Experimental Study on Paraffin Wax and Soya Wax Supported by High-Density Polyethylene and Loaded with Nano-Additives for Thermal Energy Storage
Abstract
1. Introduction
2. Materials and Methods
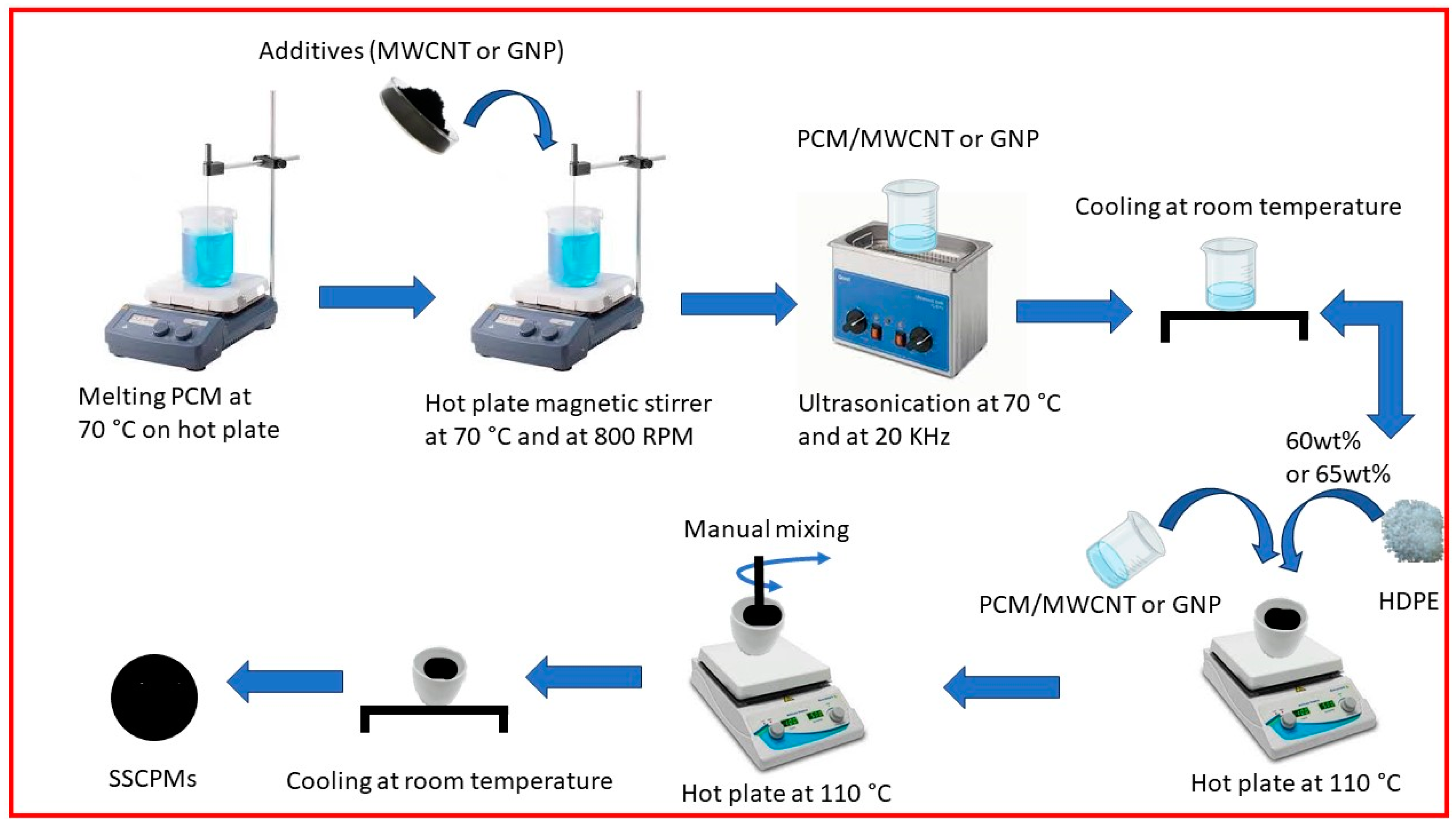
2.1. Preparation of Shape-Stable PCM
2.2. Preparation of Shape-Stable Composite PCM
3. Results
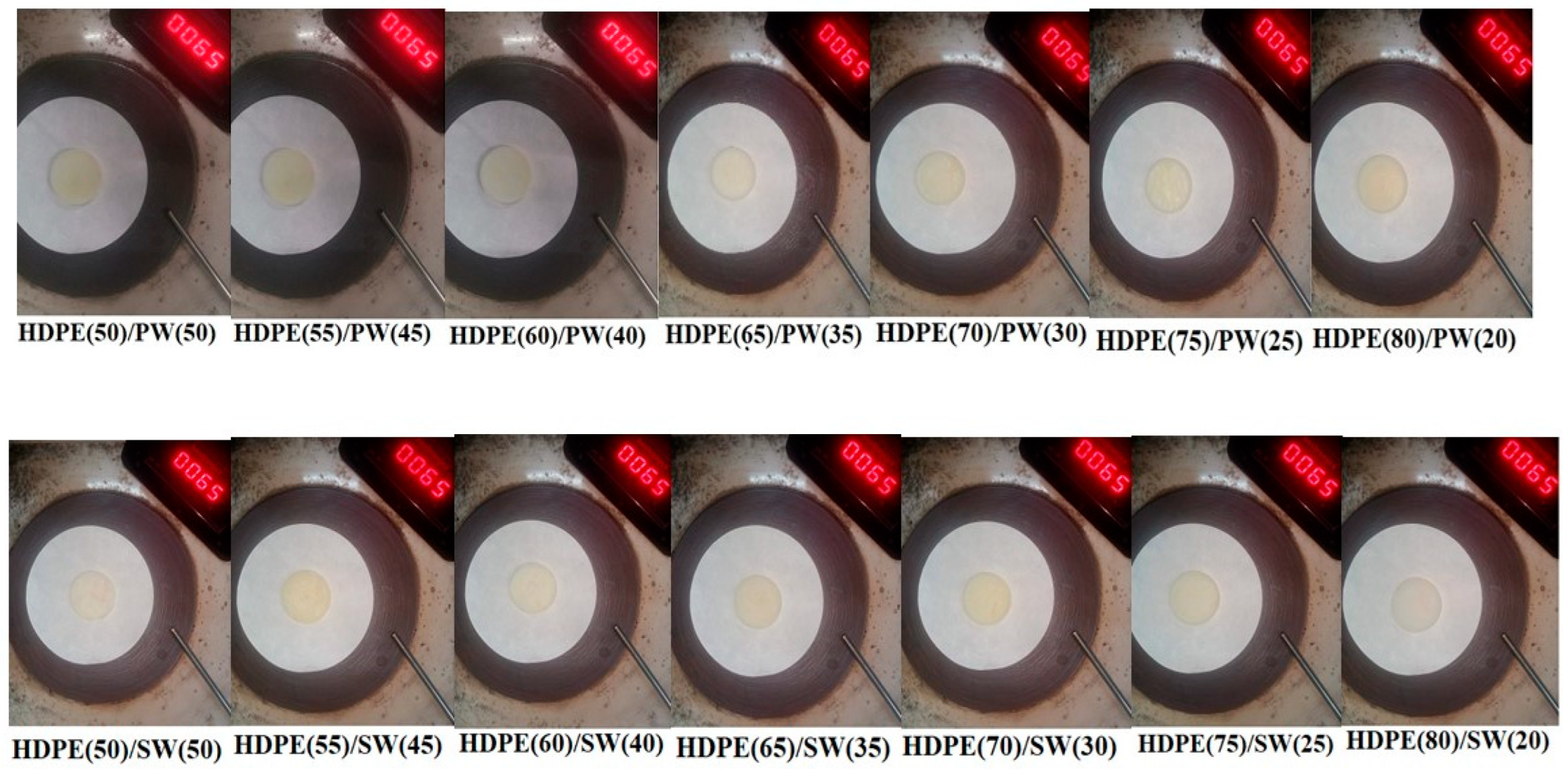
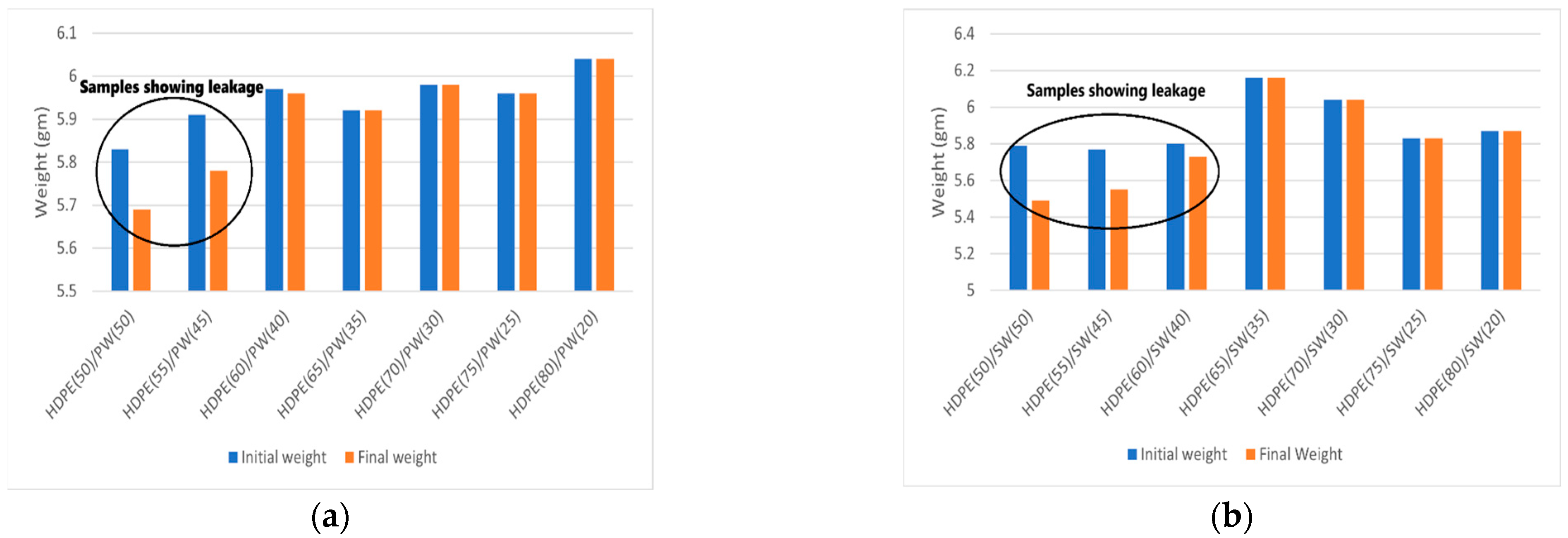
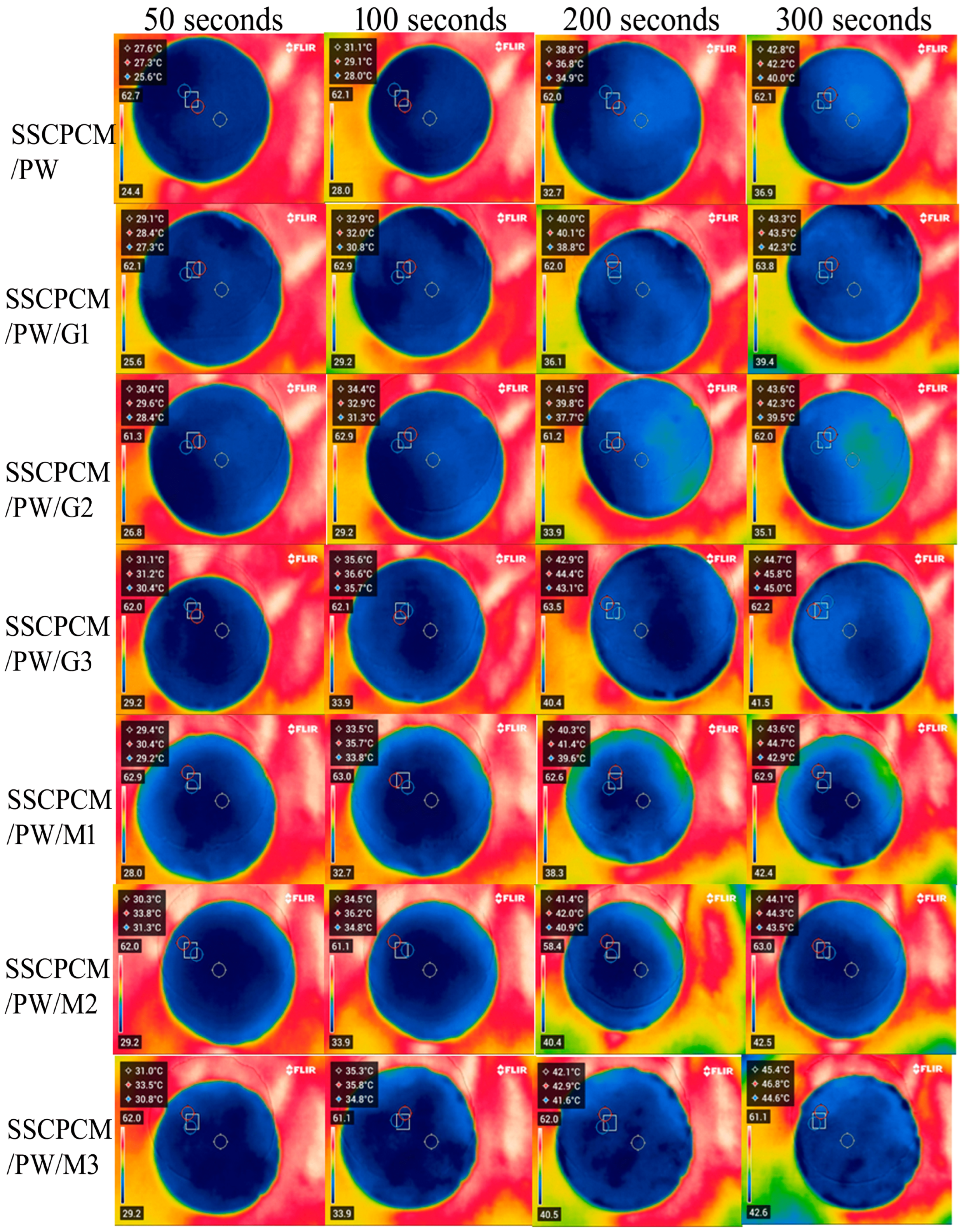
3.1. Leakage Test
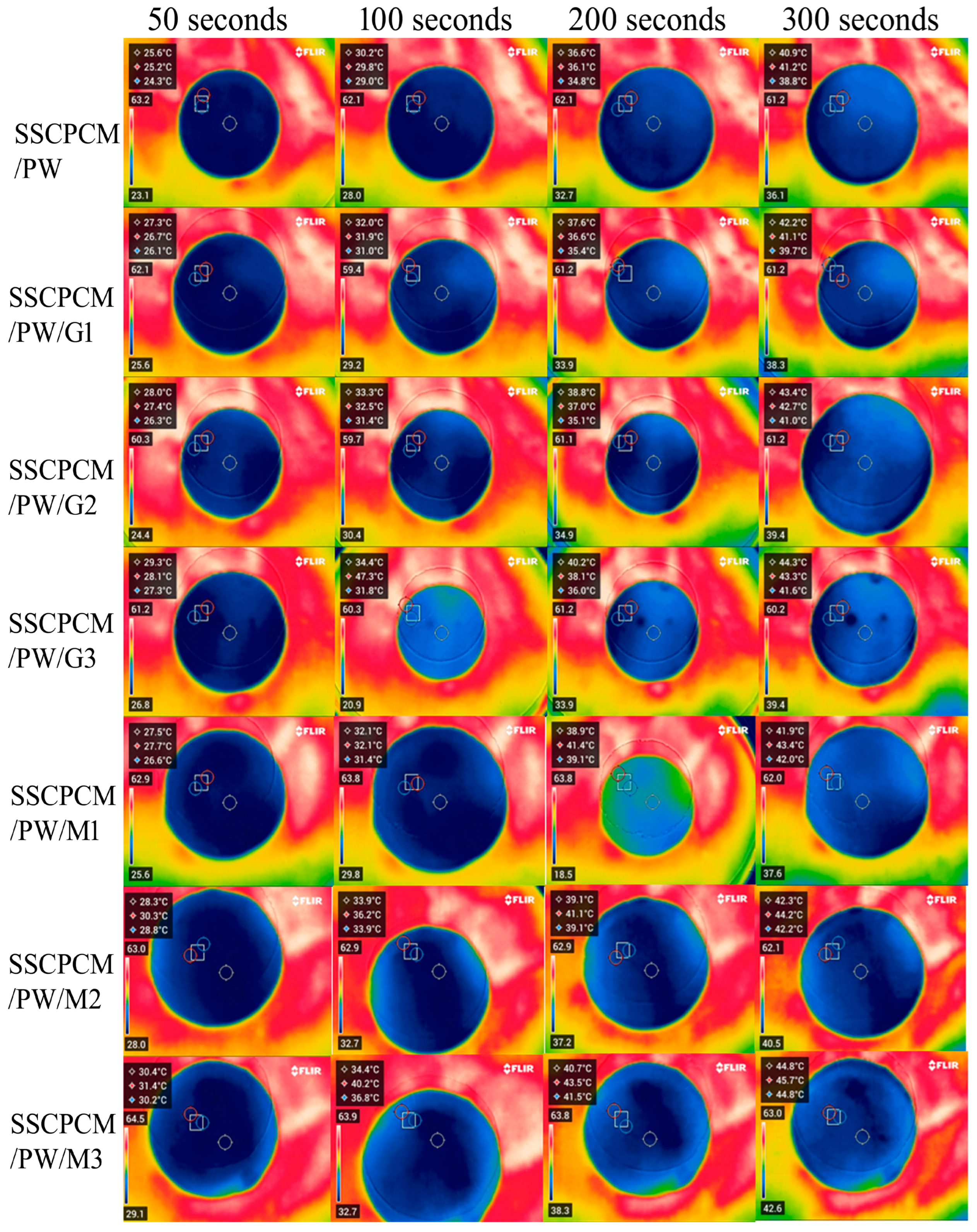
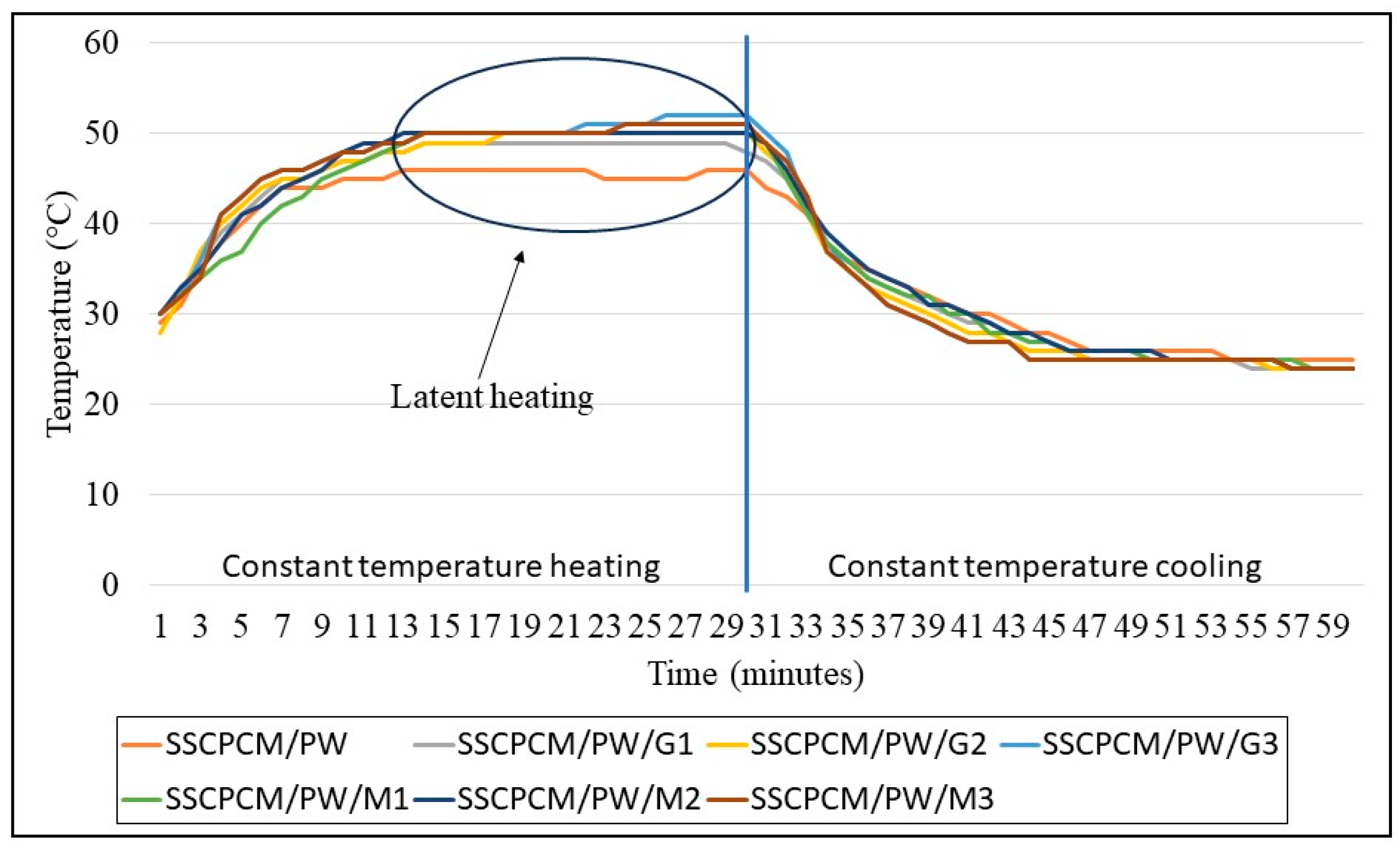
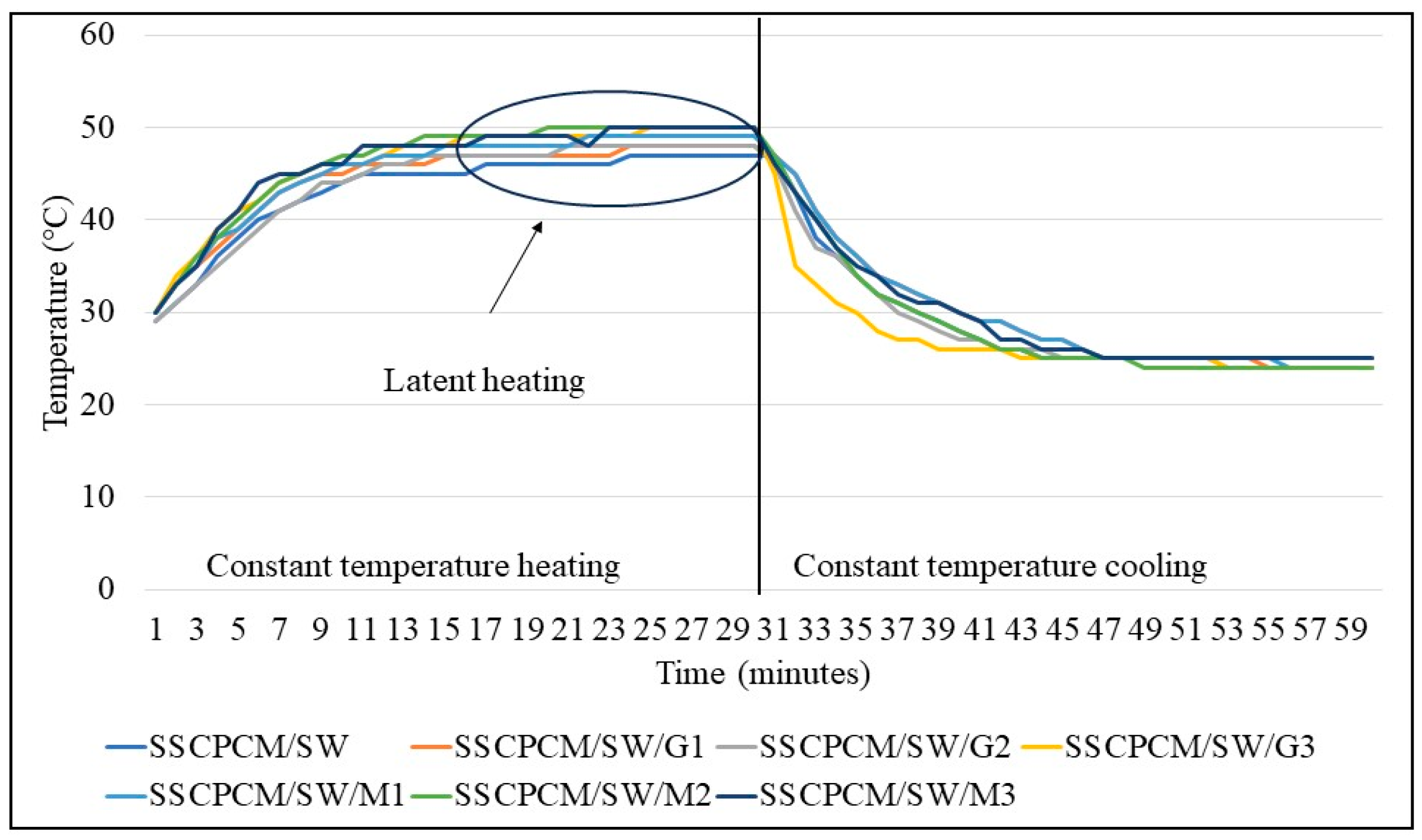
3.2. Heating and Cooling Profile
3.3. Steady-State Thermal Performance
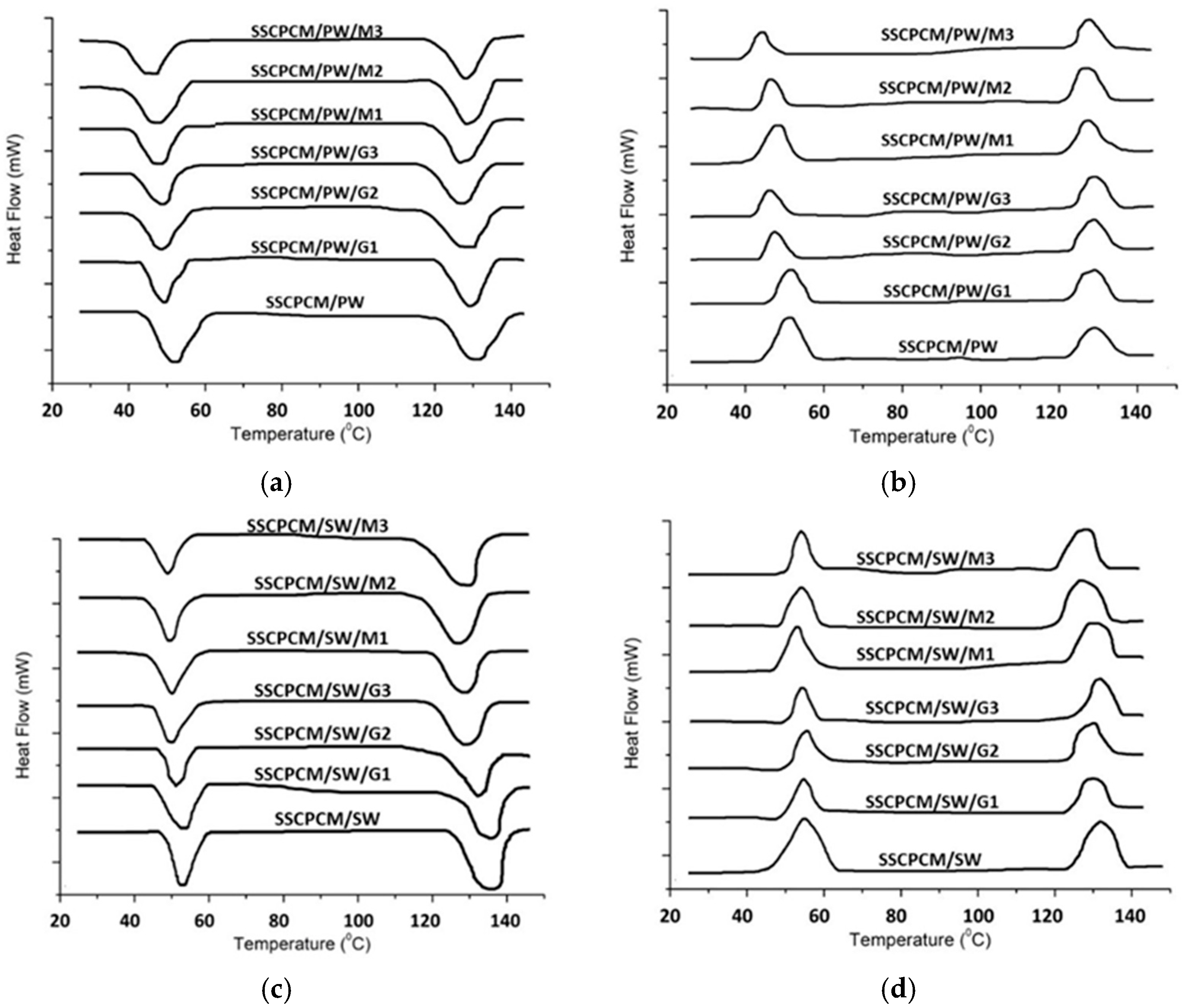
3.4. Thermal Energy Storage
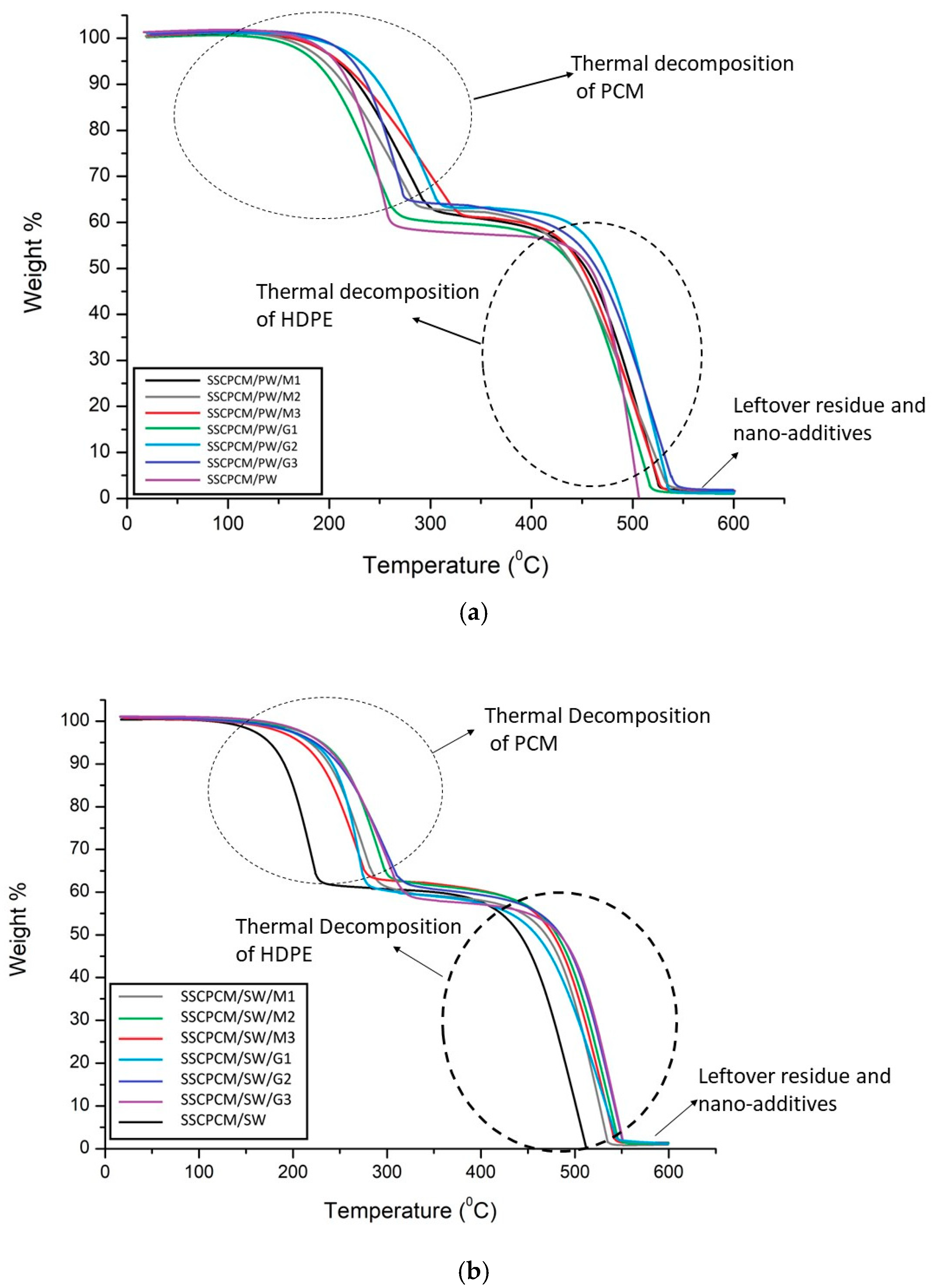
3.5. Thermal Stability
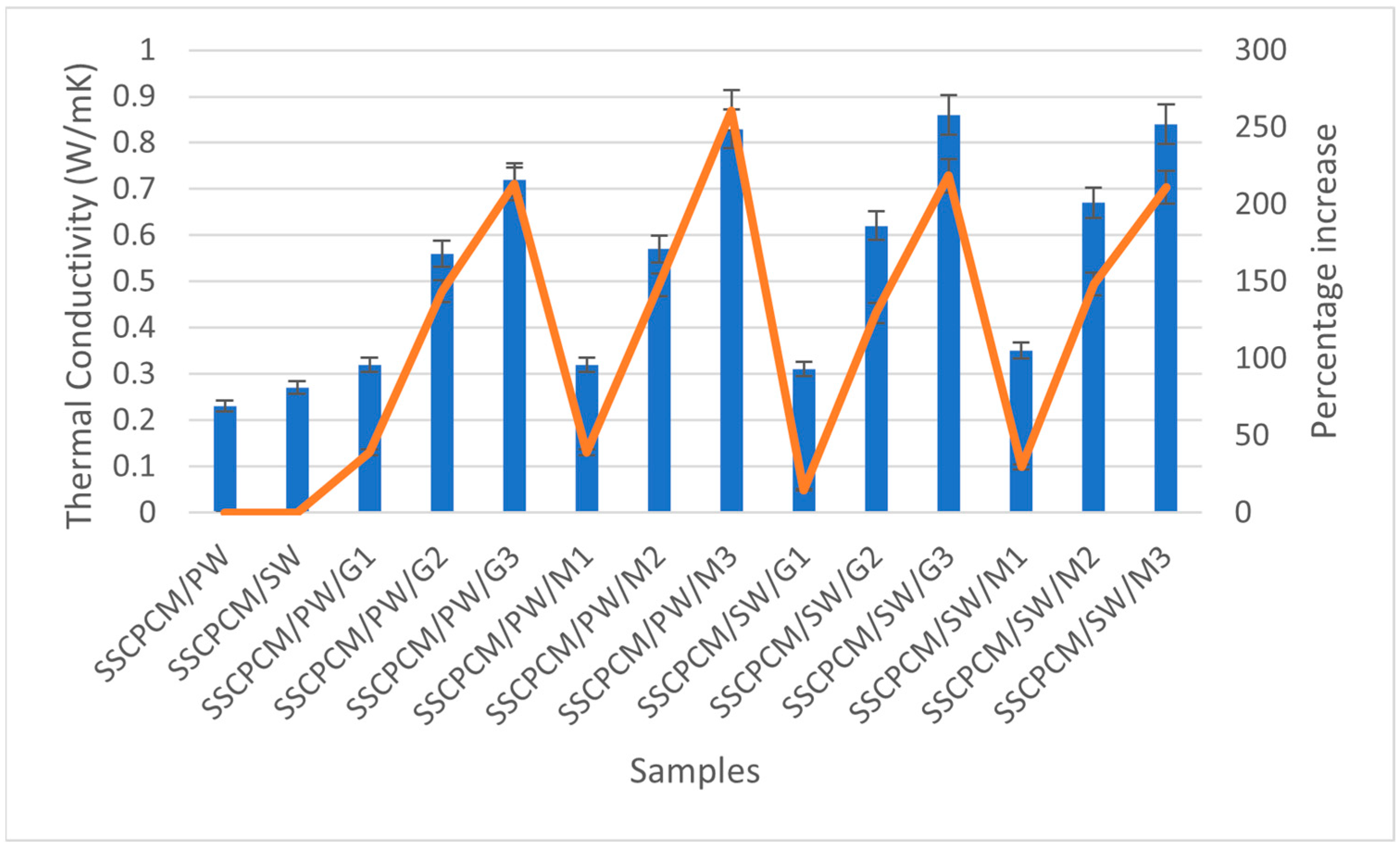
3.6. Thermal Conductivity
4. Recommendation for Future Work
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| TES | Thermal Energy Storage |
| PCM | Phase Change Material |
| SSPCM | Shape Stabilized Phase Change Material |
| SSCPCM | Shape Stabilized Composite Phase Change Material |
| HDPE | High-Density Polyethylene |
| MWCNT | multi-walled carbon nanotube |
| GO | Graphene oxide |
| PW | Paraffin wax |
| SW | Soya wax |
| DSC | Differential scanning calorimetry |
| TGA | Thermogravimetric analysis |
