Abstract
This article presents the authors’ considerations regarding the possibilities of developing fuel equipment for modern compression ignition engines used in special and non-road vehicles. The paper discusses the process of fuel combustion and atomization in the chamber of a piston combustion engine. The paper then presents the concept of modifying the atomizer of a modern fuel injector for operation using hydrogen-containing fuels of plant origin. The authors present a review of tests performed using an engine dynamometer on a modern engine with a Common Rail system running on biofuel. The CI engine operated with standard and modified fuel injectors. During the tests, the external ecological characteristics of the engine were analyzed as a function of rotational speed; the values of injection doses at individual rotational speeds and their effects on the characteristics were read from the current parameters, and the pressure and temperature in the engine’s combustion chamber were measured. The research results show that implementing the changes proposed by the authors of this work is a good direction for the development of compression ignition engines.
1. Introduction
Compression ignition (CI) engines are used in commercial, special, combat and non-road vehicles, agricultural machinery, power generators and vessels. Their development is aimed at reducing pollutants in exhaust gases. Research on reducing the toxicity of exhaust gases is carried out in various directions: the use of electronic engine control systems, the use of exhaust gas treatment systems, various modifications of engine components and the use of alternative fuels containing hydrogen.
To meet stringent diesel emission standards, a Common Rail fuel supply system was introduced. The advantage of this system is the ability to adjust the injection torque and pressure to the engine’s operating conditions by separating the fuel accumulator element (injection pump) from the fuel injectors and the pressure accumulator (rail). Various design solutions of the Common Rail system are known, but their principle of operation is the same. The essence of this solution is to divide the injection doses into several doses in one engine operation cycle and to control the desired fuel injection pressure and moment.
The aim of this solution is to appropriately organize the combustion process of the combustible mixture in the engine compartment in order to reduce the emission of toxic substances into the atmosphere, such as nitrogen oxides, carbon monoxide, unburnt hydrocarbons and solid particles. The entire system is managed by the engine controller, which collects data from sensors and appropriately controls the actuators. This solution enables multiphase fuel injection, which improves the engine’s operating parameters. The multiphase nature of fuel injection involves dividing the injected fuel dose into several doses: pilot (initial), main and final. The number of doses during one working cycle depends on the design of the electrohydraulic fuel injector. The most important dose for injection is the pilot dose, called the starting or introductory dose.
Its task is to prepare the engine’s combustion chamber in such a way that the main dose is burned at gently increasing pressure. This is characterized by the soft operation of the engine and reduces noise during its operation, eliminating the phenomenon of knocking combustion. The aim of the pilot dose is to shorten the autoignition delay time as much as possible. The shorter the time from the appearance of the first drops of fuel in the engine’s combustion chamber to the first signs of the spontaneous ignition of the flammable mixture, the smoother the entire combustion process, which affects the emission of toxins in the exhaust gases and engine performance. The injection dose is controlled by the engine computer, extending or shortening the operating time of the fuel injectors. The controller processes signals from the crankshaft position sensor: if one of the injectors begins to change its operating parameters or if the pressure in one of the cylinders changes, the computer controlling the engine operation corrects the operating time and routes a specific fuel injector to improve engine performance. Another advantage of the CR system is high system pressure, from 25 MPa at idle to 180 MPa. Thanks to such high pressures, the fuel is very well atomized and distributed in the combustion chamber, which improves its mixing with the air.
Ecological parameters can be improved by using alternative fuels and various additives, as well as conventional fuels containing hydrogen, using a duel-fuel power system and modifying the fuel supply system, exhaust system, intake system and combustion chamber. Research on alternative fuels containing hydrogen is carried out in various directions. Research is being carried out on the use of synthetic fuels, hydrorefined vegetable oil (HVO), bio-oil produced from biomass and fuel produced from biochar [1]. The search for alternative fuels for combustion engines is driven by many different motivations, including the following:
- −
- The need to reduce pollution and limit greenhouse gas emissions;
- −
- The goal of reducing dependence on one type of raw material (e.g., diesel oil);
- −
- The use of local and renewable energy sources for the production of alternative fuels, which, to some extent, may contribute to increasing the energy independence of the country (region);
- −
- The search for and introduction of various alternative fuels, which leads to increased competition between producers and stimulates innovation in the field of engines and drive technologies.
The most popular raw material in Central Europe from which biofuels are produced is rapeseed oil. There are previous works that investigated the use of pure rapeseed oil (OR) and its mixtures with diesel oil as an alternative fuel containing hydrogen in diesel engines [2]. However, whereas diesel oil does not contain oxygen molecules, the oxygen content in rapeseed oil is approximately 12%. This affects its calorific value, which is 36.7–37.7 MJ/kg, whereas the calorific value of diesel oil is 42.7–43.5 MJ/kg [3]. OR molecules are much larger than those of diesel fuel (DF) and have a molar mass of approximately 800–1000 g/mol. The molar mass of DF is 226 g/mol. This means that the viscosity of OR, which is 68–97.7 mm2/s at 20 °C, is much higher than that of DF (2.90–5.50 mm2/s at 20 °C). These factors cause reduced OR fluidity and poorer atomization, requiring a higher evaporation temperature. This causes starting problems, especially at lower temperatures.
2. Analysis of the Influence of the Hydrogen-Containing Fuel’s Physical Properties on the Parameters of the Injected Fuel Stream in Non-Road Vehicle Engines
The physical properties of the fuel affect the parameters of the injected fuel stream, such as the range, atomization, opening angle, penetration area, speed and maximum width. Spraying and distributing pure OR in the engine’s combustion chamber is unfavorable in terms of the combustion process and causes the deposition of unburnt fuel on engine components [1,2]. Therefore, the use of vegetable oils without processing causes burning and coking of hydrogen-containing fuel injector nozzles, the deterioration of injection parameters, problems with starting the engine, especially at low temperatures, blocking of precision pair elements in the injection system, the accelerated wear of components, the contamination of the non-road vehicle engine with soot (TPC system, turbocharger, valve system) and the deterioration of operating parameters. Therefore, pure rapeseed oil is not recommended as fuel for diesel engines. Analyzing the literature on the scope of considerations, it can be observed that research on the use of alternative fuels to power combustion engines has been carried out in many research centers. The works in [3,4,5] examined the macroscopic and microscopic parameters of diesel fuel injection mixed with biocomponents: methyl laurate, methyl oleate and ethyl oleate. The tests were carried out on the Common Rail system at various system pressures. SMD analysis showed that the smallest drops were obtained with a mixture of diesel oil and methyl laurate. The authors of [6] validated the computer analysis of the fuel atomization process in the combustion chamber. The mathematical model of the fuel injection process in the combustion chamber was developed based on the Euler–Lagrange equations. The pressure in the system was 250 MPa. The presented model describes the scope of penetration of the combustion chamber by the fuel stream. The validation performed showed that the mathematical model is highly similar to the real model. The model proposed by the authors can be used to analyze the spraying of various hydrogen-containing fuels as well as to modify the injection equipment. The authors of [7] describe the macroscopic fuel injection characteristics for rapeseed oil methyl ester diesel fuel. Research has shown that diesel oil has a greater tendency to undergo cavitation in the spray nozzle holes than rapeseed oil methyl ester. The penetration of the combustion chamber by the jet depends more on the pressure in the system than on the type of hydrogen-containing fuel.
The authors of [8] examined the influence of temperature on the spraying process and the flow of methanol through the atomizer. Research has shown that an increase in fuel temperature can reduce fuel droplets in the stream, which improves the injection process. A computer simulation of the fuel flow through the atomizer was carried out to investigate the cavitation phenomenon. The analysis showed that the cavitation area increases with increasing temperature and pressure in the atomizer. The average SMD and velocity of the drops in the stream were also examined. It was found that the drops reach their maximum velocity at a temperature of 60 °C. In [9,10,11], the authors analyzed the atomization of selected fuels of plant origin. In [9], the spraying characteristics of biofuels, such as hydrorefined vegetable oil (HVO), palm oil methyl ester (PME), soybean oil methyl ester (SME) and used frying oil methyl ester (UCOME), were analyzed and compared with the spraying characteristics of diesel fuel. During the tests, the fuels were heated to a temperature of 80 °C to simulate the conditions in the combustion chamber. Analyzing the test results, it was found that all fuels had a similar jet range, while the lowest combustion chamber penetration properties were demonstrated by hydrorefined vegetable oil (HVO). The study in [10] describes the atomization characteristics of modified biodiesel (MBF) in a compression ignition engine with direct fuel injection. In this work, an atomization model was prepared, and sputtering properties such as the Ohnesorge number (Oh) and the SMD formula (Sauter mean diameter) were determined. To assess the fuel dispersion and the number, diameters and surface areas of drops in the stream, the average volume diameter, dv, and the average diameter determined according to Sauter, ds, are used. The average volume diameter of the drops is used to assess the actual number of drops formed and the fineness of atomization. The average droplet diameter according to Sauter, ds, is used to calculate the heating and evaporation of drops in the fuel spray [12]. In [10], the atomization number (AI) of the tested biofuel was determined. In other research [11], the micro- and macroscopic parameters of spraying in a Common Rail engine were analyzed based on mixtures of hydrogen-containing biofuel components such as methyl laurate, methyl and ethyl oleate with diesel fuel.
The parameters tested include the range, area, opening angle, speed and maximum width of the fuel stream. The SMD formula was examined in this work. The analysis showed that diesel oil with methyl laurate has a higher SMD than methyl and ethyl oleate. The analysis of the influence of the physical properties of biofuels on the injection characteristics in modern Common Rail injection systems was discussed by the authors in [13]. In this work, using the established model, the influence of the density, viscosity and bulk elastic modulus on the injection dose and the propagation of pressure waves during the injection dose division were examined. A simulation analysis showed that the density and bulk modulus have a significant impact on fuel injection characteristics and the propagation of pressure waves. In [14], the authors presented their own method of testing small engines powered by fuels of plant origin in cities using vibroacoustic vibrations. During the tests, fuel with extremely low sulfur content and biofuel made from used frying oil were used. The study considered the impact of the use of the tested alternative fuels on engine vibrations and noise. The tests showed that the greatest vibrations were experienced by the non-road vehicle engine powered by B40 fuel (60% diesel, 40% biofuel), and the greatest noise was recorded with B10 fuel (90% diesel, 10% biofuel). In [15,16], methyl ester of karanja oil (KOME) was tested as an additive to diesel fuel in proportions of 10%, 20%, 50%, 60% and 100% in an engine with a Common Rail system (CRDI). The authors analyzed the atomization and dose rates of fuel injectors, engine characteristics, the combustion process and emissions.
The engine was tested at a constant rotational speed of 1500 min−1 and pressures of 30, 50, 75 and 100 MPa with different injection times. The tests showed that the injection time decreased with increasing KOME contents in diesel oil and with increasing injection pressure. The Sauter and arithmetic diameters of the injected fuel drops increased with a higher biocomponent content due to the higher density and viscosity of the mixture. The higher the injection pressure, the smaller the droplet diameters. Analyzing the emission of hydrocarbons and carbon oxides, it can be concluded that when the KOME content is 20% or more, there is a visible decrease in the amounts of the above-mentioned toxins in the exhaust gases. According to the authors, this is due to the shortened injection time for fuel with a higher biocomponent content.
An alternative to biofuel for diesel engines is bio-oils produced in the pyrolysis of biomass [16]. Their big advantage is their neutrality in terms of carbon dioxide emissions. However, these fuels have problems with combustion due to poor atomization. To implement bio-oils, it is necessary to improve the process of distributing and spraying them in the combustion chamber. According to the literature [17,18,19,20], bio-oil produced in the pyrolysis process can be heated or various additives can be added to it to improve atomization. Modernizing the injection equipment could improve the physical properties of this fuel and improve its injection process into the combustion chamber. Hydrorefined vegetable oil (HVO) is classified as a second-generation hydrogen-containing biofuel [21]. Research has shown that an engine powered by it emits lower amounts of nitrogen oxides, has a higher heating number and cetane number, and results in better atomization properties of the stream compared to conventional diesel oil [22,23]. A previous article [24] describes the possibilities of using biobutanol as an additive to second-generation biofuels. Studies have shown that biobutanol increases NOx emissions, but increasing the operation of the EGR valve in the engine reduces the contents of nitrogen oxides and other toxins. This happens because of the lower exhaust gas temperature. In another paper [25], the authors described the impact of second-generation biofuels on the characteristics of heat release in the combustion chamber. The research compared standard fuel, rapeseed oil methyl esters (first-generation biofuel) and babassu methyl ester (second-generation biofuel). The measurement results showed that the engine running on plant fuels was characterized by a higher combustion pressure in the chamber. This is due to the higher cetane number and the oxygen molecule content in the structural composition of the hydrogen-containing plant-based fuel. The heat release was not higher for the ester-fueled engine compared to the standard fuel. The characteristics of the heat release depend more on the engine load than on the type of fuel.
Catalytic systems in motor vehicles are mainly found in exhaust systems. Their task is to purify exhaust gases by removing toxic compounds. This type of catalytic converter does not affect the combustion process but supports exhaust gas purification [26]. The task of a liquid catalyst is to improve the organization of the combustion process. In addition, the catalyst prevents the formation of hydrocarbon bacteria in the fuel, which are responsible for the aging process. According to the literature, one of the ways to use catalytic converters is to install them in the combustion chamber of a diesel engine. In previous work [27], the authors used catalysts based on vanadium. Tests were carried out on a single-cylinder, four-stroke CI engine and showed a reduction in engine emissions and fuel consumption. The authors of [28] presented the results of a study of an engine running on biofuel from lemon oil. Engine components such as the piston, cylinder head and valves were coated with a zirconia catalyst. The modified biofuel-powered engine had lower emissions of carbon monoxide, hydrocarbons, nitrogen oxides and smoke compared to a standard conventional-fuel-fired engine. Gaseous fuels were used for research in [29]: hydrogen, methane and natural gas. The fuels were mixed with liquid fuel in a ratio of 50-50. A platinum–aluminum catalytic converter was installed in the engine. Studies have shown that the catalyst reduces nitrogen oxides. In addition, a mixture of fuel and hydrogen causes an increase in cylinder pressure and emissions of nitrogen oxides and hydrocarbons, as well as a reduction in smoke. However, the use of natural gas and methane reduces nitrogen oxide emissions. In addition, studies have shown that the use of an additional catalyst reduces the opacity of these fuels. In diesel engines of non-road vehicles, AdBlue is used to catalytically remove nitrogen oxides from exhaust gases using an ammonia-containing substance [30]. However, this substance is expensive and requires frequent replenishment. The authors of [31] considered an emulsion (W/O) as a fuel for CI engines. Research has shown that adding water to fuel reduces nitrogen oxide emissions by 19.6% and smoke emissions by 66.3%. According to the authors, water lowers the combustion temperature caused by the absorption of heat of evaporation and micro-explosions caused by the rapid evaporation of water particles. This allows for a simultaneous reduction in nitrogen oxides and smoke. It is possible to produce emulsion fuel from non-biodegradable plastic carrier bags (PGBs) [32]. Studies have shown that the use of a water–oil emulsion with PGBs reduces the emission of smoke and nitrogen oxides. The authors of [33] performed an exergetic analysis of a diesel engine running on biofuel obtained from cottonseed oil with the addition of titanium dioxide and silver oxide nanoparticles. Fuel with nanoparticles showed lower exergy losses. It should be noted that nanoparticles come at a high price. Similar studies were presented by the authors of [34]. Their results showed that the use of this type of catalytic converter reduces soot and hydrocarbon emissions by 30% at a partial engine load. However, at the maximum engine load, a slight reduction in nitrogen oxide emissions of around 5% was observed. No difference in fuel consumption was noted at either the average or maximum engine load. Another article [35] investigated the effect of mixtures of diesel fuel with methanol, ethanol, n-butanol, methanol/n-butanol and ethanol/n-butanol on combustion. The results of the study showed that the use of additives shortened the self-ignition delay and optimized the combustion process. The most favorable results were obtained with a mixture of 80% diesel, 10% methanol and 10% n-butanol. The paper describes the use of a catalytic converter in the fuel injector nozzle in an engine with direct mechanical fuel injection [36,37]. Studies have shown that the use of a platinum catalytic converter on the non-functioning part of the needle reduces the emission of toxic substances into the atmosphere. The authors of the study in [38] presented a method of partial dehydrogenation of diesel fuel to produce high-quality hydrogen. The aim of the study was to assess the possibility of using such a system in motor vehicles. The catalyst used in the experiment was a platinum base and aluminum. Studies [39,40] have shown that a suitable element used as a catalyst to activate the hydrocarbon dehydrogenation reaction is tin- and aluminum-based platinum. Development trends in internal combustion engines are aimed at reducing toxic substances in exhaust gases. The research described by the authors of [41,42] shows how an engine powered by ethanol as a fuel additive works. The content of the additive resulted in a reduction in nitrogen oxide emissions in the exhaust gases. The author of [43] describes biofuel powder as an additive to the air in the intake system. The analysis was carried out in terms of engine durability. Studies have shown that it is possible to use this type of solution in internal combustion engines.
