Removal of Nitrogen Pollutants in the Chemical Looping Process: A Review
Abstract
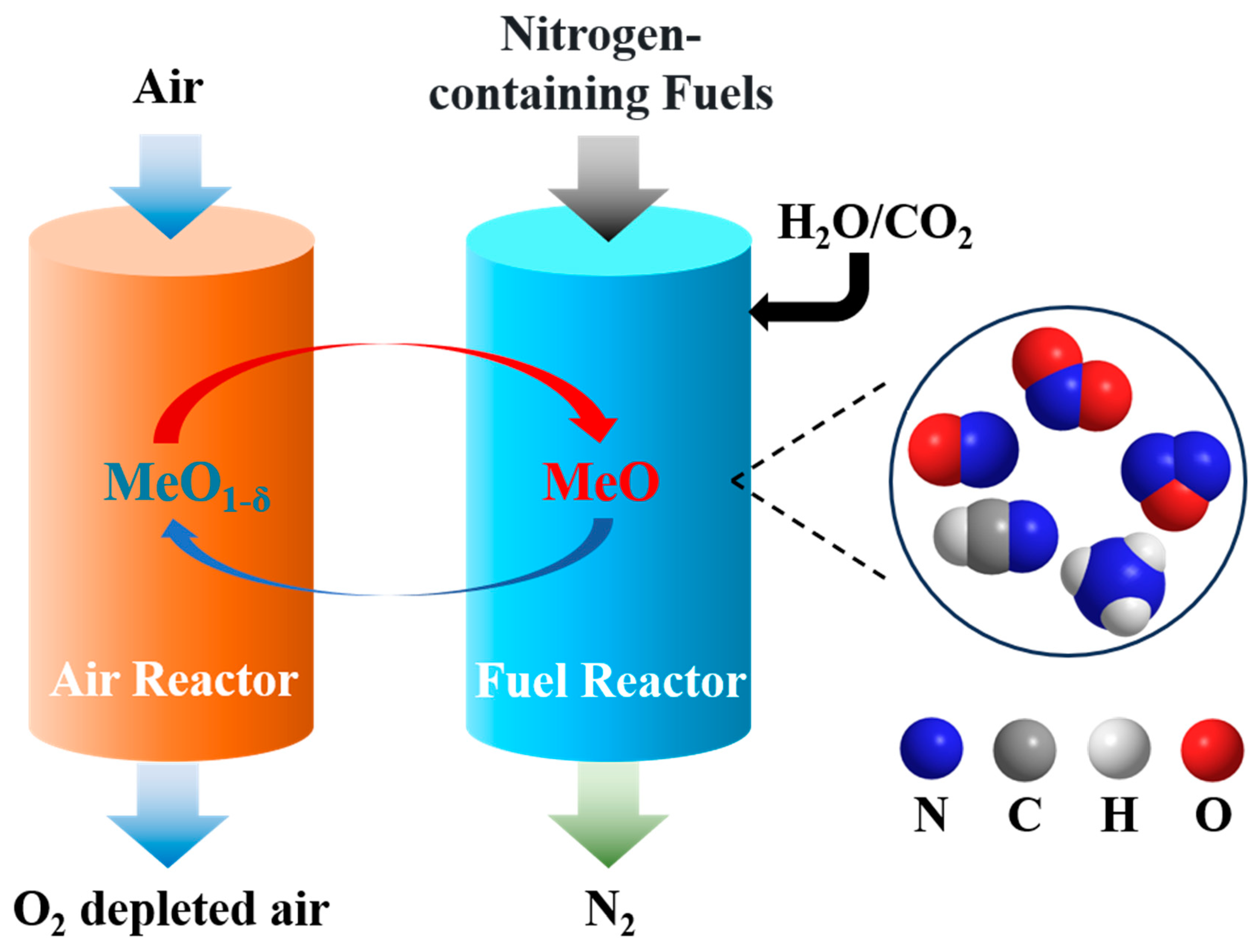
:1. Introduction
2. Formation of Nitrogen Pollutants in the Chemical Looping Process
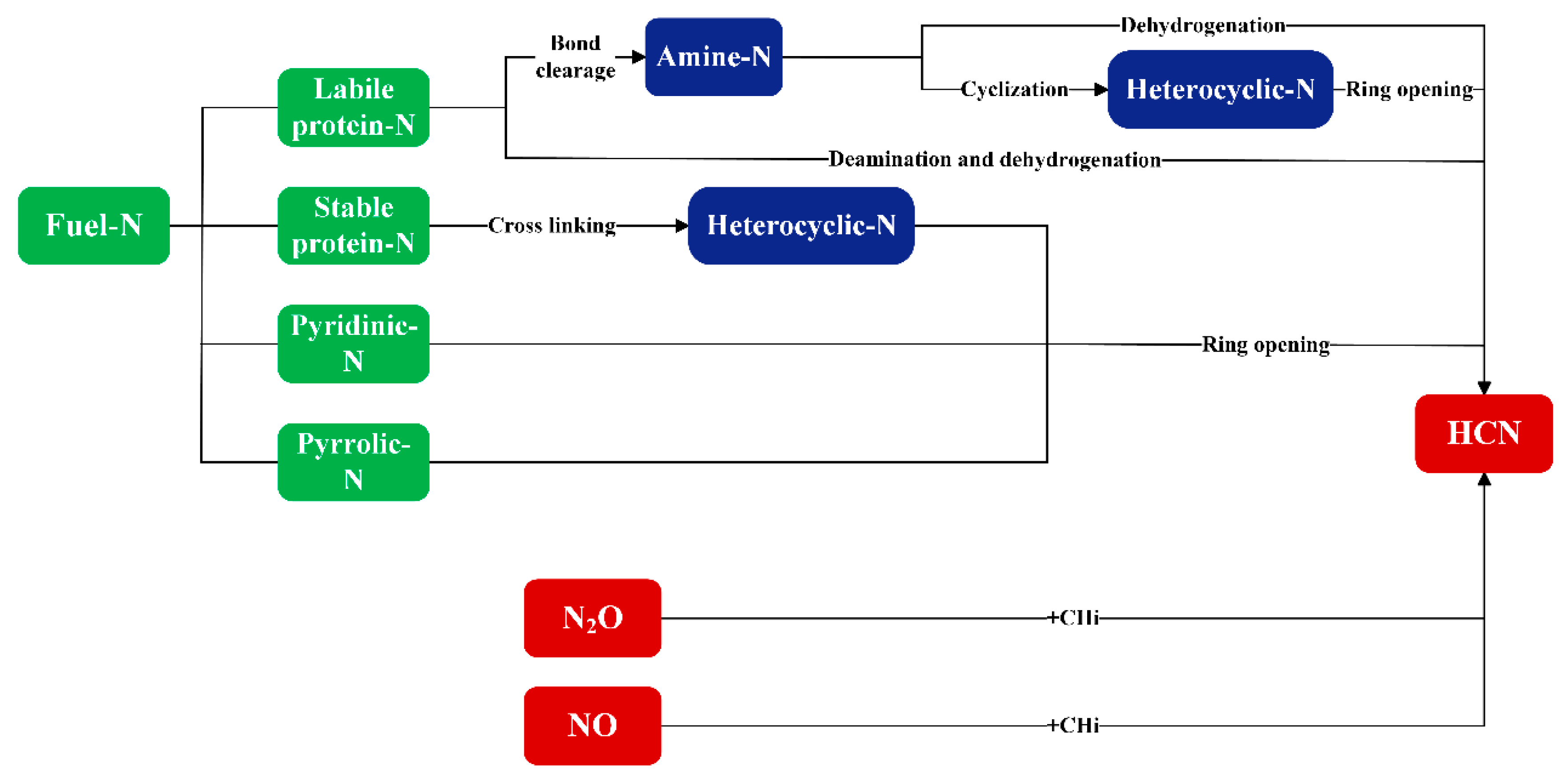
2.1. Formation of Reduced Nitrogen Pollutants
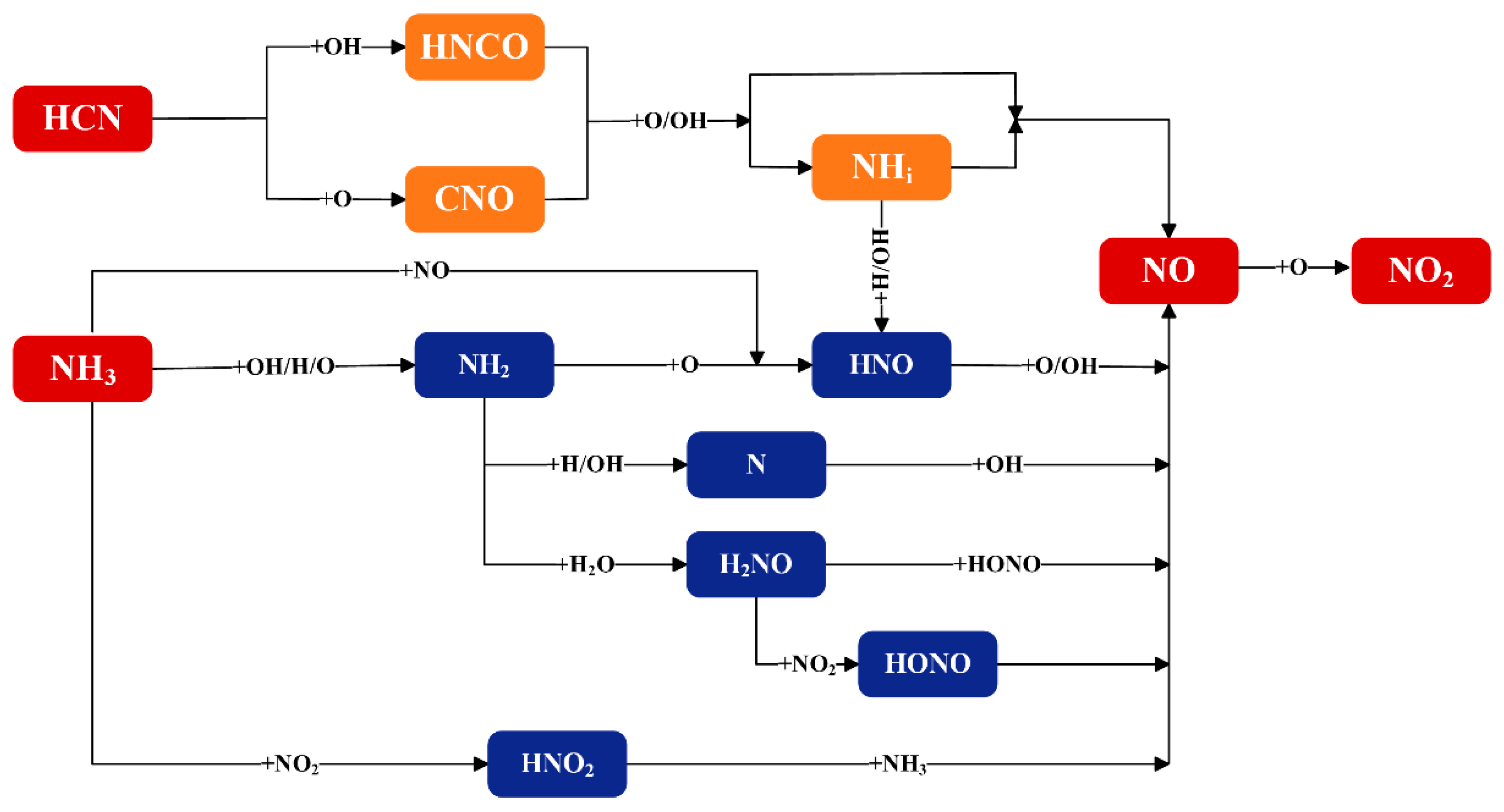
2.1.1. NH3
2.1.2. HCN
2.2. Formation of Oxidized Nitrogen Pollutants
2.2.1. NOx
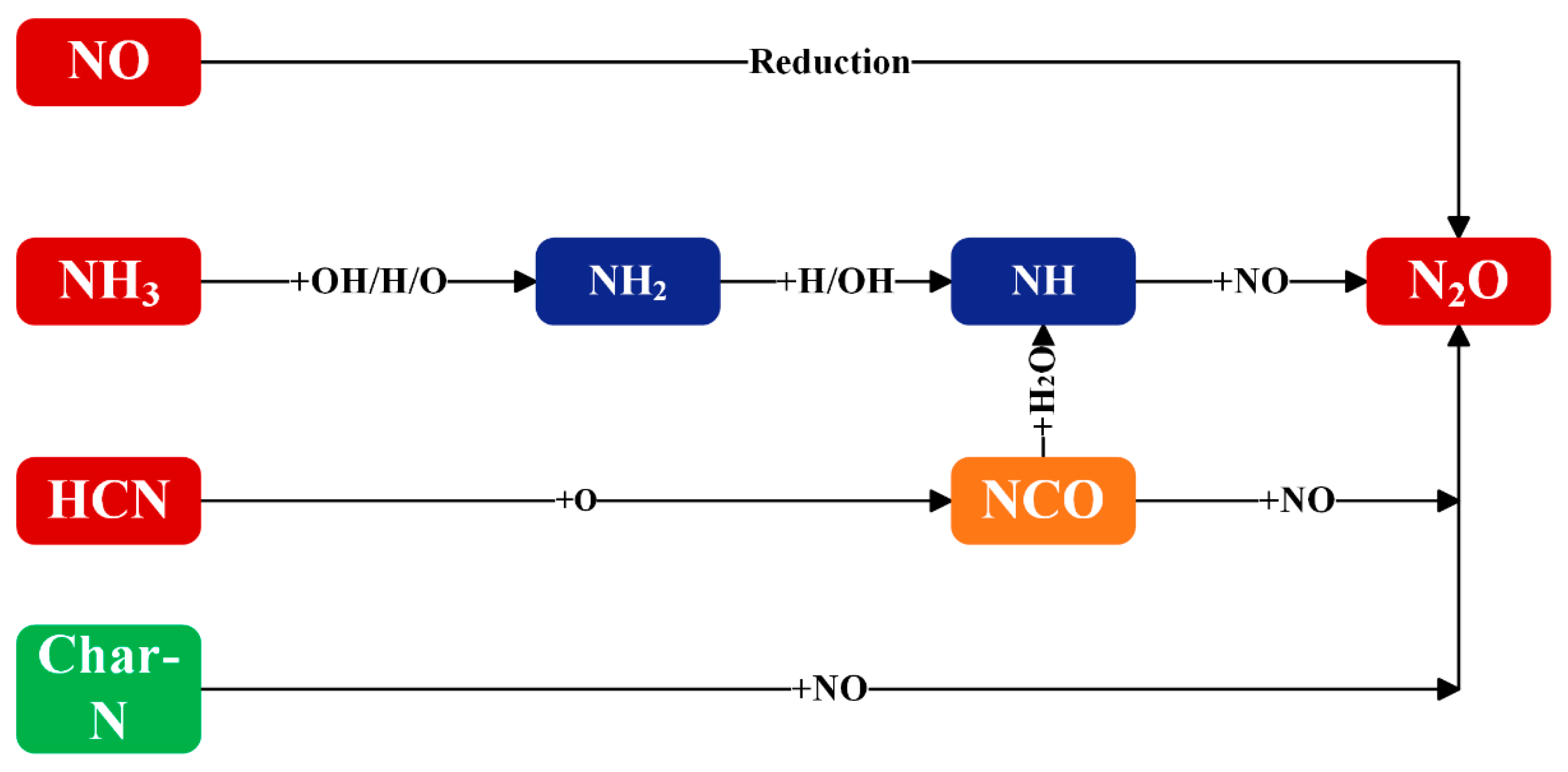
2.2.2. N2O
3. Removal of Nitrogen Pollutants in the Chemical Looping Process
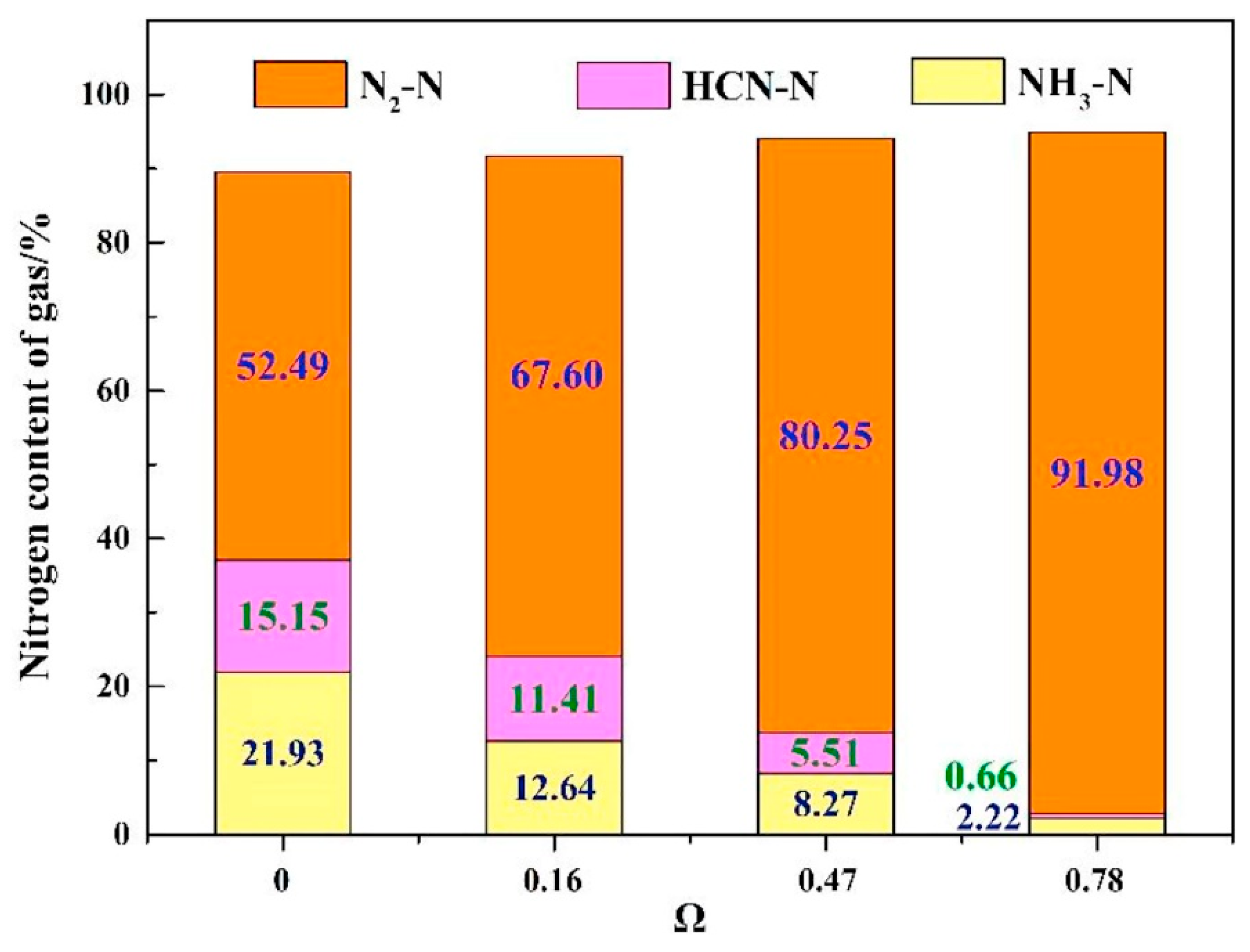
3.1. Effect of OCs
3.2. Effect of Reaction Conditions on the Conversion of Nitrogen-Containing Pollutants
3.2.1. Effect of Reaction Atmosphere
3.2.2. Effect of Reaction Temperature
3.2.3. Effect of the Cycle Number
3.3. Effect of Fuel
4. Conclusions and Outlook
Funding
Conflicts of Interest
Abbreviations
| Full name | abbreviation |
| Chemical looping process | CLP |
| Chemical looping processes | CLPs |
| nitrogen-containing | N-containing |
| oxygen carrier | OC |
| oxygen carriers | OCs |
| NO and NO2 | NOx |
| fuel nitrogen | fuel-N |
| inorganic nitrogen | inorganic-N |
| protein nitrogen | protein-N |
| pyrrole nitrogen | pyrrole-N |
| pyridine nitrogen | pyridine-N |
| amine nitrogen | amine-N |
| heterocyclic nitrogen | heterocyclic-N |
| NH3, NH2 and NH | NHi |
References
- Geng, W.; Ming, Z.; Lilin, P.; Ximei, L.; Bo, L.; Jinhui, D. China’s New Energy Development: Status, Constraints and Reforms. Renew. Sustain. Energy Rev. 2016, 53, 885–896. [Google Scholar] [CrossRef]
- Zhironkin, S.; Rybár, R. Sustainable Development Processes for Renewable Energy Technology. Processes 2022, 10, 1363. [Google Scholar] [CrossRef]
- Birol, D.F. World Energy Outlook; OECD/IEA: Paris, France, 2022. [Google Scholar]
- Kondratyev, K.Y.; Varotsos, C. Atmospheric Greenhouse Effect in the Context of Global Climate Change. Il Nuovo Cimento C 1995, 18, 123–151. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral Reefs Under Rapid Climate Change and Ocean Acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Merkel, T.C.; Lin, H.; Wei, X.; Baker, R. Power Plant Post-Combustion Carbon Dioxide Capture: An Opportunity for Membranes. J. Membr. Sci. 2010, 359, 126–139. [Google Scholar] [CrossRef]
- Kanniche, M.; Gros-Bonnivard, R.; Jaud, P.; Valle-Marcos, J.; Amann, J.-M.; Bouallou, C. Pre-Combustion, Post-Combustion and Oxy-Combustion in Thermal Power Plant for CO2 Capture. Appl. Therm. Eng. 2010, 30, 53–62. [Google Scholar] [CrossRef]
- Wang, P.; Means, N.; Shekhawat, D.; Berry, D.; Massoudi, M. Chemical-Looping Combustion and Gasification of Coals and Oxygen Carrier Development: A Brief Review. Energies 2015, 8, 10605–10635. [Google Scholar] [CrossRef]
- Chiu, P.-C.; Ku, Y. Chemical Looping Process—A Novel Technology for Inherent CO2 Capture. Aerosol Air Qual. Res. 2012, 12, 1421–1432. [Google Scholar] [CrossRef]
