Thermochemical Valorization of Plastic Waste Containing Low Density Polyethylene, Polyvinyl Chloride and Polyvinyl Butyral into Thermal and Fuel Energy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental
- Total moisture content (Wa) was determined in the analytical sample by the gravimetric method in accordance with CEN/TS 15414-1:2010 [38] based on the measurement of the weight loss of the sample while heating it at a temperature of 105–110 °C until the mass remained unchanged;
- Ash content (Ashd) was determined by the gravimetric method in accordance with PE-EN ISO 3451-1 [39]; the samples were ashed and calcined to a constant weight at 600 °C for 30 min in an FCF22S muffle furnace by CZYLOK, Jastrzębie-Zdrój, Poland;
- The content of volatile matter (VMdaf) was found based on the difference in mass of the sample placed at a temperature of 900 °C (for 7 min) in an air-free environment [40]. The weight loss, except for moisture which evaporates in the form of water vapor, is the content of volatile substances;
- The actual density of the material was detected in accordance with PN-EN ISO 1183 [41] using a liquid pycnometer placed in a thermostat together with the sample (2 g) for 30 min;
- The basic elemental composition (Cd, Hd, Nd) was determined by the method of high-temperature combustion with IR detection on a CHN 828 analyzer by Leco, St. Joseph, Michigan, USA in accordance with PN-EN ISO 21663:2021-06 [42];