Analyzing the literature on the topic under consideration, it can be observed that research on the use of alternative fuels is carried out in various directions. The most popular trend is work on second-generation biofuels and the use of various additives. These procedures are performed to improve the process of atomizing and distributing fuel in the engine compartment and to optimize the combustion process in terms of improving performance and minimizing the emission of toxic substances into the atmosphere. One way to deliver hydrogen to the combustion chamber of an engine is through the hydrocarbon dehydrogenation reaction. The storage of hydrogen in the form of a solid hydrate is presented in [44]—an innovative concept for storing hydrogen and then releasing it when needed. Diesel fuel consists mainly of paraffinic hydrocarbons. Installing the catalyst on the non-working part of the fuel injector needle can initiate the reaction of dehydrogenation of the paraffins contained in the fuel to the olefin form with the release of a free hydrogen molecule. The second method of transport is the method of injecting hydrogen into the combustion chamber of the engine with fuel in the form of a liquid catalyst, which is decomposed during the combustion process.
3. Combustion Process in Compression Ignition Engines
Combustion is a complex, rapidly occurring chemical transformation accompanied by the release of a significant amount of heat [45]. The heat of combustion is the thermal effect of the oxidation reaction of a given compound with oxygen and the formation of appropriate elements that are products of combustion. Compression ignition engines have a heterogeneous combustion mechanism. Self-ignition in such conditions is characterized by the fact that the surface temperature of the fuel in the liquid phase is lower than the temperature of autoignition. Therefore, the process of spontaneous ignition is preceded by the partial or complete evaporation of the fuel and the heating of the vapors to the temperature of autoignition [45]. As a result of the evaporation and diffusion processes of fuel and air vapors, a flammable mixture is formed. In the combustion chamber of a diesel engine, the combustion process takes place in the gas phase, because the heat of the flame is high enough to cause the liquid to degas. Due to the increased kinetic energy of the thermal motion of the vapor particles and the greater number of collisions of the fuel particles and the oxidizer, the rate of chemical reactions in the gas phase is much faster. The process of preparing a combustible mixture includes the following stages: the heating of the liquid surface, heating and evaporation, the mutual penetration of fuel vapors, and the activation of the oxidant and chemical reaction nuclei, as well as spontaneous ignition and combustion [45,46]. The combustion process is limited by the rate of fuel evaporation or the rate of chemical reactions occurring in a homogeneous mixture of hydrogen fuel and air. The phenomenon of the spontaneous ignition of fuels for internal combustion engines has been described in detail in previous papers [45,46,47,48,49,50,51,52].
The process of the combustion of the combustible mixture in the engine compartment consists of several stages, as presented in [4,45]. When analyzing the course and nature of combustion of a combustible mixture, the most important moment is the delay of spontaneous ignition. The remaining stages depend on its duration. The ignition delay period depends on the rate of fuel evaporation and the chemical reaction of the substrates. The phenomenon of spontaneous ignition is preceded by the processes of gasification of the liquid fuel and its mixture with an oxidizer. Traditionally, the self-ignition delay period can be represented by the sum of two stages: the period during which the value depends on physical factors and the period during which the value depends on the chemical agents in the fuel. Both periods run simultaneously. If the first period has a greater effect on the combustion process, it is said to occur in the diffusion region, and its speed is limited by the speed of the mutual diffusion of the fuel with the oxidizer and active reaction nuclei. However, if the second period has a larger share, the speed of the process of this stage is limited by the kinetics of the chemical reactions [45,49]. The delay of spontaneous ignition also depends on the physical and chemical properties of the fuel. The physical properties of fuels are density, viscosity and surface tension [50,51]. The viscosity and surface tension parameters affect the atomization of the fuel stream. The higher the kinematic viscosity, the smaller the spray angle of the fuel stream and the larger the droplet diameter during the injection process. This causes soot to settle in the engine’s combustion chamber and components and contaminate the engine oil, resulting in trouble starting at lower temperatures. Too high a fuel viscosity causes earlier ignition, which adversely affects the combustion process and increases emissions of nitrogen oxides into the atmosphere [50]. The chemical properties of the fuel affect the delay period of autoignition through the amount of activation energy. The value of the activation energy depends on the structure of the fuel molecule and the carbon atoms it contains [45]. The delay of spontaneous ignition increases when hydrocarbon molecules transform into molecules with fewer carbon atoms or when the bonds between carbon atoms become stronger. The ability of a fuel to spontaneously ignite depends on its cetane number. This value expresses the percentage of cetane in a mixture with α-methylnaphthalene in the reference fuel, which is compared with the tested fuel. The cetane molecule is made up of many carbon atoms, the dimensions of which are large, and the bonds between the atoms are weak. The cetane particle is easily torn apart and is rapidly transformed during the reaction in front of the flame. However, the α-methylnaphthalene particle is compact, with strong bonds between carbon atoms; therefore, it is not very active and requires high activation energy and a long autoignition time. The autoignition delay period is minimal, and the optimal composition of the combustible mixture is close to the autoignition limit.
4. Analysis of the Influence of the Needle Structure on the Combustion Process in Diesel Engines
The injector nozzle is an element influencing the shape of the fuel stream distributed in the combustion chamber. Fuel atomizers should be designed so that the flowing drops have the smallest possible diameter to increase the liquid surface [46]. There is a high temperature in the combustion chamber, so the larger the liquid surface, the more intense the heat and mass exchange process that takes place between the fuel drops and the air. The fuel flows into the combustion chamber from the atomizer, in which round holes have a diameter of several dozen micrometers. The surrounding space is filled with gas. During the spraying process, the stream splits into drops with different diameters. Disturbances occur when the fuel stream flows through the nozzle holes. The characteristics of the fuel stream depend on its flow speed. At low liquid flow speeds, disturbances that occur in the spray holes cause axially symmetric vibrations, the increase in amplitude of which causes the stream to break up and form separate drops. As the flow velocity increases, the fuel stream loses stability and its axis deforms, which leads to its disintegration. In Common Rail systems, the system pressure in the tank ranges from 25 to 200 MPa, depending on the engine load.
At such high pressures, the fuel stream breaks up into a large number of drops very close to the atomizer holes. The factors influencing the fuel atomization process are the initial forces occurring during the flow through the atomizer holes. They depend on the design of the atomizer, the speed of the flowing fuel and the properties of the fuel. Under the action of the initial excitations and aerodynamic drag forces, the stream breaks down into thicker and finer drops in the engine combustion chamber. Fuel particles moving farther are further deformed under the influence of gas forces, surface tension and viscosity. The forces of the surface tension of a liquid and its viscosity influence the processes of droplet formation. The fuel fragmentation process continues until the forces stabilizing the drops become greater than the forces causing their disintegration [46].
Parameters describing the physical properties of the stream include the length, width, opening angle and speed of movement. The distribution of drops in the stream is very uneven. The drops formed in the initial period of the injection process encounter a stationary gaseous medium and quickly lose speed. The gas surrounding them absorbs their energy and begins to move along the axis of the moving fuel. The next moving drops encounter less resistance and have higher initial velocities at the exit of the spray holes. They catch up and cut off the decelerated drops from the outer edges of the stream. They are slowed down and pushed to the periphery at the front of the injected fuel stream. As a result of this process, in the cross-section of the stream, the number and speed of movement of the drops increase as they approach the axis of the stream [45,46].
By analyzing the mechanics of a liquid flow through the atomizer, it can be inferred that the properties of the physical parameters of the fuel stream in the combustion chamber depend on the injection characteristics and fuel velocity at the outlet, the design of the atomizer, the physical properties of the fuel and the properties of the gaseous medium. The injection characteristics depend on the structure of the fuel injector, while the liquid outlet velocity depends on the pressure in the system.
The physical properties of a hydrogen-containing fuel include its density, viscosity and surface tension. An increase in viscosity causes the deterioration of grinding properties and spray uniformity. Surface tension prevents the disintegration of the liquid, so it also significantly affects the disintegration of fuel drops in the stream. However, as the surface tension and viscosity increase, the range of the stream increases. The density parameter has the least influence on the spraying process. The higher the density, the greater the range of the fuel stream. The gas medium in the combustion chamber is characterized by the following parameters: gas density, pressure and temperature. When the combustion process begins, the pressure and temperature increase. Increasing the density of a gas causes an increase in aerodynamic resistance to moving drops. They decelerate faster and may not obtain the appropriate shapes.
The density of the gaseous medium has a very significant impact on the range and angle of the stream. The design of the atomizer affects the fragmentation of the droplet and the reduction in its diameter [26]. Increasing the turbulence of the fuel flow in the atomizer channel affects the opening angle and the range of the stream [4,46], especially in systems with the Common Rail system [50]. The turbulization of the liquid stream causes an increase in swirl and pulsation turbulence in the stream, which is lost to spraying work. Therefore, atomization is improved by sharp inlet edges of the atomizer opening. Increasing the diameter of the atomizer holes increases the range of the stream, which is due to the increase in the mass and kinetic energies of the flowing fuel [46].
The authors of [4] proposed a modification of the fuel atomizer in order to improve the injection parameters. Spiral–elliptical channels were added to the non-working part of the firing pin. Based on the analysis of the models of the fuel flow through the atomizer, it was found that the channels increased the flow turbulence. Mathematical models showed that this type of channel caused an increase in the fuel temperature in the atomizer and increased the fuel swirl just before it was fed into the combustion chamber. Additionally, the modifications do not affect the quantitative operational parameters of fuel injectors, such as the injection and overflow doses [4,51].