- Guo, Q.; Hu, X.; Liu, Y.; Jia, W.; Yang, M.; Wu, M.; Tian, H.; Ryu, H.-J. Coal Chemical-Looping Gasification of Ca-Based Oxygen Carriers Decorated by CaO. Powder Technol. 2015, 275, 60–68. [Google Scholar] [CrossRef]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F. Progress in Chemical-Looping Combustion and Reforming Technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef]
- Lyngfelt, A.; Linderholm, C. Chemical-Looping Combustion of Solid Fuels—Status and Recent Progress. Energy Procedia 2017, 114, 371–386. [Google Scholar] [CrossRef]
- Lyngfelt, A. Oxygen Carriers for Chemical Looping Combustion—4000 h of Operational Experience. Oil Gas Sci. Technol.—Rev. D’IFP Energ. Nouv. 2011, 66, 161–172. [Google Scholar] [CrossRef]
- Fan, L.; Li, F.; Ramkumar, S. Utilization of Chemical Looping Strategy in Coal Gasification Processes. Particuology 2008, 6, 131–142. [Google Scholar] [CrossRef]
- Hu, Q.; Shen, Y.; Chew, J.W.; Ge, T.; Wang, C.-H. Chemical Looping Gasification of Biomass with Fe2O3/CaO as the Oxygen Carrier for Hydrogen-Enriched Syngas Production. Chem. Eng. J. 2020, 379, 122346. [Google Scholar] [CrossRef]
- He, F.; Galinsky, N.; Li, F. Chemical Looping Gasification of Solid Fuels Using Bimetallic Oxygen Carrier Particles—Feasibility Assessment and Process Simulations. Int. J. Hydrogen Energy 2013, 38, 7839–7854. [Google Scholar] [CrossRef]
- Zhao, Y.; Jin, B.; Luo, X.; Liang, Z. Thermodynamic Evaluation and Experimental Investigation of CaO-Assisted Fe-Based Chemical Looping Reforming Process for Syngas Production. Appl. Energy 2021, 288, 116614. [Google Scholar] [CrossRef]
- Rydén, M.; Lyngfelt, A.; Mattisson, T.; Chen, D.; Holmen, A.; Bjørgum, E. Novel Oxygen-Carrier Materials for Chemical-Looping Combustion and Chemical-Looping Reforming; LaxSr1−xFeyCo1−yO3−δ Perovskites and Mixed-Metal Oxides of NiO, Fe2O3 and Mn3O4. Int. J. Greenh. Gas Control 2008, 2, 21–36. [Google Scholar] [CrossRef]
- Song, D.; Lin, Y.; Fang, S.; Li, Y.; Zhao, K.; Chen, X.; Huang, Z.; He, F.; Zhao, Z.; Huang, H.; et al. Unraveling the Atomic Interdiffusion Mechanism of NiFe2O4 Oxygen Carriers during Chemical Looping CO2 Conversion. Carbon Energy 2024, e493. [Google Scholar] [CrossRef]
- Mattisson, T.; Lyngfelt, A.; Leion, H. Chemical-Looping with Oxygen Uncoupling for Combustion of Solid Fuels. Int. J. Greenh. Gas Control 2009, 3, 11–19. [Google Scholar] [CrossRef]
- Kuang, C.; Wang, S.; Luo, M.; Zhao, J. Reactivity Study and Kinetic Evaluation of CuO-Based Oxygen Carriers Modified by Three Different Ores in Chemical Looping with Oxygen Uncoupling (CLOU) Process. Chin. J. Chem. Eng. 2021, 37, 54–63. [Google Scholar] [CrossRef]
- Song, D.; Lin, Y.; Li, C.; Fang, S.; He, F.; Huang, Z.; Zhao, Z.; Xiong, Y.; Huang, H. Review on Migration and Transformation of Lattice Oxygen during Chemical Looping Conversion: Advances and Perspectives. Energy Fuels 2023, 37, 5743–5756. [Google Scholar] [CrossRef]
- Fan, L.-S.; Zeng, L.; Luo, S. Chemical-Looping Technology Platform. AIChE J. 2015, 61, 2–22. [Google Scholar] [CrossRef]
- Fan, L.-S.; Li, F. Chemical Looping Technology and Its Fossil Energy Conversion Applications. Ind. Eng. Chem. Res. 2010, 49, 10200–10211. [Google Scholar] [CrossRef]
