Phase Change Material Composite Battery Module for Thermal Protection of Electric Vehicles: An Experimental Observation
Abstract
1. Introduction
2. Materials and Methods
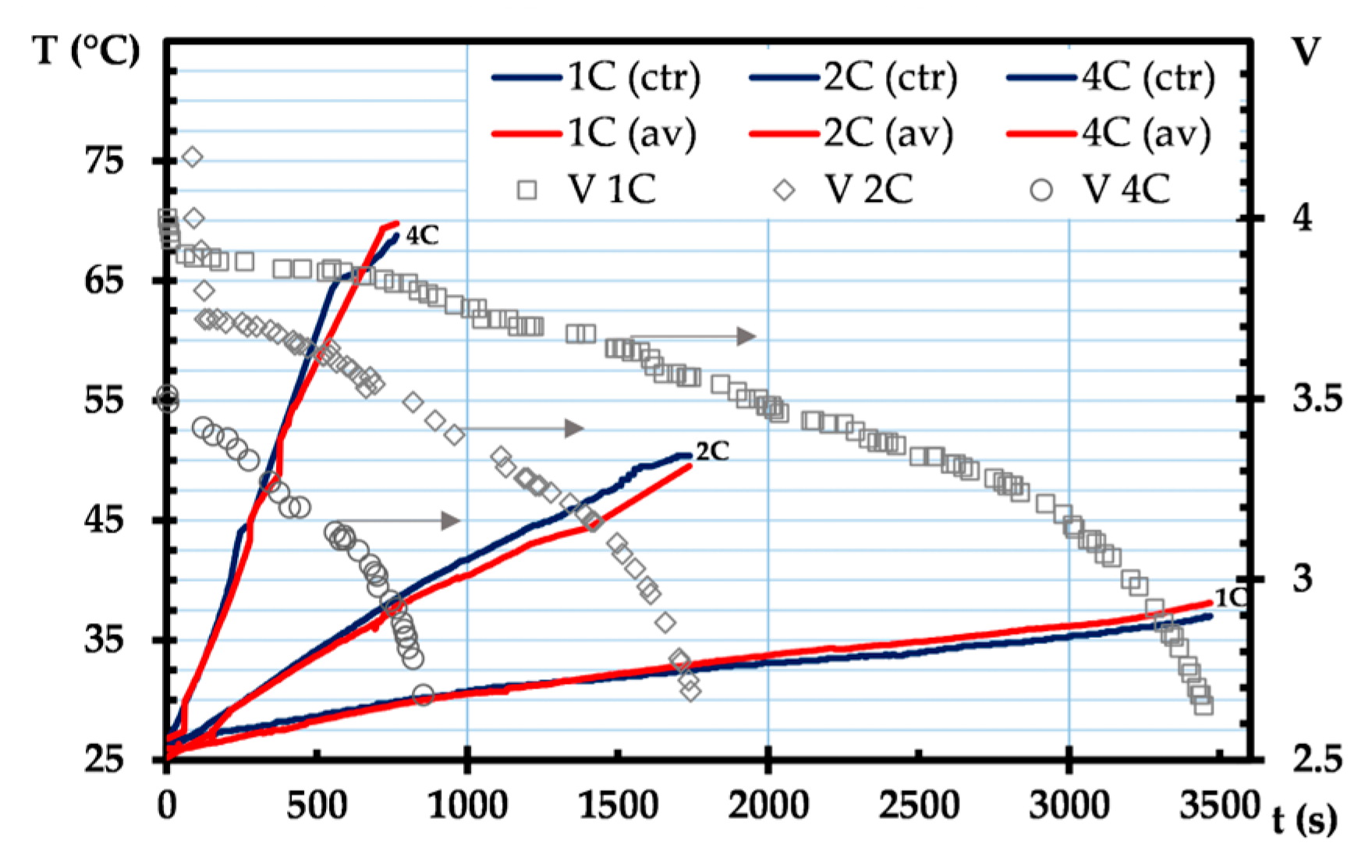
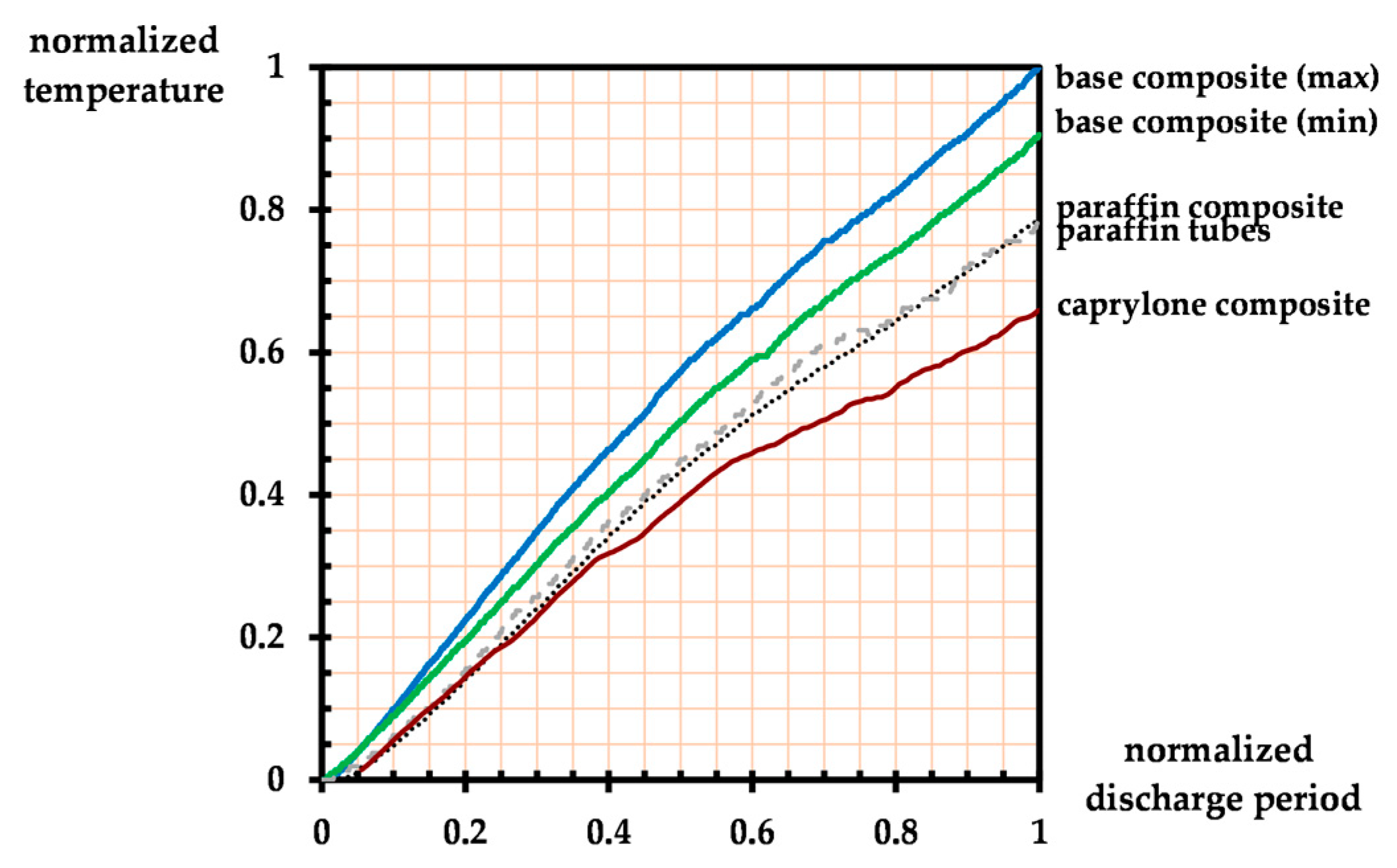
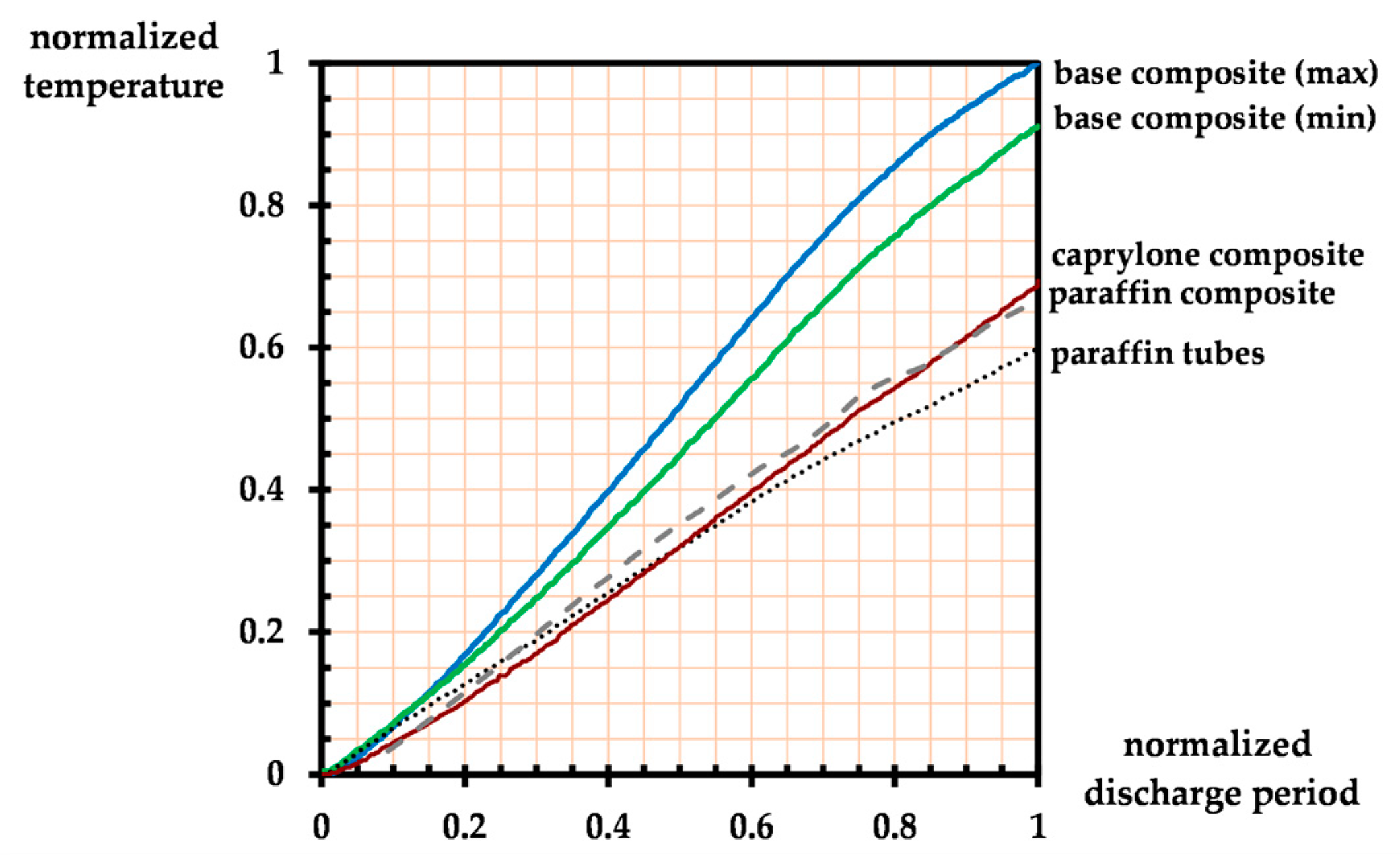
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, Z.Y.; Li, H.B.; Qu, Z.G.; Zhang, J.F. Recent Progress in Lithium-Ion Battery Thermal Management for a Wide Range of Temperature and Abuse Conditions. Int. J. Hydrogen Energy 2022, 47, 9428–9459. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal Runaway Mechanism of Lithium Ion Battery for Electric Vehicles: A Review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Kaleg, S.; Budiman, A.C.; Hapid, A.; Amin; Muharam, A.; Sudirja; Ariawan, D.; Diharjo, K. Evaluations of Aluminum Tri-Hydroxide and Pristine Montmorillonite in Glass Fiber Reinforced Polymer for Vehicle Components. Int. J. Automot. Mech. Eng. 2022, 19, 9379–9390. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, X.; Negnevitsky, M. Connecting Battery Technologies for Electric Vehicles from Battery Materials to Management. iScience 2022, 25, 103744. [Google Scholar] [CrossRef]
- Börger, A.; Mertens, J.; Wenzl, H. Thermal Runaway and Thermal Runaway Propagation in Batteries: What Do We Talk About? J. Energy Storage 2019, 24, 100649. [Google Scholar] [CrossRef]
- Shan, T.; Wang, Z.; Zhu, X.; Wang, H.; Zhou, Y.; Wang, Y.; Zhang, J.; Sun, Z. Explosion Behavior Investigation and Safety Assessment of Large-Format Lithium-Ion Pouch Cells. J. Energy Chem. 2022, 72, 241–257. [Google Scholar] [CrossRef]
- Behi, H.; Karimi, D.; Behi, M.; Jaguemont, J.; Ghanbarpour, M.; Behnia, M.; Berecibar, M.; Van Mierlo, J. Thermal Management Analysis Using Heat Pipe in the High Current Discharging of Lithium-Ion Battery in Electric Vehicles. J. Energy Storage 2020, 32, 101893. [Google Scholar] [CrossRef]
