Abstract
With the continuous growth of global energy demand and increasingly prominent environmental issues, the research and utilization of renewable energy as a substitute for traditional fossil fuels have gained significant importance. Biofuels, recognized as a key renewable energy source, are widely considered a viable alternative to fossil fuels. The primary component of biodiesel is fatty acid methyl esters (FAMEs), which are prone to oxidative degradation due to their unsaturated nature during storage and transportation. Various studies have identified several factors influencing the stability of biodiesel, including oxygen, temperature, light, water content, microbial growth, and the corrosion of metal storage tanks. This article provides a comprehensive summary of the effects of different environmental factors on the storage stability of biodiesel and explores the interrelationships between these factors. To enhance the storage stability of biodiesel, several strategies have been proposed, such as optimizing production processes, adding antioxidants, controlling storage environments, and conducting regular inspections. This review aims to provide a theoretical basis for the long-term storage of biodiesel and promote its widespread application in practical scenarios.
1. Introduction
With the continuous growth of global energy demand and the increasingly prominent environmental issues, researching and utilizing renewable energy as a substitute for traditional fossil fuels has become increasingly important. Biofuels, as an important renewable energy source, are widely regarded as one of the alternative products to fossil fuels [1,2,3]. Biofuels include bioethanol, biodiesel, bio butanol, or biogas, and their raw production materials can be specialized grain crops, economic crops, animal fats, or waste oil. From an economic and environmental perspective, the production and use of biofuels are considered to have significant sustainability advantages.
In the past decade, the growth rate of biodiesel production has been greater than that of other biofuels [4]. Biodiesel has the advantages of biodegradability and low emissions of harmful gases during combustion [5]. A palm oil methyl ester biodiesel mixture of 10% or less can operate in any diesel engine without modification, and its performance is the same as mineral diesel [6].
Unlike relatively inert fossil fuels, biodiesel has a greater tendency to degrade. Just like vegetable oils and animal fats used for extracting biodiesel, the presence of unsaturated components leads to easy oxidation and degradation, so their stable characteristics may not be maintained during transportation and storage [7]. From an environmental perspective, this oxidation sensitivity is beneficial as it makes the fuel biodegradable; however, this characteristic also makes it easy for biodiesel to degrade during storage and transportation, leading to a decrease in its quality, which is not conducive to fuel preservation and use. This is also one of the main technical barriers limiting the commercial application of biodiesel [8].
During storage, biodiesel is inevitably influenced by external factors. As storage time extends, the density, kinematic viscosity, acid value, and peroxide value of fuel samples increase, whereas the calorific value and iodine value decrease [9,10,11,12,13]. Exposure to air, high temperatures, and light intensify the oxidation reactions of biodiesel. Additionally, microbial growth and corrosion of fuel tanks significantly impact the long-term storage stability of biodiesel. These phenomena not only independently degrade biodiesel quality but also interact to accelerate its degradation, thereby reducing storage stability and service life through mutual promotion and feedback mechanisms.
This article reviews the impact of various factors on the long-term storage of biodiesel, explores the interrelationships among these factors, and discusses relevant detection methods. The goal is to provide a theoretical basis for the rational formulation of storage strategies.
2. Biodiesel
2.1. Composition and Antioxidant Properties of Biodiesel
Biodiesel, an alternative diesel fuel, is produced from biomass resources such as vegetable oil, animal oil, or waste oil through transesterification. Currently, four methods are employed for biodiesel preparation: microemulsion, dilution (blending), pyrolysis (thermal cracking), and transesterification. Among these, transesterification is the most common and widely used method due to its high conversion rate and ease of industrialization [14]. This process involves mixing triglycerides, the primary component of raw oil, with short-chain alcohols (methanol or ethanol). Under the action of a catalyst (alkaline, acidic, or enzymatic), an acyl transfer occurs, resulting in the formation of fatty acid methyl esters (biodiesel) and glycerol as a by-product, as shown in Figure 1.
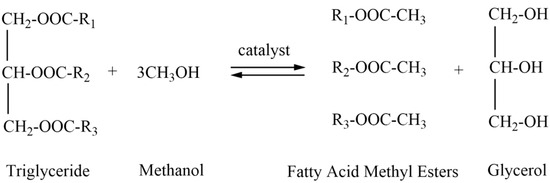
Figure 1.
Transesterification reaction principle [15].
The main components of biodiesel are fatty acid methyl esters (FAMEs), which include both saturated fatty acid methyl esters (SFAMEs) and unsaturated fatty acid methyl esters (USFAMEs). Saturated fatty acid methyl esters include methyl palmitate (C16:0) and methyl stearate (C18:0), while unsaturated fatty acid methyl esters include methyl oleate (C18:1), methyl linoleate (C18:2), and methyl linolenate (C18:3).
According to the different raw materials used, biodiesel can be divided into the first generation, second generation, and third generation [4]. The raw materials for the first generation of biodiesel are edible oil and grain crops. Common raw materials include soybean oil, sunflower seed oil, palm oil, rapeseed oil, and cottonseed oil. The second generation of biodiesel is produced from non-edible sources such as lignocellulosic materials and non-edible oils, aiming to mitigate the competition for food crops. However, non-edible crops still require land, and there may be competition for land with edible crops. Biodiesel from used cooking oils is also included in the second generation. The water content of waste cooking oil is relatively high, but after pretreatment, it can be used for biodiesel production [16]. This type of biodiesel has a relatively lower carbon footprint per ton of production, and it helps to solve the water pollution problem caused by the discharge of waste edible oil into the sewage system [17]. The third generation employs microalgae as the raw material. Compared to the first and second generations, algae-based biodiesel can alleviate concerns regarding food security and land use. However, this biodiesel type is still under development and not yet fully mature in terms of commercial production [18].
The composition of biodiesel produced from different raw materials varies. For example, biodiesel produced from vegetable oils (such as soybean oil and palm oil) typically contains a higher proportion of unsaturated fatty acid methyl esters, while biodiesel produced from animal oils (such as lard and butter) contains a higher proportion of saturated fatty acid methyl esters. Biodiesel produced from waste oil, due to its complex source, has a relatively diverse composition [19,20,21]. The composition of biodiesel will have a significant impact on its various properties [22]. For example, the viscosity of biodiesel decreases with the decrease in methyl oleate concentration and degree of unsaturation [23]. The presence of carbon–carbon double bonds in unsaturated fatty acid methyl esters makes it easier for fatty acid esters to form dense adsorption films on metal surfaces, improving the lubrication performance of biodiesel [24]. For the combustion characteristics of biodiesel, excessive unsaturated fatty acid methyl esters can reduce the calorific value of the fuel and affect combustion efficiency. At the same time, the amount of NOx exhaust emissions increases with the degree of unsaturation [25]. In addition to the composition of biodiesel itself, certain additives used in specific applications can also affect its overall performance. For example, when used in aviation engines, aeronautical additives are required, which can influence the properties and emission characteristics of the blended fuel [26].
The performance changes in biodiesel during long-term storage are closely related to its unsaturated methyl ester content. Tennison K. Jose and K. Anand [27] evaluated the degradation rate of six different fuel samples, including two pure biodiesel fuels and four mixtures, after 10 months of storage. The results indicate that samples with a higher content of unsaturated methyl esters have a higher degradation rate. This is because the oxidation rate of FAME depends on the number and position of double bonds. Unsaturated fatty acid methyl esters (USFAMEs) have lower antioxidant properties because their double bonds are easily oxidized, leading to oxidation reactions in biodiesel during storage. Oxidized biodiesel will generate peroxides, increase acidity, and affect the stability and service life of the fuel. In contrast, saturated fatty acid methyl esters (SFAMEs) have better antioxidant properties and storage stability due to the absence of double bond structures, making them suitable for use in applications that require long-term storage [28].
Oxidative stability can be used to characterize the stability of fuel under storage conditions. The oxidation of biodiesel typically proceeds in the following sequence: initially, radical initiators release hydrogen radicals, which then react with oxygen to form peroxides and carbon radicals. Subsequently, the carbon radicals further react with oxygen, ultimately yielding stable oxidation products [29]. Some stability analysis methods infer the stage of fuel oxidation by detecting oxidation products. For instance, the estimation of primary oxidation products (hydroperoxides and conjugated dienes) and secondary oxidation products (acid value, anisidine value, and deposits) is commonly employed.
