Research on Quantitative Diagnosis of Dendrites Based on Titration Gas Chromatography Technology
Abstract
1. Introduction
2. Experiment
2.1. Dendrite Detection Based on Heating DC Resistance
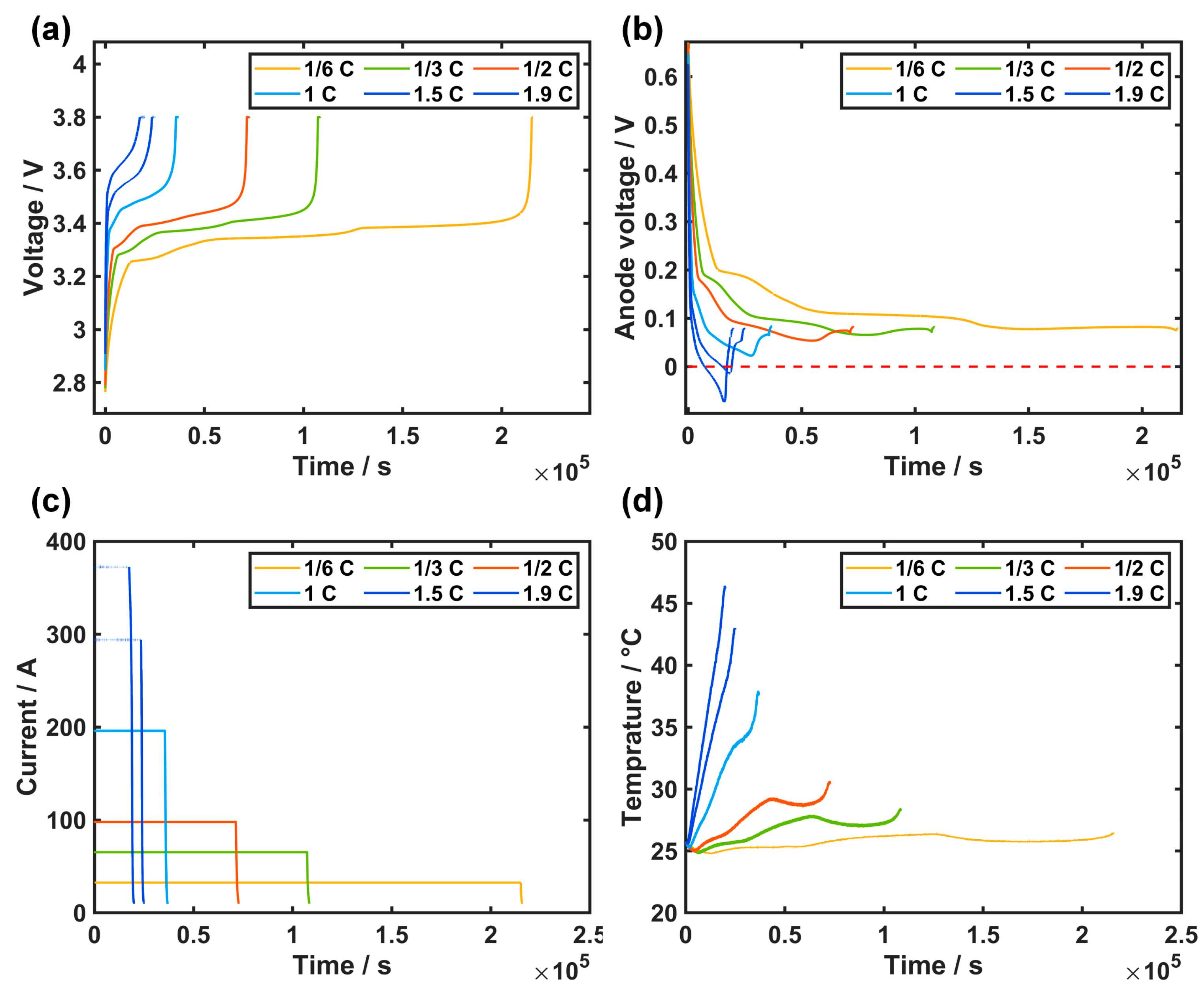
2.2. Dendrite Detection Based on Reference Electrode
2.3. Dendrite Detection Based on TGC Technology
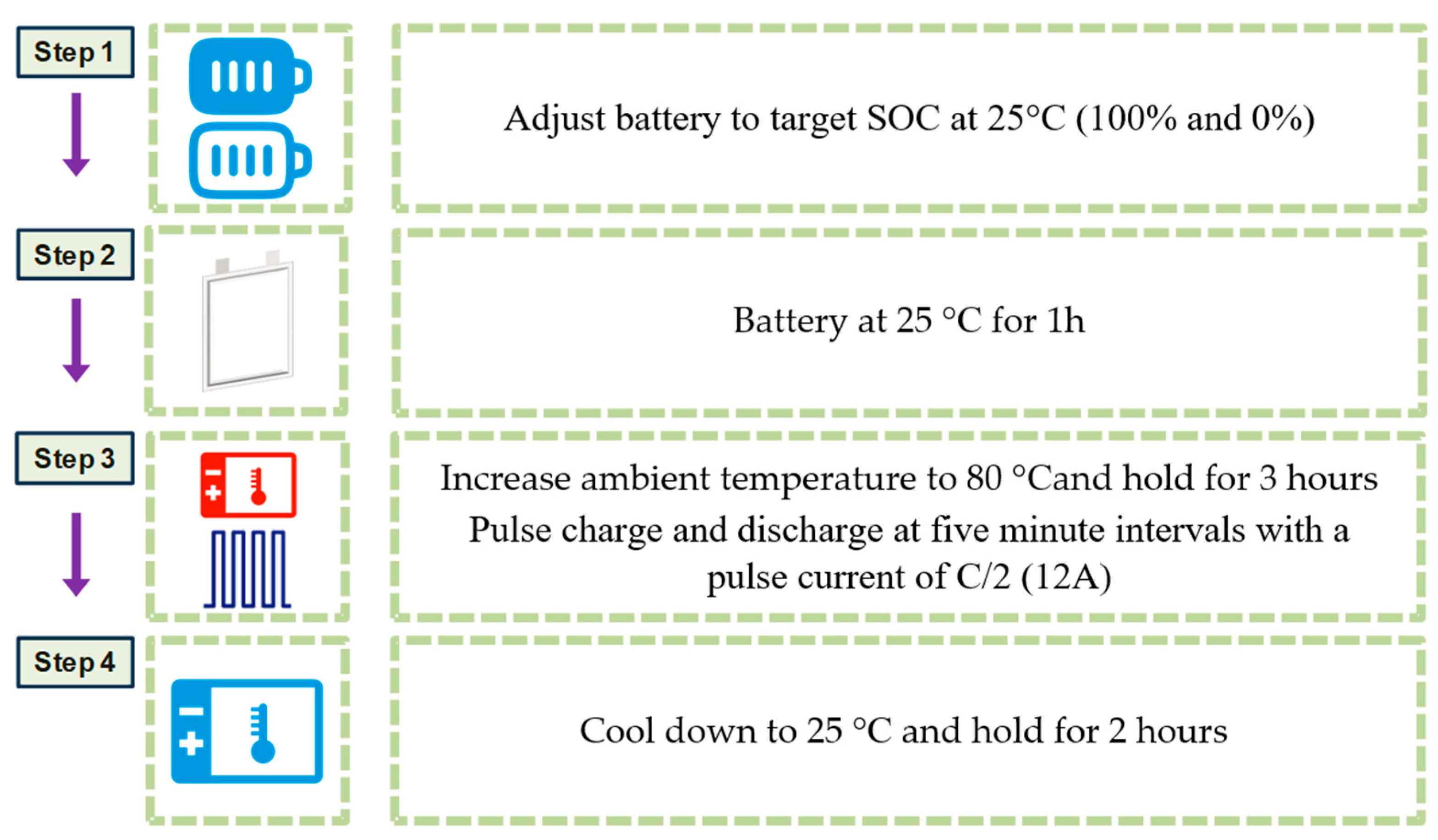
2.3.1. Battery Preparation for Measurement