- Zhi, M.; Fan, R.; Yang, X.; Zheng, L.; Yue, S.; Liu, Q.; He, Y. Recent Research Progress on Phase Change Materials for Thermal Management of Lithium-Ion Batteries. J. Energy Storage 2022, 45, 103694. [Google Scholar] [CrossRef]
- Budiman, A.C.; Hasheminejad, S.M.; Sudirja, S.; Mitayani, A.; Winoto, S.H. Visualization of Induced Counter-Rotating Vortices for Electric Vehicles Battery Module Thermal Management. Front. Heat Mass Transf. 2022, 19, 1–6. [Google Scholar] [CrossRef]
- Jarrett, A.; Kim, I.Y. Design Optimization of Electric Vehicle Battery Cooling Plates for Thermal Performance. J. Power Sources 2011, 196, 10359–10368. [Google Scholar] [CrossRef]
- Wu, W.; Wang, S.; Wu, W.; Chen, K.; Hong, S.; Lai, Y. A Critical Review of Battery Thermal Performance and Liquid Based Battery Thermal Management. Energy Convers. Manag. 2019, 182, 262–281. [Google Scholar] [CrossRef]
- Ianniciello, L.; Biwolé, P.H.; Achard, P. Electric Vehicles Batteries Thermal Management Systems Employing Phase Change Materials. J. Power Sources 2018, 378, 383–403. [Google Scholar] [CrossRef]
- Liu, C.; Xu, D.; Weng, J.; Zhou, S.; Li, W.; Wan, Y.; Jiang, S.; Zhou, D.; Wang, J.; Huang, Q. Phase Change Materials Application in Battery Thermal Management System: A Review. Materials 2020, 13, 4622. [Google Scholar] [CrossRef] [PubMed]
- Budiman, A.C.; Kaleg, S.; Sudirja; Amin; Hapid, A. Passive Thermal Management of Battery Module Using Paraffin-Filled Tubes: An Experimental Investigation. Eng. Sci. Technol. Int. J. 2022, 29, 101031. [Google Scholar] [CrossRef]
- Talluri, T.; Kim, T.H.; Shin, K.J. Analysis of a Battery Pack with a Phase Change Material for the Extreme Temperature Conditions of an Electrical Vehicle. Energies 2020, 13, 507. [Google Scholar] [CrossRef]
- Rao, Z.; Wang, S.; Wu, M.; Lin, Z.; Li, F. Experimental Investigation on Thermal Management of Electric Vehicle Battery with Heat Pipe. Energy Convers. Manag. 2013, 65, 92–97. [Google Scholar] [CrossRef]
