The Colourimetric Method for Mixing Time Measurement in Single-Use and Multi-Use Bioreactors—Methodology Overview and Practical Recommendations
Abstract
:1. Introduction
- Section 2. Applications of the Colourimetric Method for Reactor and Bioreactor Studies contains a catalogue of existing papers, which contains results based on the application of the colourimetric method, a discussion about the types of devices in which the colourimetric method has been applied, a comparison of mixing time measurement methods based on experimental data and a discussion about the advantages and shortcomings of visual and computerised image processing.
- Section 3. Laboratory Procedure Considerations contains a listing of colour-changing chemicals that can be used with the colourimetric method, recommendations regarding a typical laboratory procedure for mixing time measurement and a summary of liquids of various rheological characteristics previously analysed in colourimetric method setups.
- Section 4. Image Acquisition contains a discussion about the cases where special adaptation of the camera equipment to the reactor was necessary, with a focus on the acquisition of high-quality image data and a set of recommendations for the camera equipment, image parameters that need to be considered during image acquisition and proper lighting of the experimental setup.
- Section 5. Image Processing contains recommendations for the final step of colourimetric method application, i.e., the suggested order and description of operations during digital image processing, a summary of colour spaces that can be considered in the processing, the definitions of mixing time that have been previously applied in different studies, a discussion about local mixing time and mixing time maps and a summary of software tools that can be used for the application of the colourimetric method.
2. Applications of the Colourimetric Method for Reactor and Bioreactor Studies
2.1. Applications of the Colourimetric Method across Types of Devices
2.2. Comparisons between Mixing Time Measurement Methods
- the sensor method, based on recording temporal changes in the readings of one or more sensors placed inside the mixed liquid, including
- ○
- the thermal method, in which temperature sensors such as thermocouples monitor the changes in liquid temperature after injection of a small portion of liquid of a different temperature [46];
- ○
- the conductivity and pH methods, which rely on measuring changes in conductivity or pH after introducing a tracer with conductometric or pH electrodes [47].
- planar laser-induced fluorescence (PLIF), in which the mixing is observed through the dispersion of a fluorescent dye excited by a sheet of laser light, resulting in the emission of light at a specific wavelength [48].
2.3. Visual Observations and Computerised Image Processing
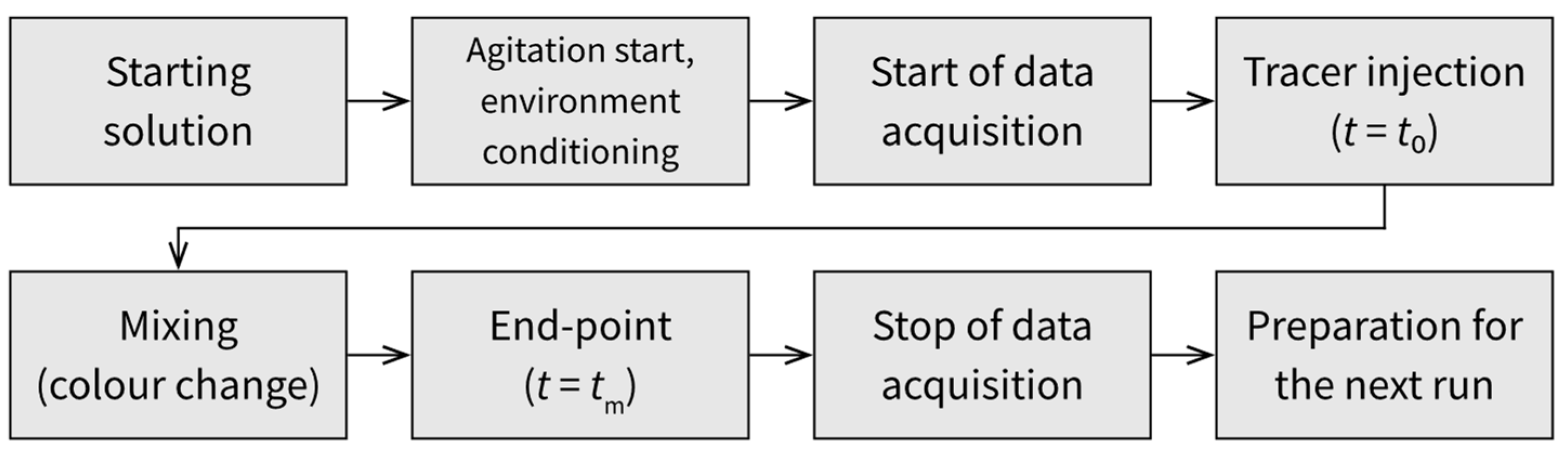
3. Laboratory Procedure Considerations
- Physical methods (without a chemical reaction)—observation of the mixing process is based on the temporal changes in concentration of a dye dissolving in the bulk of the liquid. The dye does not change its chemical structure during mixing. Examples of dyes that have been used in studies with the colourimetric method are methylene blue [26], Cochineal Red [32], Purple Drimarene R 2 RL [43] and Patent Blue V E131 [45].
- Chemical methods (with a chemical reaction)—the progress of the mixing process is assumed using the colour change in a chemical, which changes its properties based on an instantaneous chemical reaction [49]. The frequently used chemical reactions are
- ○
- A redox reaction between iodine/triiodide (i.e., / ions) and thiosulfate anions, , in a starch solution [13], usually called the iodometric reaction. During the reaction, the iodine/triiodide is reduced into an iodide, , anion, which makes the dissolved starch change from deep purple to colourless. This reaction was applied during studies of multi-use reactors [13,14,15,17,25] and single-use bag-like containers [34,35,39]. The disadvantage of the iodometric reaction with applications in SUB setups is the possible interaction between iodine and the polymer film of the single-use bag, which can lead to staining and gradual deterioration of the optical properties of the disposable container.