- Adánez, J.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Abad, A.; Palacios, J.M. Selection of Oxygen Carriers for Chemical-Looping Combustion. Energy Fuels 2004, 18, 371–377. [Google Scholar] [CrossRef]
- Williams, A. (Ed.) Combustion of Liquid Fuel Sprays; Butterworth-Heinemann: Oxford, UK, 1990; pp. 267–273. [Google Scholar] [CrossRef]
- Erisman, J.W.; Galloway, J.N.; Seitzinger, S.; Bleeker, A.; Dise, N.B.; Petrescu, A.M.R.; Leach, A.M.; de Vries, W. Consequences of Human Modification of the Global Nitrogen Cycle. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130116. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. A Review of Emerging Adsorbents for Nitrate Removal from Water. Chem. Eng. J. 2011, 168, 493–504. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, A. Evaluation of Ammonium Removal Using a Chitosan-g-Poly (Acrylic Acid)/Rectorite Hydrogel Composite. J. Hazard. Mater. 2009, 171, 671–677. [Google Scholar] [CrossRef]
- Niu, X.; Shen, L.; Jiang, S.; Gu, H.; Xiao, J. Combustion Performance of Sewage Sludge in Chemical Looping Combustion with Bimetallic Cu–Fe Oxygen Carrier. Chem. Eng. J. 2016, 294, 185–192. [Google Scholar] [CrossRef]
- Gu, H.; Shen, L.; Zhong, Z.; Niu, X.; Ge, H.; Zhou, Y.; Xiao, S.; Jiang, S. NO Release during Chemical Looping Combustion with Iron Ore as an Oxygen Carrier. Chem. Eng. J. 2015, 264, 211–220. [Google Scholar] [CrossRef]
- Xiao, S.; Shen, L.-H.; Xiao, J.; Niu, X.; Gu, H.; Song, T. NOx Release in Chemical Looping Combustion of Biomass Based on Hematite. Ranliao Huaxue Xuebao J. Fuel Chem. Technol. 2015, 43, 490–498. [Google Scholar]
- Mendiara, T.; Izquierdo, M.T.; Abad, A.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Adánez, J. Release of Pollutant Components in CLC of Lignite. Int. J. Greenh. Gas Control 2014, 22, 15–24. [Google Scholar] [CrossRef]
- Adánez, J.; Abad, A.; Mendiara, T.; Gayán, P.; de Diego, L.F.; García-Labiano, F. Chemical Looping Combustion of Solid Fuels. Prog. Energy Combust. Sci. 2018, 65, 6–66. [Google Scholar] [CrossRef]
- Jerndal, E.; Mattisson, T.; Lyngfelt, A. Thermal Analysis of Chemical-Looping Combustion. Chem. Eng. Res. Des. 2006, 84, 795–806. [Google Scholar] [CrossRef]
- Ishida, M.; Jin, H. A Novel Chemical-Looping Combustor without NOx Formation. Ind. Eng. Chem. Res. 1996, 35, 2469–2472. [Google Scholar] [CrossRef]
- Song, T.; Shen, L. Review of Reactor for Chemical Looping Combustion of Solid Fuels. Int. J. Greenh. Gas Control 2018, 76, 92–110. [Google Scholar] [CrossRef]
- Zhao, H.; Tian, X.; Ma, J.; Su, M.; Wang, B.; Mei, D. Development of Tailor-Made Oxygen Carriers and Reactors for Chemical Looping Processes at Huazhong University of Science & Technology. Int. J. Greenh. Gas Control 2020, 93, 102898. [Google Scholar] [CrossRef]
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling Nitrogen Chemistry in Combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef]
- Niu, X.; Xu, Y.; Shen, L. Effect of Torrefaction on the Evolution of Carbon and Nitrogen during Chemical Looping Gasification of Rapeseed Cake. Chem. Eng. J. 2022, 450, 138134. [Google Scholar] [CrossRef]