- Huang, Q.; Li, X.; Zhang, G.; Zhang, J.; He, F.; Li, Y. Experimental Investigation of the Thermal Performance of Heat Pipe Assisted Phase Change Material for Battery Thermal Management System. Appl. Therm. Eng. 2018, 141, 1092–1100. [Google Scholar] [CrossRef]
- Wrobel, R.; Reay, D. Heat Pipe Based Thermal Management of Electrical Machines—A Feasibility Study. Therm. Sci. Eng. Prog. 2022, 33, 101366. [Google Scholar] [CrossRef]
- Lyu, Y.; Siddique, A.R.M.; Majid, S.H.; Biglarbegian, M.; Gadsden, S.A.; Mahmud, S. Electric Vehicle Battery Thermal Management System with Thermoelectric Cooling. Energy Rep. 2019, 5, 822–827. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Zhang, Y.; Lv, S.; Ni, H.; Deng, Y.; Yuan, Y. A Review of the Power Battery Thermal Management System with Different Cooling, Heating and Coupling System. Energies 2022, 15, 1963. [Google Scholar] [CrossRef]
- Can, A.; Selimefendigil, F.; Öztop, H.F. A Review on Soft Computing and Nanofluid Applications for Battery Thermal Management. J. Energy Storage 2022, 53, 105214. [Google Scholar] [CrossRef]
- Wrobel, R. A Technology Overview of Thermal Management of Integrated Motor Drives—Electrical Machines. Therm. Sci. Eng. Prog. 2022, 29, 101222. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Li, Y.Z.; Wang, J.; Xiao, X.; Guo, W. Conception and Experimental Investigation of a Hybrid Temperature Control Method Using Phase Change Material for Permanent Magnet Synchronous Motors. Exp. Therm. Fluid Sci. 2017, 81, 9–20. [Google Scholar] [CrossRef]
- Jaguemont, J.; Omar, N.; Van den Bossche, P.; Mierlo, J. Phase-Change Materials (PCM) for Automotive Applications: A Review. Appl. Therm. Eng. 2018, 132, 308–320. [Google Scholar] [CrossRef]
