.V.; Yeruva, D.K.D.K.; Mohan, S.V.V. Regulation of Acidogenic Metabolism towards Enhanced Short Chain Fatty Acid Biosynthesis from Waste: Metagenomic Profiling. RSC Adv. 2016, 6, 18641–18653. [Google Scholar] [CrossRef]
- Dahiya, S.; Sarkar, O.; Swamy, Y.V.V.; Venkata Mohan, S. Acidogenic Fermentation of Food Waste for Volatile Fatty Acid Production with Co-Generation of Biohydrogen. Bioresour. Technol. 2015, 182, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Bolaji, I.O.; Dionisi, D. Acidogenic Fermentation of Vegetable and Salad Waste for Chemicals Production: Effect of PH Buffer and Retention Time. J. Environ. Chem. Eng. 2017, 5, 5933–5943. [Google Scholar] [CrossRef]
- Che, S.; Men, Y. Synthetic Microbial Consortia for Biosynthesis and Biodegradation: Promises and Challenges. J. Ind. Microbiol. Biotechnol. 2019, 46, 1343–1358. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Dong, W.; Xin, F.; Jiang, M. Designing Synthetic Microbial Consortia for Biofuel Production. Trends Biotechnol. 2020, 38, 828–831. [Google Scholar] [CrossRef]
- Sarkar, O.; Rova, U.; Christakopoulos, P.; Matsakas, L. Effect of Metals on the Regulation of Acidogenic Metabolism Enhancing Biohydrogen and Carboxylic Acids Production from Brewery Spent Grains: Microbial Dynamics and Biochemical Analysis. Eng. Life Sci. 2022, 22, 650–661. [Google Scholar] [CrossRef]
- Sarkar, O.; Venkata Mohan, S. Synergy of Anoxic Microenvironment and Facultative Anaerobes on Acidogenic Metabolism in a Self-Induced Electrofermentation System. Bioresour. Technol. 2020, 313, 123604. [Google Scholar] [CrossRef]
- Cardeña, R.; Cercado, B.; Buitrón, G. Microbial Electrolysis Cell for Biohydrogen Production. In Biohydrogen; Elsevier: Amsterdam, The Netherlands, 2019; pp. 159–185. [Google Scholar]
- Sravan, J.S.; Sarkar, O.; Mohan, S.V. Electron-Regulated Flux towards Biogas Upgradation–Triggering Catabolism for an Augmented Methanogenic Activity. Sustain. Energy Fuels 2020, 4, 700–712. [Google Scholar] [CrossRef]
- Savvidou, M.G.; Pandis, P.K.; Mamma, D.; Sourkouni, G.; Argirusis, C. Organic Waste Substrates for Bioenergy Production via Microbial Fuel Cells: A Key Point Review. Energies 2022, 15, 5616. [Google Scholar] [CrossRef]
- Rathore, D.; Singh, A.; Dahiya, D.; Nigam, P.S.-N. Sustainability of Biohydrogen as Fuel: Present Scenario and Future Perspective. AIMS Energy 2019, 7, 1–19. [Google Scholar] [CrossRef]
- Seruga, P.; Krzywonos, M.; den Boer, E.; Niedźwiecki, Ł.; Urbanowska, A.; Pawlak-Kruczek, H. Anaerobic Digestion as a Component of Circular Bioeconomy—Case Study Approach. Energies 2023, 16, 140. [Google Scholar] [CrossRef]
- Matsakas, L.; Sarkar, O.; Jansson, S.; Rova, U.; Christakopoulos, P. A Novel Hybrid Organosolv-Steam Explosion Pretreatment and Fractionation Method Delivers Solids with Superior Thermophilic Digestibility to Methane. Bioresour. Technol. 2020, 316, 123973. [Google Scholar] [CrossRef] [PubMed]
- Frigon, J.; Guiot, S.R. Biomethane Production from Starch and Lignocellulosic Crops: A Comparative Review. Biofuels Bioprod. Biorefin. 2010, 4, 447–458. [Google Scholar] [CrossRef]
- Daw, J.; Hallett, K.; DeWolfe, J.; Venner, I. Energy Efficiency Strategies for Municipal Wastewater Treatment Facilities; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2012. [Google Scholar]
