Characterization of the TCO Layer on a Glass Surface for PV IInd and IIIrd Generation Applications
Abstract
:1. Introduction
2. Materials and Methods
Samples Preparation
3. Results and Discussion
4. Conclusions and Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ciuła, J.; Generowicz, A.; Gronba-Chyła, A.; Wiewiórska, I.; Kwaśnicki, P.; Cygnar, M. Analysis of the Efficiency of Landfill Gas Treatment for Power Generation in a Cogeneration System in Terms of the European Green Deal. Sustainability 2024, 16, 1479. [Google Scholar] [CrossRef]
- Sciuto, G.L.; Capizzi, G.; Shikler, R.; Napoli, C. Organic solar cells defects classification by using a new feature extraction algorithm and an EBNN with an innovative pruning algorithm. Int. J. Intell. Syst. 2021, 36, 2443–2464. [Google Scholar] [CrossRef]
- Sciuto, G.L.; Coco, S. A 3D finite element model of degradation phenomena in organic solar devices affected by oxidation. Int. J. Energy Environ. Eng. 2020, 11, 431–437. [Google Scholar] [CrossRef]
- Barbusiński, K.; Kwaśnicki, P.; Gronba-Chyła, A.; Generowicz, A.; Ciuła, J.; Szeląg, B.; Fatone, F.; Makara, A.; Kowalski, Z. Influence of Environmental Conditions on the Electrical Parameters of Side Connectors in Glass–Glass Photovoltaic Modules. Energies 2024, 17, 680. [Google Scholar] [CrossRef]
- Sciuto, G.L.; Napoli, C.; Capizzi, G.; Shikler, R. Organic solar cells defects detection by means of an elliptical basis neural network and a new feature extraction technique. Optik 2019, 194, 163038. [Google Scholar] [CrossRef]
- Kwaśnicki, P.; Gronba-Chyła, A.; Generowicz, A.; Ciuła, J.; Wiewiórska, I.; Gaska, K. Alternative method of making electrical connections in the 1st and 3rd generation modules as an effective way to improve module efficiency and reduce production costs. Arch. Thermodyn. 2023, 44, 179–200. [Google Scholar] [CrossRef]
- Prete, P.; Lovergine, N. Dilute nitride III-V nanowires for high-efficiency intermediate-band photovoltaic cells: Materials requirements, self-assembly methods and properties. Prog. Cryst. Growth Charact. Mater. 2020, 66, 100510. [Google Scholar] [CrossRef]
- Ziani, N.; Belkaid, M.S. Computer Modelling Zinc Oxide/Silicon Heterojunction Solar Cells. J. Nano Electron. Phys. 2018, 10, 06002. [Google Scholar] [CrossRef]
