Study on the Effect of High-Concentration Oxygen Enrichment on Engine Performance and Exhaust Emissions Using Diesel Fuel and Palm Biodiesel Substitute Fuel
Abstract
1. Introduction
2. Experimental Setup
2.1. Specifications of the Fuel
2.2. Calculation of Oxygen Concentration
3. Simulation Model
4. Results and Discussion
4.1. Validation of Simulation and Experimental Results
4.2. Analysis of the Oxygen Enrichment Effect on Diesel Engines Using Diesel Fuel and Substitute Biodiesel
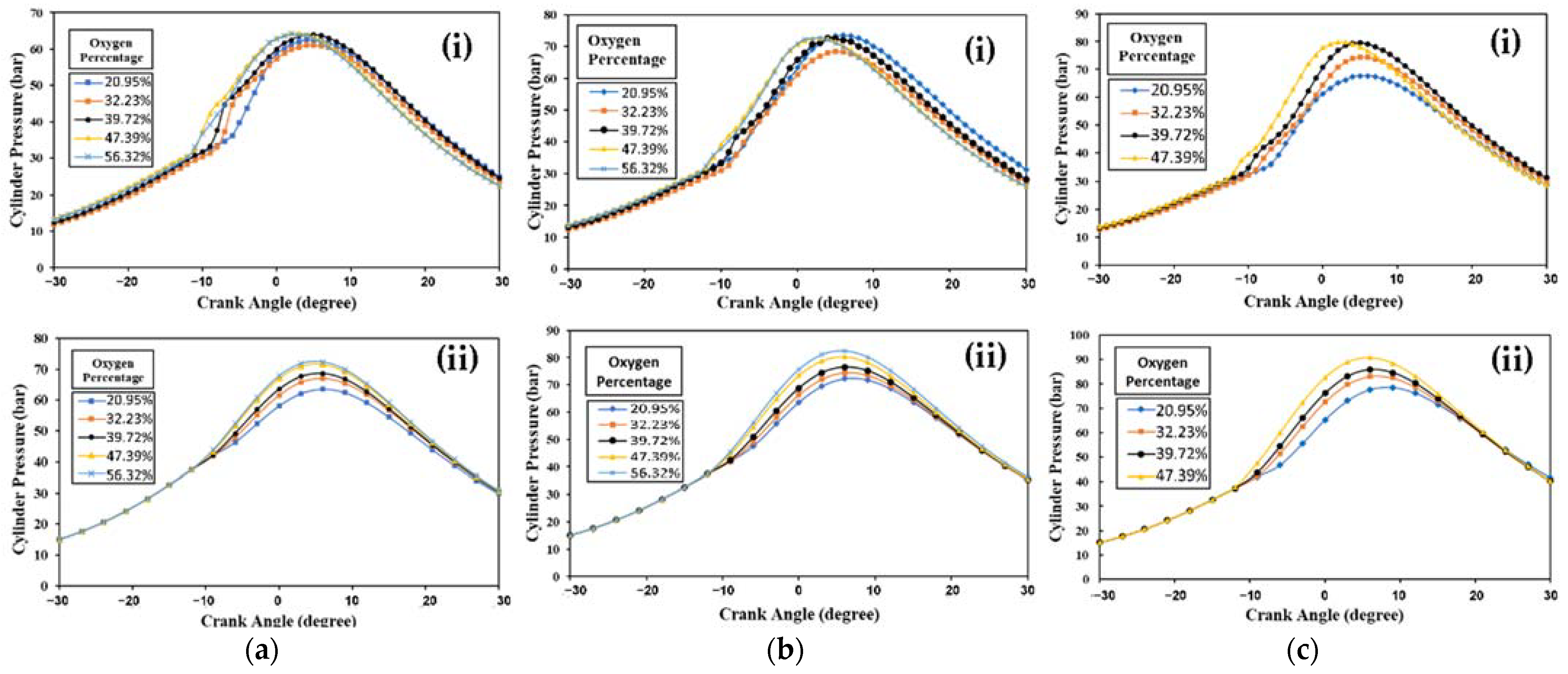
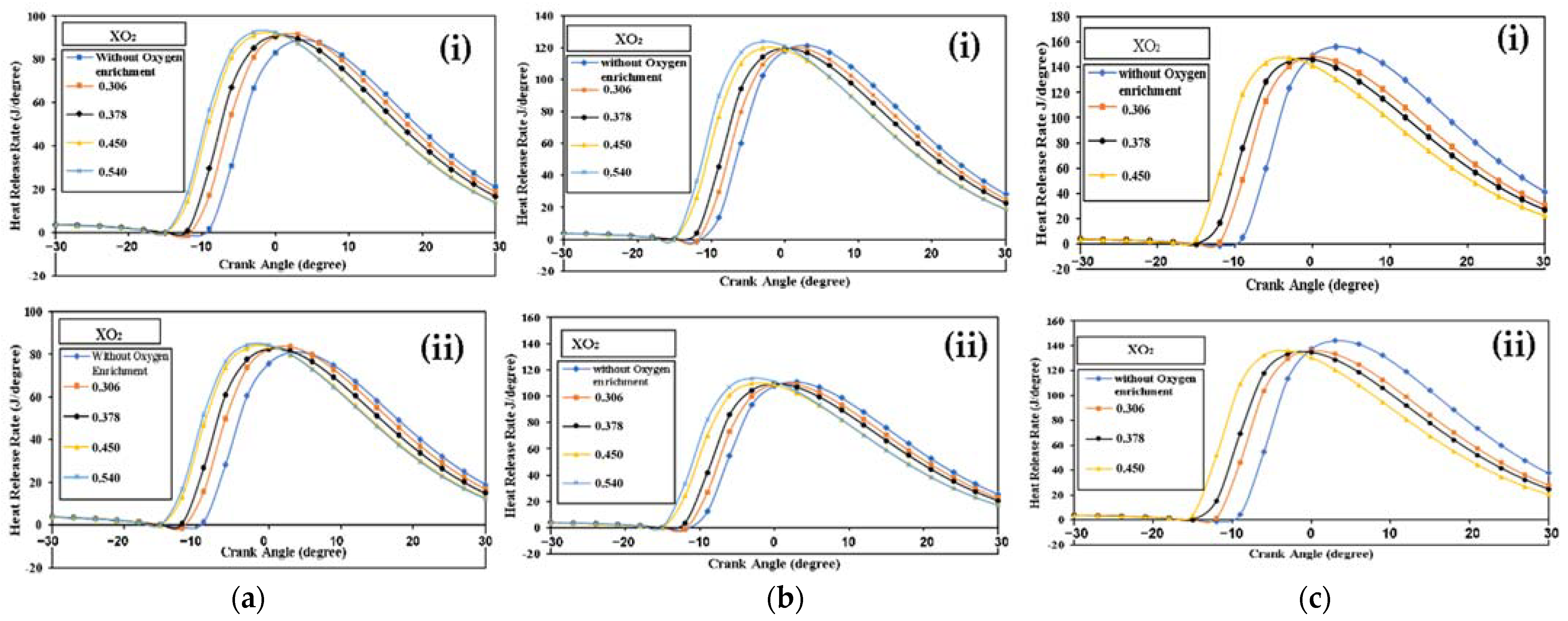
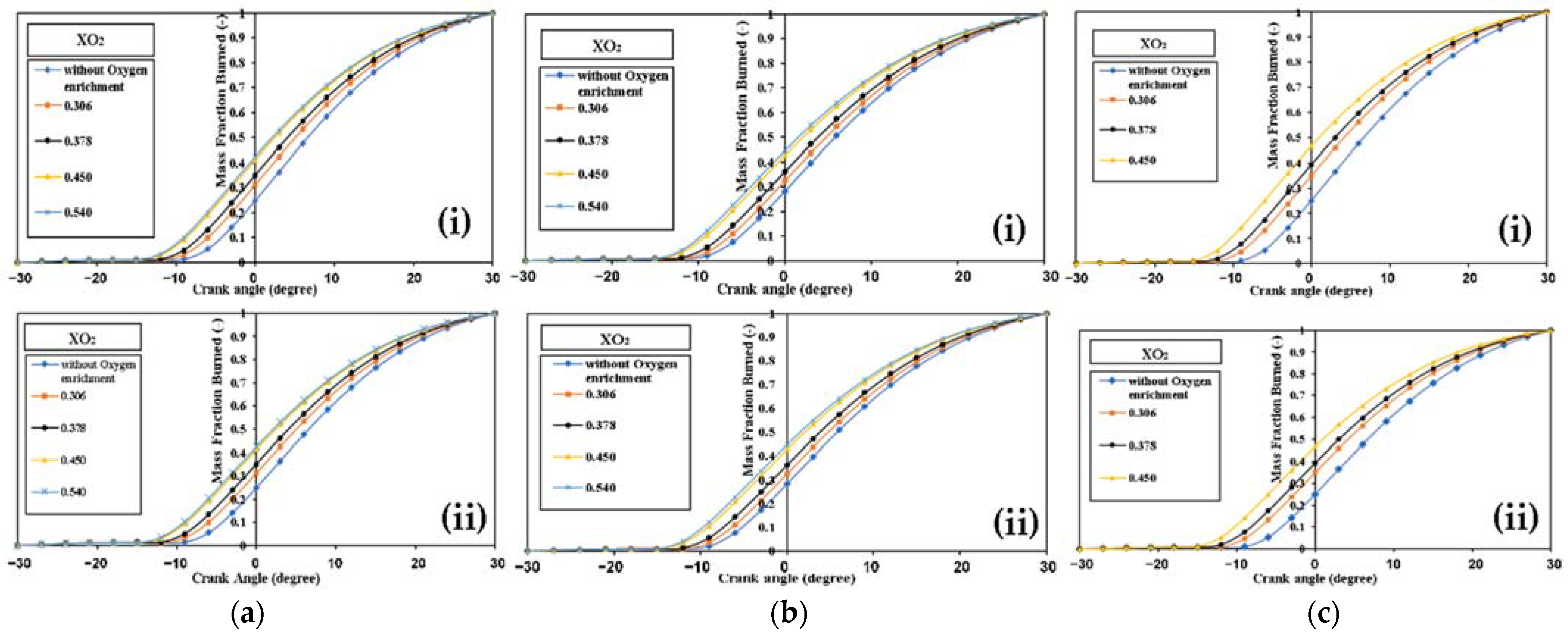
4.2.1. Diesel Engine Combustion
Diesel Engine Combustion with 50% Load
Diesel Engine Combustion with 75% Load
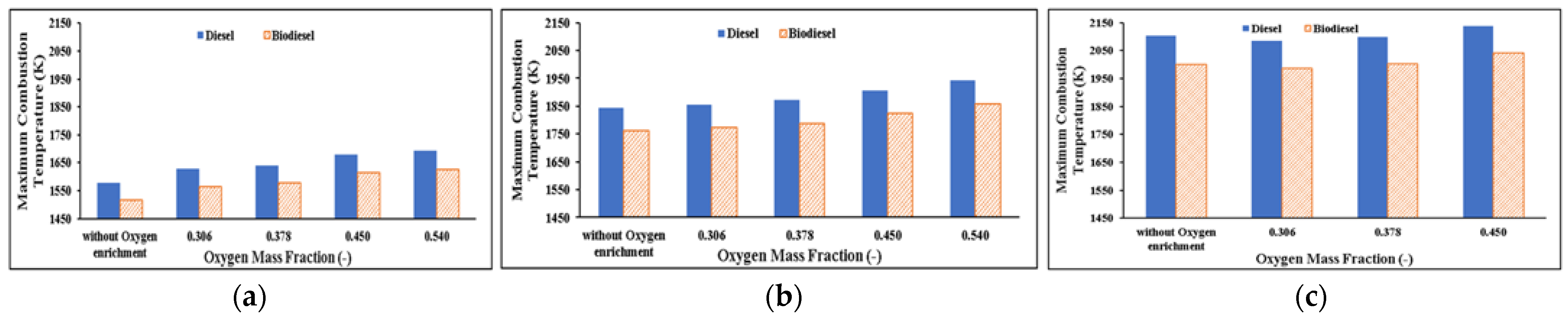
Diesel Engine Combustion with 100% Load
4.2.2. Diesel Engine Performance
Brake Specific Fuel Consumption (BSFC)
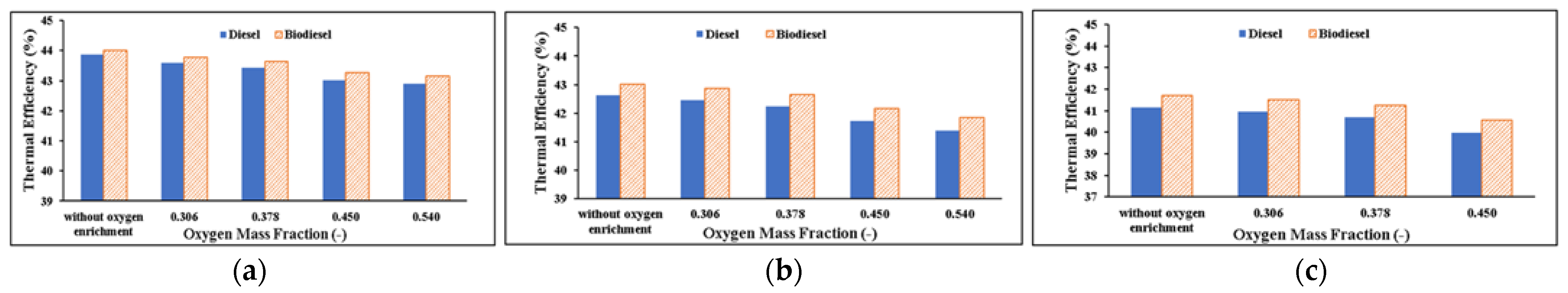
Thermal Efficiency
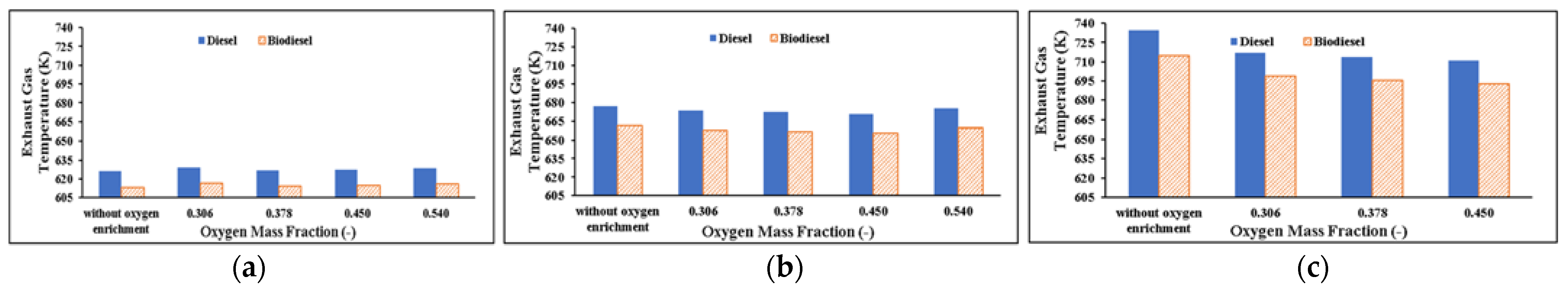