- Claus, S.; Taube, F.; Wienforth, B.; Svoboda, N.; Sieling, K.; Kage, H.; Senbayram, M.; Dittert, K.; Gericke, D.; Pacholski, A. Life-Cycle Assessment of Biogas Production under the Environmental Conditions of Northern Germany: Greenhouse Gas Balance. J. Agric. Sci. 2014, 152, 172–181. [Google Scholar] [CrossRef]
- Rathore, D.; Nizami, A.-S.; Singh, A.; Pant, D. Key Issues in Estimating Energy and Greenhouse Gas Savings of Biofuels: Challenges and Perspectives. Biofuel Res. J. 2016, 3, 380–393. [Google Scholar] [CrossRef]
- Johnson, E. New Biofuel Debut: Biopropane. Biofuels Bioprod. Biorefin. 2015, 9, 627–629. [Google Scholar] [CrossRef]
- Currie, F.; Twigg, M.S.; Huddleson, N.; Simons, K.E.; Marchant, R.; Banat, I.M. Biogenic Propane Production by a Marine Photobacterium Strain Isolated from the Western English Channel. Front. Microbiol. 2022, 13, 4084. [Google Scholar] [CrossRef]
- Hoeven, R.; Hughes, J.M.X.; Amer, M.; Wojcik, E.Z.; Tait, S.; Faulkner, M.; Yunus, I.S.; Hardman, S.J.O.; Johannissen, L.O.; Chen, G.-Q. Distributed Biomanufacturing of Liquefied Petroleum Gas. bioRxiv 2019. [Google Scholar] [CrossRef]
- Kallio, P.; Pásztor, A.; Thiel, K.; Akhtar, M.K.; Jones, P.R. An Engineered Pathway for the Biosynthesis of Renewable Propane. Nat. Commun. 2014, 5, 4731. [Google Scholar] [CrossRef]
- Razaq, I.; Simons, K.E.; Onwudili, J.A. Parametric Study of Pt/C-Catalysed Hydrothermal Decarboxylation of Butyric Acid as a Potential Route for Biopropane Production. Energies 2021, 14, 3316. [Google Scholar] [CrossRef]
- Hydrocarbons Technology, 2015 Neste’s Bio LPG Facility, Rotterdam. 2015. Available online: https://www.hydrocarbons-technology.com/projects/nestes-bio-lpg-facility-rotterdam/ (accessed on 3 January 2023).
- Onwudili, J.A.; Nouwe Edou, D.J. Process Modelling and Economic Evaluation of Biopropane Production from Aqueous Butyric Acid Feedstock. Renew. Energy 2022, 184, 80–90. [Google Scholar] [CrossRef]
- Kis, M.; Smart, J.L.; Maróti, P. Capacity and Kinetics of Light-Induced Cytochrome Oxidation in Intact Cells of Photosynthetic Bacteria. Sci. Rep. 2022, 12, 14298. [Google Scholar] [CrossRef]
- Keskin, T.; Hallenbeck, P.C. Hydrogen Production from Sugar Industry Wastes Using Single-Stage Photofermentation. Bioresour. Technol. 2012, 112, 131–136. [Google Scholar] [CrossRef]
- Hunter, C.N.; Daldal, F.; Thurnauer, M.C.; Beatty, J.T. The Purple Phototrophic Bacteria; Springer: Berlin/Heidelberg, Germany, 2009; Volume 28. [Google Scholar]
- Chandra, R.; Venkata Mohan, S. Microalgal community and their growth conditions influence biohydrogen production during integration of dark-fermentation and photo-fermentation processes. Int. J. Hydrogen Energy 2011, 36, 12211–12219. [Google Scholar] [CrossRef]
- Assawamongkholsiri, T.; Reungsang, A.; Plangkang, P.; Sittijunda, S. Repeated Batch Fermentation for Photo-Hydrogen and Lipid Production from Wastewater of a Sugar Manufacturing Plant. Int. J. Hydrogen Energy 2018, 43, 3605–3617. [Google Scholar] [CrossRef]
- Moreira, F.S.; Rodrigues, M.S.; Sousa, L.M.; Batista, F.R.X.; Ferreira, J.S.; Cardoso, V.L. Single-Stage Repeated Batch Cycles Using Co-Culture of Enterobacter Cloacae and Purple Non-Sulfur Bacteria for Hydrogen Production. Energy 2022, 239, 122465. [Google Scholar] [CrossRef]
- Borowiak, D.; Krzywonos, M. Bioenergy, Biofuels, Lipids and Pigments—Research Trends in the Use of Microalgae Grown in Photobioreactors. Energies 2022, 15, 5357. [Google Scholar] [CrossRef]
