The Effect of Residence Time during the Hydrothermal Carbonization Process of Sewage Sludge on the Properties of Hydrochar
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
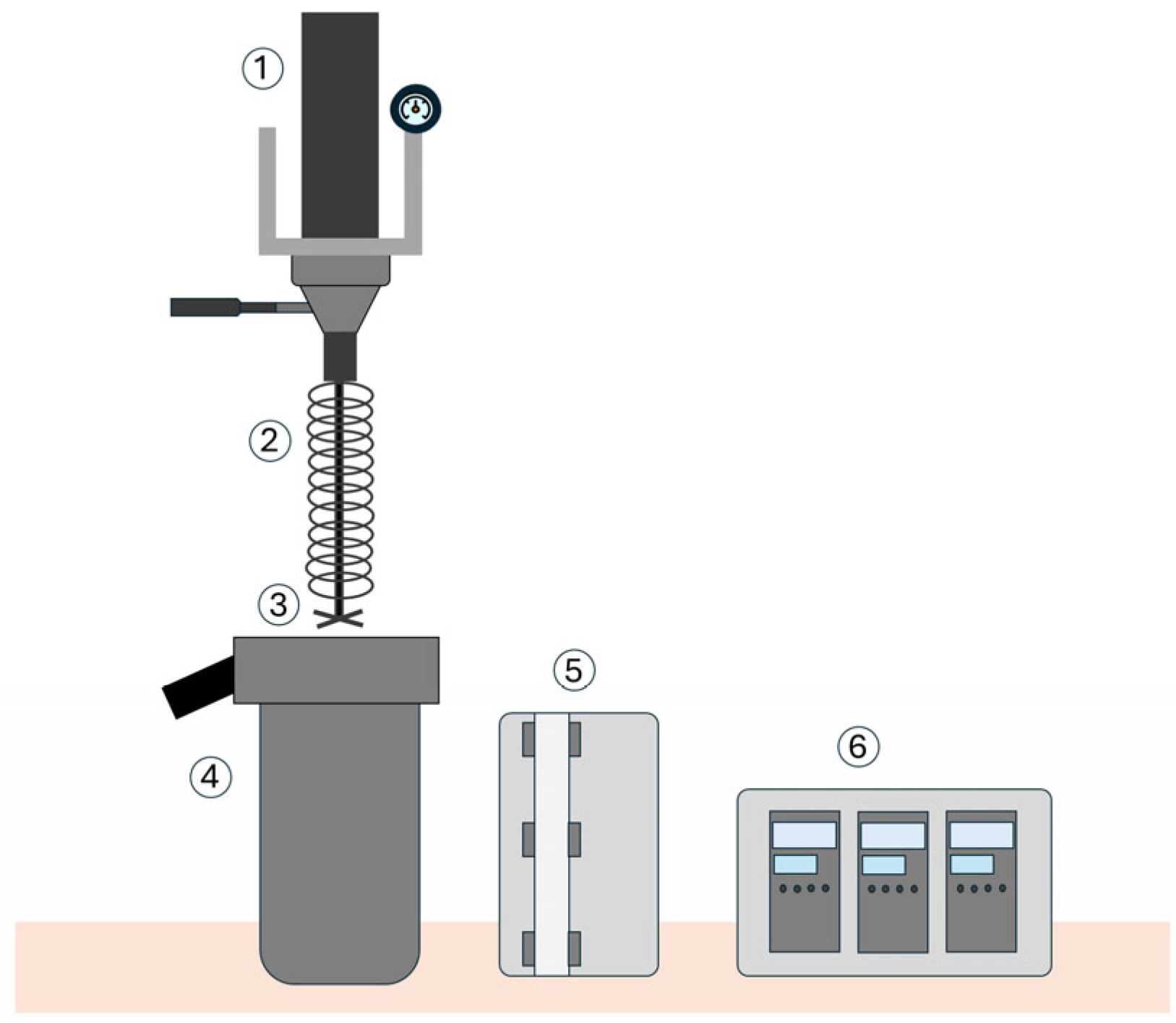
2.2. Hydrothermal Carbonization
2.3. Analytical Methods
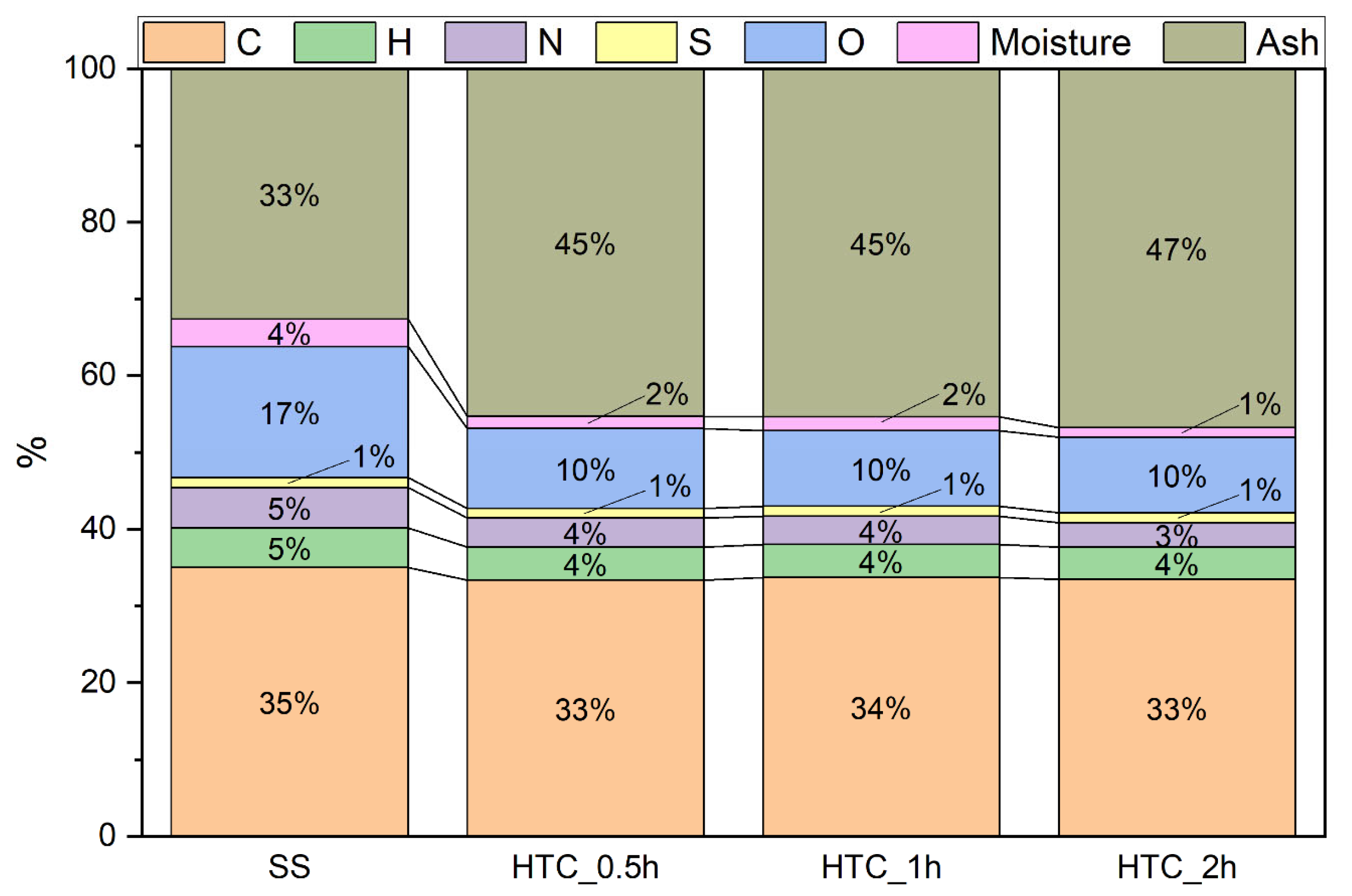
- oxygen (O) content by the differential method, taking into account the elemental analysis and the ash and moisture contents of the solid samples
- dry matter content as the difference between the total percentage of the sample and mineral matter (determined ash content)
- fixed carbon content (FC) based on the difference between 100% and volatile matter, ash, and moisture contents
- mass yield (MY)
- energy density ratio (EDR)
- energy yield (EY)
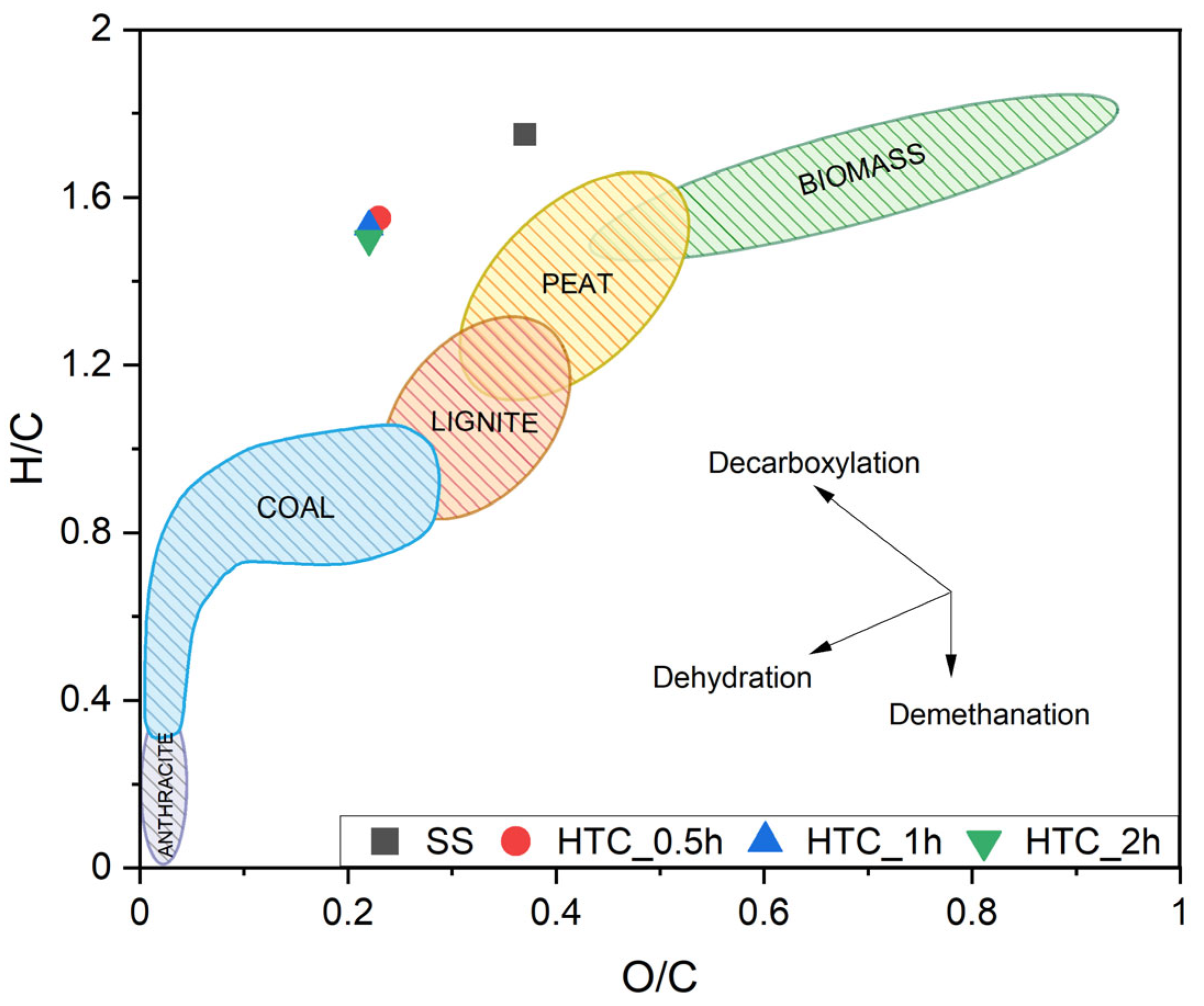
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atashi, H.; Rezaeian, F.; Mirzaei, A.A. The Green Fuel from Carbon Waste: Optimization and Product Selectivity Model Studies. Int. J. Coal Sci. Technol. 2018, 5, 399–410. [Google Scholar] [CrossRef]
- Mao, G.; Huang, N.; Chen, L.; Wang, H. Research on Biomass Energy and Environment from the Past to the Future: A Bibliometric Analysis. Sci. Total Environ. 2018, 635, 1081–1090. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.-F.; Monforti-Ferrario, F.; Nita, V. The Role of Biomass and Bioenergy in a Future Bioeconomy: Policies and Facts. Environ. Dev. 2015, 15, 3–34. [Google Scholar] [CrossRef]
- Yu, Y.; Sokhansanj, S.; Lau, A.; El-Kassaby, Y.A.; Wang, G.; Guo, Y. Hydrothermal Carbonization of Mixture Waste Gingko Leaf and Wheat Straw for Solid Biofuel Production. Ind. Crops Prod. 2023, 206, 117633. [Google Scholar] [CrossRef]