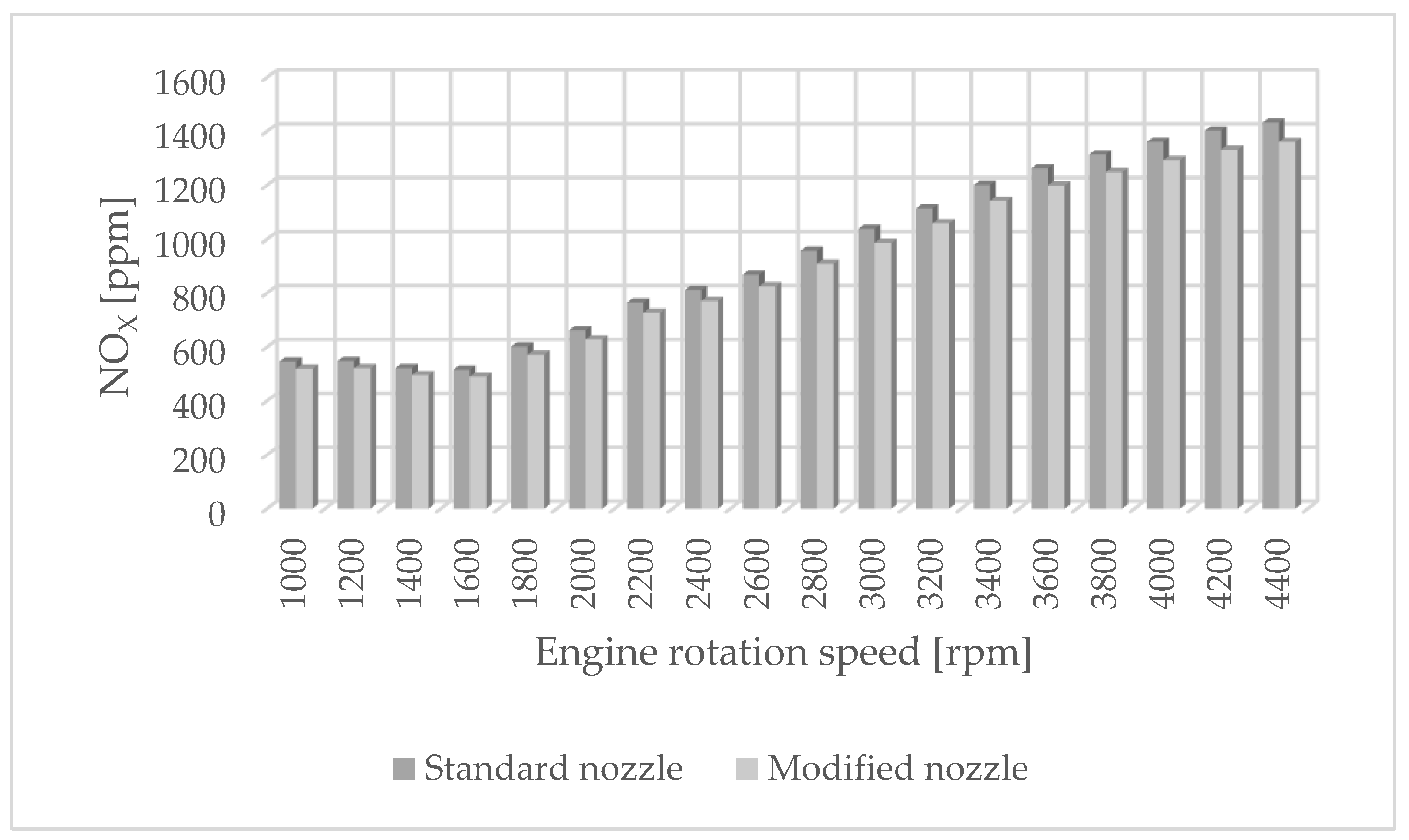
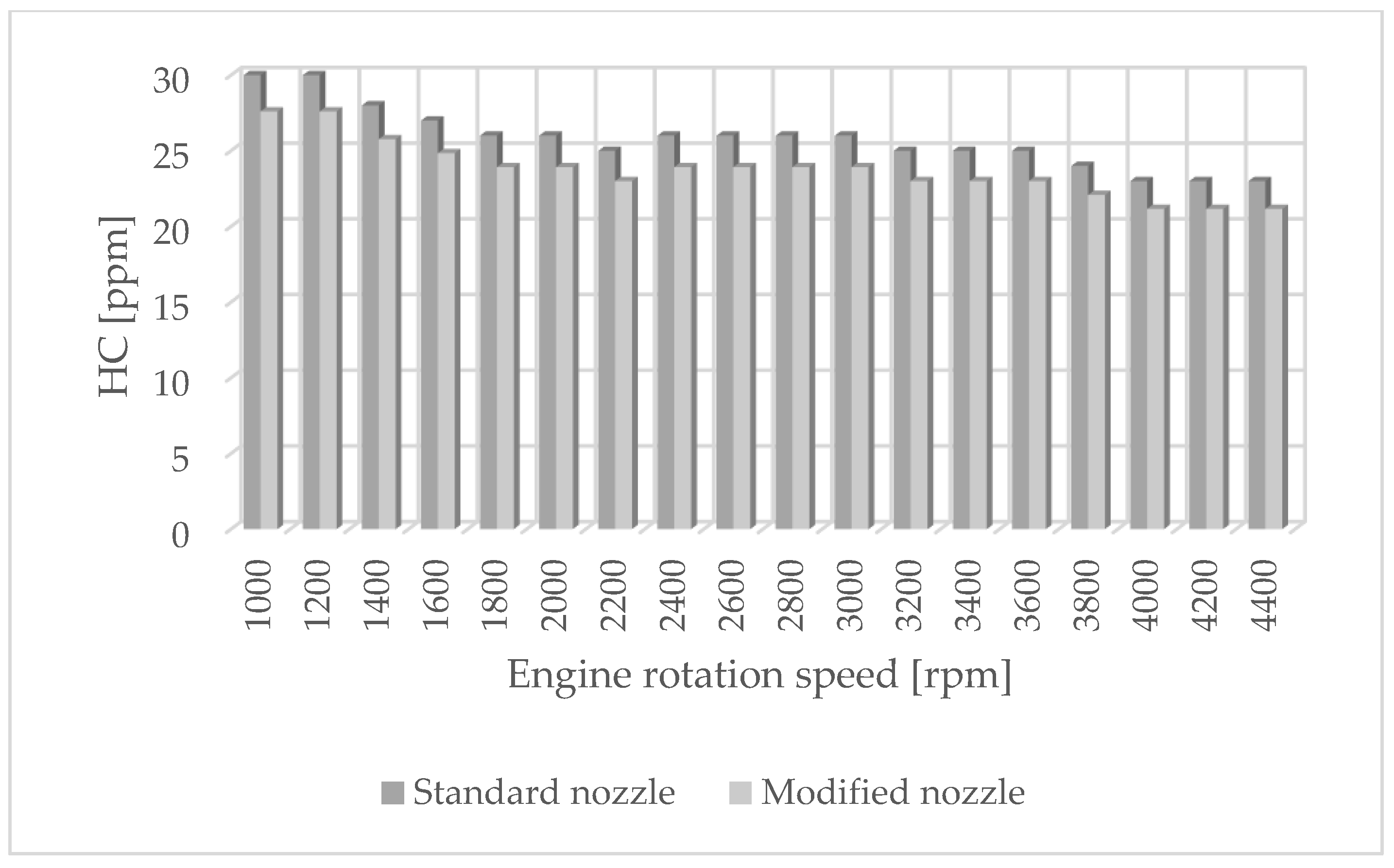
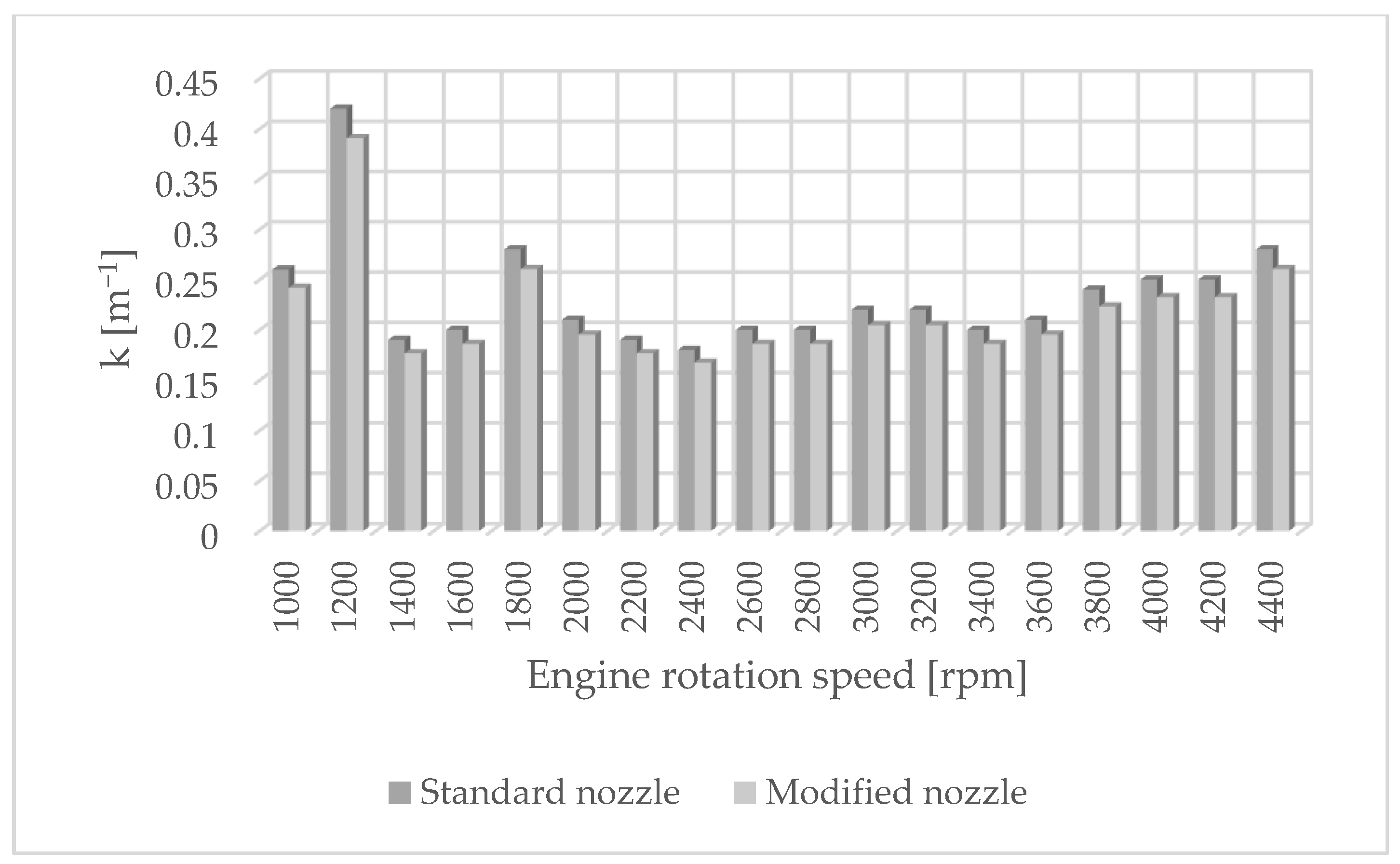
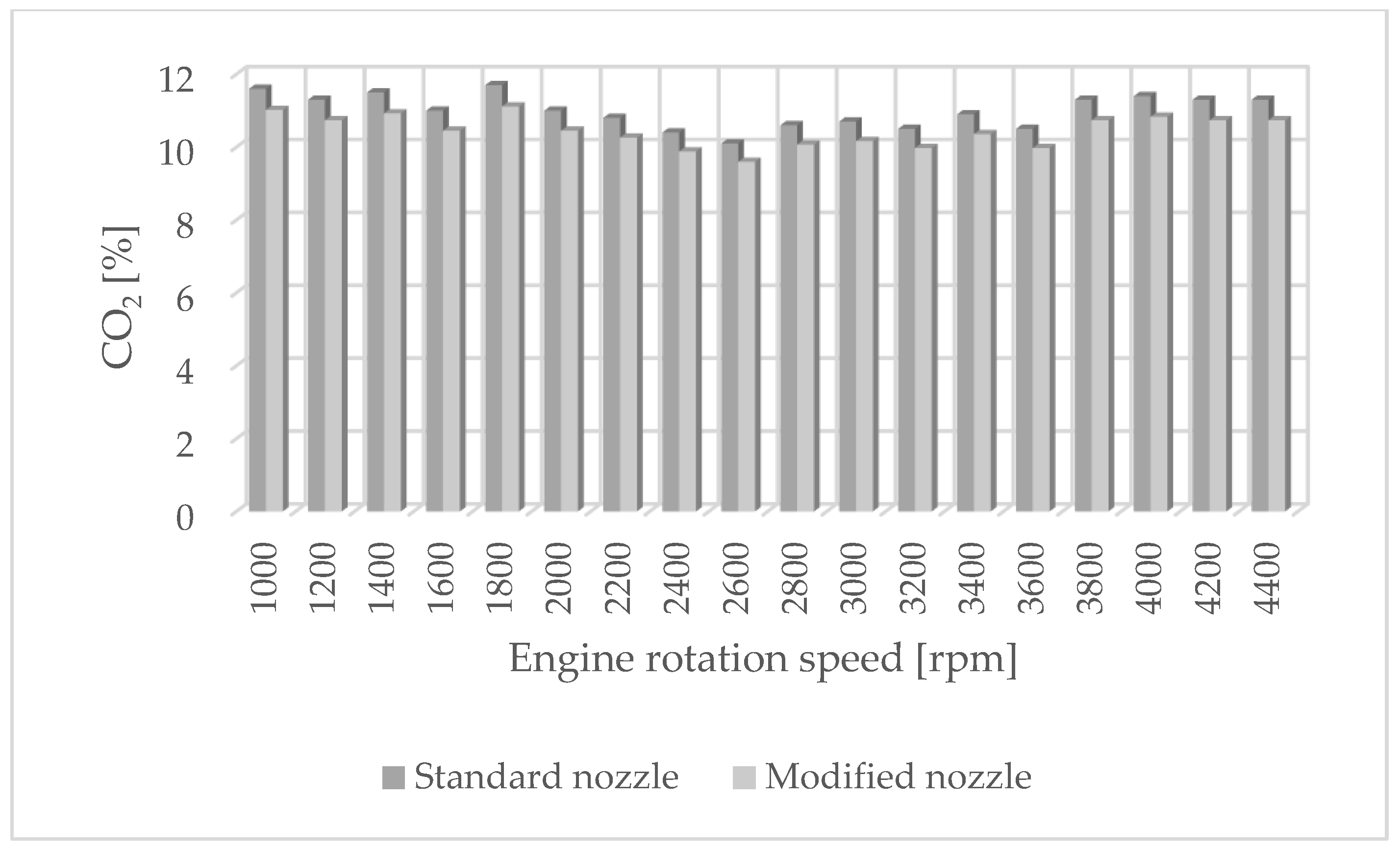
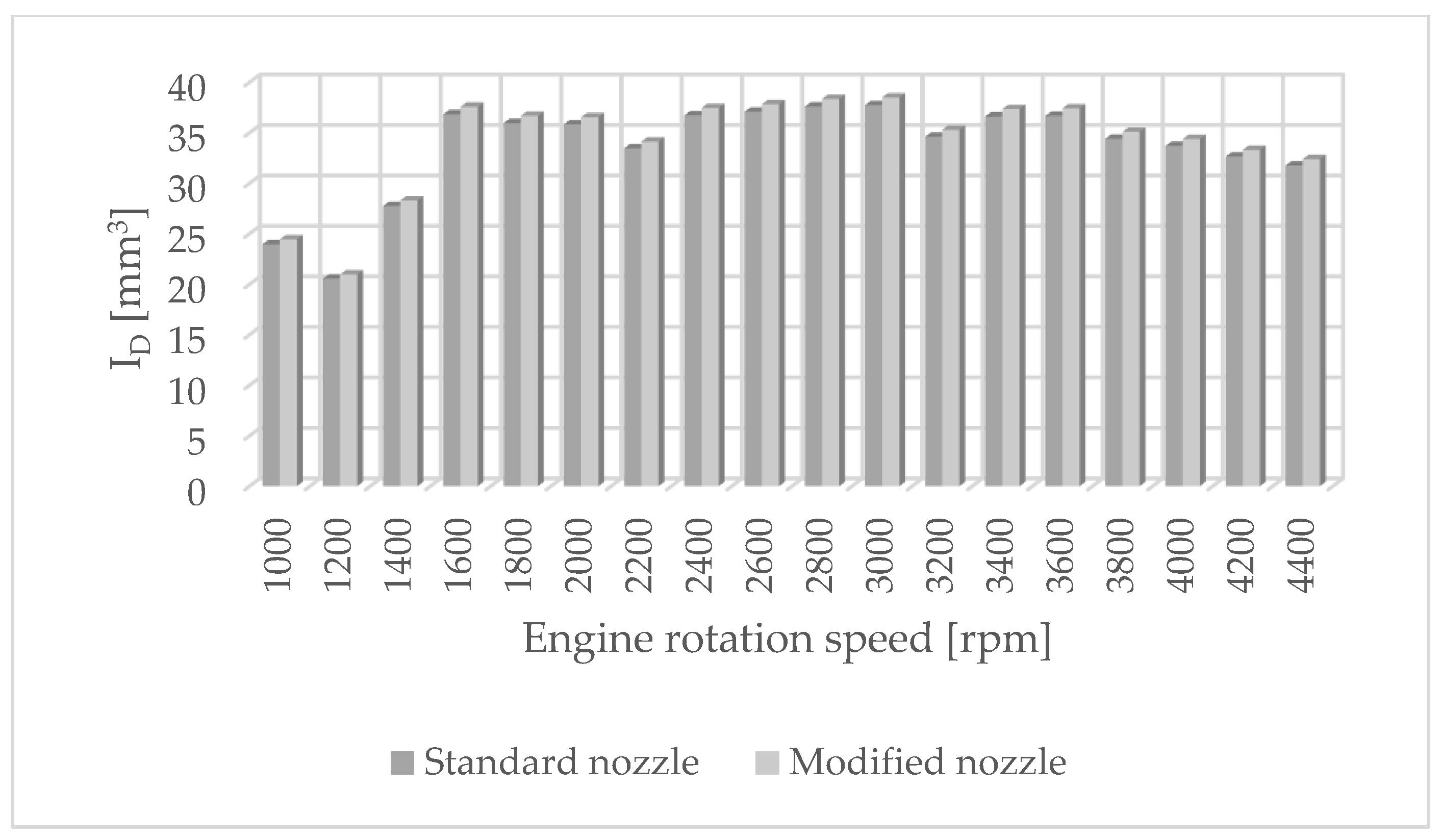
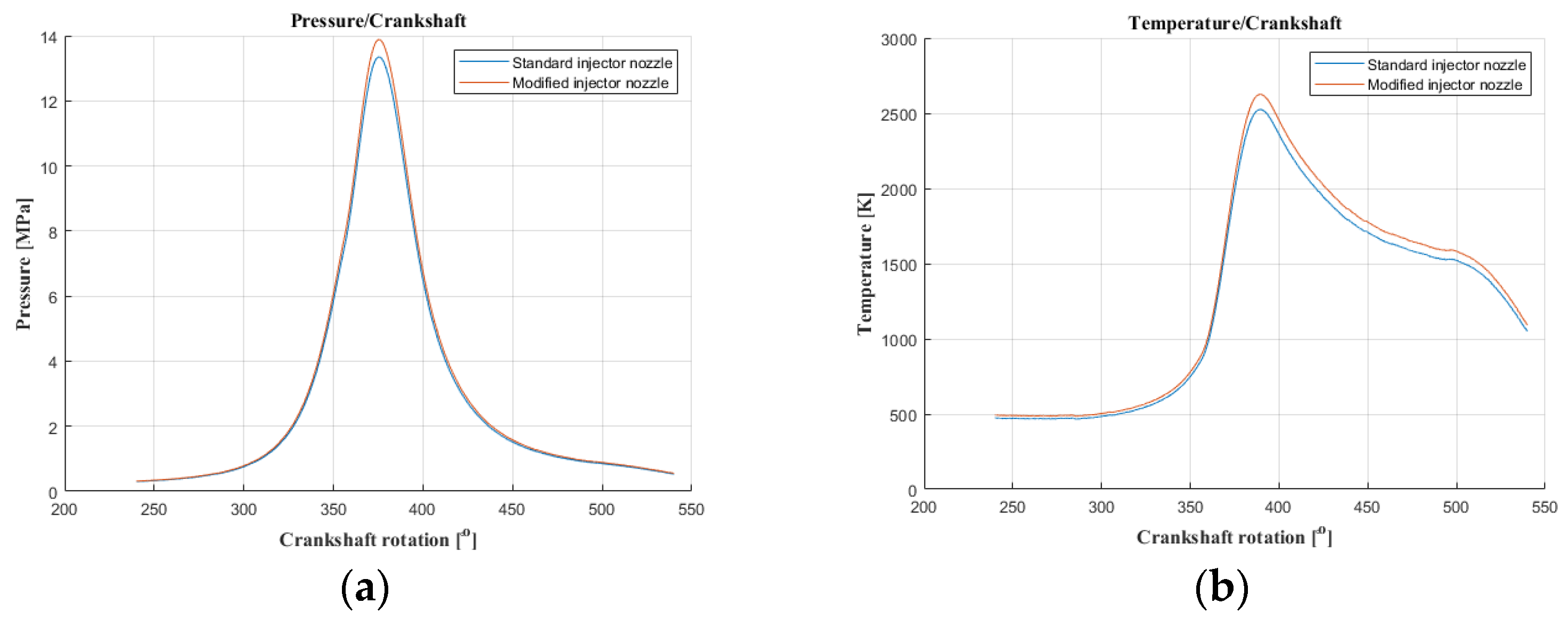
The authors of the paper performed tests using an engine dynamometer on a Fiat 1.3 JTD engine with a Common Rail system. During the tests, external ecological characteristics were measured as a function of the rotational speed of an engine powered by a fuel of plant origin—rapeseed oil methyl ester. The engine operated with standard and modified injectors. The results of measurements of ecological parameters are presented in Figure 1, Figure 2, Figure 3 and Figure 4. Figure 5 shows the injection dose values, and Figure 6 shows the pressure and temperature course in the engine combustion chamber at a rotational speed of 4000 [rpm]. This is the rotational speed at which the engine obtains maximum power.