- Xu, B.; Ordonez, J.; Rao, Z.; Xu, X.; Shabgard, H. Innovative Applications of Advanced Solar Thermal Technologies Using Phase Change Materials. Int. J. Photoenergy 2018, 2018, 5707264. [Google Scholar] [CrossRef]
- Abdulmunem, A.R. Passive Cooling by Utilizing the Combined PCM/Aluminum Foam Matrix to Improve Solar Panels Performance: Indoor Investigation. Iraqi J. Mech. Mater. Eng. 2017, 17, 712–723. [Google Scholar]
- Abdulmunem, A.R.; Mohd, P.; Abdul, H.; Hussien, H.A.; Ghazali, H. A Novel Thermal Regulation Method for Photovoltaic Panels Using Porous Metals Filled with Phase Change Material and Nanoparticle Additives. J. Energy Storage 2021, 39, 102621. [Google Scholar] [CrossRef]
- Takudzwa Muzhanje, A.; Hassan, M.A.; Hassan, H. Phase Change Material Based Thermal Energy Storage Applications for Air Conditioning: Review. Appl. Therm. Eng. 2022, 214, 118832. [Google Scholar] [CrossRef]
- Indartono, Y.S.; Suwono, A.; Pasek, A.D.; Christantho, A. Application of Phase Change Material To Save Air Conditioning Energy in Building. ASEAN Eng. J. Part A 2013, 3, 46–53. [Google Scholar]
- Cui, Y.; Xie, J.; Liu, J.; Wang, J.; Chen, S. A Review on Phase Change Material Application in Building. Adv. Mech. Eng. 2017, 9, 1–15. [Google Scholar] [CrossRef]
- Zsembinszki, G.; Fernández, A.G.; Cabeza, L.F. Selection of the Appropriate Phase Change Material for Two Innovative Compact Energy Storage Systems in Residential Buildings. Appl. Sci. 2020, 10, 2116. [Google Scholar] [CrossRef]
- Bashirpour-Bonab, H. Thermal Behavior of Lithium Batteries Used in Electric Vehicles Using Phase Change Materials. Int. J. Energy Res. 2020, 44, 12583–12591. [Google Scholar] [CrossRef]
- Kumar, R.; Mitra, A.; Srinivas, T. Role of Nano-Additives in the Thermal Management of Lithium-Ion Batteries: A Review. J. Energy Storage 2022, 48, 104059. [Google Scholar] [CrossRef]
- Chen, J.; Yang, D.; Jiang, J.; Ma, A.; Song, D. Research Progress of Phase Change Materials (PCMs) Embedded with Metal Foam (a Review). Procedia Mater. Sci. 2014, 4, 389–394. [Google Scholar] [CrossRef]