- ○
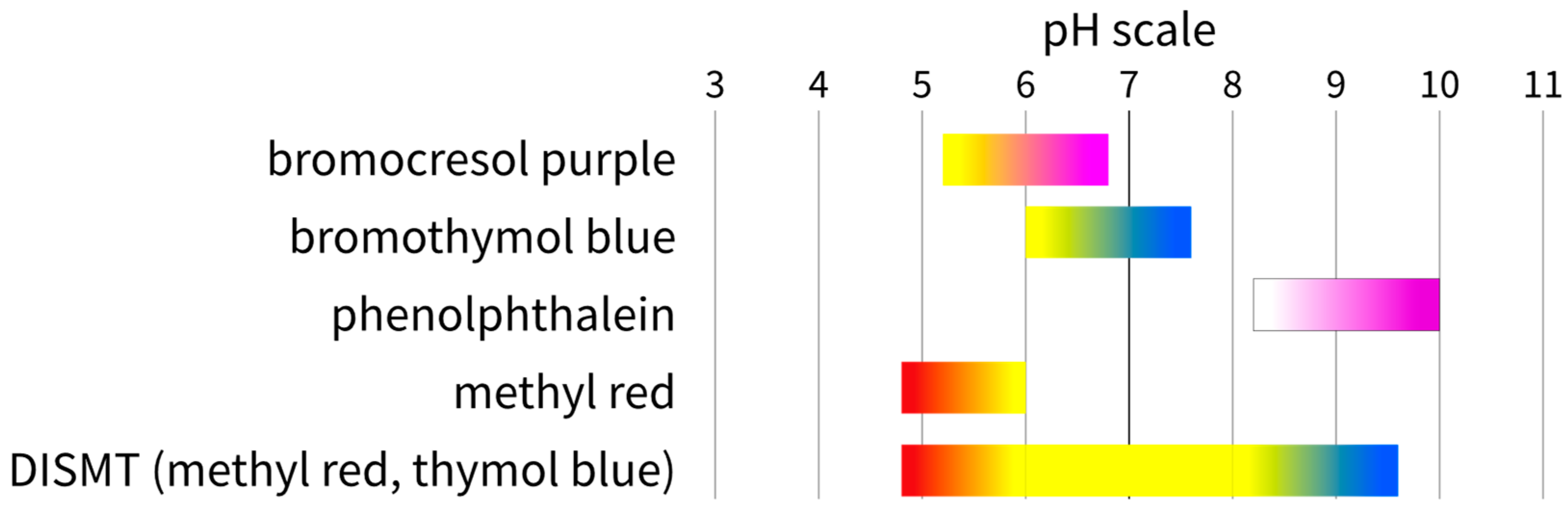
- An acid–base neutralisation reaction in the presence of one or more pH indicators. The chemicals used during the experiments were usually strong bases and acids, like sodium hydroxide and hydrochloric acid, at various concentrations depending on the setup and the indicator pH colour change range. One or more indicators in one solution can be added during the experiments. Examples of the pH indicator systems that have been used with the colourimetric method are listed in Table 2. The colour ranges of the indicator are summarized in Figure 1.
4. Image Acquisition
4.1. Properties of the Device for Characterisation
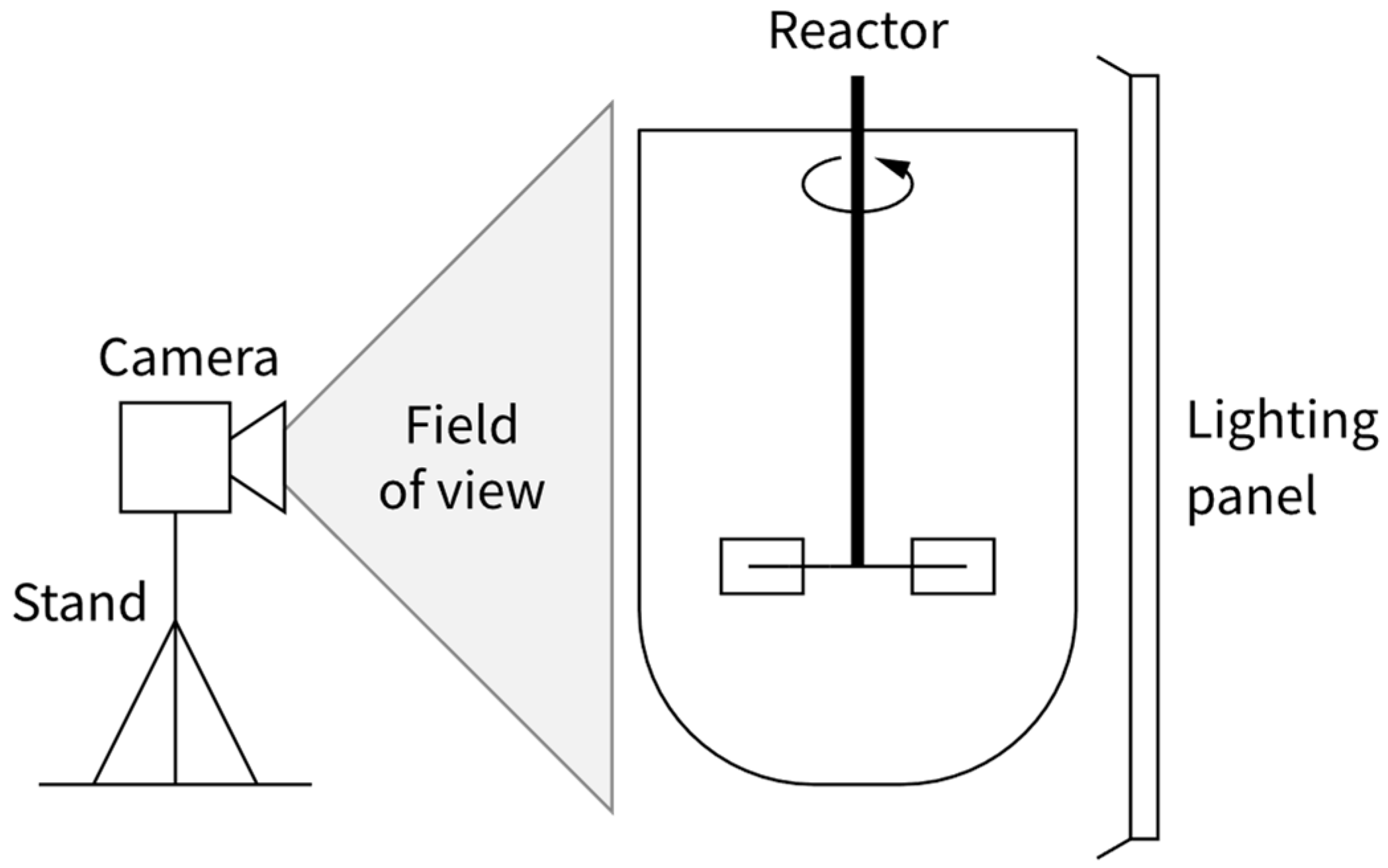
4.2. Filming Equipment (Camera, Lighting)
- Video resolution, being the number of pixels along the width and height of each frame. The resolution impacts the spatial resolution of frames related to the physical dimensions of the vessel being filmed and, generally, the amount of detail that can be captured and further retrieved from the material.
- The frame rate, equal to the number of frames captured during one second, which determines the maximum temporal resolution of the measurement. The frame rate should be a multiple of the alternating current frequency in the electrical grid to eliminate flicker from lighting equipment.