- Yan, B.; Li, Z.; Jiao, L.; Li, J.; Chen, G.; Yang, G. Chemical Looping Gasification of Chlorella: Parametric Optimization, Reaction Mechanisms, and Nitrogen-Containing Pollutants Emission. Fuel 2021, 289, 119987. [Google Scholar] [CrossRef]
- Fang, S.; Deng, Z.; Lin, Y.; Huang, Z.; Ding, L.; Deng, L.; Huang, H. Investigation of the Nitrogen Migration Characteristics in Sewage Sludge during Chemical Looping Gasification. Energy 2021, 216, 119247. [Google Scholar] [CrossRef]
- Niu, X.; Shen, L. Evolution of Carbon and Nitrogen during Chemical Looping Gasification of Rapeseed Cake with Ca-Fe Oxygen Carrier. Chem. Eng. J. 2022, 431, 134232. [Google Scholar] [CrossRef]
- Zheng, C.; Ma, J.; Yang, Q.; Zhang, T.; Luo, X.; Zhao, H. Microscopic Insight into Catalytic HCN Removal over the CuO Surface in Chemical Looping Combustion. Proc. Combust. Inst. 2023, 39, 4457–4466. [Google Scholar] [CrossRef]
- Song, T.; Guo, W.; Shen, L. Fuel Nitrogen Conversion in Chemical Looping with Oxygen Uncoupling of Coal with a CuO-Based Oxygen Carrier. Energy Fuels 2015, 29, 3820–3832. [Google Scholar] [CrossRef]
- Xie, K.-C.; Lin, J.-Y.; Li, W.-Y.; Chang, L.-P.; Feng, J.; Zhao, W. Formation of HCN and NH3 during Coal Macerals Pyrolysis and Gasification with CO2. Fuel 2005, 84, 271–277. [Google Scholar] [CrossRef]
- Ma, J.; Tian, X.; Wang, C.; Zhao, H.; Liu, Z.; Zheng, C. Fate of Fuel-nitrogen during in Situ Gasification Chemical Looping Combustion of Coal. Fuel Process. Technol. 2021, 215, 106710. [Google Scholar] [CrossRef]
- Atkinson, R.; Arey, J. Atmospheric Degradation of Volatile Organic Compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef] [PubMed]
- Calvert, J.G.; Atkinson, R.; Becker, K.H.; Kamens, R.M.; Seinfeld, J.H.; Wallington, T.H.; Yarwood, G. The Mechanisms of Atmospheric Oxidation of the Aromatic Hydrocarbons; Oxford University Press: Oxford, UK; New York, NY, USA, 2002. [Google Scholar]
- Gao, P.; Zheng, M.; Li, K.; Wang, H.; Wang, J.; Bao, G.; Wang, L. Characteristics of Nitrogen Oxide Emissions from Combustion Synthesis of a CuO Oxygen Carrier. Fuel Process. Technol. 2022, 233, 107295. [Google Scholar] [CrossRef]
- Yan, J.; Lai, J.; Yin, K.; Yan, Y.; Shen, L.; Yang, L. Syngas Production and Gas-N Evolution over Heterogeneously Doped La-Fe-O Perovskite-Type Oxygen Carriers in Chemical Looping Gasification of Microalgae. Bioresour. Technol. 2023, 369, 128507. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Bowman, C.T. Mechanism and Modeling of Nitrogen Chemistry in Combustion. Prog. Energy Combust. Sci. 1989, 15, 287–338. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Dong, N.; Lin, Y.; Chang, G.; Wei, G.; Zhao, K.; Wang, X.; Zheng, A.; Zhao, Z.; et al. Chemical Looping Gasification of High Nitrogen Wood Waste Using a Copper Slag Oxygen Carrier Modified by Alkali and Alkaline Earth Metals. Chem. Eng. J. 2021, 410, 128344. [Google Scholar] [CrossRef]