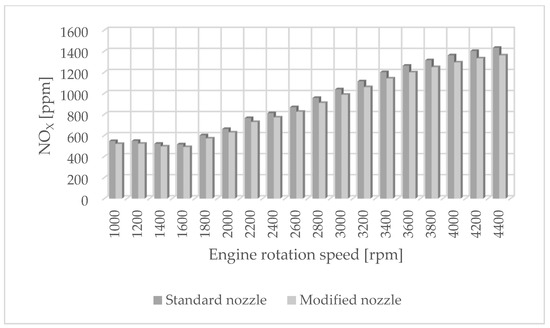
Figure 1.
NOX emissions for standard and modified nozzles at different engine rotation speeds.
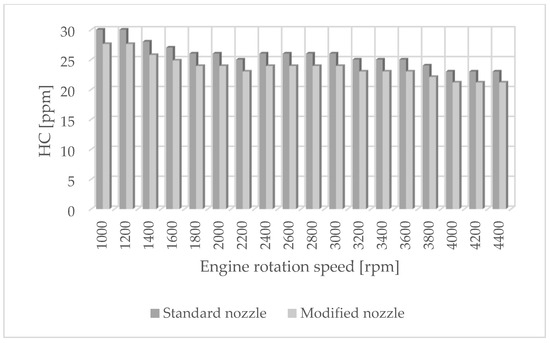
Figure 2.
HC emissions for standard and modified nozzles at different engine rotation speeds.
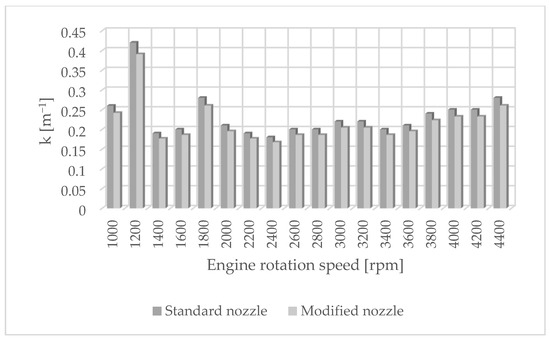
Figure 3.
Solid-particle emissions for standard and modified nozzles at different engine rotation speeds.
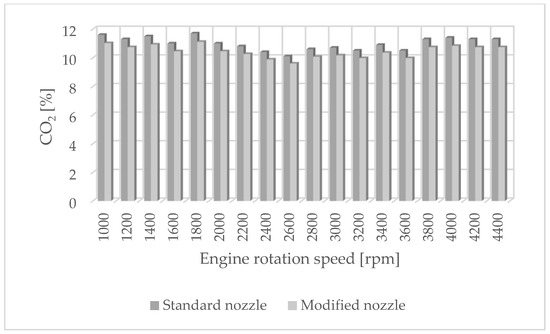
Figure 4.
CO2 emissions for standard and modified nozzles at different engine rotation speeds.
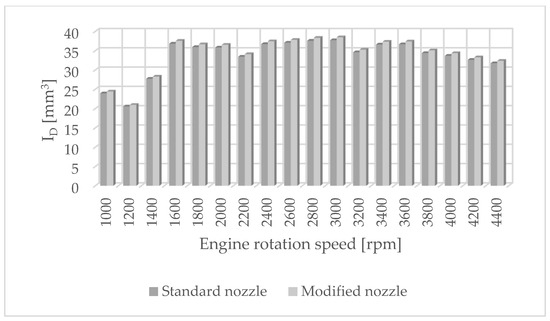
Figure 5.
Injection dosage values for standard and modified nozzles at different engine rotation speeds.
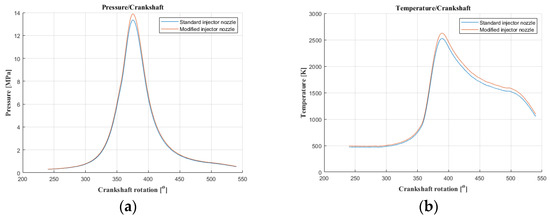
Figure 6.
The course of pressure and temperature changes in the engine chamber during combustion at an engine speed of 4000 [rpm] (pressure (a); temperature (b)).
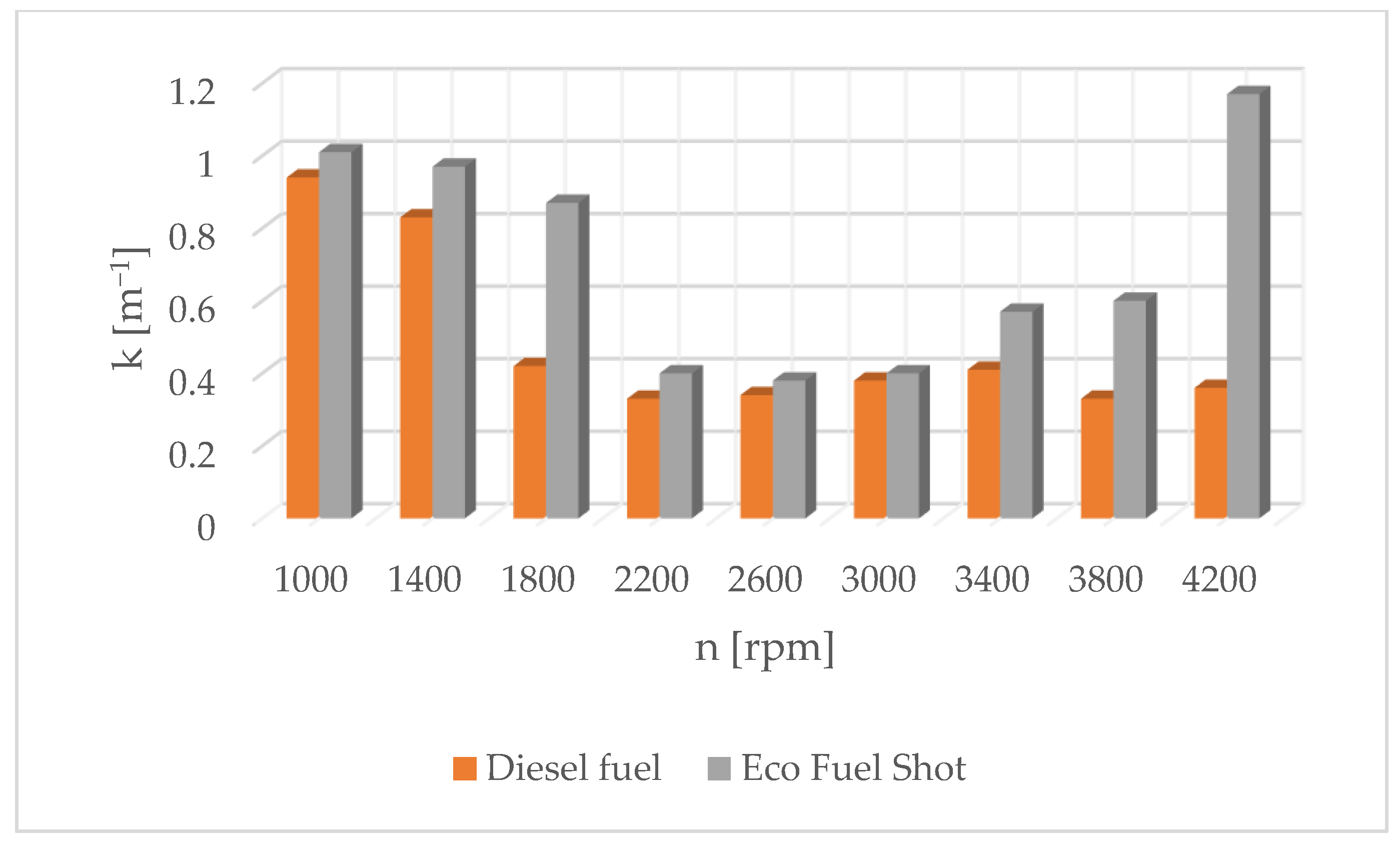
Figure 1 shows the influence of rotational speed on NOx emissions. It can be observed that as the rotational speed increases, the concentration of nitrogen oxides increases in proportion to the engine speed. On the other hand, Figure 2 shows hydrocarbon emissions. The course of this characteristic is similar to that in Figure 1. Figure 3 contains the results of measurements of the infrared radiation absorption coefficient k. The value of the coefficient is almost constant and changes only as a result of the dynamic acceleration of the engine.
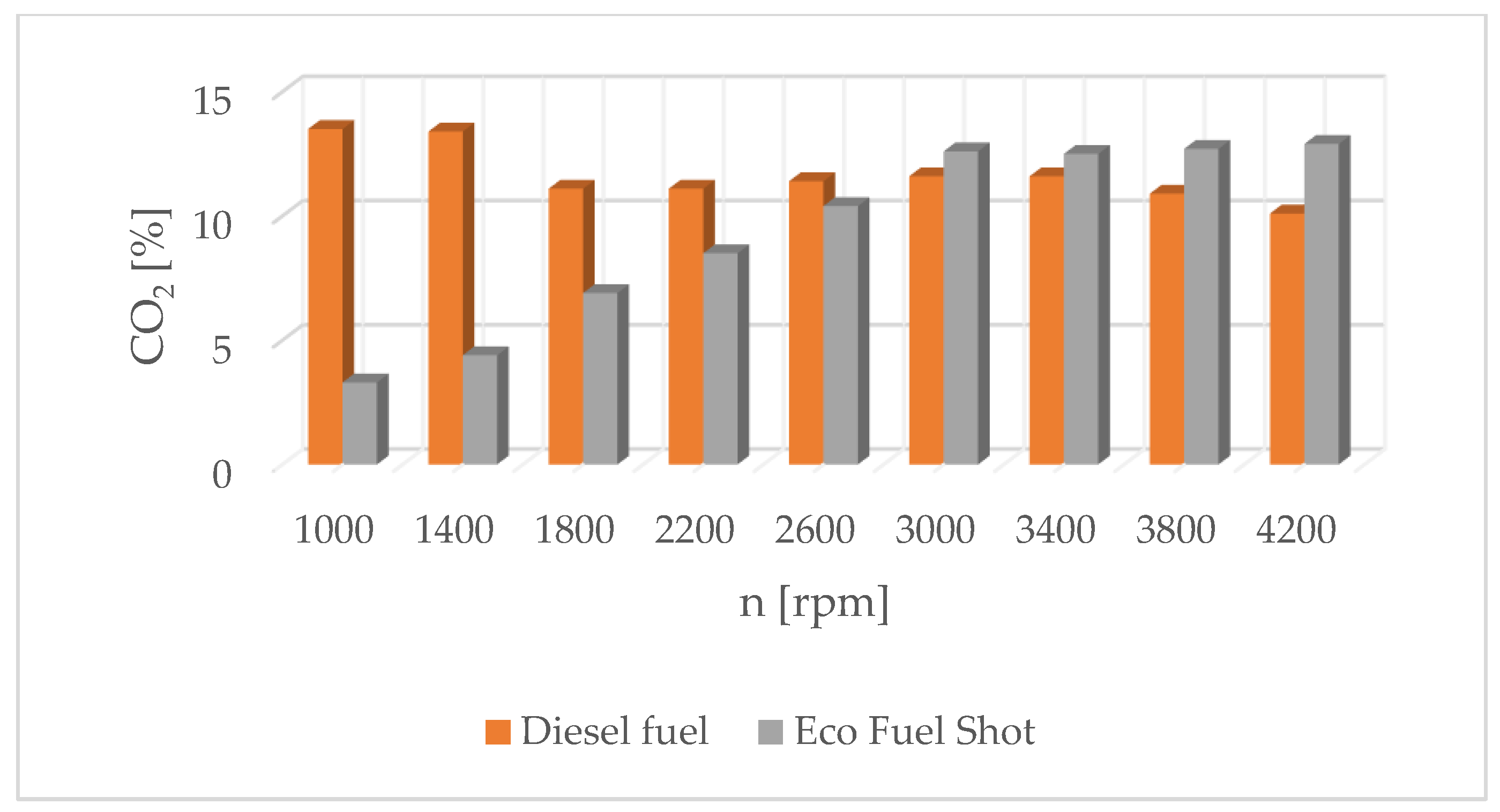
The results of carbon dioxide measurements are presented in Figure 4. CO2 emissions are at a constant level, close to 11%. Figure 5 shows the measurement results of the fuel injection dose read from the measurement interface at a given engine speed. Figure 6 shows an indicator chart made for an engine speed of n = 4000 [rpm] and for the maximum fuel dose (external characteristics).