The techniques used to evaluate biodiesel are typically based on gas chromatography (GC), high-performance liquid chromatography (HPLC), and some physical property-based spectroscopic analysis techniques [30,31,32]. But it is time-consuming and costly to regularly analyze fuel quality to monitor its storage stability. In order to detect the changes in the performance of biodiesel during storage more quickly and conveniently, researchers have proposed many alternative analysis schemes, as shown in Table 1. Due to the fact that the stability of any biodiesel sample depends on the content of unsaturated components, there are several methods that involve estimating the composition of the fuel as the data basis for predicting fuel stability. Alternatively, by detecting the acidity of biodiesel, the presence of free fatty acids and inorganic acids in biodiesel can be estimated. Some scholars have also proposed that machine learning methods can be combined to predict oxidation stability. In addition, monitoring parameters that significantly affect oxidation stability, such as moisture content, is used to assist in determining the stability of fuel storage.

Table 1.
Alternative detection methods applied to biodiesel.
2.2. Antioxidants for Biodiesel
In order to maintain the stability of biodiesel, antioxidants are often added to commercial biodiesel. The presence of antioxidants can significantly extend the storage life of biodiesel [43]. The commonly used antioxidants are phenolic compounds. Among them, artificially synthesized antioxidants include butylated hydroxytoluene (BHT), propyl gallate (PG), pyrogallol (PY), tert-butylhydroquinone (TBHQ), etc. [44], as shown in Table 2 [45].

Table 2.
Physical properties of antioxidants [45].
The hydroxyl groups (-OH) in phenolic molecules can react with free radicals to form stable phenoxyl radicals, thereby inhibiting the propagation of free radical chain reactions. This reaction not only terminates the chain oxidation process of fatty acid free radicals but also prevents the formation of new free radicals, thus protecting the fatty acids in biodiesel from oxidative degradation [46,47].
The phenoxyl radicals generated from the reaction of phenolic compounds with free radicals exhibit lower reactivity and are less likely to react with other molecules. This is because the electron distribution in phenoxyl radicals is relatively stable, making them less prone to initiating new free radical chain reactions, effectively halting the spread of oxidation reactions. This characteristic makes phenolic compounds highly effective in retarding fatty acid oxidation, making them suitable as antioxidant additives in biodiesel [48].
However, most synthetic antioxidants are toxic and costly. In contrast, natural antioxidants offer similar antioxidant performance while being biodegradable, renewable, and cost-effective. These natural antioxidants are derived from plant extracts rich in phenolic substances. Numerous studies have shown a positive correlation between the total phenolic content of plant extracts and their free radical scavenging activity [49]. Table 3 provides recent studies on natural antioxidants used in biodiesel.

Table 3.
Research on natural antioxidants for biodiesel.
Antioxidants interrupt the chain reaction by providing hydrogen atoms to neutralize the free radicals produced during the oxidation process. The presence of these antioxidants helps extend the storage life of biodiesel, with oxidation primarily involving three stages: (i) the induction stage, where free radicals preferentially react with antioxidant compounds rather than FAME; (ii) the exponential stage, when 80–90% of the antioxidants are consumed, and FAME begins to react rapidly with oxygen; (iii) the termination stage, where the rate of peroxide degradation exceeds the rate of peroxide formation, significantly altering the fuel quality [7].
3. Impact of Storage Environmental Conditions
3.1. Environmental Factors (Oxygen, Temperature, and Light)
Unsaturated fatty acids undergo oxidative degradation through a series of free radical reactions in the presence of oxygen, a process known as autoxidation. This process leads to a decline in fatty acid quality and the generation of various by-products, which adversely affect the quality and stability of biodiesel [55].
The autoxidation of fatty acid methyl esters (FAME) is a free radical chain reaction that includes three steps: initiation, propagation, and termination [56,57].
In the initiation stage, a hydrogen atom is abstracted, resulting in the formation of a free radical.
RH represents unsaturated fatty acids, and R* represents fatty acid free radicals. I* represents the initiator free radical.
Afterward, the new carbon free radicals then react with diatomic oxygen to continue propagating the cycle.
(ROO* represents peroxide radicals.)
Finally, when two free radicals react with each other to produce stable products, the chain reaction terminates.
Usually, oxygen free radicals are generated in a radioactive manner during self-oxidation reactions. These free radicals can easily extract hydrogen atoms from hydrocarbon chains and lead to polymerization. From the perspective of environmental conditions, this self-oxidation process depends on factors such as oxygen content, temperature, and light radiation intensity. The oxidation reaction continues until the reaction site or available oxygen is completely depleted.
Biodiesel fuel undergoes oxidation or self-oxidation during storage due to contact with oxygen in the ambient air. The increase in temperature will accelerate the oxidative degradation of biodiesel. The oxidation of unsaturated fatty acids and methanol can produce peroxides and other harmful byproducts [58]. This can lead to an increase in acidity, viscosity changes, and the performance degradation of biodiesel, thereby affecting its reliability and performance. As the temperature increases, the oxidation reaction rate increases exponentially, significantly shortening the storage life of biodiesel. The increase in temperature increases the kinetic energy of the molecules in biodiesel, increases the rate of chemical reactions, and accelerates the corrosion of metals by biodiesel [59].
Numerous experimental studies have shown that light irradiation can accelerate the oxidation and decomposition of biodiesel [60,61]. Biodiesel contains natural pigments such as carotenoids and chlorophyll, which act as photoreactive compounds. Under illumination conditions, these pigments can absorb light energy and transition from the ground state to the excited state [62]. Light stimulates photoreactive compounds to produce singlet oxygen species. Unsaturated fatty acid esters in biodiesel contain carbon–carbon double bonds, which are susceptible to attack by singlet oxygen species, resulting in the oxidation of the double bonds and the formation of hydroperoxides. The generated hydroperoxides are unstable and can further decompose into smaller molecules such as aldehydes, ketones, and acids or polymerize into larger molecules, thereby altering the properties of biodiesel [56]. These changes not only affect the combustion performance of biodiesel but may also cause damage to the engine.
Lighting is often considered one of the comparative parameters in numerous experimental studies [63,64,65]. However, there is relatively little research on the factor of light itself. Wang Youhao et al. [62] studied the effect of different wavelengths of light on the oxidation reaction and conducted a kinetic analysis of the oxidation process. The results indicate that the degradation rate of biodiesel varies under different wavelengths of light. Shorter-wavelength light (such as purple light) has higher energy and can more effectively excite photosensitizers, accelerating the oxidation process. Long-wavelength light (such as red light) has a weaker promoting effect on oxidation.
Overall, for biodiesel, the ideal storage location is in a non-high temperature, non-air contact, and light-shielded environment. According to Intan Shafinaz Abd Manaf et al. [66], in order to prevent biodiesel from coming into contact with oxygen, some producers choose to store biodiesel under pure nitrogen gas.
3.2. Water Content
3.2.1. Source and Impact of Water in Biodiesel
During the production, transportation, and storage of biodiesel, water ingress is possible. The moisture content in the fuel can be categorized into free water, emulsified water, and soluble water. Biodiesel exhibits significantly higher hygroscopicity compared to petroleum-based diesel, leading to an increase in soluble water content during storage. The solubility of water depends on the temperature and fuel composition. The exposure of biodiesel to environments with higher temperatures and relative humidity results in a significant increase in soluble water content after a period of storage [67]. Fregolente et al. [48] reported that biodiesel exposed for 24 h at temperatures ranging from 10 to 50 °C can absorb between 1500 and 1980 mg kg−1 of water.
The molecular structure of biodiesel contains ester bonds, which are composed of hydrocarbon groups connected by carbonyl (C=O) and oxygen atoms. The oxygen atoms can form hydrogen bonds and interact with the hydrogen atoms in water molecules [68]. This molecular structure increases the ability of biodiesel to absorb moisture in contact with air humidity during production, transportation, and storage [7,69].
In biodiesel/diesel mixtures, temperature and mixing level both affect moisture absorption. As the temperature increases, the water absorption of biodiesel also increases. As the temperature changes, the water absorbed at high temperatures will precipitate when the temperature drops. The circulation of this process may lead to water accumulation at the bottom of the storage tank. The moisture content of the mixture is not simply the sum of the moisture content of biodiesel and petroleum diesel, and the resulting mixture has a lower moisture absorption capacity [70].
For biodiesel itself, excessive moisture content can lead to incomplete combustion, generating more carbon smoke and pollutants and reducing combustion efficiency. Biodiesel with high water content is prone to detonation during combustion, which affects the smooth operation of the engine. In addition, moisture can reduce the energy density of biodiesel, reducing the energy released during combustion and thereby affecting the power output and fuel economy of the engine. The moisture in biodiesel is prone to forming emulsions with impurities in the fuel, which can cause blockages in fuel filters and injectors, affecting the normal operation of the fuel system [71,72].
It is difficult to remove all water from the fuel system in daily operations. Under temperature changes, the water dissolved in biodiesel will precipitate and form sediment, which will accumulate at the bottom of the storage tank, leading to a decrease in the effective utilization rate of the tank volume. When water is present, both chemical corrosion and microbial corrosion of the storage tank may occur. Water catalyzes the hydrolysis reaction of esters in biodiesel. For example, fatty acid methyl esters react with water to produce free fatty acids and alcohols:
The production of free fatty acids will lead to an increase in acid value, which in turn will cause the corrosion of metal structures [73].