- Nguyen, T.-B.; Nguyen, T.-K.-T.; Chen, W.-H.; Chen, C.-W.; Bui, X.-T.; Patel, A.K.; Dong, C.-D. Hydrothermal and Pyrolytic Conversion of Sunflower Seed Husk into Novel Porous Biochar for Efficient Adsorption of Tetracycline. Bioresour. Technol. 2023, 373, 128711. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.; Li, Z.; Liu, M.; Wu, D. Fed-Batch Processing of Algae Hydrothermal Carbonization Process Water Improves Anaerobic Digestion and Digestate Nutrient Content. Biomass Bioenergy 2023, 170, 106729. [Google Scholar] [CrossRef]
- Wang, C.; Lin, X.; Zhang, X.; Show, P.L. Research Advances on Production and Application of Algal Biochar in Environmental Remediation. Environ. Pollut. 2024, 348, 123860. [Google Scholar] [CrossRef] [PubMed]
- Ipiales, R.P.; Mohedano, A.F.; Diaz-Portuondo, E.; Diaz, E.; de la Rubia, M.A. Co-Hydrothermal Carbonization of Swine Manure and Lignocellulosic Waste: A New Strategy for the Integral Valorization of Biomass Wastes. Waste Manag. 2023, 169, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, J.; Hu, W.; Xie, D.; Xu, M.; Qiao, Y. In-Depth Study of the Sulfur Migration and Transformation during Hydrothermal Carbonization of Sewage Sludge. Proc. Combust. Inst. 2023, 39, 3419–3427. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Sun, R.; Cao, Y. Neutralization of Red Mud Using Bio-Acid Generated by Hydrothermal Carbonization of Waste Biomass for Potential Soil Application. J. Clean. Prod. 2020, 271, 122525. [Google Scholar] [CrossRef]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J., Jr.; Tester, J.W. Thermochemical Biofuel Production in Hydrothermal Media: A Review of Sub- and Supercritical Water Technologies. Energy Environ. Sci. 2008, 1, 32. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal Carbonization of Biomass: A Summary and Discussion of Chemical Mechanisms for Process Engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Román, S.; Libra, J.; Berge, N.; Sabio, E.; Ro, K.; Li, L.; Ledesma, B.; Álvarez, A.; Bae, S. Hydrothermal Carbonization: Modeling, Final Properties Design and Applications: A Review. Energies 2018, 11, 216. [Google Scholar] [CrossRef]