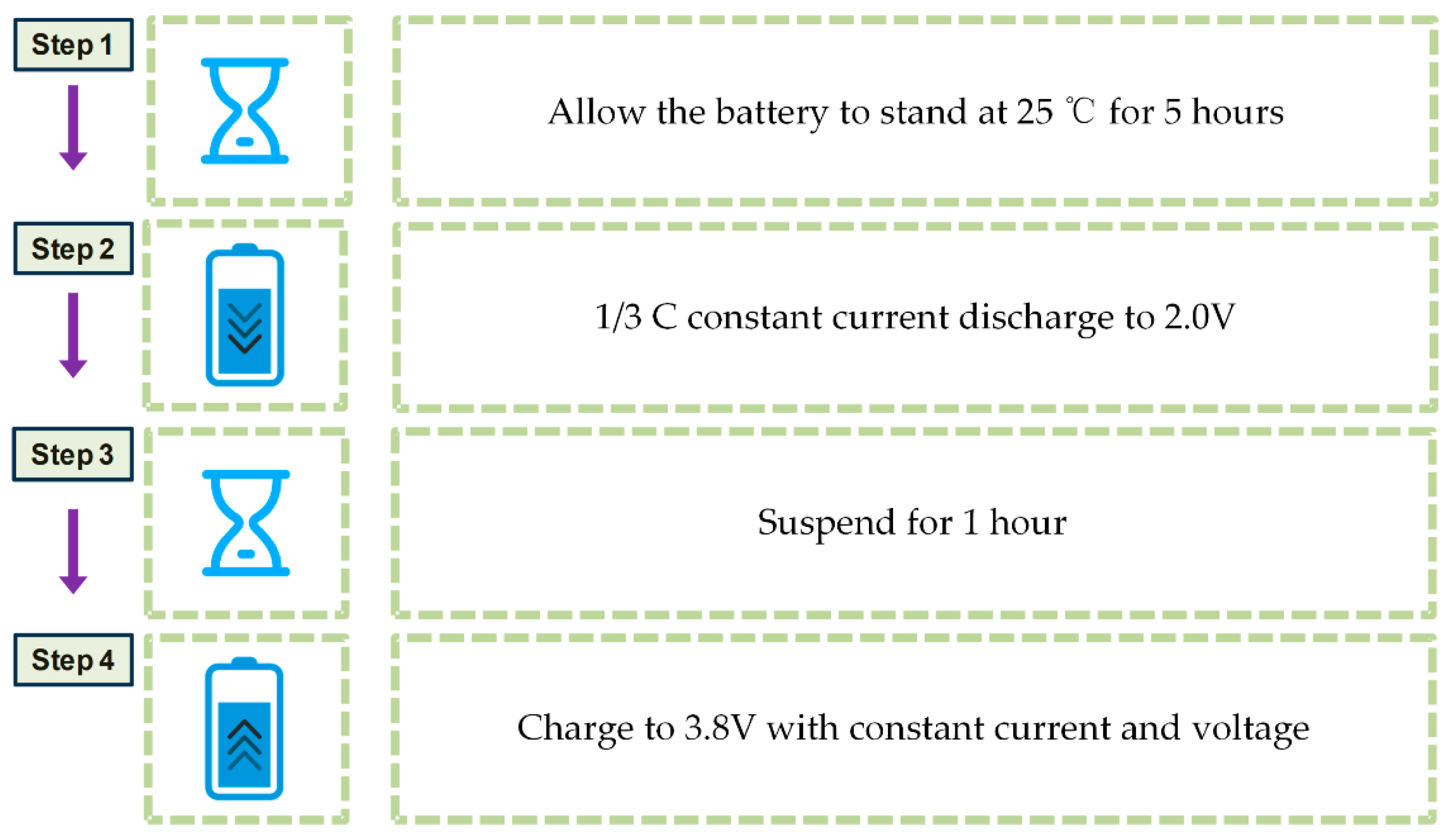
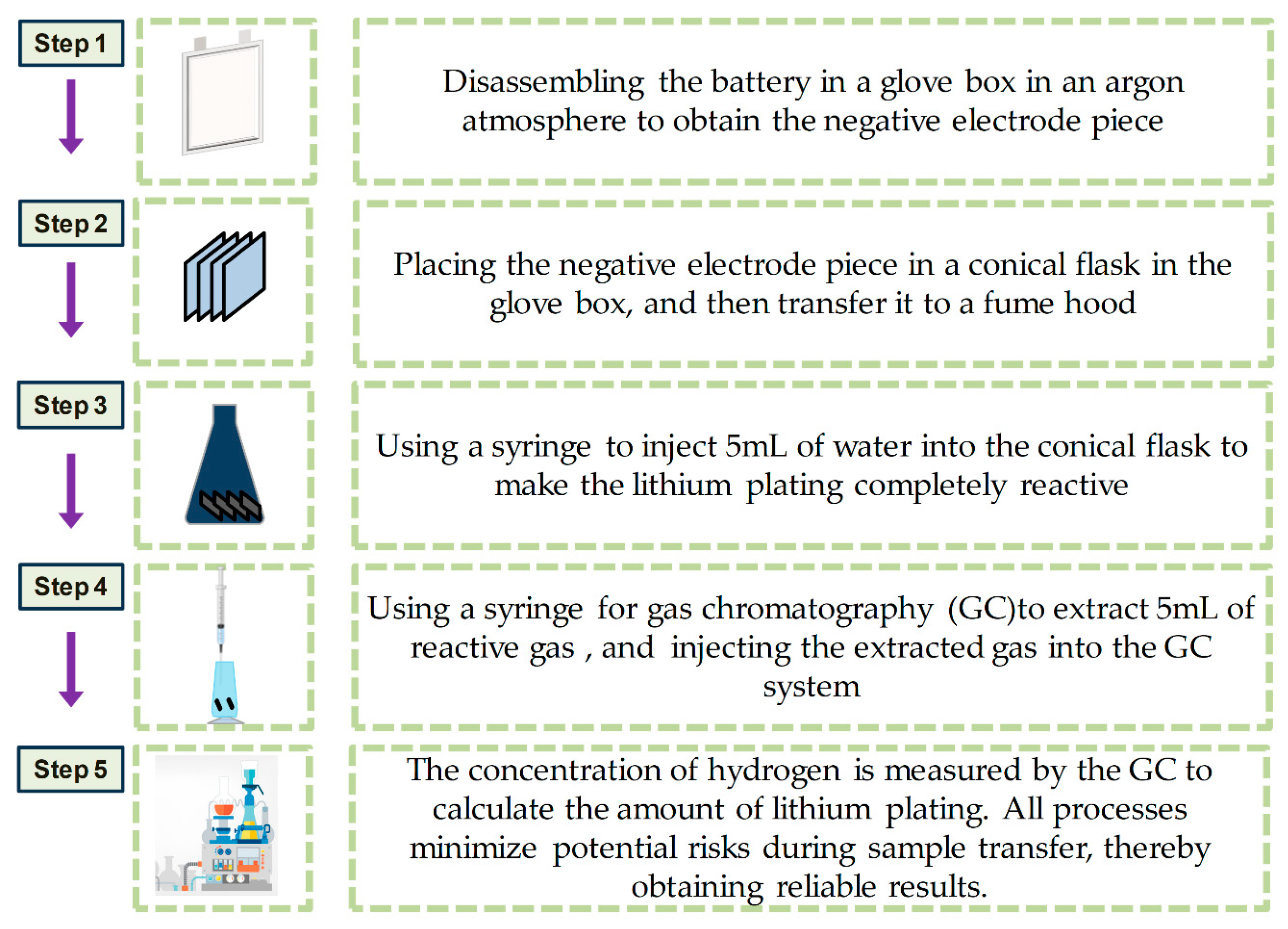
2.3.2. Titration Gas Chromatography Test
- (1)
- Disassembling the battery in a glove box in an argon atmosphere to obtain the negative electrode piece;
- (2)
- Placing the negative electrode piece in a conical flask in the glove box, and then transferring it to a fume hood;
- (3)
- Using a syringe to inject 5 mL of water into the conical flask to make the lithium plating completely reactive;
- (4)
- Using a syringe for gas chromatography (GC) to extract 5 mL of reactive gas, and injecting the extracted gas into the GC system;
- (5)
- Measuring the concentration of hydrogen via the GC to calculate the amount of lithium plating. All processes minimize potential risks during sample transfer, thereby obtaining reliable results.