- Zhao, B.; Su, Y. Emission and Conversion of NO from Algal Biomass Combustion in O2/CO2 Atmosphere. J. Environ. Manage. 2019, 250, 109419. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Mo, Y.; Fang, S.; Huang, Z.; Wei, G.; Zhao, Z.; Huang, H. A Study on the Chemical Looping Combustion of Sewage Sludge: The Emission of NOx and Its Precursors. Fuel Process. Technol. 2022, 231, 107260. [Google Scholar] [CrossRef]
- Lyngfelt, A.; Hedayati, A.; Augustsson, E. Fate of NO and Ammonia in Chemical Looping Combustion—Investigation in a 300 W Chemical Looping Combustion Reactor System. Energy Fuels 2022, 36, 9628–9647. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, H.; Fang, S.; Huang, Z.; Wei, G.; Zhang, Y.; Xia, H.; Zhao, Z.; Huang, H. Chemical Looping Combustion of Lignite Using Iron Ore: C-Gas Products (CO2, CO, CH4) and NOx Emissions. Energy 2022, 256, 124602. [Google Scholar] [CrossRef]
- Huang, Z.; Gao, N.; Liu, M.; Lin, Y.; Chang, G.; Deng, L.; Huang, H. Emissions of Nitrogenous Pollutants in Chemical Looping Gasification of High Nitrogen Wood Waste Using a K-Modified Copper Slag Oxygen Carrier. J. Therm. Anal. Calorim. 2022, 147, 9725–9735. [Google Scholar] [CrossRef]
- Fang, S.; Yan, S.; Lu, Q.; Li, C.; Wang, H.; Lin, Y.; Huang, Z.; Huang, H. Nitrogen Trade-off during Lignite Chemical Looping Combustion Using Hematite as an Oxygen Carrier. Fuel Process. Technol. 2022, 232, 107286. [Google Scholar] [CrossRef]
- Song, T.; Shen, L.; Xiao, J.; Chen, D.; Gu, H.; Zhang, S. Nitrogen Transfer of Fuel-N in Chemical Looping Combustion. Combust. Flame 2012, 159, 1286–1295. [Google Scholar] [CrossRef]
- Bayham, S.C.; Kim, H.R.; Wang, D.; Tong, A.; Zeng, L.; McGiveron, O.; Kathe, M.V.; Chung, E.; Wang, W.; Wang, A.; et al. Iron-Based Coal Direct Chemical Looping Combustion Process: 200-h Continuous Operation of a 25-kWth Subpilot Unit. Energy Fuels 2013, 27, 1347–1356. [Google Scholar] [CrossRef]
- Linderholm, C.; Knutsson, P.; Schmitz, M.; Markström, P.; Lyngfelt, A. Material Balances of Carbon, Sulfur, Nitrogen and Ilmenite in a 100 kW CLC Reactor System. Int. J. Greenh. Gas Control 2014, 27, 188–202. [Google Scholar] [CrossRef]
- Pérez-Vega, R.; Adánez-Rubio, I.; Gayán, P.; Izquierdo, M.T.; Abad, A.; García-Labiano, F.; de Diego, L.F.; Adánez, J. Sulphur, Nitrogen and Mercury Emissions from Coal Combustion with CO2 Capture in Chemical Looping with Oxygen Uncoupling (CLOU). Int. J. Greenh. Gas Control 2016, 46, 28–38. [Google Scholar] [CrossRef]
- Pérez-Astray, A.; Adánez-Rubio, I.; Mendiara, T.; Izquierdo, M.T.; Abad, A.; Gayán, P.; de Diego, L.F.; García-Labiano, F.; Adánez, J. Comparative Study of Fuel-N and Tar Evolution in Chemical Looping Combustion of Biomass under Both iG-CLC and CLOU Modes. Fuel 2019, 236, 598–607. [Google Scholar] [CrossRef]
- Penthor, S.; Mayer, K.; Pröll, T.; Hofbauer, H. Experimental Study of the Path of Nitrogen in Chemical Looping Combustion Using a Nickel-Based Oxygen Carrier. Energy Fuels 2014, 28, 6604–6609. [Google Scholar] [CrossRef]
- Cheng, M.; Normann, F.; Zhao, D.; Li, Z.; Cai, N.; Leion, H. Oxidation of Ammonia by Ilmenite under Conditions Relevant to Chemical-Looping Combustion. Energy Fuels 2015, 29, 8126–8134. [Google Scholar] [CrossRef]