- The bitrate, which is the amount of video data saved in a unit of time, usually given in kilobits per second. In general, the higher the video bitrate, the lower the compression and the more detail can be saved onto each frame. This setting is often controlled by the camera—the bitrate changes in time depending on the complexity of a given video segment.
- The lens focal length influencing the shape and relative size of objects on the focal plane depending on their placement in the frame. A lens or a camera setting that produces a rectilinear image with little barrel distortion should be used to avoid errors related to the parts of the vessel near the frame edge appearing too large relative to the centre of the frame. Understandably, there is a trade-off between the value of focal length, camera field of view and distance between the camera and the observed device.
- White balance, which is an adjustment of the relative intensity of colours and influences their temperature in relation to the light source’s colour temperature. It is recommended that the white balance parameter value is set manually to the value corresponding to the colour temperature of lighting used in the setup. If the white balance adjustment were to be left automatic, the average hue in the frame could improperly skew during the mixing process as the indicator colour gradually changes.
- The exposure value, shutter speed, f-number and ISO, i.e., the parameters that influence the exposure of the resulting images. Values of these parameters should be adjusted to obtain frames with good brightness and contrast and will depend on the intensity of light sources illuminating the frame. Values of the parameters should be set as constant during filming to prevent the camera from compensating as the image changes relative to brightness during the colour change.
5. Image Processing
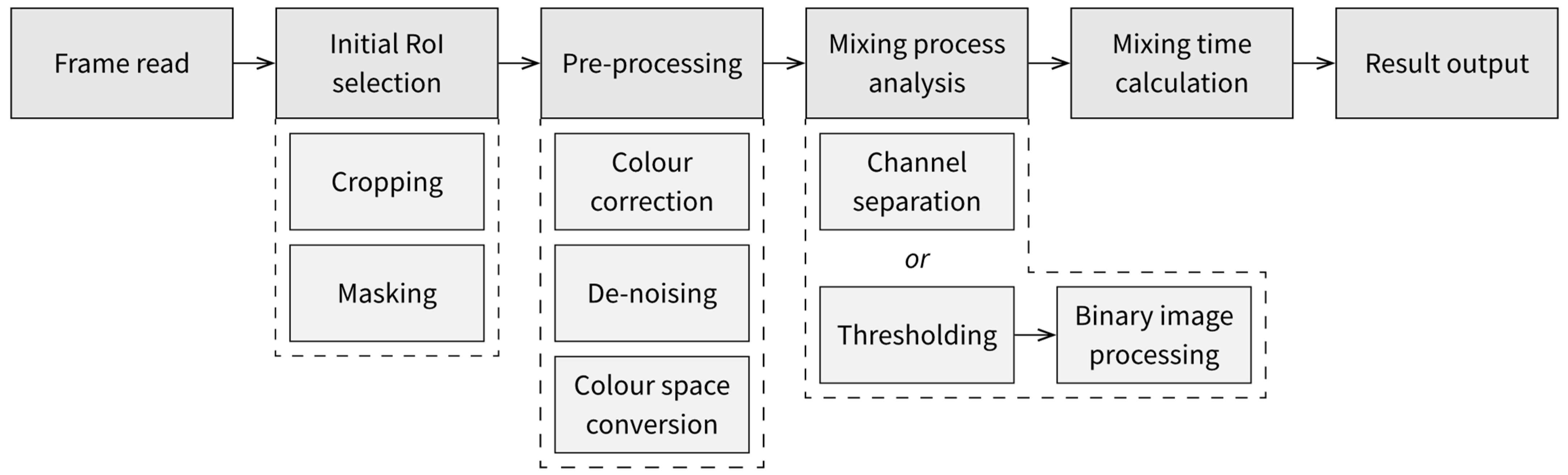
5.1. Processing Algorithm
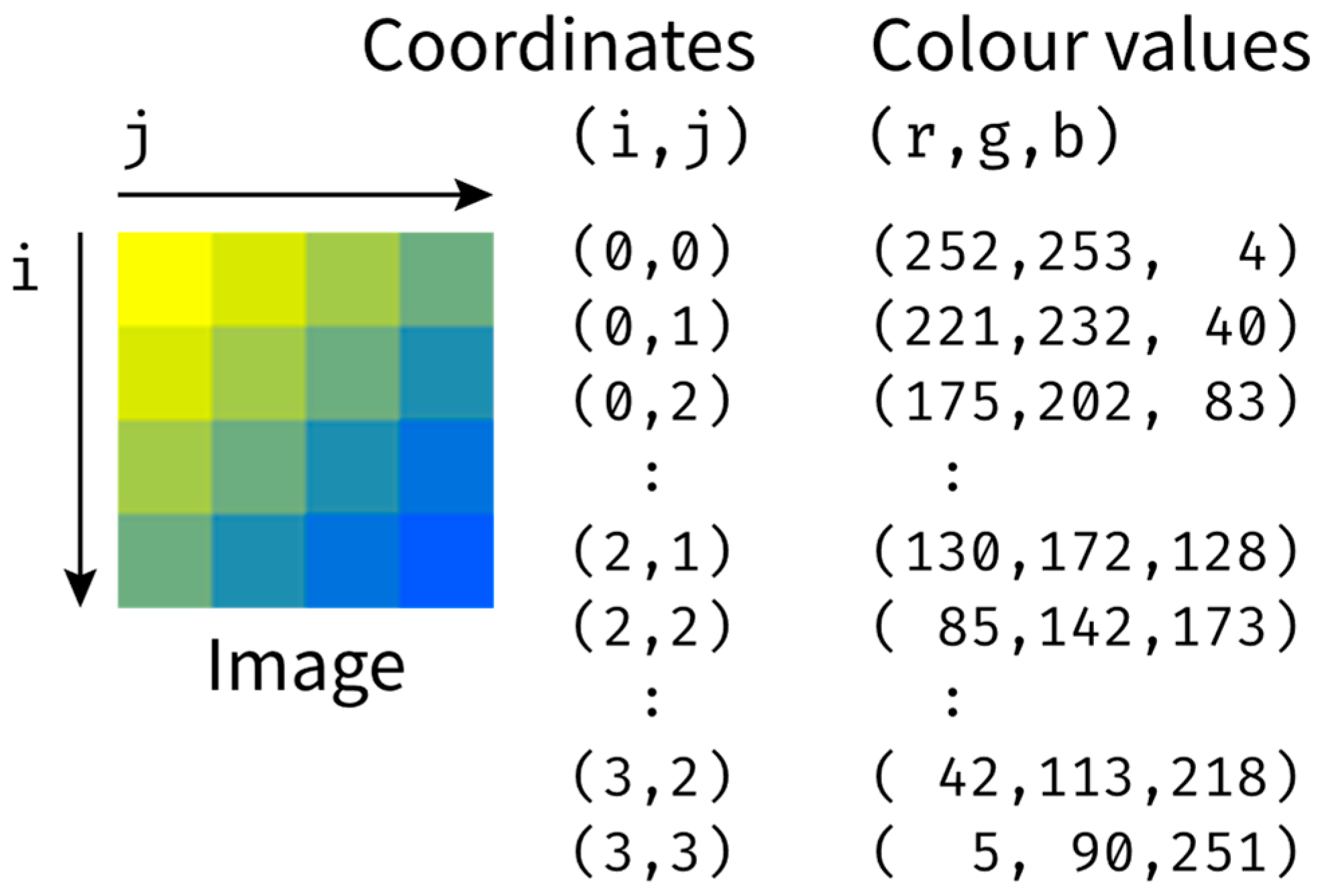
- The first approach to obtaining data about the mixing process is to perform separation of the image’s colour channels, select one of the components and directly observe changes in the values of the component, either averaged across the whole domain or divided into subdomains or individual pixels.