3.2.2. Control of Moisture Content
In the actual production process, measures should be taken to control the moisture content of biodiesel. Firstly, in the production process, it is necessary to control the moisture content of the product. Taking the production of biodiesel using the ester exchange method as an example, in the cleaning stage of the production process, there are two optional cleaning methods: water washing and dry cleaning. If water washing is used and the dehumidification process is not thorough, there may be residual moisture. Therefore, efficient water-absorbing materials and filtration equipment are needed to remove small amounts of water and impurities from biodiesel [74,75]. During water washing and subsequent dehumidification processes, some natural antioxidants may be removed [76]. Therefore, if production conditions permit it, dry cleaning can be considered [77,78,79].
For the environment in which the storage tank is located, the storage area should be kept at low relative humidity to avoid storing biodiesel in high-humidity environments. Additionally, it is necessary to ensure good sealing of the biodiesel storage tank to prevent rainwater, groundwater, or moisture from the air from entering the tank. Moreover, regular inspection and maintenance of storage tanks are also necessary. Regularly check the moisture content of biodiesel in storage tanks to ensure that the moisture content is within a safe range. In order to avoid the accumulation of free water at the bottom of the storage tank, it is also necessary to regularly discharge the accumulated water at the bottom of the storage tank.
The Karl Fisher method is the most widely used method for detecting the moisture content of biodiesel, but it requires complex titrators, expensive reagents, and qualified personnel to operate the equipment. Therefore, some scholars have developed more convenient and fast alternative solutions. Mohamed E. S. Mirghani et al. [41] proposed a rapid method for detecting the moisture content of biodiesel using Fourier transform infrared (FTIR) spectroscopy with an attended total reflection (ATR) element. Jos é Rodrigues Delfino et al. [42] found that the change in water content in biodiesel diluted with acetonitrile is directly proportional to the change in electrode/solution impedance, and based on this, developed an EIS measurement method for measuring water content in biodiesel. Nacep Suryana et al. [80] designed and constructed a phase-shifted capacitive sensor system for measuring the water content in biodiesel.
3.3. Microbial Growth
3.3.1. Generation and Impact of Microorganisms
During the storage of biodiesel, the ingress of water from the environment or during production and transportation provides necessary conditions for microbial growth [81,82,83]. Studies have indicated that biodiesel with water content exceeding 200 ppm is sufficient to initiate microbial contamination [68]. Organic acids and other oxidation products generated during oxidation reactions serve as nutrient sources for microorganisms. Moreover, suitable temperatures and oxygen concentrations within storage tanks promote microbial proliferation.
Microbial species proliferating within fuel systems include bacteria, yeast, and filamentous fungi. The typical microbial types in biodiesel storage tanks are shown in Table 4 [84]. These microorganisms form a dense biofilm on tank walls and liquid surfaces during storage. This biofilm exhibits strong adhesion and resistance, effectively protecting microorganisms from external environmental influences. In some cases, it creates anaerobic conditions between the biofilm and tank walls, facilitating the growth of anaerobic bacteria [73,85].

Table 4.
Typical microorganisms detected in biodiesel storage tanks [84].
Microbial communities metabolize hydrocarbons, non-hydrocarbon compounds, phosphorus, and nitrogen compounds present in fuels, producing numerous secondary metabolites. For biodiesel, microbial metabolism and its by-products lead to changes in its appearance, such as darkening or turbidity, primarily due to the accumulation of pigments and other chemical substances produced during microbial metabolism. Organic acids and hydroperoxides generated by microbial metabolism accelerate biodiesel oxidation, thereby reducing its stability. This oxidative reaction not only decreases the energy density of biodiesel but also impacts its combustion performance. The acidic substances produced directly increase the acid value of biodiesel, affecting both its combustion characteristics and storage lifespan [86,87]. In fuel tanks, microbial metabolites can accumulate as biomass deposits at the bottom of tanks, potentially leading to fuel line blockages, while organic acids and sulfides accelerate tank corrosion [88].
3.3.2. Assessment Methods and Control Measures
To assess microbial proliferation during biodiesel storage, quantitative PCR (qPCR) can be employed. This method allows for the rapid and effective evaluation of microbial growth at the oil–water interface in stored fuels, quantifying biomass in the biofilm and assessing contamination levels of bacteria and fungi [89].
Controlling microbial growth involves managing the water content of biodiesel, particularly by regularly removing accumulated water from tank bottoms. Additionally, biocides such as isothiazolinones, oxazolidines, thiocyanates, and morpholines can be used [90]. However, the use of biocides in biodiesel is restricted due to environmental and human health concerns. Some antioxidants also inhibit microbial growth. For instance, tert-butylhydroquinone (TBHQ) concentrations exceeding 250 ppm can inhibit the growth of various microorganisms to a certain extent [91]. Another method to prevent microbial growth is UV radiation. Germicidal ultraviolet (UV-C) radiation in the range of 200–280 nm causes irreversible damage to nucleic acids (DNA/RNA), resulting in cell death [92,93]. Regular monitoring of water content and microbial contamination levels in biodiesel, along with the periodic cleaning of tanks and transportation equipment, are essential measures to minimize opportunities for microbial proliferation.
3.4. Storage Tanks
3.4.1. Metal Storage Tanks
Corrosion is one of the main problems in metal fuel tanks. More than 80% of diesel fuel tank systems exhibit moderate to severe corrosion. On the one hand, corrosion products can cause problems such as blockage and wear; on the other hand, corrosion phenomena can also lead to the degradation of the performance of biodiesel itself, such as density, acid value, kinematic viscosity, etc. [94].
Compared to regular diesel, biodiesel has stronger corrosiveness [95]. But as for biodiesel, its corrosiveness depends on the raw materials used in its production, as the composition of biodiesel is related to the raw materials used. Biodiesel containing high concentrations of polyunsaturated fatty acids may exhibit stronger corrosiveness [96].
In general diesel engine systems, common metals include copper, carbon steel, stainless steel, aluminum alloys, etc. These metals are affected to varying degrees by the corrosiveness of biodiesel [96] (see Table 5). Among these metals, steel can provide affordable construction costs and good chemical resistance, making it the preferred material for making fuel storage tanks and transport tanks.

Table 5.
Corrosion characteristics of various materials in biodiesel [96,97,98,99].
The corrosiveness of biodiesel to metals can be characterized by corrosion rate [59]. The degree and form of corrosion can also be intuitively understood through scanning electron microscopy (SEM). By observing the morphological characteristics and distribution of corrosion products, the mechanism and process of corrosion can be inferred. The crystal structure and orientation of metals can be determined by X-ray diffraction (XRD), or the composition and characteristics of corrosion products can be determined by electrochemical methods.
During storage, due to the oxidation of dissolved oxygen, biodiesel may produce organic acids. Therefore, as the storage time of biodiesel increases, the corrosion rate of biodiesel on steel will increase [100].
Multiple sets of experimental data indicate that carbon steel will exhibit varying degrees of corrosion after being exposed to different types of biodiesel for a period of time [101,102,103]. Figure 2 shows a comparison between carbon steel and stainless steel corroded by biodiesel. The surface of carbon steel will exhibit relatively uniform corrosion, and pores or depressions can be observed [104]. As corrosion continues, Fe2O3 and Fe3O4 compounds will appear on the metal surface. The severity of corrosion may be related to the manganese content in carbon steel. Research has shown that manganese has a negative impact on the oxidation stability of biodiesel [102]. The higher the manganese content in carbon steel, the easier it is for biodiesel exposed to it to oxidize and degrade.
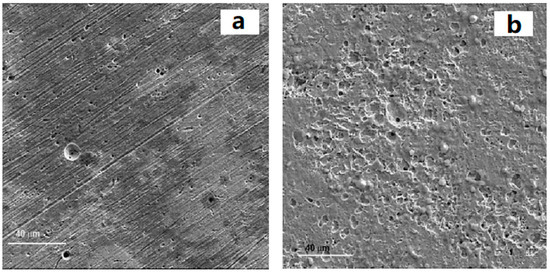
Figure 2.
Micrographs of corroded surfaces for (a) 304 type stainless steel, (b) 1018 carbon exposed to chicken fat-based biodiesel [105].
The corrosion observed on the surface of stainless steel typically manifests as localized microporous corrosion [99]. Under the same conditions, stainless steel has better corrosion resistance than copper and carbon steel [105]. This may be due to the presence of chromium in the metal. After corrosion, the Cr2O3 formed on the surface of stainless steel serves as a passivation protective film to prevent further corrosion by biodiesel, oxygen, and water [106]. Although the corrosion rate of stainless steel is extremely low, it is still affected by the mixing ratio, exposure time, and biodiesel composition. Through SEM and EDS, it can be observed that pitting corrosion occurs on the metal surface due to the dissolution and regeneration of the passivation film. After long-term exposure to biodiesel, the surface of stainless steel mainly forms oxides such as Fe2O3, Cr2O3, NiO, etc. [64].
