The Impact of Various Factors on Long-Term Storage of Biodiesel and Its Prevention: A Review
Abstract
:1. Introduction
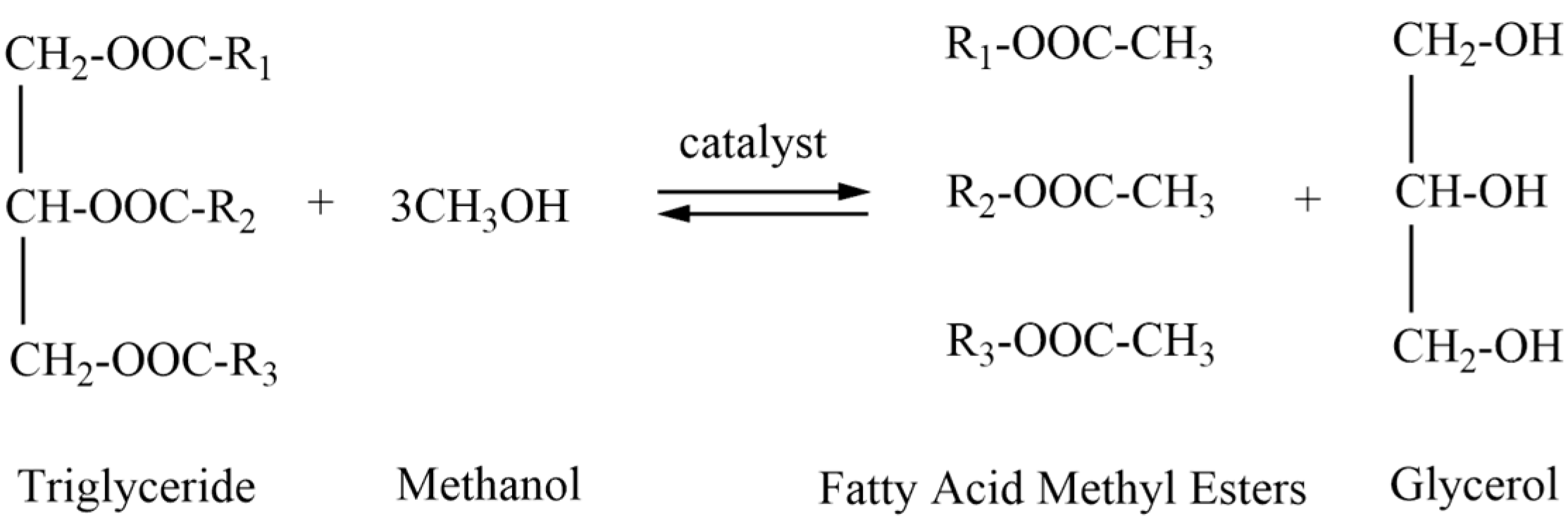
2. Biodiesel
2.1. Composition and Antioxidant Properties of Biodiesel
2.2. Antioxidants for Biodiesel
3. Impact of Storage Environmental Conditions
3.1. Environmental Factors (Oxygen, Temperature, and Light)
3.2. Water Content
3.2.1. Source and Impact of Water in Biodiesel
3.2.2. Control of Moisture Content
3.3. Microbial Growth
3.3.1. Generation and Impact of Microorganisms
3.3.2. Assessment Methods and Control Measures
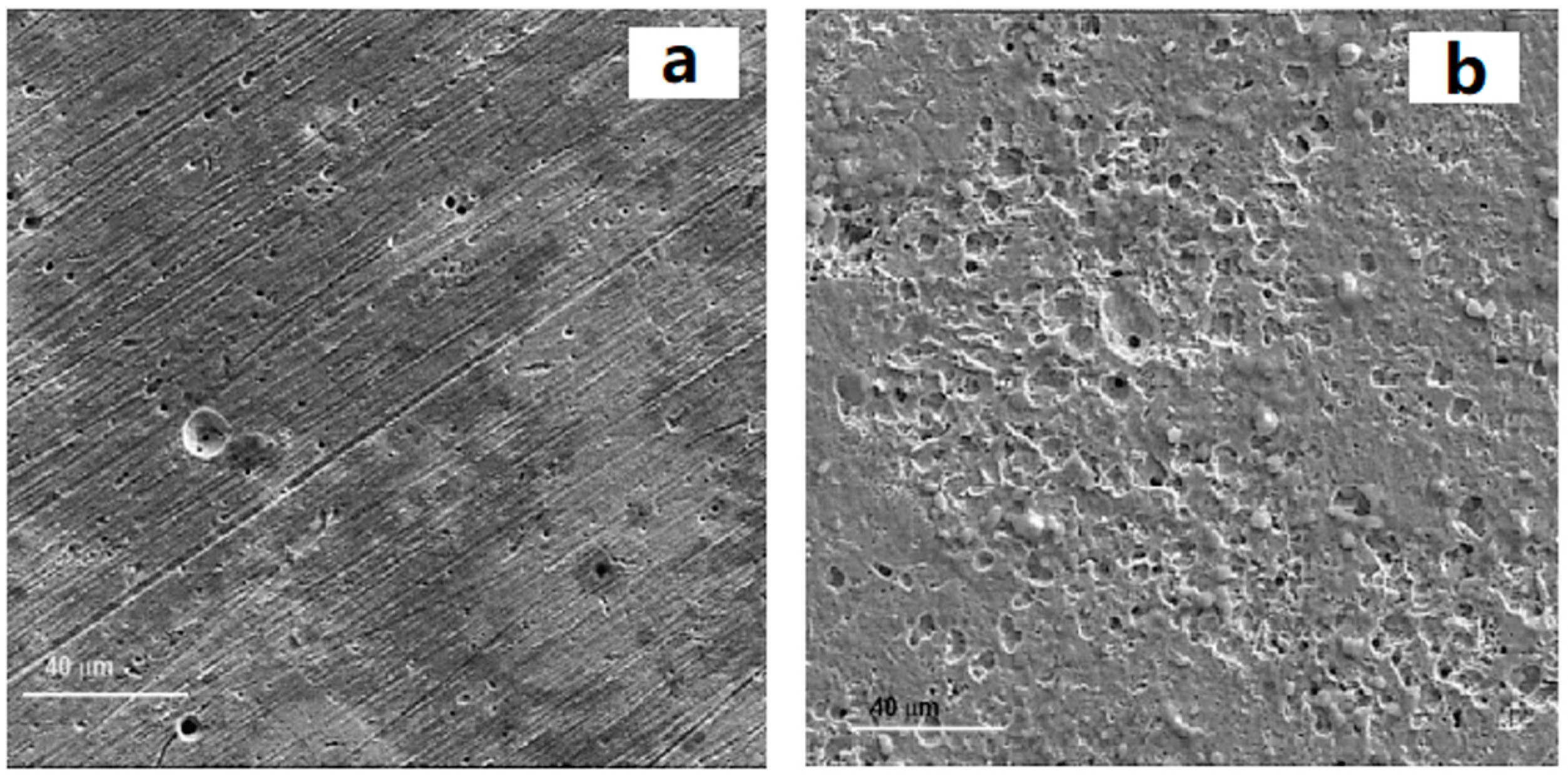
3.4. Storage Tanks
3.4.1. Metal Storage Tanks
3.4.2. Non-Metallic Storage Tanks
3.4.3. Measures to Reduce the Corrosion Impact of Metal Storage Tanks
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Y.; Cruz-Morales, P.; Zargar, A.; Belcher, M.S.; Pang, B.; Englund, E.; Dan, Q.; Yin, K.; Keasling, J.D. Biofuels for a Sustainable Future. Cell 2021, 184, 1636–1647. [Google Scholar] [CrossRef]
- Khujamberdiev, R.; Cho, H.M. Biofuels in Aviation: Exploring the Impact of Sustainable Aviation Fuels in Aircraft Engines. Energies 2024, 17, 2650. [Google Scholar] [CrossRef]
- Gheewala, S.H. Life Cycle Assessment for Sustainability Assessment of Biofuels and Bioproducts. Biofuel Res. J. 2023, 10, 1810–1815. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Syazmi, Z.A.H.S.; Mofijur, M.; Abas, A.E.P.; Bilad, M.R.; Ong, H.C.; Silitonga, A.S. Patent Landscape Review on Biodiesel Production: Technology Updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- Amaral, B.S.; Novaes, F.J.M.; Ramos, M.D.C.K.V.; de Aquino Neto, F.R.; Gioda, A. Comparative Profile of Pollutants Generated by a Stationary Engine Fueled with Diesel, Biodiesel, and Ethanol. J. Aerosol Sci. 2016, 100, 155–163. [Google Scholar] [CrossRef]
- Maawa, W.N.; Mamat, R.; Najafi, G.; De Goey, L.P.H. Performance, Combustion, and Emission Characteristics of a CI Engine Fueled with Emulsified Diesel-Biodiesel Blends at Different Water Contents. Fuel 2020, 267, 117265. [Google Scholar] [CrossRef]
- Fathurrahman, N.A.; Ginanjar, K.; Devitasari, R.D.; Maslahat, M.; Anggarani, R.; Aisyah, L.; Soemanto, A.; Solikhah, M.D.; Thahar, A.; Wibowo, E.; et al. Long-Term Storage Stability of Incorporated Hydrotreated Vegetable Oil (HVO) in Biodiesel-Diesel Blends at Highland and Coastal Areas. Fuel Commun. 2024, 18, 100107. [Google Scholar] [CrossRef]
- Christensen, E.; McCormick, R.L. Long-Term Storage Stability of Biodiesel and Biodiesel Blends. Fuel Process. Technol. 2014, 128, 339–348. [Google Scholar] [CrossRef]
- Ramos, T.C.P.M.; Santos, E.P.S.; Ventura, M.; Pina, J.C.; Cavalheiro, A.A.; Fiorucci, A.R.; Silva, M.S. Eugenol and TBHQ Antioxidant Actions in Commercial Biodiesel Obtained by Soybean Oil and Animal Fat. Fuel 2021, 286, 119374. [Google Scholar] [CrossRef]
- Lau, C.H.; Gan, S.; Lau, H.L.N.; Lee, L.Y.; Thangalazhy-Gopakumar, S.; Ng, H.K. Insights into the Effectiveness of Synthetic and Natural Additives in Improving Biodiesel Oxidation Stability. Sustain. Energy Technol. Assess. 2022, 52, 102296. [Google Scholar] [CrossRef]
- Liu, G.; Du, H.; Sailikebuli, X.; Meng, Y.; Liu, Y.; Wang, H.; Zhang, J.; Wang, B.; Saad, M.G.; Li, J.; et al. Evaluation of Storage Stability for Biocrude Derived from Hydrothermal Liquefaction of Microalgae. Energy Fuels 2021, 35, 10623–10629. [Google Scholar] [CrossRef]