- Afre, R.; Sharma, N.; Sharon, M.; Sharon, M. Transparent Conducting Oxide Films for Various Applications: A Review. Rev. Adv. Mater. Sci. 2018, 53, 79–89. [Google Scholar] [CrossRef]
- Yu, L.; O’Donnell, B.; Alet, P.-J.; Cabarrocas, P.R. All-in situ fabrication and characterization of silicon nanowires on TCO/glass substrates for photovoltaic application. Sol. Energy Mater. Sol. Cells 2010, 94, 1855–1859. [Google Scholar] [CrossRef]
- Preeti; Kumar, S. Extraction and analysis of TCO coated glass from waste amorphous silicon thin film solar module. Sol. Energy Mater. Sol. Cells 2023, 253, 112227. [Google Scholar] [CrossRef]
- He, T.; Xie, A.; Reneker, D.H.; Zhu, Y. A tough and high-performance transparent electrode from a scalable and transfer-free method, Wu. ACS Nano 2014, 8, 4782–4789. [Google Scholar] [CrossRef]
- Chiu, H.L.; Hong, K.B.; Huang, K.C.; Lu, T.C. Photonic Crystal Surface Emitting Lasers with Naturally, Formed Periodic ITO Structures. ACS Photonics 2019, 6, 684–690. [Google Scholar] [CrossRef]
- Sima, C.; Grigoriu, C.; Antohe, S. Comparison of the dye-sensitized solar cells performances based on transparent conductive ITO and FTO. Thin Solid Film. 2010, 519, 595–597. [Google Scholar] [CrossRef]
- Sang, B.; Kushiya, K.; Okumura, D.; Yamase, O. Performance improvement of CIGS-based modules by depositing high-quality Ga-doped ZnO windows with magnetron sputtering. Sol. Energy Mater. Sol. Cells 2001, 67, 237–245. [Google Scholar] [CrossRef]
- Miccoli, I.; Spampinato, R.; Marzo, F.; Prete, P.; Lovergine, N. DC-magnetron sputtering of ZnO:Al films on (00.1)Al2O3 substrates from slip-casting sintered ceramic targets. Appl. Surf. Sci. 2014, 313, 418–423. [Google Scholar] [CrossRef]
- Li, G.F.; Zhou, J.; Huang, Y.W.; Yang, M.; Feng, J.H.; Zhang, Q. Indium zinc oxide semiconductor thin films deposited by dc magnetron sputtering at room temperature. Vacuum 2010, 85, 22–25. [Google Scholar] [CrossRef]
- Lai, K.; Sun, Y.; Chen, H.; Zhi, L.; Wei, W. Effect of oxygen vacancy and Al-doping on the electronic and optical properties in SnO2. Phys. B Condens. Matter. 2013, 428, 48–52. [Google Scholar] [CrossRef]
