A Review of Thermochemical Energy Storage Systems for District Heating in the UK
Abstract
1. Introduction
2. Literature Review
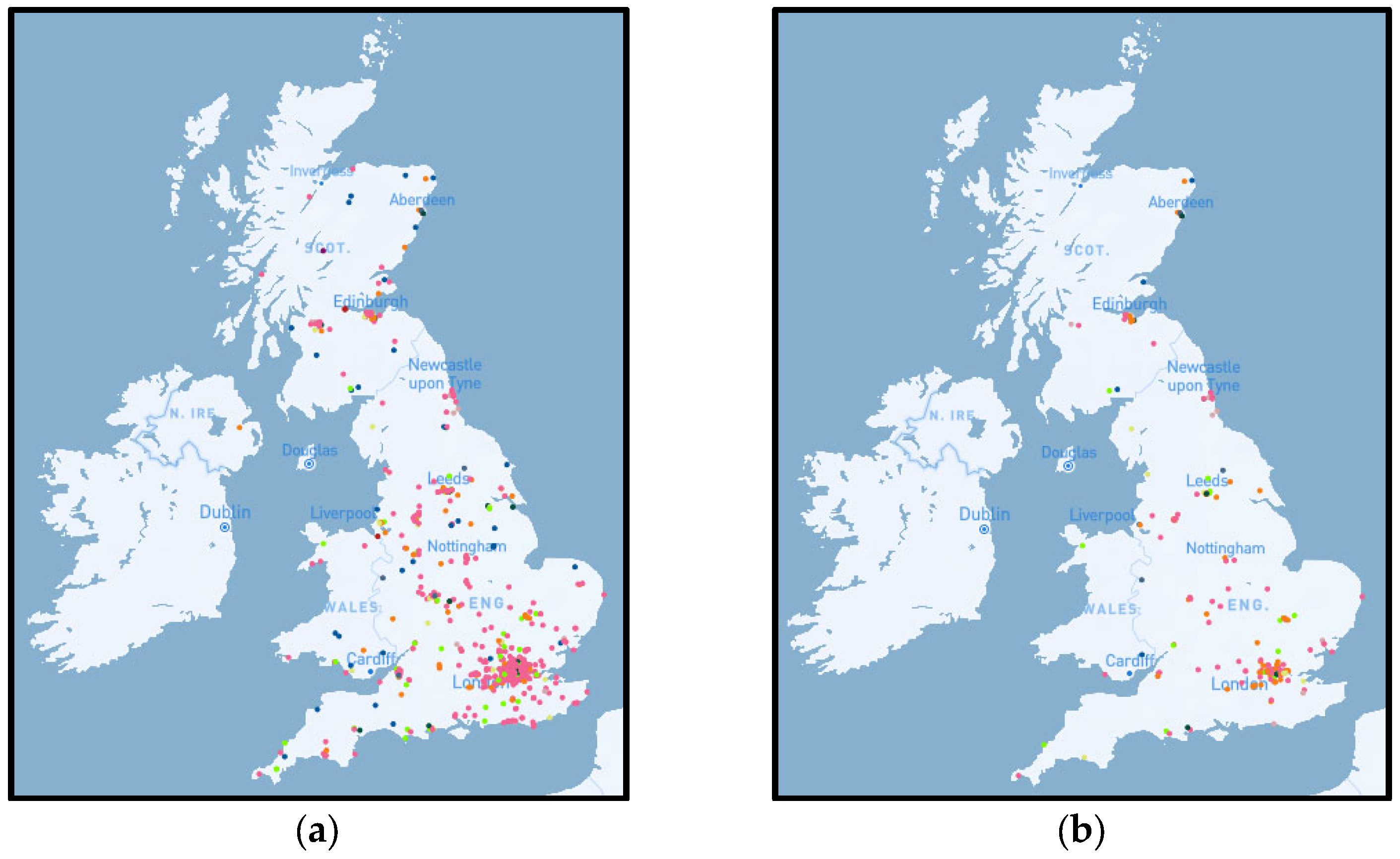
2.1. District Heating
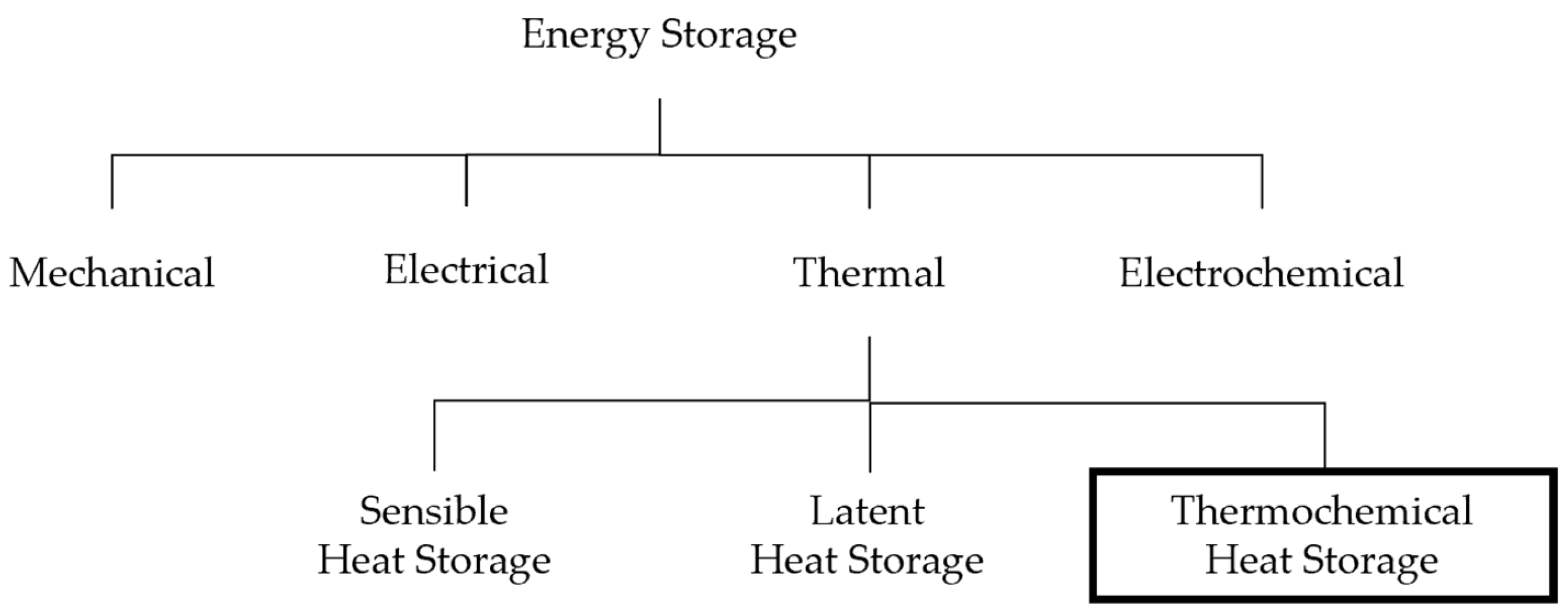
2.2. Energy Storage Technologies
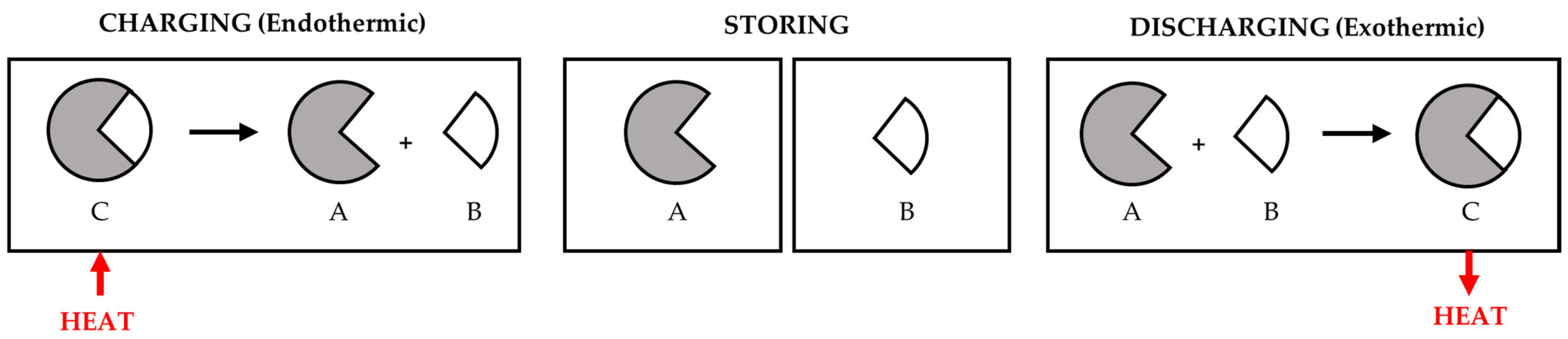
2.3. Thermochemical Energy Storage (TCES)
2.3.1. Material Selection
2.3.2. Hydroxide Systems
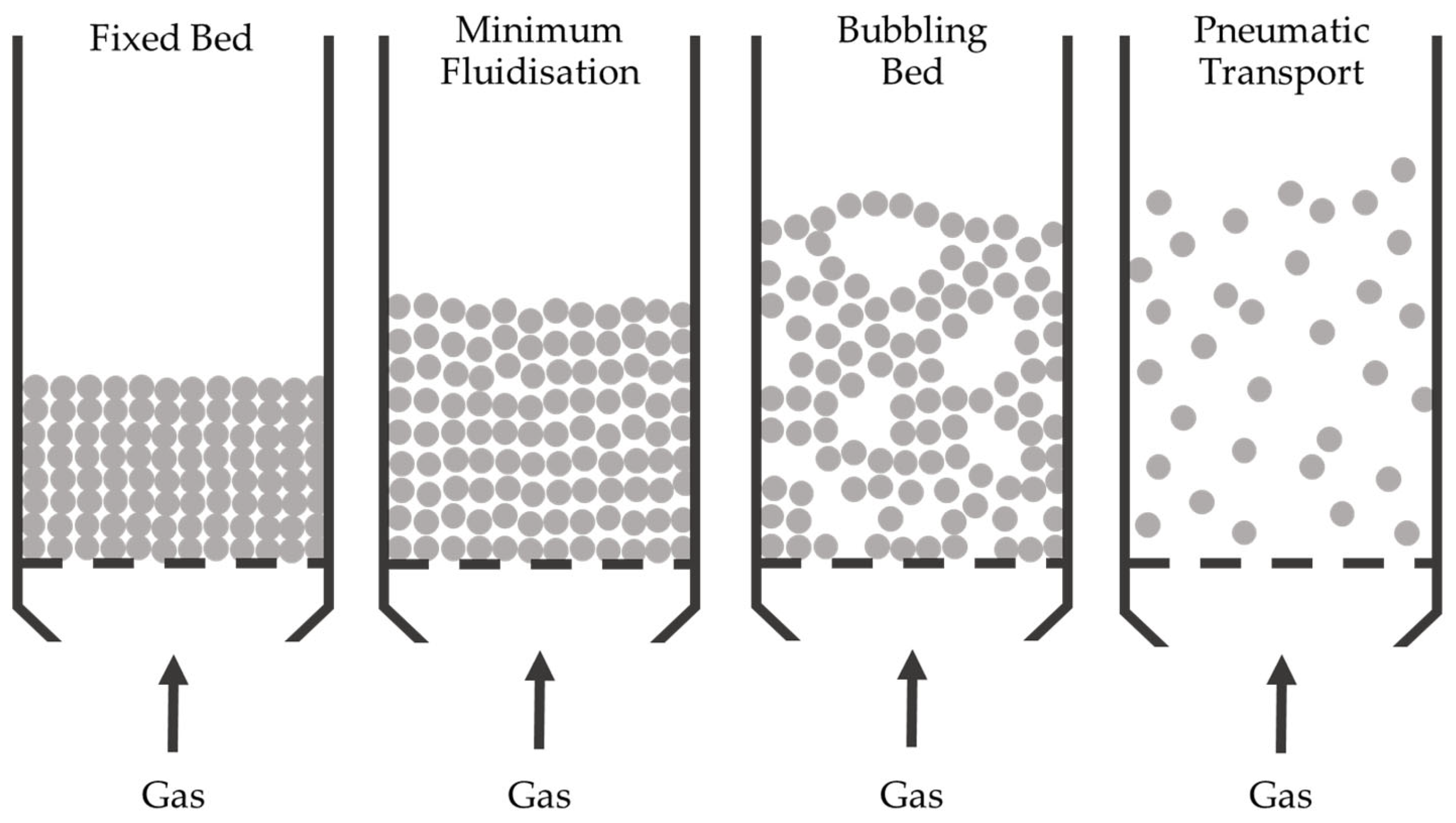
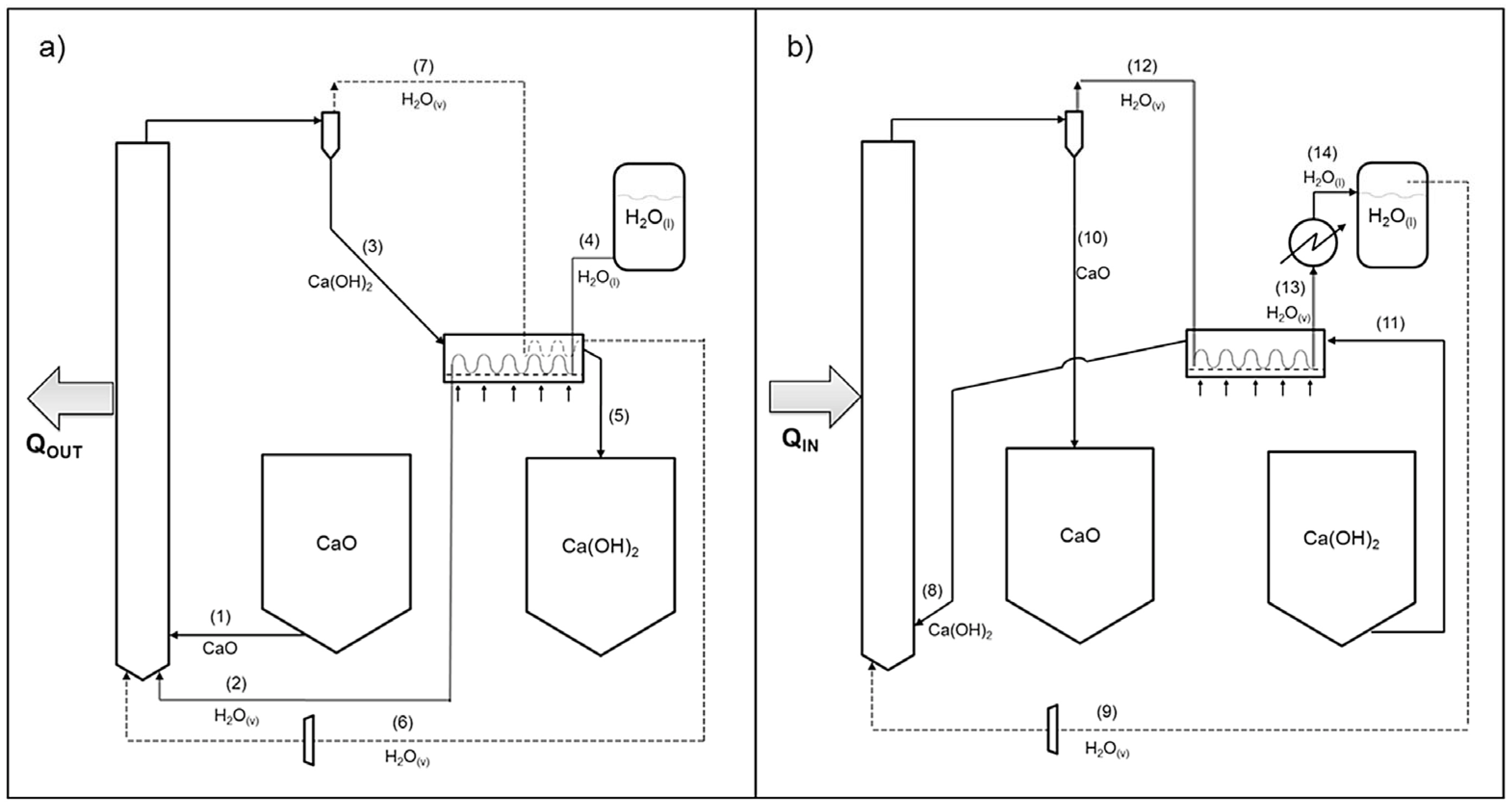
2.4. Thermochemical Reactors
2.5. Heat Exchangers
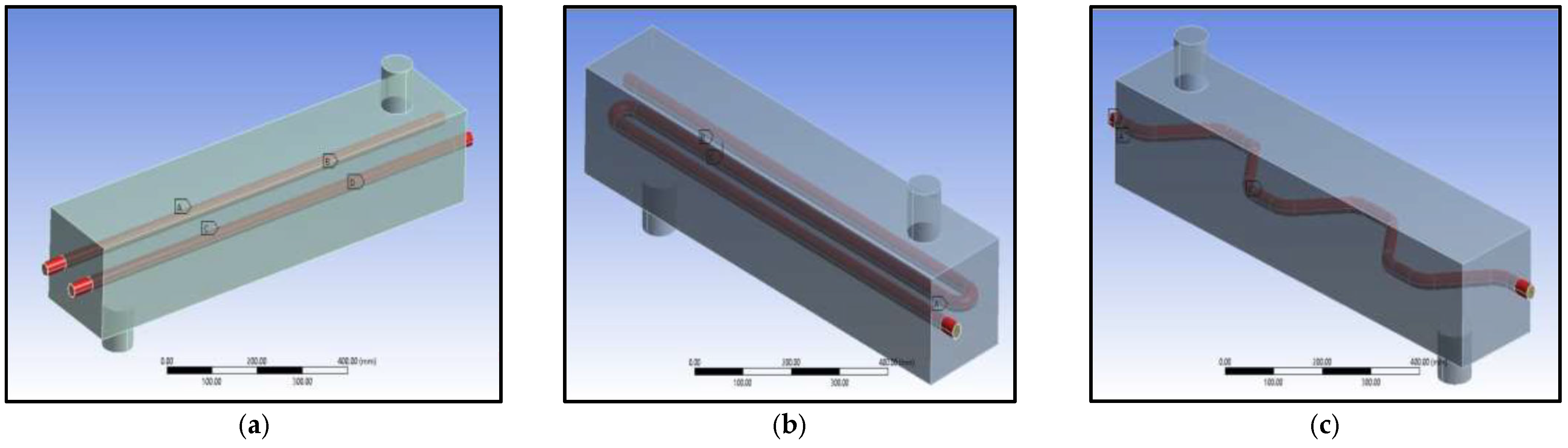
