Reuse of Lithium Iron Phosphate (LiFePO4) Batteries from a Life Cycle Assessment Perspective: The Second-Life Case Study
Abstract
:1. Introduction
1.1. Background
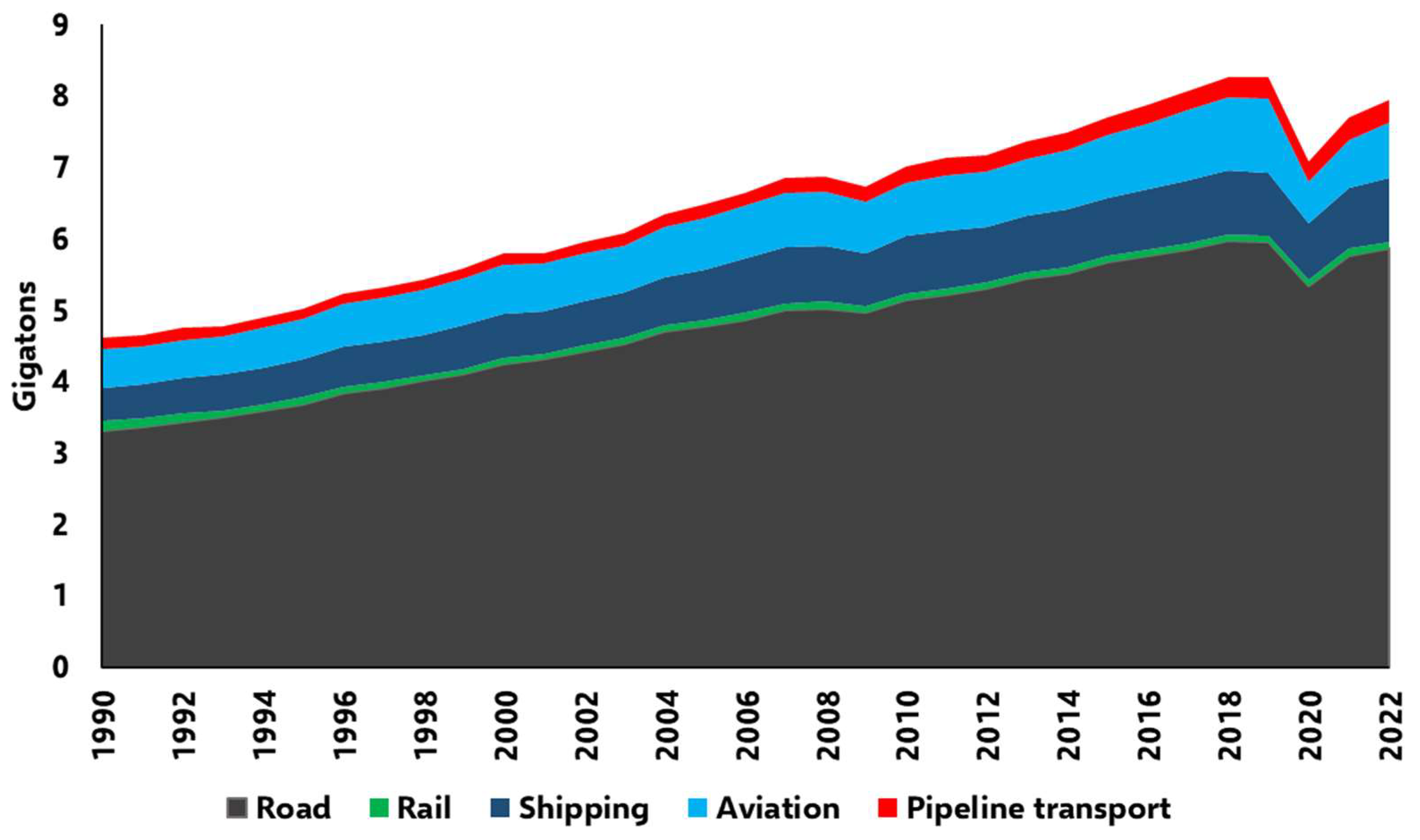
1.2. Aim of the Study
2. Materials and Methods
2.1. Case Study Description
- It could be a useful and representative example of sustainable power generation and circular economy. In fact, since through this project, it was possible to reuse materials at the end of their life, create value in a sustainable way as well as increase the reliability of the entire power grid, it could not represent just a pilot project for the future, but also a real expression of circular economy in all its forms, and therefore worthy of attention.
- The availability of information, accessibility of data, and cooperation of the company allowed the study to be conducted in an acceptable time frame.
2.2. Life Cycle Assessment
2.2.1. Goal and Scope Definition
- In the context of second life, the production phase is excluded, specifically on the assumption that the batteries are already on the market and have a different initial use than that which will be carried out within the stationary plant.
- The transport phase will also be different, as the batteries will no longer be acquired from the initial manufacturer (China) but purchased on the European market.
2.2.2. Life Cycle Inventory
- (1)
- Production
- ∅i Is the distance in km as the crow flies of commodity i from place x to place y.
