Boil-Off Gas Generation in Vacuum-Jacketed Valve Used in Liquid Hydrogen Storage Tank
Abstract
:1. Introduction
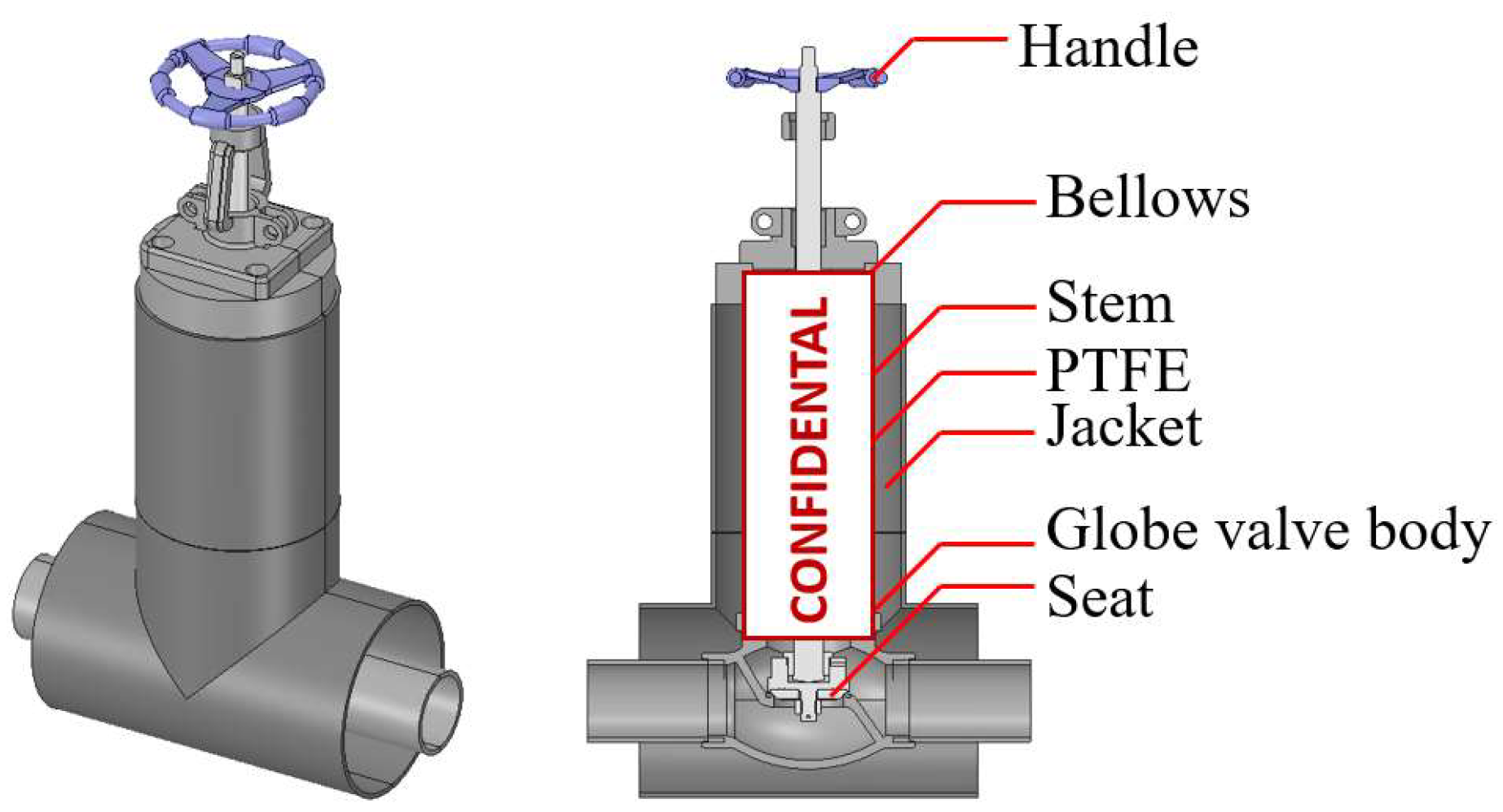
2. Vacuum-Jacketed Valve