This corrosion of storage tank materials can also have an impact on biodiesel itself. Corrosion can lead to the release of iron ions into biodiesel [98]. The presence of metals in biodiesel (as catalysts) causes metal-mediated initiation reactions, leading to accelerated free radicals and further reducing the oxidation stability of biodiesel [107]. The metal oxides and hydroxides in the corrosion products will react with water and oxygen in biodiesel to form organic acids. These organic acids will increase the acidity of biodiesel and affect its combustion performance. After the corrosion products are mixed with biodiesel, their physical and chemical properties change. For example, metal particles may increase the viscosity and density of biodiesel, affecting its spray characteristics and combustion efficiency. These changes result in incomplete combustion of the engine, increased carbon smoke and harmful emissions, and affect the power output and fuel economy of the engine. At the same time, metal corrosion products and oxides also provide abundant nutritional sources for microorganisms, promoting their growth [64,108].
In addition to its impact on storage tanks, the corrosiveness of biodiesel can also be reflected in other metal components in the system, such as pipelines, valves, etc. [109,110]. If there are no additional protective measures, these components also pose a risk of failure due to metal corrosion. At the same time, polymers produced by oxidation reactions, as well as biofilms and metabolites produced by microbial growth, may cause blockage problems in these parts.
3.4.2. Non-Metallic Storage Tanks
Compared to metal storage tanks, there is relatively less research on using non-metallic materials as containers to store biodiesel. The main materials studied are HDPE and glass.
HDPE has good corrosion resistance and chemical stability for most chemicals, including biodiesel [111,112]. However, HDPE has a certain degree of breathability. But in long-term storage, oxygen may slowly penetrate into the storage tank. If HDPE needs to be used as a storage tank, high-density, thick-walled HDPE containers need to be selected, or the containers need to be lined, which will undoubtedly greatly increase costs.
Glass has a high degree of chemical inertness, which means it will not react with the chemical components in biodiesel. However, due to the susceptibility of glass storage tanks to light exposure, it is necessary to choose dark-colored or UV-coated glass, or store glass containers in a dark environment [113]. However, the fragility and cost issues of glass have affected its potential for large-scale use as storage tanks.
According to existing research [114,115], after long-term storage under the same environmental conditions, the physical property changes in biodiesel stored in glass tanks and HDPE are significantly greater compared to stainless steel tanks. Moreover, both indoors and outdoors, the number of microorganisms growing in stainless steel tanks is lower than that in glass tanks.
Overall, compared to HDPE and glass tanks, steel tanks are safer for storing biodiesel and its mixtures, making them suitable for use as commercial tanks.
3.4.3. Measures to Reduce the Corrosion Impact of Metal Storage Tanks
To reduce the corrosion of metal storage tanks caused by biodiesel, it can be mixed with petroleum-based diesel [96]. At a certain ratio, the mixture of biodiesel and diesel exhibits good stability under various environmental conditions [7]. For various metals, the corrosiveness of biodiesel will decrease as the proportion of biodiesel in the mixed fuel decreases. Even the most corrosion-susceptible copper is almost the same in B20 (20% biodiesel and 80% diesel) as in diesel [116]. Corrosion inhibitors can also be used to protect metals exposed to biodiesel. Common corrosion inhibitors include amino amines, oxyalkylated amines, diamines, primary amines, dodecyl benzene sulfonic acids, imidazolines, naphthenic acid, phosphate esters, etc. [117]. However, corrosion inhibitors can only prolong the initial stage of corrosion and cannot completely prevent corrosion. Generally speaking, the working mechanism of inhibitors is to form a stable metal oxide layer [118]. In addition, adding antioxidants to biodiesel can also alleviate the occurrence of corrosion. Instituto de Qu í mica et al. [97] tested the effects of four common antioxidants on the degree of the corrosion of copper and carbon steel by biodiesel. The effectiveness of these antioxidants in delaying corrosion was ranked as follows: propyl gallate (PG) > butylated hydroxide (BHT) > curcumin (Curc) > tert-butylhydroquinone (TBHQ).
4. Conclusions
The proper management of biodiesel during storage is crucial. Considering the complexity of biodiesel production, logistics, and delivery in different regions, studying the stability of biodiesel under different environmental, storage, and transportation conditions is of great significance.
Existing research on the stability of biodiesel storage shows that environmental conditions, tank materials, and storage time significantly affect the quality and performance of biodiesel. Exposure to high temperature, oxygen, and light, as well as the presence of moisture and microorganisms, are key factors accelerating the oxidation and degradation process of biodiesel, leading to a decrease in its stability. The use of appropriate storage materials and testing methods is crucial for managing these challenges. Mixing biodiesel with diesel, adding antioxidants, and conducting regular quality testing are effective preventive measures to extend the storage life of biodiesel. In addition, optimizing production processes and plans based on market demand and usage plans can further ensure the stability and reliability of biodiesel as a sustainable fuel choice. Future research can focus on developing reliable predictive models and innovative storage solutions to address degradation mechanisms and improve the long-term storage stability of biodiesel.
Author Contributions
Conceptualization, W.A.; methodology, W.A.; software, W.A.; validation, W.A., H.M.C. and M.I.M.; formal analysis, W.A.; investigation, W.A.; resources, H.M.C.; data curation, W.A.; writing—original draft preparation, W.A.; writing—review and editing, W.A., H.M.C. and M.I.M.; visualization, W.A.; supervision, H.M.C.; project administration, H.M.C. and M.I.M.; funding acquisition, H.M.C. and M.I.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (NRF-2022H1A7A2A02000033).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, Y.; Cruz-Morales, P.; Zargar, A.; Belcher, M.S.; Pang, B.; Englund, E.; Dan, Q.; Yin, K.; Keasling, J.D. Biofuels for a Sustainable Future. Cell 2021, 184, 1636–1647. [Google Scholar] [CrossRef]
- Khujamberdiev, R.; Cho, H.M. Biofuels in Aviation: Exploring the Impact of Sustainable Aviation Fuels in Aircraft Engines. Energies 2024, 17, 2650. [Google Scholar] [CrossRef]