- δ Is the weight in kg of the material being transported.
- (2)
- Shipping
- (3)
- Installation
- (4)
- Use
2.2.3. Life Cycle Impact Assessment
- Atmospheric Effects: Global Warming Potential (GWP), Stratospheric Ozone Depletion (SOD), Ionizing radiation (IR), Ozone Formation-Human Health (OFHH), Fine Particulate Matter Formation (FPMP), Ozone formation-Terrestrial ecosystems (OFTE), and Terrestrial acidification Potential (TAP).
- Eutrophication: Freshwater Eutrophication Potential (FEP) and Marine Eutrophication Potential (MEP).
- Toxicity: Terrestrial Ecotoxicity (TEC), Freshwater Ecotoxicity (FEC), Marine Ecotoxicity (MEC), Human Carcinogenic Toxicity (HCT) and Human Non-Carcinogenic Toxicity (HNCT).
- Abiotic Resources: Land Use (LU), Mineral Resources Scarcity (MRS), Fossil Resources Scarcity (FRS) and Water Consumption (WC).
2.3. Scenario Analysis
3. Results and Discussion
3.1. Life Cycle Assessment
3.2. Scenario Analysis

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Environmental Agency (IEA). Transport, 2023, Paris. License: CC BY 4.0. Available online: https://www.iea.org/energy-system/transport. (accessed on 6 September 2023).
- International Environmental Agency (IEA). Greenhouse Gas Emissions from Energy. 2023. Available online: https://www.iea.org/data-and-statistics/data-product/greenhouse-gas-emissions-from-energy (accessed on 6 September 2023).
- Zhang, Y.; Ayyub, B.M. Temperature Extremes in a Changing Climate. Clim. Change Extrem. Events 2021, 9–23. [Google Scholar] [CrossRef]
- European Parliament. Regulation of the European Parliament and the Council Amending Regulation (EU) 2019/631 as Regards Strengthening the CO2 Emission Performance Standards for New Passenger Cars and New Light Commercial Vehicles in Line with the Union’s Increased Climate Ambition. 2023. Available online: https://data.consilium.europa.eu/doc/document/PE-66-2022-INIT/en/pdf (accessed on 14 December 2023).
- Vega, L.P.; Bautista, K.T.; Campos, H.; Daza, S.; Vargas, G. Biofuel Production in Latin America: A Review for Argentina, Brazil, Mexico, Chile, Costa Rica, and Colombia. Energy Rep. 2024, 11, 28–38. [Google Scholar] [CrossRef]
- Ravi, S.S.; Mazumder, J.; Sun, J.; Brace, C.; Turner, J.W. Techno-economic Assessment of Synthetic E-Fuels Derived from Atmospheric CO2 and Green Hydrogen. Energy Convers. Manag. 2023, 291, 117271. [Google Scholar] [CrossRef]
- Kittner, N.; Tsiropoulos, I.; Tarvydas, D.; Schmidt, O.; Staffell, I.; Kammen, D.M. Electric vehicles. Technological Learning in the Transition to a Low-Carbon Energy System: Conceptual Issues, Empirical Findings, and Use. In Energy Modeling; Academic Press: Cambridge, MA, USA, 2020; pp. 145–163. [Google Scholar] [CrossRef]
- Prussi, M.; Laveneziana, L.; Testa, L.; Chiaramonti, D. Comparing e-Fuels and Electrification for Decarbonization of Heavy-Duty Transports. Energies 2022, 15, 8075. [Google Scholar] [CrossRef]
- Overland, I. The Geopolitics of Renewable Energy: Debunking Four Emerging Myths. Energy Res. Soc. Sci. 2019, 49, 36–40. [Google Scholar] [CrossRef]
- Karali, N.; Shah, N. Bolstering Supplies of Critical Raw Materials for Low-Carbon Technologies through Circular Economy Strategies. Energy Res. Soc. Sci. 2022, 88, 102534. [Google Scholar] [CrossRef]
- Czerwinski, F. Critical Minerals for Zero-Emission Transportation. Materials 2022, 15, 5539. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Muralidharan, N.; Sun, Y.K.; Passerini, S.; Whittingham, M.S.; Belharouak, I. Energy and Environmental Aspects in Recycling Lithium-Ion Batteries: Concept of Battery Identity Global Passport. Mater. Today 2020, 41, 304–315. [Google Scholar] [CrossRef]
- Baum, Z.J.; Bird, R.E.; Yu, X.; Ma, J. Lithium-Ion Battery Recycling—Overview of Techniques and Trends. ACS Energy Lett. 2022, 7, 712–719. [Google Scholar] [CrossRef]
- Bae, H.; Kim, Y. Technologies of lithium recycling from waste lithium-ion batteries: A review. Mater. Adv. 2021, 2, 3234–3250. [Google Scholar] [CrossRef]
- Engel, J. Development Perspectives of Lithium-Ion Recycling Processes for Electric Vehicle Batteries. Master’s Thesis, University of Rhode Island, Kingston, RI, USA, 2016. Paper 905. Available online: https://digitalcommons.uri.edu/theses/905 (accessed on 31 March 2024).