- Chen, Y. Global Potential of Algae-Based Photobiological Hydrogen Production. Energy Environ. Sci. 2022, 15, 2843–2857. [Google Scholar] [CrossRef]
- Naschberger, A.; Mosebach, L.; Tobiasson, V.; Kuhlgert, S.; Scholz, M.; Perez-Boerema, A.; Ho, T.T.H.; Vidal-Meireles, A.; Takahashi, Y.; Hippler, M.; et al. Algal Photosystem I Dimer and High-Resolution Model of PSI-Plastocyanin Complex. Nat. Plants 2022, 8, 1191–1201. [Google Scholar] [CrossRef]
- Oey, M.; Sawyer, A.L.; Ross, I.L.; Hankamer, B. Challenges and Opportunities for Hydrogen Production from Microalgae. Plant Biotechnol. J. 2016, 14, 1487–1499. [Google Scholar] [CrossRef]
- Ban, S.; Lin, W.; Luo, Z.; Luo, J. Improving Hydrogen Production of Chlamydomonas Reinhardtii by Reducing Chlorophyll Content via Atmospheric and Room Temperature Plasma. Bioresour. Technol. 2019, 275, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Jin, L.; Zhang, C.; Tan, Y.; Jiang, P.; Ge, N.; Li, H.; Xing, X. Rapid Mutation of Spirulina Platensis by a New Mutagenesis System of Atmospheric and Room Temperature Plasmas (ARTP) and Generation of a Mutant Library with Diverse Phenotypes. PLoS ONE 2013, 8, e77046. [Google Scholar] [CrossRef]
- Ögmundarson, Ó.; Herrgård, M.J.; Forster, J.; Hauschild, M.Z.; Fantke, P.; Haus, S.; Björnsson, L.; Börjesson, P.; Frankó, B.; Galbe, M.; et al. Hydrogen Production by Clostridium Thermocellum 27405 from Cellulosic Biomass Substrates. Bioresour. Technol. 2021, 34, 125643. [Google Scholar] [CrossRef]
- Liu, C.; Yin, Y.; Chen, C.; Zhang, X.; Zhou, J.; Zhang, Q.; Chen, Y. Advances in Electricity-Steering Organic Waste Bio-Valorization for Medium Chain Carboxylic Acids Production. Energies 2023, 16, 2571. [Google Scholar] [CrossRef]
- Gomes, R.J.; de Fatima Borges, M.; de Freitas Rosa, M.; Castro-Gómez, R.J.H.; Spinosa, W.A. Acetic Acid Bacteria in the Food Industry: Systematics, Characteristics and Applications. Food Technol. Biotechnol. 2018, 56, 139. [Google Scholar] [CrossRef]
- Yang, S.; Yu, M.; Chang, W.; Tang, I. Anaerobic Fermentations for the Production of Acetic and Butyric Acids. In Bioprocessing Technologies in Biorefinery for Sustainable Production of Fuels, Chemicals, and Polymers; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 351–374. [Google Scholar]
- Naresh Kumar, A.; Sarkar, O.; Chandrasekhar, K.; Raj, T.; Narisetty, V.; Mohan, S.V.; Pandey, A.; Varjani, S.; Kumar, S.; Sharma, P.; et al. Upgrading the Value of Anaerobic Fermentation via Renewable Chemicals Production: A Sustainable Integration for Circular Bioeconomy. Sci. Total Environ. 2022, 806, 150312. [Google Scholar] [CrossRef]
- Lopes, M.S.G.; Slovic, A.M.; Gouvea, I.E.; Perez, J.R.; Parizzi, L.P. Modified Microorganisms and Methods of Making Butadiene Using Same. U.S. Patent No. 10273505, 30 April 2019. [Google Scholar]
- Williams, K.; Zheng, Y.; McGarvey, J.; Fan, Z.; Zhang, R. Ethanol and Volatile Fatty Acid Production from Lignocellulose by Clostridium Cellulolyticum. Int. Sch. Res. Not. 2013, 2013, 137835. [Google Scholar] [CrossRef]
- Ehsanipour, M.; Suko, A.V.; Bura, R. Fermentation of Lignocellulosic Sugars to Acetic Acid by Moorella Thermoacetica. J. Ind. Microbiol. Biotechnol. 2016, 43, 807–816. [Google Scholar] [CrossRef]
- Sarchami, T.; Batta, N.; Berruti, F. Production and Separation of Acetic Acid from Pyrolysis Oil of Lignocellulosic Biomass: A Review. Biofuels Bioprod. Biorefin. 2021, 15, 1912–1937. [Google Scholar] [CrossRef]