- Lazzarin, R.; Noro, M.; Righetti, G.; Mancin, S. Application of Hybrid PCM Thermal Energy Storages with and without Al Foams in Solar Heating/Cooling and Ground Source Absorption Heat Pump Plant: An Energy and Economic Analysis. Appl. Sci. 2019, 9, 1007. [Google Scholar] [CrossRef]
- Babu Sanker, S.; Baby, R. Phase Change Material Based Thermal Management of Lithium Ion Batteries: A Review on Thermal Performance of Various Thermal Conductivity Enhancers. J. Energy Storage 2022, 50, 104606. [Google Scholar] [CrossRef]
- Mehrabi-Kermani, M.; Houshfar, E.; Ashjaee, M. A Novel Hybrid Thermal Management for Li-Ion Batteries Using Phase Change Materials Embedded in Copper Foams Combined with Forced-Air Convection. Int. J. Therm. Sci. 2019, 141, 47–61. [Google Scholar] [CrossRef]
- Guimarães, T.C.; Gomes, O.d.F.M.; Oliveira de Araujo, O.M.; Tadeu Lopes, R.; da Gloria, M.Y.R.; Toledo Filho, R.D.; Koenders, E.; Caggiano, A.; Mankel, C.; Nazari Sam, M.; et al. PCM-Impregnated Textile-Reinforced Cementitious Composite for Thermal Energy Storage. Textiles 2023, 3, 98–114. [Google Scholar] [CrossRef]
- Kadam, G.; Kongi, P. Battery Thermal Management System Based on PCM with Addition of Nanoparticles. Mater. Today Proc. 2023, 72, 1543–1549. [Google Scholar] [CrossRef]
- Rathore, P.K.S.; Shukla, S.K. Potential of Macroencapsulated Pcm for Thermal Energy Storage in Buildings: A Comprehensive Review. Constr. Build. Mater. 2019, 225, 723–744. [Google Scholar] [CrossRef]
- Kheradmand, M.; Castro-Gomes, J.; Azenha, M.; Silva, P.D.; De Aguiar, J.L.B.; Zoorob, S.E. Assessing the Feasibility of Impregnating Phase Change Materials in Lightweight Aggregate for Development of Thermal Energy Storage Systems. Constr. Build. Mater. 2015, 89, 48–59. [Google Scholar] [CrossRef]