2.5.1. Geometry and Tube Pattern
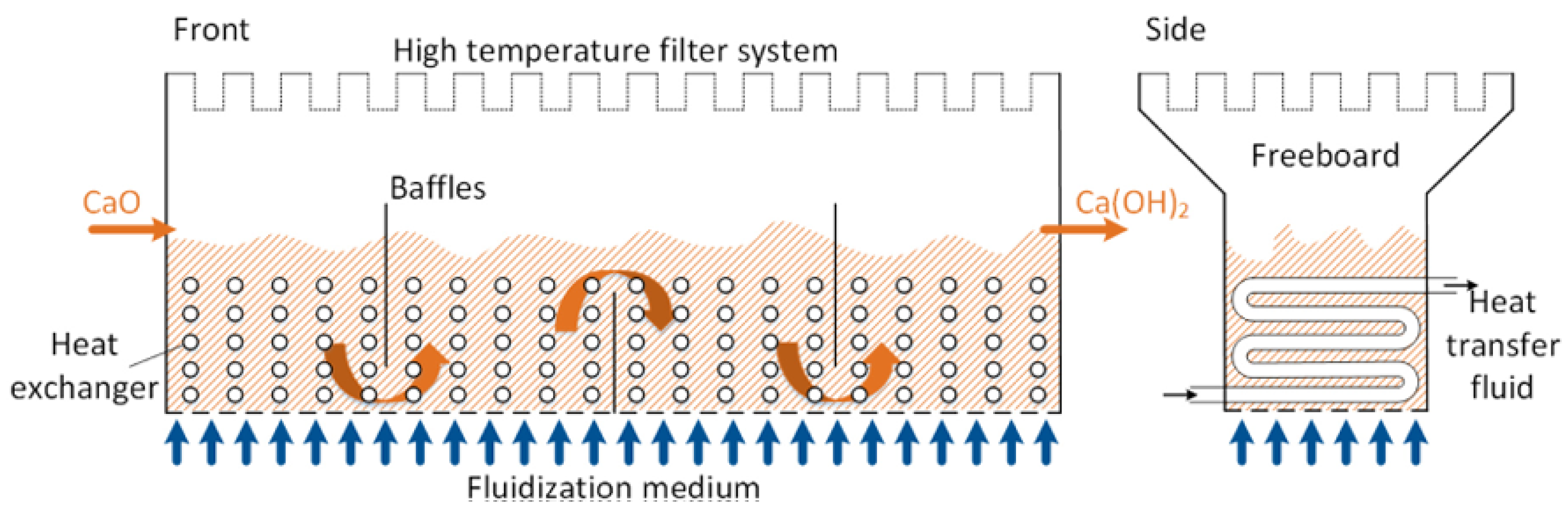
2.5.2. Heat Transfer Fluids
2.6. Enhancements
2.6.1. Nanomaterials and Coatings
2.6.2. Doping
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mahon, H.; O’Connor, D.; Friedrich, D.; Hughes, B. A review of thermal energy storage technologies for seasonal loops. Energy 2022, 239, 122207. [Google Scholar] [CrossRef]
- Asumadu-Sarkodie, S.; Owusu, P.A. Carbon dioxide emissions, GDP, energy use, and population growth: A multivariate and causality analysis for Ghana, 1971–2013. Environ. Sci. Pollut. Res. 2016, 23, 13508–13520. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strategy Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- IEA Technology Collaboration Programmes (TCPs). Available online: https://nachhaltigwirtschaften.at/en/iea/technologyprogrammes/ (accessed on 9 April 2024).
- Woolnough, D. Waste Heat Recovery. In Thermopedia; Begel House Inc.: Danbury, CT, USA, 2011. [Google Scholar] [CrossRef]
- Albert, M.D.A.; Bennett, K.O.; Adams, C.A.; Gluyas, J.G. Waste heat mapping: A UK study. Renew. Sustain. Energy Rev. 2022, 160, 112230. [Google Scholar] [CrossRef]
- Papapetrou, M.; Kosmadakis, G.; Cipollina, A.; La Commare, U.; Micale, G. Industrial waste heat: Estimation of the technically available resource in the EU per industrial sector, temperature level and country. Appl. Therm. Eng. 2018, 138, 207–216. [Google Scholar] [CrossRef]
- Werner, S. District heating and cooling in Sweden. Energy 2017, 126, 419–429. [Google Scholar] [CrossRef]
- Climate Change Committee. Net Zero—Technical Report. Available online: https://www.theccc.org.uk/publication/net-zero-technical-report/ (accessed on 9 April 2024).