- Gheewala, S.H. Life Cycle Assessment for Sustainability Assessment of Biofuels and Bioproducts. Biofuel Res. J. 2023, 10, 1810–1815. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Syazmi, Z.A.H.S.; Mofijur, M.; Abas, A.E.P.; Bilad, M.R.; Ong, H.C.; Silitonga, A.S. Patent Landscape Review on Biodiesel Production: Technology Updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- Amaral, B.S.; Novaes, F.J.M.; Ramos, M.D.C.K.V.; de Aquino Neto, F.R.; Gioda, A. Comparative Profile of Pollutants Generated by a Stationary Engine Fueled with Diesel, Biodiesel, and Ethanol. J. Aerosol Sci. 2016, 100, 155–163. [Google Scholar] [CrossRef]
- Maawa, W.N.; Mamat, R.; Najafi, G.; De Goey, L.P.H. Performance, Combustion, and Emission Characteristics of a CI Engine Fueled with Emulsified Diesel-Biodiesel Blends at Different Water Contents. Fuel 2020, 267, 117265. [Google Scholar] [CrossRef]
- Fathurrahman, N.A.; Ginanjar, K.; Devitasari, R.D.; Maslahat, M.; Anggarani, R.; Aisyah, L.; Soemanto, A.; Solikhah, M.D.; Thahar, A.; Wibowo, E.; et al. Long-Term Storage Stability of Incorporated Hydrotreated Vegetable Oil (HVO) in Biodiesel-Diesel Blends at Highland and Coastal Areas. Fuel Commun. 2024, 18, 100107. [Google Scholar] [CrossRef]
- Christensen, E.; McCormick, R.L. Long-Term Storage Stability of Biodiesel and Biodiesel Blends. Fuel Process. Technol. 2014, 128, 339–348. [Google Scholar] [CrossRef]
- Ramos, T.C.P.M.; Santos, E.P.S.; Ventura, M.; Pina, J.C.; Cavalheiro, A.A.; Fiorucci, A.R.; Silva, M.S. Eugenol and TBHQ Antioxidant Actions in Commercial Biodiesel Obtained by Soybean Oil and Animal Fat. Fuel 2021, 286, 119374. [Google Scholar] [CrossRef]
- Lau, C.H.; Gan, S.; Lau, H.L.N.; Lee, L.Y.; Thangalazhy-Gopakumar, S.; Ng, H.K. Insights into the Effectiveness of Synthetic and Natural Additives in Improving Biodiesel Oxidation Stability. Sustain. Energy Technol. Assess. 2022, 52, 102296. [Google Scholar] [CrossRef]
- Liu, G.; Du, H.; Sailikebuli, X.; Meng, Y.; Liu, Y.; Wang, H.; Zhang, J.; Wang, B.; Saad, M.G.; Li, J.; et al. Evaluation of Storage Stability for Biocrude Derived from Hydrothermal Liquefaction of Microalgae. Energy Fuels 2021, 35, 10623–10629. [Google Scholar] [CrossRef]
- de Sousa, L.S.; de Moura, C.V.R.; de Moura, E.M. Action of Natural Antioxidants on the Oxidative Stability of Soy Biodiesel during Storage. Fuel 2021, 288, 119632. [Google Scholar] [CrossRef]
- Do Amaral, B.E.; De Rezende, D.B.; Pasa, V.M.D. Aging and Stability Evaluation of Diesel/Biodiesel Blends Stored in Amber Polyethylene Bottles under Different Humidity Conditions. Fuel 2020, 279, 118289. [Google Scholar] [CrossRef]
- Gebremariam, S.N.; Marchetti, J.M. Economics of Biodiesel Production: Review. Energy Convers. Manag. 2018, 168, 74–84. [Google Scholar] [CrossRef]
- Srikumar, K.; Tan, Y.H.; Kansedo, J.; Tan, I.S.; Mubarak, N.M.; Ibrahim, M.L.; Yek, P.N.Y.; Foo, H.C.Y.; Karri, R.R.; Khalid, M. A Review on the Environmental Life Cycle Assessment of Biodiesel Production: Selection of Catalyst and Oil Feedstock. Biomass Bioenergy 2024, 185, 107239. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Inda, C.S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A Comprehensive Review of Biodiesel Production from Waste Cooking Oil and Its Use as Fuel in Compression Ignition Engines: 3rd Generation Cleaner Feedstock. J. Clean. Prod. 2021, 307, 127299. [Google Scholar] [CrossRef]
- Foteinis, S.; Chatzisymeon, E.; Litinas, A.; Tsoutsos, T. Used-Cooking-Oil Biodiesel: Life Cycle Assessment and Comparison with First- and Third-Generation Biofuel. Renew. Energy 2020, 153, 588–600. [Google Scholar] [CrossRef]
- Avinash, A.; Sasikumar, P.; Pugazhendhi, A. Analysis of the Limiting Factors for Large Scale Microalgal Cultivation: A Promising Future for Renewable and Sustainable Biofuel Industry. Renew. Sustain. Energy Rev. 2020, 134, 110250. [Google Scholar] [CrossRef]
- Hajjari, M.; Tabatabaei, M.; Aghbashlo, M.; Ghanavati, H. A Review on the Prospects of Sustainable Biodiesel Production: A Global Scenario with an Emphasis on Waste-Oil Biodiesel Utilization. Renew. Sustain. Energy Rev. 2017, 72, 445–464. [Google Scholar] [CrossRef]
- Fonseca, J.M.; Teleken, J.G.; De Cinque Almeida, V.; Da Silva, C. Biodiesel from Waste Frying Oils: Methods of Production and Purification. Energy Convers. Manag. 2019, 184, 205–218. [Google Scholar] [CrossRef]
- Wang, W.; Gowdagiri, S.; Oehlschlaeger, M.A. The High-Temperature Autoignition of Biodiesels and Biodiesel Components. Combust. Flame 2014, 161, 3014–3021. [Google Scholar] [CrossRef]
- Knothe, G. Improving Biodiesel Fuel Properties by Modifying Fatty Ester Composition. Energy Environ. Sci. 2009, 2, 759–766. [Google Scholar] [CrossRef]
- Mishra, S.; Bukkarapu, K.R.; Krishnasamy, A. A Composition Based Approach to Predict Density, Viscosity and Surface Tension of Biodiesel Fuels. Fuel 2021, 285, 119056. [Google Scholar] [CrossRef]
- Li, F.; Liu, Z.; Ni, Z.; Wang, H. Effect of Biodiesel Components on Its Lubrication Performance. J. Mater. Res. Technol. 2019, 8, 3681–3687. [Google Scholar] [CrossRef]
- Pinzi, S.; Rounce, P.; Herreros, J.M.; Tsolakis, A.; Pilar Dorado, M. The Effect of Biodiesel Fatty Acid Composition on Combustion and Diesel Engine Exhaust Emissions. Fuel 2013, 104, 170–182. [Google Scholar] [CrossRef]
- Caranton, A.R.G.; Silva, V.; Galindo, M.; Pava, J.; López, M.; Cerón, A.; Mayorga, M.A. Enhancing Performance and Emission Characteristics of Palm Based Biodiesel Blends with Aeronautical Additives: A Comprehensive Analysis in a J69 Aviation Engine. Energy Convers. Manag. 2024, 313, 118600. [Google Scholar] [CrossRef]
- Jose, T.K.; Anand, K. Effects of Biodiesel Composition on Its Long Term Storage Stability. Fuel 2016, 177, 190–196. [Google Scholar] [CrossRef]
- Lanjekar, R.D.; Deshmukh, D. A Review of the Effect of the Composition of Biodiesel on NO x Emission, Oxidative Stability and Cold Flow Properties. Renew. Sustain. Energy Rev. 2016, 54, 1401–1411. [Google Scholar] [CrossRef]
- Yaakob, Z.; Narayanan, B.N.; Padikkaparambil, S.; Unni, K.S.; Akbar, P.M. A Review on the Oxidation Stability of Biodiesel. Renew. Sustain. Energy Rev. 2014, 35, 136–153. [Google Scholar] [CrossRef]
- Naureen, R.; Tariq, M.; Yusoff, I.; Chowdhury, A.J.K.; Ashraf, M.A. Synthesis, Spectroscopic and Chromatographic Studies of Sunflower Oil Biodiesel Using Optimized Base Catalyzed Methanolysis. Saudi J. Biol. Sci. 2015, 22, 332–339. [Google Scholar] [CrossRef]
- de Matos, T.S.; dos Santos, R.C.; de Souza, C.G.; de Carvalho, R.C.; de Andrade, D.F.; D’ávila, L.A. Determination of the Biodiesel Content on Biodiesel/Diesel Blends and Their Adulteration with Vegetable Oil by High-Performance Liquid Chromatography. Energy Fuels 2019, 33, 11310–11317. [Google Scholar] [CrossRef]
- Kaisan, M.U.; Abubakar, S.; Ashok, B.; Balasubramanian, D.; Narayan, S.; Grujic, I.; Stojanovic, N. Comparative Analyses of Biodiesel Produced from Jatropha and Neem Seed Oil Using a Gas Chromatography–Mass Spectroscopy Technique. Biofuels 2021, 12, 757–768. [Google Scholar] [CrossRef]