- de Sousa, L.S.; de Moura, C.V.R.; de Moura, E.M. Action of Natural Antioxidants on the Oxidative Stability of Soy Biodiesel during Storage. Fuel 2021, 288, 119632. [Google Scholar] [CrossRef]
- Do Amaral, B.E.; De Rezende, D.B.; Pasa, V.M.D. Aging and Stability Evaluation of Diesel/Biodiesel Blends Stored in Amber Polyethylene Bottles under Different Humidity Conditions. Fuel 2020, 279, 118289. [Google Scholar] [CrossRef]
- Gebremariam, S.N.; Marchetti, J.M. Economics of Biodiesel Production: Review. Energy Convers. Manag. 2018, 168, 74–84. [Google Scholar] [CrossRef]
- Srikumar, K.; Tan, Y.H.; Kansedo, J.; Tan, I.S.; Mubarak, N.M.; Ibrahim, M.L.; Yek, P.N.Y.; Foo, H.C.Y.; Karri, R.R.; Khalid, M. A Review on the Environmental Life Cycle Assessment of Biodiesel Production: Selection of Catalyst and Oil Feedstock. Biomass Bioenergy 2024, 185, 107239. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Inda, C.S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A Comprehensive Review of Biodiesel Production from Waste Cooking Oil and Its Use as Fuel in Compression Ignition Engines: 3rd Generation Cleaner Feedstock. J. Clean. Prod. 2021, 307, 127299. [Google Scholar] [CrossRef]
- Foteinis, S.; Chatzisymeon, E.; Litinas, A.; Tsoutsos, T. Used-Cooking-Oil Biodiesel: Life Cycle Assessment and Comparison with First- and Third-Generation Biofuel. Renew. Energy 2020, 153, 588–600. [Google Scholar] [CrossRef]
- Avinash, A.; Sasikumar, P.; Pugazhendhi, A. Analysis of the Limiting Factors for Large Scale Microalgal Cultivation: A Promising Future for Renewable and Sustainable Biofuel Industry. Renew. Sustain. Energy Rev. 2020, 134, 110250. [Google Scholar] [CrossRef]
- Hajjari, M.; Tabatabaei, M.; Aghbashlo, M.; Ghanavati, H. A Review on the Prospects of Sustainable Biodiesel Production: A Global Scenario with an Emphasis on Waste-Oil Biodiesel Utilization. Renew. Sustain. Energy Rev. 2017, 72, 445–464. [Google Scholar] [CrossRef]
- Fonseca, J.M.; Teleken, J.G.; De Cinque Almeida, V.; Da Silva, C. Biodiesel from Waste Frying Oils: Methods of Production and Purification. Energy Convers. Manag. 2019, 184, 205–218. [Google Scholar] [CrossRef]
- Wang, W.; Gowdagiri, S.; Oehlschlaeger, M.A. The High-Temperature Autoignition of Biodiesels and Biodiesel Components. Combust. Flame 2014, 161, 3014–3021. [Google Scholar] [CrossRef]
- Knothe, G. Improving Biodiesel Fuel Properties by Modifying Fatty Ester Composition. Energy Environ. Sci. 2009, 2, 759–766. [Google Scholar] [CrossRef]
- Mishra, S.; Bukkarapu, K.R.; Krishnasamy, A. A Composition Based Approach to Predict Density, Viscosity and Surface Tension of Biodiesel Fuels. Fuel 2021, 285, 119056. [Google Scholar] [CrossRef]
- Li, F.; Liu, Z.; Ni, Z.; Wang, H. Effect of Biodiesel Components on Its Lubrication Performance. J. Mater. Res. Technol. 2019, 8, 3681–3687. [Google Scholar] [CrossRef]
- Pinzi, S.; Rounce, P.; Herreros, J.M.; Tsolakis, A.; Pilar Dorado, M. The Effect of Biodiesel Fatty Acid Composition on Combustion and Diesel Engine Exhaust Emissions. Fuel 2013, 104, 170–182. [Google Scholar] [CrossRef]
- Caranton, A.R.G.; Silva, V.; Galindo, M.; Pava, J.; López, M.; Cerón, A.; Mayorga, M.A. Enhancing Performance and Emission Characteristics of Palm Based Biodiesel Blends with Aeronautical Additives: A Comprehensive Analysis in a J69 Aviation Engine. Energy Convers. Manag. 2024, 313, 118600. [Google Scholar] [CrossRef]
