Influence of γ-Fe2O3 Nanoparticles Added to Gasoline–Bio-Oil Blends Derived from Plastic Waste on Combustion and Emissions Generated in a Gasoline Engine
Abstract
:1. Introduction
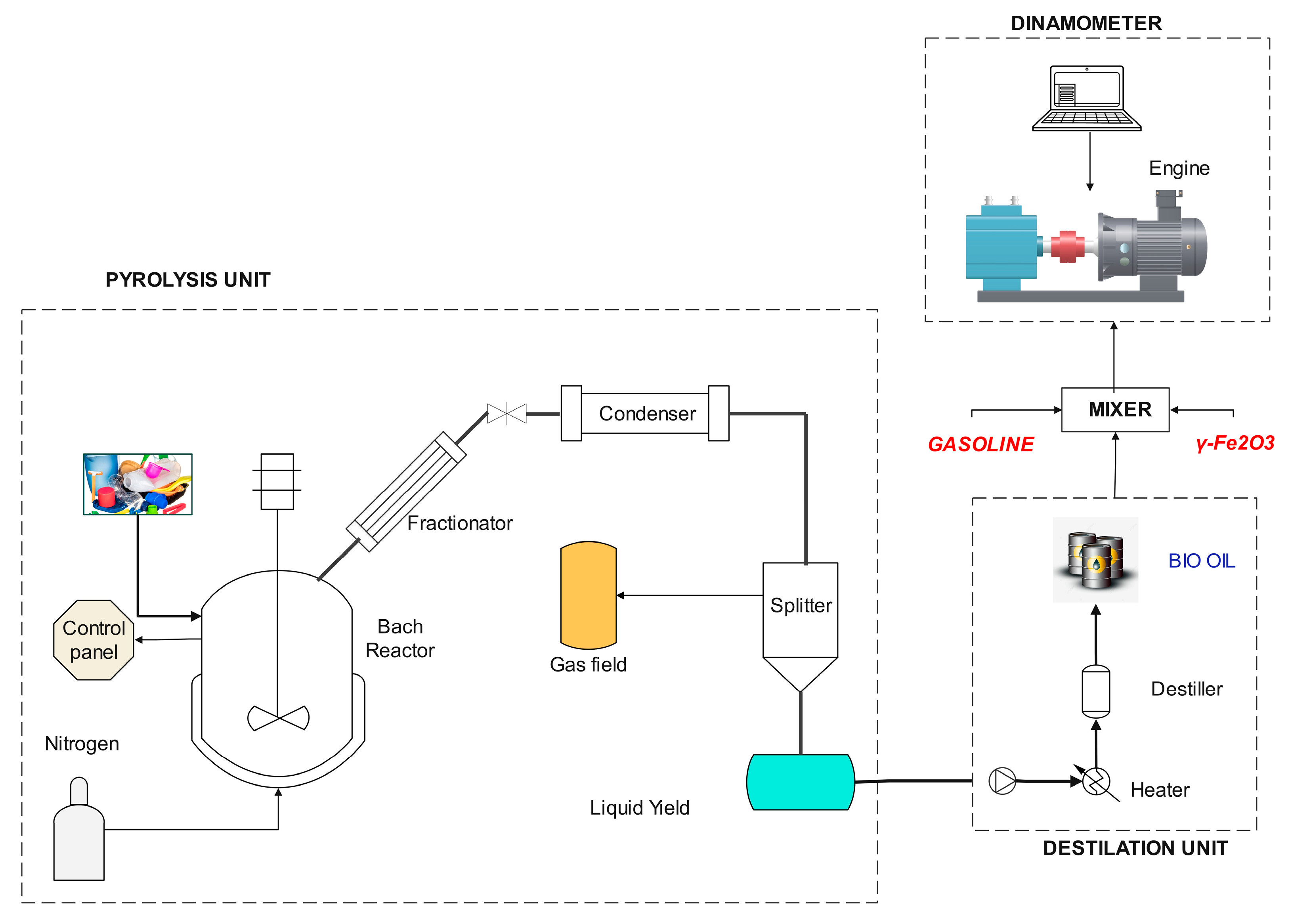
2. Materials and Methods
2.1. Materials
2.2. Production of Bio-Oil
2.3. Samples
2.4. Power/Torque Tests
2.5. Combustion Emissions
3. Results
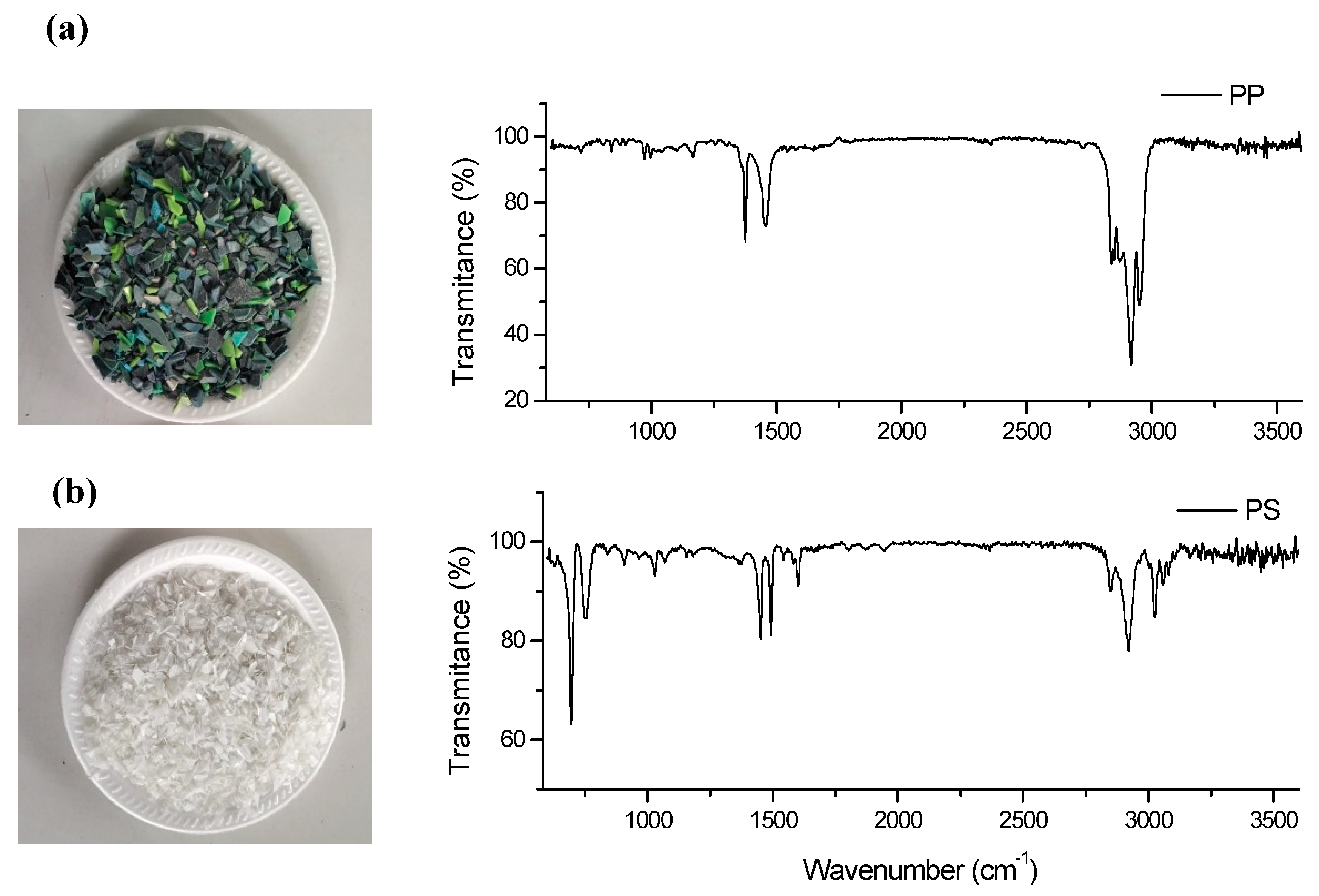
3.1. Characterization of Plastic Waste
3.2. Characterization of Samples
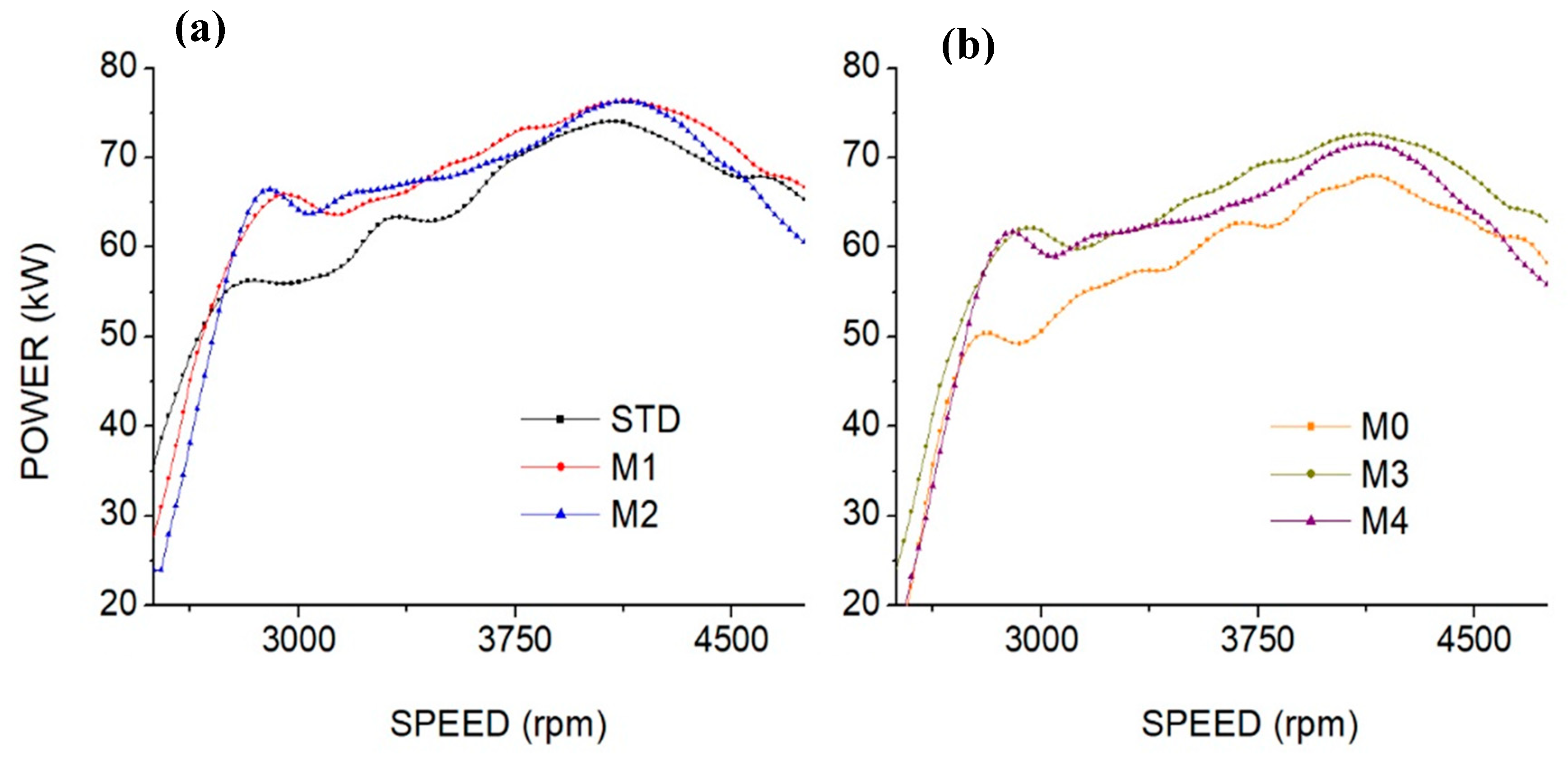
3.3. Engine Performance
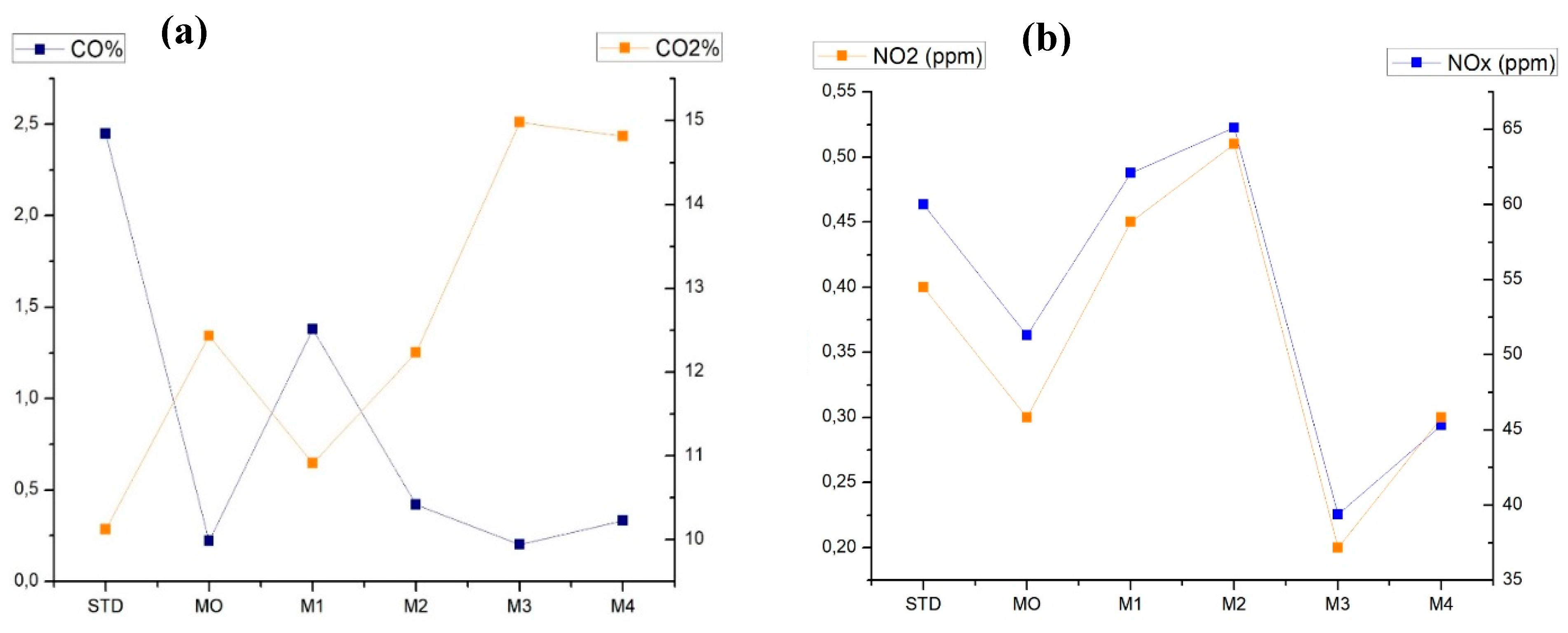
3.4. Evaluation of Emissions in Blended Fuels
3.5. Research Octane Number (RON) and Efficiency
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elsharkawy, E.A.; Al-Sood, M.M.A.; El-Fakharany, M.K.; Ahmed, M. Comparative study of combustion, performance, and emissions of a diesel engine fuelled with biodiesel blend with metallic and organic nano-particles. Int. J. Glob. Warm. 2020, 20, 133–159. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Kibria, M.G.; Masuk, N.I.; Safayet, R.; Nguyen, H.Q.; Mourshed, M. Plastic Waste: Challenges and Opportunities to Mitigate Pollution and Effective Management. Int. J. Environ. Res. 2023, 17, 20. [Google Scholar] [CrossRef]
- Czajczyńska, D.; Anguilano, L.; Ghazal, H.; Krzyżyńska, R.; Reynolds, A.J.; Spencer, N.; Jouhara, H. Potential of pyrolysis processes in the waste management sector. Therm. Sci. Eng. Prog. 2017, 3, 171–197. [Google Scholar] [CrossRef]