- Jin, F. (Ed.) Application of Hydrothermal Reactions to Biomass Conversion; Green Chemistry and Sustainable Technology; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-54457-6. [Google Scholar]
- Hoekman, S.K.; Broch, A.; Robbins, C. Hydrothermal Carbonization (HTC) of Lignocellulosic Biomass. Energy Fuels 2011, 25, 1802–1810. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal Carbonization of Biomass Residuals: A Comparative Review of the Chemistry, Processes and Applications of Wet and Dry Pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. Strength, Storage, and Combustion Characteristics of Densified Lignocellulosic Biomass Produced via Torrefaction and Hydrothermal Carbonization. Appl. Energy 2014, 135, 182–191. [Google Scholar] [CrossRef]
- Pauline, A.L.; Joseph, K. Hydrothermal Carbonization of Organic Wastes to Carbonaceous Solid Fuel—A Review of Mechanisms and Process Parameters. Fuel 2020, 279, 118472. [Google Scholar] [CrossRef]
- Fakudze, S.; Chen, J. A Critical Review on Co-Hydrothermal Carbonization of Biomass and Fossil-Based Feedstocks for Cleaner Solid Fuel Production: Synergistic Effects and Environmental Benefits. Chem. Eng. J. 2023, 457, 141004. [Google Scholar] [CrossRef]
- Sharma, A.K.; Ghodke, P.K.; Chen, W.-H. Progress in Green Adsorbent Technologies from Sewage Sludge for Wastewater Remediation and Carbon Capture: A Sustainable Approach towards Clean Environment. Curr. Opin. Green Sustain. Chem. 2024, 46, 100883. [Google Scholar] [CrossRef]
- Xu, H.; Li, M.; Ou, Y.; Li, S.; Zheng, X.; Li, X.; Tang, C.; Chen, D. The Reconstitution of Reed Cellulose by the Hydrothermal Carbonization and Acid Etching to Improve the Performance of Photocatalytic Degradation of Antibiotics. Int. J. Biol. Macromol. 2023, 236, 123976. [Google Scholar] [CrossRef]