- Jose, T.K.; Anand, K. Effects of Biodiesel Composition on Its Long Term Storage Stability. Fuel 2016, 177, 190–196. [Google Scholar] [CrossRef]
- Lanjekar, R.D.; Deshmukh, D. A Review of the Effect of the Composition of Biodiesel on NO x Emission, Oxidative Stability and Cold Flow Properties. Renew. Sustain. Energy Rev. 2016, 54, 1401–1411. [Google Scholar] [CrossRef]
- Yaakob, Z.; Narayanan, B.N.; Padikkaparambil, S.; Unni, K.S.; Akbar, P.M. A Review on the Oxidation Stability of Biodiesel. Renew. Sustain. Energy Rev. 2014, 35, 136–153. [Google Scholar] [CrossRef]
- Naureen, R.; Tariq, M.; Yusoff, I.; Chowdhury, A.J.K.; Ashraf, M.A. Synthesis, Spectroscopic and Chromatographic Studies of Sunflower Oil Biodiesel Using Optimized Base Catalyzed Methanolysis. Saudi J. Biol. Sci. 2015, 22, 332–339. [Google Scholar] [CrossRef]
- de Matos, T.S.; dos Santos, R.C.; de Souza, C.G.; de Carvalho, R.C.; de Andrade, D.F.; D’ávila, L.A. Determination of the Biodiesel Content on Biodiesel/Diesel Blends and Their Adulteration with Vegetable Oil by High-Performance Liquid Chromatography. Energy Fuels 2019, 33, 11310–11317. [Google Scholar] [CrossRef]
- Kaisan, M.U.; Abubakar, S.; Ashok, B.; Balasubramanian, D.; Narayan, S.; Grujic, I.; Stojanovic, N. Comparative Analyses of Biodiesel Produced from Jatropha and Neem Seed Oil Using a Gas Chromatography–Mass Spectroscopy Technique. Biofuels 2021, 12, 757–768. [Google Scholar] [CrossRef]
- Pichler, J.; Frauscher, M.; Marchetti-Deschmann, M. Advanced Method for the Detection of Saturated Monoglycerides in Biodiesel Using GC-EI-MS/MS. ACS Omega 2024, 9, 23476–23484. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.G.; de Araújo, M.T.; dos Santos, R.C.; de Andrade, D.F.; da Silva, B.V.; D’Avila, L.A. Analysis and Quantitation of Fatty Acid Methyl Esters in Biodiesel by High-Performance Liquid Chromatography. Energy Fuels 2018, 32, 11547–11554. [Google Scholar] [CrossRef]
- Tercini, A.C.B.; Pinesi, M.; Cyntia Calera, G.; Sequinel, R.; Hatanaka, R.R.; de Oliveira, J.E.; Flumignan, D.L. Ultrafast Gas Chromatographic Method for Quantitative Determination of Total FAMEs in Biodiesel: An Analysis of 90 s. Fuel 2018, 222, 792–799. [Google Scholar] [CrossRef]
- Doudin, K.I. Quantitative and Qualitative Analysis of Biodiesel by NMR Spectroscopic Methods. Fuel 2021, 284, 119114. [Google Scholar] [CrossRef]
- Batista, A.D.; Amais, R.S.; Rocha, F.R.P. Liquid–Liquid Microextraction in Sequential Injection Analysis for the Direct Spectrophotometric Determination of Acid Number in Biodiesel. Microchem. J. 2016, 124, 55–59. [Google Scholar] [CrossRef]
- Xie, W.-Q.; Gong, Y.-X.; Yu, K.-X. A Rapid Method for the Quantitative Analysis of Total Acid Number in Biodiesel Based on Headspace GC Technique. Fuel 2017, 210, 236–240. [Google Scholar] [CrossRef]
- Valeriano, M.C.; Morais Neto, A.; Batista, A.C.F.; Mamián López, M.B. Fast Determination of Oxidative Stability of Biodiesel by Merging Raman Spectroscopy & Machine Learning. SSRN 2024. [Google Scholar] [CrossRef]
- De Almeida Cozendey, D.; De Oliveira Muniz, R.; Cavalcante Dos Santos, R.; Gimenes De Souza, C.; França De Andrade, D.; Antonio d’Avila, L. Quantitative Analysis of Free Glycerol in Biodiesel Using Solid-Phase Extraction and High-Performance Liquid Chromatography. Microchem. J. 2021, 168, 106347. [Google Scholar] [CrossRef]