- Nayak, J.; Pal, P. Transforming Waste Cheese-Whey into Acetic Acid through a Continuous Membrane-Integrated Hybrid Process. Ind. Eng. Chem. Res. 2013, 52, 2977–2984. [Google Scholar] [CrossRef]
- Sim, J.H.; Kamaruddin, A.H. Optimization of Acetic Acid Production from Synthesis Gas by Chemolithotrophic Bacterium–Clostridium Aceticum Using Statistical Approach. Bioresour. Technol. 2008, 99, 2724–2735. [Google Scholar] [CrossRef]
- Ravinder, T.; Ramesh, B.; Seenayya, G.; Reddy, G. Fermentative Production of Acetic Acid from Various Pure and Natural Cellulosic Materials by Clostridium Lentocellum SG6. World J. Microbiol. Biotechnol. 2000, 16, 507–512. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, M.; Chen, X.; Li, D.; Qin, L.; Li, Z.; Yao, J.; Liang, X. Mixed Culture of Saccharomyces Cerevisiae and Acetobacter Pasteurianus for Acetic Acid Production. Biochem. Eng. J. 2013, 79, 41–45. [Google Scholar] [CrossRef]
- Tang, I.-C.; Yang, S.-T.; Okos, M.R. Acetic Acid Production from Whey Lactose by the Co-Culture of Streptococcus Lactis and Clostridium Formicoaceticum. Appl. Microbiol. Biotechnol. 1988, 28, 138–143. [Google Scholar] [CrossRef]
- Murali, N.; Srinivas, K.; Ahring, B.K. Increasing the Production of Volatile Fatty Acids from Corn Stover Using Bioaugmentation of a Mixed Rumen Culture with Homoacetogenic Bacteria. Microorganisms 2021, 9, 337. [Google Scholar] [CrossRef]
- Mostafa, N.A. Production of Acetic Acid and Glycerol from Salted and Dried Whey in a Membrane Cell Recycle Bioreactor. Energy Convers. Manag. 2001, 42, 1133–1142. [Google Scholar] [CrossRef]
- Morinaga, T.; Kawada, N. The Production of Acetic Acid from Carbon Dioxide and Hydrogen by an Anaerobic Bacterium. J. Biotechnol. 1990, 14, 187–194. [Google Scholar] [CrossRef]
- Singh, S.; Gosu, V.; Upadhyaya, S.; Kumar, U.K.A. Process Intensification of Propionic Acid Separation–Effect of Channel Geometry on Microchannel Distillation. Chem. Eng. Process.-Process Intensif. 2021, 169, 108599. [Google Scholar] [CrossRef]
- Mordor Intelligence Reports, 2020 Mordorintelligence. Available online: https://www.mordorintelligence.com/industry-reports/propionic-acid-market (accessed on 10 February 2023).
- Bhatia, S.K.; Yang, Y.-H. Microbial Production of Volatile Fatty Acids: Current Status and Future Perspectives. Rev. Environ. Sci. Bio/Technol. 2017, 16, 327–345. [Google Scholar] [CrossRef]
- Feng, X.; Chen, F.; Xu, H.; Wu, B.; Li, H.; Li, S.; Ouyang, P. Green and Economical Production of Propionic Acid by Propionibacterium Freudenreichii CCTCC M207015 in Plant Fibrous-Bed Bioreactor. Bioresour. Technol. 2011, 102, 6141–6146. [Google Scholar] [CrossRef]
- Guan, N.; Li, J.; Shin, H.; Wu, J.; Du, G.; Shi, Z.; Liu, L.; Chen, J. Comparative Metabolomics Analysis of the Key Metabolic Nodes in Propionic Acid Synthesis in Propionibacterium Acidipropionici. Metabolomics 2015, 11, 1106–1116. [Google Scholar] [CrossRef]
- Ali, R.; Saravia, F.; Hille-Reichel, A.; Gescher, J.; Horn, H. Propionic Acid Production from Food Waste in Batch Reactors: Effect of PH, Types of Inoculum, and Thermal Pre-Treatment. Bioresour. Technol. 2021, 319, 124166. [Google Scholar] [CrossRef] [PubMed]