- Adesina, A. Use of Phase Change Materials in Concrete: Current Challenges. Renew. Energy Environ. Sustain. 2019, 4, 9. [Google Scholar] [CrossRef]
- Wang, F.; Nasajpour-Esfahani, N.; Alizadeh, A.; Fadhil Smaisim, G.; Abed, A.M.; Hadrawi, S.K.; Aminian, S.; Sabetvand, R.; Toghraie, D. Thermal Performance of a Phase Change Material (PCM) Microcapsules Containing Au Nanoparticles in a Nanochannel: A Molecular Dynamics Approach. J. Mol. Liq. 2023, 373, 121128. [Google Scholar] [CrossRef]
- Memon, S.A.; Cui, H.; Lo, T.Y.; Li, Q. Development of Structural-Functional Integrated Concrete with Macro-Encapsulated PCM for Thermal Energy Storage. Appl. Energy 2015, 150, 245–257. [Google Scholar] [CrossRef]
- Liu, T.; Hu, J.; Tao, C.; Zhu, X.; Wang, X. Effect of Parallel Connection on 18650-Type Lithium Ion Battery Thermal Runaway Propagation and Active Cooling Prevention with Water Mist. Appl. Therm. Eng. 2021, 184, 116291. [Google Scholar] [CrossRef]
- Huda, N.; Kaleg, S.; Hapid, A.; Kurnia, M.R.; Budiman, A.C. The Influence of the Regenerative Braking on the Overall Energy Consumption of a Converted Electric Vehicle. SN Appl. Sci. 2020, 2, 606. [Google Scholar] [CrossRef]
- Deng, Y.; Feng, C.; E, J.; Zhu, H.; Chen, J.; Wen, M.; Yin, H. Effects of Different Coolants and Cooling Strategies on the Cooling Performance of the Power Lithium Ion Battery System: A Review. Appl. Therm. Eng. 2018, 142, 10–29. [Google Scholar] [CrossRef]
- Zhao, Y.; Patel, Y.; Zhang, T.; Offer, G.J. Modeling the Effects of Thermal Gradients Induced by Tab and Surface Cooling on Lithium Ion Cell Performance. J. Electrochem. Soc. 2018, 165, A3169–A3178. [Google Scholar] [CrossRef]
- Budiman, A.C.; Kaleg, S.; Hidayat, N.A.; Silalahi, G.N.; Gani, M.N.; Sudirja; Amin; Muharam, A.; Hapid, A. Experimental Study of Two Organic Phase Change Materials in Cylindrical Containers for Battery Module Thermal Management: A Comparative Analysis. J. Phys. Conf. Ser. 2021, 2047, 012018. [Google Scholar] [CrossRef]