2.4. Dendrite Detection Based on NMR Technology
3. Results
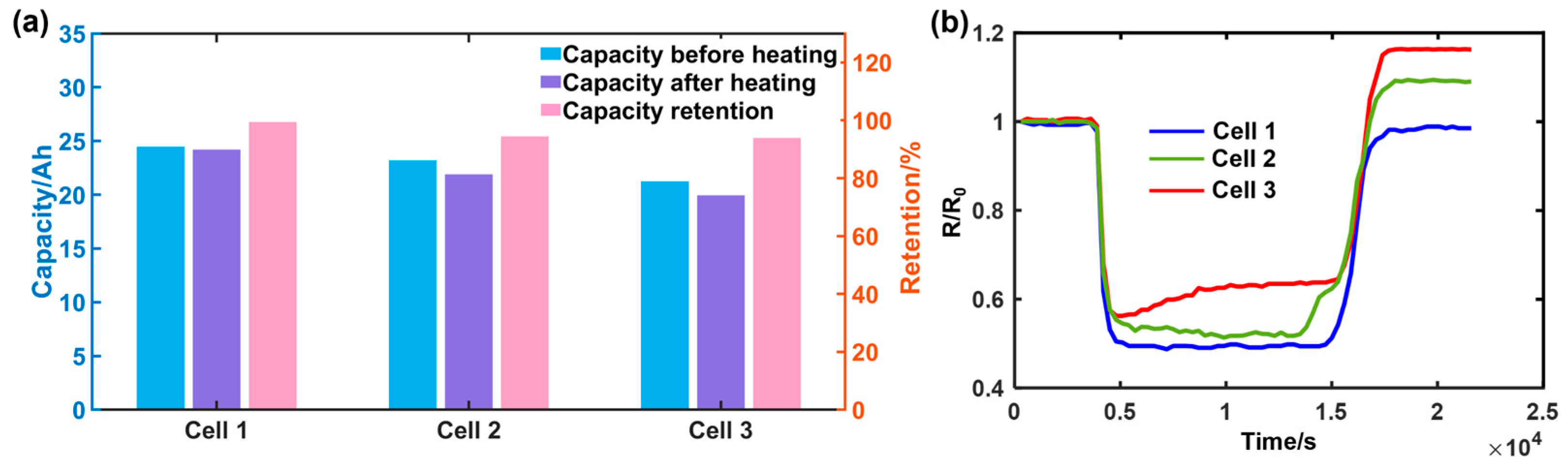
3.1. Experimental Results of Heating DC Resistance
3.2. Experimental Results of Reference Electrode
3.3. Experimental Results of TGC
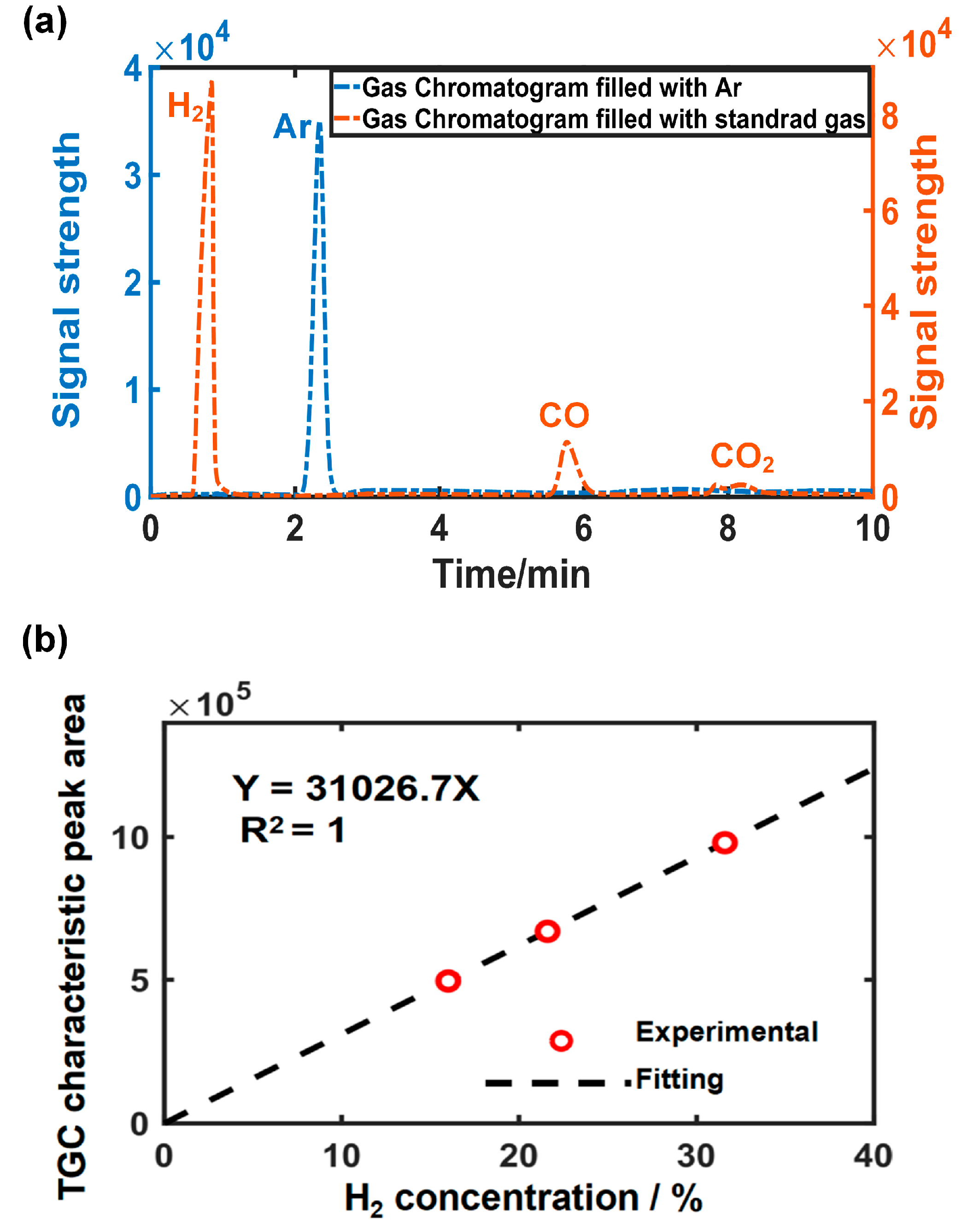
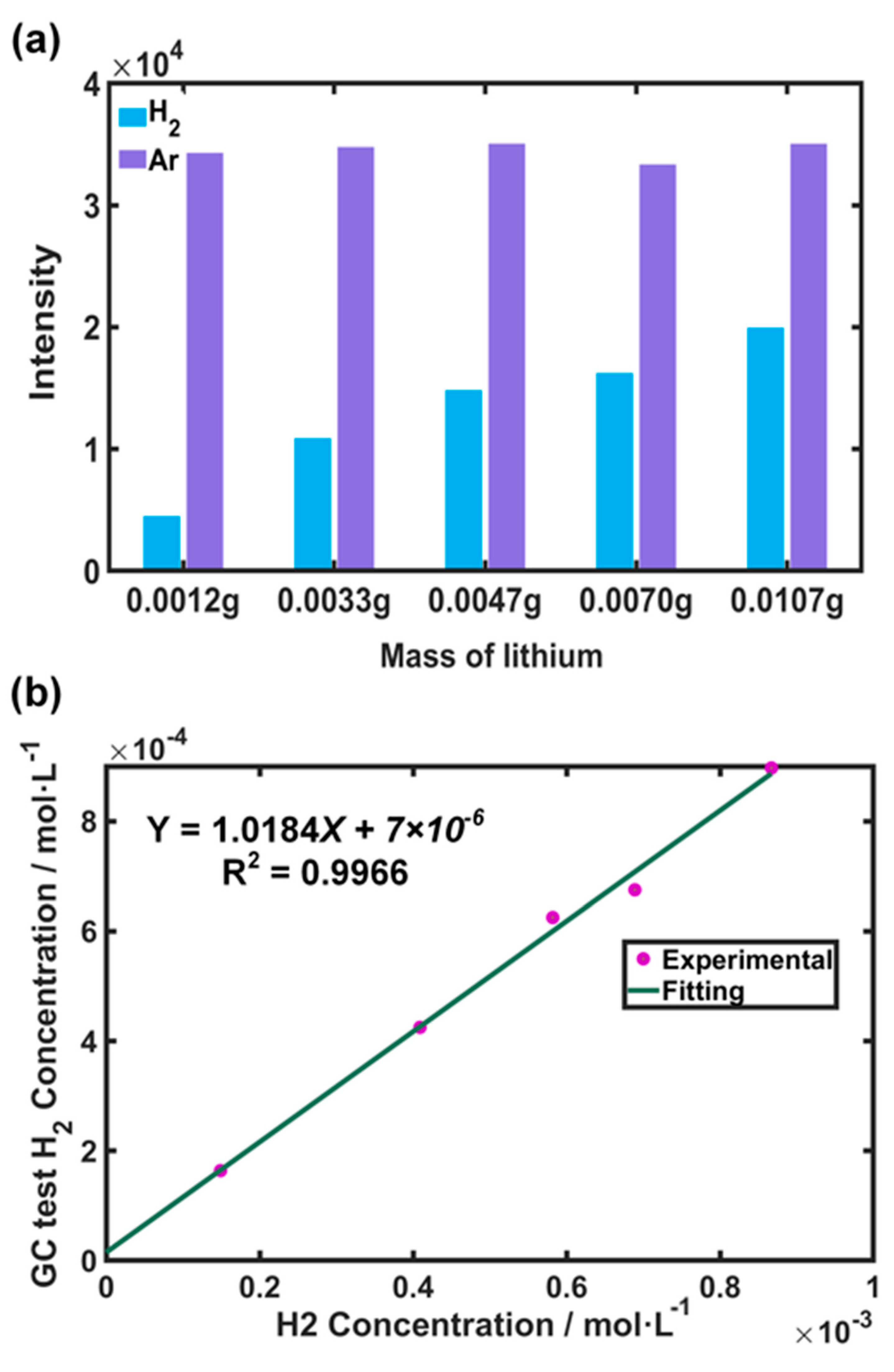
3.3.1. TGC Calibration Results
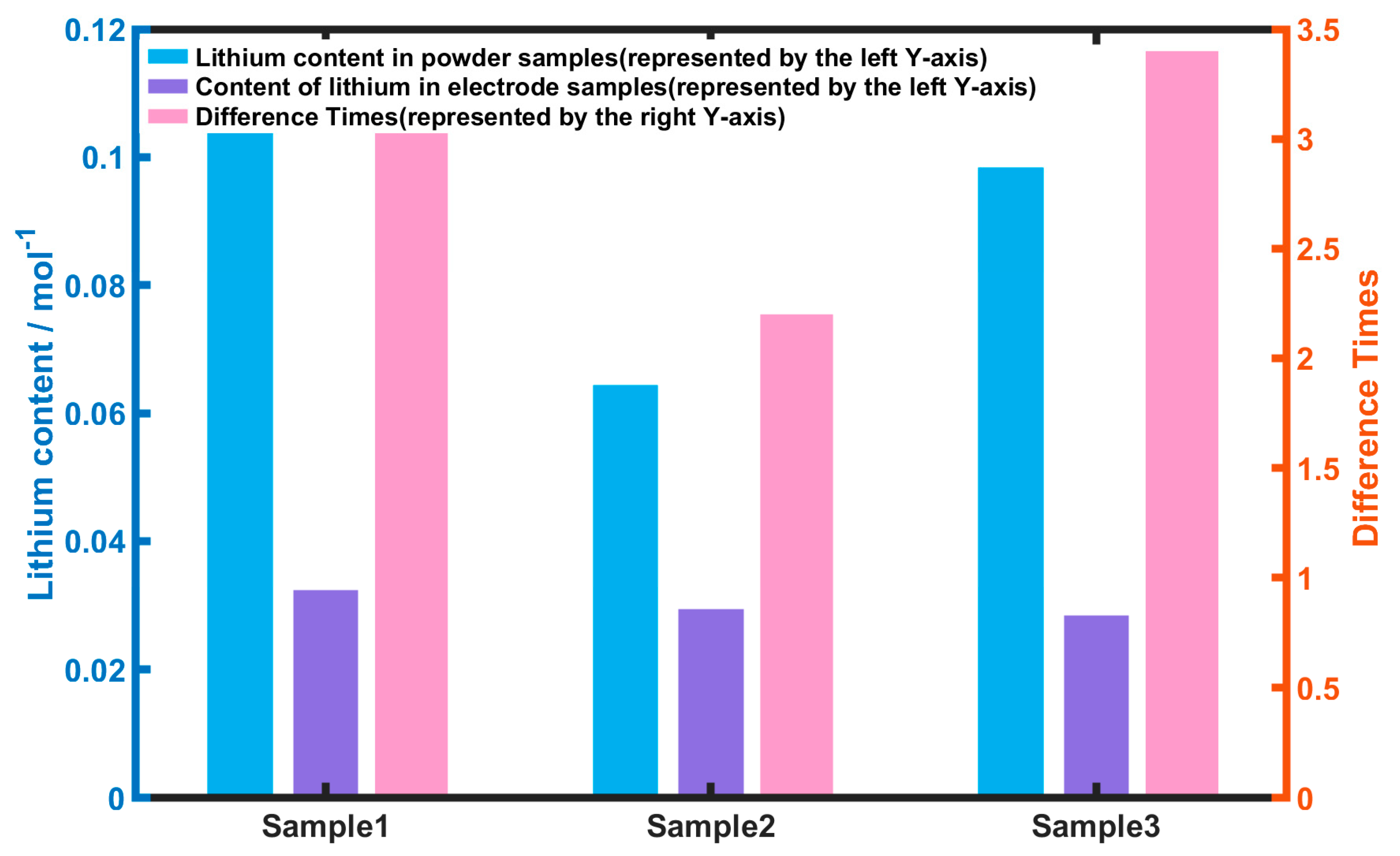
3.3.2. Testing of Lithium Plating in Battery Samples
3.3.3. Fresh Cell Background Noise Analysis
3.3.4. Battery Sample Calibration Results
3.4. Experimental Results of NMR
3.5. Discussion
4. Conclusions and Outlook
4.1. Conclusions
4.2. Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rangarajan, S.S.; Sunddararaj, S.P.; Sudhakar, A.; Shiva, C.K.; Subramaniam, U.; Collins, E.R.; Senjyu, T. Lithium-Ion Batteries—The Crux of Electric Vehicles with Opportunities and Challenges. Clean Technol. 2022, 4, 908–930. [Google Scholar] [CrossRef]
- Hu, G.; Huang, P.; Bai, Z.; Wang, Q.; Qi, K. Comprehensively analysis the failure evolution and safety evaluation of automotive lithium ion battery. eTransportation 2021, 10, 100140. [Google Scholar] [CrossRef]
- Tomaszewska, A.; Chu, Z.; Feng, X.; O’Kane, S.; Liu, X.; Chen, J.; Ji, C.; Endler, E.; Li, R.; Liu, L.; et al. Lithium-ion battery fast charging: A review. eTransportation 2019, 1, 100011. [Google Scholar] [CrossRef]
- Piao, N.; Gao, X.; Yang, H.; Guo, Z.; Hu, G.; Cheng, H.-M.; Li, F. Challenges and development of lithium-ion batteries for low temperature environments. eTransportation 2022, 11, 100145. [Google Scholar] [CrossRef]
- Tanim, T.R.; Dufek, E.J.; Dickerson, C.C.; Wood, S.M. Electrochemical Quantification of Lithium Plating: Challenges and Considerations. J. Electrochem. Soc. 2019, 166, A2689–A2696. [Google Scholar] [CrossRef]
- Li, Z.; Fang, R.; Ge, H.; Liu, Z.; Spingler, F.B.; Jossen, A.; Zhang, J.; Liaw, B. Multiphysics Footprint of Li Plating for Li-Ion Battery and Challenges for High-Accuracy Detection. J. Electrochem. Soc. 2022, 169, 080530. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.; Jia, W.; Yu, Y.; Zhang, R.; Li, T.; Fu, X.; Niu, X.; Li, J.; Kang, Y. Three-dimensional carbon material as stable host for dendrite-free lithium metal anodes. Electrochim. Acta 2019, 301, 251–257. [Google Scholar] [CrossRef]