- Guelpa, E.; Verda, V. Thermal energy storage in district heating and cooling systems: A review. Appl. Energy 2019, 252, 113474. [Google Scholar] [CrossRef]
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A review on high temperature thermochemical heat energy storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Martorell, I.; Miró, L.; Fernández, A.I.; Barreneche, C. 1—Introduction to thermal energy storage (TES) systems. In Advances in Thermal Energy Storage Systems; Cabeza, L.F., Ed.; Woodhead Publishing Series in Energy; Woodhead Publishing: Sawston, UK, 2015; pp. 1–28. [Google Scholar] [CrossRef]
- Xu, C.; Yu, Z.; Xie, Y.; Ren, Y.; Ye, F.; Ju, X. Study of the hydration behavior of zeolite-MgSO4 composites for long-term heat storage. Appl. Therm. Eng. 2018, 129, 250–259. [Google Scholar] [CrossRef]
- Gbenou, T.R.S.; Fopah-Lele, A.; Wang, K. Macroscopic and microscopic investigations of low-temperature thermochemical heat storage reactors: A review. Renew. Sustain. Energy Rev. 2022, 161, 112152. [Google Scholar] [CrossRef]
- GOV.UK. Energy Consumption in the UK 2020. Available online: https://www.gov.uk/government/statistics/energy-consumption-in-the-uk-2020 (accessed on 11 April 2024).
- District Energy|Enviroenergy. Available online: https://enviroenergy.co.uk/about-us/district-energy/ (accessed on 11 April 2024).
- Albert, D. District Heating in Norway: How Norwegian Cities Keep Warm, Life in Norway. Available online: https://www.lifeinnorway.net/district-heating-in-norway/ (accessed on 11 April 2024).
- Fjernvarme, 2015—Fjernvarmebalanse. GWh. ssb.no. Available online: https://www.ssb.no/energi-og-industri/statistikker/fjernvarme/aar/2021-05-26 (accessed on 11 April 2024).
- Di Lucia, L.; Ericsson, K. Low-carbon district heating in Sweden—Examining a successful energy transition. Energy Res. Soc. Sci. 2014, 4, 10–20. [Google Scholar] [CrossRef]
- Hall, T.; Vidén, S. The Million Homes Programme: A review of the great Swedish planning project. Plan. Perspect. 2005, 20, 301–328. [Google Scholar] [CrossRef]
- Heat Networks Planning Database|BEIS & Barbour ABI. Available online: https://data.barbour-abi.com/smart-map/repd/beis/?type=heat_network (accessed on 23 May 2024).
- GOV.UK. Heat Networks. Available online: https://www.gov.uk/government/collections/heat-networks (accessed on 11 April 2024).
- Ambrose, J.; Correspondent, J.A.E. UK Government Backs Plan to Ban Gas and “Hydrogen-Ready” Boilers. The Guardian. 13 December 2023. Available online: https://www.theguardian.com/business/2023/dec/13/uk-government-backs-plan-ban-gas-hydrogen-ready-boilers-newbuilds-2025 (accessed on 11 April 2024).
- Nottingham City District Heating Scheme. Available online: https://www.vitalenergi.co.uk/our-work/nottingham-city-district-heating/ (accessed on 11 April 2024).
- Desai, F.; Prasad, J.S.; Muthukumar; Rahman, M.M. Thermochemical energy storage system for cooling and process heating applications: A review. Energy Convers. Manag. 2021, 229, 113617. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, Z.; Ghafoor, A. A review of performance enhancement of PCM based latent heat storage system within the context of materials, thermal stability and compatibility. Energy Convers. Manag. 2016, 115, 132–158. [Google Scholar] [CrossRef]
- Kalaiselvam, S.; Parameshwaran, R. Thermal Energy Storage Technologies for Sustainability: Systems Design, Assessment and Applications; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Abedin, A.H.; Rosen, M.A. A Critical Review of Thermochemical Energy Storage Systems. Open Renew. Energy J. 2011, 4, 42–46. Available online: https://benthamopen.com/ABSTRACT/TOREJ-4-42 (accessed on 9 April 2024). [CrossRef]
- Böhm, H.; Lindorfer, J. Techno-economic assessment of seasonal heat storage in district heating with thermochemical materials. Energy 2019, 179, 1246–1264. [Google Scholar] [CrossRef]
- Xu, J.; Wang, R.Z.; Li, Y. A review of available technologies for seasonal thermal energy storage. Sol. Energy 2014, 103, 610–638. [Google Scholar] [CrossRef]
- Marie, L.F.; Landini, S.; Bae, D.; Francia, V.; O’Donovan, T.S. Advances in thermochemical energy storage and fluidised beds for domestic heat. J. Energy Storage 2022, 53, 105242. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Moya, J.; Bayón, A.; Jana, P.; Romero, M.; Gonzalez-Aguilar, J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Thermochemical energy storage at high temperature via redox cycles of Mn and Co oxides: Pure oxides versus mixed ones. Sol. Energy Mater. Sol. Cells 2014, 123, 47–57. [Google Scholar] [CrossRef]