- Mirghani, M.E.S.; Kabbashi, N.A.; Alam, M.Z.; Qudsieh, I.Y.; Alkatib, M.F.R. Rapid Method for the Determination of Moisture Content in Biodiesel Using FTIR Spectroscopy. J. Am. Oil Chem. Soc. 2011, 88, 1897–1904. [Google Scholar] [CrossRef]
- Delfino, J.R.; Pereira, T.C.; Costa Viegas, H.D.; Marques, E.P.; Pupim Ferreira, A.A.; Zhang, L.; Zhang, J.; Brandes Marques, A.L. A Simple and Fast Method to Determine Water Content in Biodiesel by Electrochemical Impedance Spectroscopy. Talanta 2018, 179, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Chrysikou, L.P.; Litinas, A.; Bezergianni, S. Assessment of Biodiesel Stability under Long-Term Storage and Dynamic Accelerated Oxidation: A Comparison Approach. Clean Technol. Environ. Policy 2022, 24, 2583–2593. [Google Scholar] [CrossRef]
- Jeyakumar, N.; Huang, Z.; Balasubramanian, D.; Le, A.T.; Nguyen, X.P.; Pandian, P.L.; Hoang, A.T. Experimental Evaluation over the Effects of Natural Antioxidants on Oxidation Stability of Binary Biodiesel Blend. Int. J. Energy Res. 2022, 46, 20437–20461. [Google Scholar] [CrossRef]
- Rajamohan, S.; Hari Gopal, A.; Muralidharan, K.R.; Huang, Z.; Paramasivam, B.; Ayyasamy, T.; Nguyen, X.P.; Le, A.T.; Hoang, A.T. Evaluation of Oxidation Stability and Engine Behaviors Operated by Prosopis Juliflora Biodiesel/Diesel Fuel Blends with Presence of Synthetic Antioxidant. Sustain. Energy Technol. Assess. 2022, 52, 102086. [Google Scholar] [CrossRef]
- Longanesi, L.; Pereira, A.P.; Johnston, N.; Chuck, C.J. Oxidative Stability of Biodiesel: Recent Insights. Biofuels Bioprod. Biorefining 2022, 16, 265–289. [Google Scholar] [CrossRef]
- Pahlavan, F.; Lamanna, A.; Park, K.-B.; Kabir, S.F.; Kim, J.-S.; Fini, E.H. Phenol-Rich Bio-Oils as Free-Radical Scavengers to Hinder Oxidative Aging in Asphalt Binder. Resour. Conserv. Recycl. 2022, 187, 106601. [Google Scholar] [CrossRef]
- Afailal, Z.; Gil-Lalaguna, N.; Torrijos, M.T.; Gonzalo, A.; Arauzo, J.; Sánchez, J.L. Antioxidant Additives Produced from Argan Shell Lignin Depolymerization. Energy Fuels 2021, 35, 17149–17166. [Google Scholar] [CrossRef]
- Jain, S.; Purohit, S.; Kumar, D.; Goud, V.V. Passion Fruit Seed Extract as an Antioxidant Additive for Biodiesel; Shelf Life and Consumption Kinetics. Fuel 2021, 289, 119906. [Google Scholar] [CrossRef]
- Karunanithi, G.; Varadappan, A.M.S. Exploring the Effectiveness of Novel Coffea Arabica Leaf Pigment as a Natural Antioxidant Additive for Date Seed Biodiesel. Fuel 2022, 324, 124561. [Google Scholar] [CrossRef]
- Kimura, M.; Savada, F.Y.; Tashima, D.L.M.; Romagnoli, É.S.; Chendynski, L.T.; Silva, L.R.C.; Borsato, D. Application of the Self-Organizing Map in the Classification of Natural Antioxidants in Commercial Biodiesel. Biofuels 2021, 12, 673–678. [Google Scholar] [CrossRef]
- Khounani, Z.; Hosseinzadeh-Bandbafha, H.; Moustakas, K.; Talebi, A.F.; Goli, S.A.H.; Rajaeifar, M.A.; Khoshnevisan, B.; Salehi Jouzani, G.; Peng, W.; Kim, K.-H.; et al. Environmental Life Cycle Assessment of Different Biorefinery Platforms Valorizing Olive Wastes to Biofuel, Phosphate Salts, Natural Antioxidant, and an Oxygenated Fuel Additive (Triacetin). J. Clean. Prod. 2021, 278, 123916. [Google Scholar] [CrossRef]
- A Novel Application of Mangifera Indica L and Eugenia Uniflora L Extracts as Antioxidants to Control Biodiesel Oxidation Stability—Neuana—2021—Environmental Progress & Sustainable Energy—Wiley Online Library. Available online: https://aiche.onlinelibrary.wiley.com/doi/full/10.1002/ep.13540 (accessed on 4 July 2024).