- Coral, J.; Karp, S.G.; Porto de Souza Vandenberghe, L.; Parada, J.L.; Pandey, A.; Soccol, C.R. Batch Fermentation Model of Propionic Acid Production by Propionibacterium Acidipropionici in Different Carbon Sources. Appl. Biochem. Biotechnol. 2008, 151, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, J.; Tan, M.; Liu, L.; Jiang, L.; Sun, J.; Lee, P.; Du, G.; Chen, J. Optimization and Scale-up of Propionic Acid Production by Propionic Acid-Tolerant Propionibacterium Acidipropionici with Glycerol as the Carbon Source. Bioresour. Technol. 2010, 101, 8902–8906. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, J.A.; Hassan, M.-C.A.; Ramsay, B.A. Biological Conversion of Hemicellulose to Propionic Acid. Enzym. Microb. Technol. 1998, 22, 292–295. [Google Scholar] [CrossRef]
- Gupta, A.; Srivastava, A.K. Continuous Propionic Acid Production from Cheese Whey Using In Situ Spin Filter. Biotechnol. Bioprocess Eng. BBE 2001, 6, 1–5. [Google Scholar] [CrossRef]
- Feng, X.-H.; Chen, F.; Xu, H.; Wu, B.; Yao, J.; Ying, H.-J.; Ouyang, P.-K. Propionic Acid Fermentation by Propionibacterium Freudenreichii CCTCC M207015 in a Multi-Point Fibrous-Bed Bioreactor. Bioprocess Biosyst. Eng. 2010, 33, 1077–1085. [Google Scholar] [CrossRef]
- Himmi, E.H.; Bories, A.; Boussaid, A.; Hassani, L. Propionic Acid Fermentation of Glycerol and Glucose by Propionibacterium Acidipropionici and Propionibacterium Freudenreichii Ssp. Shermanii. Appl. Microbiol. Biotechnol. 2000, 53, 435–440. [Google Scholar] [CrossRef]
- Coral, J. Propionic Acid Production by Propionibacterium Sp. Using Low-Cost Carbon Sources in Submerged Fermentation; Biotechnology and Bioprocesses Engineering Division Federal University of Parana: Curitiba, Brazil, 2008. [Google Scholar]
- Sabra, W.; Dietz, D.; Zeng, A.-P. Substrate-Limited Co-Culture for Efficient Production of Propionic Acid from Flour Hydrolysate. Appl. Microbiol. Biotechnol. 2013, 97, 5771–5777. [Google Scholar] [CrossRef]
- Zhang, A.; Yang, S.-T. Propionic Acid Production from Glycerol by Metabolically Engineered Propionibacterium Acidipropionici. Process Biochem. 2009, 44, 1346–1351. [Google Scholar] [CrossRef]
- He, G.; Kong, Q.; Chen, Q.; Ruan, H. Batch and Fed-Batch Production of Butyric Acid by Clostridium Butyricum ZJUCB. J. Zhejiang Univ. Sci. B 2005, 6, 1076. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, Z.; Yang, S.-T. Butyric Acid Production from Acid Hydrolysate of Corn Fibre by Clostridium Tyrobutyricum in a Fibrous-Bed Bioreactor. Process Biochem. 2002, 38, 657–666. [Google Scholar] [CrossRef]
- Lee, K.M.; Min, K.; Choi, O.; Kim, K.-Y.; Woo, H.M.; Kim, Y.; Han, S.O.; Um, Y. Electrochemical Detoxification of Phenolic Compounds in Lignocellulosic Hydrolysate for Clostridium Fermentation. Bioresour. Technol. 2015, 187, 228–234. [Google Scholar] [CrossRef]
- Wang, L.; Ou, M.S.; Nieves, I.; Erickson, J.E.; Vermerris, W.; Ingram, L.O.; Shanmugam, K.T. Fermentation of Sweet Sorghum Derived Sugars to Butyric Acid at High Titer and Productivity by a Moderate Thermophile Clostridium Thermobutyricum at 50 °C. Bioresour. Technol. 2015, 198, 533–539. [Google Scholar] [CrossRef]
- Shahab, R.L.; Brethauer, S.; Davey, M.P.; Smith, A.G.; Vignolini, S.; Luterbacher, J.S.; Studer, M.H. A Heterogeneous Microbial Consortium Producing Short-Chain Fatty Acids from Lignocellulose. Science 2020, 369, eabb1214. [Google Scholar] [CrossRef]