- Regulation (EU) 2023/1542 of the European Parliament and of the Council of 12 July 2023 Concerning Batteries and Waste Batteries, Amending Directive 2008/98/EC and Regulation (EU) 2019/1020 and Repealing Directive 2006/66/EC (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/eli/reg/2023/1542/oj (accessed on 12 January 2024).
- Ruggieri, R.; Ruggeri, M.; Vinci, G.; Poponi, S. Electric Mobility in a Smart City: European Overview. Energies 2021, 14, 315. [Google Scholar] [CrossRef]
- Mowri, S.T.; Barai, A.; Moharana, S.; Gupta, A.; Marco, J. Assessing the Impact of First-Life Lithium-Ion Battery Degradation on Second-Life Performance. Energies 2024, 17, 501. [Google Scholar] [CrossRef]
- Kebede, A.A.; Kalogiannis, T.; van Mierlo, J.; Berecibar, M. A comprehensive review of stationary energy storage devices for large-scale renewable energy sources grid integration. Renew. Sustain. Energy Rev. 2022, 159, 112213. [Google Scholar] [CrossRef]
- ISO 14040; Environmental Management—Life Cycle Assessment—Principle and Framework. International Organisation for Standardisation (ISO): Geneva, Switzerland, 2006. Available online: https://www.iso.org/standard/37456.html (accessed on 31 March 2024).
- ISO 14044; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. International Organisation for Standardisation (ISO): Geneva, Switzerland, 2006. Available online: https://www.iso.org/standard/38498.html (accessed on 31 March 2024).
- Hu, X.; Xu, L.; Lin, X.; Pecht, M. Battery Lifetime Prognostics. Joule 2020, 4, 310–346. [Google Scholar] [CrossRef]
- Philippot, M.; Costa, D.; Hosen, M.S.; Senécat, A.; Brouwers, E.; Nanini-Maury, E.; van Mierlo, J.; Messagie, M. Environmental Impact of the Second Life of an Automotive Battery: Reuse and Repurpose Based on Ageing Tests. J. Clean. Prod. 2022, 366, 132872. [Google Scholar] [CrossRef]
- Kotak, Y.; Fernández, C.M.; Casals, L.C.; Kotak, B.S.; Koch, D.; Geisbauer, C.; Trilla, L.; Gómez-Núñez, A.; Schweiger, H.-G. End of Electric Vehicle Batteries: Reuse vs. Recycle. Energies 2021, 14, 2217. [Google Scholar] [CrossRef]
- Porzio, J.; Scown, C.D. Life-Cycle Assessment Considerations for Batteries and Battery Materials. Adv. Energy Mater. 2021, 11, 2100771. [Google Scholar] [CrossRef]
- Siret, C.; Tytgat, J.; Ebert, T.; Mistry, M.; Thirlaway, C.; Schutz, B.; Xhantopoulos, D.; Wiaux, J.-P.; Chanson, C.; Tomboy, W.; et al. PEFCR—Product Environmental Footprint Category Rules for High Specific Energy Rechargeable Batteries for Mobile Applications; Version: H.; Time of Validity: 31 December 2020. Available online: https://ec.europa.eu/environment/eussd/smgp/pdf/PEFCR_Batteries.pdf (accessed on 27 March 2021).
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The Ecoinvent Database Version 3 (Part I): Overview and Methodology. Int. J. Life Cycle Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- Pre-Consulting. LCA Software for Informed Changemakers. 2024. Available online: https://simapro.com/ (accessed on 21 February 2024).
- SNE Research. LIB Manufacturing Equipment Development Status and Mid/Long-Term Outlook (~2030). 2023. Available online: https://www.sneresearch.com/en/business/report/ (accessed on 25 September 2023).
- European Commission. Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs. In Study on the Critical Raw Materials for the EU 2023—Final Report; Grohol, M., Veeh, C., Eds.; Publications Office of the European Union: Luxembourg, 2023; Available online: https://data.europa.eu/doi/10.2873/725585 (accessed on 31 March 2024).
- Garcia, L.V.; Ho, Y.-C.; Myo Thant, M.M.; Han, D.S.; Lim, J.W. Lithium in a Sustainable Circular Economy: A Comprehensive Review. Processes 2023, 11, 418. [Google Scholar] [CrossRef]
- Hocking, M. Deutsche Bank. Market Research. Lithium 101: Welcome to the Lithium Age. 2016. Available online: http://www.belmontresources.com (accessed on 2 October 2023).
- USGS. Mineral Commodity Summary. Antimony [Online]. 2018. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/antimony/ (accessed on 21 February 2024).
- CRU. Lithium Prices Crash through $10,000 as Hype Meets Reality, CRU Insight. 2019. Available online: https://www.crugroup.com/knowledge-andinsights/insights/2019/lithium-prices-crash-through-10-000-as-hype-meets-reality (accessed on 2 October 2023).