- Si, H.; Zhao, C.; Wang, B.; Liang, X.; Gao, M.; Jiang, Z.; Yu, H.; Yang, Y.; Gu, Z.; Ogino, K.; et al. Liquid-Solid Ratio during Hydrothermal Carbonization Affects Hydrochar Application Potential in Soil: Based on Characteristics Comparison and Economic Benefit Analysis. J. Environ. Manag. 2023, 335, 117567. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, D.; Xu, S.; Wang, Z.; Chen, X.; Ding, Y.; Chu, Q.; Sha, Z. Hydrothermal Carbonization of Biogas Slurry and Cattle Manure into Soil Conditioner Mitigates Ammonia Volatilization from Paddy Soil. Chemosphere 2023, 344, 140378. [Google Scholar] [CrossRef] [PubMed]
- Parshetti, G.K.; Liu, Z.; Jain, A.; Srinivasan, M.P.; Balasubramanian, R. Hydrothermal Carbonization of Sewage Sludge for Energy Production with Coal. Fuel 2013, 111, 201–210. [Google Scholar] [CrossRef]
- Zheng, C.; Ma, X.; Yao, Z.; Chen, X. The Properties and Combustion Behaviors of Hydrochars Derived from Co-Hydrothermal Carbonization of Sewage Sludge and Food Waste. Bioresour. Technol. 2019, 285, 121347. [Google Scholar] [CrossRef]
- Gaur, R.Z.; Khoury, O.; Zohar, M.; Poverenov, E.; Darzi, R.; Laor, Y.; Posmanik, R. Hydrothermal Carbonization of Sewage Sludge Coupled with Anaerobic Digestion: Integrated Approach for Sludge Management and Energy Recycling. Energy Convers. Manag. 2020, 224, 113353. [Google Scholar] [CrossRef]
- Zhang, J.H.; Lin, Q.M.; Zhao, X.R. The Hydrochar Characters of Municipal Sewage Sludge under Different Hydrothermal Temperatures and Durations. J. Integr. Agric. 2014, 13, 471–482. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Shama, G.; Wheatley, A.D.; Martin, S.J.; Holdich, R.G. Hydrothermal Carbonisation of Sewage Sludge: Effect of Process Conditions on Product Characteristics and Methane Production. Bioresour. Technol. 