- Nagarajan, J.; Narayanasamy, B. Effects of Natural Antioxidants on the Oxidative Stability of Waste Cooking Oil Biodiesel. Biofuels 2021, 12, 485–494. [Google Scholar] [CrossRef]
- Varghese, G.; Saeed, K.; Rutt, K.J. Determination of the Oxidative Stability of Biodiesel Fuels by Near-Infrared Spectroscopy. Fuel 2021, 290, 120015. [Google Scholar] [CrossRef]
- Jemima Romola, C.V.; Meganaharshini, M.; Rigby, S.P.; Ganesh Moorthy, I.; Shyam Kumar, R.; Karthikumar, S. A Comprehensive Review of the Selection of Natural and Synthetic Antioxidants to Enhance the Oxidative Stability of Biodiesel. Renew. Sustain. Energy Rev. 2021, 145, 111109. [Google Scholar] [CrossRef]
- Fazal, M.A.; Rubaiee, S.; Al-Zahrani, A.; Ghazali, S. Biodiesel Degradation Mechanism upon Exposure of Metal Surfaces: A Study on Biodiesel Sustainability. Fuel 2022, 310, 122341. [Google Scholar] [CrossRef]
- Dunn, R.O. Effect of Temperature on the Oil Stability Index (OSI) of Biodiesel. Energy Fuels 2008, 22, 657–662. [Google Scholar] [CrossRef]
- Ateeq, M.; Li, L.; Abdullah, M.; Ahmed, A.; Gohar, G.A.; Rafiq, M.; Rauf, S.; Ali, A.; Saleem, H. Evaluating Corrosion Effect of Biodiesel Produced from Neem Oil on Automotive Materials. Mater. Today Sustain. 2022, 18, 100130. [Google Scholar] [CrossRef]
- Berrios, M.; Martín, M.A.; Chica, A.F.; Martín, A. Storage Effect in the Quality of Different Methyl Esters and Blends with Diesel. Fuel 2012, 91, 119–125. [Google Scholar] [CrossRef]
- Bouaid, A.; Martinez, M.; Aracil, J. Long Storage Stability of Biodiesel from Vegetable and Used Frying Oils. Fuel 2007, 86, 2596–2602. [Google Scholar] [CrossRef]
- Wang, W.; Li, F.; Wang, H. Study of Light Wavelength on the Oxidative Stability of Jatropha Biodiesel. Fuel 2021, 292, 120230. [Google Scholar] [CrossRef]
- Aquino, I.P.; Hernandez, R.P.B.; Chicoma, D.L.; Pinto, H.P.F.; Aoki, I.V. Influence of Light, Temperature and Metallic Ions on Biodiesel Degradation and Corrosiveness to Copper and Brass. Fuel 2012, 102, 795–807. [Google Scholar] [CrossRef]
- Alves, S.M.; Dutra-pereira, F.K.; Bicudo, T.C. Influence of Stainless Steel Corrosion on Biodiesel Oxidative Stability during Storage. Fuel 2019, 249, 73–79. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, F.; Zhang, H.; Wang, S.; Zhao, Z.; Wang, W.; Chen, R. Fishhook Characteristics of Biodiesel Lubricity during Autoxidation. Fuel 2023, 331, 125897. [Google Scholar] [CrossRef]
- Manaf, I.S.A.; Embong, N.H.; Khazaai, S.N.M.; Rahim, M.H.A.; Yusoff, M.M.; Lee, K.T.; Maniam, G.P. A Review for Key Challenges of the Development of Biodiesel Industry. Energy Convers. Manag. 2019, 185, 508–517. [Google Scholar] [CrossRef]
- Kolobeng, R.; Ketlogetswe, C.; Jonas, M.; Mautle, D. Effects of Moisture Content on Biodiesel (B100) Properties during Storage: A Comparative Analysis between Biodiesel Produced from Used Cooking Oil and Beef Tallow. Sustain. Energy Technol. Assess. 2022, 54, 102844. [Google Scholar] [CrossRef]
- Cazarolli, J.C.; Silva, T.L.; Lobato, M.R.; Brito, J.R.D.; Rampelotto, P.H.; Rocha, J.V.D.S.; Azambuja, A.O.D.; Mann, M.B.; Ferrão, M.F.; Peralba, M.D.C.R.; et al. Impact of Water Content on Microbial Growth in Brazilian Biodiesel during Simulated Storage. Fuel 2021, 297, 120761. [Google Scholar] [CrossRef]
- Fregolente, P.B.L.; Fregolente, L.V.; Wolf Maciel, M.R. Water Content in Biodiesel, Diesel, and Biodiesel–Diesel Blends. J. Chem. Eng. Data 2012, 57, 1817–1821. [Google Scholar] [CrossRef]
- He, B.B.; Thompson, J.C.; Routt, D.W.; Van Gerpen, J.H. Moisture Absorption in Biodiesel and Its Petro-Diesel Blends. Appl. Eng. Agric. 2007, 23, 71–76. [Google Scholar] [CrossRef]
- Khalife, E.; Kazerooni, H.; Mirsalim, M.; Roodbar Shojaei, T.; Mohammadi, P.; Salleh, A.M.; Najafi, B.; Tabatabaei, M. Experimental Investigation of Low-Level Water in Waste-Oil Produced Biodiesel-Diesel Fuel Blend. Energy 2017, 121, 331–340. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Ma, L. Influences of Water Content in Feedstock Oil on Burning Characteristics of Fatty Acid Methyl Esters. Processes 2020, 8, 1130. [Google Scholar] [CrossRef]
- Sørensen, G.; Pedersen, D.V.; Nørgaard, A.K.; Sørensen, K.B.; Nygaard, S.D. Microbial Growth Studies in Biodiesel Blends. Bioresour. Technol. 2011, 102, 5259–5264. [Google Scholar] [CrossRef] [PubMed]
- Fregolente, P.B.L.; Maciel, M.R.W. Water Absorbing Material to Removal Water from Biodiesel and Diesel. Procedia Eng. 2012, 42, 1983–1988. [Google Scholar] [CrossRef]
- Okumuş, Z.Ç.; Doğan, T.H.; Temur, H. Removal of Water by Using Cationic Resin during Biodiesel Purification. Renew. Energy 2019, 143, 47–51. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Khurana, D. Long-Term Storage Oxidation Stability of Karanja Biodiesel with the Use of Antioxidants. Fuel Process. Technol. 2013, 106, 447–452. [Google Scholar] [CrossRef]
- Atadashi, I.M. Purification of Crude Biodiesel Using Dry Washing and Membrane Technologies. Alex. Eng. J. 2015, 54, 1265–1272. [Google Scholar] [CrossRef]
