Exploring Hydrogen Embrittlement: Mechanisms, Consequences, and Advances in Metal Science
Abstract
:1. Introduction
1.1. The Purpose and Methodology of the Review
1.2. Relevance and Knowledge Gap Statements
2. Hydrogenation Process and Its Effects
2.1. Hydrogenation Process
- Electrochemical processes (electrochemical corrosion, etching, electroplating), when hydrogen is ionized in the electrolyte and absorbed by the material, passing into the state of a quasi-ion—a proton of a metal shielded by electrons [15]. It is important to note that this process can take place at room temperature. As a result of electrochemical corrosion and cathodic treatment [16,17], internal delamination [18], as well as bubbles and cracks emerging on the surface, can occur in a metal.
- The contact of a metal with a hydrogen-containing gaseous medium at elevated temperatures and pressures as a result of thermal dissociation of hydrogen [19]. In this process, hydrogen can also enter into chemical reactions with the structural components of technical metals and alloys, for example, carbides.
- The interaction with reactive hydrogen-containing substances (for example, hydrogen sulfide). In this case, chemical reactions of metals with hydrogen compounds produce hydrogen, which is absorbed by the material. This phenomenon is widespread in the chemical and oil and gas industries [20].
- Adsorption. It consists of the accumulation of hydrogen atoms on the surface of materials. There is physical (low-temperature) and chemical (high-temperature) adsorption. During physical adsorption, Van der Waals forces act on hydrogen atoms; during chemical adsorption, the forces of chemical interaction act on these atoms.
- Absorption–dissolution of adsorbed hydrogen atoms in materials. Absorption can occur both with the release and absorption of heat—it depends solely on the nature of the material under study.
- Diffusion. Hydrogen, due to its small atomic radius, diffuses much more easily than, for example, carbon and nitrogen. The diffusion coefficient of hydrogen for pure iron is 1.5 × 10−5 cm2/s, for ferritic steel 10−6 cm2/s, and for austenitic steel 2.3 × 10−8 cm2/s.
2.2. Effects of Hydrogenation
- penetration
- diffusion process
- absorption
- destruction of material in a local place
- changing material parameters
- inverse influence on the nature of penetration with corresponding diffusion transfer
2.3. Factors Contributing to Hydrogenation
- Parts’ safety factor. For safety-relevant parts, hydrogen purification should be increased.
- Parts with small cross-sectional area, such as small springs, thinner springs, etc.
- Toothed parts, which are prone to stress concentration.
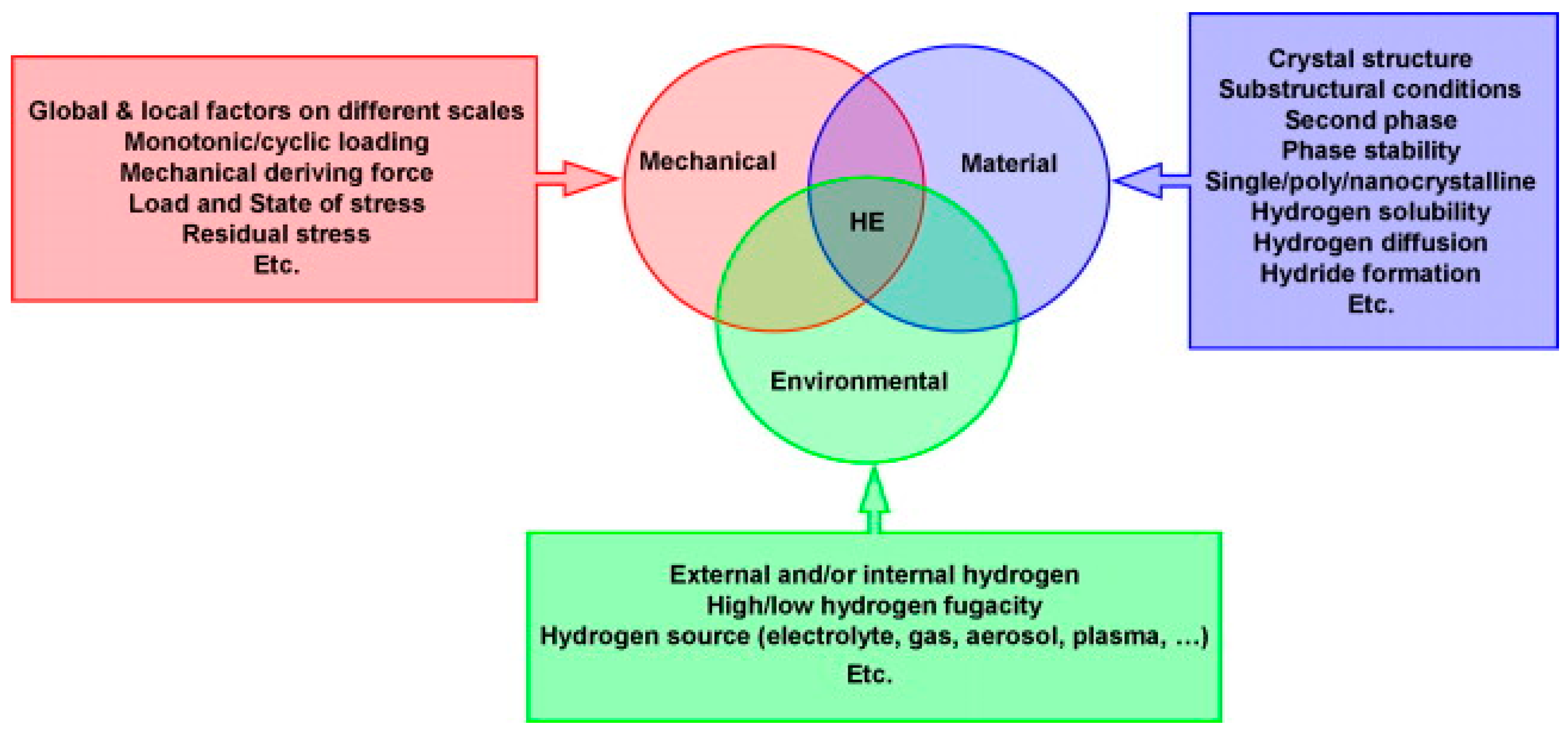
3. Mechanisms, Types, and Models of HE
3.1. Mechanisms and Models
3.2. Reversible and Irreversible Embrittlement
- Brittleness of this kind manifests itself in a certain temperature range, which depends on the rate of deformation, the nature of the alloys, and their chemical composition.
- With an increase in the strain rate, the temperature interval for the drop in plasticity decreases, plastic characteristics increase, and if the rate exceeds a certain limit, then brittleness is not detected.
- The transition temperature from ductile to brittle fracture increases with increasing hydrogen content.
- Fracture at a low strain rate occurs along grain boundaries.
- Hydrogen has little effect on yield strength and elongation until necking occurs and greatly reduces lateral contraction.