5. Analysis of the Possibility of Using a Liquid Catalytic Converter and Hydrogen-Containing Environments in Diesel Engine Fuel Infrastructure
Catalytic converters in motor vehicles are mainly found in exhaust systems. Their task is to neutralize toxic substances such as nitrogen oxides, hydrocarbons, carbon monoxide and particulate matter. This type of catalytic converter does not affect the combustion process but only supports the purification of exhaust gases [26,53]. The task of the liquid catalyst is to support combustion processes with hydrogen and prevent the formation and neutralization of toxic substances during the combustion process. In addition, the catalyst prevents the formation of hydrocarbon bacteria in the fuel, which are responsible for the aging process.
Plant-based fuels are characterized by the fact that they age faster due to the content of oxygen molecules. This promotes the multiplication of bacteria in the fuel. The fuel uses the Eco Fuel Shot catalyst. According to the literature, one of the ways to use catalytic converters is to install them in the combustion chamber of a diesel engine. The authors used catalysts based on vanadium. The tests were carried out on a single-cylinder, four-stroke CI engine. The results of the analysis showed that nitrogen oxides and hydrocarbons were reduced by 70%, carbon monoxide by 60% and carbon dioxide by 50% [27,28,54,55]. A platinum–aluminum catalytic converter was installed in the engine. Studies have shown that the catalytic converter reduces nitrogen oxides from exhaust gases. The mixture of fuel and hydrogen increases the pressure in the cylinder and the emission of nitrogen oxides and hydrocarbons and reduces smoke [29,56] can initiated the hydrogen embrittlement of infrastructure structural materials. However, the use of natural gas and methane reduces nitrogen oxide emissions. In addition, studies have shown that the use of an additional catalyst reduces the opacity of these fuels. In diesel engines, the removal of nitrogen oxides from the exhaust gases is carried out by urea AdBlue, a substance containing ammonia [30,57]. However, this substance is expensive and requires frequent replenishment. The authors of [31,58] considered an emulsion (W/O) as a hydrogen-containing fuel for CI engines and behavior of solid lubricant component properties. Test results showed that adding water to the fuel reduces nitrogen oxide emissions by 19.6% and smoke emissions by 66.3%. According to the authors, this is because water lowers the combustion temperature caused by the absorption of evaporation heat and micro-explosions caused by the rapid evaporation of water particles. This allows nitrogen oxides and exhaust gas opacity to be reduced at the same time. It is possible to produce an emulsion fuel from non-biodegradable plastic carrier bags (PGBs) [32,59]. Studies have shown that the use of a water–oil emulsion with PGBs reduces the emission of smoke and nitrogen oxides. The emulsion with 10% diesel oil was characterized by the highest exergetic efficiency [33,60]. Nanoparticles in the form of cerium oxide can be added to diesel fuel, and the results of such a study are presented in [34,61]. Their results showed that the use of this type of catalytic converter reduces soot and hydrocarbon emissions by 30% at a partial engine load. However, at the maximum engine load, a slight reduction in nitrogen oxide emissions of around 5% was observed. No difference in hydrogen-containing fuel consumption was noticed at either the average or maximum engine load. A previous article [35,62] analyzed the effect of mixtures of diesel fuel with methanol, ethanol, n-butanol, methanol/n-butanol and ethanol/n-butanol on combustion. The results of the study showed that, compared to pure diesel, the use of admixtures shortened the delay period of spontaneous ignition and improved the combustion process. The most favorable results were obtained with a mixture of 80% diesel, 10% methanol and 10% n-butanol.
Previous research describes the use of a catalytic converter in the fuel injector nozzle (and its tribotechnical properties) in an engine with direct mechanical hydrogen-containing fuel injection [36,37,63,64]. Studies have shown that the use of a platinum catalytic converter on the non-functioning part of the needle reduced the emission of toxic substances into the atmosphere. Another paper [38,65] presents a method for the partial dehydrogenation of diesel fuel to produce high-quality hydrogen. The aim of the study was to assess the possibility of using such a system in motor vehicles. The catalyst used in the experiment was a platinum base and aluminum. Studies [39,40,66,67] have shown that a suitable element used as a catalyst to activate the hydrocarbon dehydrogenation reaction is tin–aluminum-based platinum. Development trends in internal combustion engines are aimed at reducing toxic substances in exhaust gases. In [39,40,41,42,68,69], the results of tests on an engine powered by ethanol as a hydrogen-containing fuel additive are presented. The content of the additive reduced the emission of nitrogen oxides in the exhaust gases. In [43,70], the author proposed powdered biofuel as an additive to the air in the intake system. An analysis was carried out in terms of engine durability. Studies have shown that it is possible to use this type of solution in internal combustion engines.
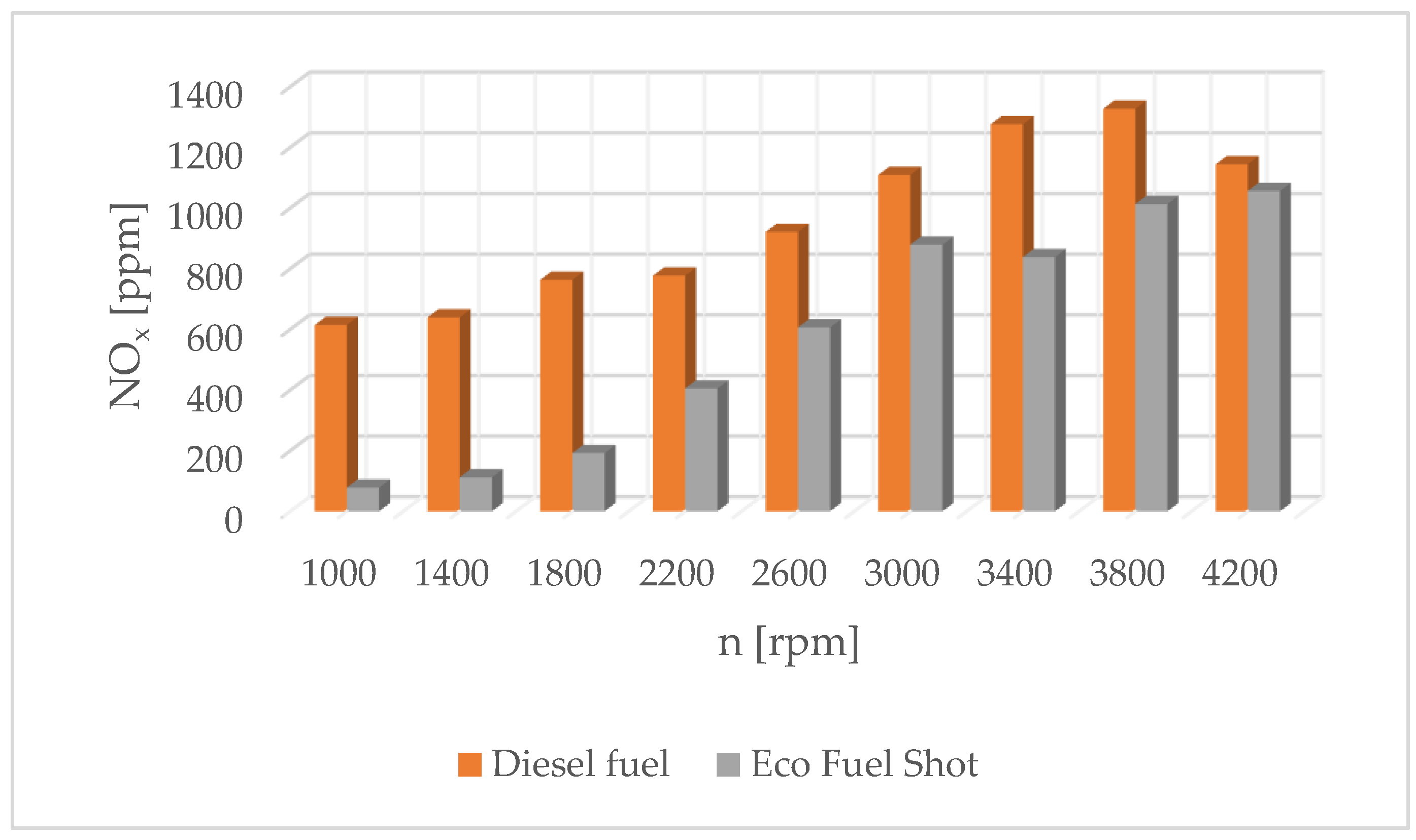
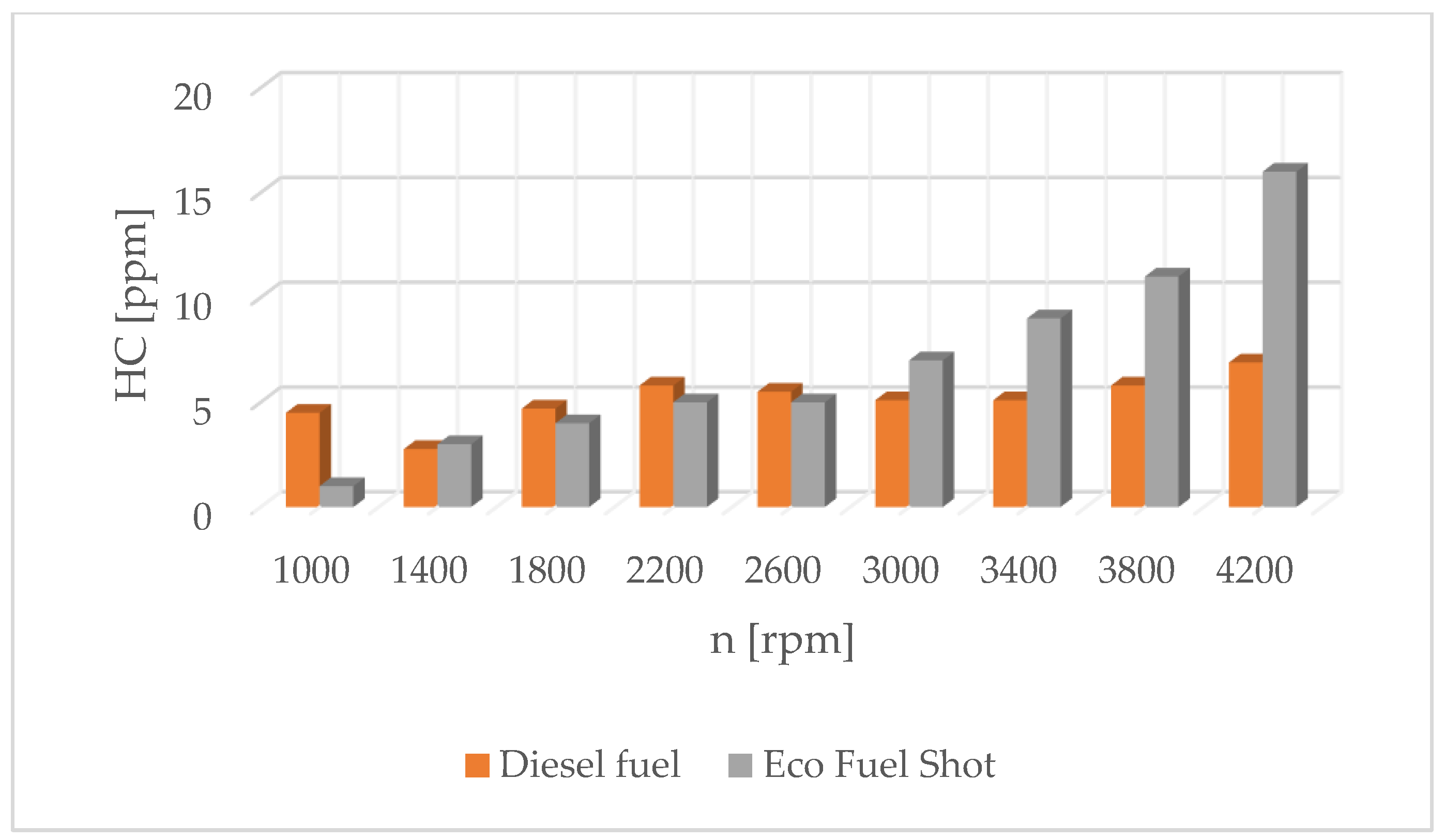
Analyzing the literature on the scope of the research carried out, it can be concluded that research on the use of catalytic converters in combustion engines is carried out in various directions. One of the possibilities to reduce emissions and fuel consumption is to use the Eco Fuel Shot liquid catalyst [71]. The authors’ research results are presented in Figure 7, Figure 8, Figure 9 and Figure 10.
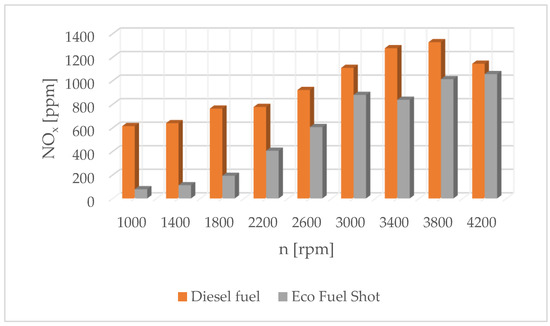
Figure 7.
Concentration of nitrogen oxides in exhaust gases for diesel and Eco Fuel Shot.