3. Vaporization Analysis
3.1. Mathematical Model
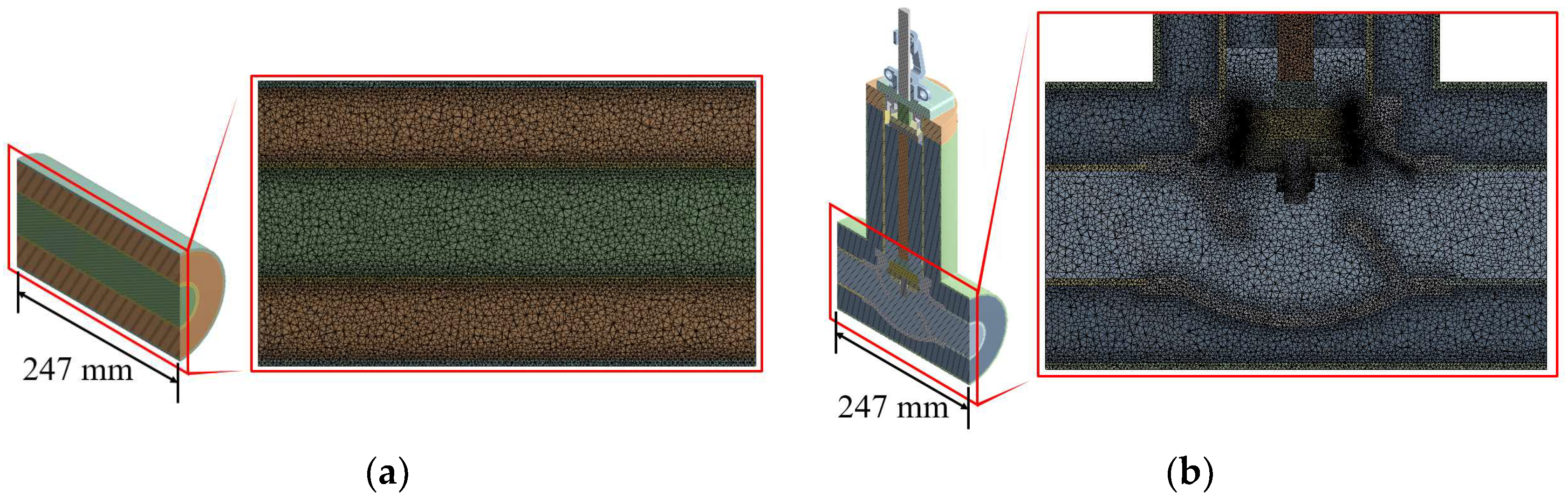
3.2. Grid Configuration and Load and Boundary Conditions
4. Analysis Results
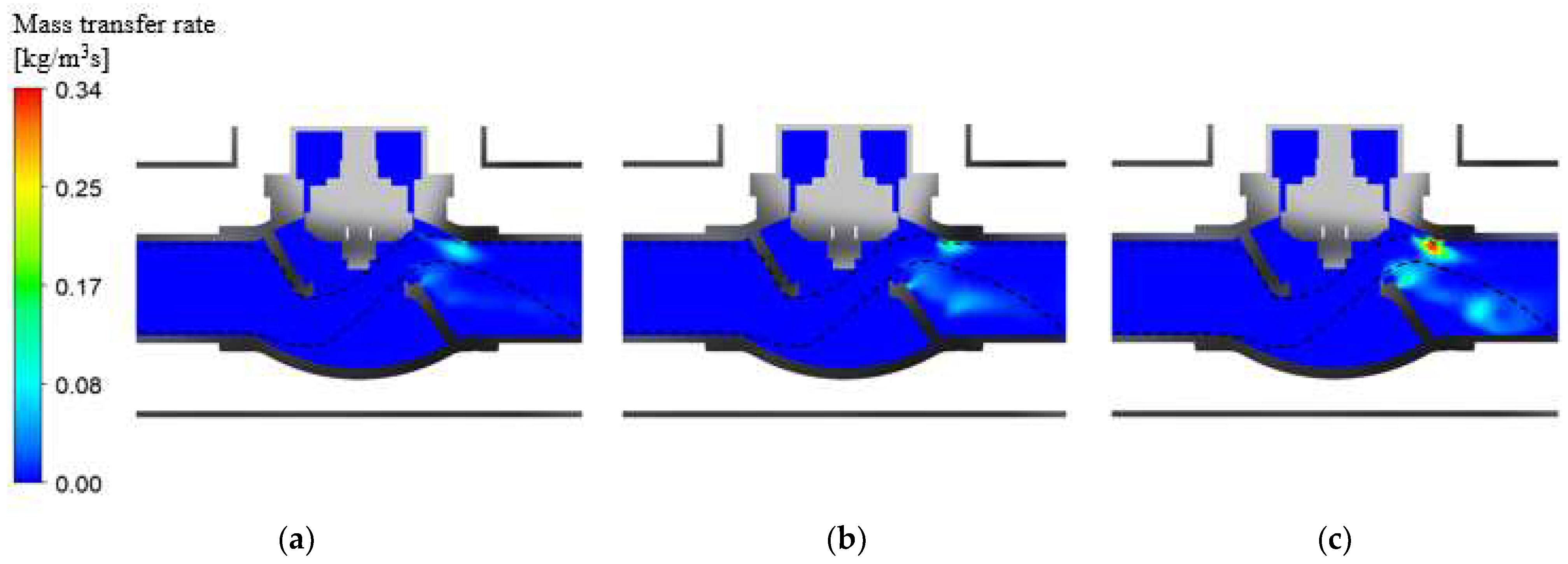