2015, 177, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Merzari, F.; Goldfarb, J.; Andreottola, G.; Mimmo, T.; Volpe, M.; Fiori, L. Hydrothermal Carbonization as a Strategy for Sewage Sludge Management: Influence of Process Withdrawal Point on Hydrochar Properties. Energies 2020, 13, 2890. [Google Scholar] [CrossRef]
- Wilk, M. A Novel Method of Sewage Sludge Pre-Treatment-HTC. E3S Web Conf. 2016, 10, 00103. [Google Scholar] [CrossRef]
- Jenkins, B.; Baxter, L.; Miles, T.; Miles, T. Combustion Properties of Biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Miles, T.R.; Miles, T.R.; Baxter, L.L.; Bryers, R.W.; Jenkins, B.M.; Oden, L.L. Boiler Deposits from Firing Biomass Fuels. Biomass Bioenergy 1996, 10, 125–138. [Google Scholar] [CrossRef]
- Czerwińska, K.; Wierońska-Wiśniewska, F.; Bytnar, K.; Mikusińska, J.; Śliz, M.; Wilk, M. The effect of an acidic environment during the hydrothermal carbonization of sewage sludge on solid and liquid products: The fate of heavy metals, phosphorus and other compounds. J. Environ. Manag. 2024, 365, 121637. [Google Scholar] [CrossRef] [PubMed]
- ASTM D7582; Standard Test Methods for Proximate Analysis of Coal and Coke by Macro Thermogravimetric Analysis. ASTM: West Conshohocken, PA, USA, 2023.
- ISO 17246:2010; Coal—Proximate Analysis. ISO: Geneva, Switzerland, 2010.
- PKN-ISO/TS 12902:2007; Solid Mineral Fuels-Determination of Total Carbon, Hydrogen and Nitrogen-Instrumental Methods. Polish Committee for Standardization: Warsaw, Poland, 2013.
- DIN 51 900; Determining the Gross Calorific Value of Solid and Liquid Fuels Using the Bomb Calorimeter, and Calculation of Net Calorific Value-Part 1: General Information. European Standards: Brussels, Belgium, 2000.
- ISO 1928; Coal and Coke—Determination of Gross Calorific Value. ISO: Geneva, Switzerland, 2020.