Exhaust Gas Temperature
4.2.3. Diesel Engine Emissions
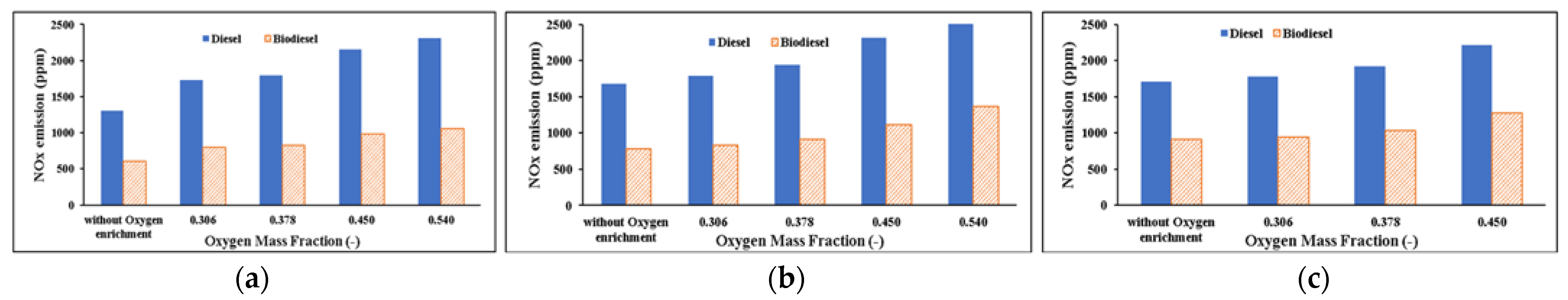
NOx Emissions
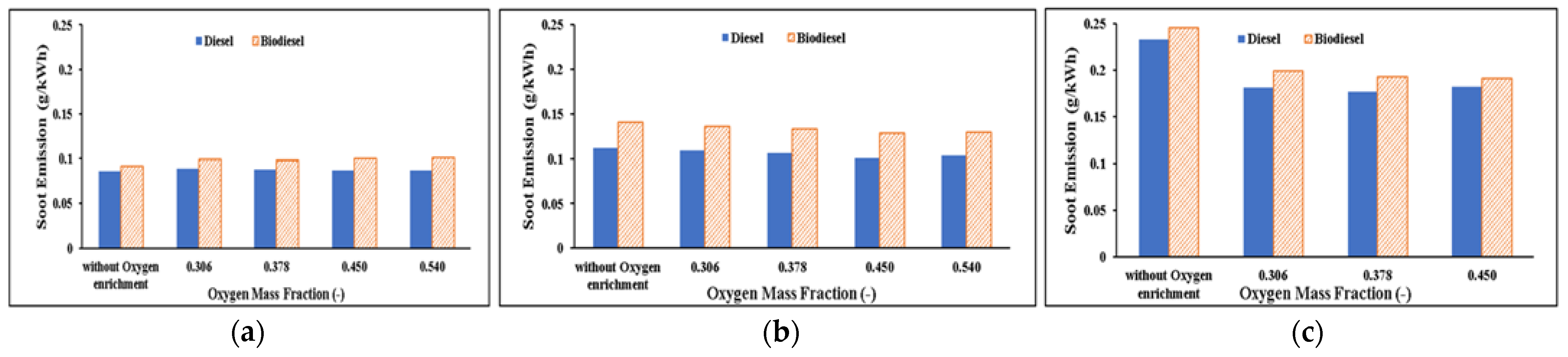
Soot Emissions
5. Conclusions
- Based on the diesel engine’s combustion parameters:
- While the peak HRR rises when the mass fraction of oxygen increases when using both fuels, the peak falls when using biodiesel substitution because of the lower heating value.
- The 90% condition of the fuel MFB is earlier due to an increase in combustion quality.
- Considering the diesel engine’s performance parameters:
- BSFC increases as the oxygen mass fraction increases because the ignition delay is shorter. Due to its lower calorific value, the biodiesel substitute has a greater BSFC than that of diesel fuel.
- When using both fuels, efficiency drops as the oxygen mass percentage rises because of the rise in BSFC. Due to its reduced calorific value compared to diesel fuel, biodiesel can be used more efficiently.