- Muresanu, A.D.; Dudescu, M.C. Numerical and Experimental Evaluation of a Battery Cell under Impact Load. Batteries 2022, 8, 48. [Google Scholar] [CrossRef]
- Arora, S.; Kapoor, A.; Shen, W. Application of Robust Design Methodology to Battery Packs for Electric Vehicles: Identification of Critical Technical Requirements for Modular Architecture. Batteries 2018, 4, 30. [Google Scholar] [CrossRef]
- Bulla, M.; Schmandt, C.; Kolling, S.; Kisters, T.; Sahraei, E. An Experimental and Numerical Study on Charged 21700 Lithium-Ion Battery Cells under Dynamic and High Mechanical Loads. Energies 2023, 16, 211. [Google Scholar] [CrossRef]
- Kim, K.M.; Jeong, Y.S.; Bang, I.C. Thermal Analysis of Lithium Ion Battery-Equipped Smartphone Explosions. Eng. Sci. Technol. Int. J. 2019, 22, 610–617. [Google Scholar] [CrossRef]
- Zhao, Y.; Zou, B.; Zhang, T.; Jiang, Z.; Ding, J.; Ding, Y. A Comprehensive Review of Composite Phase Change Material Based Thermal Management System for Lithium-Ion Batteries. Renew. Sustain. Energy Rev. 2022, 167, 112667. [Google Scholar] [CrossRef]
- Mevawalla, A.; Panchal, S.; Tran, M.K.; Fowler, M.; Fraser, R. Mathematical Heat Transfer Modeling and Experimental Validation of Lithium-Ion Battery Considering: Tab and Surface Temperature, Separator, Electrolyte Resistance, Anode-Cathode Irreversible and Reversible Heat. Batteries 2020, 6, 61. [Google Scholar] [CrossRef]
- Sobianowska-Turek, A.; Urbańska, W.; Janicka, A.; Zawiślak, M.; Matla, J. The Necessity of Recycling of Waste Li-ion Batteries Used in Electric Vehicles as Objects Posing a Threat to Human Health and the Environment. Recycling 2021, 6, 35. [Google Scholar] [CrossRef]






| Parameter | Value |
|---|---|
| Rated capacity | 4800 mAh |
| Nominal voltage | 3.7 V |
| Max. charge voltage | 4.2 V |
| Discharge cut-off voltage | 2.75 V |