- Li, L.; Wang, Y.; Xu, J.; Flora, J.R.V.; Hoque, S.; Berge, N.D. Quantifying the Sensitivity of Feedstock Properties and Process Conditions on Hydrochar Yield, Carbon Content, and Energy Content. Bioresour. Technol. 2018, 262, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, D.W.; Chiou, C.T.; Kile, D.E. Influence of Soil Organic Matter Composition on the Partition of Organic Compounds. Environ. Sci. Technol. 1992, 26, 336–340. [Google Scholar] [CrossRef]
- Pronobis, M. Evaluation of the Influence of Biomass Co-Combustion on Boiler Furnace Slagging by Means of Fusibility Correlations. Biomass Bioenergy 2005, 28, 375–383. [Google Scholar] [CrossRef]
- Cong, K.; Han, F.; Zhang, Y.; Li, Q. The Investigation of Co-Combustion Characteristics of Tobacco Stalk and Low Rank Coal Using a Macro-TGA. Fuel 2019, 237, 126–132. [Google Scholar] [CrossRef]
- Huang, J.; Liu, J.; Kuo, J.; Xie, W.; Zhang, X.; Chang, K.; Buyukada, M.; Evrendilek, F. Kinetics, Thermodynamics, Gas Evolution and Empirical Optimization of (Co-)Combustion Performances of Spent Mushroom Substrate and Textile Dyeing Sludge. Bioresour. Technol. 2019, 280, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Song, X.; Shan, M.; Yang, X. Effect of Pelletization on Biomass Thermal Degradation in Combustion: A Case Study of Peanut Shell and Wood Sawdust Using Macro-TGA. Energy Built Environ. 2024. [Google Scholar] [CrossRef]
- Sieradzka, M.; Gao, N.; Quan, C.; Mlonka-Mędrala, A.; Magdziarz, A. Biomass Thermochemical Conversion via Pyrolysis with Integrated CO2 Capture. Energies 2020, 13, 1050. [Google Scholar] [CrossRef]
- Mureddu, M.; Dessì, F.; Orsini, A.; Ferrara, F.; Pettinau, A. Air- and Oxygen-Blown Characterization of Coal and Biomass by Thermogravimetric Analysis. Fuel 2018, 212, 626–637. [Google Scholar] [CrossRef]
- Saetea, P.; Tippayawong, N. Recovery of Value-Added Products from Hydrothermal Carbonization of Sewage Sludge. ISRN Chem. Eng. 2013, 2013, 268947. [Google Scholar] [CrossRef]
- Cao, Z.; Hülsemann, B.; Wüst, D.; Oechsner, H.; Lautenbach, A.; Kruse, A. Effect of Residence Time during Hydrothermal Carbonization of Biogas Digestate on the Combustion Characteristics of Hydrochar and the Biogas Production of Process Water. Bioresour. Technol. 2021, 333, 125110. [Google Scholar] [CrossRef]
- Lin, H.; Li, C.; Jiang, Y.; Zhang, L.; Zhang, S.; Wang, D.; Leng, C.; Hu, X. Hydrothermal Carbonization of Pretreated Pine Needles: The Impacts of Temperature and Atmosphere in Pretreatment on Structural Evolution of Hydrochar. J. Anal. Appl. Pyrolysis 2024, 178, 106421. [Google Scholar] [CrossRef]
- Hartulistiyoso, E.; Farobie, O.; Anis, L.A.; Syaftika, N.; Bayu, A.; Amrullah, A.; Moheimani, N.R.; Karnjanakom, S.; Matsumura, Y. Co-Production of Hydrochar and Bioactive Compounds from Ulva Lactuca via a Hydrothermal Process. Carbon Resour. Convers. 2024, 7, 100183. [Google Scholar] [CrossRef]
- Wenzel, R.N. Surface Roughness and Contact Angle. J. Phys. Colloid Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- He, C.; Giannis, A.; Wang, J.-Y. Conversion of Sewage Sludge to Clean Solid Fuel Using Hydrothermal Carbonization: Hydrochar Fuel Characteristics and Combustion Behavior. Appl. Energy 2013, 111, 257–266. [Google Scholar] [CrossRef]