4. Current Knowledge Regarding HE in Specific Metals and Alloys
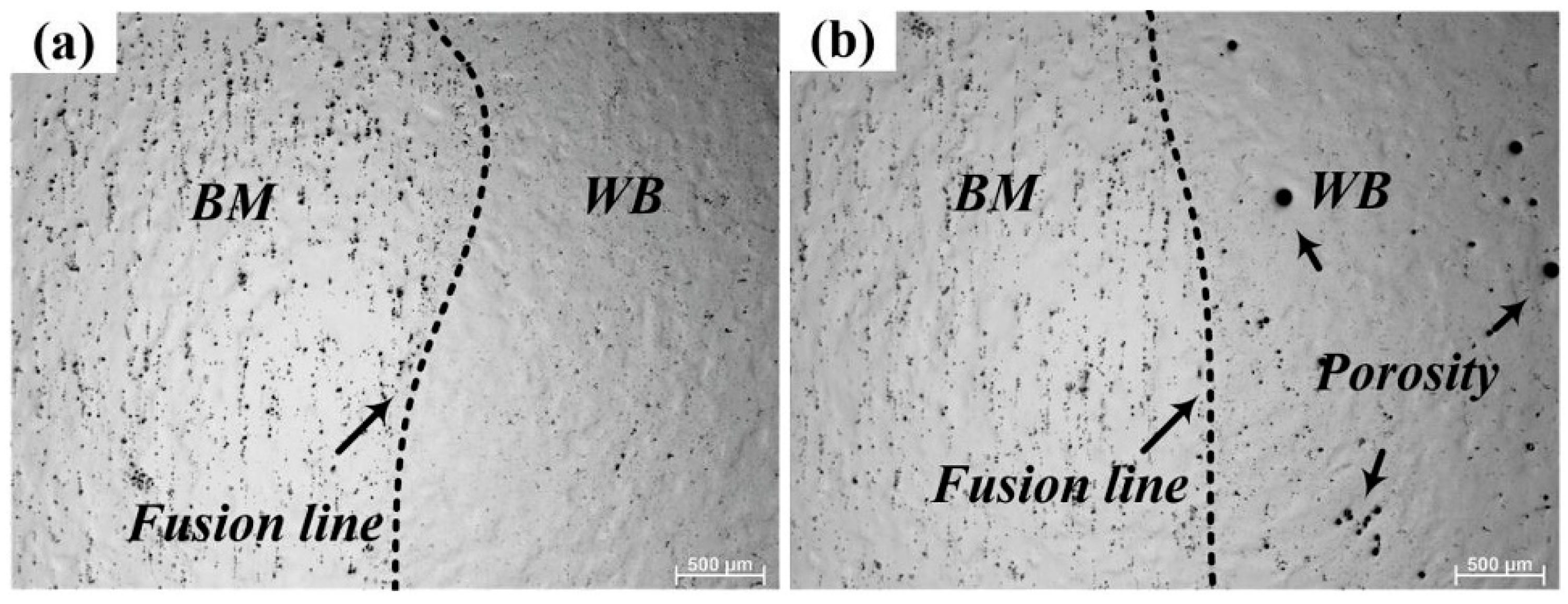
4.1. Steels
- The second is the Troiano theory [81], which proceeds from the initiation of secondary cracks in front of the top of the main crack and their subsequent merging.
- high pressure of molecular hydrogen in microvolumes;
- decrease in surface energy;
- decohesion of the lattice, intergranular, and interphase boundaries.
4.2. Aluminum and Its Alloys
4.3. Titanium and Its Alloys
4.4. Zirconium and Its Alloys
4.5. Nickel and Its Alloys
4.6. Tantalum
4.7. Vanadium and Its Alloys
4.8. Niobium and Its Alloys
4.9. Copper and Its Alloys
4.10. Uranium
4.11. Other Metals
5. Hydrogen Trap and Crack Formation
6. Impact of HE in Different Areas and Industries
6.1. HE in Construction
6.2. HE in Plumbing
6.3. HE in the Industries of Oil and Gas
7. HE Test Methods and Mitigation
7.1. Testing Methods
- Isolating hydrogen from the inert gas.
- Employing mass spectrometry.
- Analyzing the conductivity of the emitted gas.
- Measuring the volume of hydrogen gas.
- Conducting gas chromatography.
- Utilizing a heated Palladium filter.
7.2. Mitigation of HE
8. Key Findings and Future Implications
8.1. Key Findings
- Hydrogen Accumulation Mechanisms: This review paper has presented multiple mechanisms through which hydrogen accumulates in metals. These mechanisms encompass metallurgical processes, product manufacturing, and external environmental factors. Understanding these mechanisms is crucial for effective mitigation strategies. Discussions on mechanisms such as hydrogen-enhanced localized plasticity (HELP) and hydrogen-enhanced decohesion (HEDE), along with the exploration of various models, contribute to a nuanced understanding of HE.
- Hydrogenation Process: By delving into the hydrogenation process and elucidating different types of HE, the paper provides a valuable context for researchers, engineers, and professionals dealing with high-strength materials in diverse applications. This comprehensive discussion aids in identifying specific challenges and tailoring approaches to address them effectively.
- Hydrogen Effects on Metals: The paper has comprehensively outlined the diverse effects of hydrogen on metals. It established that HE can significantly reduce the mechanical properties of metals, leading to an increased susceptibility to fractures and failures.
- Metal-Specific Responses: The review paper systematically examines the influence of hydrogen on a diverse range of materials and alloys, providing a comprehensive overview of its effects in a structured manner. The paper has explored how different metals and their alloys exhibit distinct responses to hydrogen exposure. This knowledge is invaluable for tailoring materials for specific applications and ensuring structural integrity.
- Modeling and Classification: The paper has presented various models and classifications of HE, providing a framework for understanding the complexities of this phenomenon. These models aid in predicting, preventing, and mitigating HE in different contexts.
8.2. Future Implications
- Advanced Detection Methods: Future research should focus on developing more sensitive and accurate methods for detecting and quantifying hydrogen within metals. This will enable early identification of embrittlement risks.
- Mitigation Strategies: Research should continue to explore innovative mitigation strategies to counteract HE. This includes the development of coatings, materials, and manufacturing processes that are less susceptible to HE.
- Metal-Specific Studies: Investigating the HE behavior of specific metals and alloys remains crucial. Future studies can delve deeper into the underlying mechanisms, enabling the development of tailored solutions.
- Hydrogen Storage and Transportation: Given the importance of hydrogen in emerging energy technologies, research should focus on materials and methods for safe hydrogen storage and transportation, minimizing the risk of embrittlement in critical infrastructure.
- Environmental Factors: As environmental factors can contribute to HE, research should address the impact of environmental conditions on HE, allowing for better risk assessment and management.
- Multiscale Modeling: Advancements in multiscale modeling techniques can provide a more accurate understanding of HE. Future research can employ these models to predict and prevent embrittlement in complex systems.
- Standardization: Establishing standardized testing protocols and guidelines for evaluating HE susceptibility can aid industries in ensuring the safety and reliability of metal components.
- Interdisciplinary Collaboration: Promoting cooperation among engineers, materials scientists, and environmental specialists is crucial for tackling the complex issues presented by (HE).
9. Conclusions
Funding
Conflicts of Interest
References
- Dwivedi, S.K.; Vishwakarma, M. Hydrogen embrittlement in different materials: A review. Int. J. Hydrogen Energy 2018, 43, 21603–21616. [Google Scholar] [CrossRef]
- Cottis, R.A. 2.10—Hydrogen Embrittlement. In Shreir’s Corrosion; Elsevier: Amsterdam, The Netherlands, 2010; Volume 2, pp. 902–922. [Google Scholar] [CrossRef]
- Lynch, S. Hydrogen embrittlement phenomena and mechanisms. Corros. Rev. 2012, 30, 105–123. [Google Scholar] [CrossRef]
- Laadel, N.E.; El Mansori, M.; Kang, N.; Marlin, S.; Boussant-Roux, Y. Permeation Barriers for Hydrogen Embrittlement Prevention in Metals—A Review on Mechanisms, Materials Suitability and Efficiency. Int. J. Hydrogen Energy 2022, 47, 32707–32731. [Google Scholar] [CrossRef]
- Spiridonov, N.V.; Ivashko, V.S.; Kudina, A.V.; Kurash, V.V. Hydrogenation and Destruction of the Structure of Steel Parts of Machines and Mechanisms in Hydrogen-Containing Media. Sci. Technol. 2014. Available online: https://cyberleninka.ru/article/n/navodorozhivanie-i-razrushenie-struktury-stalnyh-detaley-mashin-i-mehanizmov-v-vodorodsoderzhaschih-sredah (accessed on 30 May 2024).