- Börner, M.; Friesen, A.; Grützke, M.; Stenzel, Y.; Brunklaus, G.; Haetge, J.; Nowak, S.; Schappacher, F.; Winter, M. Correlation of aging and thermal stability of commercial 18650-type lithium ion batteries. J. Power Sources 2017, 342, 382–392. [Google Scholar] [CrossRef]
- Waldmann, T.; Wohlfahrt-Mehrens, M. Effects of rest time after Li plating on safety behavior—ARC tests with commercial high-energy 18650 Li-ion cells. Electrochim. Acta 2017, 230, 454–460. [Google Scholar] [CrossRef]
- Liu, W.; Qiu, B.; Yan, J.; He, C.; Zhang, P.; Mi, H. An aqueous polyethylene oxide-based solid-state electrolyte with high voltage stability for dendrite-free lithium deposition via a self-healing electrostatic shield. Dalton Trans. 2021, 50, 14296–14302. [Google Scholar] [CrossRef]
- Friesen, A.; Horsthemke, F.; Mönnighoff, X.; Brunklaus, G.; Krafft, R.; Börner, M.; Risthaus, T.; Winter, M.; Schappacher, F.M. Impact of cycling at low temperatures on the safety behavior of 18650-type lithium ion cells: Combined study of mechanical and thermal abuse testing accompanied by post-mortem analysis. J. Power Sources 2016, 334, 1–11. [Google Scholar] [CrossRef]
- Verma, P.; Maire, P.; Novák, P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochim. Acta 2010, 55, 6332–6341. [Google Scholar] [CrossRef]
- Chen, K.-H.; Wood, K.N.; Kazyak, E.; LePage, W.S.; Davis, A.L.; Sanchez, A.J.; Dasgupta, N.P. Dead lithium: Mass transport effects on voltage, capacity, and failure of lithium metal anodes. J. Mater. Chem. A 2017, 5, 11671–11681. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, S.; Weng, S.; Li, X.; Wang, X.; Wang, Z.; Chen, L. Anionic Effect on Enhancing the Stability of a Solid Electrolyte Interphase Film for Lithium Deposition on Graphite. Nano Lett. 2021, 21, 5316–5323. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Liu, B.; Mecklenburg, M.; Li, Y. Ultrafast deposition of faceted lithium polyhedra by outpacing SEI formation. Nature 2023, 620, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, D.; Liu, M.; Shen, C.; Hu, Y.; Liu, Y.; Guo, B. Improving the Durability of Lithium-Metal Anode via In situ Constructed Multilayer SEI. ACS Appl. Mater. Interfaces 2021, 13, 49445–49452. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Zhang, C.; Guo, Y.; Peng, L.; Zhang, X.; Qian, W.; Zhang, L.; Zhang, S. Advanced Nonflammable Localized High-Concentration Electrolyte for High Energy Density Lithium Battery. Energy Environ. Mater. 2021, 5, 1294–1302. [Google Scholar] [CrossRef]