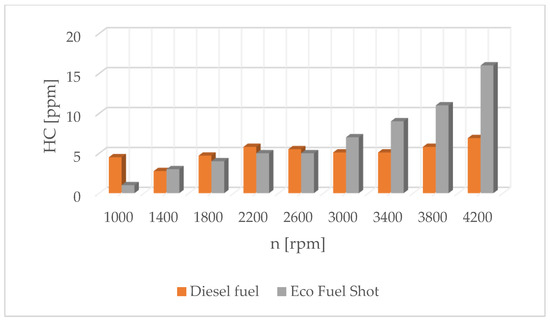
Figure 8.
Concentration of hydrocarbons in exhaust gases for diesel and Eco Fuel Shot.
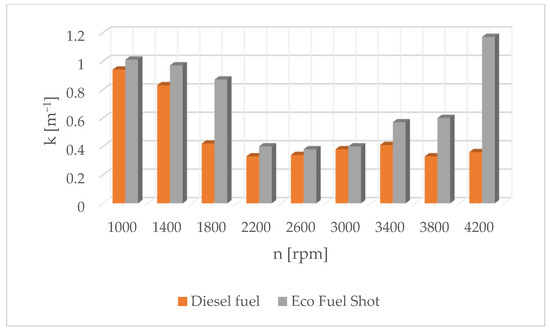
Figure 9.
Soot content in exhaust gases for diesel and Eco Fuel Shot.
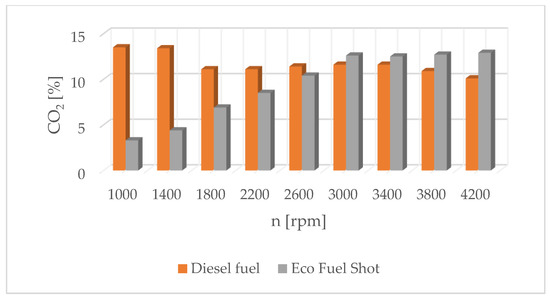
Figure 10.
Carbon dioxide content in exhaust gases for diesel and Eco Fuel Shot.
Combustion in a CI engine is a complex, fast-occurring chemical reaction that is accompanied by the release of a large amount of heat. The heat of combustion is the thermal effect of the oxidation reaction of a given compound with oxygen and the formation of elements that are products of combustion. The heat of combustion of a chemical compound and the amounts and types of combustion products depend on the temperature and pressure prevailing during combustion. During the combustion of the fuel–air mixture, toxic substances are produced, which are ejected from the combustion chamber into the environment. The concentration of toxins and their type depend on the conditions in the cylinder. An internal combustion engine is an energy device in which a heterogeneous combustion process occurs. It is based on the fact that the surface temperature of the fuel in the liquid or solid phase is lower than the temperature of autoignition. The process of the evaporation and gasification of the fuel just before spontaneous ignition is very important, as it affects all stages of combustion. As a result of the evaporation and diffusion of gases, a fuel–air mixture is formed. If the composition and temperature of the mixture and the duration of pre-ignition processes are sufficient for spontaneous ignition, the combustion process occurs, which intensifies because of the heat released. During heterogeneous combustion, the combustion process takes place in the vapor phase. The heat of the flame is sufficient for the intensive degassing of the liquid, and the speed of chemical reactions in the gas phase is much higher than at the surface due to the higher kinetic energy of the thermal motion of the vapor particles and the greater number of probable collisions of the fuel particles with the oxidizer. Alternative fuels containing hydrogen combustion involve a series of successive or simultaneously occurring processes: heat supply to the surface of the liquid, its heating and evaporation, the mutual diffusion of fuel vapors, the oxidizer and active seeds of the chemical reaction, spontaneous ignition and combustion. The combustion speed is limited by the time required for the evaporation of the fuel and the diffusive mixing of the vapors with the surrounding fuel. If the chemical reactions in the combustible mixture occur quickly, the combustion speed is limited by the evaporation rate as the slowest process, while if the charge temperature is not too high, the combustion is limited by the speed of chemical reactions occurring in the homogeneous fuel–air mixture. Fuel is supplied to the engine’s combustion chamber by means of injectors. The stream flowing out of the nozzle holes breaks down into a collection of chaotic droplets. They have different dynamics and structure. Their evaporation and combustion depend on the conditions in the engine compartment and the physical and chemical properties of the fuel, such as its viscosity, density, surface tension and cetane number. The evaporation process of a fuel droplet can be divided into initial and non-stationary stages, when evaporation occurs under superheated conditions from the initial temperature to the equilibrium evaporation temperature. The ambient temperature affects the evaporation stage through the surface temperature of the droplet. Studies have shown that as the temperature increases, the temperature of the droplets increases, but not symmetrically: the higher the temperature of the droplets, the smaller the effect of ambient temperature. The influence of pressure in the cylinder is similar. As the pressure increases, the temperature of the droplets increases, but the higher the pressure, the lower the temperature increase. An accurate mathematical description of the presented phenomenon is impossible due to the nature of the course. The droplets ejected from the injector have different dimensions. Therefore, evaporation is referred to as the evaporation of individual droplets, and statistical methods are used to determine its course. Eco Fuel Shot is a liquid catalyst added to the fuel whose task is to reduce the emission of toxic substances in exhaust gases. Studies have shown that the use of a liquid catalyst increases the cetane number of the fuel by 3–5 units. In addition, the catalytic converter affects the physical parameters of fuels, which changes the characteristics of fuel droplets leaving the injector. The advantage of the Eco Fuel Shot catalyst is its low activation energy. This allows the carbon and hydrogen bonds in the fuel to break down more quickly, which improves the combustion process overall. The catalyst reacts efficiently with the substrates, thanks to which the chemical compound is quickly formed as the final product [71]. This leads to reduced emissions of toxins in exhaust gases. Substances that reduce the interfacial tension between two phases are called surfactants. These are active substances on the surface that influence the amount of activation energy between the reacting substrates in hydrogen, their influence as surface active environment in the course of hydrogen degradation of energetic, transport turbine and engine equipment’s, which need modification their hydrogen resistant properties for adaptation to work on greener alternative fuels [70,71,72,73,74,75,76,77,78,79,80,81,82,83].
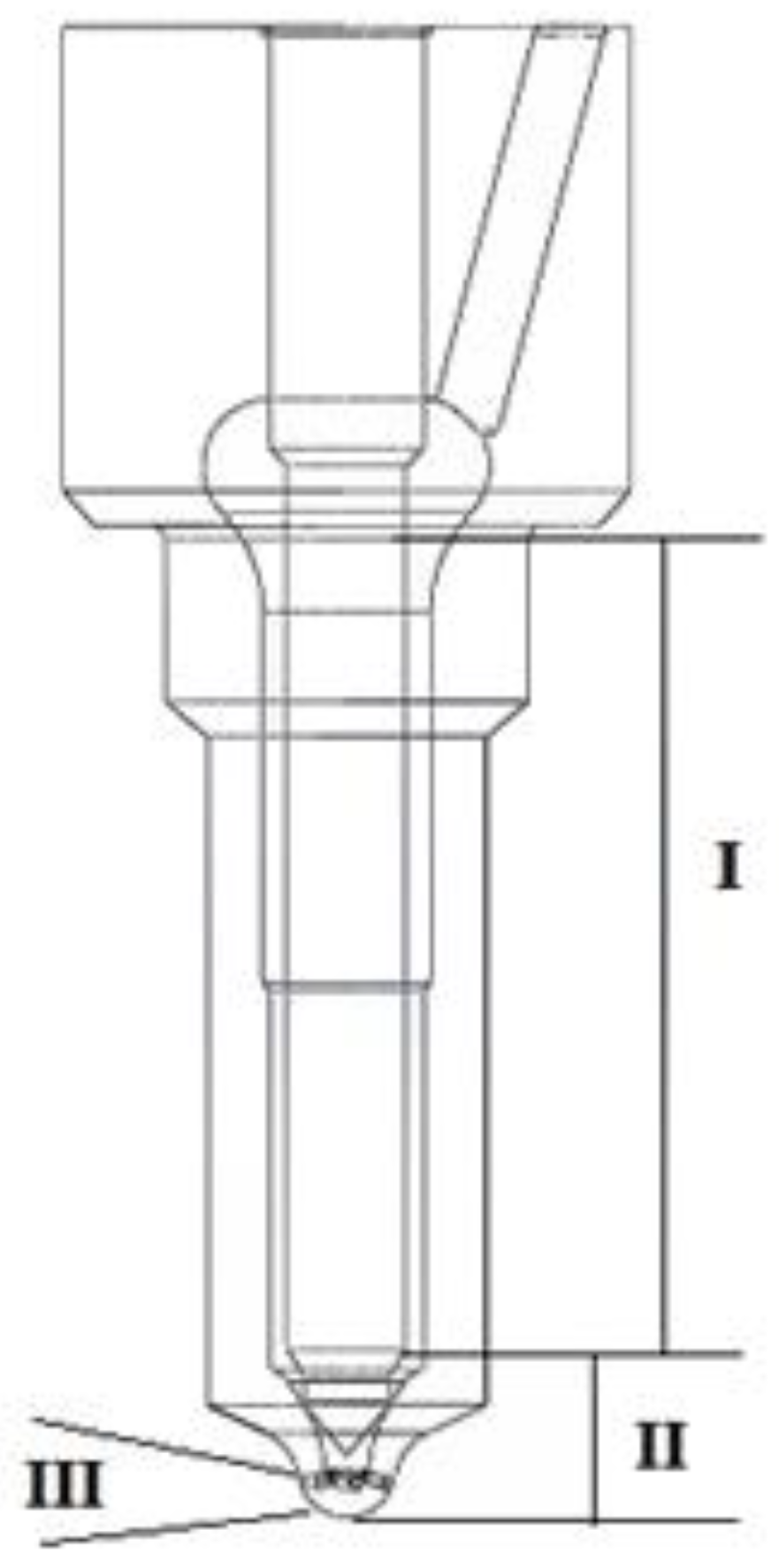
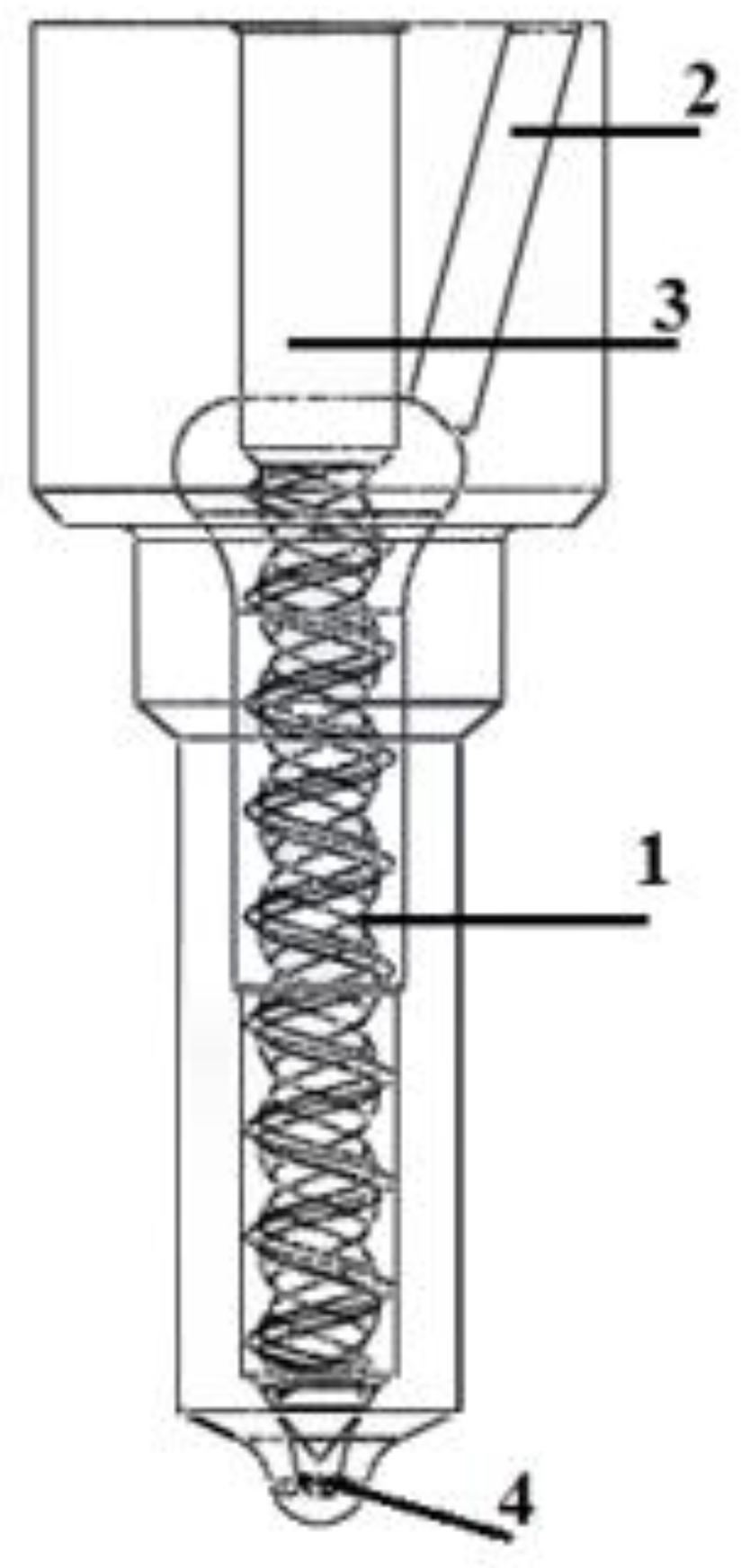
The application of a hydrogen-containing fuel required a sprayer modification with spiral–elliptical channels (Figure 11 and Figure 12).
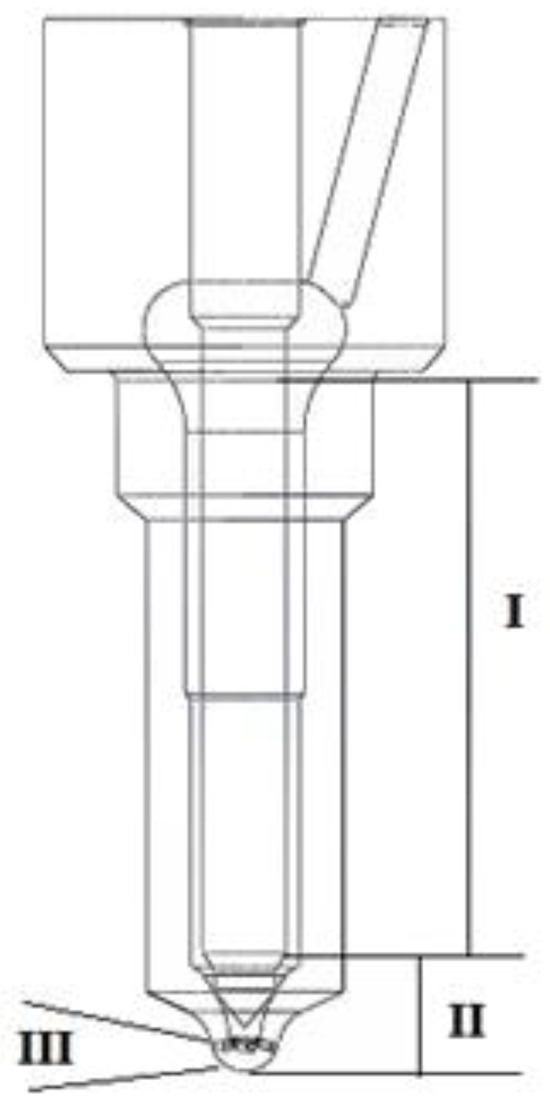
Figure 11.