- Park, J.H.; Buurma, C.; Sivananthan, S.; Kodama, R.; Gao, W.; Gessert, T.A. The effect of postannealing on Indium Tin Oxide thin films by magnetron sputtering method. Appl. Surf. Sci. 2014, 307, 388–392. [Google Scholar] [CrossRef]
- Chavan, G.T.; Kim, Y.; Khokhar, M.Q.; Hussain, S.Q.; Cho, E.-C.; Yi, J.; Ahmad, Z.; Rosaiah, P.; Jeon, C.-W. A Brief Review of Transparent Conducting Oxides (TCO): The Influence of Different Deposition Techniques on the Efficiency of Solar Cells. Nanomaterials 2023, 13, 1226. [Google Scholar] [CrossRef]
- Hussain, S.Q.; Kim, S.; Ahn, S.; Balaji, N.; Lee, Y.; Lee, J.H.; Yi, J. Influence of high work function ITO:Zr films for the barrier height modification in a-Si:H/c-Si heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2014, 122, 130–135. [Google Scholar] [CrossRef]
- Lee, J.Y.; Connor, S.T.; Cui, Y.; Peumans, P. Solution-processed metal nanowire mesh transparent electrodes. Nano Lett. 2008, 8, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Madaria, A.R.; Kumar, A.; Ishikawa, F.N.; Zhou, C. Uniform, highly conductive and patterned transparent films of a percolating silver nanowire network on rigid and flexible substrates using a dry transfer technique. Nano Res. 2010, 3, 564–573. [Google Scholar] [CrossRef]
- Rathmell, A.R.; Bergin, S.M.; Hua, Y.L.; Li, Z.Y.; Wiley, B.J. The growth mechanism of copper nanowires and their properties in flexible, transparent conducting films. Adv. Mater. 2010, 22, 3558–3563. [Google Scholar] [CrossRef] [PubMed]
- Mohl, M.; Dombovari, A.; Vajtai, R.; Ajayan, P.M.; Kordas, K. Self-assembled large scale metal alloy grid patterns as flexible transparent conductive layers. Sci. Rep. 2015, 5, 13710. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Park, Y.S.; Kim, K.H.; Lee, W.J. Properties of AZO/Ag/AZO multilayer thin film deposited on polyethersulfone substrate. Trans. Electr. Electron. Mater. 2013, 14, 9–11. [Google Scholar] [CrossRef]
- Rosli, N.N.; Ibrahima, M.A.; Ludin, N.A.; Teridi, M.A.M.; Sopian, K. A review of graphene based transparent conducting films for use in solar photovoltaic applications. Renew. Sustain. Energy Rev. 2019, 99, 83–99. [Google Scholar] [CrossRef]
- Sun, J.; Jasieniak, J.J. Semitransparent solar cells. Phys. D Aplik. Fiz. 2017, 50, 093001. [Google Scholar] [CrossRef]