- Beltran, S.P.; Balbuena, P.B. Lithium Dissolution and SEI Formation on Lithium Metal Anodes: Electrolyte and Surface Effects. ECS Meet. Abstr. 2022, MA2022-02, 145. [Google Scholar] [CrossRef]
- Luo, H. Lithium-Ion Battery Cathode-Electrolyte Interface. ECS Meet. Abstr. 2023, MA2023-01, 2828. [Google Scholar] [CrossRef]
- Li, R.; O’kane, S.; Marinescu, M.; Offer, G.J. Modelling Solvent Consumption from SEI Layer Growth in Lithium-Ion Batteries. J. Electrochem. Soc. 2022, 169, 060516. [Google Scholar] [CrossRef]
- Ren, Y.; Widanage, D.; Marco, J. A Plating-Free Charging Scheme for Battery Module Based on Anode Potential Estimation to Prevent Lithium Plating. Batteries 2023, 9, 294. [Google Scholar] [CrossRef]
- Carter, R.; Klein, E.J.; Kingston, T.A.; Love, C.T. Detection of Lithium Plating During Thermally Transient Charging of Li-Ion Batteries. Front. Energy Res. 2019, 7, 00144. [Google Scholar] [CrossRef]
- Yang, X.-G.; Ge, S.; Liu, T.; Leng, Y.; Wang, C.-Y. A look into the voltage plateau signal for detection and quantification of lithium plating in lithium-ion cells. J. Power Sources 2018, 395, 251–261. [Google Scholar] [CrossRef]
- Smart, M.C.; Ratnakumar, B.V. Effects of Electrolyte Composition on Lithium Plating in Lithium-Ion Cells. J. Electrochem. Soc. 2011, 158, A379–A389. [Google Scholar] [CrossRef]
- Petzl, M.; Danzer, M.A. Nondestructive detection, characterization, and quantification of lithium plating in commercial lithium-ion batteries. J. Power Sources 2014, 254, 80–87. [Google Scholar] [CrossRef]
- Zeng, Z.; Liang, W.-I.; Liao, H.-G.; Xin, H.L.; Chu, Y.-H.; Zheng, H. Visualization of Electrode–Electrolyte Interfaces in LiPF6/EC/DEC Electrolyte for Lithium Ion Batteries via in Situ TEM. Nano Lett. 2014, 14, 1745–1750. [Google Scholar] [CrossRef] [PubMed]
- Zhe, D.; Zhenyu, H.; Lei, L.; Huang, Y.; Shen, Y. Applications of ultrasound technique in characterization of lithium-ion batteries. Energy Storage Sci. Technol. 2019, 8, 1033. (In Chinese) [Google Scholar]
- Hsieh, Y.-C.; Leißing, M.; Nowak, S.; Hwang, B.-J.; Winter, M.; Brunklaus, G. Quantification of Dead Lithium via In Situ Nuclear Magnetic Resonance Spectroscopy. Cell Rep. Phys. Sci. 2020, 1, 100139. [Google Scholar] [CrossRef]
- Gotoh, K.; Izuka, M.; Arai, J.; Okada, Y.; Sugiyama, T.; Takeda, K.; Ishida, H. In situ 7Li nuclear magnetic resonance study of the relaxation effect in practical lithium ion batteries. Carbon 2014, 79, 380–387. [Google Scholar] [CrossRef]