- Catarino, M.; Ferreira, E.; Soares Dias, A.P.; Gomes, J. Dry Washing Biodiesel Purification Using Fumed Silica Sorbent. Chem. Eng. J. 2020, 386, 123930. [Google Scholar] [CrossRef]
- Arthus, L.; Ramos Estevam, B.; Jova Aguila, Z.; Regina Wolf Maciel, M.; Vasconcelos Fregolente, L. Facile Tuning of Hydrogel Properties for Efficient Water Removal from Biodiesel: An Assessment of Alkaline Hydrolysis and Drying Techniques. Chem. Eng. Sci. 2023, 282, 119224. [Google Scholar] [CrossRef]
- Suryana, N.; Huda, M.A.; Choirunnisak, O.; Mulyono, A.E.; Samanhudi, R.D.; Sukra, K.F.A.; Dzulfiqar, F.; Yuliarto, B.; Nuryadi, R. Optimization of Phase Shift-Based Capacitive Sensor for Water Content Detection in Biodiesel. IEEE Sens. J. 2022, 22, 16131–16140. [Google Scholar] [CrossRef]
- Cazarolli, J.C.; De Quadros, P.D.; Bücker, F.; Santiago, M.R.F.; Piatnicki, C.M.S.; Peralba, M.D.C.R.; Cavalcanti, E.H.D.S.; Bento, F.M. Microbial Growth in Acrocomia Aculeata Pulp Oil, Jatropha Curcas Oil, and Their Respective Biodiesels under Simulated Storage Conditions. Biofuel Res. J. 2016, 3, 514–520. [Google Scholar] [CrossRef]
- Martin-Sanchez, P.M.; Gorbushina, A.A.; Toepel, J. Quantification of Microbial Load in Diesel Storage Tanks Using Culture- and qPCR-Based Approaches. Int. Biodeterior. Biodegrad. 2018, 126, 216–223. [Google Scholar] [CrossRef]
- de Azambuja, A.O.; Bücker, F.; de Quadros, P.D.; Zhalnina, K.; Dias, R.; Vacaro, B.B.; Correa, C.; Ferrão, M.F.; de Oliveira Camargo, F.A.; Triplett, E.; et al. Microbial Community Composition in Brazilian Stored Diesel Fuel of Varying Sulfur Content, Using High-Throughput Sequencing. Fuel 2017, 189, 340–349. [Google Scholar] [CrossRef]
- Komariah, L.N.; Arita, S.; Rendana, M.; Ramayanti, C.; Suriani, N.L.; Erisna, D. Microbial Contamination of Diesel-Biodiesel Blends in Storage Tank; an Analysis of Colony Morphology. Heliyon 2022, 8, e09264. [Google Scholar] [CrossRef] [PubMed]
- Longinos, S.N.; Zannikos, F. The Effect of Microbial Growth on Physicochemical Properties of Biodiesel–Diesel Mixtures. Braz. J. Chem. Eng. 2022, 39, 345–360. [Google Scholar] [CrossRef]
- Zimmer, A.R.; Oliboni, A.; Viscardi, S.L.C.; Teixeira, R.M.; Ferrão, M.F.; Bento, F.M. Biodiesel Blend (B10) Treated with a Multifunctional Additive (Biocide) under Simulated Stored Conditions: A Field and Lab Scale Monitoring. Biofuel Res. J. 2017, 4, 627–636. [Google Scholar] [CrossRef]
- Stamps, B.W.; Bojanowski, C.L.; Drake, C.A.; Nunn, H.S.; Lloyd, P.F.; Floyd, J.G.; Emmerich, K.A.; Neal, A.R.; Crookes-Goodson, W.J.; Stevenson, B.S. In Situ Linkage of Fungal and Bacterial Proliferation to Microbiologically Influenced Corrosion in B20 Biodiesel Storage Tanks. Front. Microbiol. 2020, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Ching, T.H.; Yoza, B.A.; Wang, R.; Masutani, S.; Donachie, S.; Hihara, L.; Li, Q.X. Biodegradation of Biodiesel and Microbiologically Induced Corrosion of 1018 Steel by Moniliella Wahieum Y12. Int. Biodeterior. Biodegrad. 2016, 108, 122–126. [Google Scholar] [CrossRef]
- Bücker, F.; De Moura, T.M.; Da Cunha, M.E.; De Quadros, P.D.; Beker, S.A.; Cazarolli, J.C.; Caramão, E.B.; Frazzon, A.P.G.; Bento, F.M. Evaluation of the Deteriogenic Microbial Community Using qPCR, n-Alkanes and FAMEs Biodegradation in Diesel, Biodiesel and Blends (B5, B10, and B50) during Storage. Fuel 2018, 233, 911–917. [Google Scholar] [CrossRef]
- Luz, G.; Sousa, B.; Guedes, A.; Barreto, C.; Brasil, L. Biocides Used as Additives to Biodiesels and Their Risks to the Environment and Public Health: A Review. Molecules 2018, 23, 2698. [Google Scholar] [CrossRef]
- Beker, S.A.; Da Silva, Y.P.; Bücker, F.; Cazarolli, J.C.; De Quadros, P.D.; Peralba, M.D.C.R.; Piatnicki, C.M.S.; Bento, F.M. Effect of Different Concentrations of Tert-Butylhydroquinone (TBHQ) on Microbial Growth and Chemical Stability of Soybean Biodiesel during Simulated Storage. Fuel 2016, 184, 701–707. [Google Scholar] [CrossRef]
- Neves, A.C.; Polinarski, M.A.; Rosado, F.R.; Fiorini, A.; da Silva, E.A.; Alves, H.J. Effect of Ultraviolet Radiation on Inactivation of Microorganisms Present in Brazilian Diesel Fuel. Biofuels Bioprod. Biorefining 2020, 14, 1152–1162. [Google Scholar] [CrossRef]
- Polinarski, M.A.; Neves, A.C.; Fiorini, A.; Rosado, F.R.; Silva, E.A.D.; Alves, H.J. Ultraviolet Radiation as an Antimicrobial Treatment in Brazilian Diesel Oil: Effect of Biodiesel, Sulfur, and Water Contents. Fuel 2022, 308, 122076. [Google Scholar] [CrossRef]
- Oni, B.A.; Sanni, S.E.; Ezurike, B.O.; Okoro, E.E. Effect of Corrosion Rates of Preheated Schinzochytrium Sp. Microalgae Biodiesel on Metallic Components of a Diesel Engine. Alex. Eng. J. 2022, 61, 7509–7528. [Google Scholar] [CrossRef]
- Cestari, A.; de Araújo, M.; de Oliveira, D.C. Corrosion Behavior of Metallic Surfaces in Biodiesel Evaluated by Atomic Force Microscopy, Vickers Micro Hardness, and Copper Strip Test. Eng. Fail. Anal. 2021, 124, 105329. [Google Scholar] [CrossRef]
- Kugelmeier, C.L.; Monteiro, M.R.; Da Silva, R.; Kuri, S.E.; Sordi, V.L.; Della Rovere, C.A. Corrosion Behavior of Carbon Steel, Stainless Steel, Aluminum and Copper upon Exposure to Biodiesel Blended with Petrodiesel. Energy 2021, 226, 120344. [Google Scholar] [CrossRef]