- Felderhoff, M.; Bogdanović, B. High Temperature Metal Hydrides as Heat Storage Materials for Solar and Related Applications. Int. J. Mol. Sci. 2009, 10, 325. [Google Scholar] [CrossRef]
- Barker, R. The reversibility of the reaction CaCO3 ⇄ CaO+CO2. J. Appl. Chem. Biotechnol. 1973, 23, 733–742. [Google Scholar] [CrossRef]
- Aihara, M.; Nagai, T.; Matsushita, J.; Negishi, Y.; Ohya, H. Development of porous solid reactant for thermal-energy storage and temperature upgrade using carbonation/decarbonation reaction. Appl. Energy 2001, 69, 225–238. [Google Scholar] [CrossRef]
- Ervin, G. Solar heat storage using chemical reactions. J. Solid State Chem. 1977, 22, 51–61. [Google Scholar] [CrossRef]
- Criado, Y.A.; Alonso, M.; Abanades, J.C. Kinetics of the CaO/Ca(OH)2 Hydration/Dehydration Reaction for Thermochemical Energy Storage Applications. Ind. Eng. Chem. Res. 2014, 53, 12594–12601. [Google Scholar] [CrossRef]
- Schaube, F.; Wörner, A.; Tamme, R. High Temperature Thermochemical Heat Storage for Concentrated Solar Power Using Gas–Solid Reactions. J. Sol. Energy Eng. 2011, 133, 031006. [Google Scholar] [CrossRef]
- Mejia, A.C.; Afflerbach, S.; Linder, M.; Schmidt, M. Experimental analysis of encapsulated CaO/Ca(OH)2 granules as thermochemical storage in a novel moving bed reactor. Appl. Therm. Eng. 2020, 169, 114961. [Google Scholar] [CrossRef]
- Fahim, M.A.; Ford, J.D. Energy storage using the BaO/sub 2/-BaO reaction cycle. Chem. Eng. J. Lausanne Switz. 1983, 27, 21–28. [Google Scholar] [CrossRef]
- Neises, M.; Tescari, S.; de Oliveira, L.; Roeb, M.; Sattler, C.; Wong, B. Solar-heated rotary kiln for thermochemical energy storage. Sol. Energy 2012, 86, 3040–3048. [Google Scholar] [CrossRef]
- Wentworth, W.E.; Chen, E. Simple thermal decomposition reactions for storage of solar thermal energy. Sol. Energy 1976, 18, 205–214. [Google Scholar] [CrossRef]
- Chen, X.; Jin, X.; Liu, Z.; Ling, X.; Wang, Y. Experimental investigation on the CaO/CaCO3 thermochemical energy storage with SiO2 doping. Energy 2018, 155, 128–138. [Google Scholar] [CrossRef]
- Azpiazu, M.N.; Morquillas, J.M.; Vazquez, A. Heat recovery from a thermal energy storage based on the Ca(OH)2/CaO cycle. Appl. Therm. Eng. 2003, 23, 733–741. [Google Scholar] [CrossRef]
- Irabien, A.; Toquero, A.; Ortiz, M.I. Kinetic behaviour of non-isothermal lime hydration. Chem. Eng. J. 1989, 40, 93–99. [Google Scholar] [CrossRef]
- Schaube, F.; Koch, L.; Wörner, A.; Müller-Steinhagen, H. A thermodynamic and kinetic study of the de- and rehydration of Ca(OH)2 at high H2O partial pressures for thermo-chemical heat storage. Thermochim. Acta 2012, 538, 9–20. [Google Scholar] [CrossRef]
- Galwey, A.K.; Laverty, G.M. A kinetic and mechanistic study of the dehydroxylation of calcium hydroxide. Thermochim. Acta 1993, 228, 359–378. [Google Scholar] [CrossRef]
- Prasad, J.S.; Muthukumar; Desai, F.; Basu, D.N.; Rahman, M.M. A critical review of high-temperature reversible thermochemical energy storage systems. Appl. Energy 2019, 254, 113733. [Google Scholar] [CrossRef]
- Kato, Y.; Nakahata, J.; Yoshizawa, Y. Durability characteristics of the hydration of magnesium oxide under repetitive reaction. J. Mater. Sci. 1999, 34, 475–480. [Google Scholar] [CrossRef]
- Kato, Y.; Kobayashi, K.; Yoshizawa, Y. Durability to repetitive reaction of magnesium oxide/water reaction system for a heat pump. Appl. Therm. Eng. 1998, 18, 85–92. [Google Scholar] [CrossRef]
- Filippou, D.; Katiforis, N.; Papassiopi, N.; Adam, K. On the kinetics of magnesia hydration in magnesium acetate solutions. J. Chem. Technol. Biotechnol. 1999, 74, 322–328. [Google Scholar] [CrossRef]
- Pan, Z.; Zhao, C.Y. Dehydration/hydration of MgO/H2O chemical thermal storage system. Energy 2015, 82, 611–618. [Google Scholar] [CrossRef]
- Yan, J.; Yang, B.W.; Zhao, C.Y. Investigation of hydration/dehydration processes in a fluidized bed reactor using MgO/Mg(OH)2 thermochemical energy storage system. Sol. Energy 2022, 231, 630–645. [Google Scholar] [CrossRef]
- Kato, Y.; Yamashita, N.; Kobayashi, K.; Yoshizawa, Y. Kinetic study of the hydration of magnesium oxide for a chemical heat pump. Appl. Therm. Eng. 1996, 16, 853–862. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, C.Y. Experimental study of CaO/Ca(OH)2 in a fixed-bed reactor for thermochemical heat storage. Appl. Energy 2016, 175, 277–284. [Google Scholar] [CrossRef]