- Chen, Y.S.; Huang, C.; Liu, P.Y.; Yen, H.W.; Niu, R.; Burr, P.; Moore, K.L.; Martínez-Pañeda, E.; Atrens, A.; Cairney, J.M. Hydrogen trapping and embrittlement in metals—A review. Int. J. Hydrogen Energy 2024, in press. [Google Scholar] [CrossRef]
- Kolachev, B.A. Hydrogen Embrittlement of Non-Ferrous Metals; Publishing House “Metallurgy”: Moscow, Russia, 1966; 160p. [Google Scholar]
- Kolachev, B.A. Hydrogen Brittleness of Metals; Metallurgy: Moscow, Russia, 1985; 216p. [Google Scholar]
- Glazkova, S.M.; Pastoev, A.V.; Sarrak, V.I.; Filippov, G.A.; Shlyafirner, A.M. Studies of the influence of hydrogen on the ductility and nature of destruction of structural steel 38 XC. Phys.-Chem. Mech. Mater. 1976, 5, 21–26. [Google Scholar]
- Ovchinnikov, I.I. Study of the behavior of shell structures operating in environments that cause corrosion cracking. Bull. Eurasian Sci. 2012, 13. Available online: https://cyberleninka.ru/article/n/issledovanie-povedeniya-obolochechnyh-konstruktsiy-ekspluatiruyuschihsya-v-sredah-vyzyvayuschih-korrozionnoe-rastreskivanie (accessed on 30 May 2024).
- Sheleg, V.K.; Prisevok, A.F. HydrogenResistant Protective Materials for Friction Parts of Machines and Equipment, Operating in Technogenic Hydrogen-Containing Media. Vestn. BNTU [Bull. Belarusian Natl. Tech. Univ.] 2007, 3, 15–22. [Google Scholar]
- Kurash, V.V.; Kudina, A.V.; Lisai, N.K.; Lisai, A.N. Investigation on Composition of Corrosion Resistant Metal Coating with Low Hydrogen Permeability Formed with the Help of Welding Deposition Method. Agropanorama 2011, 2, 35–39. [Google Scholar]
- Ivashko, V.S.; Kurash, V.V.; Kudina, A.V. Theoretical Kinetics Aspects of Machine Part and Mechanism Surface Wear. Vestn. BNTU [Bull. Belarusian Natl. Tech. Univ.] 2005, 5, 59–63. [Google Scholar]
- Barrera, O.; Bombac, D.; Chen, Y.; Daff, T.D.; Galindo-Nava, E.; Gong, P.; Haley, D.; Horton, R.; Katzarov, I.; Kermode, J.R.; et al. Understanding and mitigating hydrogen embrittlement of steels: A review of experimental, modelling and design progress from atomistic to continuum. J. Mater. Sci. 2018, 53, 6251–6290. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, H. Chapter 13—Hydrogen storage materials. In New and Future Developments in Catalysis; Suib, S.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 377–405. [Google Scholar]
- Lu, X.; Wang, D.; Zhang, Z.; Kheradmand, N.; Barnoush, A. Effect of electrochemical charging on the hydrogen embrittlement susceptibility of alloy 718. Acta Mater. 2019, 179, 36–48. [Google Scholar] [CrossRef]
- Peral, L.B.; Díaz, A.; Colombo, C.; Alegre, J.; Cuesta, I.I. Effect of electrochemical charging on the hydrogen embrittlement susceptibility of a low-alloyed tempered martensitic steel submitted to high internal pressure. Int. J. Hydrogen Energy 2024, 63, 657–667. [Google Scholar] [CrossRef]
- Gu, C.; Hu, J.; Zhong, X. Evidence of hydrogen gas evolution accelerating the cathodic coating delamination. Corros. Commun. 2022, 7, 63–69. [Google Scholar] [CrossRef]
- Bar, R.; Dabah, E.; Eliezer, D.; Kannengiesser, T.; Boellinghaus, T. The influence of hydrogen on thermal desorption processes in structural materials. Procedia Eng. 2011, 10, 3668–3676. [Google Scholar] [CrossRef]
- Myagkikh, P.N. Hydrogenation and Hydrogen Embrittlement. Master’s Thesis, Toglyatti State University, Institute of Me-chanical Engineering, Togliatti, Russia, 2016. [Google Scholar]
- Moroz, L.S.; Chechulin, B.B. Hydrogen Brittleness of Metals; Metallurgy: Moscow, Russia, 1967; 255p. [Google Scholar]
- Van Leeuwen, H.P. The kinetics of hydrogen embrittlement: A quantitative diffusion model. Eng. Fract. Mech. 1974, 6, 141–161. [Google Scholar] [CrossRef]
- Shishvan, S.S.; Csányi, G.; Deshpande, V.S. Strain rate sensitivity of the hydrogen embrittlement of ferritic steels. Acta Mater. 2023, 257, 119173. [Google Scholar] [CrossRef]
- Kadyrbekov, B.A.; Kolesnikov, V.A.; Pechersky, V.N. Evaluation of the resistance of steels to corrosion cracking when tested at a constant strain rate. Phys.-Chem. Mater. 1989, 1, 39–43. [Google Scholar]
- Momotani, Y.; Shibata, A.; Terada, D.; Tsuji, N. Effect of strain rate on hydrogen embrittlement in low-carbon martensitic steel. Int. J. Hydrogen Energy 2017, 42, 3371–3379. [Google Scholar] [CrossRef]
- Ustolin, F.; Paltrinieri, N.; Berto, F. Loss of integrity of hydrogen technologies: A critical review. Int. J. Hydrogen Energy 2020, 45, 23809–23840. [Google Scholar] [CrossRef]
- Rodnikov, S.N.; Ovchinnikova, T.M. The influence of hydrogen on the strength and plastic characteristics of high-strength steels. Phys.-Chem. Mater. 1984, 20, 103–104. [Google Scholar]
- Karpenko, G.V. Strength of Steel in a Corrosive Environment; Mashgiz: Moscow, Russia, 1963; 187p. [Google Scholar]
- Azhogin, F.F. Corrosion Cracking and Protection of High-Strength Steels; Metallurgy: Moscow, Russia, 1974; 256p. [Google Scholar]
- Hardie, D.; Liu, S. The effect of stress concentration on hydrogen embrittlement of a low alloy steel. Corros. Sci. 1996, 38, 721–733. [Google Scholar] [CrossRef]
- Korchagin, A.P. Study of the plastic properties of steel in various stress states after exposure to hydrogenating media. Probl. Strength 1975, 7, 114–117. [Google Scholar]
- Yakovlev Yu, A. Changing the Structure and Destruction of Materials Containing Hydrogen. Abstract. UDC 539.3 St. Petersburg 2013. Available online: https://www.dissercat.com/content/izmenenie-struktury-i-razrushenie-materialov-soderzhashchikh-vodorod (accessed on 2 June 2024).
- Skripchuk, G.A. Hydrogen fragility. Young Sci. 2009, 11, 13–15. Available online: https://moluch.ru/archive/11/825/ (accessed on 30 May 2024).