- Normann, F.; Wismer, A.O.; Müller, C.R.; Leion, H. Oxidation of Ammonia by Iron, Manganese and Nickel Oxides—Implications on NOx Formation in Chemical-Looping Combustion. Fuel 2019, 240, 57–63. [Google Scholar] [CrossRef]
- Pachler, R.F.; Penthor, S.; Mayer, K.; Hofbauer, H. Investigation of the Fate of Nitrogen in Chemical Looping Combustion of Gaseous Fuels Using Two Different Oxygen Carriers. Energy 2020, 195, 116926. [Google Scholar] [CrossRef]
- Fang, S.; Deng, Z.; Lin, Y.; Huang, Z.; Ding, L.; Deng, L.; Huang, H. Nitrogen Migration in Sewage Sludge Chemical Looping Gasification Using Copper Slag Modified by NiO as an Oxygen Carrier. Energy 2021, 228, 120448. [Google Scholar] [CrossRef]
- Mayrhuber, S.; Normann, F.; Yilmaz, D.; Leion, H. Effect of the Oxygen Carrier Ilmenite on NOX Formation in Chemical-Looping Combustion. Fuel Process. Technol. 2021, 222, 106962. [Google Scholar] [CrossRef]
- Jiang, H.; Huo, R.; Zhang, Z.; Lin, Y.; Zhao, Z.; Huang, Z.; Fang, Y.; Li, H. Removal of Pollution from the Chemical Looping Process: A Mini Review. Fuel Process. Technol. 2021, 221, 106937. [Google Scholar] [CrossRef]
- Han, J.; Kim, H. The Reduction and Control Technology of Tar during Biomass Gasification/Pyrolysis: An Overview. Renew. Sustain. Energy Rev. 2008, 12, 397–416. [Google Scholar] [CrossRef]
- Yi, L.; Liu, H.; Lu, G.; Zhang, Q.; Wang, J.; Hu, H.; Yao, H. Effect of Mixed Fe/Ca Additives on Nitrogen Transformation during Protein and Amino Acid Pyrolysis. Energy Fuels 2017, 31, 9484–9490. [Google Scholar] [CrossRef]
- Xu, C.; Tsubouchi, N.; Hashimoto, H.; Ohtsuka, Y. Catalytic Decomposition of Ammonia Gas with Metal Cations Present Naturally in Low Rank Coals. Fuel 2005, 84, 1957–1967. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, J.; Zuo, W.; Chen, L.; Cui, Y.; Tan, T. Nitrogen Conversion in Relation to NH3 and HCN during Microwave Pyrolysis of Sewage Sludge. Environ. Sci. Technol. 2013, 47, 3498–3505. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Guan, R.; Yang, J.; Li, H.; Yu, Z.; Liang, S.; Yu, W.; Hu, J.; Hou, H.; Liu, B. Effects of Red Mud on Emission Control of NOx Precursors during Sludge Pyrolysis: A Protein Model Compound Study. Waste Manag. 2019, 85, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Zhao, Y.; Zhang, Y.; Sun, S.; Meng, S.; Guo, Y.; Huang, Y. Effects of K and Ca on Reforming of Model Tar Compounds with Pyrolysis Biochars under H2O or CO2. Chem. Eng. J. 2016, 306, 422–432. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, S.; Wang, Y.; Su, S.; Sun, L.; Xu, B.; He, L.; Xiang, J. Catalytic Effects of Inherent Alkali and Alkaline Earth Metallic Species on Steam Gasification of Biomass. Int. J. Hydrogen Energy 2015, 40, 15460–15469. [Google Scholar] [CrossRef]
- Hu, Z.; Miao, Z.; Wu, J.; Jiang, E. Nickel-Iron Modified Natural Ore Oxygen Carriers for Chemical Looping Steam Methane Reforming to Produce Hydrogen. Int. J. Hydrogen Energy 2021, 46, 39700–39718. [Google Scholar] [CrossRef]