- Criado, Y.A.; Alonso, M.; Abanades, J.C.; Anxionnaz-Minvielle, Z. Conceptual process design of a CaO/Ca(OH)2 thermochemical energy storage system using fluidized bed reactors. Appl. Therm. Eng. 2014, 73, 1087–1094. [Google Scholar] [CrossRef]
- Shirzad, M.; Karimi, M.; Silva, J.A.C.; Rodrigues, A.E. Moving Bed Reactors: Challenges and Progress of Experimental and Theoretical Studies in a Century of Research. Ind. Eng. Chem. Res. 2019, 58, 9179–9198. [Google Scholar] [CrossRef]
- Pardo, P.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cognet; Cabassud, M. Ca(OH)2/CaO reversible reaction in a fluidized bed reactor for thermochemical heat storage. Sol. Energy 2014, 107, 605–616. [Google Scholar] [CrossRef]
- Valverde, J.M.; Pontiga, F.; Soria-Hoyo, C.; Quintanilla, M.A.; Moreno, H.; Duran, F.J.; Espin, M.J. Improving the gas–solids contact efficiency in a fluidized bed of CO2 adsorbent fine particles. Phys. Chem. Chem. Phys. 2011, 13, 14906–14909. [Google Scholar] [CrossRef] [PubMed]
- Kunii, D.; Levenspiel, O. Fluidization Engineering; Butterworth-Heinemann: Oxford, UK, 1991. [Google Scholar]
- Siedlecki, M.; De Jong, W.; Verkooijen, A.H.M. Fluidized Bed Gasification as a Mature And Reliable Technology for the Production of Bio-Syngas and Applied in the Production of Liquid Transportation Fuels—A Review. Energies 2011, 4, 389. [Google Scholar] [CrossRef]
- Geldart, D. Types of gas fluidization. Powder Technol. 1973, 7, 285–292. [Google Scholar] [CrossRef]
- Rosa, T.; Lin, J.; Pfeffer, R.; Nielsen, D.; Dai, L. Synthesis of Amine-Modified Aerogel Sorbents and Metal-Organic Framework-5 (MOF-5) Membranes for Carbon Dioxide Separation; Arizona State University: Tempe, AZ, USA, 2010. [Google Scholar]
- Trung Bui, T.; Anh Duc, L. Determination on Fluidization Velocity Types of the Continuous Refined Salt Fluidized Bed Drying. In Current Drying Processes; InTech Open: London, UK, 2020. [Google Scholar] [CrossRef]
- Cot-Gores, J.; Castell, A.; Cabeza, L.F. Thermochemical energy storage and conversion: A-state-of-the-art review of the experimental research under practical conditions. Renew. Sustain. Energy Rev. 2012, 16, 5207–5224. [Google Scholar] [CrossRef]
- Kishan, R.; Singh, D.; Sharma, A.K. CFD Analysis of Heat Exchanger Models Design Using ANSYS Fluent. Int. J. Mech. Eng. Technol. 2020, 11, 1–9. Available online: https://papers.ssrn.com/abstract=3540077 (accessed on 5 May 2024). [CrossRef]
- Escobar, M.; Trierweiler, J.O. Optimal heat exchanger network synthesis: A case study comparison. Appl. Therm. Eng. 2013, 51, 801–826. [Google Scholar] [CrossRef]
- Prabhanjan, D.G.; Raghavan, G.S.V.; Rennie, T.J. Comparison of heat transfer rates between a straight tube heat exchanger and a helically coiled heat exchanger. Int. Commun. Heat Mass Transf. 2002, 29, 185–191. [Google Scholar] [CrossRef]
- Ruthven, D.M. The residence time distribution for ideal laminar flow in helical tube. Chem. Eng. Sci. 1971, 26, 1113–1121. [Google Scholar] [CrossRef]
- Chokphoemphun, S.; Eiamsa-ard, S.; Promvonge; Thongdaeng, S.; Hongkong, S. Heat transfer of a coil-tube heat exchanger in the freeboard zone of a rice husk fluidized-bed combustor. Int. Commun. Heat Mass Transf. 2021, 127, 105462. [Google Scholar] [CrossRef]
- Angerer, M.; Becker, M.; Härzschel, S.; Kröper, K.; Gleis, S.; Vandersickel, A.; Spliethoff, H. Design of a MW-scale thermo-chemical energy storage reactor. Energy Rep. 2018, 4, 507–519. [Google Scholar] [CrossRef]
- Vignarooban, K.; Xu, X.; Arvay, A.; Hsu, K.; Kannan, A.M. Heat transfer fluids for concentrating solar power systems—A review. Appl. Energy 2015, 146, 383–396. [Google Scholar] [CrossRef]
- Liu, M.; Belusko, M.; Tay, N.H.S.; Bruno, F. Impact of the heat transfer fluid in a flat plate phase change thermal storage unit for concentrated solar tower plants. Sol. Energy 2014, 101, 220–231. [Google Scholar] [CrossRef]
- Feldhoff, J.F.; Benitez, D.; Eck, M.; Riffelmann, K.-J. Economic potential of solar thermal power plants with direct steam generation compared with HTF plants. J. Sol. Energy Eng. Trans. ASME 2010, 132, 041001. [Google Scholar] [CrossRef]
- Fernández, A.G.; Ushak, S.; Galleguillos, H.; Pérez, F.J. Development of new molten salts with LiNO3 and Ca(NO3)2 for energy storage in CSP plants. Appl. Energy 2014, 119, 131–140. [Google Scholar] [CrossRef]
- CAS Common Chemistry. Available online: https://commonchemistry.cas.org/detail?cas_rn=57-55-6 (accessed on 30 May 2024).