- Mihai, F.C.; Gündogdu, S.; Markley, L.A.; Olivelli, A.; Khan, F.R.; Gwinnett, C.; Gutberlet, J.; Reyna-Bensusan, N.; Llanquileo-Melgarejo, P.; Meidiana, C.; et al. Plastic Pollution, Waste Management Issues, and Circular Economy Opportunities in Rural Communities. Sustainability 2022, 14, 20. [Google Scholar] [CrossRef]
- Chaves, I.C.; Allen, V.B.Q.; Puente, A.; Profesor, U.; Ronald, M.E.; Salas, J. Evaluación de las Técnicas SEM y EDS en la Investigación Nanotecnológica de Catalizadores para la Producción de Biocombustibles. 2013. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/. Available online: https://core.ac.uk/download/pdf/60992329.pdf (accessed on 4 June 2024).
- Al-Rumaihi, A.; Shahbaz, M.; Mckay, G.; Mackey, H.; Al-Ansari, T. A review of pyrolysis technologies and feedstock: A blending approach for plastic and biomass towards optimum biochar yield. Renew. Sustain. Energy Rev. 2022, 167, 112715. [Google Scholar] [CrossRef]
- Klaimy, S.; Lamonier, J.F.; Casetta, M.; Heymans, S.; Duquesne, S. Recycling of plastic waste using flash pyrolysis—Effect of mixture composition. Polym. Degrad. Stab. 2021, 187, 109540. [Google Scholar] [CrossRef]
- Dai, L.; Zhou, N.; Lv, Y.; Cheng, Y.; Wang, Y.; Liu, Y.; Cobb, K.; Chen, P.; Lei, H.; Ruan, R. Pyrolysis technology for plastic waste recycling: A state-of-the-art review. Prog. Energy Combust. Sci. 2022, 93, 101021. [Google Scholar] [CrossRef]
- Jaafar, Y.; Abdelouahed, L.; El Hage, R.; El Samrani, A.; Taouk, B. Pyrolysis of common plastics and their mixtures to produce valuable petroleum-like products. Polym. Degrad. Stab. 2022, 195, 109770. [Google Scholar] [CrossRef]
- Gebre, S.H.; Sendeku, M.G.; Bahri, M. Recent Trends in the Pyrolysis of Non-Degradable Waste Plastics. Chem. Open 2021, 10, 1202–1226. [Google Scholar] [CrossRef]