- McShane, E.J.; Colclasure, A.M.; McCloskey, B.D. (Industrial Electrochemistry and Electrochemical Engineering Division Student Achievement Award) Mass Spectrometry Titration for Quantitative Probing of Lithium Plating and Solid-Electrolyte Interphase Formation. ECS Meet. Abstr. 2021, MA2021-01, 945. [Google Scholar] [CrossRef]
- Pecher, O.; Carretero-González, J.; Griffith, K.J.; Grey, C.P. Materials’ Methods: NMR in Battery Research. Chem. Mater. 2016, 29, 213–242. [Google Scholar] [CrossRef]
- Arai, J.; Okada, Y.; Sugiyama, T.; Izuka, M.; Gotoh, K.; Takeda, K. In Situ Solid State7Li NMR Observations of Lithium Metal Deposition during Overcharge in Lithium Ion Batteries. J. Electrochem. Soc. 2015, 162, A952–A958. [Google Scholar] [CrossRef]
- Chang, H.J.; Trease, N.M.; Ilott, A.J.; Zeng, D.; Du, L.-S.; Jerschow, A.; Grey, C.P. Investigating Li Microstructure Formation on Li Anodes for Lithium Batteries by in Situ 6Li/7Li NMR and SEM. J. Phys. Chem. C 2015, 119, 16443–16451. [Google Scholar] [CrossRef]
- Fang, C.; Li, J.; Zhang, M.; Zhang, Y.; Yang, F.; Lee, J.Z.; Lee, M.-H.; Alvarado, J.; Schroeder, M.A.; Yang, Y.; et al. Quantifying inactive lithium in lithium metal batteries. Nature 2019, 572, 511–515. [Google Scholar] [CrossRef] [PubMed]
- McShane, E.J.; Colclasure, A.M.; Brown, D.E.; Konz, Z.M.; Smith, K.; McCloskey, B.D. Quantification of Inactive Lithium and Solid–Electrolyte Interphase Species on Graphite Electrodes after Fast Charging. ACS Energy Lett. 2020, 5, 2045–2051. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, S.; Chen, Q.; Chen, F.; Zhang, X.; Ouyang, M.; Han, X.; Zheng, Y. Online Fast Charging Model without Lithium Plating for Long-Dimensional Cells in Automotive Applications. Batteries 2023, 9, 563. [Google Scholar] [CrossRef]
- Liu, J.; Chu, Z.; Li, H.; Ren, D.; Zheng, Y.; Lu, L.; Han, X.; Ouyang, M. Lithium-plating-free fast charging of large-format lithium-ion batteries with reference electrodes. Int. J. Energy Res. 2021, 45, 7918–7932. [Google Scholar] [CrossRef]
- Zhao, T.; Zheng, Y.; Liu, J.; Zhou, X.; Chu, Z.; Han, X. A study on half-cell equivalent circuit model of lithium-ion battery based on reference electrode. Int. J. Energy Res. 2020, 45, 4155–4169. [Google Scholar] [CrossRef]


| Parameters | Values/Types |
|---|---|
| Battery type | Lix(NiCoMn)1/3O2/graphite |
| Rated capacity (Ah) | 24 |