- Pichler, J.; Frauscher, M.; Marchetti-Deschmann, M. Advanced Method for the Detection of Saturated Monoglycerides in Biodiesel Using GC-EI-MS/MS. ACS Omega 2024, 9, 23476–23484. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.G.; de Araújo, M.T.; dos Santos, R.C.; de Andrade, D.F.; da Silva, B.V.; D’Avila, L.A. Analysis and Quantitation of Fatty Acid Methyl Esters in Biodiesel by High-Performance Liquid Chromatography. Energy Fuels 2018, 32, 11547–11554. [Google Scholar] [CrossRef]
- Tercini, A.C.B.; Pinesi, M.; Cyntia Calera, G.; Sequinel, R.; Hatanaka, R.R.; de Oliveira, J.E.; Flumignan, D.L. Ultrafast Gas Chromatographic Method for Quantitative Determination of Total FAMEs in Biodiesel: An Analysis of 90 s. Fuel 2018, 222, 792–799. [Google Scholar] [CrossRef]
- Doudin, K.I. Quantitative and Qualitative Analysis of Biodiesel by NMR Spectroscopic Methods. Fuel 2021, 284, 119114. [Google Scholar] [CrossRef]
- Batista, A.D.; Amais, R.S.; Rocha, F.R.P. Liquid–Liquid Microextraction in Sequential Injection Analysis for the Direct Spectrophotometric Determination of Acid Number in Biodiesel. Microchem. J. 2016, 124, 55–59. [Google Scholar] [CrossRef]
- Xie, W.-Q.; Gong, Y.-X.; Yu, K.-X. A Rapid Method for the Quantitative Analysis of Total Acid Number in Biodiesel Based on Headspace GC Technique. Fuel 2017, 210, 236–240. [Google Scholar] [CrossRef]
- Valeriano, M.C.; Morais Neto, A.; Batista, A.C.F.; Mamián López, M.B. Fast Determination of Oxidative Stability of Biodiesel by Merging Raman Spectroscopy & Machine Learning. SSRN 2024. [Google Scholar] [CrossRef]
- De Almeida Cozendey, D.; De Oliveira Muniz, R.; Cavalcante Dos Santos, R.; Gimenes De Souza, C.; França De Andrade, D.; Antonio d’Avila, L. Quantitative Analysis of Free Glycerol in Biodiesel Using Solid-Phase Extraction and High-Performance Liquid Chromatography. Microchem. J. 2021, 168, 106347. [Google Scholar] [CrossRef]
- Mirghani, M.E.S.; Kabbashi, N.A.; Alam, M.Z.; Qudsieh, I.Y.; Alkatib, M.F.R. Rapid Method for the Determination of Moisture Content in Biodiesel Using FTIR Spectroscopy. J. Am. Oil Chem. Soc. 2011, 88, 1897–1904. [Google Scholar] [CrossRef]
- Delfino, J.R.; Pereira, T.C.; Costa Viegas, H.D.; Marques, E.P.; Pupim Ferreira, A.A.; Zhang, L.; Zhang, J.; Brandes Marques, A.L. A Simple and Fast Method to Determine Water Content in Biodiesel by Electrochemical Impedance Spectroscopy. Talanta 2018, 179, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Chrysikou, L.P.; Litinas, A.; Bezergianni, S. Assessment of Biodiesel Stability under Long-Term Storage and Dynamic Accelerated Oxidation: A Comparison Approach. Clean Technol. Environ. Policy 2022, 24, 2583–2593. [Google Scholar] [CrossRef]
- Jeyakumar, N.; Huang, Z.; Balasubramanian, D.; Le, A.T.; Nguyen, X.P.; Pandian, P.L.; Hoang, A.T. Experimental Evaluation over the Effects of Natural Antioxidants on Oxidation Stability of Binary Biodiesel Blend. Int. J. Energy Res. 2022, 46, 20437–20461. [Google Scholar] [CrossRef]
- Rajamohan, S.; Hari Gopal, A.; Muralidharan, K.R.; Huang, Z.; Paramasivam, B.; Ayyasamy, T.; Nguyen, X.P.; Le, A.T.; Hoang, A.T. Evaluation of Oxidation Stability and Engine Behaviors Operated by Prosopis Juliflora Biodiesel/Diesel Fuel Blends with Presence of Synthetic Antioxidant. Sustain. Energy Technol. Assess. 2022, 52, 102086. [Google Scholar] [CrossRef]
- Longanesi, L.; Pereira, A.P.; Johnston, N.; Chuck, C.J. Oxidative Stability of Biodiesel: Recent Insights. Biofuels Bioprod. Biorefining 2022, 16, 265–289. [Google Scholar] [CrossRef]
- Pahlavan, F.; Lamanna, A.; Park, K.-B.; Kabir, S.F.; Kim, J.-S.; Fini, E.H. Phenol-Rich Bio-Oils as Free-Radical Scavengers to Hinder Oxidative Aging in Asphalt Binder. Resour. Conserv. Recycl. 2022, 187, 106601. [Google Scholar] [CrossRef]
- Afailal, Z.; Gil-Lalaguna, N.; Torrijos, M.T.; Gonzalo, A.; Arauzo, J.; Sánchez, J.L. Antioxidant Additives Produced from Argan Shell Lignin Depolymerization. Energy Fuels 2021, 35, 17149–17166. [Google Scholar] [CrossRef]
- Jain, S.; Purohit, S.; Kumar, D.; Goud, V.V. Passion Fruit Seed Extract as an Antioxidant Additive for Biodiesel; Shelf Life and Consumption Kinetics. Fuel 2021, 289, 119906. [Google Scholar] [CrossRef]
- Karunanithi, G.; Varadappan, A.M.S. Exploring the Effectiveness of Novel Coffea Arabica Leaf Pigment as a Natural Antioxidant Additive for Date Seed Biodiesel. Fuel 2022, 324, 124561. [Google Scholar] [CrossRef]
- Kimura, M.; Savada, F.Y.; Tashima, D.L.M.; Romagnoli, É.S.; Chendynski, L.T.; Silva, L.R.C.; Borsato, D. Application of the Self-Organizing Map in the Classification of Natural Antioxidants in Commercial Biodiesel. Biofuels 2021, 12, 673–678. [Google Scholar] [CrossRef]
- Khounani, Z.; Hosseinzadeh-Bandbafha, H.; Moustakas, K.; Talebi, A.F.; Goli, S.A.H.; Rajaeifar, M.A.; Khoshnevisan, B.; Salehi Jouzani, G.; Peng, W.; Kim, K.-H.; et al. Environmental Life Cycle Assessment of Different Biorefinery Platforms Valorizing Olive Wastes to Biofuel, Phosphate Salts, Natural Antioxidant, and an Oxygenated Fuel Additive (Triacetin). J. Clean. Prod. 2021, 278, 123916. [Google Scholar] [CrossRef]
- A Novel Application of Mangifera Indica L and Eugenia Uniflora L Extracts as Antioxidants to Control Biodiesel Oxidation Stability—Neuana—2021—Environmental Progress & Sustainable Energy—Wiley Online Library. Available online: https://aiche.onlinelibrary.wiley.com/doi/full/10.1002/ep.13540 (accessed on 4 July 2024).
- Nagarajan, J.; Narayanasamy, B. Effects of Natural Antioxidants on the Oxidative Stability of Waste Cooking Oil Biodiesel. Biofuels 2021, 12, 485–494. [Google Scholar] [CrossRef]
- Varghese, G.; Saeed, K.; Rutt, K.J. Determination of the Oxidative Stability of Biodiesel Fuels by Near-Infrared Spectroscopy. Fuel 2021, 290, 120015. [Google Scholar] [CrossRef]
- Jemima Romola, C.V.; Meganaharshini, M.; Rigby, S.P.; Ganesh Moorthy, I.; Shyam Kumar, R.; Karthikumar, S. A Comprehensive Review of the Selection of Natural and Synthetic Antioxidants to Enhance the Oxidative Stability of Biodiesel. Renew. Sustain. Energy Rev. 2021, 145, 111109. [Google Scholar] [CrossRef]
- Fazal, M.A.; Rubaiee, S.; Al-Zahrani, A.; Ghazali, S. Biodiesel Degradation Mechanism upon Exposure of Metal Surfaces: A Study on Biodiesel Sustainability. Fuel 2022, 310, 122341. [Google Scholar] [CrossRef]
- Dunn, R.O. Effect of Temperature on the Oil Stability Index (OSI) of Biodiesel. Energy Fuels 2008, 22, 657–662. [Google Scholar] [CrossRef]
- Ateeq, M.; Li, L.; Abdullah, M.; Ahmed, A.; Gohar, G.A.; Rafiq, M.; Rauf, S.; Ali, A.; Saleem, H. Evaluating Corrosion Effect of Biodiesel Produced from Neem Oil on Automotive Materials. Mater. Today Sustain. 2022, 18, 100130. [Google Scholar] [CrossRef]
- Berrios, M.; Martín, M.A.; Chica, A.F.; Martín, A. Storage Effect in the Quality of Different Methyl Esters and Blends with Diesel. Fuel 2012, 91, 119–125. [Google Scholar] [CrossRef]
- Bouaid, A.; Martinez, M.; Aracil, J. Long Storage Stability of Biodiesel from Vegetable and Used Frying Oils. Fuel 2007, 86, 2596–2602. [Google Scholar] [CrossRef]