- Zhangjiagang Guotai Huarong New Chemical Materials Co., Ltd. (ZGHNCM). About. 2023. Available online: http://www.gthr.com.cn/about-e.html (accessed on 3 October 2023).
- Contemporary Amperex Technology Co., Limited (CATL). Supplier Portal. 2023. Available online: https://nsrm.catl.com/#/supplier-portal/home-page?lang=en (accessed on 2 October 2023).
- Aluminium Stewardship Initiative. 2023. Available online: https://aluminium-stewardship.org/about-asi/members/Jiangsu-Dingsheng-New-Materials-Joint-Stock-Co---Ltd (accessed on 21 February 2024).
- Statista. Major China Seaborne Bauxite Receiving Ports. 2023. Available online: https://www.statista.com/statistics/1325319/major-china-seaborne-bauxite-receiving-ports/ (accessed on 21 February 2024).
- IHS. Chemical Economics Handbook: Phosphorus and Phosphorus Chemicals; S&P Global: New York, NY, USA, 2017. [Google Scholar]
- Asian Metal. 2023. Available online: https://www.asianmetal.com/news/1774466/Jiayuan-Technology-and-CATL-to-build-100,000tpy-electrolytic-copper-foil-project (accessed on 2 October 2023).
- Markets and Markets. Dimethyl Carbonate Market. 2022. Available online: https://www.marketsandmarkets.com/ResearchInsight/dimethyl-carbonate-market.asp (accessed on 2 October 2023).
- Shao, Z.; He, H.; Mao, S.; Liang, S.; Liu, S.; Tan, X.; Gao, M.; Xin, Y. Toward Greener and More Sustainable Freight Systems. Comparing Freight Strategies in the United States and China. International Council on Clean Transportation (ICCT). 2022. Available online: https://theicct.org/wp-content/uploads/2022/01/China-US-freight_final.pdf (accessed on 4 October 2023).
- Cui, S.; Pittman, R.; Zhao, J. Restructuring the Chinese Freight Railway: Two Scenarios. Asia Glob. Econ. 2020, 1, 100002. [Google Scholar] [CrossRef]
- Vinci, G.; Maddaloni, L.; Ruggeri, M.; Vieri, S. Environmental life cycle assessment of rice production in northern Italy: A case study from Vercelli. Int. J. Life Cycle Assess. 2022, 1–18. [Google Scholar] [CrossRef]
- Xu, Q.; Dai, L.; Gao, P.; Dou, Z. The Environmental, Nutritional, and Economic Benefits of Rice-Aquaculture Animal Coculture in China. Energy 2022, 249, 123723. [Google Scholar] [CrossRef]
- de Schryver, A.M.; van Zelm, R.; Humbert, S.; Pfister, S.; McKone, T.E.; Huijbregts, M.A.J. Value choices in life cycle impact the assessment of stressors causing human health damage. J. Ind. Ecol. 2011, 15, 796–815. [Google Scholar] [CrossRef]
- Wolf, M.A.; Pant, R.; Chomkhamsri, K.; Sala, S.; Pennington, D. International Reference Life Cycle Data System (ILCD) Handbook—Towards More Sustainable Production and Consumption for a Resource-Efficient Europe. JRC Reference Report, EUR 24982 EN. European Commission—Joint Research Centre; Publications Office of the European Union: Luxembourg, 2012; Available online: https://eplca.jrc.ec.europa.eu/uploads/JRC-Reference-Report-ILCD-Handbook-Towards-more-sustainable-production-and-consumption-for-a-resource-efficient-Europe.pdf (accessed on 21 February 2024).