- Roßkopf, C.; Afflerbach, S.; Schmidt, M.; Görtz, B.; Kowald, T.; Linder, M.; Trettin, R. Investigations of nano coated calcium hydroxide cycled in a thermochemical heat storage. Energy Convers. Manag. 2015, 97, 94–102. [Google Scholar] [CrossRef]
- Shkatulov, A.; Ryu, J.; Kato, Y.; Aristov, Y. Composite material “Mg(OH)2/vermiculite”: A promising new candidate for storage of middle temperature heat. Energy 2012, 44, 1028–1034. [Google Scholar] [CrossRef]
- Funayama, S.; Takasu, H.; Zamengo, M.; Kariya, J.; Kim, S.T.; Kato, Y. Performance of thermochemical energy storage of a packed bed of calcium hydroxide pellets. Energy Storage 2019, 1, e40. [Google Scholar] [CrossRef]
- Slimani, H.; Ousaleh, H.A.; El Harrak, A.; Linder, M.; Faik, A. Doping effects on magnesium hydroxide: Enhancing dehydration and hydration performance for thermochemical energy storage applications. Chem. Eng. J. 2024, 488, 151048. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, C.Y. Thermodynamic and kinetic study of the dehydration process of CaO/Ca(OH)2 thermochemical heat storage system with Li doping. Chem. Eng. Sci. 2015, 138, 86–92. [Google Scholar] [CrossRef]



| Sensible TES | Latent TES | Thermochemical TES | |
|---|---|---|---|
| Fundamental principle |
|
|
|
| Amount of energy stored | |||
| Energy density | Small (~50 kWh/m3) | Medium (~100 kWh/m3) | High (~500 kWh/m3) |
| Storage temperature | Charging step temperature | Charging step temperature | Ambient temperature |
| Storage period | Limited (thermal losses) | Limited (thermal losses) | Theoretically unlimited |
| Technology | Simple | Medium | Complex |
| Pros/cons |
|
|
|
| TCES System | Example | Equation | Energy Density (MJ/kg) | Operating Temperature (°C) |
|---|---|---|---|---|
| Metal hydrides | Magnesium hydride | 2.85 | 250–500 | |
| Carbonates | Calcium carbonate | 1.78 | 700–1000 | |
| Hydroxides | Calcium hydroxide | 1.86 | 450–500 | |
| Magnesium hydroxide | 1.98 | 250–450 | ||
| Metal oxides | Barium peroxide | 0.45 | 400–1030 | |
| Cobalt (II,III) oxide | 0.85 | 350–1100 | ||
| Ammonia compounds | Ammonium bisulphate | 2.01 | 430–930 |
| TCES System | Reaction | Advantages | Disadvantages |
|---|---|---|---|
| Metal hydrides | MgH2 |
| |
| Carbonates | CaCO3 |
|
|
| Hydroxides | Mg(OH)2 |
| |
| Ca(OH)2 | |||
| Metal oxides | BaO2 |
| |
| Co3O4 |
| ||
| Ammonia compounds | NH4HSO4 |
|
| HTF Group | Name | Composition (wt%) | Melting Point (°C) | Stability Limit (°C) | Viscosity (Pa·s) | Thermal Conductivity (W/m·K) | Heat Capacity (kJ/kg·K) | Cost ($/kg) |
|---|---|---|---|---|---|---|---|---|
| Air | Air | Air | - | - | 0.00003 (at 600 °C) | 0.06 (at 600 °C) | 1.12 (at 600 °C) | 0 |
| Water/steam | Water/steam | H2O | 0 | - | 0.00133 (at 600 °C) | 0.08 (at 600 °C) | 2.42 (at 600 °C) | ~0 |
| Thermal oils | Mineral oil | N/A | −20 | 300 | N/A | ~0.1 | N/A | 0.3 |
| Synthetic oil | N/A | −20 | 350 | N/A | ~0.1 | N/A | 3 | |
| Organics | Biphenyl/ diphenyl oxide | N/A | 12 | 393 | 0.00059 (at 300 °C) | ~0.01 (at 300 °C) | 1.93 (at 300 °C) | 100 |
| Molten salts | Hitec | NaNO3 (7) KNO3 (53) NaNO2 (40) | 142 | 535 | 0.00316 (at 300 °C) | ~0.2 (at 300 °C) | 1.56 (at 300 °C) | 0.93 |
| Na-K-Li nitrates | NaNO3 (28) KNO3 (52) LiNO3 (20) | 130 | 600 | 0.03 (at 300 °C) | N/A | 1.091 | ~1.1 | |
| Na-K-Li carbonates | Li2CO3 (32.1) Na2CO3 (33.4) K2CO3 (34.5) | ~400 | 800–850 | 0.0043 (at 800 °C) | N/A | ~1.4 to 1.5 | ~1.2 to 1.3 | |
| Liquid metals | Na | - | 98 | 883 | 0.00021 (at 600 °C) | 46.0 (at 600 °C) | 1.25 (at 600 °C) | 2 |
| Na-K | Na (22.2) K (77.8) | −12 | 785 | 0.00018 (at 600 °C) | 26.2 (at 600 °C) | 0.87 (at 600 °C) | 2 | |
| Pb-Bi | Pb (44.5) Bi (55.45) | 125 | 1533 | 0.00108 (at 600 °C) | 12.8 (at 600 °C) | 0.15 (at 600 °C) | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roger-Lund, S.; Darkwa, J.; Worall, M.; Calautit, J.; Boukhanouf, R. A Review of Thermochemical Energy Storage Systems for District Heating in the UK. Energies 2024, 17, 3389. https://doi.org/10.3390/en17143389
Roger-Lund S, Darkwa J, Worall M, Calautit J, Boukhanouf R. A Review of Thermochemical Energy Storage Systems for District Heating in the UK. Energies. 2024; 17(14):3389. https://doi.org/10.3390/en17143389
Chicago/Turabian StyleRoger-Lund, Sarah, Jo Darkwa, Mark Worall, John Calautit, and Rabah Boukhanouf. 2024. "A Review of Thermochemical Energy Storage Systems for District Heating in the UK" Energies 17, no. 14: 3389. https://doi.org/10.3390/en17143389
APA StyleRoger-Lund, S., Darkwa, J., Worall, M., Calautit, J., & Boukhanouf, R. (2024). A Review of Thermochemical Energy Storage Systems for District Heating in the UK. Energies, 17(14), 3389. https://doi.org/10.3390/en17143389