4.1. BOG Generation
4.2. BOG Generation Regarding Tank Pressures
5. Discussion
6. Conclusions
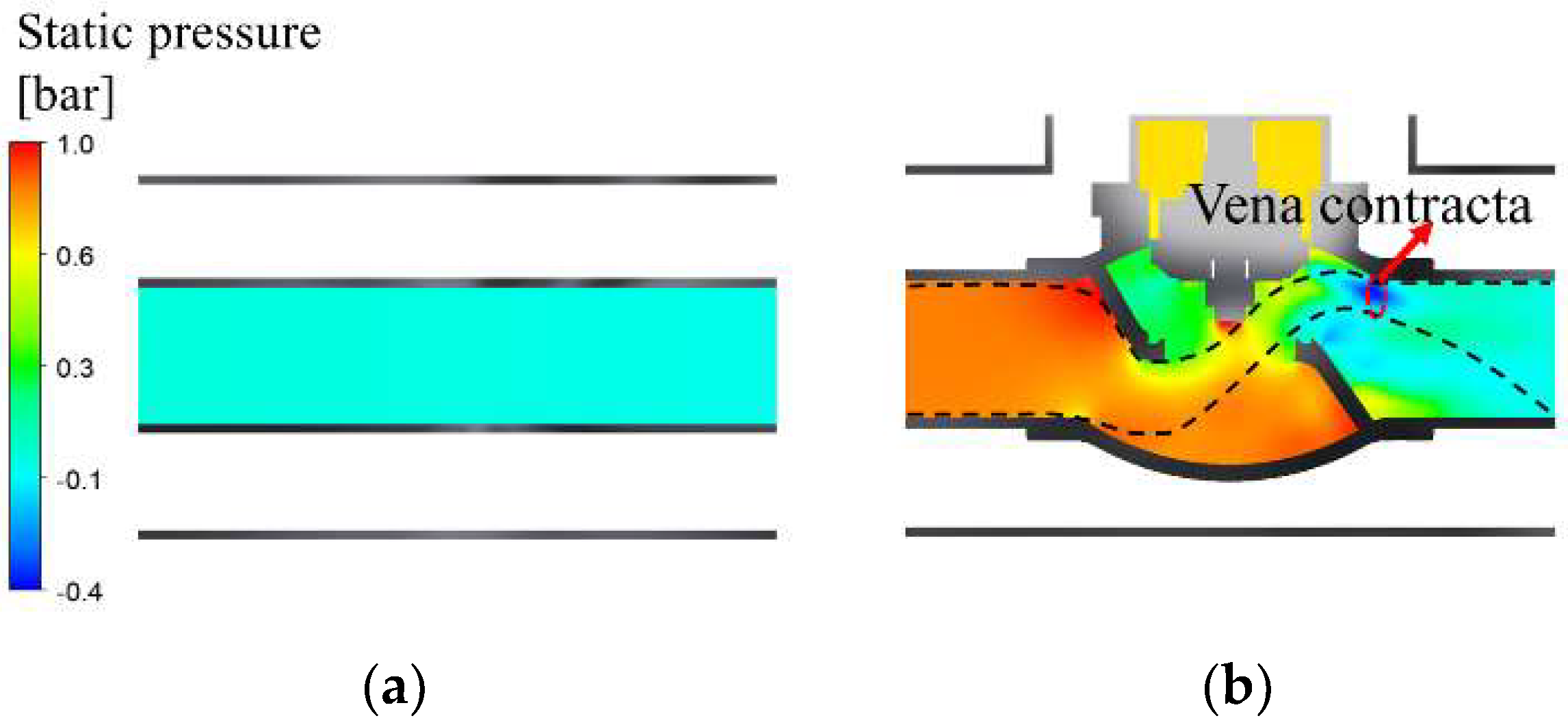
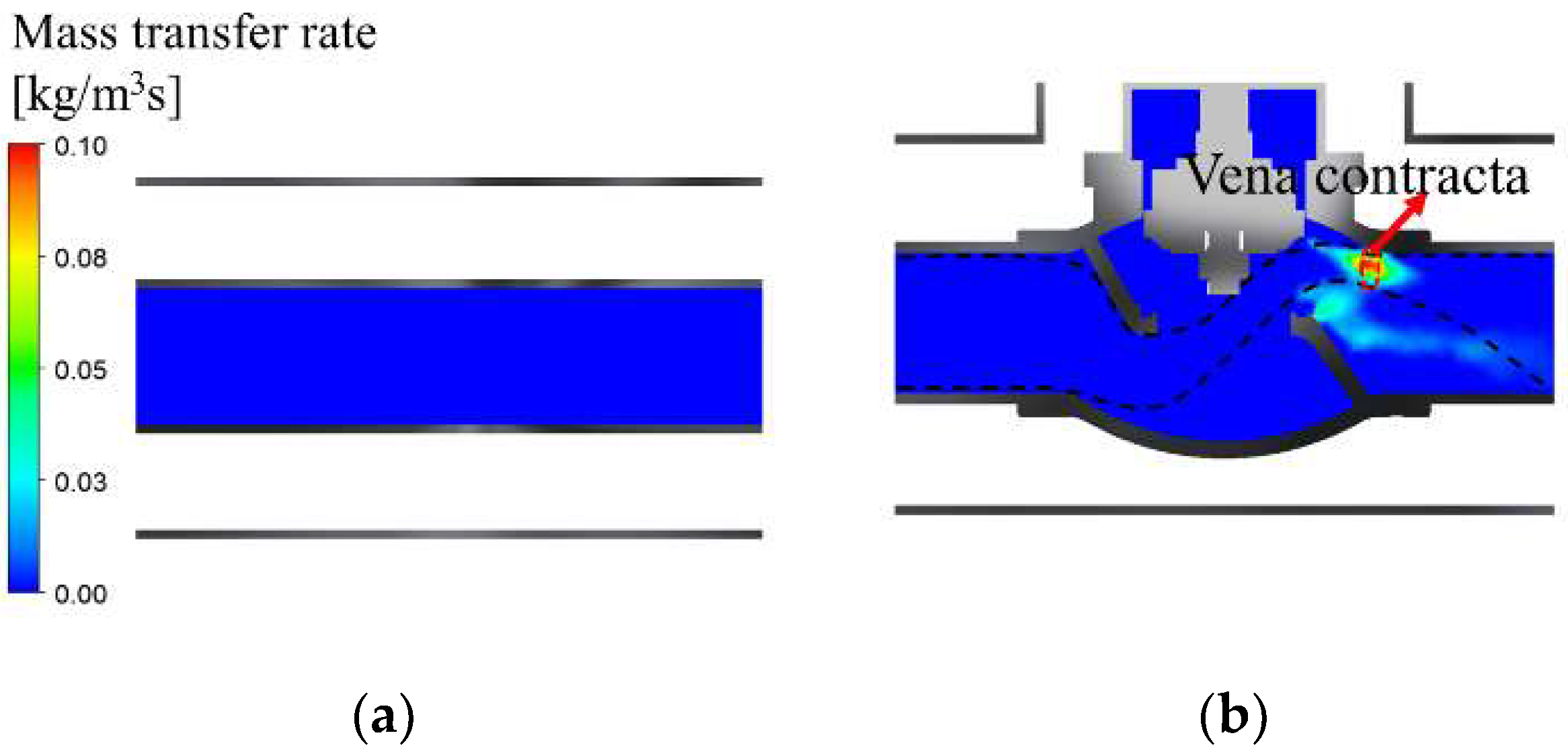