- Jang, J.S.; Kim, J.; Ghorpade, U.; Shin, H.H.; Gang, M.G.; Park, S.D.; Kim, H.J.; Lee, D.S.; Kim, J.H. Comparison study of ZnO-based quaternary TCO materials for photovoltaic application. J. Alloys Compd. 2019, 793, 499–504. [Google Scholar] [CrossRef]
- Kim, I.Y.; Shin, S.W.; Gang, M.G.; Lee, S.H.; Gurav, K.V.; Patil, P.S.; Yun, J.H.; Lee, J.Y.; Kim, J.H. Comparative study of quaternary Mg and Group III element codoped ZnO thin films with transparent conductive characteristics. Thin Solid Film. 2014, 570, 321–325. [Google Scholar] [CrossRef]
- Karakaya, S.; Kaba, L. Wrinkle type nanostructured of Al-Ce codoped ZnO thin films for photocatalytic applications. Surf. Interfaces 2024, 44, 103655. [Google Scholar] [CrossRef]
- Saarenpaa, H.; Niemi, T.; Tukiainen, A.; Lemmetyinen, H.; Tkachenko, N. Aluminum doped zinc oxide films grown by atomic layer deposition for organic photovoltaic devices. Sol. Energy Mater. Sol. Cells 2010, 94, 1379–1383. [Google Scholar] [CrossRef]
- Gultepe, O.; Atay, F. The effect of Al element on structural, optical, electrical, surface and photocatalytic properties of Solgel derived ZnO films. Appl. Phys. A 2022, 128, 25. [Google Scholar] [CrossRef]
- Kumawat, A.; Sharma, A.; Chattopadhyay, S.; Misra, K.P. Temperature dependent photoluminescence in Sol-gel derived Ce doped ZnO nanoparticles. Mater. Today Proc. 2021, 43, 2965–2969. [Google Scholar] [CrossRef]
- Kumawat, A.; Chattopadhyay, S.; Misra, R.D.K.; Misra, K.P.; Valiyaneerilakkal, U. Significance of microstrain in impacting band gap and photoluminescence behavior of Ce-doped ZnO thin films deposited via sol-gel process. Phys. Scr. 2023, 98, 025816. [Google Scholar] [CrossRef]
- Anand, V.; Sakthivelu, A.; Kumar, K.D.A.; Valanarasu, S.; Ganesh, V.; Shkir, M.; Kathalingam, A.; AlFaify, S. Novel rare earth Gd and Al co-doped ZnO thin films prepared by nebulizer spray method for optoelectronic applications. Superlattices Microstruct. 2018, 123, 311–322. [Google Scholar] [CrossRef]
- Anand, V.; Sakthivelu, A.; Kumar, K.D.A.; Valanarasu, S.; Ganesh, V.; Shkir, M.; AlFaify, S.; Algarni, H. Rare earth Eu3+ codoped AZO thin films prepared by nebulizer spray pyrolysis technique for optoelectronics. J. Solgel Sci. Technol. 2018, 86, 293–304. [Google Scholar] [CrossRef]