- Xiao, Z.; Cheng, C.; Bao, T.; Liu, L.; Wang, B.; Tao, W.; Pei, X.; Yang, S.-T.; Wang, M. Production of Butyric Acid from Acid Hydrolysate of Corn Husk in Fermentation by Clostridium Tyrobutyricum: Kinetics and Process Economic Analysis. Biotechnol. Biofuels 2018, 11, 164. [Google Scholar] [CrossRef]
- Stein, U.H.; Wimmer, B.; Ortner, M.; Fuchs, W.; Bochmann, G. Maximizing the Production of Butyric Acid from Food Waste as a Precursor for ABE-Fermentation. Sci. Total Environ. 2017, 598, 993–1000. [Google Scholar] [CrossRef]
- Zigová, J.; Šturdík, E.; Vandák, D.; Schlosser, Š. Butyric Acid Production by Clostridium Butyricum with Integrated Extraction and Pertraction. Process Biochem. 1999, 34, 835–843. [Google Scholar] [CrossRef]
- Canganella, F.; Wiegel, J. Continuous Cultivation of Clostridium Thermobutyricum in a Rotary Fermentor System. J. Ind. Microbiol. Biotechnol. 2000, 24, 7–13. [Google Scholar] [CrossRef]
- Wei, D.; Liu, X.; Yang, S.-T. Butyric Acid Production from Sugarcane Bagasse Hydrolysate by Clostridium Tyrobutyricum Immobilized in a Fibrous-Bed Bioreactor. Bioresour. Technol. 2013, 129, 553–560. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Liang, S.; Wang, X.; Cen, P.; Xu, Z. Butyric Acid Fermentation in a Fibrous Bed Bioreactor with Immobilized Clostridium Tyrobutyricum from Cane Molasses. Bioresour. Technol. 2009, 100, 3403–3409. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Han, H.-J.; Zhang, C.H. Continuous Butyric Acid Production by Corn Stalk Immobilized Clostridium Thermobutyricum Cells. Afr. J. Microbiol. Res. 2011, 5, 661–666. [Google Scholar]
- Huang, J.; Cai, J.; Wang, J.; Zhu, X.; Huang, L.; Yang, S.-T.; Xu, Z. Efficient Production of Butyric Acid from Jerusalem Artichoke by Immobilized Clostridium Tyrobutyricum in a Fibrous-Bed Bioreactor. Bioresour. Technol. 2011, 102, 3923–3926. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, O.; Rova, U.; Christakopoulos, P.; Matsakas, L. Ethanol Addition Promotes Elongation of Short-Chain Fatty Acids to Medium-Chain Fatty Acids Using Brewery Spent Grains as Substrate. J. Environ. Chem. Eng. 2021, 9, 105990. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mohan, S.V.; Chang, Y.C. Sustainable Production of Medium Chain Fatty Acids (MCFA) with an Enriched Mixed Bacterial Culture: Microbial Characterization Using Molecular Methods. Sustain. Energy Fuels 2018, 2, 372–380. [Google Scholar] [CrossRef]
- Menon, A.; Lyng, J.G. Circular Bioeconomy Solutions: Driving Anaerobic Digestion of Waste Streams towards Production of High Value Medium Chain Fatty Acids. Rev. Environ. Sci. Bio/Technol. 2021, 20, 189–208. [Google Scholar] [CrossRef]
- Kallscheuer, N.; Polen, T.; Bott, M.; Marienhagen, J. Reversal of β-Oxidative Pathways for the Microbial Production of Chemicals and Polymer Building Blocks. Metab. Eng. 2017, 42, 33–42. [Google Scholar] [CrossRef]
- Chen, W.-S.; Strik, D.P.; Buisman, C.J.N.; Kroeze, C. Production of Caproic Acid from Mixed Organic Waste: An Environmental Life Cycle Perspective. Environ. Sci. Technol. 2017, 51, 7159–7168. [Google Scholar] [CrossRef]
- Candry, P.; Van Daele, T.; Denis, K.; Amerlinck, Y.; Andersen, S.J.; Ganigué, R.; Arends, J.B.A.; Nopens, I.; Rabaey, K.; Chen, W.-S.; et al. A Novel High-Throughput Method for Kinetic Characterisation of Anaerobic Bioproduction Strains, Applied to Clostridium Kluyveri. Sci. Rep. 2018, 8, 9724. [Google Scholar] [CrossRef]
- Stamatopoulou, P.; Malkowski, J.; Conrado, L.; Brown, K.; Scarborough, M. Fermentation of Organic Residues to Beneficial Chemicals: A Review of Medium-Chain Fatty Acid Production. Processes 2020, 8, 1571. [Google Scholar] [CrossRef]