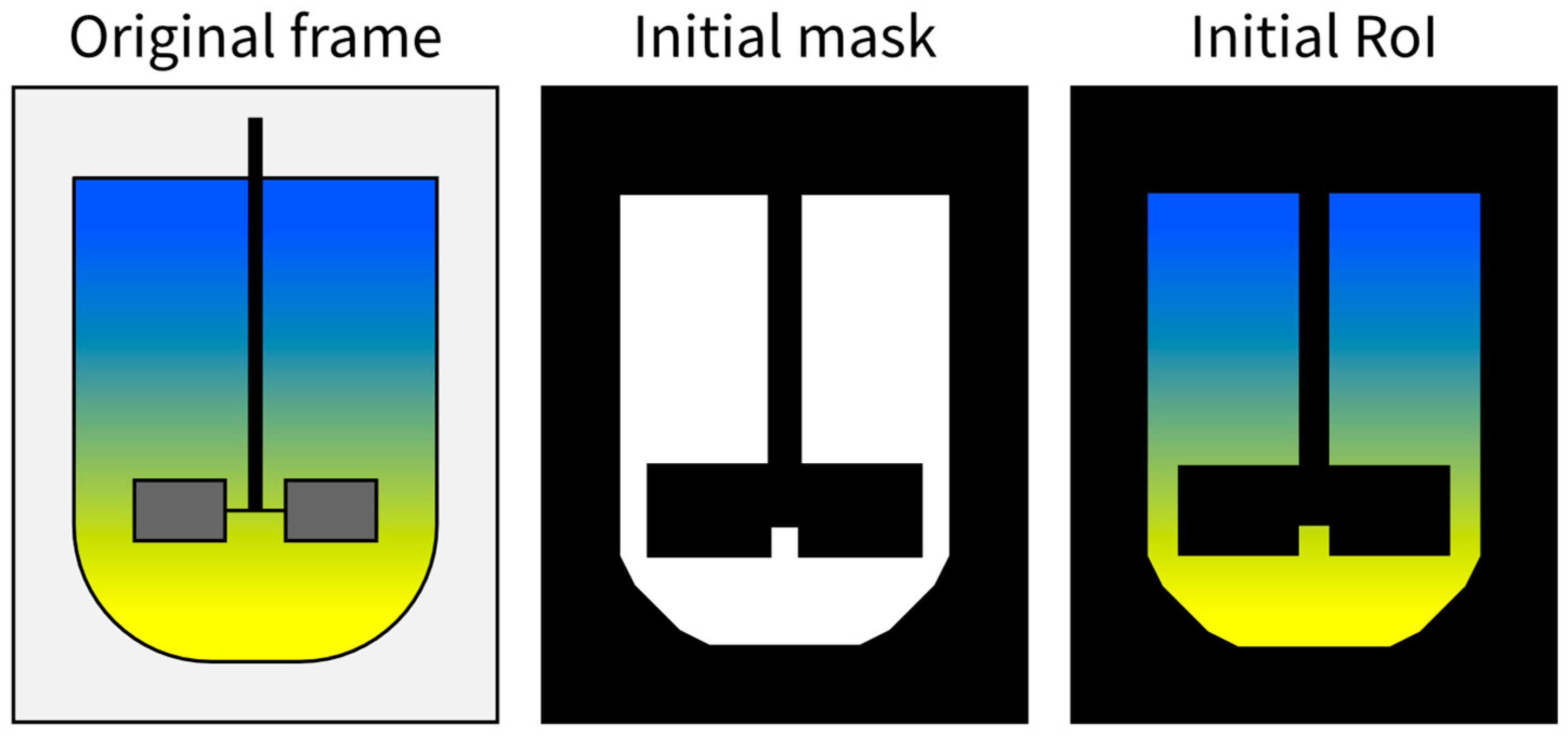
- The second approach, which could be used when the apparent shape of the observed liquid changes in time, is to find the areas corresponding to each discernible state of mixedness through thresholding. The thresholding will require the selection of colour ranges corresponding to each state of the applied indicators, which, if the video material is filmed at consistent filming parameters, should be performed once for the whole series of measurements. The binary masks resulting from thresholding can be improved with binary image operations, such as erosion and dilation or opening and closing [59].