- Anand, V.; Sakthivelu, A.; Deva Arun Kumar, K.; Valanarasu, S.; Kathalingam, A.; Ganesh, V.; Shkir, M.; AlFaify, S.; Yahia, I.S. Rare earth Sm3+ codoped AZO thin films for opto-electronic application prepared by spray pyrolysis. Ceram. Int. 2018, 44, 6730–6738. [Google Scholar] [CrossRef]
- Üzar, N.; Algün, G.; Akçay, N.; Akcan, D.; Arda, L. Structural, optical, electrical and humudity sensing properties of (Y/Al) codoped ZnO thin films. J. Mater. Sci. Mater. Electron. 2017, 28, 11861–11870. [Google Scholar] [CrossRef]
- Mereu, R.A.; Mesaros, A.; Vasilescu, M.; Popa, M.; Gabor, M.S.; Ciontea, L.; Petrisor, T. Synthesis and characterization of undoped, Al and/or Ho doped ZnO thin Films. Ceram. Int. 2013, 39, 5535–5543. [Google Scholar] [CrossRef]
- Ahmed, M.A.M.; Meyer, W.E.; Nel, J.M. Effect of (Ce, Al) codoped ZnO thin films on the Schottky diode properties fabricated using the sol-gel spin coating. Mater. Sci. Semicond. Process. 2019, 103, 104612. [Google Scholar] [CrossRef]
- Kumar, K.D.A.; Thomas, R.; Valanarasu, S.; Ganesh, V.; Shkir, M.; AlFaify, S.; Thirumalai, J. Analysis of Pr codoped Al: ZnO thin flms using feasible nebulizer spray technique for optoelectronic technology. Appl. Phys. A 2019, 125, 712. [Google Scholar] [CrossRef]
- Sharma, A.; Kumawat, A.; Chattopadhyay, S.; Khangarot, R.K.; Halder, N.; Misra, R.D.K.; Misra, K.P. Band gap reduction and Zn related defects enhancement in Zn(Al, Ce)O nanoparticles. Mater. Today Proc. 2022, 60, 21–25. [Google Scholar] [CrossRef]
- Kambe, M.; Fukawa, M.; Taneda, N.; Sato, K. Improvement of a-Si solar cell properties by using SnO2: F TCO films coated with an ultrathin TiO2 layer prepared by APCVD. Sol. Energy Mater. Sol. Cells 2006, 90, 3014–3020. [Google Scholar] [CrossRef]
- Consonni, V.; Rey, G.; Roussel, H.; Doisneau, B.; Blanquet, E.; Bellet, D. Preferential orientation of fluorine-doped SnO2 thin films: The effects of growth temperature. Acta Mater. 2013, 61, 22–31. [Google Scholar] [CrossRef]
- Yu, S.; Li, L.; Lyu, X.; Zhang, W. Preparation and investigation of nanothick FTO/Ag/FTO multilayer transparent electrodes with high figure of merit. Sci. Rep. 2016, 6, 20399. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Huang, L.; Ren, N.; Kong, X.; Cai, Y.; Zhang, J. Superhydrophobic and anti-reflective ZnO nanorod-coated FTO transparent conductive thin films prepared by a three-step method. J. Alloys Compd. 2016, 674, 368–375. [Google Scholar] [CrossRef]