- Maafa, I.M. Pyrolysis of Polystyrene Waste: A Review. Polymers 2021, 13, 225. [Google Scholar] [CrossRef]
- Wang, Z.; Burra, K.G.; Lei, T.; Gupta, A.K. Co-pyrolysis of waste plastic and solid biomass for synergistic production of biofuels and chemicals-A review. Prog. Energy. Combust. Sci. 2021, 84, 100899. [Google Scholar] [CrossRef]
- Yaqoob, H.; Tan, E.S.; Ali, H.M.; Ong, H.C.; Jamil, M.A.; Farooq, M.U. Sustainable energy generation from plastic waste: An in-depth review of diesel engine application. Environ. Technol. Innov. 2024, 34, 103467. [Google Scholar] [CrossRef]
- Babayemi, J.O.; Nnorom, I.C.; Osibanjo, O.; Weber, R. Ensuring sustainability in plastics use in Africa: Consumption, waste generation, and projections. Environ. Sci. Eur. 2019, 31, 60. [Google Scholar] [CrossRef]
- Mustayen, A.G.M.B.; Rasul, M.G.; Wang, X.; Hazrat, M.A.; Negnevitsky, M.; Jahirul, M.I. Impact of waste-plastic-derived diesel on the performance and emission characteristics of a diesel engine under low load conditions. Energy Convers. Manag. 2023, 283, 116936. [Google Scholar] [CrossRef]
- Szwaja, M.; Chwist, M.; Szwaja, S.; Juknelevičius, R. Impact of Pyrolysis Oil Addition to Ethanol on Combustion in the Internal Combustion Spark Ignition Engine. Clean Technol. 2021, 3, 450–461. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B.; Sadhukhan, A.K.; Gupta, P.; Tigga, V.P. Waste plastic to pyrolytic oil and its utilization in CI engine: Performance analysis and combustion characteristics. Fuel 2020, 262, 116539. [Google Scholar] [CrossRef]