- Ge, H.; Shen, L.; Gu, H.; Jiang, S. Effect of Co-Precipitation and Impregnation on K-Decorated Fe2O3/Al2O3 Oxygen Carrier in Chemical Looping Combustion of Bituminous Coal. Chem. Eng. J. 2015, 262, 1065–1076. [Google Scholar] [CrossRef]
- Liu, T.; Yu, Z.; Li, G.; Guo, S.; Shan, J.; Li, C.; Fang, Y. Performance of Potassium-Modified Fe2O3/Al2O3 Oxygen Carrier in Coal-Direct Chemical Looping Hydrogen Generation. Int. J. Hydrogen Energy 2018, 43, 19384–19395. [Google Scholar] [CrossRef]
- Bao, J.; Li, Z.; Cai, N. Reduction Kinetics of Foreign-Ion-Promoted Ilmenite Using Carbon Monoxide (CO) for Chemical Looping Combustion. Ind. Eng. Chem. Res. 2013, 52, 10646–10655. [Google Scholar] [CrossRef]
- Yu, Z.; Li, C.; Fang, Y.; Huang, J.; Wang, Z. Reduction Rate Enhancements for Coal Direct Chemical Looping Combustion with an Iron Oxide Oxygen Carrier. Energy Fuels 2012, 26, 2505–2511. [Google Scholar] [CrossRef]
- Gu, H.; Shen, L.; Xiao, J.; Zhang, S.; Song, T.; Chen, D. Iron Ore as Oxygen Carrier Improved with Potassium for Chemical Looping Combustion of Anthracite Coal. Combust. Flame 2012, 159, 2480–2490. [Google Scholar] [CrossRef]
- Liu, T.; Yu, Z.; Mei, Y.; Feng, R.; Yang, S.; Wang, Z.; Fang, Y. Potassium Migration and Transformation during the Deep Reduction of Oxygen Carrier (OC) by Char in Coal-Direct Chemical Looping Hydrogen Generation Using Potassium-Modified Fe2O3/Al2O3 OC. Fuel 2019, 256, 115883. [Google Scholar] [CrossRef]
- Ma, J.; Mei, D.; Tian, X.; Zhang, S.; Yang, J.; Wang, C.; Chen, G.; Zhao, Y.; Zheng, C.; Zhao, H. Fate of Mercury in Volatiles and Char during in Situ Gasification Chemical-Looping Combustion of Coal. Environ. Sci. Technol. 2019, 53, 7887–7892. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.-J.; Yu, J.; McKenzie, L.J.; Hayashi, J.; Li, C.-Z. Conversion of Fuel-N into HCN and NH3 during the Pyrolysis and Gasification in Steam: A Comparative Study of Coal and Biomass. Energy Fuels 2007, 21, 517–521. [Google Scholar] [CrossRef]
- Wang, P.; Yan, J.; Wang, S.; Xu, P.; Shen, L.; Song, T. Synergistic Effects of Lanthanum Ferrite Perovskite and Hydrogen to Promote Ammonia Production during Microalgae Catalytic Pyrolysis Process. Bioresour. Technol. 2021, 340, 125641. [Google Scholar] [CrossRef]
- Deng, J.; Wang, X.; Wei, Z.; Wang, L.; Wang, C.; Chen, Z. A Review of NOx and SOx Emission Reduction Technologies for Marine Diesel Engines and the Potential Evaluation of Liquefied Natural Gas Fuelled Vessels. Sci. Total Environ. 2021, 766, 144319. [Google Scholar] [CrossRef]
- Bao, W.; Zhang, J.; Li, F.; Chang, L. Formation of NOx Precursors During Chinese Pulverized Coal Pyrolysis in an Arc Plasma Jet. Energy Fuels 2007, 21, 2082–2087. [Google Scholar] [CrossRef]
- Niu, X.; Xiao, J. Nitrogen Transformation in Chemical Looping Combustion of Sewage Sludge. J. Fuel Chem. Technol. 2017, 45, 505. [Google Scholar]
- Ksepko, E.; Lysowski, R. Extremely Stable and Durable Mixed Fe–Mn Oxides Supported on ZrO2 for Practical Utilization in CLOU and CLC Processes. Catalysts 2021, 11, 1285. [Google Scholar] [CrossRef]