- Mishima, R.; Hino, M.; Uzu, H.; Meguro, T.; Yamamoto, K. High-current perovskite solar cells fabricated with optically enhanced transparent conductive oxides. Appl. Phys. Express 2017, 10, 062301. [Google Scholar] [CrossRef]
- Shibayama, N.; Fukumoto, S.; Sugita, H.; Kanda, H.; Ito, S. Influence of transparent conductive oxide layer on the inverted perovskite solar cell using PEDOT: PSS for hole transport layer. Mater. Res. Bull. 2018, 106, 433–438. [Google Scholar] [CrossRef]
- Zhang, S.-T.; Foldyna, M.; Roussel, H.; Consonni, V.; Pernot, E.; Schmidt-Mende, L.; Rapenne, L.; Jiménez, C.; Deschanvres, J.-L.; Muñoz-Rojas, D.; et al. Tuning the properties of F:SnO2 (FTO) nanocomposites with S:TiO2 nanoparticles—Promising hazy transparent electrodes for photovoltaics applications. J. Mater. Chem. C 2017, 5, 91–102. [Google Scholar] [CrossRef]
- Kim, H.; Auyeung, R.C.Y.; Pique, A. Transparent conducting F-doped SnO2 thin films grown by pulsed laser deposition. Thin Solid Film. 2008, 516, 5052–5056. [Google Scholar] [CrossRef]
- Qia, F.; Chu, H.; Xie, Y.; Weng, Z. Recent progress of transparent conductive electrodes in the construction of efficient flexible organic solar cells. Int. J. Energy Res. 2022, 46, 4071–4087. [Google Scholar] [CrossRef]
- Way, A.; Luke, J.; Evans, A.D.; Li, Z.; Kim, J.-S.; Durrant, J.R.; Ka Hin Lee, H.; Tsoi, W.C. Fluorine doped tin oxide as an alternative of indium tin oxide for bottom electrode of semi-transparent organic photovoltaic devices. AIP Adv. 2019, 9, 085220. [Google Scholar] [CrossRef]
- Yang, Z.; Song, J.; Zeng, H.; Wang, M. Organic composition tailored perovskite solar cells and light-emitting diodes: Perspectives and advances. Mater. Today Energy 2019, 14, 100338. [Google Scholar] [CrossRef]
- Pulli, E.; Rozzi, E.; Bella, F. Transparent photovoltaic technologies: Current trends towards upscaling. Energy Convers. Manag. 2020, 219, 112982. [Google Scholar] [CrossRef]
- Husain, A.A.F.; Hasan, W.Z.W.; Shafie, S.; Hamidon, M.N.; Pandey, S.S. A review of transparent solar photovoltaic technologies. Renew. Sustain. Energy Rev. 2018, 94, 779–791. [Google Scholar] [CrossRef]