- Exhaust gas temperature (EGT) decreases as increased oxygen reduces combustion after TDC. EGT in the use of biodiesel substitute is lower. Based on the diesel engine emission parameters:
- NOx emissions increase as the oxygen mass fraction increases due to an increase in the maximum combustion temperature due to increased fuel oxidation, resulting in thermal NOx formation. In the use of biodiesel substitutes, NOx emissions are lower because the maximum combustion temperature is also lower.
- As the mass percentage of oxygen increases, soot production is reduced because there is more oxygen available for fuel oxidation. As the lower peak HRR indicates, using biodiesel results in more soot emissions than using diesel because of poorer combustion.
- The BSFC value and soot emissions from the use of diesel fuel are lower than those with the use of biodiesel substitutes. Diesel fuel is used less frequently than biodiesel fuel when it comes to thermal efficiency and NOx emissions.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mustayen, A.G.M.B.; Rasul, M.G.; Wang, X.; Negnevitsky, M.; Hamilton, J.M. Remote areas and islands power generation: A review on diesel engine performance and emission improvement techniques. Energy Convers. Manag. 2022, 260, 115614. [Google Scholar] [CrossRef]
- Dahham, R.Y.; Wei, H.; Pan, J. Improving Thermal Efficiency of Internal Combustion Engines: Recent Progress and Remaining Challenges. Energies 2022, 15, 6222. [Google Scholar] [CrossRef]
- Dhahad, H.A.; Hasan, A.M.; Chaichan, M.T.; Kazem, H.A. Prognostic of diesel engine emissions and performance based on an intelligent technique for nanoparticle additives. Energy 2022, 238, 121855. [Google Scholar] [CrossRef]
- Kozina, A.; Radica, G.; Nižetić, S. Emission analysis of diesel vehicles in circumstances of emission regulation system failure: A case study. J. Energy Resour. Technol. 2022, 144, 082307. [Google Scholar] [CrossRef]
- Fayyazbakhsh, A.; Bell, M.L.; Zhu, X.; Mei, X.; Koutný, M.; Hajinajaf, N.; Zhang, Y. Engine emissions with air pollutants and greenhouse gases and their control technologies. J. Clean. Prod. 2022, 376, 134260. [Google Scholar] [CrossRef]
- Palani, Y.; Devarajan, C.; Manickam, D.; Thanikodi, S. Performance and emission characteristics of biodiesel-blend in diesel engine: A review. Environ. Eng. Res. 2022, 27, 200338. [Google Scholar] [CrossRef]
- Baskar, P.; dan Senthilkumar, A. Effects of oxygen enriched combustion on pollution and performance characteristics of a diesel engine. Eng. Sci. Technol. Int. J. 2016, 19, 438–443. [Google Scholar] [CrossRef]