- Dong, N.; Huo, R.; Liu, M.; Deng, L.; Deng, Z.; Chang, G.; Huang, Z.; Huang, H. Chemical Looping Gasification of Sewage Sludge Using Copper Slag Modified by NiO as an Oxygen Carrier. Chin. J. Chem. Eng. 2021, 29, 335–343. [Google Scholar] [CrossRef]
- Miettinen, H. The in fluence of some oxide and sulphate surfaces on N_2O decomposition. In Proceedings of the 12th International Conference on Fluidized Bed Combustion, Montreal, Canada, 21–24 April 1991; pp. 999–1003. [Google Scholar]
- Wang, Z.; Zhou, J.; Wen, Z.; Liu, J.; Cen, K. Effect of Mineral Matter on NO Reduction in Coal Reburning Process. Energy Fuels 2007, 21, 2038–2043. [Google Scholar] [CrossRef]
- Zong, P.; Jiang, Y.; Tian, Y.; Li, J.; Yuan, M.; Ji, Y.; Chen, M.; Li, D.; Qiao, Y. Pyrolysis Behavior and Product Distributions of Biomass Six Group Components: Starch, Cellulose, Hemicellulose, Lignin, Protein and Oil. Energy Convers. Manag. 2020, 216, 112777. [Google Scholar] [CrossRef]




| No. | OC Type | Fuel | NOx Characteristics | Ref. |
|---|---|---|---|---|
| 1 | NiO/Al2O3 | Coal | XNO: 16.98–8.85% (AR); XN2: 100% (FR) | 2012 [60] |
| 2 | Iron ore | Coal | XNO: 3.8–7.1% (AR); XNO: 0.5–0.8% (FR) | 2015 [31] |
| 3 | Fe-Based | Coal | XNO: 10–15% (FR) | 2013 [61] |
| 4 | Ilmenite | Coal | XNO: 11%; XN2: 62% (FR) | 2014 [62] |
| 5 | Ilmenite | Coal | XN2: 100% (FR) | 2014 [33] |
| 6 | CuO/Fe2O3/MgAl2O4 | Coal | XNO: 20% (FR) | 2016 [63] |
| 7 | CuO/Cement | Coal | XNOX: 6–18% (FR) | 2015 [45] |
| 8 | Hematite | Biomass | XN2: 94% (FR) | 2019 [64] |
| 9 | NiO | NH3 + Natural Gas | XNO: <0.01% (AR); XN2: 99.99% (FR) | 2014 [65] |
| 10 | Ilmenite | NH3 | XNO: 18% (FR) | 2015 [66] |
| 11 | NiO/Al2O3 | Coal | XNO: 45%, 63% and 0% for Fe/Mn/Ni-based OC (FR) | 2019 [67] |
| 12 | Hematite | Coal | XNO: 21.2% XN2: 35.9% | 2021 [47] |
| 13 | Iron ore | Coal | XN2: 92.91% and 99.99% for perovskite/Cu-based (FR) | 2020 [68] |
| 14 | Hematite | Coal | XN2: 76% for blank and 94–99% for others | 2022 [59] |
| 15 | Ilmenite | Coal | XN2: 68.10–75.33% | 2021 [69] |
| 16 | Ilmenite | Coal | XN2: 39.74–80.40% | 2021 [42] |
| 17 | Ilmenite | Coal | XN2: 91–98% | 2021 [70] |
| N-Containing Pollutant | Generation Rate (%) | Removal Rate (%) | |
|---|---|---|---|
| Pyrolysis | CLC | ||
| NH3 | 11.7 ± 0.00 | 4.52 ± 2.16 | 61.37 |
| HCN | 9.93 ± 0.00 | 0.73 ± 0.12 | 92.64 |
| NO | 0 | 3.10 ± 0.93 | / |
| Total N-containing pollutants | 21.63 | 8.72 | 59.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Chen, X.; Lin, Y.; Song, D.; Mao, M.; Wang, X.; Mo, S.; Li, Y.; Huang, Z.; He, F. Removal of Nitrogen Pollutants in the Chemical Looping Process: A Review. Energies 2024, 17, 3432. https://doi.org/10.3390/en17143432
Zhou Y, Chen X, Lin Y, Song D, Mao M, Wang X, Mo S, Li Y, Huang Z, He F. Removal of Nitrogen Pollutants in the Chemical Looping Process: A Review. Energies. 2024; 17(14):3432. https://doi.org/10.3390/en17143432
Chicago/Turabian StyleZhou, Yuchao, Xinfei Chen, Yan Lin, Da Song, Min Mao, Xuemei Wang, Shengwang Mo, Yang Li, Zhen Huang, and Fang He. 2024. "Removal of Nitrogen Pollutants in the Chemical Looping Process: A Review" Energies 17, no. 14: 3432. https://doi.org/10.3390/en17143432