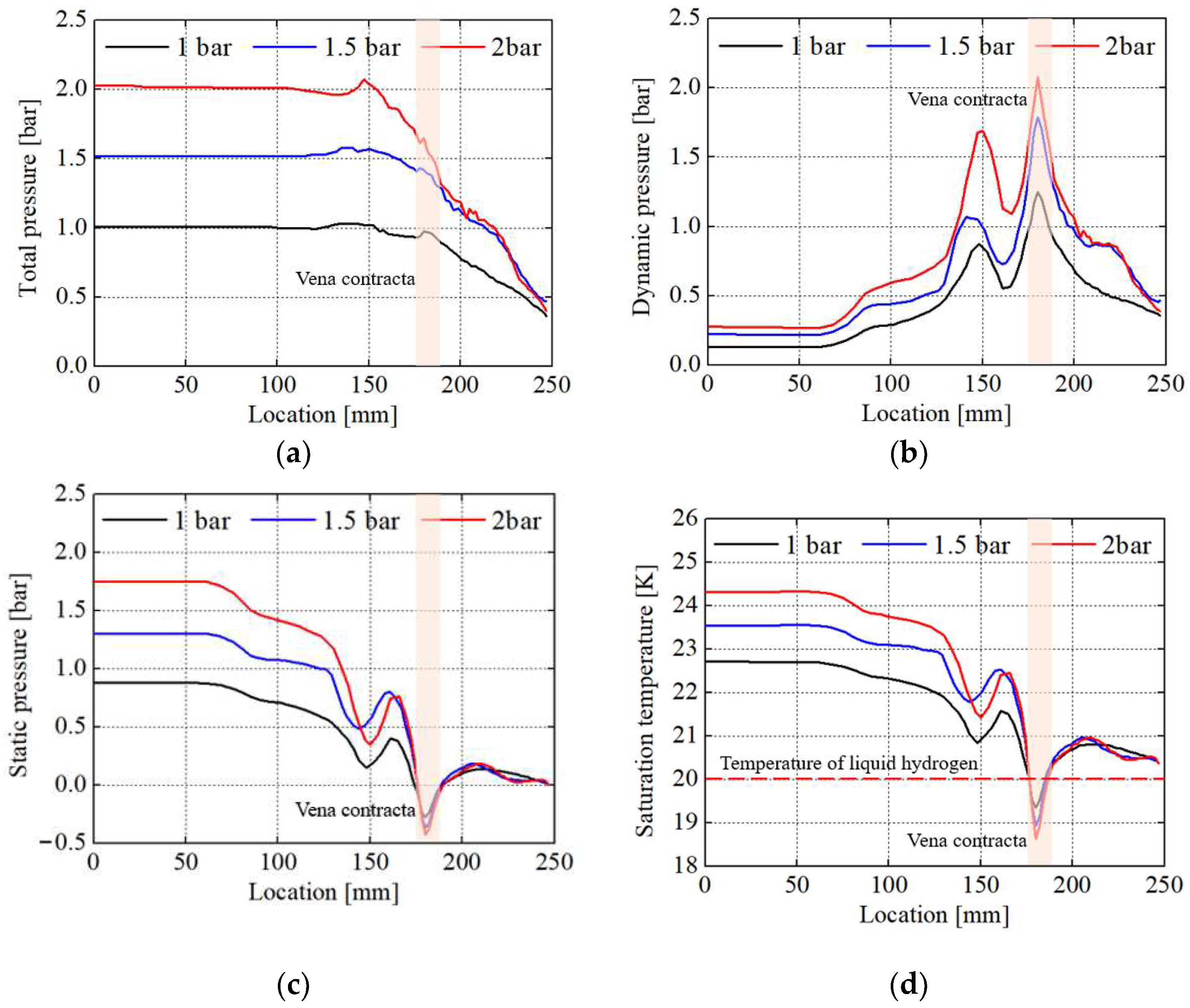
- Under the condition where the pressure of the liquid hydrogen storage tank is 1 bar, the maximum velocity and minimum static pressure of 62.9 m/s and −0.4 bar, respectively, occurred at the vena contracta in the vacuum-jacketed valve. The saturation temperature reached a minimum of 19.3 K, and the mass transfer rate of BOG was 0.132 .