- Zein, S.H.; Grogan, C.T.; Yansaneh, O.Y.; Putranto, A. Pyrolysis of High-Density Polyethylene Waste Plastic to Liquid Fuels—Modelling and Economic Analysis. Processes 2022, 10, 1503. [Google Scholar] [CrossRef]
- Yakın, A.; Behçet, R. Effect of different types of fuels tested in a gasoline engine on engine performance and emissions. Int. J. Hydrogen Energy 2021, 46, 33325–33338. [Google Scholar] [CrossRef]
- Yaqoob, H.; Ali, H.M. Sustainability analysis of neat waste tire oil powered diesel engine: A thermodynamics approach. Process Saf. Environ. Protection 2024, 182, 1121–1129. [Google Scholar] [CrossRef]
- Al-Mashhadani, H.; Fernando, S.; Al-Mashhadani, H.; Fernando, S. Properties, performance, and applications of biofuel blends: A review. AIMS 2017, 5, 735–767. [Google Scholar] [CrossRef]
- Abdallah, A.M.; Abdel-Rahman, A.A.; Elwardany, A.E. Analysis of the impact of different nanoparticle metal oxides as fuel additives in compression ignition engine performance. Energy Rep. 2020, 6, 99–105. [Google Scholar] [CrossRef]
- Valihesari, M.; Pirouzfar, V.; Ommi, F.; Zamankhan, F. Investigating the effect of Fe2O3 and TiO2 nanoparticle and engine variables on the gasoline engine performance through statistical analysis. Fuel 2019, 254, 115618. [Google Scholar] [CrossRef]
- Lamore, M.T.; Zeleke, D.S.; Kassa, B.Y. A comparative study on the effect of nano-additives on performance and emission characteristics of CI engine run on castor biodiesel blended fuel. Energy Convers. Manag. X 2023, 20, 100493. [Google Scholar] [CrossRef]