- Acero, A.P.; Rodríguez, C.; Ciroth, A. LCIA Methods: Impact Assessment Methods in Life Cycle Assessment and Their Impact Categories; GreenDelta: Berlin, Germany, 2014. [Google Scholar]
- Bare, J.C. Tool for the Reduction and Assessment of Chemical and Other Environmental Impacts (TRACI), Version 2.1—User’s Manual; EPA/600/R-12/554; US Environmental Protection Agency: Washington, DC, USA, 2012. [Google Scholar]
- Milian, Y.E.; Jamett, N.; Cruz, C.; Herrera-León, S.; Chacana-Olivares, J. A Comprehensive Review of Emerging Technologies for Recycling Spent Lithium-Ion Batteries. Sci. Total Environ. 2024, 910, 168543. [Google Scholar] [CrossRef] [PubMed]
- Dobó, Z.; Dinh, T.; Kulcsár, T. A review on recycling of spent lithium-ion batteries. Energy Rep. 2023, 9, 6362–6395. [Google Scholar] [CrossRef]
- Akhmetov, N.; Manakhov, A.; Al-Qasim, A.S. Li-Ion Battery Cathode Recycling: An Emerging Response to Growing Metal Demand and Accumulating Battery Waste. Electronics 2023, 12, 1152. [Google Scholar] [CrossRef]
- Toro, L.; Moscardini, E.; Baldassari, L.; Forte, F.; Falcone, I.; Coletta, J.; Toro, L. A Systematic Review of Battery Recycling Technologies: Advances, Challenges, and Future Prospects. Energies 2023, 16, 6571. [Google Scholar] [CrossRef]
- Kallitsis, E.; Korre, A.; Kelsall, G.H. Life cycle assessment of recycling options for automotive Li-ion battery packs. J. Clean. Prod. 2022, 371, 133636. [Google Scholar] [CrossRef]
- Chalaris, M.; Gkika, D.A.; Tolkou, A.K.; Kyzas, G.S. Advancements and sustainable strategies for the treatment and management of wastewaters from metallurgical industries: An overview. Env. Sci. Pollut. Res. 2023, 30, 119627–119653. [Google Scholar] [CrossRef]
- Conejo, A.N.; Birat, J.P.; Dutta, A. A review of the current environmental challenges of the steel industry and its value chain. J. Environ. Manag. 2020, 259, 109782. [Google Scholar] [CrossRef]
- Sonter, L.J.; Barrett, D.J.; Soares-Filho, B.S.; Moran, C.J. Global Demand for Steel Drives Extensive Land-Use Change in Brazil’s Iron Quadrangle. Glob. Environ. Change 2014, 26, 63–72. [Google Scholar] [CrossRef]
- Georgitzikis, K.; Mancini, L.; d’Elia, E.; Vidal-Legaz, B. Sustainability Aspects of Bauxite and Aluminium—Climate Change, Environmental, Socio-Economic, and Circular Economy Considerations, EUR 30760 EN; Publications Office of the European Union: Luxembourg, 2021; ISBN 978-92-76-40039-4. [Google Scholar] [CrossRef]
- Ahmadi, L.; Young, S.B.; Fowler, M.; Fraser, R.A.; Achachlouei, M.A. A cascaded life cycle: Reuse of electric vehicle lithium-ion battery packs in energy storage systems. Int. J. Life Cycle Assess. 2017, 22, 111–124. [Google Scholar] [CrossRef]
- Ahmadi, L.; Yip, A.; Fowler, M.; Young, S.B.; Fraser, R.A. Environmental feasibility of re-use of electric vehicle batteries. Sustain Energy Technol. Assess. 2014, 6, 64–74. [Google Scholar] [CrossRef]
- Wilson, N.; Meiklejohn, E.; Overton, B.; Robinson, F.; Farjana, S.H.; Li, W.; Staines, J. A physical allocation method for comparative life cycle assessment: A case study of repurposing Australian electric vehicle batteries. Resour. Conserv. Recycl. 2021, 174, 105759. [Google Scholar] [CrossRef]
- Marchese, D.; Giosuè, C.; Staffolani, A.; Conti, M.; Orcioni, S.; Soavi, F.; Cavalletti, M.; Stipa, P. An Overview of the Sustainable Recycling Processes Used for Lithium-Ion Batteries. Batteries 2024, 10, 27. [Google Scholar] [CrossRef]
- Wrålsen, B.; O’Born, R. Use of Life Cycle Assessment to Evaluate Circular Economy Business Models in the Case of Li-ion Battery Remanufacturing. Int. J. Life Cycle Assess. 2023, 28, 554–565. [Google Scholar] [CrossRef]