- Qayyum, F.; Umar, M.; Dölling, J.; Guk, S.; Prahl, U. Mechanics of New-Generation Metals and Alloys. Compr. Mech. Mater. 2024, 3, 31–57. [Google Scholar] [CrossRef]
- Djukic, M.B.; Bakic, G.M.; Sijacki Zeravcic, V.; Sedmak, A.; Rajicic, B. The Synergistic Action and Interplay of Hydrogen Embrittlement Mechanisms in Steels and Iron: Localized Plasticity and Decohesion. Eng. Fract. Mech. 2019, 216, 106528. [Google Scholar] [CrossRef]
- Barnoush, A.; Vehoff, H. Recent Developments in the Study of Hydrogen Embrittlement: Hydrogen Effect on Dislocation Nucleation. Acta Mater. 2010, 58, 5274–5285. [Google Scholar] [CrossRef]
- Bobkov, G.O. Analysis of hydrogen embrittlement and hydride destruction of core components of nuclear power plants. Politech. Stud. J. 2020. [Google Scholar] [CrossRef]
- Zhang, C.; Alavi, A. First-principles study of superabundant vacancy formation in metal hydrides. J. Am. Chem. Soc. 2005, 127, 9808–9817. [Google Scholar] [CrossRef]
- Kartamyshev, A.I.; Vo, D.D.; Lipnitskii, A.G. The interaction between light impurities and vacancies in titanium and aluminum metals: A DFT study. St. Petersburg Polytech. Univ. J. Phys. Math. 2016, 2, 96–102. [Google Scholar] [CrossRef]
- Fukai, Y. Superabundant Vacancies Formed in Metal Hydrogen Alloys. Phys. Scr. 2003, T103, 11. [Google Scholar] [CrossRef]
- Buckley, C.E.; Birnbaum, H.K.; Lin, J.S.; Spooner, S.; Bellmann, D.; Staron, P.; Udovic, T.J.; Hollar, E. Characterization of H defects in the aluminum–hydrogen system using small-angle scattering techniques. J. Appl. Crystallogr. Int. Union Crystallogr. 2001, 34, 119–129. [Google Scholar]
- Takai, K. Lattice defects dominating hydrogen-related failure of metals. Acta Mater. 2008, 56, 5158–5167. [Google Scholar] [CrossRef]
- Doshida, T.; Nakamura, M.; Saito, H.; Sawada, T.; Takai, K. Hydrogen-enhanced lattice defect formation and hydrogen embrittlement of cyclically prestressed tempered martensitic steel. Acta Mater. 2013, 61, 7755–7766. [Google Scholar] [CrossRef]
- Hatano, M.; Hatano, M.; Fujinami, M.; Arai, K.; Nagumo, M. Hydrogen embrittlement of austenitic stainless steels revealed by deformation microstructures and strain-induced creation of vacancies. Acta Mater. 2014, 67, 342–353. [Google Scholar] [CrossRef]
- Momida, H.; Asari, Y.; Nakamura, Y.; Tateyama, Y.; Ohno, T. Hydrogen-enhanced vacancy embrittlement of grain boundaries in iron. Phys. Rev. B Am. Phys. Soc. 2013, 88, 144107. [Google Scholar] [CrossRef]
- Neeraj, T.; Srinivasan, R.; Li, J. Hydrogen embrittlement of ferritic steels: Observations on deformation microstructure, nanoscale dimples and failure by nanovoiding. Acta Mater. 2012, 60, 5160–5171. [Google Scholar] [CrossRef]
- Nagumo, M. Hydrogen related failure of steels—A new aspect. Mater. Sci. Technol. 2004, 20, 940–950. [Google Scholar] [CrossRef]
- Song, J.; Curtin, W.A. A nanoscale mechanism of hydrogen embrittlement in metals. Acta Mater. 2011, 59, 1557–1569. [Google Scholar] [CrossRef]
- Bystrzejewski, M.; Łabedź, O.; Kaszuwara, W.; Huczko, A.; Lange, H. Controlling the diameter and magnetic properties of carbon-encapsulated iron nanoparticles produced by carbon arc discharge. Powder Technol. 2013, 246, 7–15. [Google Scholar] [CrossRef]
- El-khatib, A.M.; Bondouk, I.I.; Omar, K.M.; Hamdy, A.; El-khatib, M. Impact of changing electrodes dimensions and different ACs on the characteristics of nano composites NZnO/MWCNTs prepared by the arc discharge method. Surf. Interfaces 2022, 29, 101736. [Google Scholar] [CrossRef]
- Todera¸s, M.; Aljohani, F.S.; El-Khatib, M. Impact of electrical current on cluster nucleation production: Phase, structure, and nanosize for Al2O3. J. Cryst. Growth 2024, 625, 127437. [Google Scholar] [CrossRef]
- Yin, S.; Cheng, G.; Chang, T.-H.; Richter, G.; Zhu, Y.; Gao, H. Hydrogen embrittlement in metallic nanowires. Nat. Commun. 2019, 10, 2004. [Google Scholar] [CrossRef]
- Li, J.; Wu, Z.; Wang, F.; Zhang, L.; Zhou, C.; Lu, C.; Teng, L.; Lin, Q. Study on the Hydrogen Embrittlement of Nanograined Materials with Different Grain Sizes by Atomistic Simulation. Materials 2022, 15, 4589. [Google Scholar] [CrossRef]
- Beachem, C.D. A New Model for Hydrogen-Assisted Cracking (Hydrogen “Embrittlement”). Metall. Trans. 1972, 3, 441–455. [Google Scholar] [CrossRef]
- Hirth, J.P. Effects of hydrogen on the properties of iron and steel. Metall. Trans. A 1980, 11, 861–890. [Google Scholar] [CrossRef]
- Sanchez, J.; Lee, S.F.; Martin-Rengel, M.A.; Fullea, J.; Andrade, C.; Ruiz-Hervías, J. Measurement of hydrogen and embrittlement of high strength steels. Eng. Fail. Anal. 2016, 59, 467–477. [Google Scholar] [CrossRef]
- Lynch, S.P. Hydrogen embrittlement (HE) phenomena and mechanisms. In Stress Corrosion Cracking: Theory and Practice; Woodhead Publishing: Shaston, UK, 2011. [Google Scholar] [CrossRef]
- Ogorodnikova, S.K. (Ed.) Handbook of the Petrochemist; 1978; Volume T.2/L.1. Chemistry; 592p, Available online: https://www.geokniga.org/books/12449 (accessed on 2 June 2024).
- Prisevok, A.F. The mechanism of hydrogen wear of metals and alloys. Bull. BSPA 2002, 3, 23–25. [Google Scholar]
- Moroz, L.S.; Mingin, T.E. Metal Science and Heat Treatment of Metals; Springer: Berlin/Heidelberg, Germany, 1962; pp. 2–6. [Google Scholar]
- Cotterill, P. The hydrogen embrittlement of metals. Prog. Mater. Sci. 1961, 9, 205–301. [Google Scholar] [CrossRef]
- Hinotani, S.; Ohmori, Y.; Terasaki, F. Effect of nickel on hydride formation and hydrogen embrittlement in NiCrFe alloys. Mater. Sci. Eng. 1985, 74, 119–131. [Google Scholar] [CrossRef]
- Boniszewski, T.; Smith, G.C. The influence of hydrogen on the plastic deformation ductility, and fracture of nickel in tension. Acta Metall. 1963, 11, 165–178. [Google Scholar] [CrossRef]
- Nechay, E.P.; Popov, K.V. Study of Steels and Alloys; Nauka: Moscow, Russia, 1964; pp. 227–229. [Google Scholar]
- Zhang, L.; Imade, M.; An, B.; Wen, M.; Iijima, T.; Fukuyama, S.; Yokogawa, K. Internal reversible hydrogen embrittlement of austenitic stainless steels based on type 316 at low temperatures. ISIJ Int. 2012, 52, 240–246. [Google Scholar] [CrossRef]
- Nechay, E.P. Influence of Hydrogen on the Service Properties of Steel; Irkutsk: Irkutsk, Russia, 1963; pp. 131–139. [Google Scholar]
- Kim, T.K.; Baek, J.H.; Choi, B.S.; Jeong, Y.H.; Lee, D.J.; Chang, M.H. Characteristics of hydriding and hydrogen embrittlement of the Ti-Al-Zr alloy. Ann. Nucl. Energy 2002, 29, 2041–2053. [Google Scholar] [CrossRef]
- Williams, D.N.; Jaffee, R.I. Relationships between impact and low-strain-rate hydrogen embrittlement of titanium alloys. J. Less-Common. Met. 1960, 2, 42–48. [Google Scholar] [CrossRef]
- Livanov, V.A.; Bukhanova, A.A.; Kolachev, B.A.; Guselnikov, N.Y. Titanium and Its Alloys; Academy of Sciences of the USSR: Moscow, Russia, 1963; pp. 307–316. [Google Scholar]
- Arkhangelskaya, E.A.; Lepov, V.V.; Larionov, V.P. Coherent model of delayed destruction of a damaged environment. Phys. Mesomech. 2001, 4, 81–87. [Google Scholar]
- Baranov, V.P. Kinetics of delayed fracture of high-strength steels in inactive and hydrogen-containing media. Izvestia Tulsk. State Univ. 2004, 4, 3–19. [Google Scholar]
- Shvachko, V.I. Model of hydrogen embrittlement of structural steels. Met. New Technol. 2001, 23, 1501–1512. [Google Scholar]
- Cavaliere, P.; Perrone, A.; Marsano, D.; Marzanese, A.; Sadeghi, B. Modelling of the hydrogen embrittlement in austenitic stainless steels. Materialia 2023, 30, 101855. [Google Scholar] [CrossRef]
- Nechaev, Y.S. The role of hydride-like segregations at dislocations and grain boundaries in the delayed fracture of steels. In Proceedings of the 3rd International Conference “Hydrogen Treatment of Materials-2001”, Donetsk, Ukraine, 14–18 May 2001. [Google Scholar]
- Shashkova, L.V. Synergetic law of damage to metals and alloys by hydrogen. MNIZH 2013, 14, 106–113. Available online: https://cyberleninka.ru/article/n/sinergeticheskiy-zakon-povrezhdaemosti-metallov-i-splavov-vodorodom (accessed on 30 August 2023).