5.2. Colour Spaces
5.3. Mixing Time Value Calculation
5.3.1. Global Mixing Time
5.3.2. Local Mixing Time, Mixing Time Maps
5.4. Software Tools
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Godleski, E.S.; Smith, J.C. Power Requirements and Blend Times in the Agitation of Pseudoplastic Fluids. AIChE J. 1962, 8, 617–620. [Google Scholar] [CrossRef]
- Gaugler, L.; Mast, Y.; Fitschen, J.; Hofmann, S.; Schlüter, M.; Takors, R. Scaling-down Biopharmaceutical Production Processes via a Single Multi-compartment Bioreactor (SMCB). Eng. Life Sci. 2023, 23, e2100161. [Google Scholar] [CrossRef] [PubMed]
- Rosseburg, A.; Fitschen, J.; Wutz, J.; Wucherpfennig, T.; Schlüter, M. Hydrodynamic Inhomogeneities in Large Scale Stirred Tanks—Influence on Mixing Time. Chem. Eng. Sci. 2018, 188, 208–220. [Google Scholar] [CrossRef]
- Ascanio, G. Mixing Time in Stirred Vessels: A Review of Experimental Techniques. Chin. J. Chem. Eng. 2015, 23, 1065–1076. [Google Scholar] [CrossRef]
- Hillig, F.; Pilarek, M.; Junne, S.; Neubauer, P. Cultivation of Marine Microorganisms in Single-Use Systems. In Disposable Bioreactors II; Eibl, D., Eibl, R., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2013; Volume 138, pp. 179–206. ISBN 978-3-642-45157-7. [Google Scholar]
- Tan, R.-K.; Eberhard, W.; Büchs, J. Measurement and Characterization of Mixing Time in Shake Flasks. Chem. Eng. Sci. 2011, 66, 440–447. [Google Scholar] [CrossRef]
- Li, L.; Wang, K.; Zhao, Q.; Gao, Q.; Zhou, H.; Jiang, J.; Mei, W. A Critical Review of Experimental and CFD Techniques to Characterize the Mixing Performance of Anaerobic Digesters for Biogas Production. Rev. Environ. Sci. Bio/Technol. 2022, 21, 665–689. [Google Scholar] [CrossRef]
- Brown, D.A.R.; Jones, P.N.; Middleton, J.C.; Papadopoulos, G.; Arik, E.B. Experimental Methods. In Handbook of Industrial Mixing; Paul, E.L., Atiemo-Obeng, V.A., Kresta, S.M., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2003; pp. 145–256. ISBN 978-0-471-26919-9. [Google Scholar]
- Löffelholz, C.; Husemann, U.; Greller, G.; Meusel, W.; Kauling, J.; Ay, P.; Kraume, M.; Eibl, R.; Eibl, D. Bioengineering Parameters for Single-Use Bioreactors: Overview and Evaluation of Suitable Methods. Chem. Ing. Tech. 2013, 85, 40–56. [Google Scholar] [CrossRef]
- Cabaret, F.; Bonnot, S.; Fradette, L.; Tanguy, P.A. Mixing Time Analysis Using Colorimetric Methods and Image Processing. Ind. Eng. Chem. Res. 2007, 46, 5032–5042. [Google Scholar] [CrossRef]
- Fox, E.A.; Gex, V.E. Single-Phase Blending of Liquids. AIChE J. 1956, 2, 539–544. [Google Scholar] [CrossRef]
- Norwood, K.W.; Metzner, A.B. Flow Patterns and Mixing Rates in Agitated Vessels. AIChE J. 1960, 6, 432–437. [Google Scholar] [CrossRef]
- Carreau, P.J.; Patterson, I.; Yap, C.Y. Mixing of Viscoelastic Fluids with Helical-Ribbon Agitators. I—Mixing Time and Flow Patterns. Can. J. Chem. Eng. 1976, 54, 135–142. [Google Scholar] [CrossRef]
- Haß, V.C.; Nienow, A.W. Ein Neuer, Axial Fördernder Rührer Zum Dispergieren von Gas in Flüssigkeiten: Ein Neuer, Axial Fördernder Rührer Zum Dispergieren von Gas in Flüssigkeiten. Chem. Ing. Tech. 1989, 61, 152–154. [Google Scholar] [CrossRef]
- Saito, F.; Nienow, A.W.; Chatwin, S.; Moore, I.P.T. Power, Gas Dispersion and Homogenisation Characteristics of Scaba SRGT and Rushton Turbine Impellers. J. Chem. Eng. Jpn. JCEJ 1992, 25, 281–287. [Google Scholar] [CrossRef]
- Lamberto, D.J.; Muzzio, F.J.; Swanson, P.D.; Tonkovich, A.L. Using Time-Dependent RPM to Enhance Mixing in Stirred Vessels. Chem. Eng. Sci. 1996, 51, 733–741. [Google Scholar] [CrossRef]
- Kraume, M.; Zehner, P. Experience with Experimental Standards for Measurements of Various Parameters in Stirred Tanks. Chem. Eng. Res. Des. 2001, 79, 811–818. [Google Scholar] [CrossRef]
- Delaplace, G.; Bouvier, L.; Moreau, A.; Guérin, R.; Leuliet, J.-C. Determination of Mixing Time by Colourimetric Diagnosis—Application to a New Mixing System. Exp. Fluids 2004, 36, 437–443. [Google Scholar] [CrossRef]
- Foucault, S.; Ascanio, G.; Tanguy, P.A. Mixing Times in Coaxial Mixers with Newtonian and Non-Newtonian Fluids. Ind. Eng. Chem. Res. 2006, 45, 352–359. [Google Scholar] [CrossRef]
- Bonnot, S.; Cabaret, F.; Fradette, L.; Tanguy, P.A. Characterization of Mixing Patterns in a Coaxial Mixer. Chem. Eng. Res. Des. 2007, 85, 1129–1135. [Google Scholar] [CrossRef]
- Delaplace, G.; Thakur, R.K.; Bouvier, L.; André, C.; Torrez, C. Dimensional Analysis for Planetary Mixer: Mixing Time and Reynolds Numbers. Chem. Eng. Sci. 2007, 62, 1442–1447. [Google Scholar] [CrossRef]
- Fradette, L.; Thomé, G.; Tanguy, P.A.; Takenaka, K. Power and Mixing Time Study Involving a Maxblend® Impeller with Viscous Newtonian and Non-Newtonian Fluids. Chem. Eng. Res. Des. 2007, 85, 1514–1523. [Google Scholar] [CrossRef]
- Iranshahi, A.; Devals, C.; Heniche, M.; Fradette, L.; Tanguy, P.A.; Takenaka, K. Hydrodynamics Characterization of the Maxblend Impeller. Chem. Eng. Sci. 2007, 62, 3641–3653. [Google Scholar] [CrossRef]
- Gabelle, J.-C.; Augier, F.; Carvalho, A.; Rousset, R.; Morchain, J. Effect of Tank Size on k L a and Mixing Time in Aerated Stirred Reactors with Non-newtonian Fluids. Can. J. Chem. Eng. 2011, 89, 1139–1153. [Google Scholar] [CrossRef]