| Dimensions | 21.2 ± 0.3 mm (diameter), 70.3 ± 0.5 mm (height) |
| Mass | 0.07 kg |
| Discharge temperature range | −20–60 °C |
| Expected cycle life | 500 cycles > 80% |
| Parameter | No PCM | Paraffin | Paraffin + Graphite |
|---|---|---|---|
| Ultimate force (N) | 604 ± 16 | 225 ± 16 | 140 ± 35 |
| Ultimate stress (MPa) | 62.2 ± 0.9 | 23.2 ± 3.8 | 10.1 ± 2.5 |
| Modulus (MPa) | 1835 ± 135 | 598 ± 101 | 349 ± 36 |
| Total elongation (%) | 3.56 ± 0.02 | 3.75 ± 0.54 | 3.63 ± 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budiman, A.C.; Azzopardi, B.; Sudirja; Perdana, M.A.P.; Kaleg, S.; Hadiastuti, F.S.; Hasyim, B.A.; Amin; Ristiana, R.; Muharam, A.; et al. Phase Change Material Composite Battery Module for Thermal Protection of Electric Vehicles: An Experimental Observation. Energies 2023, 16, 3896. https://doi.org/10.3390/en16093896
Budiman AC, Azzopardi B, Sudirja, Perdana MAP, Kaleg S, Hadiastuti FS, Hasyim BA, Amin, Ristiana R, Muharam A, et al. Phase Change Material Composite Battery Module for Thermal Protection of Electric Vehicles: An Experimental Observation. Energies. 2023; 16(9):3896. https://doi.org/10.3390/en16093896
Chicago/Turabian StyleBudiman, Alexander C., Brian Azzopardi, Sudirja, Muhammad A. P. Perdana, Sunarto Kaleg, Febriani S. Hadiastuti, Bagus A. Hasyim, Amin, Rina Ristiana, Aam Muharam, and et al. 2023. "Phase Change Material Composite Battery Module for Thermal Protection of Electric Vehicles: An Experimental Observation" Energies 16, no. 9: 3896. https://doi.org/10.3390/en16093896
APA StyleBudiman, A. C., Azzopardi, B., Sudirja, Perdana, M. A. P., Kaleg, S., Hadiastuti, F. S., Hasyim, B. A., Amin, Ristiana, R., Muharam, A., & Hapid, A. (2023). Phase Change Material Composite Battery Module for Thermal Protection of Electric Vehicles: An Experimental Observation. Energies, 16(9), 3896. https://doi.org/10.3390/en16093896