- Wang, W.; Li, F.; Wang, H. Study of Light Wavelength on the Oxidative Stability of Jatropha Biodiesel. Fuel 2021, 292, 120230. [Google Scholar] [CrossRef]
- Aquino, I.P.; Hernandez, R.P.B.; Chicoma, D.L.; Pinto, H.P.F.; Aoki, I.V. Influence of Light, Temperature and Metallic Ions on Biodiesel Degradation and Corrosiveness to Copper and Brass. Fuel 2012, 102, 795–807. [Google Scholar] [CrossRef]
- Alves, S.M.; Dutra-pereira, F.K.; Bicudo, T.C. Influence of Stainless Steel Corrosion on Biodiesel Oxidative Stability during Storage. Fuel 2019, 249, 73–79. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, F.; Zhang, H.; Wang, S.; Zhao, Z.; Wang, W.; Chen, R. Fishhook Characteristics of Biodiesel Lubricity during Autoxidation. Fuel 2023, 331, 125897. [Google Scholar] [CrossRef]
- Manaf, I.S.A.; Embong, N.H.; Khazaai, S.N.M.; Rahim, M.H.A.; Yusoff, M.M.; Lee, K.T.; Maniam, G.P. A Review for Key Challenges of the Development of Biodiesel Industry. Energy Convers. Manag. 2019, 185, 508–517. [Google Scholar] [CrossRef]
- Kolobeng, R.; Ketlogetswe, C.; Jonas, M.; Mautle, D. Effects of Moisture Content on Biodiesel (B100) Properties during Storage: A Comparative Analysis between Biodiesel Produced from Used Cooking Oil and Beef Tallow. Sustain. Energy Technol. Assess. 2022, 54, 102844. [Google Scholar] [CrossRef]
- Cazarolli, J.C.; Silva, T.L.; Lobato, M.R.; Brito, J.R.D.; Rampelotto, P.H.; Rocha, J.V.D.S.; Azambuja, A.O.D.; Mann, M.B.; Ferrão, M.F.; Peralba, M.D.C.R.; et al. Impact of Water Content on Microbial Growth in Brazilian Biodiesel during Simulated Storage. Fuel 2021, 297, 120761. [Google Scholar] [CrossRef]
- Fregolente, P.B.L.; Fregolente, L.V.; Wolf Maciel, M.R. Water Content in Biodiesel, Diesel, and Biodiesel–Diesel Blends. J. Chem. Eng. Data 2012, 57, 1817–1821. [Google Scholar] [CrossRef]
- He, B.B.; Thompson, J.C.; Routt, D.W.; Van Gerpen, J.H. Moisture Absorption in Biodiesel and Its Petro-Diesel Blends. Appl. Eng. Agric. 2007, 23, 71–76. [Google Scholar] [CrossRef]
- Khalife, E.; Kazerooni, H.; Mirsalim, M.; Roodbar Shojaei, T.; Mohammadi, P.; Salleh, A.M.; Najafi, B.; Tabatabaei, M. Experimental Investigation of Low-Level Water in Waste-Oil Produced Biodiesel-Diesel Fuel Blend. Energy 2017, 121, 331–340. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Ma, L. Influences of Water Content in Feedstock Oil on Burning Characteristics of Fatty Acid Methyl Esters. Processes 2020, 8, 1130. [Google Scholar] [CrossRef]
- Sørensen, G.; Pedersen, D.V.; Nørgaard, A.K.; Sørensen, K.B.; Nygaard, S.D. Microbial Growth Studies in Biodiesel Blends. Bioresour. Technol. 2011, 102, 5259–5264. [Google Scholar] [CrossRef] [PubMed]
- Fregolente, P.B.L.; Maciel, M.R.W. Water Absorbing Material to Removal Water from Biodiesel and Diesel. Procedia Eng. 2012, 42, 1983–1988. [Google Scholar] [CrossRef]
- Okumuş, Z.Ç.; Doğan, T.H.; Temur, H. Removal of Water by Using Cationic Resin during Biodiesel Purification. Renew. Energy 2019, 143, 47–51. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Khurana, D. Long-Term Storage Oxidation Stability of Karanja Biodiesel with the Use of Antioxidants. Fuel Process. Technol. 2013, 106, 447–452. [Google Scholar] [CrossRef]
- Atadashi, I.M. Purification of Crude Biodiesel Using Dry Washing and Membrane Technologies. Alex. Eng. J. 2015, 54, 1265–1272. [Google Scholar] [CrossRef]
- Catarino, M.; Ferreira, E.; Soares Dias, A.P.; Gomes, J. Dry Washing Biodiesel Purification Using Fumed Silica Sorbent. Chem. Eng. J. 2020, 386, 123930. [Google Scholar] [CrossRef]
- Arthus, L.; Ramos Estevam, B.; Jova Aguila, Z.; Regina Wolf Maciel, M.; Vasconcelos Fregolente, L. Facile Tuning of Hydrogel Properties for Efficient Water Removal from Biodiesel: An Assessment of Alkaline Hydrolysis and Drying Techniques. Chem. Eng. Sci. 2023, 282, 119224. [Google Scholar] [CrossRef]
- Suryana, N.; Huda, M.A.; Choirunnisak, O.; Mulyono, A.E.; Samanhudi, R.D.; Sukra, K.F.A.; Dzulfiqar, F.; Yuliarto, B.; Nuryadi, R. Optimization of Phase Shift-Based Capacitive Sensor for Water Content Detection in Biodiesel. IEEE Sens. J. 2022, 22, 16131–16140. [Google Scholar] [CrossRef]
- Cazarolli, J.C.; De Quadros, P.D.; Bücker, F.; Santiago, M.R.F.; Piatnicki, C.M.S.; Peralba, M.D.C.R.; Cavalcanti, E.H.D.S.; Bento, F.M. Microbial Growth in Acrocomia Aculeata Pulp Oil, Jatropha Curcas Oil, and Their Respective Biodiesels under Simulated Storage Conditions. Biofuel Res. J. 2016, 3, 514–520. [Google Scholar] [CrossRef][Green Version]
- Martin-Sanchez, P.M.; Gorbushina, A.A.; Toepel, J. Quantification of Microbial Load in Diesel Storage Tanks Using Culture- and qPCR-Based Approaches. Int. Biodeterior. Biodegrad. 2018, 126, 216–223. [Google Scholar] [CrossRef]
- de Azambuja, A.O.; Bücker, F.; de Quadros, P.D.; Zhalnina, K.; Dias, R.; Vacaro, B.B.; Correa, C.; Ferrão, M.F.; de Oliveira Camargo, F.A.; Triplett, E.; et al. Microbial Community Composition in Brazilian Stored Diesel Fuel of Varying Sulfur Content, Using High-Throughput Sequencing. Fuel 2017, 189, 340–349. [Google Scholar] [CrossRef]
- Komariah, L.N.; Arita, S.; Rendana, M.; Ramayanti, C.; Suriani, N.L.; Erisna, D. Microbial Contamination of Diesel-Biodiesel Blends in Storage Tank; an Analysis of Colony Morphology. Heliyon 2022, 8, e09264. [Google Scholar] [CrossRef] [PubMed]
- Longinos, S.N.; Zannikos, F. The Effect of Microbial Growth on Physicochemical Properties of Biodiesel–Diesel Mixtures. Braz. J. Chem. Eng. 2022, 39, 345–360. [Google Scholar] [CrossRef]
- Zimmer, A.R.; Oliboni, A.; Viscardi, S.L.C.; Teixeira, R.M.; Ferrão, M.F.; Bento, F.M. Biodiesel Blend (B10) Treated with a Multifunctional Additive (Biocide) under Simulated Stored Conditions: A Field and Lab Scale Monitoring. Biofuel Res. J. 2017, 4, 627–636. [Google Scholar] [CrossRef]
- Stamps, B.W.; Bojanowski, C.L.; Drake, C.A.; Nunn, H.S.; Lloyd, P.F.; Floyd, J.G.; Emmerich, K.A.; Neal, A.R.; Crookes-Goodson, W.J.; Stevenson, B.S. In Situ Linkage of Fungal and Bacterial Proliferation to Microbiologically Influenced Corrosion in B20 Biodiesel Storage Tanks. Front. Microbiol. 2020, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Ching, T.H.; Yoza, B.A.; Wang, R.; Masutani, S.; Donachie, S.; Hihara, L.; Li, Q.X. Biodegradation of Biodiesel and Microbiologically Induced Corrosion of 1018 Steel by Moniliella Wahieum Y12. Int. Biodeterior. Biodegrad. 2016, 108, 122–126. [Google Scholar] [CrossRef]
- Bücker, F.; De Moura, T.M.; Da Cunha, M.E.; De Quadros, P.D.; Beker, S.A.; Cazarolli, J.C.; Caramão, E.B.; Frazzon, A.P.G.; Bento, F.M. Evaluation of the Deteriogenic Microbial Community Using qPCR, n-Alkanes and FAMEs Biodegradation in Diesel, Biodiesel and Blends (B5, B10, and B50) during Storage. Fuel 2018, 233, 911–917. [Google Scholar] [CrossRef]
- Luz, G.; Sousa, B.; Guedes, A.; Barreto, C.; Brasil, L. Biocides Used as Additives to Biodiesels and Their Risks to the Environment and Public Health: A Review. Molecules 2018, 23, 2698. [Google Scholar] [CrossRef]