- When the tank pressures changed from 1 to 1.5 and 2 bar, the dynamic and static pressures exhibited a negative correlation, and the maximum dynamic and minimum static pressures at the vena contracta were observed to be 1.25, 1.79, and 2.08 bar and −0.27, −0.37, and −0.43 bar, respectively.
- The due to the increase in tank pressures shows a similar trend to that of the static pressure. The minimum observed at the vena contracta was 19.3, 18.9, and 18.6 K, respectively. Because the BOG generation reached its maximum at the vena contracta where both the static pressure and were lowest, the change in the amount of BOG regarding the tank pressures was significantly influenced by the static pressure and .
- The total amount of BOG generated in the vacuum-jacketed valve per hour at tank pressures of 1, 1.5, and 2 bar was 0.132, 0.221, and 0.283 , respectively. It was observed that the increase in the amount of BOG slowed down after the tank pressure reached 1.5 bar.
- Using the vaporization analysis method proposed in this study, it is possible to estimate the amount of BOG generated during the unloading process of a liquid hydrogen storage tank. It is anticipated that this estimation can be utilized for the design of BOG capture and re-liquefaction processes.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abe, A.; Nakamura, M.; Sato, I.; Uetani, H.; Fujitani, T. Studies of the Large-Scale Sea Transportation of Liquid Hydrogen. Int. J. Hydrogen Energy 1998, 23, 115–121. [Google Scholar] [CrossRef]
- Smith, J.R.; Gkantonas, S.; Mastorakos, E. Modelling of Boil-Off and Sloshing Relevant to Future Liquid Hydrogen Carriers. Energies 2022, 15, 2046. [Google Scholar] [CrossRef]
- Al Ghafri, S.Z.S.; Swanger, A.; Jusko, V.; Siahvashi, A.; Perez, F.; Johns, M.L.; May, E.F. Modelling of Liquid Hydrogen Boil-Off. Energies 2022, 15, 1149. [Google Scholar] [CrossRef]
- Oh, S.-J.; Kwon, J.-Y.; Jeon, K.-S.; Yoon, J.-H. A Numerical Analysis Study on the Characteristics of Evaporation in Liquid Hydrogen Tank with Vacuum Layer According to Changes in Heat Flux and Vacuum Pressure. Int. J. Hydrogen Energy 2024, 50, 542–557. [Google Scholar] [CrossRef]
- Lee, W.H. A Pressure Iteration Scheme for Two-Phase Flow Modeling. Multiph. Transp. Fundam. React. Saf. Appl. 1980, 1, 407–432. [Google Scholar] [CrossRef]
- Seo, Y.-M.; Noh, H.-W.; Ha, D.-W.; Koo, T.-H.; Ko, R.-K. Numerical Study on the Effects of Gravity Direction and Hydrogen Filling Rate on BOG in the Liquefied Hydrogen Storage Tank. J. Hydrog. New Energy 2023, 34, 342–349. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, S.-P.; Lee, G.-H. Numerical Analysis and Experimental Verification Based on Design Parameter Variation of Vacuum-Insulated Pipe for Cryogenics. ISMEMS 2016 2016, 93, 488–493. [Google Scholar] [CrossRef]
- Nichita, B.A.; Thome, J.R. A Level Set Method and a Heat Transfer Model Implemented into FLUENT for Modeling of Microscale Two Phase Flows; AVT-178 Specialists’ Meeting on System Level Thermal Management for Enhanced Platform Efficiency: Bucharest, Romania, 2010. [Google Scholar]
- Sun, D.-L.; Xu, J.-L.; Wang, L. Development of a Vapor-Liquid Phase Change Model for Volume-of-Fluid Method in FLUENT. Int. Commun. Heat Mass Transf. 2012, 39, 1101–1106. [Google Scholar] [CrossRef]
- Aydelott, J.C. Normal Gravity Self-Pressurization of 9-Inch (23-cm) Diameter Spherical Liquid Hydrogen Tankage; National Aeronautics and Space Administration: Washington, DC, USA, 1967.