The fuel atomizer divided into zones: I—the non-working part of the needle; II—the needle well; III—the outlet of the atomizer.
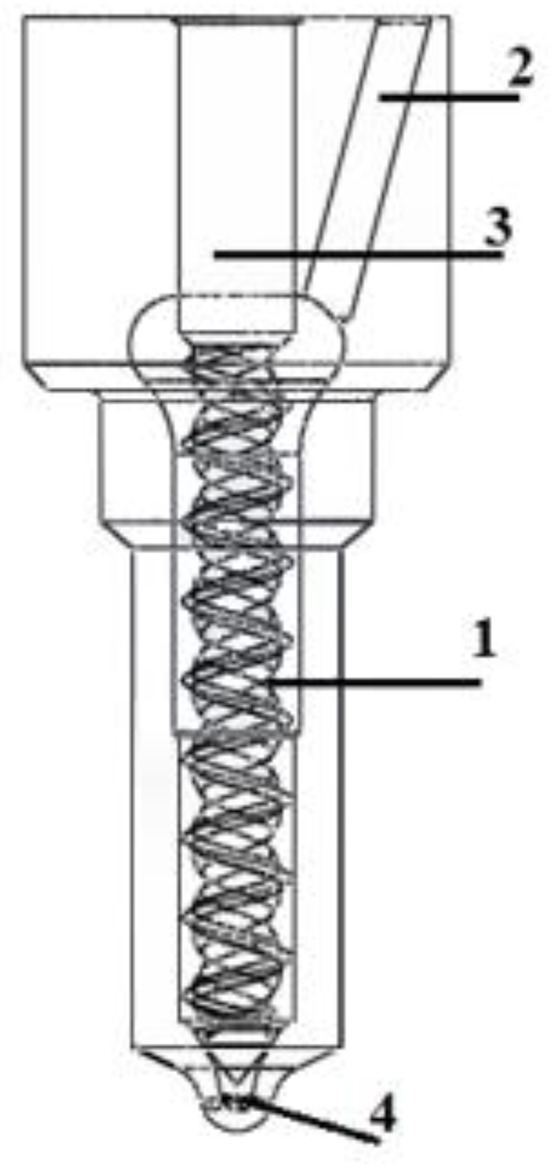
Figure 12.
The modified fuel atomizer: 1—channels in the non-working part of the needle; 2—the fuel supply channel; 3—the precision atomizer pair; and 4—the injection holes [4,14,81].
6. Conclusions
Combustion engines will continue to be used for the purpose of transport for years. City vehicles are equipped with electric drives, but commercial vehicles will continue to use combustion engines for many years to come. One of the directions for reducing toxic emissions from combustion vehicles is the use of alternative fuels. First-, second- and third-generation biofuels are known. However, first- and second-generation fuels are currently widely used. Development work on third-generation biofuels is still ongoing. The biggest problem is obtaining them on a large scale.
Analyzing the measurement results and the processes occurring during the formation of the fuel stream and the combustion of the flammable mixture, it can be concluded that one of the directions of the development of modern fuel supply equipment in combustion engines is the modification of atomizers aimed at increasing the turbulization of the fuel flow through them. The use of a catalytic additive in the fuel reduces emissions, mainly of nitrogen oxides and hydrocarbons. This happens because the catalytic converter increases the cetane number of the fuel, affecting the chemical properties of the fuel.
Vegetable fuels have a higher viscosity, surface tension and density compared to conventional fuel. This worsens the atomization process in the engine’s combustion chamber, which translates into less effective combustion of the flammable mixture. One of the possibilities to improve atomization and the entire combustion process is to modify the fuel injector atomizer needle. Research conducted by the authors shows that, in modern systems with the Common Rail system, this solution affects the physical parameters of the sprayed fuel stream and engine operation. The results of measurements of ecological engine parameters showed that all indicators for an engine operating with modified injectors were lower, especially hydrocarbon emissions and exhaust opacity.
This is because the physical parameters of the fuel stream and hydrogen-containing environments mainly influence the formation of carbon-based pollutants in the exhaust gases, while the emission of nitrogen oxides depends mainly on the combustion process and the temperature in the engine chamber. Engine tests have shown that despite the increased pressure and temperature in the combustion chamber, nitrogen oxide emissions slightly decrease, especially at higher rotational speeds. This may be because the higher temperature increases the efficiency of the exhaust gas catalytic converter in the engine exhaust system. It should be noted that the injection dose values read from the current engine parameters were at a similar level. This means that the changes made only affect the qualitative operating parameters of the fuel injector, not the quantitative ones.
Author Contributions
The scope of work of the individual authors during the performance of this project was the same. The authors performed the study together and then analyzed its findings. The authors equally contributed to the paper assembly. Partially: conceptualization, A.I.B. and K.F.A.; data curation, T.K.O. and M.M.; formal analysis, J.J.E. and A.I.B.; investigation, T.K.O. and M.M.; methodology, J.J.E., A.I.B. and K.F.A.; writing—original draft, A.I.B.; writing—review and editing, A.I.B. and K.F.A., software, T.K.O.; validation, A.I.B. and K.F.A.; resources, K.F.A. and A.I.B.; visualization, T.K.O.; supervision, A.I.B.; project administration, A.I.B.; funding acquisition, A.I.B. All authors have read and agreed to the published version of the manuscript.
Funding
The Polish National Agency for Academic Exchange (NAWA) and the Ministry of Education and Science of Ukraine for partial support in the framework of project BPN/BUA/2021/1/00003/U/00001 (Contract M/66-2024), “Evaluation of the long-term new materials durability for structural elements of green hydrogen production and transportation infrastructure”.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the Polish National Agency for Academic Exchange (NAWA) and the Ministry of Education and Science of Ukraine for partial support.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Reitz, R.; Ogawa, H.; Payri, R.; Fansler, T.; Kokjohn, S.; Moriyoshi, Y.; Agarwal, A.; Arcoumanis, D.; Assanis, D.; Bae, C.; et al. The Future of the Internal Combustion Engine. Int. J. Engine Res. 2020, 21, 3–10. [Google Scholar] [CrossRef]
- Bocheński, C.I. Biodiesel Paliwo Rolnicze; Wydawnictwo SGGW: Warsaw, Poland, 2003. [Google Scholar]
- Bocheński, C.I. Paliwa i Oleje Smarujące w Rolnictwie; Wydawnictwo SGGW: Warsaw, Poland, 2005. [Google Scholar]
- Eliasz, J.; Osipowicz, T.; Abramek, K.F.; Mozga, Ł. Model Issues Regarding Modification of Fuel Injector Components to Improve the Injection Parameters of a Modern Compression Ignition Engine Powered by Biofuel. Appl. Sci. 2019, 9, 5479. [Google Scholar] [CrossRef]
- Han, D.; Zhai, J.; Duan, Y.; Ju, D.; Lin, H.; Huang, Z. Macroscopic and microscopic spray characteristics of fatty acid esters on a common rail injection system. Fuel 2017, 203, 370–379. [Google Scholar] [CrossRef]
- Marcic, S.; Marcic, M.; Wensing, M.; Vogel, T.; Praunseis, Z. A simplified model for a diesel spray. Fuel 2018, 222, 485–495. [Google Scholar] [CrossRef]
- Kegl, B.; Lesnik, L. Modeling of macroscopic mineral diesel and biodiesel spray characteristics. Fuel 2018, 222, 810–820. [Google Scholar] [CrossRef]
- Chen, Z.; Yao, A.; Yao, C.; Yin, Z.; Xu, H.; Geng, P.; Dou, Z.; Hu, J.; Wu, T.; Ma, M. Effect of fuel temperature on the methanol spray and nozzle internal flow. Appl. Therm. Eng. 2017, 114, 673–684. [Google Scholar] [CrossRef]
- Bohl, T.; Tian, G.; Smallbone, A.; Roskilly, A.P. Macroscopic spray characteristic of next-generation bio-derived diesel fuels in comparison to mineral. Appl. Energy 2017, 186, 562–573. [Google Scholar] [CrossRef]
- Ghahremani, A.R.; Saidi, M.H.; Hajinezhad, A.; Mozafari, A.A. Experimental investigation of spray characteristics of a modified bio-diesel in a direct injection combustion chamber. Exp. Therm. Fluid Sci. 2017, 81, 445–453. [Google Scholar] [CrossRef]
- Dmytrakh, I.M.; Leshchak, R.L.; Syrotyuk, A.M.; Lutyts’kyi, O.L. Influence of the bulk concentration of hydrogen in the metal on the specific features of deformation of low-alloy pipe steel. Mater. Sci. 2014, 50, 170–178. [Google Scholar] [CrossRef]
- Wang, L.; Lowrie, J.; Ngaile, G.; Fang, T. High injection pressure diesel sprays from a piezoelectric fuel injector. Appl. Therm. Eng. 2019, 152, 807–824. [Google Scholar] [CrossRef]
- Han, D.; Li, K.; Duan, Y.; Lin, H.; Huang, Z. Numerical study on fuel physical effects on the split injection process on a Common Rail injection system. Energy Convers. Manag. 2017, 134, 47–58. [Google Scholar] [CrossRef]
- Chiatti, G.; Chiavola, O.; Palmieri, F. Vibration and acoustic characteristics of a city-car engine fueled with biodiesels blends. Appl. Energy 2017, 185, 664–670. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Dhar, A.; Gupta, J.G.; Kim, W.I.; Lin, C.S.; Park, S. Effect of fuel injection pressure and injection timing on spray characteristics and particulate size–number distribution in a biodiesel fuelled common rail direct injection diesel engine. Appl. Energy 2014, 130, 212–221. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Dhar, A.; Gupta, J.G.; Kim, W.I.; Lin, C.S.; Park, S. Effect of fuel injection pressure and injection timing of Karanja biodiesel blends on fuel spray, engine performance, emissions and combustion characteristics. Energy Convers. Manag. 2015, 91, 302–314. [Google Scholar] [CrossRef]
- Panchasara, H.; Ashwath, N. Effects of Pyrolysis Bio-Oils on Fuel Atomisation—A Review. Energies 2021, 14, 794. [Google Scholar] [CrossRef]
- Dabros, T.M.; Stummann, M.Z.; Høj, M.; Jensen, P.A.; Grunwaldt, J.D.; Gabrielsen, J.; Mortensen, P.M.; Jensen, A.D. Transportation fuels from biomass fast pyrolysis, catalytic hydrodeoxygenation, and catalytic fast hydropyrolysis. Prog. Energy Combust. Sci. 2018, 68, 268–309. [Google Scholar] [CrossRef]
- Hossain, A.; Davies, P. Pyrolysis liquids and gases as alternative fuels in internal combustion engines—A review. Renew. Sustain. Energy Rev. 2013, 21, 165–189. [Google Scholar] [CrossRef]
- Mueller, C.J. The feasibility of using raw liquids from fast pyrolysis of woody biomass as fuels for compression-ignition engines: A literature review. SAE Int. J. Fuels Lubr. 2013, 6, 251–262. [Google Scholar] [CrossRef]
- Berlini, R.; Da Costa, R.; Roque, L.F.A.; De Souza, T.A.Z.; Coronado, C.J.R.; Pinto, G.M.; Cintra, A.J.A.; Raats, O.O.; Oliveira, B.M.; Frez, G.V.; et al. Experimental assessment of renewable diesel fuels (HVO/Farnesane) and bioethanol on dual-fuel mode. Energy Convers. Manag. 2022, 258, 115554. [Google Scholar]
- De Souza, T.; Pinto, G.M.; Julio, A.A.V.; Coronado, C.J.R.; Perez-Herrera, R.; Siqueira, S.; Da Costa, R.B.R.; Roberts, J.J.; Palacio, J.C.E. Biodiesel in South American countries: A review on policies, stages of development and imminent competition with hydrotreated vegetable oil. Renew Sustain Energy Rev. 2022, 153, 11175. [Google Scholar] [CrossRef]
- Dimitriadis, A.; Seljak, T.; Vihar, R.; Zvar Baskovic, U.; Dimaratos, A.; Bezergianni, S.; Samaras, Z.; Katrašnik, T. Improving PM-NOx trade-off with paraffinic fuels: A study towards diesel engine optimization with HVO. Fuel 2020, 265, 116921. [Google Scholar] [CrossRef]
- Valeika, G.; Matijosius, J.; Rimkus, A. Research of the impact of EGR rate on energy and environmental parameters of compression ignition internal combustion engine fuelled by hydrogenated vegetable oil (HVO) and biobutanol—Castor oil fuel mixtures. Energy Convers. Manag. 2022, 270, 116198. [Google Scholar] [CrossRef]
- Łagowski, P.; Wcisło, G.; Kurczyński, D. Comparison of the Combustion Process Parameters in a Diesel Engine Powered by Second-Generation Biodiesel Compared to the First-Generation Biodiesel. Energies 2022, 15, 6835. [Google Scholar] [CrossRef]
- Vembathu Rajesh, A.; Mathalai Sundaram, C.; Sivaganesan, V.; Nagarajan, B.; Harikishore, S. Emission reduction techniques in CI engine with catalytic converter. Mater. Today Proc. 2020, 21, 98–103. [Google Scholar] [CrossRef]
- Udhayakumar, N.; Mani, M.; Ramesh Babu, S.; Karthikkeyan, G.; Peace John Samuel, K.; Karna, V. An experimental investigation on emission characteristics in CI engine with zinc and vanadium coated catalytic converter. Mater. Today Proc. 2022, 62, 2250–2255. [Google Scholar] [CrossRef]
- Karthickeyan, V.; Thiyagarajan, S.; Edwin Geo, V.; Ashok, B.; Nanthagopal, K.; Chyuan, O.H.; Vignesh, R. Simultaneous reduction of NOx and and smoke emissions with low viscous biofuel in low heat rejection engine using selective catalytic reduction technique. Fuel 2019, 255, 115854. [Google Scholar] [CrossRef]
- Abu-Jrai, A.M.; Al-Muhtaseb, A.H.; Hasan, A.O. Combustion, performance, and selective catalytic reduction of NOx for a diesel engine operated with combined tri fuel (H2, CH4, and conventional diesel). Energy 2017, 115, 901–910. [Google Scholar] [CrossRef]
- Guziałkowska Tic, J.; Tic, W.J. Modyfikatory stosowane w procesie spalania olejów opałowych i paliw stałych. CHEMIK 2012, 66, 1203–1210. [Google Scholar]
- Park, J.; Oh, J. Study on the characteristics of performance, combustion, and emissions for a diesel water emulsion fuel on a combustion visualization engine and a commercial diesel engine. Fuel 2022, 311, 122520. [Google Scholar] [CrossRef]