| Operating voltage (V) | 2.5~4.2 |
| Charging operating temperature (°C) | −25~55 |
| Discharging operating temperature (°C) | −30~55 |
| Parameters | Specifications |
|---|---|
| Size (mm) | 620 × 102 × 22.9 |
| Rated capacity (Ah) | 190.6 |
| Working voltage (V) | 2.0~3.8 |
| Operation temperature (°C) | −30~60 |
| Weight (Kg) | 3.2 |
| Types | Product Classification | Normal Capacity (Ah) | Charge/Discharge Cut-Off Voltage |
|---|---|---|---|
| Cell | Pouch cell | 1 Ah | 4.2/3.0 |
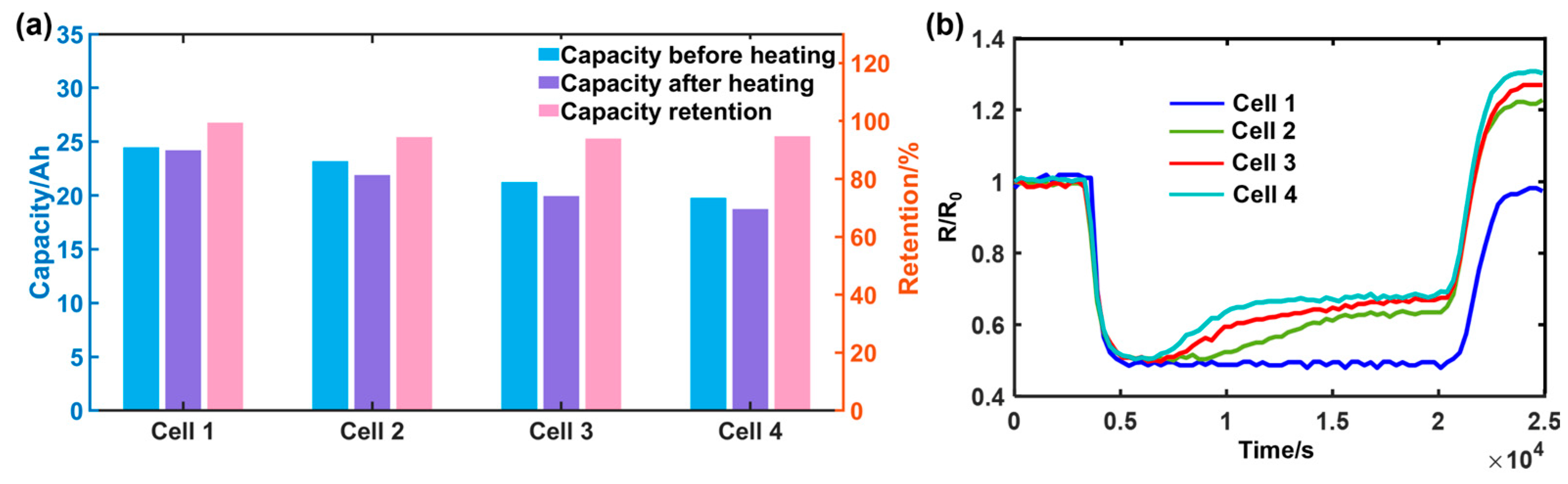
| Cell | Capacity Change before Heating (Ah) | Capacity Change after Heating (Ah) | Capacity Retention Rate % |
|---|---|---|---|
| 1 | 24.62 | 24.59 | 99.87 |
| 2 | 24.30 | 24.28 | 99.91 |
| 3 | 22.30 | 20.76 | 93.00 |
| 4 | 20.74 | 18.40 | 90.01 |
| Sample | Lithium Metal Content/g | Converted Residual Capacity/mAh |
|---|---|---|
| 1 | 0.014 | 54 |
| 2 | 0.011 | 42 |
| 3 | 0.013 | 50 |
| Mean value | 0.0127 | 49 |
| Sample | Initial Capacity/Ah | Capacity Decay/Ah | Based on Capacity Decay/g | TGC/g | Error/% | Remove Noise/g | Error/% |
|---|---|---|---|---|---|---|---|
| A | 1.097 | 0.687 | 0.178 | 0.179 | 0.6 | 0.167 | 6.2 |
| B | 1.110 | 0.720 | 0.186 | 0.204 | 9.7 | 0.191 | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Cai, H.; Li, S.; Wang, Y.; Zhang, X.; Wu, Z.; Lai, Y.; Bezha, M.; Bezha, K.; Nagaoka, N.; et al. Research on Quantitative Diagnosis of Dendrites Based on Titration Gas Chromatography Technology. Energies 2024, 17, 2409. https://doi.org/10.3390/en17102409
Yang K, Cai H, Li S, Wang Y, Zhang X, Wu Z, Lai Y, Bezha M, Bezha K, Nagaoka N, et al. Research on Quantitative Diagnosis of Dendrites Based on Titration Gas Chromatography Technology. Energies. 2024; 17(10):2409. https://doi.org/10.3390/en17102409
Chicago/Turabian StyleYang, Kai, Hongchang Cai, Suran Li, Yu Wang, Xue Zhang, Zhenxuan Wu, Yilin Lai, Minella Bezha, Klara Bezha, Naoto Nagaoka, and et al. 2024. "Research on Quantitative Diagnosis of Dendrites Based on Titration Gas Chromatography Technology" Energies 17, no. 10: 2409. https://doi.org/10.3390/en17102409
APA StyleYang, K., Cai, H., Li, S., Wang, Y., Zhang, X., Wu, Z., Lai, Y., Bezha, M., Bezha, K., Nagaoka, N., Zheng, Y., & Feng, X. (2024). Research on Quantitative Diagnosis of Dendrites Based on Titration Gas Chromatography Technology. Energies, 17(10), 2409. https://doi.org/10.3390/en17102409