- Bhuvaneswari, P.V.; Velusamy, P.; Babu, R.R.; Babu, S.M.; Ramamurthi, K.; Arivanandhan, M. Effect of fluorine doping on the structural, optical and electrical properties of spray depositedcadmium stannate thin films. Mater. Sci. Semicond. Proc. 2013, 16, 1964–1970. [Google Scholar] [CrossRef]
- Arefi-Khonsari, F.; Bauduin, N.; Donsanti, F.; Amouroux, J. Deposition of transparent conductivetin oxide thin films doped with fluorine by PACVD. Thin Solid Film. 2003, 427, 208–214. [Google Scholar] [CrossRef]
- Elangovan, E.; Ramamurthi, K. Studies on microstructural and electrical properties of spray-deposited fluorine-doped tin oxide thin films from low-cost precursor. Thin Solid Film. 2005, 476, 231–236. [Google Scholar] [CrossRef]
- Elangovan, E.; Ramamurthi, K. A study on low cost-high conducting fluorine and antimony-doped tin oxide thin films. Appl. Surf. Sci. 2005, 249, 183–196. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeon, K.A.; Kim, G.H.; Lee, S.Y. Lee Electrical, structural, and optical properties of ITO thin films prepared at room temperature by pulsed laser deposition. Appl. Surf. Sci. 2006, 252, 4834. [Google Scholar] [CrossRef]
- Martinez, A.I.; Acosta, D.R. Effect of the fluorine content on the structural and electrical properties of SnO2 and ZnO–SnO2 thin films prepared by spray pyrolysis. Thin Solid Film. 2005, 483, 107. [Google Scholar] [CrossRef]

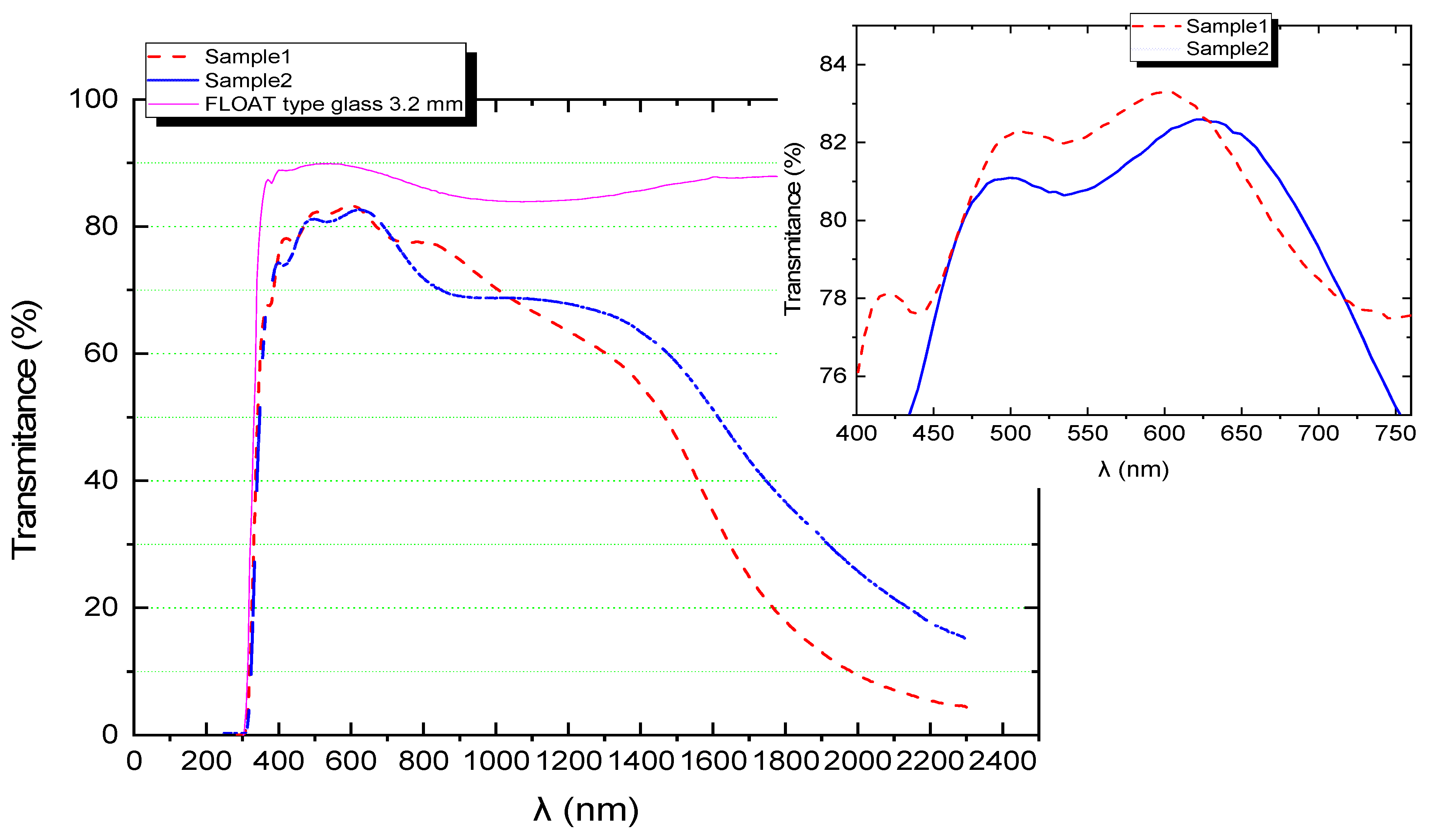
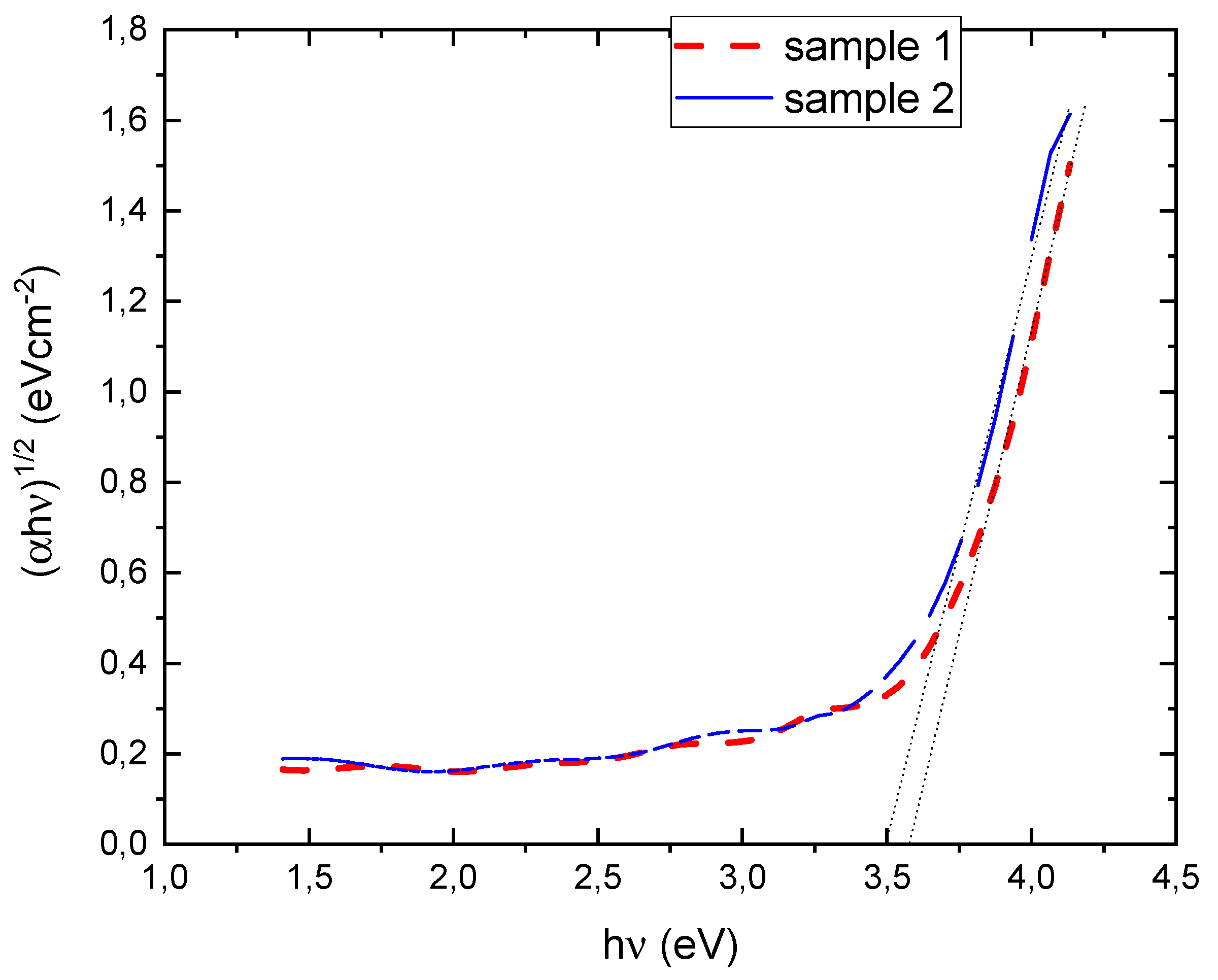
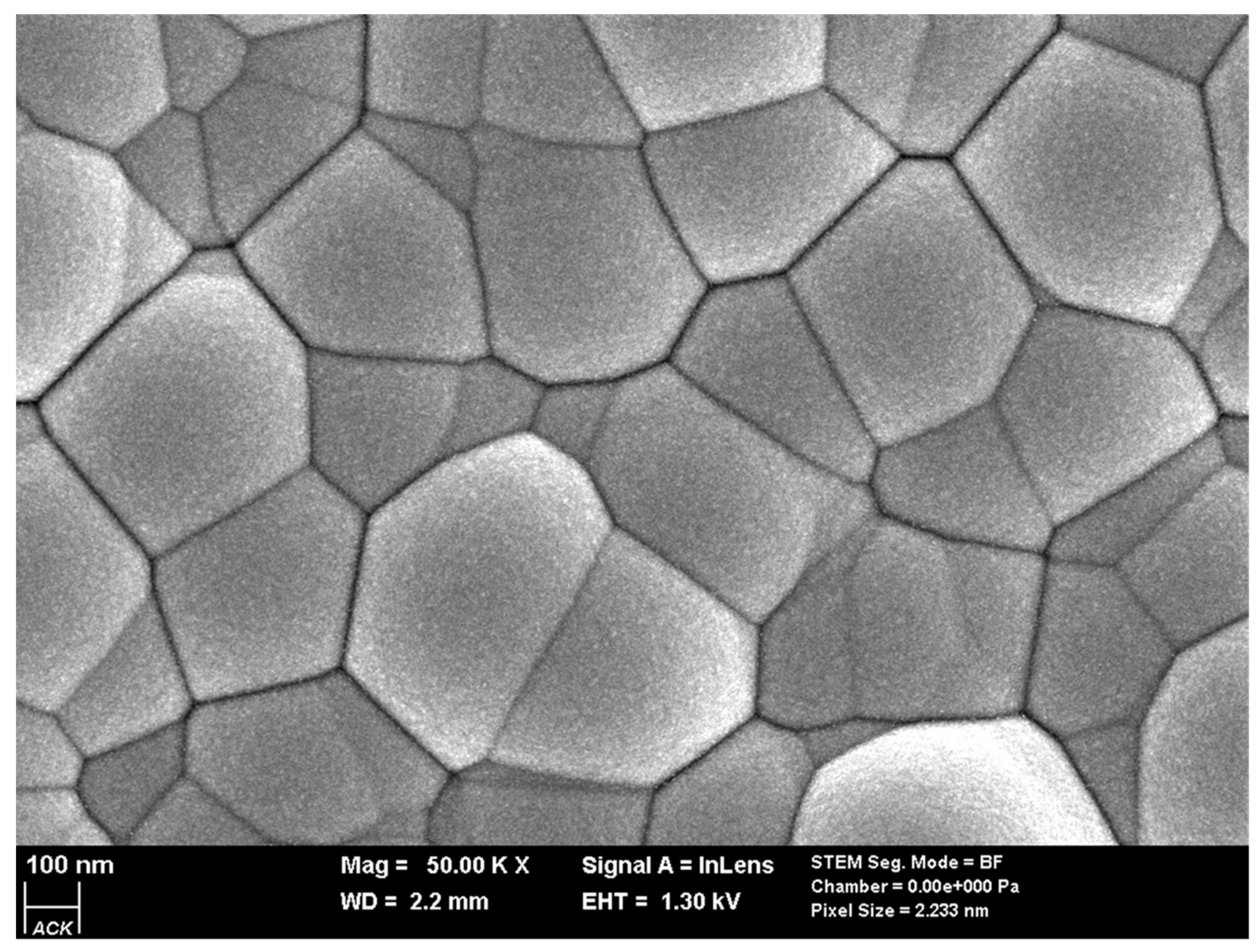

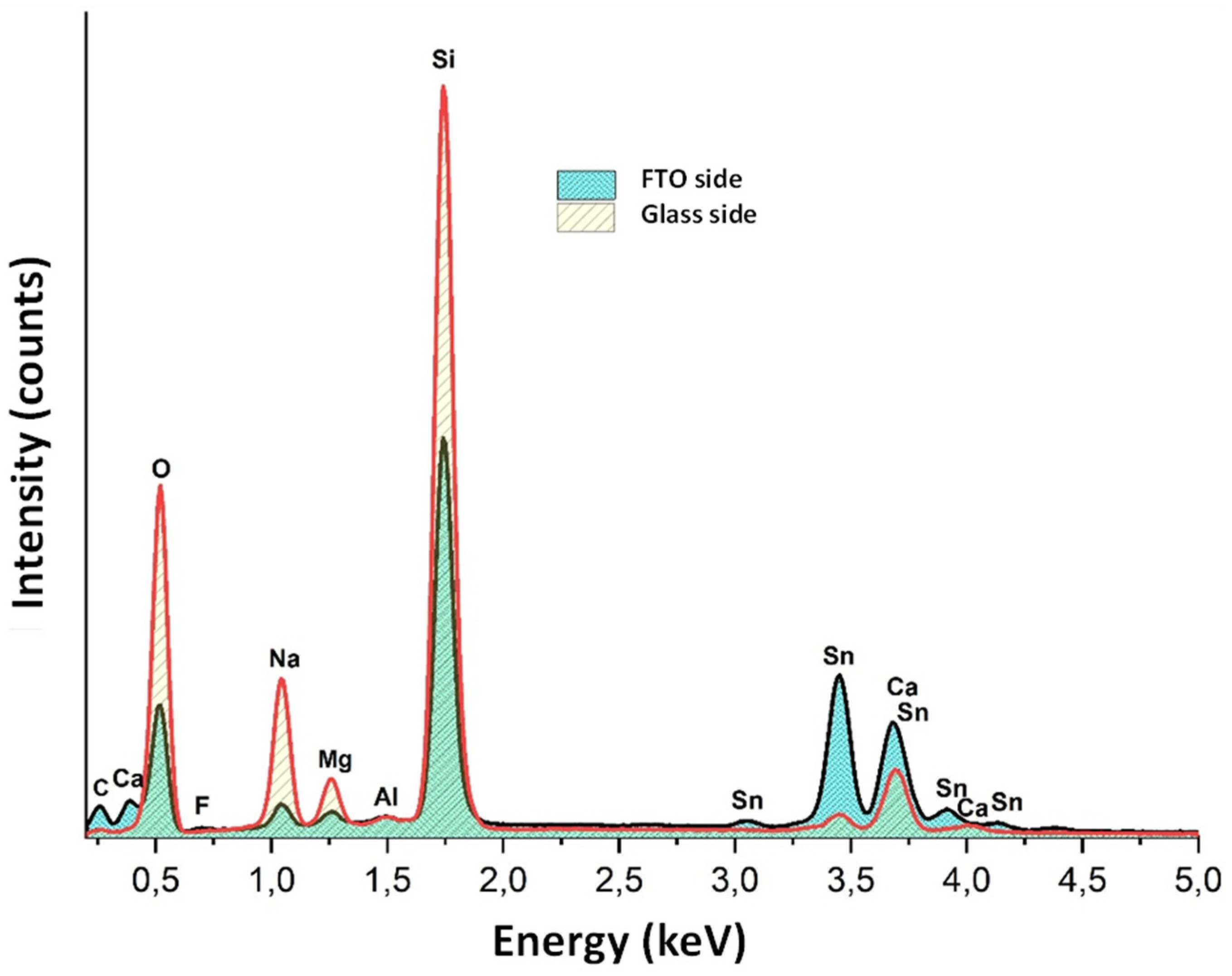
| SAMPLE1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample ID | Sheet Resistance (Ω−1) ±0.01 | Resistivity (Ω cm) ±0.02 × 10−0.4 | Conductivity (Ω−1 cm−1) ±0.02 × 102 | CCC Bulk (cm−3) ±0.05 × 1020 | CCC Sheet (cm−2) ±0.05 × 1020 | BD Cross Hall Coef. (cm3 C−1) ±0.01 × 10−2 | AC Cross Hall Coef. (cm3 C−1) ±0.01 × 10−2 | Avg. Hall Coef. (cm3 C−1) ±0.01 × 10−2 | Mobility (cm2 V−1 s−1) ±0.01 |
| 1.1 | 7.47 | 4.03 × 10−4 | 2480 | −4.18 × 1020 | −2.26 × 1020 | −0.0150 | −0.0149 | −0.0149 | −37.06 |
| 1.2 | 7.50 | 4.05 × 10−4 | 2470 | −3.91 × 1020 | −2.11 × 1020 | −0.0161 | −0.0159 | −0.0160 | −39.44 |
| 1.3 | 7.54 | 4.07 × 10−4 | 2456 | −3.98 × 1020 | −2.15 × 1020 | −0.0158 | −0.0156 | −0.0157 | −38.54 |