- Śliz, M.; Tuci, F.; Czerwińska, K.; Fabrizi, S.; Lombardi, L.; Wilk, M. Hydrothermal Carbonization of the Wet Fraction from Mixed Municipal Solid Waste: Hydrochar Characteristics and Energy Balance. Waste Manag. 2022, 151, 39–48. [Google Scholar] [CrossRef]
- Wilk, M.; Śliz, M.; Czerwińska, K.; Śledź, M. The Effect of an Acid Catalyst on the Hydrothermal Carbonization of Sewage Sludge. J. Environ. Manag. 2023, 345, 118820. [Google Scholar] [CrossRef]
- Bryers, R.W. Fireside Slagging, Fouling, and High-Temperature Corrosion of Heat-Transfer Surface due to Impurities in Steam-Raising Fuels. Prog. Energy Combust. Sci. 1996, 22, 29–120. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G.; Morgan, T.J. An Overview of the Organic and Inorganic Phase Composition of Biomass. Fuel 2012, 94, 1–33. [Google Scholar] [CrossRef]
- Song, E.; Park, S.; Kim, H. Upgrading Hydrothermal Carbonization (HTC) Hydrochar from Sewage Sludge. Energies 2019, 12, 2383. [Google Scholar] [CrossRef]

| Parameter | Equation |
|---|---|
| Oxygen content, % | O = 100 − C–H − N − S − Ash − M |
| Dry matter content, % | Dry matter content = 100 − Ash |
| Fixed carbon content, % | FC = 100 − VM − Ash − M |
| Mass yield, % | where: masshydrochar—is the mass of hydrochar, kg massSS—is the mass of sewage sludge, kg |
| Energy density ratio | where: HHVhydrocha—is the HHV of hydrochar, MJ/kg HHVSS—is the HHV of sewage sludge, MJ/kg |
| Energy yield, % | |
| Polarity index |
| Parameter | SS | HTC_0.5h | HTC_1h | HTC_2h |
|---|---|---|---|---|
| PROXIMATE ANALYSIS | ||||
| Dry organic matter, % | 67.4 | 54.7 | 54.6 | 53.2 |
| VM, % | 61.8 | 48.9 | 48.6 | 47.0 |
| FC, % | 2.0 | 4.2 | 4.2 | 4.9 |
| ENERGY PARAMETERS | ||||
| HHV, MJ/kg | 14.74 | 14.07 | 14.22 | 14.09 |
| LHV, MJ/kg | 13.63 | 13.13 | 13.30 | 13.17 |
| MY, % | - | 60.31 | 61.64 | 63.02 |
| EDR | - | 0.95 | 0.97 | 0.96 |
| EY, % | - | 57.54 | 59.49 | 60.21 |
| PRODUCT DISTRIBUTION | ||||
| solid fraction, %wt. | - | 5.73 | 5.86 | 5.99 |
| liquid fraction, %wt. | - | 91.91 | 89.33 | 89.26 |
| gas and losses, %wt. | - | 5.24 | 4.52 | 4.32 |
| Parameter | SS | HTC_0.5h | HTC_1h | HTC_2h |
|---|---|---|---|---|
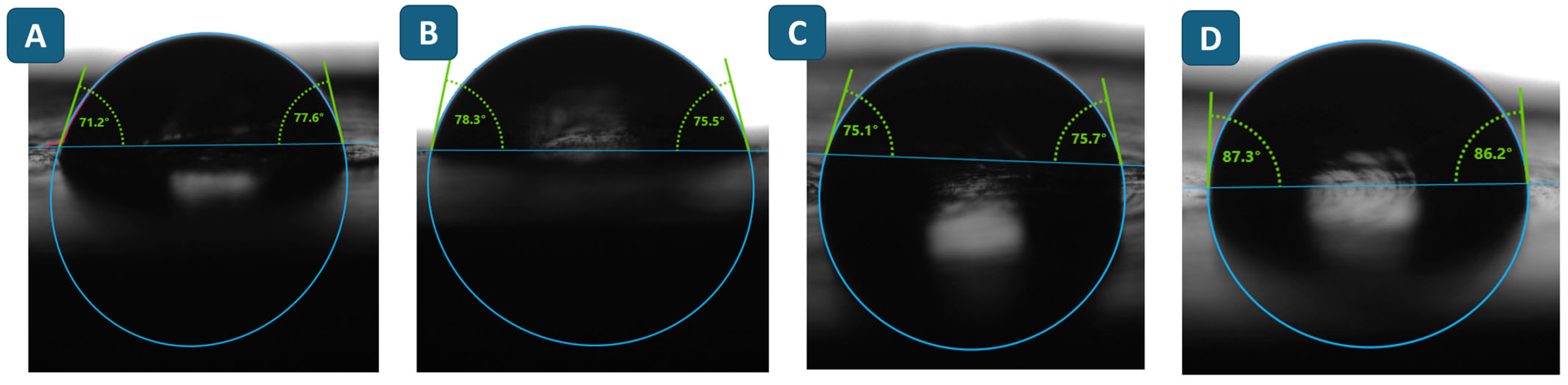
| Contact angle with water, ° | 75.7 ± 6.0 | 75.7 ± 3.7 | 75.5 ± 3.5 | 81.8 ± 4.8 |
| Polarity index | 0.64 | 0.42 | 0.40 | 0.38 |
| Oxide | SS | HTC_0.5h | HTC_1h | HTC_2h |
|---|---|---|---|---|
| Na2O | 0.7367 | 0.3982 | 0.4549 | 0.3546 |
| MgO | 4.3358 | 4.1074 | 4.1114 | 4.1368 |
| Al2O3 | 7.5850 | 7.8706 | 7.9795 | 8.0470 |
| SiO2 | 26.5249 | 27.0726 | 26.9042 | 26.3887 |
| P2O5 | 21.8986 | 21.5918 | 21.8070 | 21.5085 |
| SO3 | 4.0171 | 4.4008 | 3.6788 | 4.6645 |
| Cl | 0.0549 | 0.0340 | 0.0267 | 0.0304 |
| K2O | 1.9380 | 1.3713 | 1.3655 | 1.2909 |
| CaO | 20.8553 | 21.0838 | 21.361 | 21.4059 |
| Fe2O3 | 9.2019 | 9.1599 | 9.3642 | 9.1782 |
| ZnO | 0.6566 | 0.6768 | 0.6972 | 0.6919 |
| PbO | 0.0385 | 0.0353 | 0.0385 | 0.0375 |
| Cr2O3 | 0.0647 | 0.0525 | 0.0454 | 0.0429 |
| NiO | 0.0791 | 0.0798 | 0.0777 | 0.0824 |
| CuO | 0.1170 | 0.1144 | 0.1241 | 0.1124 |