- Ma, J.; Wang, J.; Jia, K.; Liang, Z.; Ji, G.; Zhuang, Z.; Zhou, G.; Cheng, H.M. Adaptable Eutectic Salt for the Direct Recycling of Highly Degraded Layer Cathodes. J. Am. Chem. Soc. 2022, 144, 20306–20314. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Abdullah, M.F.; Dawood, M.K.; Wei, B.; Subramanian, Y.; Azad, A.T.; Nourin, S.; Afroze, S.; Taweekun, J.; Azad, A.K. Innovative Lithium-Ion Battery Recycling: Sustainable Process for Recovery of Critical Materials from Lithium-Ion Batteries. J. Energy Storage 2023, 67, 107551. [Google Scholar] [CrossRef]
- Svetkina, O.Y.; Koveria, A.S.; Ovcharenko, A.O.; Tarasova, H.v.; Panteleieva, O.S. Development of a Scheme for the Utilisation of Spent Lithium-Ion Batteries by Bioleaching. J. Chem. Technol. 2023, 31, 590–600. [Google Scholar] [CrossRef]
- Anwani, S.; Methekar, R.; Ramadesigan, V. Resynthesizing of Lithium Cobalt Oxide from Spent Lithium-Ion Batteries Using an Environmentally Benign and Economically Viable Recycling Process. Hydrometallurgy 2020, 197, 105430. [Google Scholar] [CrossRef]
- Ma, G.; Cheng, M. Hydrothermal Method Preparing Lithium-Ion Battery Cathode Material Li4Ti5O12 Using Metatitanic Acid. Ferroelectrics 2018, 536, 181–186. [Google Scholar] [CrossRef]
| 1st Life | 2nd Life | |
|---|---|---|
| (1) GOAL AND SCOPE DEFINITION | ||
| Functional Unit | 1 kWh | 1 kWh |
| System boundaries | From cradle to grave | From gate to grave |
| Life Cycle Phases | Transportation of raw materials, manufacturing, shipping, use | Shipping, use |
| (2) LIFE CYCLE INVENTORY (LCI) | ||
| Data quality | Primary data were obtained through interviews with the company managers, secondary data from gray literature, and scientific literature | |
| Database | Ecoinvent v3.8 | |
| (3) LIFE CYCLE IMPACT ASSESSMENT (LCIA) | ||
| Calculation method | Recipe 2016 MidPoint (H) | |
| Impact categories |
| |
| Software | Simapro 9.5. | |
| Characteristics | Amount | Unit |
|---|---|---|
| Lifetime | 15 | years |
| Cycle per lifetime | 5475 | n cycles |
| BEES installed power | 20 | MW |
| BEES installed energy capacity | 40 | MWh |
| BEES round-trip efficiency | 85 | % |
| BESS one way efficiency | 92 | % |
| Depth of discharge | 80 | % |
| Delivered energy during the lifetime | 161,526 | MWh |
| Electricity losses (discharge) | 13,674 | |
| Electricity losses (charge) | 14,831 | |
| Electricity losses (total) | 28,505 |
| Component | Anode | Battery Container | Cathode | Cell Container | Cooling System | Electrolyte | Module Container | Separator | Transportation | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Categories/Unit | Value | % | Value | % | Value | % | Value | % | Value | % | Value | % | Value | % | Value | % | Value | % | Value | % | |
| Atmospheric | |||||||||||||||||||||
| GWP | kg CO2 eq | 5.65 × 100 | 11% | 1.49 × 100 | 3% | 9.49 × 100 | 18% | 1.27 × 101 | 25% | 2.93 × 100 | 6% | 6.20 × 100 | 12% | 8.94 × 100 | 17% | 4.04 × 10−1 | 1% | 3.60 × 100 | 7% | 5.14 × 101 | 100% |
| SOD | kg CFC11 eq | 2.24 × 10−6 | 18% | 3.06 × 10−7 | 3% | 1.67 × 10−6 | 14% | 2.15 × 10−6 | 18% | 7.24 × 10−7 | 6% | 1.65 × 10−6 | 14% | 2.84 × 10−6 | 23% | 3.92 × 10−8 | 1% | 5.47 × 10−7 | 4% | 1.22 × 10−5 | 100% |
| IR | kBq Co-60 eq | 5.97 × 10−2 | 35% | 2.97 × 10−3 | 2% | 1.91 × 10−2 | 11% | 2.16 × 10−2 | 13% | 7.75 × 10−3 | 5% | 3.04 × 10−2 | 18% | 2.30 × 10−2 | 13% | 5.39 × 10−4 | 1% | 6.03 × 10−3 | 4% | 1.71 × 10−1 | 100% |
| OFHH | kg NOx eq | 1.91 × 10−2 | 12% | 4.17 × 10−3 | 3% | 2.27 × 10−2 | 14% | 3.15 × 10−2 | 19% | 1.17 × 10−2 | 7% | 1.39 × 10−2 | 8% | 3.50 × 10−2 | 21% | 6.18 × 10−4 | 1% | 2.69 × 10−2 | 16% | 1.66 × 10−1 | 100% |
| FPMP | kg PM2.5 eq | 5.33 × 10−3 | 12% | 1.06 × 10−3 | 3% | 5.78 × 10−3 | 14% | 7.37 × 10−3 | 19% | 2.04 × 10−3 | 7% | 2.57 × 10−3 | 8% | 9.70 × 10−3 | 21% | 9.34 × 10−5 | 1% | 9.82 × 10−5 | 16% | 3.40 × 10−2 | 100% |