- Giuliano, M.; Valsania, M.C.; Ticali, P.; Sartoretti, E.; Morandi, S.; Bensaid, S.; Ricchiardi, G.; Sgroi, M. Characterization of the evolution of noble metal particles in a commercial three-way catalyst: Correlation between real and simulated ageing. Catalysts 2021, 11, 247. [Google Scholar] [CrossRef]
- Palmay, P.; Haro, C.; Huacho, I.; Barzallo, D.; Bruno, J.C. Production and Analysis of the Physicochemical Properties of the Pyrolytic Oil Obtained from Pyrolysis of Different Thermoplastics and Plastic Mixtures. Molecules 2022, 27, 3287. [Google Scholar] [CrossRef]
- Barzallo, D.; Lazo, R.; Medina, C.; Guashpa, C.; Tacuri, C.; Palmay, P. Synthesis and Application of ZSM-5 Catalyst Supported with Zinc and/or Nickel in the Conversion of Pyrolytic Gases from Recycled Polypropylene and Polystyrene Mixtures under Hydrogen Atmosphere. Polymers 2023, 15, 3329. [Google Scholar] [CrossRef]
- Egbosiuba, T.C. Biochar and bio-oil fuel properties from nickel nanoparticles assisted pyrolysis of cassava peel. Heliyon 2022, 8, e10114. [Google Scholar] [CrossRef]
- Quesada, L.; Calero, M.; Martín-Lara, M.Á.; Pérez, A.; Blázquez, G. Production of an Alternative Fuel by Pyrolysis of Plastic Wastes Mixtures. Energy Fuels 2020, 34, 1781–1790. [Google Scholar] [CrossRef]
- Torres, J.; Molina, D.; Pinto, C.; Rueda, F. Study of the blending of petrol with 10% anhydrous ethanol. Evaluation of physicochemical properties. CT&F-Cienc. Tecnol. Y Futuro 2002, 2, 71–82. [Google Scholar] [CrossRef]
- Abiyazani, N.K.; Pirouzfar, V.; Su, C.H. Enhancing engine power and torque and reducing exhaust emissions of blended fuels derived from gasoline-propanol-nano particles. Energy 2022, 241, 122924. [Google Scholar] [CrossRef]