- Chin, J.-S. Analysis of the Effect of Oxygen Addition on Minimum Ignition Energy. J. Energy 1983, 7, 710–715. [Google Scholar] [CrossRef]
- Donahue, R.J.; dan Foster, D.E. Effects of oxygen enhancement on the emissions from a di diesel via manipulation of fuels and combustion chamber 75 gas composition. SAE Tech. Pap. 2000, 109, 334–350. [Google Scholar] [CrossRef]
- Wai, P.; Kanokkhanarat, P.; Oh, B.S.; Wongpattharaworakul, V.; Depaiwa, N.; Po-ngaen, W.; Chollacoop, N.; Srisurangkul, C.; Kosaka, H.; Yamakita, M.; et al. Experimental investigation of the influence of ethanol and biodiesel on common rail direct injection diesel Engine’s combustion and emission characteristics. Case Stud. Therm. Eng. 2022, 39, 102430. [Google Scholar] [CrossRef]
- Zhang, X.; Duan, Y.; Zhang, R.; Wei, H.; Chen, L. Optical study of oxygen enrichment on methane combustion characteristics under high compression-ratio conditions. Fuel 2022, 328, 125251. [Google Scholar] [CrossRef]
- Kapusuz, M.; Çakmak, A.; Özcan, H. Application of oxygen enrichment and adiabatic humidification to suction air for reducing exhaust emissions in a gasoline engine. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 194–211. [Google Scholar] [CrossRef]
- Gad, M.S.; El-Araby, R.; Abed, K.A.; El-Ibiari, N.N.; El Morsi, A.K.; dan ElDiwani, G.I. Performance and emissions characteristics of C.I. engine fueled with palm oil/palm oil methyl ester blended with diesel fuel. Egypt. J. Pet. 2018, 27, 215–219. [Google Scholar] [CrossRef]
- Al Qubeissi, M.; Mahmoud, A.; Al-Damook, M.; Almshahy, A.; Khatir, Z.; Soyhan, H.S.; Raja Ahsan Shah, R.M. Comparative analysis of battery thermal management system using biodiesel fuels. Energies 2023, 16, 565. [Google Scholar] [CrossRef]
- Kesharvani, S.; Dwivedi, G.; Verma, T.N.; Verma, P. The experimental investigation of a diesel engine using ternary blends of algae biodiesel, ethanol and diesel fuels. Energies 2023, 16, 229. [Google Scholar] [CrossRef]
- Giakoumis, E.G. Analysis of 22 vegetable oils’ physico-chemical properties and fatty acid composition on a statistical basis, and correlation with the degree of unsaturation. Renew. Energy 2018, 126, 403–419. [Google Scholar] [CrossRef]
- Belousov, A.S.; Esipovich, A.L.; Kanakov, E.A.; Otopkova, K.V. Recent advances in sustainable production and catalytic transformations of fatty acid methyl esters. Sustain. Energy Fuels 2021, 5, 4512–4545. [Google Scholar] [CrossRef]