- Colombo, C.; Fumagalli, G.; Bolzoni, F.; Gobbi, G.; Vergani, L. Fatigue behavior of hydrogen pre-charged low alloy Cr-Mo steel. Int. J. Fatigue 2016, 83, 2–9. [Google Scholar] [CrossRef]
- Şeşen, B.M.; Mansoor, M.; Örnek, C. Elucidating the dynamics of hydrogen embrittlement in duplex stainless steel. Corros. Sci. 2023, 225, 111549. [Google Scholar] [CrossRef]
- Zappfe, S.A.; Sims, C. Hydrogen Embrittlement, Internal Stress and Defects in Steel. Trans. Am. Inst. Min (Metall) Engrs. 1941, 145, 225–259. [Google Scholar]
- Pfeil, L.B. The Effect of Occluded Hydrogen on the Tensile Strength of Iron. Proc. R. Soc. Lond. A 1926, 112, 182–195. [Google Scholar]
- Oriani, R.A. Hydrogen Embrittlement of Steels. Annu. Rev. Mater. Sci. 1978, 8, 327–357. [Google Scholar] [CrossRef]
- Troiano, A.R. The role of hydrogen and other interstitials in the mechanical behavior of metals. Trans. ASM 1960, 52, 54–80. [Google Scholar] [CrossRef]
- Álvarez, G.; Zafra, A.; Belzunce, F.J.; Rodríguez, C. Hydrogen embrittlement testing procedure for the analysis of structural steels with Small Punch Tests using notched specimens. Eng. Fract. Mech. 2021, 253, 1–14. [Google Scholar] [CrossRef]
- Galaktionova, N.A. Hydrogen in Metals; Metallurgy: Moscow, Russia, 1967; 304p. [Google Scholar]
- Dobatkin, V.N. Gases and Oxides in Wrought Aluminum Alloys; Metallurgy: Moscow, Russia, 1976; 264p. [Google Scholar]
- Lunarska, E. Effect of precipitations on hydrogen transport and hydrogen embrittlement of aluminum alloys. Mater. Sci. 2004, 40, 399–407. [Google Scholar] [CrossRef]
- Kannan, M.; Raja, V.S. Hydrogen embryo susceptibility of over aged 7010 Al-alloy. J. Mater. Sci. 2006, 41, 5495–5499. [Google Scholar]
- Kim, S.J. Electrochemical characteristics of Al-Mg alloy in seawater for leisure ship: Stress corrosion cracking and hydrogen embrittlement. Korean J. Chem. Eng. 2009, 26, 250–257. [Google Scholar] [CrossRef]
- Kumar, S.; Namboodhiri, T. Precipitation hardening and hydrogen embrittlement of aluminum alloy AA7020. Bull. Mater. Sci. 2011, 34, 311–321. [Google Scholar] [CrossRef]
- Nykyforchyn, H.M.; Ostash, O.P.; Tsyrul’nyk, O.T.; Andreiko, I.M. Electrochemical evaluation of the in-service degradation of an aircraft aluminum alloy. Mater. Sci. 2008, 44, 254–259. [Google Scholar] [CrossRef]
- Han, Y.; Xue, S.; Fu, R.; Lin, L.; Lin, Z.; Pei, Y.; Sun, H. Influence of hydrogen embrittlement on impact property and microstructural characteristics in aluminum alloy weld. Vacuum 2020, 172, 109073. [Google Scholar] [CrossRef]
- Nosov, V.K. Hydrogen Plasticization during Hot Deformation of Titanium Alloys; Metallurgy: Moscow, Russia, 1986; 120p. [Google Scholar]
- Williams, D.N. The hydrogen embrittlement of titanium alloys. J. Inst. Met. 1962, 91, 147–152. [Google Scholar]
- Livanov, V.A.; Bukhanova, A.A.; Kolachev, B.A. Scientific Reports of Higher Education; Metallurgy: Moscow, Russia, 1958; pp. 248–254. [Google Scholar]
- Tal-Gutelmacher, E.; Eliezer, D. Hydrogen-assisted degradation of titanium based alloys. Mater. Trans. 2004, 45, 1594–1600. [Google Scholar] [CrossRef]
- Lenning, G.A.; Spretnak, J.W.; Jaffee, R.I. Effect of Hydrogen on Alpha Titanium Alloy. J. Met. 1956, 8, 1235–1240. [Google Scholar] [CrossRef]
- Burte, H.M.; Erbin, E.F.; Hahn, G.T.; Seeger, J.W.; Kotfila, R.J.; Wruck, D.A. Hydrogen Embrittlement of Titanium Alloys. Met. Prog. 1955, 67, 115. [Google Scholar]
- Madina, V.; Azkarate, I. Compatibility of materials with hydrogen. Particular case: Hydrogen embrittlement of titanium alloys. Int. J. Hydrogen Energy 2009, 34, 5976–5980. [Google Scholar] [CrossRef]
- Lee, H.-H.; Lee, K.-Y.; Lee, J.-Y. The Ti-based metal hydride electrode for Ni-MH rechargeable batteries. J. Alloys Compd. 1996, 239, 63–70. [Google Scholar] [CrossRef]
- Luan, B.; Liu, H.K.; Cui, N.; Liu, H.K.; Dou, S.X. Effect of cobalt addition on the performance of titanium-based hydrogenstorage electrodes. J. Power Sources 1995, 55, 197–203. [Google Scholar] [CrossRef]
- Huang, T.; Wu, Z.; Yu, X.; Chen, J.; Xia, B.; Huang, T.; Xu, N. Hydrogen absorption–desorption behavior of zirconium-substituting Ti–Mn based hydrogen storage alloys. Intermetallics 2004, 12, 91–96. [Google Scholar]
- Sinha, V.K.; Yu, G.Y.; Wallace, W.E. Hydrogen storage in some ternary and quaternary zirconium-based alloys with the C14 structure. J. Less-Common. Met. 1985, 106, 67–77. [Google Scholar] [CrossRef]
- Geld, P.V.; Ryabov, R.A. Hydrogen in Metals and Alloys; Metallurgy: Moscow, Russia, 1974; 271p. [Google Scholar]
- Parfenov, B.G.; Gerasimov, V.V.; Venediktov, G.I. Corrosion of Zirconium and Its Alloys; Atomizdat: Moscow, Russia, 1967; 257p. [Google Scholar]
- Vlasov, N.M.; Fedik, I.I. Hydrogen embrittlement of zirconium alloys. Met. Sci. Heat Treat. 2003, 45, 328–331. [Google Scholar] [CrossRef]
- Kalish, H.S. The Preparation of Zirconium Powder. Professional Degree Theses, Missouri School of Mines and Metallurgy, Rolla, MO, USA, 1953. Available online: https://scholarsmine.mst.edu/professional_theses/180 (accessed on 2 June 2024).