| OFTE | kg NOx eq | 1.92 × 10−2 | 12% | 4.18 × 10−3 | 3% | 2.27 × 10−2 | 14% | 3.15 × 10−2 | 19% | 1.17 × 10−2 | 7% | 1.40 × 10−2 | 8% | 3.51 × 10−2 | 21% | 6.20 × 10−4 | 1% | 2.70 × 10−2 | 16% | 1.66 × 10−1 | 100% |
| TAP | kg SO2 eq | 3.84 × 10−2 | 15% | 6.52 × 10−3 | 3% | 3.97 × 10−2 | 16% | 5.09 × 10−2 | 20% | 1.52 × 10−2 | 6% | 3.38 × 10−2 | 13% | 5.17 × 10−2 | 20% | 8.44 × 10−4 | 1% | 1.85 × 10−2 | 7% | 2.55 × 10−1 | 100% |
| Eutrophication | |||||||||||||||||||||
| FEP | kg P eq | 5.20 × 10−4 | 19% | 7.36 × 10−5 | 3% | 3.45 × 10−4 | 13% | 4.30 × 10−4 | 16% | 1.36 × 10−4 | 5% | 2.93 × 10−4 | 11% | 8.53 × 10−4 | 31% | 7.86 × 10−6 | 1% | 6.13 × 10−5 | 2% | 2.72 × 10−3 | 100% |
| MEP | kg N eq | 2.16 × 10−4 | 20% | 2.04 × 10−5 | 2% | 7.64 × 10−5 | 7% | 9.15 × 10−5 | 9% | 2.57 × 10−5 | 2% | 4.33 × 10−4 | 40% | 2.08 × 10−4 | 19% | 1.46 × 10−6 | 1% | 1.14 × 10−6 | 1% | 1.07 × 10−3 | 100% |
| Toxicity | |||||||||||||||||||||
| TEC | kg 1,4-DCB | 5.36 × 10−1 | 14% | 7.70 × 10−2 | 2% | 4.57 × 10−1 | 12% | 5.80 × 10−1 | 15% | 1.87 × 10−1 | 5% | 1.19 × 100 | 31% | 6.00 × 10−1 | 16% | 7.14 × 10−3 | 1% | 2.32 × 10−1 | 6% | 3.86 × 100 | 100% |
| FEC | 2.12 × 10−4 | 8% | 9.20 × 10−5 | 3% | 4.79 × 10−4 | 18% | 6.18 × 10−4 | 23% | 1.71 × 10−4 | 6% | 3.03 × 10−4 | 11% | 7.94 × 10−4 | 29% | 4.35 × 10−6 | 1% | 2.37 × 10−5 | 1% | 2.70 × 10−3 | 100% | |
| MEC | 7.17 × 10−4 | 16% | 1.33 × 10−4 | 3% | 6.70 × 10−4 | 15% | 8.55 × 10−4 | 19% | 2.49 × 10−4 | 6% | 2.98 × 10−4 | 7% | 1.37 × 10−3 | 31% | 6.08 × 10−6 | 1% | 9.87 × 10−5 | 2% | 4.40 × 10−3 | 100% | |
| HCT | 2.13 × 10−3 | 16% | 2.47 × 10−4 | 2% | 1.46 × 10−3 | 11% | 2.05 × 10−3 | 16% | 1.05 × 10−3 | 8% | 1.66 × 10−3 | 13% | 1.55 × 10−3 | 12% | 2.16 × 10−5 | 1% | 2.75 × 10−3 | 21% | 1.29 × 10−2 | 100% | |
| HNCT | 5.65 × 10−2 | 2% | 1.22 × 10−2 | 4% | 1.88 × 10−2 | 1% | 2.13 × 10−2 | 1% | 1.28 × 10−2 | 0% | 4.97 × 10−2 | 1% | 3.14 × 100 | 90% | 9.58 × 10−4 | 1% | 5.44 × 10−2 | 2% | 3.48 × 100 | 100% | |
| Abiotic resources | |||||||||||||||||||||
| LU | m2a crop eq | 4.71 × 10−1 | 12% | 1.11 × 10−1 | 3% | 6.77 × 10−1 | 17% | 8.23 × 10−1 | 21% | 2.31 × 10−1 | 6% | 5.92 × 10−1 | 15% | 9.51 × 10−1 | 24% | 8.28 × 10−3 | 1% | 3.00 × 10−2 | 1% | 3.89 × 100 | 100% |
| MRS | kg Cu eq | 5.21 × 10−1 | 34% | 3.41 × 10−2 | 2% | 9.49 × 10−2 | 6% | 9.38 × 10−2 | 6% | 2.49 × 10−2 | 2% | 1.02 × 10−1 | 7% | 6.51 × 10−1 | 43% | 4.34 × 10−4 | 1% | 3.40 × 10−4 | 1% | 1.52 × 100 | 100% |
| FRS | kg oil eq | 1.67 × 100 | 15% | 2.88 × 10−1 | 3% | 1.84 × 100 | 16% | 2.41 × 100 | 21% | 7.86 × 10−1 | 7% | 1.68 × 100 | 15% | 1.79 × 100 | 16% | 2.26 × 10−1 | 1% | 7.28 × 10−1 | 6% | 1.14 × 101 | 100% |
| WC | m3 | 3.09 × 10−1 | 42% | 1.15 × 10−2 | 2% | 8.13 × 10−2 | 11% | 7.16 × 10−2 | 10% | 4.33 × 10−2 | 6% | 9.11 × 10−2 | 12% | 1.21 × 10−1 | 16% | 3.25 × 10−3 | 1% | 5.34 × 10−4 | 0% | 7.32 × 10−1 | 100% |
| Impact Categories | Unit | 1st Life | 2nd Life | Difference |
|---|---|---|---|---|
| Atmospheric | ||||
| Global warming | kg CO2 eq | 5.14 × 101 | 8.16 × 10−1 | −5.06 × 101 |
| Stratospheric ozone depletion | kg CFC11 eq | 1.22 × 10−5 | 2.60 × 10−7 | −1.19 × 10−5 |
| Ionizing radiation | kBq Co-60 eq | 1.71 × 10−1 | 2.92 × 10−3 | −1.68 × 10−1 |
| Ozone formation, Human health | kg NOx eq | 1.66 × 10−1 | 7.69 × 10−3 | −1.58 × 10−1 |
| Fine particulate matter formation | kg PM2.5 eq | 3.40 × 10−2 | 4.45 × 10−5 | −3.40 × 10−2 |
| Ozone formation, Terrestrial ecosystems | kg NOx eq | 1.66 × 10−1 | 7.74 × 10−3 | −1.58 × 10−1 |
| Terrestrial acidification | kg SO2 eq | 2.55 × 10−1 | 3.75 × 10−3 | −2.52 × 10−1 |
| Eutrophication | ||||
| Freshwater eutrophication | kg P eq | 2.72 × 10−3 | 3.01 × 10−5 | −2.69 × 10−3 |
| Marine eutrophication | kg N eq | 1.07 × 10−3 | 4.12 × 10−7 | −1.07 × 10−3 |
| Toxicity | ||||
| Terrestrial ecotoxicity | kg 1.4 DCB | 3.86 × 100 | 6.77 × 10−2 | −3.79 × 100 |