- Wood, B.M.; Kirwan, K.; Maggs, S.; Meredith, J.; Coles, S.R. Study of combustion performance of biodiesel for potential application in motorsport. J. Clean. Prod. 2015, 93, 167–173. [Google Scholar] [CrossRef]
- Awad, O.I.; Mamat, R.; Ibrahim, T.K.; Ali, O.M.; Kadirgama, K.; Leman, A.M. Performance and combustion characteristics of an SI engine fueled with fusel oil-gasoline at different water content. Appl. Therm. Eng. 2017, 123, 1374–1385. [Google Scholar] [CrossRef]
- Nouri, M.; Isfahani, A.H.M.; Shirneshan, A. Effects of Fe2O3 and Al2O3 nanoparticle-diesel fuel blends on the combustion, performance and emission characteristics of a diesel engine. Clean Technol. Environ. Policy 2021, 23, 2265–2284. [Google Scholar] [CrossRef]
- Yusof, S.N.A.; Sidik, N.A.C.; Asako, Y.; Japar, W.M.A.A.; Mohamed, S.B.; Muhammad, N.M.A. A comprehensive review of the influences of nanoparticles as a fuel additive in an internal combustion engine (ICE). Nanotechnol. Rev. 2021, 9, 1326–1349. [Google Scholar] [CrossRef]
- Varatharajan, K.; Cheralathan, M. Influence of fuel properties and composition on NOx emissions from biodiesel powered diesel engines: A review. Renew. Sustain. Energy Rev. 2012, 16, 3702–3710. [Google Scholar] [CrossRef]
- Ozgur, T.; Tuccar, G.; Uludamar, E.; Yilmaz, A.C.; Güngör, C.; Ozcanli, M.; Serin, H.; Aydin, K. Effect of nanoparticle additives on NOx emissions of diesel fuelled compression ignition engine. Int. J. Glob. Warm. 2015, 7, 487–498. [Google Scholar] [CrossRef]
- Yuan, C.; Rodionov, N.; Mehrabi-Kalajahi, S.; Emelianov, D.A.; Zinnatullin, A.L.; Varfolomeev, M.A.; Zairov, R.; Stepanov, A.; Mustafina, A.R.; Al-Muntaser, A.; et al. Catalytic combustion of heavy oil using γ-Fe2O3 nanocatalyst in in-situ combustion process. J. Pet. Sci. Eng. 2022, 209, 109819. [Google Scholar] [CrossRef]