- Abdelbasset, W.K.; Alrawaili, S.M.; Elsayed, S.H.; Diana, T.; Ghazali, S.; Felemban, B.F.; Zwawi, M.; Algarni, M.; Su, C.H.; Nguyen, H.C.; et al. Optimization of heterogeneous Catalyst-assisted fatty acid methyl esters biodiesel production from Soybean oil with different Machine learning methods. Arab. J. Chem. 2022, 15, 103915. [Google Scholar] [CrossRef]
- Ramos, M.; Dias, A.P.S.; Puna, J.F.; Gomes, J.; Bordado, J.C. Biodiesel production processes and sustainable raw materials. Energies 2019, 12, 4408. [Google Scholar] [CrossRef]
- Khalid, A.; Osman, S.A.; Jaat, M.N.M.; Mustaffa, N.; Basharie, S.M.; dan Manshoor, B. Performance and emissions characteristics of diesel engine fuelled by biodiesel derived from palm oil. Appl. Mech. Mater. 2013, 315, 517–522. [Google Scholar] [CrossRef]
- ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. Available online: https://www.iso.org/standard/66912.html (accessed on 27 December 2023).
- ISO 15550:2016; Internal Combustion Engines Determination and Method for the Measurement of Engine Power. Available online: https://www.iso.org/standard/70030.html (accessed on 27 December 2023).
- Heywood, J.B. Internal Combustion Engine Fundamentals, 2nd ed.; McGraw-Hill Education: New York, NY, USA, 2018; p. 1056. [Google Scholar]
- Poon, H.M.; Ng, H.K.; Gan, S.; dan Chong, W.T. Development of a Reduced Biodiesel Surrogate Fuel Model for Multi-Dimensional Computational Fluid Dynamic Simulations. IOP Conf. Ser. Earth Environ. Sci. 2019, 268, 012080. [Google Scholar] [CrossRef]
- Baskar, P.; dan Senthil Kumar, A. Experimental investigation on performance characteristics of a diesel engine using diesel-water emulsion with oxygen enriched air. Alex. Eng. J. 2017, 56, 137–146. [Google Scholar] [CrossRef]
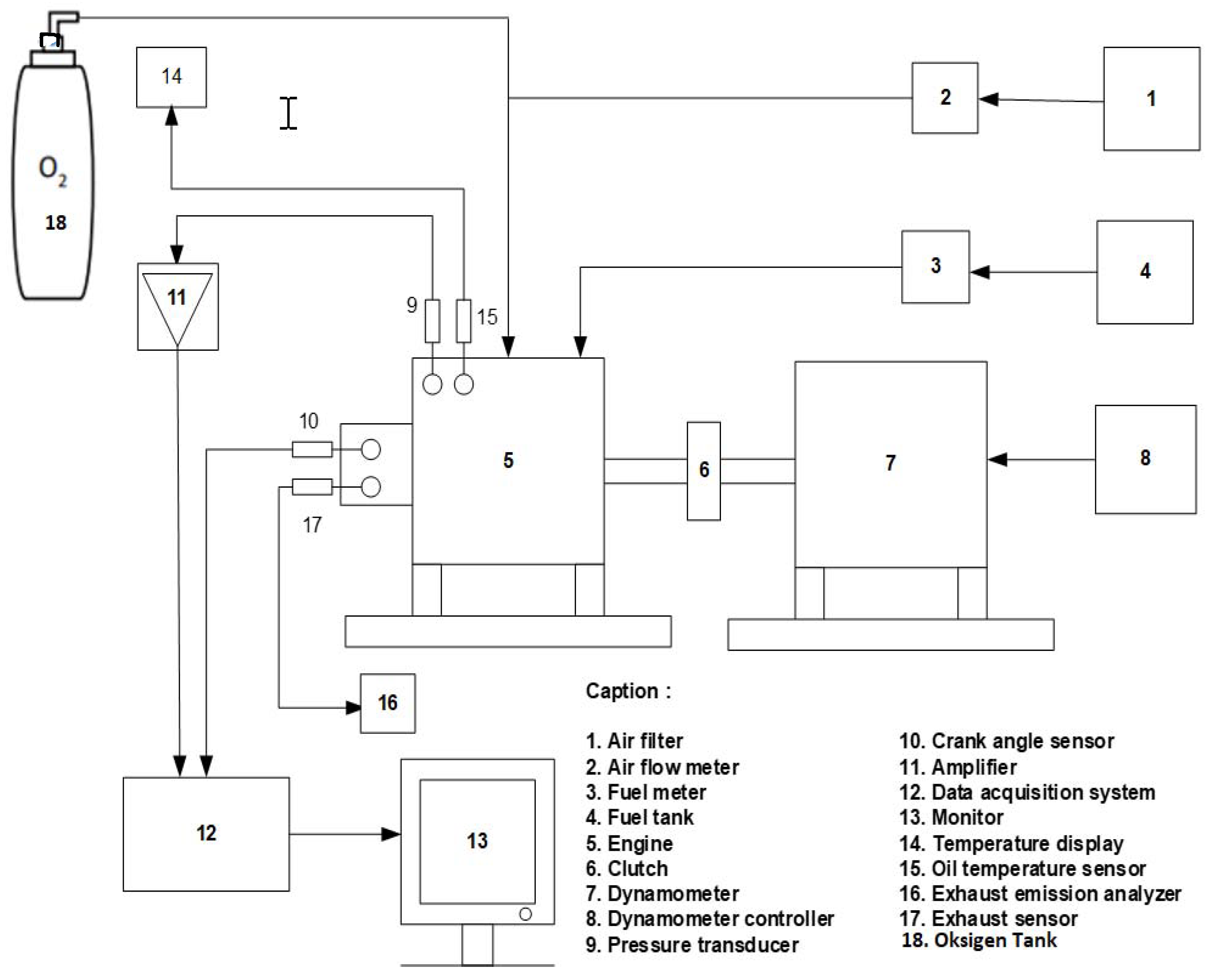
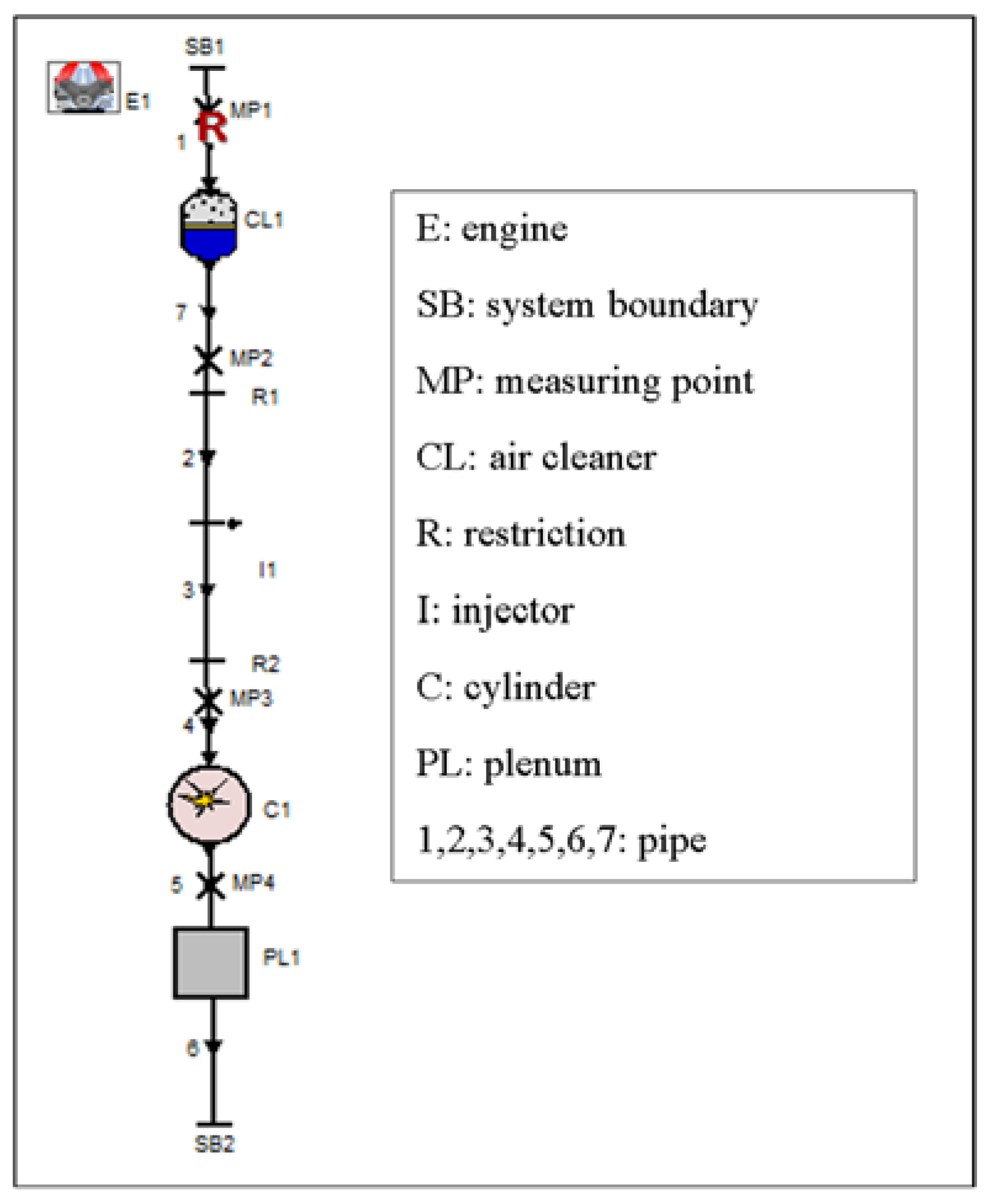


| Parameter | Specification |
|---|---|
| Bore × stroke | 102 mm × 105 mm |
| Displacement | 857 cc |
| Maximum power | 15.5 (PS)/2400 rpm |
| Compression ratio | 17.8 |
| Number of cylinders | 1 |
| Injection system | direct injection |
| Parameter | Value |
|---|---|
| Density (kg/m3) | 820 |
| Viscosity (mm2/s) | 4.5 |
| Cetane Number | 53 |
| Flash point (°C) | 55 |
| Boiling point (°C) | 370 |
| Calorific value (kJ/kg) | 47,314 |
| Pour point | 18 |
| Carbon residue (% m/m) | 0.3 |
| (kg/h) | Oxygen Percentage (%) |
|---|---|
| 0 | 20.95% |
| 3 | 32.23% |
| 5 | 39.72% |
| 7 | 47.39% |
| 10 | 56.32% |
| Input Data | Unit | Output Data | Unit |
|---|---|---|---|
| Bore, Stroke, and Connecting rod length | Mm | Engine power | kW |
| Fuel type | - | IMEP, BMEP, FMEP | bar |