- Wu, S.-L.; Luo, G.; Sun, J.; Wei, W.; Song, L.; Ni, B.-J. Medium Chain Fatty Acids Production from Anaerobic Fermentation of Waste Activated Sludge. J. Clean. Prod. 2021, 279, 123482. [Google Scholar] [CrossRef]
- Bao, S.; Wang, Q.; Zhang, P.; Zhang, Q.; Wu, Y.; Li, F.; Tao, X.; Wang, S.; Nabi, M.; Zhou, Y. Effect of Acid/Ethanol Ratio on Medium Chain Carboxylate Production with Different VFAs as the Electron Acceptor: Insight into Carbon Balance and Microbial Community. Energies 2019, 12, 3720. [Google Scholar] [CrossRef]
- Reddy, M.V.; Chang, Y.-C. Production of Biofuel Precursor Molecules (Monocarboxylic Acids, Biohydrogen) from Apple and Pumpkin Waste through an Anaerobic Fermentation Process. Sustain. Energy Fuels 2021, 5, 4133–4140. [Google Scholar] [CrossRef]
- Grootscholten, T.I.M.; Strik, D.P.B.T.B.; Steinbusch, K.J.J.; Buisman, C.J.N.; Hamelers, H.V.M. Two-Stage Medium Chain Fatty Acid (MCFA) Production from Municipal Solid Waste and Ethanol. Appl. Energy 2014, 116, 223–229. [Google Scholar] [CrossRef]
- Khor, W.C.; Andersen, S.; Vervaeren, H.; Rabaey, K. Electricity-Assisted Production of Caproic Acid from Grass. Biotechnol. Biofuels 2017, 10, 180. [Google Scholar] [CrossRef]
- Nzeteu, C.O.; Trego, A.C.; Abram, F.; O’Flaherty, V. Reproducible, High-Yielding, Biological Caproate Production from Food Waste Using a Single-Phase Anaerobic Reactor System. Biotechnol. Biofuels 2018, 11, 108. [Google Scholar] [CrossRef]
- Roghair, M.; Liu, Y.; Strik, D.P.; Weusthuis, R.A.; Bruins, M.E.; Buisman, C.J.N. Development of an Effective Chain Elongation Process from Acidified Food Waste and Ethanol into N-Caproate. Front. Bioeng. Biotechnol. 2018, 6, 50. [Google Scholar] [CrossRef]
- Zhu, X.; Tao, Y.; Liang, C.; Li, X.; Wei, N.; Zhang, W.; Zhou, Y.; Yang, Y.; Bo, T. The Synthesis of N-Caproate from Lactate: A New Efficient Process for Medium-Chain Carboxylates Production. Sci. Rep. 2015, 5, 14360. [Google Scholar] [CrossRef]
- Fan, G.; Liu, P.; Chang, X.; Yin, H.; Cheng, L.; Teng, C.; Gong, Y.; Li, X. Isolation and Identification of a High-Yield Ethyl Caproate-Producing Yeast from Daqu and Optimization of Its Fermentation. Front. Microbiol. 2021, 12, 663744. [Google Scholar] [CrossRef]
- Sengupta, A.; Roy, S.; Mukherjee, S.; Ghosh, M. Production of Medium Chain Fatty Acid Rich Mustard Oil Using Packed Bed Bioreactor. J. Oleo Sci. 2015, 64, 153–159. [Google Scholar] [CrossRef]
- Zhu, Z.; Hu, Y.; Teixeira, P.G.; Pereira, R.; Chen, Y.; Siewers, V.; Nielsen, J. Multidimensional Engineering of Saccharomyces Cerevisiae for Efficient Synthesis of Medium-Chain Fatty Acids. Nat. Catal. 2020, 3, 64–74. [Google Scholar] [CrossRef]
- Narisetty, V.; Adlakha, N.; Kumar Singh, N.; Dalei, S.K.; Prabhu, A.A.; Nagarajan, S.; Naresh Kumar, A.; Amruthraj Nagoth, J.; Kumar, G.; Singh, V.; et al. Integrated Biorefineries for Repurposing of Food Wastes into Value-Added Products. Bioresour. Technol. 2022, 363, 127856. [Google Scholar] [CrossRef] [PubMed]
- Venkata Mohan, S.; Amulya, K.; Annie Modestra, J.; Sarkar, O.; Nareshkumar, A.; Rohit, M.V.; Nagendranatha Reddy, C. Bioenergy from Waste Remediation: Recent Advances towards Environmental Biorefinery. JUET Res. J. Sci. Technol. 2014, 1, 73–84. [Google Scholar]