- Küçükosman, R.; Yontar, A.A.; Ocakoglu, K. Nanoparticle additive fuels: Atomization, combustion and fuel characteristics. J. Anal. Appl. Pyrolysis 2022, 165, 105575. [Google Scholar] [CrossRef]
- Aklouche, F.Z.; Hadhoum, L.; Loubar, K.; Tazerout, M. A Comprehensive Study on Effect of Biofuel Blending Obtained from Hydrothermal Liquefaction of Olive Mill Waste Water in Internal Combustion Engine. Energies 2023, 16, 2534. [Google Scholar] [CrossRef]
- Ding, H.; Wang, C.; Zhu, X. Estimation of the kinematic viscosities of bio-oil/alcohol blends: Kinematic viscosity-temperature formula and mixing rules. Fuel 2019, 254, 115687. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Martins-Vieira, J.C.; Missau, J.; Anshu, K.; Siakpebru, O.K.; Thengane, S.K.; Morais, A.R.C.; Tanabe, E.H.; Bertuol, D.A. Review on Biomass Pyrolysis with a Focus on Bio-Oil Upgrading Techniques. Analytica 2023, 4, 182–205. [Google Scholar] [CrossRef]




| Parameters | Specifications |
|---|---|
| Engine Type | Turbocharged Inline Three-Cylinder |
| Engine Model | T-GDi—1.0 L |
| Fuel | Gasoline |
| Nominal power | 120 hp or 88 kW |
| Maximum torque | 200 Nm |
| Nominal power | 6.000 rpm |
| Nominal voltage | 48 V |
| Comprehension ratio | 10, 5:1 |
| Feeding | Direct injection. Turbo. Intercooler. |
| Displacement | 998 cm3 |
| Samples | API Density (°) | Relative Density | Viscosity (cSt) at 25 °C | Flash Point (°C) | Distillation (%) | ||
|---|---|---|---|---|---|---|---|