- Borkov, N.V. Sat. “Metallurgy and Metallurgy of Pure Metals”; Gosatomizdat: Moscow, Russia, 1961; pp. 46–63. [Google Scholar]
- Geld, P.V.; Ryabov, R.A.; Kodes, E.S. Hydrogen and Imperfections of Metal Structure; Metallurgy: Moscow, Russia, 1979; p. 221. [Google Scholar]
- Glazunov, G.P.; Azhazha, V.M.; Andreev, A.A.; Baron, D.I.; Volkov, E.D.; Konotopsky, A.L.; Neklyudov, I.M.; Svinarenko, A.P. Kinetics of hydrogen penetration in two-layer diffusion systems based on zirconium and palladium. In Questions of Atomic Science and Technology; Series: Physics of Radiation Damage and Radiation Materials Science; Ural-Press: Moscow, Russia, 2007; pp. 13–18. [Google Scholar]
- Ivanova, S.V. Study of the processes of absorption, diffusion and solubility of hydrogen in zirconium products of fuel as-semblies of VVER and RBMK reactors. Mater. Sci. 2002, 7, 42–49. [Google Scholar]
- Kalin, B.A. (Ed.) Physical Material Science. T. 6. Konstruktsionnye Materialy Yadernoy Tekhniki [Physical Material Science, 6. Construction Materials of Nuclear Technique]; MIFI Publ.: Moscow, Russia, 2012. (In Russian) [Google Scholar]
- Deng, Y.; Liao, H.; He, Y.; Yin, Y.; Pellegrini, M.; Su, G.; Okamoto, K.; Wu, Y. Investigation on hydrogen embrittlement and failure characteristics of Zr-4 cladding based on the GTN method. Nucl. Mater. Energy 2023, 36, 101463. [Google Scholar] [CrossRef]
- Boniszewski, T.; Smith, G.H. A note on nickel hydride. J. Phys. Chem. Solids 1961, 21, 115–118. [Google Scholar] [CrossRef]
- Glikman, L.A.; Kolgatin, N.N.; Teodorovich, V.P.; Deryabina, V.I. Destruction of Steels under the Influence of Hydrogen at High Temperatures and Pressures; Folium: Moscow, Russia, 1959; pp. 16–21. [Google Scholar]
- Mihelich, J.L.; Troiano, A.R. Delayed Failure in a Hydrogenated Face-centred Cubic Alloy of Nickel. Nature 1963, 197, 996–997. [Google Scholar] [CrossRef]
- Blanchard, P.; Troiano, A.R. Embrittlement of metals by hydrogen. influence of the crystallographic and electronic structure. Mem. Sci. Rev. Met. 1960, 57, 409–422. [Google Scholar]
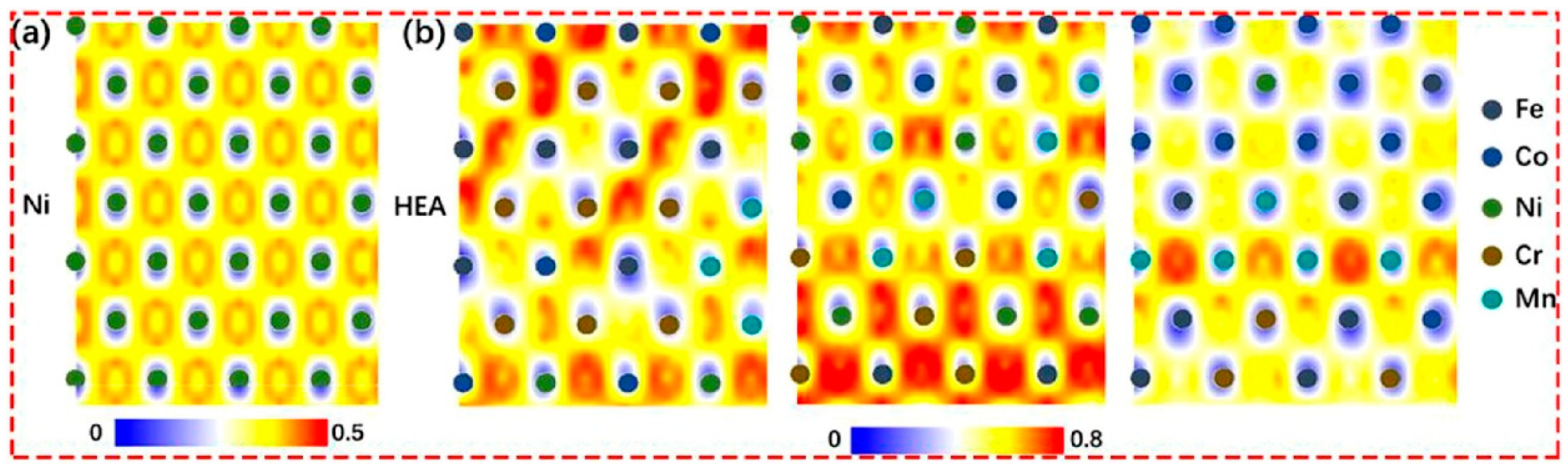
- Zhou, X.Y.; Zhu, J.H.; Wu, Y.; Yang, X.S.; Lookman, T.; Wu, H.H. Machine Learning Assisted Design of FeCoNiCrMn High-Entropy Alloys with Ultra-Low Hydrogen Diffusion Coefficients. Acta Mater. 2022, 224, 117535. [Google Scholar] [CrossRef]
- Gypen, L.A.; Brabers, M.; Deruyttere, A. Corrosion resistance of tantalum base alloys. Elimination of hydrogen embrittlement in tantalum by substitutional alloying. Mater. Corros. 1984, 35, 37–46. [Google Scholar] [CrossRef]
- Galaktionova, N.A. Hydrogen in Metals; Metallurgizdat: Moscow, Russia, 1959. [Google Scholar]
- Bishop, C.R.; Stern, M. Preventing Hydrogen Embrittlement of Tantalum. M. J. Metals 1961, 13, 144–145. [Google Scholar] [CrossRef]
- Ensinger, W.; Flege, S.; Baba, K. Platinum implantation into tantalum for protection against hydrogen embrittlement during corrosion. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2012, 272, 441–445. [Google Scholar] [CrossRef]
- Rostoker, W.; Kolodney, M. The Metallurgy of Vanadium. J. Electrochem. Soc. 1958, 105, 197C. [Google Scholar] [CrossRef]
- Ko, W.S.; Jeon, J.B.; Shim, J.H.; Lee, B.J. Origin of Hydrogen Embrittlement in Vanadium-Based Hydrogen Separation Membranes. Int. J. Hydrogen Energy 2012, 37, 13583–13593. [Google Scholar] [CrossRef]
- Nishimura, C.; Komaki, M.; Amano, M. Hydrogen Permeation Characteristics of Vanadium-Nickel Alloys. Mater. Trans. JIM 1991, 32, 501–507. [Google Scholar] [CrossRef]
- Komjathy, S.J. The niobium-hydrogen system. Less-Common. Met. 1960, 2, 466–480. [Google Scholar] [CrossRef]
- Peterson, D.T.; Hull, A.B.; Loomis, B.A. Hydrogen embrittlement considerations in niobium-base alloys for application in the ITER divertor. J. Nucl. Mater. 1992, 191–194, 430–432. [Google Scholar] [CrossRef]
- Watanabe, N.; Yukawa, H.; Nambu, T.; Matsumoto, Y.; Zhang, G.; Morinaga, M. Alloying effects of Ru and W on the resistance to hydrogen embrittlement and hydrogen permeability of niobium. J. Alloys Compd. 2009, 477, 851–854. [Google Scholar] [CrossRef]
- Veleckis, E.; Edwards, R.K. Thermodynamic properties in the systems vanadium-hydrogen, niobium-hydrogen, and tantalum-hydrogen. J. Phys. Chem. 1969, 73, 683–692. [Google Scholar] [CrossRef]
- Murphy, A.D. (Ed.) Melting and Casting of Non-Ferrous Metals and Alloys; Metallurgizdat: Moscow, Russia, 1959. [Google Scholar]
- Lee, P.D.; Hunt, J.D. Hydrogen porosity in directionally solidified aluminium-copper alloys: A mathematical model. Acta Mater. 2001, 49, 1383–1398. [Google Scholar] [CrossRef]