References
- Zhu, C.; Hao, Y.; Wu, H.; Chen, M.; Quan, B.; Liu, S.; Hu, X.; Liu, S.; Ji, Q.; Lu, X.; et al. Self-Assembly of Binderless MXene Aerogel for Multiple-Scenario and Responsive Phase Change Composites with Ultrahigh Thermal Energy Storage Density and Exceptional Electromagnetic Interference Shielding. Nanomicro Lett. 2024, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Alkhedher, M.; Ramadan, M.; Ghazal, M. Hybrid PCM-based thermal management for lithium-ion batteries: Trends and challenges. J. Energy Storage 2023, 73, 108775. [Google Scholar] [CrossRef]
- Al-Yasiri, Q.; Szabó, M. Energetic and thermal comfort assessment of phase change material passively incorporated building envelope in severe hot Climate: An experimental study. Appl. Energy 2022, 314, 118957. [Google Scholar] [CrossRef]
- Patel, B.; Rathore, P.K.S.; Gupta, N.K.; Sikarwar, B.S.; Sharma, R.K.; Kumar, R.; Pandey, A.K. Location optimization of phase change material for thermal energy storage in concrete block for development of energy efficient buildings. Renew Energy 2023, 218, 119306. [Google Scholar] [CrossRef]
- Balasundaram, P.; Baranidharan, B.; Sivaram, N.M. A VIKOR based selection of phase change material for thermal energy storage in solar dryer system. Mater. Today Proc. 2023, 90, 245–249. [Google Scholar] [CrossRef]