- Saha, D.; Roy, B. Effects of plastic-grocery-bag derived oil-water-diesel emulsions on combustion, performance and emission characteristics, and exergo economic aspects of compression ignition engine. Sustain. Energy Technol. Assess. 2022, 54, 102877. [Google Scholar]
- Dogan, B.; Celik, M.; Bayindirli, C.; Erol, D. Exergy, exergyeconomic, and sustainability analyses of a diesel engine using biodiesel fuel blends containing nanoparticles. Energy 2023, 274, 127278. [Google Scholar] [CrossRef]
- Leach, F.C.P.; Davy, M.; Terry, B. Combustion and emissions from cerium oxide nanoparticle dosed diesel fuel in a high speed diesel research engine under low temperature combustion (LTC) conditions. Fuel 2021, 288, 119636. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, J.; Li, J.; Lv, J.; Wang, S.; Zhong, Y.; Dong, R.; Gao, S.; Cao, C.; Tan, D. Investigation on combustion, performance and emission characteristics of a diesel engine fueled with diesel/alcohol/n-butanol blended fuels. Fuel 2022, 320, 123975. [Google Scholar] [CrossRef]
- Klyus, O. The use of turbulization in preliminary fuel treatment in self-ignition engines. Combust. Engines 2009, 138, 49–53. [Google Scholar] [CrossRef]
- Sa, B.; Klyus, O.; Markov, V.; Kamaltdinov, V. A numerical study of the effect of spiral counter grooves on a needle on flow turbulence in a diesel injector. Fuel 2021, 290, 120013. [Google Scholar] [CrossRef]
- Gianotti, E.; Taillades-Jacquin, M.; Carmona, A.R.; Taillades, G.; Rozière, J.; Jones, D.J. Hydrogen generation via catalytic partial dehydrogenation of gasoline and diesel fuels. Appl. Catal. B Environ. 2016, 185, 233–241. [Google Scholar] [CrossRef]
- Wang, N.; Qiu, J.; Wu, J.; Yuan, X.; You, K.; Luo, H. Microwave assisted synthesis of Sn–modified MgAlO as support for platinum catalyst in cyclohexane dehydrogenation to cyclohexene. Appl. Catal. A Gen. 2016, 516, 9–16. [Google Scholar] [CrossRef]
- Echeverri, A.; Gomez, T.; Hadad, C. Ammonia borane dehydrogenation tendencies using Pt4, Au4, and Pt2Au2 clusters as catalysts. Mol. Catal. 2019, 471, 9–20. [Google Scholar] [CrossRef]
- Lodi, F.; Zare, A.; Arora, P.; Stevanovic, S.; Ristovski, Z.; Brown, R.J.; Bodisco, T. Combustion characteristics of microalgae-based dioctyl phthalate biofuel during ambient, preheated and hot engine operation. Fuel 2023, 331, 125890. [Google Scholar] [CrossRef]
- Mendiburu, A.Z.; Lauermann, C.H.; Hayashi, T.C.; Marinos, D.J.; Rodrigues da Costa, R.B.; Coronado, C.J.R.; Roberst, J.J.; De Carvahlo, J.A., Jr. Ethanol as a renewable biofuel: Combustion characteristics and application in engines. Energy 2022, 257, 124288. [Google Scholar] [CrossRef]
- Elfasakhany, A. Investigation of biomass powder as a direct solid biofuel in combustion engines: Modelling assessment and comparisons. Aim Shams Eng. J. 2021, 12, 2991–2998. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Yin, Z.; Linga, P.; He, T.; Zheng, X.Y. Coupling amino acid L-Val with THF for superior hydrogen hydrate kinetics: Implication for hydrate-based hydrogen storage. Chem. Eng. J. 2023, 467, 143459. [Google Scholar] [CrossRef]
- Ambrozik, A. Wybrane Zagadnienia Procesów Cieplnych w Tłokowych Silnikach Spalinowych; Wydawnictwo Politechniki Świętokrzyskiej: Kielce, Poland, 2003. [Google Scholar]
- Ambrozik, A. Analiza Cykli Pracy Czterosuwowych Silników Spalinowych; Wydawnictwo Politechniki Świętokrzyskiej: Kielce, Poland, 2010. [Google Scholar]
- Kurczyński, D.; Łagowski, P. Performance indices of a common rail-system CI engine powered by diesel oil and biofuel blends. J. Energy Inst. 2019, 92, 1897–1913. [Google Scholar] [CrossRef]
- Chaudhari, V.D.; Kulkarni, A.; Deshmukh, D. Spray characteristics of biofuels for advance combustion engines. Clean. Eng. Technol. 2021, 5, 100265. [Google Scholar] [CrossRef]
- Pandey, R.K.; Rehman, A.; Sarviya, R.M. Impact of alternative fuel properties on fuel spray behavior and atomization. Renew. Sustain. Energy Rev. 2012, 16, 1762–1778. [Google Scholar] [CrossRef]
- Knothe, G.; Matheaus, A.C.; Ryan, T.W. Cetane numbers of branched and straight-chain fatty esters determined in an quality ignition tester. Fuel 2003, 82, 971–975. [Google Scholar] [CrossRef]
- Köten, H.; Parlakyigit, A.S. Effects of the diesel engine parameters on the ignition delay. Fuel 2018, 216, 23–28. [Google Scholar] [CrossRef]
- Stoeck, T.; Osipowicz, T.; Abramek, K.F. Methodology for the repair of Denso Common Rail solenoid injectors. Eksploat. Niezawodn. Maint. Reliab. 2014, 16, 270–275. [Google Scholar]
- Akid, R.; Dmytrakh, I.M.; Gonzalez-Sanchez, J. Fatigue damage accumulation: The role of corrosion on the early stages of crack development. Corros. Eng. Sci. Technol. 2006, 41, 328–335. [Google Scholar] [CrossRef]
- Aksimentyeva, O.I.; Demchenko, P.Y.; Savchyn, V.P.; Balitskii, O.A. The chemical exfoliation phenomena in layered GaSe polyaniline composite. Nanoscale Res. Lett. 2013, 8, 2. [Google Scholar] [CrossRef]
- Wasim, M.; Djukic, M.; Ngo, T.D. Influence of hydrogen-enhanced plasticity and decohesion mechanisms of hydrogen embrittlement on the fracture resistance of steel. Eng. Fail. Anal. 2021, 123, 1053. [Google Scholar] [CrossRef]
- Djukic, M.; Curtin, W.A.; Zhang, Z.; Sedmak, A. Recent advances on hydrogen embrittlement understanding and future research framework. Eng. Fract. Mech. 2021, 241, 107439. [Google Scholar] [CrossRef]
- Moustabchir, H.; Azari, Z.; Hairi, S.; Dmytrakh, I. Experimental and computed stress distribution ahead of notch in pressure vessel: Application of T-stress conception. Comput. Mater. Sci. 2012, 58, 59–66. [Google Scholar] [CrossRef]
- Balyts’kyi, O.O. Elastic characteristics of laminated gallium and indium chalcogenides. Mater. Sci. 2004, 40, 706–709. [Google Scholar] [CrossRef]
- Capelle, J.; Dmytrakh, I.; Gilgert, J.; Jodin, P.; Pluvinage, G. A comparison of experimental results and computations for cracked tubes subjected to internal pressure. Mater. Technol. 2006, 40, 233–237. [Google Scholar]
- Bihun, R.I.; Stasyuk, Z.V.; Balitskii, O.A. Crossover from quantum to classical electron transport in ultrathin metal films. Phys. B Condens. Matter. 2016, 487, 73–77. [Google Scholar] [CrossRef]
- Balitskii, O.A.; Savchyn, V.P.; Savchyn, P.V. Thermal oxidation of indium and gallium sulphides. Phys. B Condens. Matter. 2005, 355, 365–369. [Google Scholar] [CrossRef]
- Romaniv, O.N.; Nikiforchin, G.N.; Kozak, L.Y. Cyclic rack resistance of constructional steel in gaseous hydrogen. Sov. Mater. Sci. 1987, 23, 439–450. [Google Scholar] [CrossRef]
- Balitskii, A.A.; Kolesnikov, V.A.; Vus, O.B. Tribotechnical properties of nitrogen manganese steels under rolling friction at addition of (GaSe)xIn1-x, powders into contact zone. Metallofiz. Noveishie Tekhnologii. 2010, 32, 685–695. Available online: https://www.scopus.com/record/display.uri?eid=2-s2.0-77957864676&origin=resultslist&sort=plf-f#metrics (accessed on 26 April 2024).
- Dmytrakh, I.M.; Akid, R.; Miller, K.J. Electrochemistry of deformed smooth surfaces and short corrosion fatigue crack growth behaviour. Br. Corros. J. 1997, 32, 138–144. [Google Scholar] [CrossRef]
- Balitska, V.; Filipecki, J.; Shpotyuk, O.; Swiatek, J.; Vakiv, M. Dynamic radiation-induced effects in chalcogenide vitreous compounds. J. Non-Cryst. Solids 2001, 287, 216–221. [Google Scholar] [CrossRef]
- Balitska, V.; Shpotyuk, Y.; Filipecki, J.; Shpotyuk, O.; Iovu, M. Post-irradiation relaxation in vitreous arsenic/antimony trisulphides. J. Non-Cryst. Solids 2011, 357, 487–489. [Google Scholar] [CrossRef]
- Balitskii, O.A.; Kolesnikov, V.O.; Balitskii, A.I.; Eliasz, J.; Havrylyuk, M.R. Hydrogen effect on the high-nickel surface steel properties during machining and wear with lubricants. Arch. Mater. Sci. Eng. 2020, 104, 49–57. [Google Scholar] [CrossRef]
- Balitska, V.O.; Golovchak, R.; Kovalskiy, A.; Skordeva, E.; Shpotyuk, O. Effect of Co60 γ-irradiation on the optical properties of As-Ge-S glasses. J. Non-Cryst. Solids 2003, 326–327, 130–134. [Google Scholar] [CrossRef]
- Dmytrakh, I.M.; Syrotyuk, A.M.; Leshchak, R.L. Effect of preliminary hydrogenation–dehydrogenation of low-alloy steel on its ability to absorb electrochemical hydrogen. Mater. Sci. 2021, 57, 387–396. [Google Scholar] [CrossRef]
- Balitskii, O.; Kolesnikov, V. Identification of wear products in the automotive tribotechnical system using computer vision methods, artificial intelligence and Big Data. In Proceedings of the 2019 XIth International Scientific and Practical Conference on Electronics and Information Technologies (ELIT), Lviv, Ukraine, 16–18 September 2019; pp. 24–27. [Google Scholar] [CrossRef]
- Osipowicz, T.; Koniuszy, A.; Taustyka, V.; Abramek, K.F.; Mozga, Ł. Evaluation of Ecological Parameters of a compression ignition Engine Fueled by Diesel Oil with an Eco Fuel Shot Liquid Catalyst. Catalyst 2023, 13, 1513. [Google Scholar] [CrossRef]
- Shpotyuk, O.I.; Balitska, V.O.; Vakiv, M.M.; Shpotyuk, L.I. Sensors of high-energy radiation based on amorphous chalcogenides. Sens. Actuators A Phys. 1998, 68, 356–358. [Google Scholar] [CrossRef]
- Kindrachuk, M.; Volchenko, D.; Balitskii, A.; Abramek, K.F.; Volchenko, M.; Balitskii, O.; Skrypnyk, V.; Zhuravlev, D.; Yurchuk, A.; Kolesnikov, V. Wear resistance of spark ignition engine piston rings in hydrogen-containing environments. Energies 2021, 14, 4801. [Google Scholar] [CrossRef]
- Balitskii, O.I.; Kvasnytska, Y.H.; Ivaskevych, L.M.; Mialnitsa, H.P.; Kvasnytska, K.H. Fatigue fracture of the blades of gas turbine engine made of a new refractory nickel alloy. Mater. Sci. 2022, 57, 475–483. [Google Scholar] [CrossRef]
- Balitskii, O.I.; Ivaskevich, L.M.; Mochulskyi, V.M. Temperature dependences of age-hardening austenitic steels mechanical properties in gaseous hydrogen. In Proceedings of the 12th International Conference on Fracture, ICF-12, Ottawa, ON, Canada, 12–17 July 2009; Elboujdaini, M., Ed.; NRC: Ottawa, ON, Canada, 2009. Paper No. T19.001; Code 93954. Volume 8, pp. 5786–5792. Available online: https://www.researchgate.net/publication/281269596_Temperature_Dependences_of_Age-hardening_Austenitic_Steels_Mechanical_Properties_in_Gaseous_Hydrogen (accessed on 26 April 2024).
- Balyts’kyi, O.I.; Ivas’kevych, L.M.; Mochul’s’kyi, V.M.; Holiyan, O.M. Influence of hydrogen on the crack resistance of 10Kh15N27T3V2MR steel. Mater. Sci. 2009, 45, 258–267. [Google Scholar] [CrossRef]
- Balyts’kyi, O.I.; Ivas’kevych, L.M.; Mochul’s’kyi, V.M. Mechanical properties of martensitic steels in gaseous hydrogen. Strength Mater. 2012, 44, 64–71. [Google Scholar] [CrossRef]
- Balitskii, A.I.; Ivaskevich, L.M. Hydrogen effect on cumulation of failure, mechanical properties, and fracture toughness of Ni-Cr alloys. Adv. Mat. Sci. Eng. 2019, 2019, 3680253. [Google Scholar] [CrossRef]
- Tkachov, V.I.; Ivas’kevych, L.M.; Mochul’s’kyi, V.M. Temperature dependences of the mechanical properties of austenitic and martensitic steels in hydrogen. Mater. Sci. 2007, 43, 654–666. [Google Scholar] [CrossRef]
- Tkachov, V.I.; Ivas’kevych, L.M.; Vytvyts’kyi, V.I. Methodological aspects of determination of hydrogen resistance of steels. Mater. Sci. 2002, 38, 484–493. [Google Scholar] [CrossRef]
- Patent of Ukraine 149899 (Patent for Utility Model), Hydrogen-containing fuel sprayer with spiral-elliptical channels/Balitskii, A.I., Abramek, K.F., Osipowicz, T., Mozga, L., Eliasz, J.J., Balitskii, O.A. Valid from 16.12.2021.-4 p. Publ. in Bullletin No 50, 15.12.2021. Available online: https://base.uipv.org/searchINV/search.php?action=search (accessed on 26 April 2024).
- Glassman, I.; Yetter, R.A. Chapter 4—Flame Phenomena in Premixed Combustible Gases. In Combustion, 4th ed.; Glassman, I., Yetter, R.A., Eds.; Academic Press: Cambridge, MA, USA, 2008; pp. 147–260. ISBN 9780120885732. [Google Scholar] [CrossRef]
- Williams, F.A. Combustion Theory the Fundamental Theory of Chemically Reacting Flow Systems, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1985; 1934p. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).