- Yamabe, J.; Takagoshi, D.; Matsunaga, H.; Matsuoka, S.; Ishikawa, T.; Ichigi, T. High-Strength Copper-Based Alloy with Excellent Resistance to Hydrogen Embrittlement. Int. J. Hydrogen Energy 2016, 41, 15089–15094. [Google Scholar] [CrossRef]
- Mulford, R.N.; Ellinger, F.N.; Zacharaisen, W.H. A New Form of Uranium Hydride. J. Am. Chem. Soc. 1954, 76, 297–300. [Google Scholar] [CrossRef]
- Beevers, C.J.; Newman, G.T. Hydrogen Embrittlement in Uranium. J. Nucl. Mater. 1967, 23, 10–18. [Google Scholar] [CrossRef]
- Cotterill, R.; Goosey, R.E.; Martin, A.J. Metallurgy Beryllium London. In Proceedings of the An International Conference Organized by the Institute of Metals and Held at the Royal Commonwealth Society, London, UK, 16–18 October 1961. [Google Scholar]
- Blanchard, R.; Bochirol, L. In Proceedings of the 5th Collogue Metallurg. Gaz dans Metaux, Saclay, France, 26–27 June 1961; pp. 157–162. [Google Scholar]
- Bobby Kannan, M.; Dietzel, W.; Blawert, C.; Atrens, A.; Lyon, P. Stress corrosion cracking of rare-earth containing magnesium alloys ZE41, QE22 and Elektron 21 (EV31A) compared with AZ80. Mater. Sci. Eng. A 2008, 480, 529. [Google Scholar] [CrossRef]
- Fairman, L.; Bray, H.J. Transgranular see in Mg-Al alloys. Corros. Sci. 1971, 11, 533. [Google Scholar] [CrossRef]
- Kappes, M.; Iannuzzi, M.; Carranza, R.M. Hydrogen Embrittlement of Magnesium and Magnesium Alloys: A Review. J. Electrochem. Soc. 2013, 160, C168–C178. [Google Scholar] [CrossRef]
- Hondros, E.D.; Moore, A.J.W. The influence of an electric potential gradient on the thermal etching of silver. Acta Metall. 1960, 8, 751–757. [Google Scholar] [CrossRef]
- Martin, D.L.; Parker, E.R. Papers—Miscellaneous Heavy Metals and Alloys—Embrittlement of Silver by Oxygen and Hydrogen. Trans. Am. Inst. Min. Metall. Engrs. 1943, 152, 269. [Google Scholar]
- Dadfarnia, M.; Sofronis, P.; Neeraj, T. Hydrogen Interaction with Multiple Traps: Can It Be Used to Mitigate Embrittlement? Int. J. Hydrogen Energy 2011, 36, 10141–10148. [Google Scholar] [CrossRef]
- Gotalsky, N.; Yu, N. The problem of welding hardening steels and known methods for solving it. Automat. Svaka. 1994, 36–40. [Google Scholar]
- Mishin, V.M.; Filippov, G.A. Physics of Delayed Fracture of Steels; Poligrafprom: Krasnodar, Russia, 2013; 455p. [Google Scholar]
- Sofronis, P.; McMeeking, P.M. Numerical analysis of hydrogen transport near a blunting cracks tip. J. Mech. Phys. Solids 1989, 37, 317–350. [Google Scholar] [CrossRef]
- Lufrano, J.; Sofronis, P. Enhanced hydrogen concentrations ahead of rounded notches and cracks competition between plastic strain and hydrostatic strees. Acta Mater. 1998, 46, 1519–1526. [Google Scholar] [CrossRef]
- Tetelman, A. Destruction of Solids; Metallurgy: Moscow, Russia, 1967; pp. 261–301, 463–499. [Google Scholar]
- Mikhailov, V.E.; Lepov, V.V.; Alymov, V.T.; Larionov, V.P. Delayed Destruction of Structures under the Action of Hydrogen; Publishing House of the Siberian Branch of the Russian Academy of Sciences: Novosibirsk, Russia, 1999; 242p. [Google Scholar]
- Arkhangelskaya, E.A.; Lepov, V.V.; Larionov, V.P. A Connected Model of Delayed Destruction of a Damaged Medium. Fiz. Mesomekh. 2001. Available online: https://cyberleninka.ru/article/n/svyaznaya-model-zamedlennogo-razrusheniya-povrezhdaemoy-sredy (accessed on 30 August 2023).
- Kholtobina, A.S.; Pippan, R.; Romaner, L.; Scheiber, D.; Ecker, W.; Razumovskiy, V.I. Hydrogen Trapping in bcc Iron. Materials 2020, 13, 2288. [Google Scholar] [CrossRef]
- Suvage, W.F.; Nippes, E.F.; Tokwnga, Y. Hydrogen induced cracking in HY-130 steel weldments. Weld. J. 1978, 118–126. [Google Scholar]
- Goldstein, R.V.; Entov, V.M.; Pavlovsky, B.R. Dokl; Academy of Sciences of the USSR: Moscow, Russia, 1977; Volume 237, pp. 828–831. [Google Scholar]
- Panasyuk, V.V.; Andreikiv, A.E.; Kharin, V.S. Model of crack growth in deformed metals under the influence of hydrogen. FHMM 1987, 3–17. [Google Scholar]
- Wasim, M.; Djukic, M.B.; Ngo, T.D. Influence of hydrogen-enhanced plasticity and decohesion mechanisms of hydrogen embrittlement on the fracture resistance of steel. Eng. Fail. Anal. 2021, 123, 105312. [Google Scholar] [CrossRef]
- Wise, M.L.H.; Farr, J.P.G.; Harris, I.R.; Hirst, J.R. Hydrogen dans métaux. Congr. Intern. 1972, 2, 541. [Google Scholar]
- Campari, A.; Ustolin, F.; Alvaro, A.; Paltrinieri, N. A review on hydrogen embrittlement and risk-based inspection of hydrogen technologies. Int. J. Hydrogen Energy 2023, 48, 35316–35346. [Google Scholar] [CrossRef]
- Arutyunyan, R.A. The Problem of Embrittlement in the Mechanics of Materials. Bull. St. Petersburg Univ. Math. Mech. Astron. 2009. Available online: https://cyberleninka.ru/article/n/problema-ohrupchivaniya-v-mehanike-materialov (accessed on 30 May 2024).