- Sieblist, C.; Jenzsch, M.; Pohlscheidt, M.; Lübbert, A. Insights into Large-Scale Cell-Culture Reactors: I. Liquid Mixing and Oxygen Supply. Biotechnol. J. 2011, 6, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Allonneau, C.; Olmos, E.; Guyot, S.; Ferret, E.; Gervais, P.; Cachon, R. Hydrodynamic Characterization of a New Small-Scale Reactor Mixed by a Magnetic Bar. Biochem. Eng. J. 2015, 96, 29–37. [Google Scholar] [CrossRef]
- Trad, Z.; Fontaine, J.-P.; Larroche, C.; Vial, C. Experimental and Numerical Investigation of Hydrodynamics and Mixing in a Dual-Impeller Mechanically-Stirred Digester. Chem. Eng. J. 2017, 329, 142–155. [Google Scholar] [CrossRef]
- Chezeau, B.; Fontaine, J.P.; Vial, C. Analysis of Liquid-to-Gas Mass Transfer, Mixing and Hydrogen Production in Dark Fermentation Process. Chem. Eng. J. 2019, 372, 715–727. [Google Scholar] [CrossRef]
- Amiraftabi, M.; Khiadani, M.; Mohammed, H.A. Performance of a Dual Helical Ribbon Impeller in a Two-Phase (Gas-Liquid) Stirred Tank Reactor. Chem. Eng. Process. Process Intensif. 2020, 148, 107811. [Google Scholar] [CrossRef]
- Samaras, J.J.; Ducci, A.; Micheletti, M. Flow, Suspension and Mixing Dynamics in DASGIP Bioreactors, Part 2. AIChE J. 2020, 66, e16999. [Google Scholar] [CrossRef]
- Fitschen, J.; Hofmann, S.; Wutz, J.; Kameke, A.V.; Hoffmann, M.; Wucherpfennig, T.; Schlüter, M. Novel Evaluation Method to Determine the Local Mixing Time Distribution in Stirred Tank Reactors. Chem. Eng. Sci. X 2021, 10, 100098. [Google Scholar] [CrossRef]
- Vivek, V.; Eka, F.N.; Chew, W. Mixing Studies in an Unbaffled Bioreactor Using a Computational Model Corroborated with In-Situ Raman and Imaging Analyses. Chem. Eng. J. Adv. 2022, 9, 100232. [Google Scholar] [CrossRef]
- Tregidgo, M.; Dorn, M.; Lucas, C.; Micheletti, M. Design and Characterization of a Novel Perfusion Reactor for Biopharmaceuticals Production. Chem. Eng. Res. Des. 2023, 194, 344–357. [Google Scholar] [CrossRef]
- Kaiser, S.C.; Eibl, R.; Eibl, D. Engineering Characteristics of a Single-Use Stirred Bioreactor at Bench-Scale: The Mobius CellReady 3L Bioreactor as a Case Study. Eng. Life Sci. 2011, 11, 359–368. [Google Scholar] [CrossRef]
- Nienow, A.W.; Rielly, C.D.; Brosnan, K.; Bargh, N.; Lee, K.; Coopman, K.; Hewitt, C.J. The Physical Characterisation of a Microscale Parallel Bioreactor Platform with an Industrial CHO Cell Line Expressing an IgG4. Biochem. Eng. J. 2013, 76, 25–36. [Google Scholar] [CrossRef]
- Delbridge, J.N.; Barrett, T.A.; Ducci, A.; Micheletti, M. Power, Mixing and Flow Dynamics of the Novel AllegroTM Stirred Tank Reactor. Chem. Eng. Sci. 2023, 271, 118545. [Google Scholar] [CrossRef]
- Tissot, S.; Farhat, M.; Hacker, D.L.; Anderlei, T.; Kühner, M.; Comninellis, C.; Wurm, F. Determination of a Scale-up Factor from Mixing Time Studies in Orbitally Shaken Bioreactors. Biochem. Eng. J. 2010, 52, 181–186. [Google Scholar] [CrossRef]
- Monteil, D.T.; Tontodonati, G.; Ghimire, S.; Baldi, L.; Hacker, D.L.; Bürki, C.A.; Wurm, F.M. Disposable 600-mL Orbitally Shaken Bioreactor for Mammalian Cell Cultivation in Suspension. Biochem. Eng. J. 2013, 76, 6–12. [Google Scholar] [CrossRef]
- Werner, S.; Greulich, J.; Geipel, K.; Steingroewer, J.; Bley, T.; Eibl, D. Mass Propagation of Helianthus annuus Suspension Cells in Orbitally Shaken Bioreactors: Improved Growth Rate in Single-Use Bag Bioreactors. Eng. Life Sci. 2014, 14, 676–684. [Google Scholar] [CrossRef]
- Rodriguez, G.; Anderlei, T.; Micheletti, M.; Yianneskis, M.; Ducci, A. On the Measurement and Scaling of Mixing Time in Orbitally Shaken Bioreactors. Biochem. Eng. J. 2014, 82, 10–21. [Google Scholar] [CrossRef]
- Rodriguez, G.; Micheletti, M.; Ducci, A. Macro- and Micro-Scale Mixing in a Shaken Bioreactor for Fluids of High Viscosity. Chem. Eng. Res. Des. 2018, 132, 890–901. [Google Scholar] [CrossRef]
- Jones, S.M.J.; Louw, T.M.; Harrison, S.T.L. Energy Consumption Due to Mixing and Mass Transfer in a Wave Photobioreactor. Algal Res. 2017, 24, 317–324. [Google Scholar] [CrossRef]
- Plais, C.; Augier, F. Effect of Liquid Viscosity on Mixing Times in Bubble Columns. Theor. Found. Chem. Eng. 2016, 50, 969–974. [Google Scholar] [CrossRef]
- Xiao, J.; Zou, C.; Liu, M.; Zhang, G.; Delaplace, G.; Jeantet, R.; Chen, X.D. Mixing in a Soft-Elastic Reactor (SER) Characterized Using an RGB Based Image Analysis Method. Chem. Eng. Sci. 2018, 181, 272–285. [Google Scholar] [CrossRef]
- Wurm, H.; Sandmann, M. Establishment of a Simple Method to Evaluate Mixing Times in a Plastic Bag Photobioreactor Using Image Processing Based on Freeware Tools. BMC Res. Notes 2021, 14, 470. [Google Scholar] [CrossRef] [PubMed]
- Hoogendoorn, C.J.; Den Hartog, A.P. Model Studies on Mixers in the Viscous Flow Region. Chem. Eng. Sci. 1967, 22, 1689–1699. [Google Scholar] [CrossRef]
- Bartczak, M.; Wierzchowski, K.; Pilarek, M. Mixing Performance in a Litre-Scale Rocking Disposable Bioreactor: DoE-Based Investigation of Mixing Time Dependence on Operational Parameters. Chem. Eng. J. 2022, 431, 133288. [Google Scholar] [CrossRef]
- Guillard, F.; Trägårdh, C.; Fuchs, L. A Study of Turbulent Mixing in a Turbine-Agitated Tank Using a Fluorescence Technique. Exp. Fluids 2000, 28, 225–235. [Google Scholar] [CrossRef]
- Paul, E.L.; Atiemo-Obeng, V.A.; Kresta, S.M. (Eds.) Handbook of Industrial Mixing: Science and Practice; Wiley-Interscience: Hoboken, NJ, USA, 2004; ISBN 978-0-471-26919-9. [Google Scholar]