- Yang, X.; Sun, L.; Yuan, Y.; Zhao, X.; Cao, X. Experimental investigation on performance comparison of PV/T-PCM system and PV/T system. Renew Energy 2018, 119, 152–159. [Google Scholar] [CrossRef]
- Iqbal, K.; Khan, A.; Sun, D.; Ashraf, M.; Rehman, A.; Safdar, F.; Basit, A.; Maqsood, H.S. Phase change materials, their synthesis and application in textiles—A review. J. Text. Inst. 2019, 110, 625–638. [Google Scholar] [CrossRef]
- Calati, M.; Hooman, K.; Mancin, S. Thermal storage based on phase change materials (PCMs) for refrigerated transport and distribution applications along the cold chain: A review. Int. J. Thermofluids 2022, 16, 100224. [Google Scholar] [CrossRef]
- Mert, M.S.; Mert, H.H.; Arıcı, M. Development and properties of n-octadecane/kaolinite composites as form-stabilized phase change materials for energy storage. J. Clean. Prod. 2023, 410, 137304. [Google Scholar] [CrossRef]
- Gupta, K.K.; Rathore, P.K.S.; Sikarwar, B.S.; Pandey, A.K. Solar to thermal energy storage performance of composite phase change material supported by copper foam loaded with graphite and boron nitride. Sol. Energy 2024, 272, 112459. [Google Scholar] [CrossRef]
- Cheng, P.; Chen, X.; Gao, H.; Zhang, X.; Tang, Z.; Li, A.; Wang, G. Different dimensional nanoadditives for thermal conductivity enhancement of phase change materials: Fundamentals and applications. Nano Energy 2021, 85, 105948. [Google Scholar] [CrossRef]