| Diameter of the intake and exhaust ports | Mm | Engine brake torque | Nm |
| Input data | Unit | Output data | Unit |
| Lifting the intake and exhaust valves | Mm | Peak pressure rise | bar/deg |
| Exhaust pipes’ length and diameter | Mm | Peak pressure | bar |
| The diameter and length of intake pipes | Mm | Peak temperature | K |
| Diameter of the throttle | Mm | Heat losses | kJ |
| Air-fuel ratio, CR, Air flow coefficient | - | Volumetric efficiency | - |
| Engine friction | Bar | Exhaust gas pressure | bar |
| Ignition timing | Deg | NOx emission | g/kWh |
| Surface area of the cylinder head, piston, and liner | mm2 | HC emission | g/kWh |
| Ambient temperature and pressure | degC | CO emission | g/kWh |
| Air cleaner volume | Liter | Exhaust gas pressure | bar |
| Throttle angle | Deg | Exhaust gas temperature | K |
| Compounds | Composition of Substitute Biodiesel | LHV (MJ/kg) | Density (kg/mm3) |
|---|---|---|---|
| C11H22O2 (MD) | 0.24 | 34.46 | 887 ± 100 |
| C11H20O2 (MD9D) | 0.26 | 34.20 | 900 ± 100 |
| C7H16 | 0.5 | 44.95 | 700 ± 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghany, F.A.; Wahono, B.; Praptijanto, A.; Putrasari, Y.; Dimyani, A.; Nur, A.; Suherman; Pratama, M.; Wardana, M.K.A. Study on the Effect of High-Concentration Oxygen Enrichment on Engine Performance and Exhaust Emissions Using Diesel Fuel and Palm Biodiesel Substitute Fuel. Energies 2024, 17, 244. https://doi.org/10.3390/en17010244
Ghany FA, Wahono B, Praptijanto A, Putrasari Y, Dimyani A, Nur A, Suherman, Pratama M, Wardana MKA. Study on the Effect of High-Concentration Oxygen Enrichment on Engine Performance and Exhaust Emissions Using Diesel Fuel and Palm Biodiesel Substitute Fuel. Energies. 2024; 17(1):244. https://doi.org/10.3390/en17010244
Chicago/Turabian StyleGhany, Fariza Almira, Bambang Wahono, Achmad Praptijanto, Yanuandri Putrasari, Ahmad Dimyani, Arifin Nur, Suherman, Mulia Pratama, and Muhammad Khristamto Aditya Wardana. 2024. "Study on the Effect of High-Concentration Oxygen Enrichment on Engine Performance and Exhaust Emissions Using Diesel Fuel and Palm Biodiesel Substitute Fuel" Energies 17, no. 1: 244. https://doi.org/10.3390/en17010244
APA StyleGhany, F. A., Wahono, B., Praptijanto, A., Putrasari, Y., Dimyani, A., Nur, A., Suherman, Pratama, M., & Wardana, M. K. A. (2024). Study on the Effect of High-Concentration Oxygen Enrichment on Engine Performance and Exhaust Emissions Using Diesel Fuel and Palm Biodiesel Substitute Fuel. Energies, 17(1), 244. https://doi.org/10.3390/en17010244