- Kim, S.-Y.; Kang, B.-H. Thermal Analysis of a Liquid Hydrogen Vessel. J. Korean Hydrog. Energy Soc. 1997, 8, 57–65. [Google Scholar] [CrossRef]
- Kim, S.-Y. Liquefied Hydrogen Production and Storage Technology and Its Application. J. Soc. Air-Cond. Refrig. Eng. Korea 2023, 52, 56–64. [Google Scholar]
- Kim, M.J. Heat Transfer Characteristics of Vacuum Insulated Pipe Due to Vacuum Degree; Dong-A University: Busan, Korea, 2022. [Google Scholar]
- Bae, K.-H.; Park, J.-H.; Kang, S.-M. A Study on the Flow Characteristics inside a Glove Valve for Ships. J. Korean Soc. Mar. Eng. 2008, 32, 110–118. [Google Scholar] [CrossRef]
- Technical Center for High-Performance Valve. Available online: http://www.dau-valve.com/ (accessed on 4 May 2023).

| Density [kg/m3] | Thermal Conductivity [W/mK] | Specific Heat Capacity [J/kgK] | |
|---|---|---|---|
| SUS 316L | 7900 | 16 | 470 |
| PTFE | 2175 | 0.24 | 1000 |
| EPDM | 860 | 0.15 | 2010 |
| Boundary Condition | Value |
|---|---|
| Working fluid | Liquid and gaseous hydrogen |
| Inlet total pressure [bar] | 1, 1.5, 2 |
| Outlet static pressure [bar] | 0 |
| Operating pressure [bar] | 1 |
| Turbulence model | k-ω SST |
| Thermal conductivity (vacuum 10−3 Torr) [W/mK] | 0.0014 |
| Heat transfer coefficient [W/m2K] | 10 |
| Ambient temperature [K] | 300 |
| Tank Pressures [bar] | BOG Generation per Hour [m3/h] |
|---|---|
| 1 | 0.132 |
| 1.5 | 0.221 |
| 2 | 0.283 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, H.-S.; Woo, S.-U.; Han, S.-H. Boil-Off Gas Generation in Vacuum-Jacketed Valve Used in Liquid Hydrogen Storage Tank. Energies 2024, 17, 2341. https://doi.org/10.3390/en17102341
Hwang H-S, Woo S-U, Han S-H. Boil-Off Gas Generation in Vacuum-Jacketed Valve Used in Liquid Hydrogen Storage Tank. Energies. 2024; 17(10):2341. https://doi.org/10.3390/en17102341
Chicago/Turabian StyleHwang, Hae-Seong, Seong-Un Woo, and Seung-Ho Han. 2024. "Boil-Off Gas Generation in Vacuum-Jacketed Valve Used in Liquid Hydrogen Storage Tank" Energies 17, no. 10: 2341. https://doi.org/10.3390/en17102341
APA StyleHwang, H.-S., Woo, S.-U., & Han, S.-H. (2024). Boil-Off Gas Generation in Vacuum-Jacketed Valve Used in Liquid Hydrogen Storage Tank. Energies, 17(10), 2341. https://doi.org/10.3390/en17102341