- Rathore, P.K.S.; Gupta, K.K.; Patel, B.; Sharma, R.K.; Gupta, N.K. Beeswax as a potential replacement of paraffin wax as shape stabilized solar thermal energy storage material: An experimental study. J. Energy Storage 2023, 68, 107714. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Xi, S.; Xie, H.; Yu, W. 3D porous copper foam-based shape-stabilized composite phase change materials for high photothermal conversion, thermal conductivity and storage. Renew Energy 2021, 175, 307–317. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, P.; Li, M. Preparation and thermal characterization of paraffin/metal foam composite phase change material. Appl. Energy 2013, 112, 1357–1366. [Google Scholar] [CrossRef]
- Yao, C.; Kong, X.; Li, Y.; Du, Y.; Qi, C. Numerical and experimental research of cold storage for a novel expanded perlite-based shape-stabilized phase change material wallboard used in building. Energy Convers Manag. 2018, 155, 20–31. [Google Scholar] [CrossRef]
- Huo, Y.; Yan, T.; Chang, X.; Pan, W. Expanded graphite@octadecanol composite phase change material with photothermal conversion interface. Solar Energy 2023, 263, 111922. [Google Scholar] [CrossRef]
- Zauner, C.; Hengstberger, F.; Etzel, M.; Lager, D.; Hofmann, R.; Walter, H. Experimental characterization and simulation of a fin-tube latent heat storage using high density polyethylene as PCM. Appl. Energy 2016, 179, 237–246. [Google Scholar] [CrossRef]
- Tang, Y.; Su, D.; Huang, X.; Alva, G.; Liu, L.; Fang, G. Synthesis and thermal properties of the MA/HDPE composites with nano-additives as form-stable PCM with improved thermal conductivity. Appl. Energy 2016, 180, 116–129. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, S.; Tian, Y.; Zhou, D. Comprehensive evaluation of Paraffin-HDPE shape stabilized PCM with hybrid carbon nano-additives. Appl. Therm. Eng. 2019, 163, 114404. [Google Scholar] [CrossRef]
- Cheng, W.; Zhang, R.; Xie, K.; Liu, N.; Wang, J. Heat conduction enhanced shape-stabilized paraffin/HDPE composite PCMs by graphite addition: Preparation and thermal properties. Sol. Energy Mater. Sol. Cells 2010, 94, 1636–1642. [Google Scholar] [CrossRef]
- Wang, H.; Rao, Z.; Li, L.; Liao, S. A novel composite phase change material of high-density polyethylene/d-mannitol/expanded graphite for medium-temperature thermal energy storage: Characterization and thermal properties. J. Energy Storage 2023, 60, 106603. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Xu, C.; Cong, R.; Fang, G. Thermal properties of 1-hexadecanol/high density polyethylene/graphene nanoplates composites as form-stable heat storage materials. Sol. Energy Mater. Sol. Cells 2022, 237, 111580. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, Y.; Liu, Y.; Wang, S.; Guo, X.; Wang, H.; Cao, D. Paraffin/polyethylene/graphite composite phase change materials with enhanced thermal conductivity and leakage-proof. Adv. Compos. Hybrid Mater. 2021, 4, 543–551. [Google Scholar] [CrossRef]
- Wu, S.; Yan, T.; Kuai, Z.; Pan, W. Thermal conductivity enhancement on phase change materials for thermal energy storage: A review. Energy Storage Mater. 2020, 25, 251–295. [Google Scholar] [CrossRef]

| Material | Density (g/cm3) | Thermal Conductivity (W/m-K) | Specific Heat (J/g-K) | Latent Heat (J/g) | Melting Point (°C) |
|---|---|---|---|---|---|
| HDPE | 0.965 | 0.288–0.480 | 1.8–2.7 | 174 | 118–137 |
| Paraffin wax | 0.820 | 0.210 | 2.1 | 187 | 51–53 |
| Soya wax | 0.90 | 0.205 | 2.7 | 201 | 52–55 |
| Sample | HDPE | Paraffin Wax (PW) | Soya Wax (SW) |
|---|---|---|---|
| HDPE/PW (50/50) | 50 | 50 | 0 |
| HDPE/PW (55/45) | 55 | 45 | 0 |
| HDPE/PW (60/40) | 60 | 40 | 0 |
| HDPE/PW (65/35) | 65 | 35 | 0 |
| HDPE/PW (70/30) | 70 | 30 | 0 |
| HDPE/PW (75/25) | 75 | 25 | 0 |
| HDPE/PW (80/20) | 80 | 20 | 0 |
| HDPE/SW (50/50) | 50 | 0 | 50 |
| HDPE/SW (55/45) | 55 | 0 | 45 |
| HDPE/SW (60/40) | 60 | 0 | 40 |
| HDPE/SW (65/35) | 65 | 0 | 35 |