| Freshwater ecotoxicity | 2.70 × 10−3 | 1.12 × 10−5 | −2.69 × 10−3 | |
| Marine ecotoxicity | 4.40 × 10−3 | 4.54 × 10−5 | −4.35 × 10−3 | |
| Human carcinogenic toxicity | 1.29 × 10−2 | 1.21 × 10−3 | −1.17 × 10−2 | |
| Human non-carcinogenic toxicity | 3.48 × 100 | 1.80 × 10−2 | −3.46 × 100 | |
| Abiotic resources | ||||
| Land use | m2a crop eq | 3.89 × 100 | 1.03 × 10−2 | −3.88 × 100 |
| Mineral resource scarcity | kg Cu eq | 1.52 × 100 | 1.66 × 10−4 | −1.52 × 100 |
| Fossil resource scarcity | kg oil eq | 1.14 × 101 | 2.54 × 10−1 | −1.12 × 101 |
| Water consumption | m3 | 7.32 × 101 | 2.59 × 10−4 | −7.32 × 10−1 |
| Tipology | Characteristics | Disadvantages | Ref. |
|---|---|---|---|
| Pyrometallurgical | After being crushed and separated, the graphite and active cathode materials are heat-treated to eliminate the binders and carbon. Then, the remaining constituents undergo burning at around 1600 °C, yielding an alloy containing CO, Ni, and other metals. Following that, the other metals are removed from the lithium carbonate. | Energetic emissions of dioxins, carbon dioxide, sulfides, and furans, loss of material | [64,65] |
| Hydrometallurgical | Rendering agents that precipitate, extract, or adsorb different metals like Co, Mn, and Ni are used to dissolve the crushed matter. In the solution left behind, lithium is still dissolved to create lithium carbonate by further filtration. Pretreatments along the hydrometallurgical route include discharging and dismantling. It uses less energy and produces less harmful gasses, allowing for higher purity than pyrometallurgical. | Strong acids, such as sulfuric acid, are used, which poses a problem with the waste generated because it requires downstream treatment | [14] |
| Bioleaching | Bacteria and fungi are used to produce organic acids that leach metals. Compared with the traditional hydrometallurgical process, acids are replaced with microorganisms, producing lower environmental impacts and material costs. | extended leaching cycle, slow kinetics, low bacterial activity, and challenging operating conditions | [66] |
| Ultrasonic treatment | Aluminum is subjected to agitation and ultrasonic washing to extract all electrode components. Ultrasonic waves could generate more pressure due to the cavitation effect, which would enable the dissolution and disintegration of substances that are insoluble in water. | The type of polymer binder used has a significant impact on the delamination process’ efficiency. | [67] |
| Eutectic Salt | Lithium iodide (LiI) and lithium hydroxide (LiOH) are mixed in a eutectic mixture for the recovery of spent materials. This combination melts at temperatures below 200 °C, turning it into a liquid at comparatively low temperatures while consuming less energy and resources than conventional methods. | Operational difficulties related to the non-uniformity of various batteries, | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinci, G.; Arangia, V.C.; Ruggieri, R.; Savastano, M.; Ruggeri, M. Reuse of Lithium Iron Phosphate (LiFePO4) Batteries from a Life Cycle Assessment Perspective: The Second-Life Case Study. Energies 2024, 17, 2544. https://doi.org/10.3390/en17112544
Vinci G, Arangia VC, Ruggieri R, Savastano M, Ruggeri M. Reuse of Lithium Iron Phosphate (LiFePO4) Batteries from a Life Cycle Assessment Perspective: The Second-Life Case Study. Energies. 2024; 17(11):2544. https://doi.org/10.3390/en17112544
Chicago/Turabian StyleVinci, Giuliana, Vittorio Carobene Arangia, Roberto Ruggieri, Marco Savastano, and Marco Ruggeri. 2024. "Reuse of Lithium Iron Phosphate (LiFePO4) Batteries from a Life Cycle Assessment Perspective: The Second-Life Case Study" Energies 17, no. 11: 2544. https://doi.org/10.3390/en17112544