| AVG | 7.50 | 4.05 × 10−4 | 2469 | −4.02 × 1020 | −2.17 × 1020 | −0.0156 | −0.0155 | −0.0155 | −38.34 |
| SD | 0.04 | 0.02 × 10−4 | 12 | 0.14 × 1020 | 0.08 × 1020 | 0.0006 | 0.0005 | 0.0005 | 1.20 |
| SAMPLE2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample ID | Sheet Resistance (Ω−1) ±0.01 | Resistivity (Ω cm) ±0.02 × 10−0.4 | Conductivity (Ω−1 cm−1) ±0.02 × 102 | CCC Bulk (cm−3) ±0.05 × 1020 | CCC Sheet (cm−2) ±0.05 × 1020 | BD Cross Hall Coef. (cm3 C−1) ±0.01 × 10−2 | AC Cross Hall Coef. (cm3 C−1) ±0.01 × 10−2 | Avg. Hall Coef. (cm3 C−1) ±0.01 × 10−2 | Mobility (cm2 V−1 s−1) ±0.01 |
| 2.1 | 13.79 | 4.69 × 10−4 | 2133 | −4.34 × 1020 | −1.48 × 1020 | −0.0144 | −0.0144 | −0.0144 | −30.67 |
| 2.2 | 14.44 | 4.91 × 10−4 | 2037 | −4.35 × 1020 | −1.48 × 1020 | −0.0143 | −0.0144 | −0.0143 | −29.23 |
| 2.3 | 13.16 | 4.47 × 10−4 | 2235 | −4.67 × 1020 | −1.59 × 1020 | −0.0134 | −0.0134 | −0.0134 | −29.85 |
| AVG | 13.80 | 4.69 × 10−4 | 2135 | −4.45 × 1020 | −1.51 × 1020 | −0.0140 | −0.0141 | −0.0140 | −29.92 |
| SD | 0.64 | 0.22 × 10−4 | 99 | 0.19 × 1020 | 0.07 × 1020 | 0.0006 | 0.0006 | 0.0006 | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwaśnicki, P.; Gronba-Chyła, A.; Generowicz, A.; Ciuła, J.; Makara, A.; Kowalski, Z. Characterization of the TCO Layer on a Glass Surface for PV IInd and IIIrd Generation Applications. Energies 2024, 17, 3122. https://doi.org/10.3390/en17133122
Kwaśnicki P, Gronba-Chyła A, Generowicz A, Ciuła J, Makara A, Kowalski Z. Characterization of the TCO Layer on a Glass Surface for PV IInd and IIIrd Generation Applications. Energies. 2024; 17(13):3122. https://doi.org/10.3390/en17133122
Chicago/Turabian StyleKwaśnicki, Paweł, Anna Gronba-Chyła, Agnieszka Generowicz, Józef Ciuła, Agnieszka Makara, and Zygmunt Kowalski. 2024. "Characterization of the TCO Layer on a Glass Surface for PV IInd and IIIrd Generation Applications" Energies 17, no. 13: 3122. https://doi.org/10.3390/en17133122
APA StyleKwaśnicki, P., Gronba-Chyła, A., Generowicz, A., Ciuła, J., Makara, A., & Kowalski, Z. (2024). Characterization of the TCO Layer on a Glass Surface for PV IInd and IIIrd Generation Applications. Energies, 17(13), 3122. https://doi.org/10.3390/en17133122