- Melton, L.A.; Lipp, C.W.; Spradling, R.W.; Paulson, K.A. Dismt—Determination of Mixing Time through Color Changes. Chem. Eng. Commun. 2002, 189, 322–338. [Google Scholar] [CrossRef]
- Vanhamel, S.; Masy, C. Production of Disposable Bags: A Manufacturer’s Report. In Single-Use Technology in Biopharmaceutical Manufacture; Eibl, R., Eibl, D., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011; pp. 113–134. ISBN 978-0-470-90999-7. [Google Scholar]
- Alvarez, M.M.; Guzmán, A.; Elías, M. Experimental Visualization of Mixing Pathologies in Laminar Stirred Tank Bioreactors. Chem. Eng. Sci. 2005, 60, 2449–2457. [Google Scholar] [CrossRef]
- Vega-Alvarado, L.; Taboada, B.; Hidalgo-Millán, A.; Ascanio, G. An Image Analysis Method for the Measurement of Mixing Times in Stirred Vessels. Chem. Eng. Technol. 2011, 34, 859–866. [Google Scholar] [CrossRef]
- Seletzky, J.; Otten, K.; Lotter, S.; Fricke, J.; Peter, C.; Maier, H.; Büchs, J. A Simple and Inexpensive Method for Investigating Microbiological, Enzymatic, or Inorganic Catalysis Using Standard Histology and Microbiology Laboratory Equipment: Assembly, Mass Transfer Properties, Hydrodynamic Conditions and Evaluation. Biotech. Histochem. 2006, 81, 133–138. [Google Scholar] [CrossRef]
- Jossen, V.; Eibl, R.; Eibl, D. Single-Use Bioreactors—An Overview. In Single-Use Technology in Biopharmaceutical Manufacture; Eibl, R., Eibl, D., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 37–52. ISBN 978-1-119-47789-1. [Google Scholar]
- Bartczak, M.; Wierzchowski, K.; Pilarek, M. Mass Transfer in a Liter-Scale Wave Mixed Single-Use Bioreactor: Influence of Viscosity and Antifoaming Agent. Ind. Eng. Chem. Res. 2023, 62, 10893–10902. [Google Scholar] [CrossRef]
- Sharma, R.; Collair, W.; Williams, A.; Harrison, S.T.L.; Tai, S.L. Design and Engineering Characterization of a Horizontal Tubular Bioreactor with Spiral Impeller for Cell Cultivation. Biochem. Eng. J. 2023, 191, 108794. [Google Scholar] [CrossRef]
- Burger, W.; Burge, M.J. Digital Image Processing: An Algorithmic Introduction Using Java; Texts in Computer Science; Springer: London, UK, 2008; ISBN 978-1-84628-379-6. [Google Scholar]
- Russ, J.C. The Image Processing Handbook; CRC Press: Boca Raton, FL, USA, 2006; ISBN 978-0-203-88109-5. [Google Scholar]
- Tkalcic, M.; Tasic, J.F. Colour Spaces: Perceptual, Historical and Applicational Background. In Proceedings of the The IEEE Region 8 EUROCON 2003. Computer as a Tool, Ljubljana, Slovenia, 22–24 September 2003; IEEE: Ljubljana, Slovenia, 2003; Volume 1, pp. 304–308. [Google Scholar]
- Kahu, S.Y.; Raut, R.B.; Bhurchandi, K.M. Review and Evaluation of Color Spaces for Image/Video Compression. Color Res. Appl. 2019, 44, 8–33. [Google Scholar] [CrossRef]
- Cheng, H.D.; Jiang, X.H.; Sun, Y.; Wang, J. Color Image Segmentation: Advances and Prospects. Pattern Recognit. 2001, 34, 2259–2281. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Yang, R.; Jia, K.; Zhang, H. Research on Segmentation of Weed Images Based on Computer Vision. J. Electron. 2007, 24, 285–288. [Google Scholar] [CrossRef]
- Sural, S.; Qian, G.; Pramanik, S. Segmentation and Histogram Generation Using the HSV Color Space for Image Retrieval. In Proceedings of the International Conference on Image Processing, Rochester, NY, USA, 22–25 September 2002; IEEE: Rochester, NY, USA, 2002; Volume 2, pp. II-589–II-592. [Google Scholar]
- Bora, D.J.; Gupta, A.K.; Khan, F.A. Comparing the Performance of L*A*B* and HSV Color Spaces with Respect to Color Image Segmentation. arXiv 2015, arXiv:1506.01472. [Google Scholar] [CrossRef]
- Alata, O.; Quintard, L. Is There a Best Color Space for Color Image Characterization or Representation Based on Multivariate Gaussian Mixture Model? Comput. Vis. Image Underst. 2009, 113, 867–877. [Google Scholar] [CrossRef]
- Lee, S.-M.; Lee, K.-T.; Lee, S.-H.; Song, J.-K. Origin of Human Colour Preference for Food. J. Food Eng. 2013, 119, 508–515. [Google Scholar] [CrossRef]
- Afshari-Jouybari, H.; Farahnaky, A. Evaluation of Photoshop Software Potential for Food Colorimetry. J. Food Eng. 2011, 106, 170–175. [Google Scholar] [CrossRef]
- Cai, J.; Goshtasby, A. Detecting Human Faces in Color Images. Image Vis. Comput. 1999, 18, 63–75. [Google Scholar] [CrossRef]
- Suzuki, K.; Hirayama, E.; Sugiyama, T.; Yasuda, K.; Okabe, H.; Citterio, D. Ionophore-Based Lithium Ion Film Optode Realizing Multiple Color Variations Utilizing Digital Color Analysis. Anal. Chem. 2002, 74, 5766–5773. [Google Scholar] [CrossRef]
- Trujillo-de Santiago, G.; Rojas-de Gante, C.; García-Lara, S.; Ballescá-Estrada, A.; Alvarez, M.M. Studying Mixing in Non-Newtonian Blue Maize Flour Suspensions Using Color Analysis. PLoS ONE 2014, 9, e112954. [Google Scholar] [CrossRef]
- Bauer, I.; Dreher, T.; Eibl, D.; Glöckler, R.; Husemann, U.; John, G.T.; Kaiser, S.C.; Kampeis, P.; Kauling, J.; Kleebank, S.; et al. Recommendations for Process Engineering Characterisation of Single-Use Bioreactors and Mixing Systems by Using Experimental Methods, 2nd ed.; Dechema Biotechnologie: Frankfurt am Main, Germany, 2020; ISBN 978-3-89746-227-4. [Google Scholar]