- Giuliano, A. The Transition of Scientific Research from Biomass-to-Energy/Biofuels to Biomass-to-Biochemicals in a Biorefinery Systems Framework. Energies 2023, 16, 2261. [Google Scholar] [CrossRef]
- Soleymani Angili, T.; Grzesik, K.; Salimi, E.; Loizidou, M. Life Cycle Analysis of Food Waste Valorization in Laboratory-Scale. Energies 2022, 15, 7000. [Google Scholar] [CrossRef]
- Clark, J.; Deswarte, F. The Biorefinery Concept. In Introduction to Chemicals from Biomass; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 1–29. ISBN 9781118714478. [Google Scholar]
- Zetterholm, J.; Bryngemark, E.; Ahlström, J.; Söderholm, P.; Harvey, S.; Wetterlund, E. Economic Evaluation of Large-Scale Biorefinery Deployment: A Framework Integrating Dynamic Biomass Market and Techno-Economic Models. Sustainability 2020, 12, 7126. [Google Scholar] [CrossRef]
- Takkellapati, S.; Li, T.; Gonzalez, M.A. An Overview of Biorefinery-Derived Platform Chemicals from a Cellulose and Hemicellulose Biorefinery. Clean Technol. Environ. policy 2018, 20, 1615–1630. [Google Scholar] [CrossRef]
- Isah, S.; Ozbay, G. Valorization of Food Loss and Wastes: Feedstocks for Biofuels and Valuable Chemicals. Front. Sustain. Food Syst. 2020, 4, 82. [Google Scholar] [CrossRef]
- Chandel, A.K.; Forte, M.B.S.; Gonçalves, I.S.; Milessi, T.S.; Arruda, P.V.; Carvalho, W.; Mussatto, S.I. Brazilian Biorefineries from Second Generation Biomass: Critical Insights from Industry and Future Perspectives. Biofuels Bioprod. Biorefin. 2021, 15, 1190–1208. [Google Scholar] [CrossRef]
- Potrč, S.; Čuček, L.; Martin, M.; Kravanja, Z. Synthesis of European Union Biorefinery Supply Networks Considering Sustainability Objectives. Processes 2020, 8, 1588. [Google Scholar] [CrossRef]
- Gargalo, C.L.; Udugama, I.; Pontius, K.; Lopez, P.C.; Nielsen, R.F.; Hasanzadeh, A.; Mansouri, S.S.; Bayer, C.; Junicke, H.; Gernaey, K.V. Towards Smart Biomanufacturing: A Perspective on Recent Developments in Industrial Measurement and Monitoring Technologies for Bio-Based Production Processes. J. Ind. Microbiol. Biotechnol. Off. J. Soc. Ind. Microbiol. Biotechnol. 2020, 47, 947–964. [Google Scholar] [CrossRef]
- Levidow, L. Eco-Efficient Biorefineries: Techno-Fix for Resource Constraints? Econ. Rural. 2015, 349, 32–55. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iragavarapu, G.P.; Imam, S.S.; Sarkar, O.; Mohan, S.V.; Chang, Y.-C.; Reddy, M.V.; Kim, S.-H.; Amradi, N.K. Bioprocessing of Waste for Renewable Chemicals and Fuels to Promote Bioeconomy. Energies 2023, 16, 3873. https://doi.org/10.3390/en16093873
Iragavarapu GP, Imam SS, Sarkar O, Mohan SV, Chang Y-C, Reddy MV, Kim S-H, Amradi NK. Bioprocessing of Waste for Renewable Chemicals and Fuels to Promote Bioeconomy. Energies. 2023; 16(9):3873. https://doi.org/10.3390/en16093873
Chicago/Turabian StyleIragavarapu, Gayathri Priya, Syed Shahed Imam, Omprakash Sarkar, Srinivasula Venkata Mohan, Young-Cheol Chang, Motakatla Venkateswar Reddy, Sang-Hyoun Kim, and Naresh Kumar Amradi. 2023. "Bioprocessing of Waste for Renewable Chemicals and Fuels to Promote Bioeconomy" Energies 16, no. 9: 3873. https://doi.org/10.3390/en16093873
APA StyleIragavarapu, G. P., Imam, S. S., Sarkar, O., Mohan, S. V., Chang, Y.-C., Reddy, M. V., Kim, S.-H., & Amradi, N. K. (2023). Bioprocessing of Waste for Renewable Chemicals and Fuels to Promote Bioeconomy. Energies, 16(9), 3873. https://doi.org/10.3390/en16093873