- Serqueira, D.S.; Pereira, J.F.S.; Squissato, A.L.; Rodrigues, M.A.; Lima, R.C.; Faria, A.M.; Richter, E.M.; Munoz, R.A.A. Oxidative Stability and Corrosivity of Biodiesel Produced from Residual Cooking Oil Exposed to Copper and Carbon Steel under Simulated Storage Conditions: Dual Effect of Antioxidants. Renew. Energy 2021, 164, 1485–1495. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Squissato, A.L.; Lima, A.F.; Richter, E.M.; Munoz, R.A.A. Corrosive Character of Moringa Oleifera Lam Biodiesel Exposed to Carbon Steel under Simulated Storage Conditions. Renew. Energy 2019, 139, 1263–1271. [Google Scholar] [CrossRef]
- Chandran, D.; Raviadaran, R.; Lau, H.L.N.; Numan, A.; Elumalai, P.V.; Samuel, O.D. Corrosion Characteristic of Stainless Steel and Galvanized Steel in Water Emulsified Diesel, Diesel and Palm Biodiesel. Eng. Fail. Anal. 2023, 147, 107129. [Google Scholar] [CrossRef]
- Thangarasu, V.; Balaji, B.; Ramanathan, A. Experimental Investigation of Tribo-Corrosion and Engine Characteristics of Aegle Marmelos Correa Biodiesel and Its Diesel Blends on Direct Injection Diesel Engine. Energy 2019, 171, 879–892. [Google Scholar] [CrossRef]
- Adama, K.K.; Onyeachu, I.B.; Modebe, L.U.; Chukwuike, V.I.; Oghuma, P.O.; Akhabue, C.E. Comparative Evaluation of Corrosion Behaviour of Carbon Steel (C1020) in Different Biodiesels: Synthesis, Characterization and Electrochemical Investigations. Results Eng. 2024, 21, 101695. [Google Scholar] [CrossRef]
- Kivevele, T.; Huan, Z. Influence of Metal Contaminants and Antioxidant Additives on Storage Stability of Biodiesel Produced from Non-Edible Oils of Eastern Africa Origin (Croton megalocarpus and Moringa oleifera Oils). Fuel 2015, 158, 530–537. [Google Scholar] [CrossRef]
- Adama, K.K.; Ukhurebor, K.E.; Pal, K.; Hossain, I. Effect of Neem Oil Biodiesel on the Surface and Structural Integrity of Carbon Steel Alloy: Chromatographic, Spectroscopic, and Morphological Investigations. Int. J. Biol. Macromol. 2024, 269, 132199. [Google Scholar] [CrossRef] [PubMed]
- Komariah, L.N.; Arita, S.; Prianda, B.E.; Dewi, T.K. Technical Assessment of Biodiesel Storage Tank; A Corrosion Case Study. J. King Saud Univ. Eng. Sci. 2023, 35, 232–237. [Google Scholar] [CrossRef]
- Rocabruno-Valdés, C.I.; Hernández, J.A.; Muñoz-Ledo, R.; Salinas-Bravo, V.M.; Lucio-Garcia, M.A.; Lopez-Sesenes, R.; Porcayo-Calderon, J.; González-Rodriguez, J.G. Corrosion Behavior of Metallic Materials in Chicken Fat-Based Biodiesel. Int. J. Electrochem. Sci. 2020, 15, 334–349. [Google Scholar] [CrossRef]
- Olsson, C.-O.A.; Landolt, D. Passive Films on Stainless Steels—Chemistry, Structure and Growth. Electrochim. Acta 2003, 48, 1093–1104. [Google Scholar] [CrossRef]
- Sarin, A.; Arora, R.; Singh, N.P.; Sharma, M.; Malhotra, R.K. Influence of Metal Contaminants on Oxidation Stability of Jatropha Biodiesel. Energy 2009, 34, 1271–1275. [Google Scholar] [CrossRef]
- Dharma, S.; Silitonga, A.S.; Shamsuddin, A.H.; Sebayang, A.H.; Milano, J.; Sebayang, R.; Sarjianto; Ibrahim, H.; Bahri, N.; Ginting, B.; et al. Properties and Corrosion Behaviors of Mild Steel in Biodiesel-Diesel Blends. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 3887–3899. [Google Scholar] [CrossRef]
- Groysman, A. Corrosion in Systems for Storage and Transportation of Petroleum Products and Biofuels; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Hosseinabadi, N.; Moheimani, N.R.; Javaherdashti, R. Biofuels-Related Materials Deterioration in Biorefineries, Transportation and Internal Combustion Engines: A Technical Review. Corros. Eng. Sci. Technol. 2022, 57, 178–194. [Google Scholar] [CrossRef]
- Maru, M.M.; Lucchese, M.M.; Legnani, C.; Quirino, W.G.; Balbo, A.; Aranha, I.B.; Costa, L.T.; Vilani, C.; de Sena, L.Á.; Damasceno, J.C.; et al. Biodiesel Compatibility with Carbon Steel and HDPE Parts. Fuel Process. Technol. 2009, 90, 1175–1182. [Google Scholar] [CrossRef]
- Thompson, M.R.; Mu, B.; Ewaschuk, C.M.; Cai, Y.; Oxby, K.J.; Vlachopoulos, J. Long Term Storage of Biodiesel/Petrol Diesel Blends in Polyethylene Fuel Tanks. Fuel 2013, 108, 771–779. [Google Scholar] [CrossRef]
- Nurul Komariah, L.; Marwani; Aprisah, S.; Rosa, Y.S.L. Storage Tank Materials for Biodiesel Blends; the Analysis of Fuel Property Changes. MATEC Web Conf. 2017, 101, 02012. [Google Scholar] [CrossRef]
- Narasimmanaidu, S.R.; Zini, N.H.M.; Saadun, M.N.A.; Anuar, F.S.; Yaakob, M.Y.; Nor, M.K.M. Effect of Storage Tank Material on Biodiesel Stability under Different Environmental Conditions. J. Tribol. 2023, 36, 32–42. [Google Scholar]
- Khalid, A.; Manshoor, B.; Sapit, A.; Razali, M.A.; Zaman, I.; Anuar, M.D. The Effect of Storage Container and Light Exposure on Biodiesel Characteristics Derived by Crude Palm Oil. Appl. Mech. Mater. 2015, 773–774, 486–490. [Google Scholar] [CrossRef]