- Kong, J.; Dimitrov, M.; Yang, Y.; Liyanage, J.; Cao, L.; Staples, J.; Mantor, M.; Zhou, H. Accelerating MATLAB Image Processing Toolbox Functions on GPUs. In Proceedings of the 3rd Workshop on General-Purpose Computation on Graphics Processing Units, Pittsburgh, PA, USA, 14 March 2010; ACM: Pittsburgh, PA, USA, 2010; pp. 75–85. [Google Scholar]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Culjak, I.; Abram, D.; Pribanic, T.; Dzapo, H.; Cifrek, M. A Brief Introduction to OpenCV. In Proceedings of the 2012 Proceedings of the 35th International Convention MIPRO, Opatija, Croatia, 21–25 May 2012; IEEE: Opatija, Croatia, 2012. [Google Scholar]
- Howse, J. OpenCV Computer Vision with Python; Packt Publishing: Birmingham, UK, 2013; ISBN 978-1-78216-392-3. [Google Scholar]

| Working Volume | Commercial Name (If Applicable) | Colour-Changing Reagents | Image Analysis | Notes | Reference | |
|---|---|---|---|---|---|---|
| Software (If Applicable, Version No. If Specified by Authors) | Colour Space (If Applicable) | |||||
| Stirred tank reactors (multi-use) | ||||||
| 22–280 L | phenolphthalein | n/a (visual) | [11] | |||
| 25–37 L | methyl red | n/a (visual) | [12] | |||
| 3 L, 13 L | iodine + starch | n/a (visual) | [13] | |||
| 140 L | iodine + starch | n/a (visual) | [14] | |||
| 180 L | iodine + starch | n/a (visual) | [15] | |||
| 7 L | bromothymol blue | n/a (visual) | no quantitative data | [16] | ||
| 50 L | iodine + starch | n/a (visual) | [17] | |||
| 30 L | DISMT * | OPTIMAS 6.5 | RGB (G channel) | [18] | ||
| 750 L | bromocresol purple | n/a (visual) | [19] | |||
| 46 L | bromocresol purple | n/s | RGB (G channel) | [20] | ||
| 7.8 L, 14.5 L, 200 L | various (see footer) ** | n/s (in-house) | RGB (channel depending on indicator) | [10] | ||
| 30 L | DISMT * | OPTIMAS 6.5 | RGB (G channel) | [21] | ||
| 35 L, 77 L, 190 L | bromocresol purple | n/s (in-house) | RGB (G channel) | [22] | ||
| 4 L | bromocresol purple | Image-Pro Plus 4.5.1 | n/s | [23] | ||
| 42 L, 340 L | n/s | n/s (in-house) | grayscale | [24] | ||
| 200 L, 400 L | iodine + starch | n/s | n/s | [25] | ||
| 10 mL | methylene blue | ImageJ 1.48b6, MATLAB R2012a | grayscale | [26] | ||
| 5 L | bromocresol purple | n/s | n/s | [27] | ||
| 15 m3 | phenolphthalein | ImageJ | grayscale | [3] | ||
| 2 L | bromocresol purple | MATLAB | HSV | [28] | ||
| 12 L | phenolphthalein | ImageJ | RGB | [29] | ||
| 200 mL | DASGIP® Cellferm-pro | DISMT * | MATLAB | RGB (G channel) | with microcarriers | [30] |
| 3 L | bromothymol blue | n/s | grayscale | [31] | ||
| 10 L | Cochineal Red | MATLAB R2020a | RGB (R channel) | [32] | ||
| 3.8 L | bromothymol blue | MATLAB | grayscale | single multi-compartment bioreactor | [2] | |
| 250 mL | DISMT * | MATLAB | RGB (G channel) | [33] | ||
| Stirred tank reactors (SUBs) | ||||||
| 3 L | Mobius CellReady™ | iodine + starch | n/s | n/s | [34] | |
| 15 mL | Ambr™ | iodine + starch | n/a (visual) | [35] | ||
| 1 L | Allegro™ STR 50 | DISMT * | MATLAB | RGB (G channel) | scale-down prototype of a 50 L bioreactor | [36] |
| Shaken or rocked vessels (shake flasks, orbitally shaken or wave-mixed SUBs) | ||||||
| 2 L, 3 L, 30 L, 1500 L | Kühner™ ES-W shaker | DISMT * | n/s (in-house) | RGB (G channel) | orbitally shaken | [37] |
| 100 mL, 250 mL, 500 mL | bromothymol blue | n/a (visual) | n/a | shake flasks | [6] | |
| 600 mL | TubeSpin® 600 | DISMT * | n/s | RGB (G channel) | orbitally shaken | [38] |
| 2 L, 20 L | BIOSTAT® CultiBag™ RM | iodine + starch | n/a (visual) | shake flasks and rocking bioreactor bags (orbitally shaken or rocked) | [39] | |
| 2 L | DISMT * | MATLAB (version n/s) | RGB (G channel) | orbitally shaken | [40,41] | |
| 10 L | phenolphthalein | n/a (visual) | rocking (wave-mixed) single-use bioreactor; no quantitative data | [42] | ||
| Others | ||||||
| 37 L | Purple Drimarene R 2 RL | n/s (in-house) | grayscale | bubble column | [43] | |
| 120 mL | bromophenol blue | n/s | RGB | soft elastic reactor | [44] | |
| 10 L | Patent Blue V (E131) dye | VLC 3.0.16, IrfanView 4.58, GIMP 2.10.24 | grayscale | plastic bag bubble photobioreactor | [45] | |
| Indicator | Colour at Low pH | pH Colour Change Range | Colour at High pH | Reference |
|---|---|---|---|---|
| Single indicator systems | ||||
| bromocresol purple | yellow | 5.2–6.8 | purple | [19,20,22,23,27,28] |
| bromothymol blue | yellow | 6.0–7.6 | blue | [2,6,16,31] |
| phenolphthalein | colourless | 8.2–10.0 | pink | [3,11,29,42] |
| methyl red | red | 4.8–6.0 | yellow | [12] |
| Double indicator systems | ||||
| DISMT (methyl red, thymol blue) | red | 4.8–9.6 | blue | [18,21,30,33,36,37,38,40,41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartczak, M.; Pilarek, M. The Colourimetric Method for Mixing Time Measurement in Single-Use and Multi-Use Bioreactors—Methodology Overview and Practical Recommendations. Energies 2024, 17, 221. https://doi.org/10.3390/en17010221
Bartczak M, Pilarek M. The Colourimetric Method for Mixing Time Measurement in Single-Use and Multi-Use Bioreactors—Methodology Overview and Practical Recommendations. Energies. 2024; 17(1):221. https://doi.org/10.3390/en17010221
Chicago/Turabian StyleBartczak, Mateusz, and Maciej Pilarek. 2024. "The Colourimetric Method for Mixing Time Measurement in Single-Use and Multi-Use Bioreactors—Methodology Overview and Practical Recommendations" Energies 17, no. 1: 221. https://doi.org/10.3390/en17010221