| HDPE/SW (70/30) | 70 | 0 | 30 |
| HDPE/SW (75/25) | 75 | 0 | 25 |
| HDPE/SW (80/20) | 80 | 0 | 20 |
| Sample | HDPE | PW | SW | MWCNT | GNP |
|---|---|---|---|---|---|
| SSCPCM/PW | 60 | 40 | - | - | - |
| SSCPCM/SW | 65 | - | 35 | - | - |
| SSCPCM/PW/M1 | 60 | 39 | - | 1 | - |
| SSCPCM/PW/M2 | 60 | 38 | - | 2 | - |
| SSCPCM/PW/M3 | 60 | 37 | - | 3 | - |
| SSCPCM/PW/G1 | 60 | 39 | - | - | 1 |
| SSCPCM/PW/G2 | 60 | 38 | - | - | 2 |
| SSCPCM/PW/G3 | 60 | 37 | - | - | 3 |
| SSCPCM/SW/M1 | 65 | - | 39 | 1 | - |
| SSCPCM/SW/M2 | 65 | - | 38 | 2 | - |
| SSCPCM/SW/M3 | 65 | - | 37 | 3 | - |
| SSCPCM/SW/G1 | 65 | - | 39 | - | 1 |
| SSCPCM/SW/G2 | 65 | - | 38 | - | 2 |
| SSCPCM/SW/G3 | 65 | - | 37 | - | 3 |
| Samples | Melting Point | Freezing Point | Melting Enthalpy | Freezing Enthalpy |
|---|---|---|---|---|
| SSCPCM/PW | 44.4 | 57.6 | 93.5 | 90.7 |
| SSCPCM/SW | 47.1 | 62.2 | 86.8 | 82.6 |
| SSCPCM/PW/G1 | 43.1 | 54.3 | 90.3 | 84.3 |
| SSCPCM/PW/G2 | 42.8 | 54.6 | 84.4 | 80.8 |
| SSCPCM/PW/G3 | 42.3 | 52.2 | 82.1 | 76.8 |
| SSCPCM/PW/M1 | 44.2 | 52.7 | 88.5 | 74.3 |
| SSCPCM/PW/M2 | 43.7 | 53.4 | 85.6 | 72.1 |
| SSCPCM/PW/M3 | 42.7 | 54.6 | 80.4 | 74.7 |
| SSCPCM/SW/G1 | 46.7 | 58.7 | 81.2 | 76.3 |
| SSCPCM/SW/G2 | 45.2 | 59.6 | 78.9 | 73.6 |
| SSCPCM/SW/G3 | 44.8 | 57.4 | 75.3 | 70.2 |
| SSCPCM/SW/M1 | 46.2 | 58.3 | 82.8 | 78.6 |