| TiO2 | 1.0303 | 1.1142 | 1.0801 | 1.1633 |
| MnO | 0.3377 | 0.3548 | 0.3565 | 0.3640 |
| Rb2O | 0.0055 | 0.0066 | 0.0069 | 0.0066 |
| SrO | 0.1879 | 0.1884 | 0.1947 | 0.1923 |
| ZrO2 | 0.1879 | 0.0647 | 0.0565 | 0.0631 |
| SnO2 | 0.0143 | 0.0132 | 0.0185 | 0.0182 |
| BaO | 0.2043 | 0.1951 | 0.2360 | 0.2095 |
| Index | SS | HTC_0.5h | HTC_1h | HTC_2h | ||||
|---|---|---|---|---|---|---|---|---|
| Value | Evaluation | Value | Evaluation | Value | Evaluation | Value | Evaluation | |
| RB | 37.07 | Low melting point | 36.12 | Low melting point | 36.66 | Low melting point | 36.37 | Low melting point |
| RB/A | 1.05 | Medium slagging tendency | 1.00 | Medium slagging tendency | 1.02 | Medium slagging tendency | 1.02 | Medium slagging tendency |
| SR | 43.54 | High slagging tendency | 44.08 | High slagging tendency | 43.58 | High slagging tendency | 43.18 | High slagging tendency |
| RS | 1.36 | Medium slagging tendency | 1.28 | Medium slagging tendency | 1.31 | Medium slagging tendency | 1.35 | Medium slagging tendency |
| Fu | 2.82 | High slagging tendency | 1.77 | High slagging tendency | 1.86 | High slagging tendency | 1.68 | High slagging tendency |
| LF | 2.74 | - | 2.75 | - | 2.72 | - | 2.78 | - |
| Fe2O3/CaO | 0.44 | Formation of eutectics | 0.43 | Formation of eutectics | 0.44 | Formation of eutectics | 0.43 | Formation of eutectics |
| Parameter | SS | HTC_0.5h | HTC_1h | HTC_2h |
|---|---|---|---|---|
| Ti, °C | 205 | 247 | 243 | 247 |
| ti, min | 17.97 | 22.05 | 21.65 | 22.02 |
| Tb, °C | 526 | 478 | 472 | 475 |
| tb, min | 49.83 | 45.05 | 44.48 | 44.80 |
| t0.5, min | 19.62 | 23.87 | 20.64 | 23.63 |
| t1, min | 25.00 | 27.77 | 27.58 | 27.53 |
| T1, °C | 276 | 306 | 304 | 304 |
| DTG1, %/min | 2.34 | 2.87 | 2.91 | 2.67 |
| T2, °C | 499 | 401 | 399 | 398 |
| DTG2, %/min | 3.86 | 2.03 | 2.17 | 2.19 |
| DTGmean, %/min | 1.00 | 0.80 | 0.81 | 0.76 |
| Di, %·min−3 | 0.0052 | 0.0047 | 0.0049 | 0.0044 |
| Db, %·min−4·10−5 | 9.6 | 9.6 | 11.5 | 9.1 |
| S, %·min−2·°C−3·10−8 | 10.6 | 7.9 | 8.5 | 7.1 |
| Hf, °C | 822.4 | 1038.9 | 984.2 | 1041.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czerwińska, K.; Mikusińska, J.; Błoniarz, A.; Śliz, M.; Wilk, M. The Effect of Residence Time during the Hydrothermal Carbonization Process of Sewage Sludge on the Properties of Hydrochar. Energies 2024, 17, 3380. https://doi.org/10.3390/en17143380
Czerwińska K, Mikusińska J, Błoniarz A, Śliz M, Wilk M. The Effect of Residence Time during the Hydrothermal Carbonization Process of Sewage Sludge on the Properties of Hydrochar. Energies. 2024; 17(14):3380. https://doi.org/10.3390/en17143380
Chicago/Turabian StyleCzerwińska, Klaudia, Joanna Mikusińska, Aleksandra Błoniarz, Maciej Śliz, and Małgorzata Wilk. 2024. "The Effect of Residence Time during the Hydrothermal Carbonization Process of Sewage Sludge on the Properties of Hydrochar" Energies 17, no. 14: 3380. https://doi.org/10.3390/en17143380
APA StyleCzerwińska, K., Mikusińska, J., Błoniarz, A., Śliz, M., & Wilk, M. (2024). The Effect of Residence Time during the Hydrothermal Carbonization Process of Sewage Sludge on the Properties of Hydrochar. Energies, 17(14), 3380. https://doi.org/10.3390/en17143380