- Beker, S.A.; Da Silva, Y.P.; Bücker, F.; Cazarolli, J.C.; De Quadros, P.D.; Peralba, M.D.C.R.; Piatnicki, C.M.S.; Bento, F.M. Effect of Different Concentrations of Tert-Butylhydroquinone (TBHQ) on Microbial Growth and Chemical Stability of Soybean Biodiesel during Simulated Storage. Fuel 2016, 184, 701–707. [Google Scholar] [CrossRef]
- Neves, A.C.; Polinarski, M.A.; Rosado, F.R.; Fiorini, A.; da Silva, E.A.; Alves, H.J. Effect of Ultraviolet Radiation on Inactivation of Microorganisms Present in Brazilian Diesel Fuel. Biofuels Bioprod. Biorefining 2020, 14, 1152–1162. [Google Scholar] [CrossRef]
- Polinarski, M.A.; Neves, A.C.; Fiorini, A.; Rosado, F.R.; Silva, E.A.D.; Alves, H.J. Ultraviolet Radiation as an Antimicrobial Treatment in Brazilian Diesel Oil: Effect of Biodiesel, Sulfur, and Water Contents. Fuel 2022, 308, 122076. [Google Scholar] [CrossRef]
- Oni, B.A.; Sanni, S.E.; Ezurike, B.O.; Okoro, E.E. Effect of Corrosion Rates of Preheated Schinzochytrium Sp. Microalgae Biodiesel on Metallic Components of a Diesel Engine. Alex. Eng. J. 2022, 61, 7509–7528. [Google Scholar] [CrossRef]
- Cestari, A.; de Araújo, M.; de Oliveira, D.C. Corrosion Behavior of Metallic Surfaces in Biodiesel Evaluated by Atomic Force Microscopy, Vickers Micro Hardness, and Copper Strip Test. Eng. Fail. Anal. 2021, 124, 105329. [Google Scholar] [CrossRef]
- Kugelmeier, C.L.; Monteiro, M.R.; Da Silva, R.; Kuri, S.E.; Sordi, V.L.; Della Rovere, C.A. Corrosion Behavior of Carbon Steel, Stainless Steel, Aluminum and Copper upon Exposure to Biodiesel Blended with Petrodiesel. Energy 2021, 226, 120344. [Google Scholar] [CrossRef]
- Serqueira, D.S.; Pereira, J.F.S.; Squissato, A.L.; Rodrigues, M.A.; Lima, R.C.; Faria, A.M.; Richter, E.M.; Munoz, R.A.A. Oxidative Stability and Corrosivity of Biodiesel Produced from Residual Cooking Oil Exposed to Copper and Carbon Steel under Simulated Storage Conditions: Dual Effect of Antioxidants. Renew. Energy 2021, 164, 1485–1495. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Squissato, A.L.; Lima, A.F.; Richter, E.M.; Munoz, R.A.A. Corrosive Character of Moringa Oleifera Lam Biodiesel Exposed to Carbon Steel under Simulated Storage Conditions. Renew. Energy 2019, 139, 1263–1271. [Google Scholar] [CrossRef]
- Chandran, D.; Raviadaran, R.; Lau, H.L.N.; Numan, A.; Elumalai, P.V.; Samuel, O.D. Corrosion Characteristic of Stainless Steel and Galvanized Steel in Water Emulsified Diesel, Diesel and Palm Biodiesel. Eng. Fail. Anal. 2023, 147, 107129. [Google Scholar] [CrossRef]
- Thangarasu, V.; Balaji, B.; Ramanathan, A. Experimental Investigation of Tribo-Corrosion and Engine Characteristics of Aegle Marmelos Correa Biodiesel and Its Diesel Blends on Direct Injection Diesel Engine. Energy 2019, 171, 879–892. [Google Scholar] [CrossRef]
- Adama, K.K.; Onyeachu, I.B.; Modebe, L.U.; Chukwuike, V.I.; Oghuma, P.O.; Akhabue, C.E. Comparative Evaluation of Corrosion Behaviour of Carbon Steel (C1020) in Different Biodiesels: Synthesis, Characterization and Electrochemical Investigations. Results Eng. 2024, 21, 101695. [Google Scholar] [CrossRef]
- Kivevele, T.; Huan, Z. Influence of Metal Contaminants and Antioxidant Additives on Storage Stability of Biodiesel Produced from Non-Edible Oils of Eastern Africa Origin (Croton megalocarpus and Moringa oleifera Oils). Fuel 2015, 158, 530–537. [Google Scholar] [CrossRef]
- Adama, K.K.; Ukhurebor, K.E.; Pal, K.; Hossain, I. Effect of Neem Oil Biodiesel on the Surface and Structural Integrity of Carbon Steel Alloy: Chromatographic, Spectroscopic, and Morphological Investigations. Int. J. Biol. Macromol. 2024, 269, 132199. [Google Scholar] [CrossRef] [PubMed]
- Komariah, L.N.; Arita, S.; Prianda, B.E.; Dewi, T.K. Technical Assessment of Biodiesel Storage Tank; A Corrosion Case Study. J. King Saud Univ. Eng. Sci. 2023, 35, 232–237. [Google Scholar] [CrossRef]
- Rocabruno-Valdés, C.I.; Hernández, J.A.; Muñoz-Ledo, R.; Salinas-Bravo, V.M.; Lucio-Garcia, M.A.; Lopez-Sesenes, R.; Porcayo-Calderon, J.; González-Rodriguez, J.G. Corrosion Behavior of Metallic Materials in Chicken Fat-Based Biodiesel. Int. J. Electrochem. Sci. 2020, 15, 334–349. [Google Scholar] [CrossRef]
- Olsson, C.-O.A.; Landolt, D. Passive Films on Stainless Steels—Chemistry, Structure and Growth. Electrochim. Acta 2003, 48, 1093–1104. [Google Scholar] [CrossRef]
- Sarin, A.; Arora, R.; Singh, N.P.; Sharma, M.; Malhotra, R.K. Influence of Metal Contaminants on Oxidation Stability of Jatropha Biodiesel. Energy 2009, 34, 1271–1275. [Google Scholar] [CrossRef]
- Dharma, S.; Silitonga, A.S.; Shamsuddin, A.H.; Sebayang, A.H.; Milano, J.; Sebayang, R.; Sarjianto; Ibrahim, H.; Bahri, N.; Ginting, B.; et al. Properties and Corrosion Behaviors of Mild Steel in Biodiesel-Diesel Blends. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 3887–3899. [Google Scholar] [CrossRef]
- Groysman, A. Corrosion in Systems for Storage and Transportation of Petroleum Products and Biofuels; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Hosseinabadi, N.; Moheimani, N.R.; Javaherdashti, R. Biofuels-Related Materials Deterioration in Biorefineries, Transportation and Internal Combustion Engines: A Technical Review. Corros. Eng. Sci. Technol. 2022, 57, 178–194. [Google Scholar] [CrossRef]
- Maru, M.M.; Lucchese, M.M.; Legnani, C.; Quirino, W.G.; Balbo, A.; Aranha, I.B.; Costa, L.T.; Vilani, C.; de Sena, L.Á.; Damasceno, J.C.; et al. Biodiesel Compatibility with Carbon Steel and HDPE Parts. Fuel Process. Technol. 2009, 90, 1175–1182. [Google Scholar] [CrossRef]
- Thompson, M.R.; Mu, B.; Ewaschuk, C.M.; Cai, Y.; Oxby, K.J.; Vlachopoulos, J. Long Term Storage of Biodiesel/Petrol Diesel Blends in Polyethylene Fuel Tanks. Fuel 2013, 108, 771–779. [Google Scholar] [CrossRef]
- Nurul Komariah, L.; Marwani; Aprisah, S.; Rosa, Y.S.L. Storage Tank Materials for Biodiesel Blends; the Analysis of Fuel Property Changes. MATEC Web Conf. 2017, 101, 02012. [Google Scholar] [CrossRef]
- Narasimmanaidu, S.R.; Zini, N.H.M.; Saadun, M.N.A.; Anuar, F.S.; Yaakob, M.Y.; Nor, M.K.M. Effect of Storage Tank Material on Biodiesel Stability under Different Environmental Conditions. J. Tribol. 2023, 36, 32–42. [Google Scholar]
- Khalid, A.; Manshoor, B.; Sapit, A.; Razali, M.A.; Zaman, I.; Anuar, M.D. The Effect of Storage Container and Light Exposure on Biodiesel Characteristics Derived by Crude Palm Oil. Appl. Mech. Mater. 2015, 773–774, 486–490. [Google Scholar] [CrossRef]
- Fazal, M.A.; Suhaila, N.R.; Haseeb, A.S.M.A.; Rubaiee, S.; Al-Zahrani, A. Influence of Copper on the Instability and Corrosiveness of Palm Biodiesel and Its Blends: An Assessment on Biodiesel Sustainability. J. Clean. Prod. 2018, 171, 1407–1414. [Google Scholar] [CrossRef]
- Jakeria, M.R.; Fazal, M.A.; Haseeb, A.S.M.A. Effect of Corrosion Inhibitors on Corrosiveness of Palm Biodiesel. Corros. Eng. Sci. Technol. 2015, 50, 56–62. [Google Scholar] [CrossRef]
- Hoang, A.T.; Tabatabaei, M.; Aghbashlo, M. A Review of the Effect of Biodiesel on the Corrosion Behavior of Metals/Alloys in Diesel Engines. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 42, 2923–2943. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).