- Fazal, M.A.; Suhaila, N.R.; Haseeb, A.S.M.A.; Rubaiee, S.; Al-Zahrani, A. Influence of Copper on the Instability and Corrosiveness of Palm Biodiesel and Its Blends: An Assessment on Biodiesel Sustainability. J. Clean. Prod. 2018, 171, 1407–1414. [Google Scholar] [CrossRef]
- Jakeria, M.R.; Fazal, M.A.; Haseeb, A.S.M.A. Effect of Corrosion Inhibitors on Corrosiveness of Palm Biodiesel. Corros. Eng. Sci. Technol. 2015, 50, 56–62. [Google Scholar] [CrossRef]
- Hoang, A.T.; Tabatabaei, M.; Aghbashlo, M. A Review of the Effect of Biodiesel on the Corrosion Behavior of Metals/Alloys in Diesel Engines. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 42, 2923–2943. [Google Scholar] [CrossRef]
| Detection Item | Method | Reference |
|---|---|---|
| Biodiesel component content | Gas chromatography electron ionization tandem mass spectrometry method | J Pichler et al. [33] |
| High-performance liquid chromatography | C Gimenes de Souza et al. [34] | |
| Ultrafast gas chromatography | ACB Tercini et al. [35] | |
| NMR spectroscopic methods | KI Doudin [36] | |
| Total acid number | Direct spectrophotometric | Alex D. Batista et al. [37] |
| Reaction headspace gas chromatography | WQ Xie al. [38] | |
| Oxidative stability | Merging Raman spectroscopy and machine learning | MC Valeriano et al. [39] |
| Water content | Solid-phase extraction and high-performance liquid chromatography | D de Almeida Cozendey et al. [40] |
| Fourier transform infrared (FTIR) spectroscopy with an attenuated total reflectance (ATR) element | MES Mirghani et al. [41] | |
| Electrochemical impedance spectroscopy | JR Delfino et al. [42] |
| Antioxidant | Molecular Formula | Molar Mass (g/mol) | Density (g/mL) | Melting Point (°C) | Boiling Point (°C) |
|---|---|---|---|---|---|
| Butylated hydroxytoluene (BHT) | C15H24O | 220.35 | 1.048 | 69–73 | 265 |
| Propyl gallate (PG) | C10H12O5 | 212.2 | 1.21 | 146–149 | 312.03 |
| Pyrogallol (PY) | C6H6O3 | 126.11 | 1.112 | 43–47 | 309 |
| tert-Butylhydroquinone (TBHQ) | C10H14O2 | 166.2 | 1.05 | 127–129 | 295 |
| No. | Source | Plant Material | Type of Oil | Extraction Method | Solvent | Reference |
|---|---|---|---|---|---|---|
| 1 | Yellow passion fruits | Seeds | Waste cooking oil (soybean) biodiesel | Soxhlet extraction and solvent extraction | Methanol | [49] |
| 2 | Bilberry | Leaves | Soy biodiesel | Solvent extraction combined with freeze-drying | Synth | [12] |
| Oregano | Leaves | |||||
| Basil | Leaves | |||||
| 3 | Coffea (Coffea arabica L.) | Leaves | Date-seed biodiesel | Soxhlet extraction | Synth | [50] |
| 4 | Blackberries | Fruits | Commercial methyl biodiesel | Solvent impregnation method | Synth | [51] |
| Hibiscus | Flowers | |||||
| Senna | Leaves | |||||
| 5 | Olive | Leaves | Olive pomace oil biodiesel | Sonication and rotary evaporation | Methanol | [52] |
| 6 | Mango (Mangifera indica L.) | Leaves | Soybean biodiesel | Soxhlet extraction | Synth | [53] |
| Pitanga (Eugenia uniflora L.) | Leaves | |||||
| 7 | Turmeric | Rhizomes | Waste cooking oil biodiesel | Soxhlet extraction | Synth | [54] |
| Cinnamon | Bark | |||||
| Black pepper | Fruits | |||||
| Red bell pepper | Fruits | |||||
| Watermelon | Seeds |
| Bacteria | Yeasts | Moulds/Fungi |
|---|---|---|
| Actinetobacter, Bacillus sp., Clostridium sporogenes, Flavofacterium diffusum, Micrococcus sp., Pseudomonas sp., Pseudomonas aeruginosa, Serratia marcescens, Sarcina sp., Hydrogenomonas sp., Clostiridum sp., Gordonia sp., etc. | Candida sp., Candida famata, Candida lypolytica, Candida silvícola, Candida tropicalis, Rhodotorula sp., Saccharomyces sp., etc. | Acremonium sp., Aspergillus sp., Aspergillus fumigatus, Cladosporium sp., Fusarium oxysporum, Penicillium sp., Penicillium citrinum, Penicillium funiculosm, Trichiderma sp., Paecilomyces sp., Moniliella and Byssochlamys, Phyla sp., Pseudallescheria boydii, Hormoconis resinae, Fusarium sp., Aureobasidium pullulans, Moniliella wahieum, Byssochlamys nivea, etc. |
| Material | Corrosion Resistance | Corrosion Phenomenon | Main Surface Products |
|---|---|---|---|
| Copper | Very Weak | Uniform Corrosion | CuCO3, Cu(OH)2 |
| Carbon Steel | Weak | Uniform or Localized Corrosion | Fe2O3, Fe3O4 |
| Stainless Steel | Strong | Localized Corrosion or Pitting | Fe2O3, Cr2O3, NiO |
| Aluminum | Strong | Pitting | Al2O3, Al(OH)3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ai, W.; Cho, H.M.; Mahmud, M.I. The Impact of Various Factors on Long-Term Storage of Biodiesel and Its Prevention: A Review. Energies 2024, 17, 3449. https://doi.org/10.3390/en17143449
Ai W, Cho HM, Mahmud MI. The Impact of Various Factors on Long-Term Storage of Biodiesel and Its Prevention: A Review. Energies. 2024; 17(14):3449. https://doi.org/10.3390/en17143449
Chicago/Turabian StyleAi, Wenbo, Haeng Muk Cho, and Md. Iqbal Mahmud. 2024. "The Impact of Various Factors on Long-Term Storage of Biodiesel and Its Prevention: A Review" Energies 17, no. 14: 3449. https://doi.org/10.3390/en17143449
APA StyleAi, W., Cho, H. M., & Mahmud, M. I. (2024). The Impact of Various Factors on Long-Term Storage of Biodiesel and Its Prevention: A Review. Energies, 17(14), 3449. https://doi.org/10.3390/en17143449