- Krening, X.-M.; Krening, X.-M.; Tyurin, Y.I.; Baumbach, H. Non-Equilibrium Metal-Hydrogen Systems. Titanium, Stainless Steel; Publishing House of Tomsk University: Tomsk, Russia, 2002; 350p. [Google Scholar]
- Chernov, I.P.; Cherdantsev, Y.P.; Tyurin, Y.I. Methods for Studying Metal-Hydrogen Systems; Ener-goatomizdat: Moscow, Russia, 2004; 270p. [Google Scholar]
- Chernov, I.P.; Krening, M.; Cherdantsev, Y.P.; Surkov, A.S.; Tyurin, Y.I.; Nikitenkov, N.N.; Lider, A.M. Non-destructive testing of hydrogen-helium embrittlement of structural materials. Bull. Tomsk. Polytech. Univ. 2008, 321, 14–19. [Google Scholar]
- Chernov, I.P.; Cherdantsev, Y.P.; Mamontov, A.P.; Panin, A.V.; Nikitenkov, N.N.; Lider, A.M.; Garanin, G.V.; Pushilina, N.S.; Ivanova, S.V. Non-Destructive Methods of Control of Hydrogen Embrittlement of Structural Materials Alternative Energy and Ecology, 2009; No. 2, pp. 15–21. Available online: https://cyberleninka.ru/article/n/nerazrushayuschie-metody-kontrolya-vodorodnogo-ohrupchivaniya-konstruktsionnyh-materialov (accessed on 2 June 2024).
- Nelson, G.G. Hydrogen embrittlement. In Embrittlement of Structural Steels and Alloys; Metallurgy: Moscow, Russia, 1988; pp. 256–333. [Google Scholar]
- Tsvetkov, A.S. Hydrogen in Metals, Review Lecture. Corrosion in the Oil and Gas Industry Scientific and Technical Conference 09/06/2022 Samara. Available online: https://corr-conf.ru/articles/2022 (accessed on 2 June 2024).
- Ivanova, S.V. Diffusion of hydrogen absorbed during manufacture and operation in zirconium products of active zones of thermal neutron reactors. In Proceedings of the 5th International Conference “Hydrogen Economics and Hydrogen Processing of Materials”, Donetsk, Ukraine, 21–25 May 2007; pp. 796–800. [Google Scholar]
- Merson, E.; Myagkikh, P.; Poluyanov, V.; Dorogov, M.; Merson, D.; Vinogradov, A. The Fundamental Difference between Cleavage and Hydrogen-Assisted Quasi-Cleavage in Ferritic Materials Revealed by Multiscale Quantitative Fractographic and Side Surface Characterization. Mater. Sci. Eng. A 2021, 824, 141826. [Google Scholar] [CrossRef]
- Schrader, A.V. Hydrogen in Metals. Chemistry; Knowledge: Moscow, Russia, 1979; 64p. [Google Scholar]
- Fassina, P.; Brunella, M.F.; Lazzari, L.; Re, G.; Vergani, L.; Sciuccati, A. Effect of Hydrogen and Low Temperature on Fa-tigue Crack Growth of Pipeline Steels. Eng. Fract. Mech. 2013, 103, 10–25. [Google Scholar] [CrossRef]
- Kondrashova, O.G.; Nazarova, M.N. Causal Analysis of Accidents in Vertical Steel Tanks. Oil and Gas Business. 2004. Available online: http://ogbus.ru/files/ogbus/authors/Kondrashova (accessed on 2 June 2024).
- Sinyutina, S.E.; Vigdorovich, V.I. Some aspects of metal hydrogenation. Bull. Russ. Univ. Math. 2002, 7, 129–140. Available online: https://cyberleninka.ru/article/n/nekotorye-aspekty-navodorazhivaniya-metallov (accessed on 30 May 2024).
- Zhao, C.; Song, X.; Yang, Y.; Zhang, B. Hydrogen absorption cracking of zirconium alloy in the application of nuclear industry. Int. J. Hydrogen Energy 2013, 38, 10903–10911. [Google Scholar] [CrossRef]
- Lider, A.M.; Pushilina, N.S.; Kudiyarov, V.N.; Leader, A.M.; Teresov, A.D. Influence of surface structure on hydrogen interaction with Zr–1Nb alloy. J. Alloys Compd. 2015, 645 (Suppl. S1), 476–479. [Google Scholar]
- Matysina, Z.A. Hydrogen and Solid-Phase Transformations in Metals, Alloys and Fullerites: Monograph; Science and Education: Dnepropetrovsk, Ukraine, 2002; 420p. [Google Scholar]
- Pushilina, N.S.; Lider, A.M.; Kudiyarov, V.N.; Chernov, I.P.; Ivanova, S. Hydrogen effect on zirconium alloy surface treated by pulsed electron beam. J. Nucl. Mater. 2015, 456, 311–315. [Google Scholar] [CrossRef]
- Parkins, R.N. Development of Strain-Rate Testing and Its Implications. In ASTM Special Technical Publication; ASTM: West Conshohocken, PA, USA, 1979. [Google Scholar] [CrossRef]
- Wang, M.; Akiyama, E.; Tsuzaki, K. Effect of hydrogen on the fracture behavior of high strength steel during slow strain rate test. Corros. Sci. 2007, 49, 4081–4097. [Google Scholar] [CrossRef]
- Pérez Escobar, D.; Verbeken, K.; Duprez, L.; Verhaege, M. Evaluation of hydrogen trapping in high strength steels by thermal desorption spectroscopy. Mater. Sci. Eng. A 2012, 551, 50–58. [Google Scholar] [CrossRef]
- Chitani, K.; Kanno, M.; Kuramoto, S. Recent development in hydrogen microprint technique and its application to hydrogen embrittlement. ISIJ Int. 2003, 43, 496–504. [Google Scholar] [CrossRef]
- Matsuda, S.; Ichitani, K.; Kanno, M. Visualization of hydrogen diffusion path by a high sensitivity hydrogen microprint technique. Environ.-Induc. Crack. Mater. 2008, 1, 239–248. [Google Scholar] [CrossRef]
- Nakatani, M.; Fujihara, H.; Sakihara, M.; Minoshima, K. Influence of irreversible hydrogen and stress cycle frequency on the fatigue crack growth property in high-strength steel and hydrogen visualization. Procedia Eng. 2011, 10, 2381–2386. [Google Scholar] [CrossRef]
- Rudomilova, D.; Prošek, T.; Luckeneder, G. Techniques for investigation of hydrogen embrittlement of advanced high strength steels. Corros. Rev. 2018, 36, 413–434. [Google Scholar] [CrossRef]
- Tyurin, Y.I.; Baumbach, H.; Crening, X.-M. Radiation-Stimulated Release of Hydrogen from Metals; Publishing House of the Tomsk Polytechnic University: Tomsk, Russia, 2000; 264p. [Google Scholar]
- Garanin, G.V.; Larionov, V.V.; Lider, A.M. Laboratory installation for measuring the propagation velocity of ultrasonic waves in hydrogenated metals. Bull. Sci. Sib. 2012, 4, 55–60. Available online: http://sjs.tpu.ru/journal/article/view/353 (accessed on 2 June 2024).
- Lider, A.M. Dynamics of hydrogen accumulation and defects in titanium and stainless steel during electrolytic saturation with hydrogen. Phys.-Math. Sci. 2002, 149, 230. [Google Scholar]
- Lider, A.M.; Larionov, V.V.; Garanin, G.V.; Krening, H.V. Method of ultrasonic determination of hydrogen in materials and products based on titanium. J. Tech. Phys. 2013, 83, 157–158. [Google Scholar]
- Lider, A.M.; Chernov, I.P.; Cherdantsev, Y.P. Defects in titanium initiated by hydrogen. Phys. Mesome-Chanics 2000, 3, 97–100. [Google Scholar]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobola, D.; Dallaev, R. Exploring Hydrogen Embrittlement: Mechanisms, Consequences, and Advances in Metal Science. Energies 2024, 17, 2972. https://doi.org/10.3390/en17122972
Sobola D, Dallaev R. Exploring Hydrogen Embrittlement: Mechanisms, Consequences, and Advances in Metal Science. Energies. 2024; 17(12):2972. https://doi.org/10.3390/en17122972
Chicago/Turabian StyleSobola, Dinara, and Rashid Dallaev. 2024. "Exploring Hydrogen Embrittlement: Mechanisms, Consequences, and Advances in Metal Science" Energies 17, no. 12: 2972. https://doi.org/10.3390/en17122972