| SSCPCM/SW/M2 | 45.8 | 57.7 | 76.8 | 74.3 |
| SSCPCM/SW/M3 | 44.6 | 57.2 | 74.2 | 70.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, D.K.; Rathore, P.K.S.; Singh, R.K.; Gupta, A.K.; Sikarwar, B.S. Experimental Study on Paraffin Wax and Soya Wax Supported by High-Density Polyethylene and Loaded with Nano-Additives for Thermal Energy Storage. Energies 2024, 17, 2461. https://doi.org/10.3390/en17112461
Yadav DK, Rathore PKS, Singh RK, Gupta AK, Sikarwar BS. Experimental Study on Paraffin Wax and Soya Wax Supported by High-Density Polyethylene and Loaded with Nano-Additives for Thermal Energy Storage. Energies. 2024; 17(11):2461. https://doi.org/10.3390/en17112461
Chicago/Turabian StyleYadav, Deepak Kumar, Pushpendra Kumar Singh Rathore, Rajeev Kumar Singh, Arvind Kumar Gupta, and Basant Singh Sikarwar. 2024. "Experimental Study on Paraffin Wax and Soya Wax Supported by High-Density Polyethylene and Loaded with Nano-Additives for Thermal Energy Storage" Energies 17, no. 11: 2461. https://doi.org/10.3390/en17112461
APA StyleYadav, D. K., Rathore, P. K. S., Singh, R. K., Gupta, A. K., & Sikarwar, B. S. (2024). Experimental Study on Paraffin Wax and Soya Wax Supported by High-Density Polyethylene and Loaded with Nano-Additives for Thermal Energy Storage. Energies, 17(11), 2461. https://doi.org/10.3390/en17112461