| T10 | T50 | T90 | |||||
| STD | 58.56 | 0.744 | 1.17 | 18 | 45 | 81 | 130 |
| M0 | 58.56 | 0.744 | 1.17 | 18 | 45 | 88 | 133 |
| M1 | 58.84 | 0.747 | 1.061 | 18 | 50 | 99 | 161 |
| M2 | 57.50 | 0.752 | 1.033 | 18 | 51 | 99 | 156 |
| M3 | 57.46 | 0.748 | 1.058 | 18 | 51 | 98 | 150 |
| M4 | 56.46 | 0.753 | 1.025 | 18 | 59 | 103 | 167 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmay, P.; Barzallo, D.; Puente, C.; Robalino, R.; Quinaluisa, D.; Bruno, J.C. Influence of γ-Fe2O3 Nanoparticles Added to Gasoline–Bio-Oil Blends Derived from Plastic Waste on Combustion and Emissions Generated in a Gasoline Engine. Energies 2024, 17, 2843. https://doi.org/10.3390/en17122843
Palmay P, Barzallo D, Puente C, Robalino R, Quinaluisa D, Bruno JC. Influence of γ-Fe2O3 Nanoparticles Added to Gasoline–Bio-Oil Blends Derived from Plastic Waste on Combustion and Emissions Generated in a Gasoline Engine. Energies. 2024; 17(12):2843. https://doi.org/10.3390/en17122843
Chicago/Turabian StylePalmay, Paul, Diego Barzallo, Cesar Puente, Ricardo Robalino, Dayana Quinaluisa, and Joan Carles Bruno. 2024. "Influence of γ-Fe2O3 Nanoparticles Added to Gasoline–Bio-Oil Blends Derived from Plastic Waste on Combustion and Emissions Generated in a Gasoline Engine" Energies 17, no. 12: 2843. https://doi.org/10.3390/en17122843
APA StylePalmay, P., Barzallo, D., Puente, C., Robalino, R., Quinaluisa, D., & Bruno, J. C. (2024). Influence of γ-Fe2O3 Nanoparticles Added to Gasoline–Bio-Oil Blends Derived from Plastic Waste on Combustion and Emissions Generated in a Gasoline Engine. Energies, 17(12), 2843. https://doi.org/10.3390/en17122843