- Sulfur content (Sd) was found using the high-temperature combustion method with IR detection on the CS 580 analyzer by Eltra, Bydgoszcz, Poland in accordance with PN-EN ISO 21663:2021-06 [42];
- The oxygen content was calculated as follows: Od = 100% − (Ashd + Cd + Nd + Hd + Sd), where: O—oxygen content (%), Ashd—ash content (%, dry basis), C—carbon content (%), N—nitrogen content (%), H—hydrogen content (%), and S—sulfur content (%);
- Heat of combustion () was measured using the calorimetric method on an isoperiobolic calorimeter (6400 Calorimeter by Parr, Moline, IL, USA) in accordance with PN-EN ISO 21654:2021-12 [43]; the measurement consisted of determining the heat of combustion of the tested sample at a constant volume and temperature of 25 °C by burning certified benzoic acid;
- Total chlorine content (Cld) on a dry basis was detected using the method of high-temperature combustion in an HF-210 Horizontal Furnace (Mitsubishi Chemical Analytech, Tokyo, Japan), coupled with the Dionex Aquion ion chromatograph (Thermo Scientific, Waltham, MA, USA) in accordance with PN-EN 15408:2011 [44].
2.3. Synergy Effects
2.4. Kinetic Model for a Multi-Step Mechanism
2.5. Activation Energy
2.6. Comprehensive Pyrolysis Index (CPI)
3. Results and Discussion
3.1. Material Characteristics
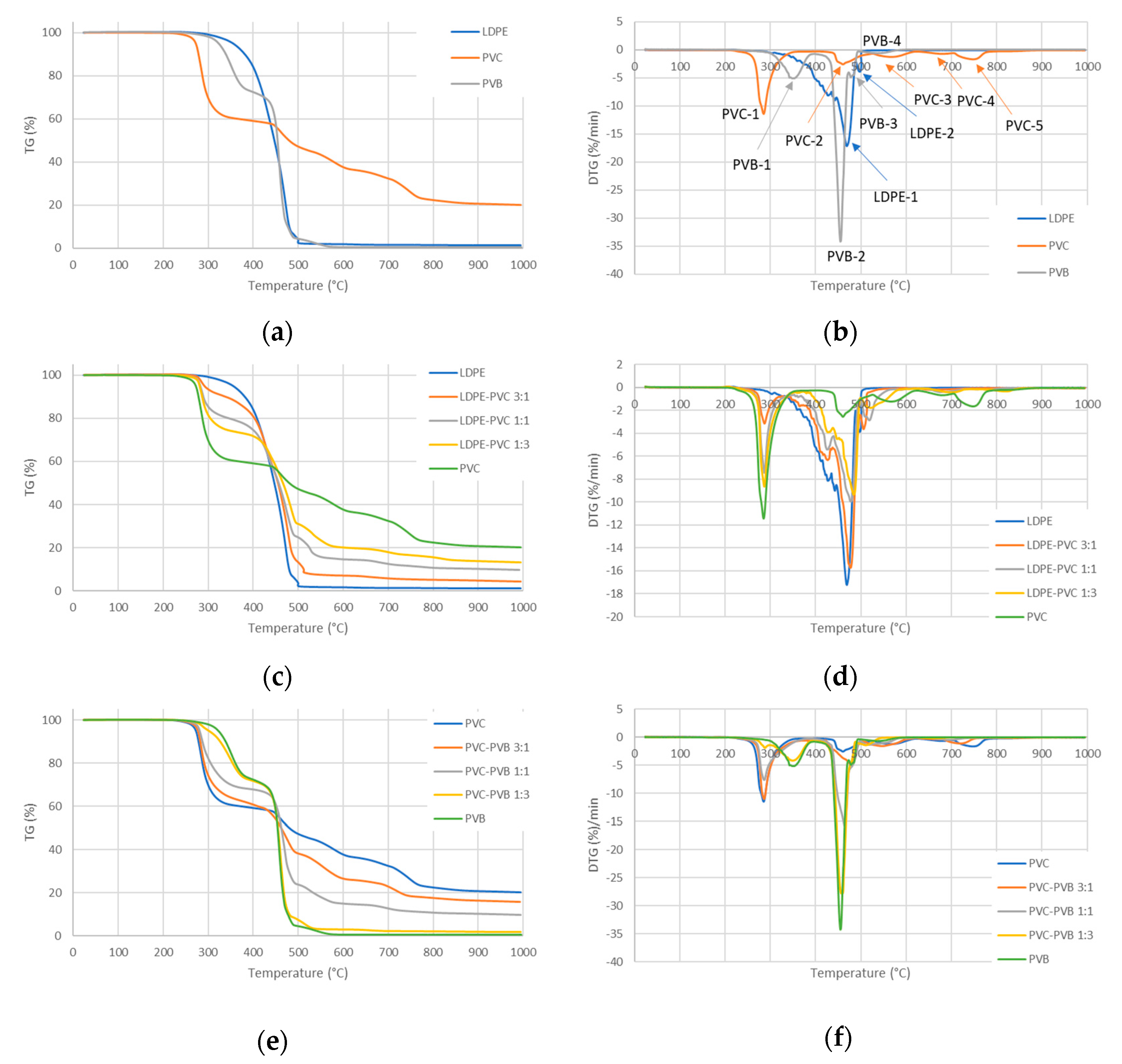
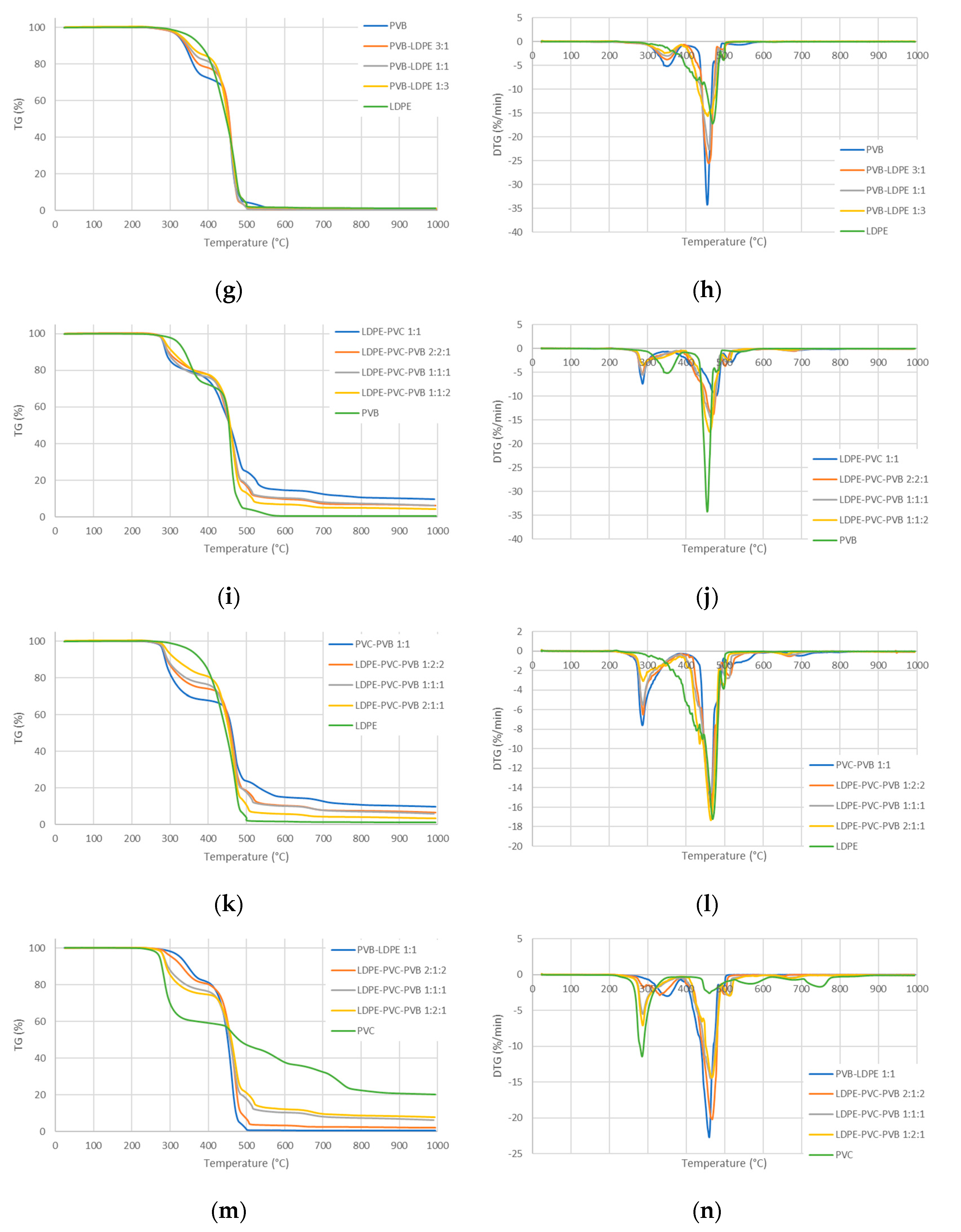
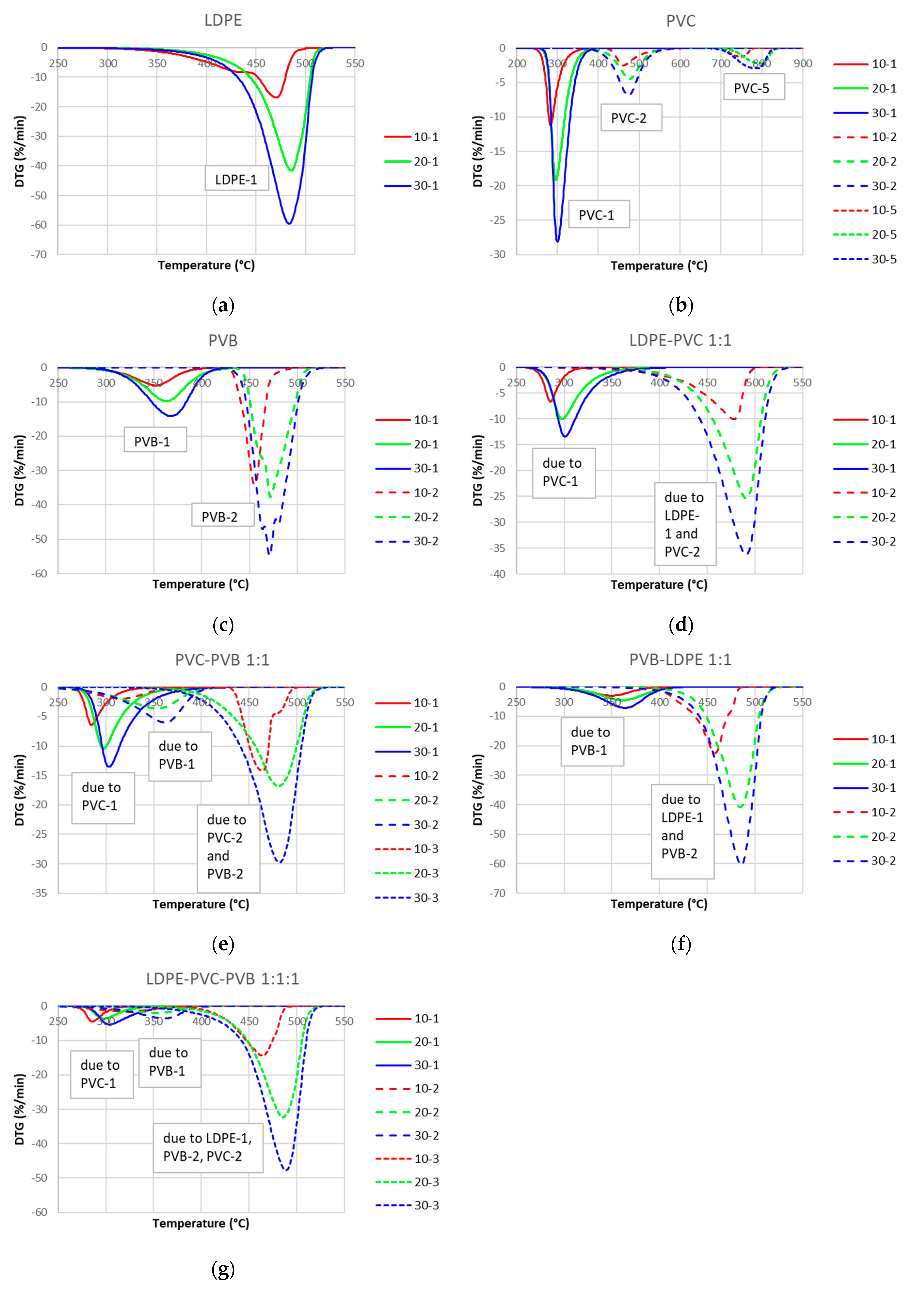
3.2. TG/DTG Analysis
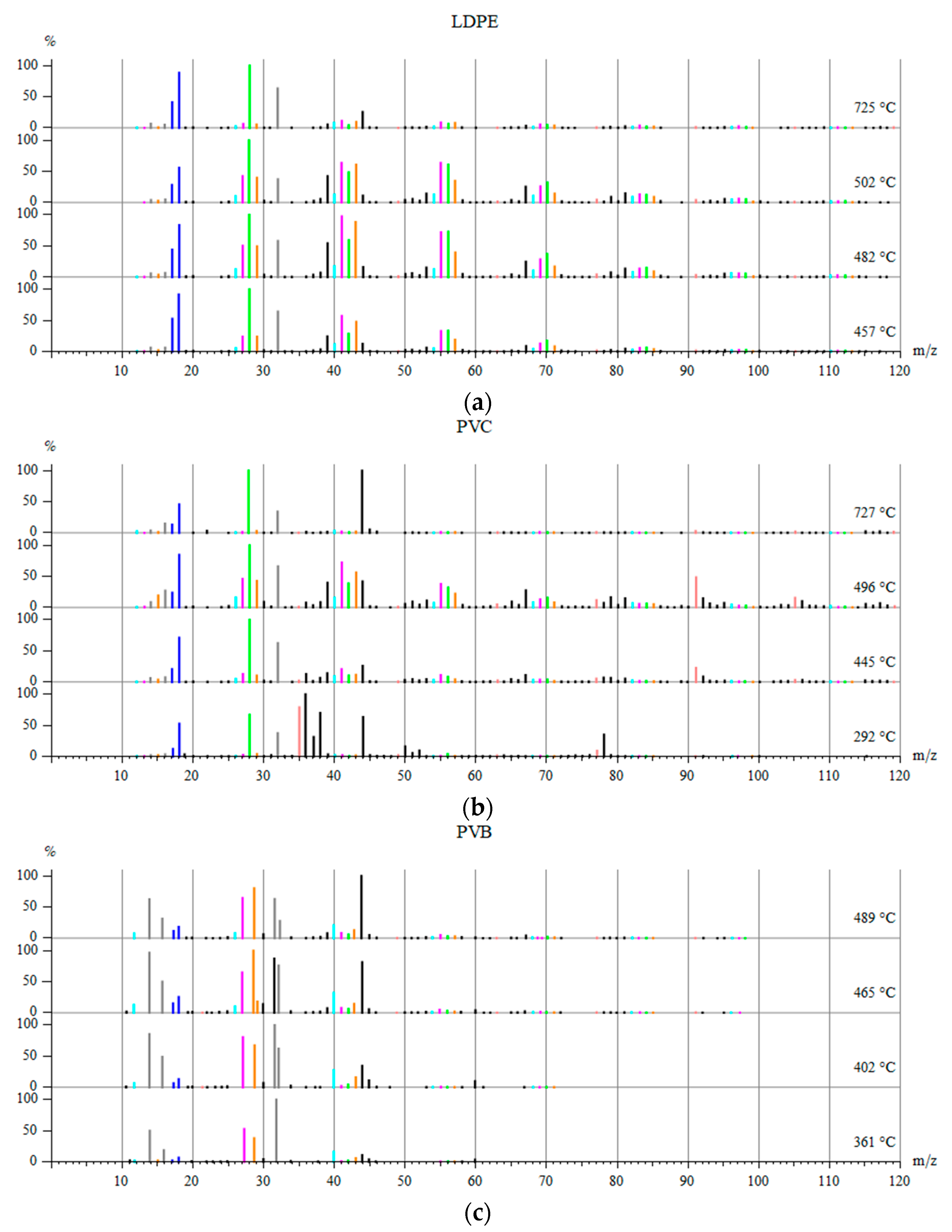
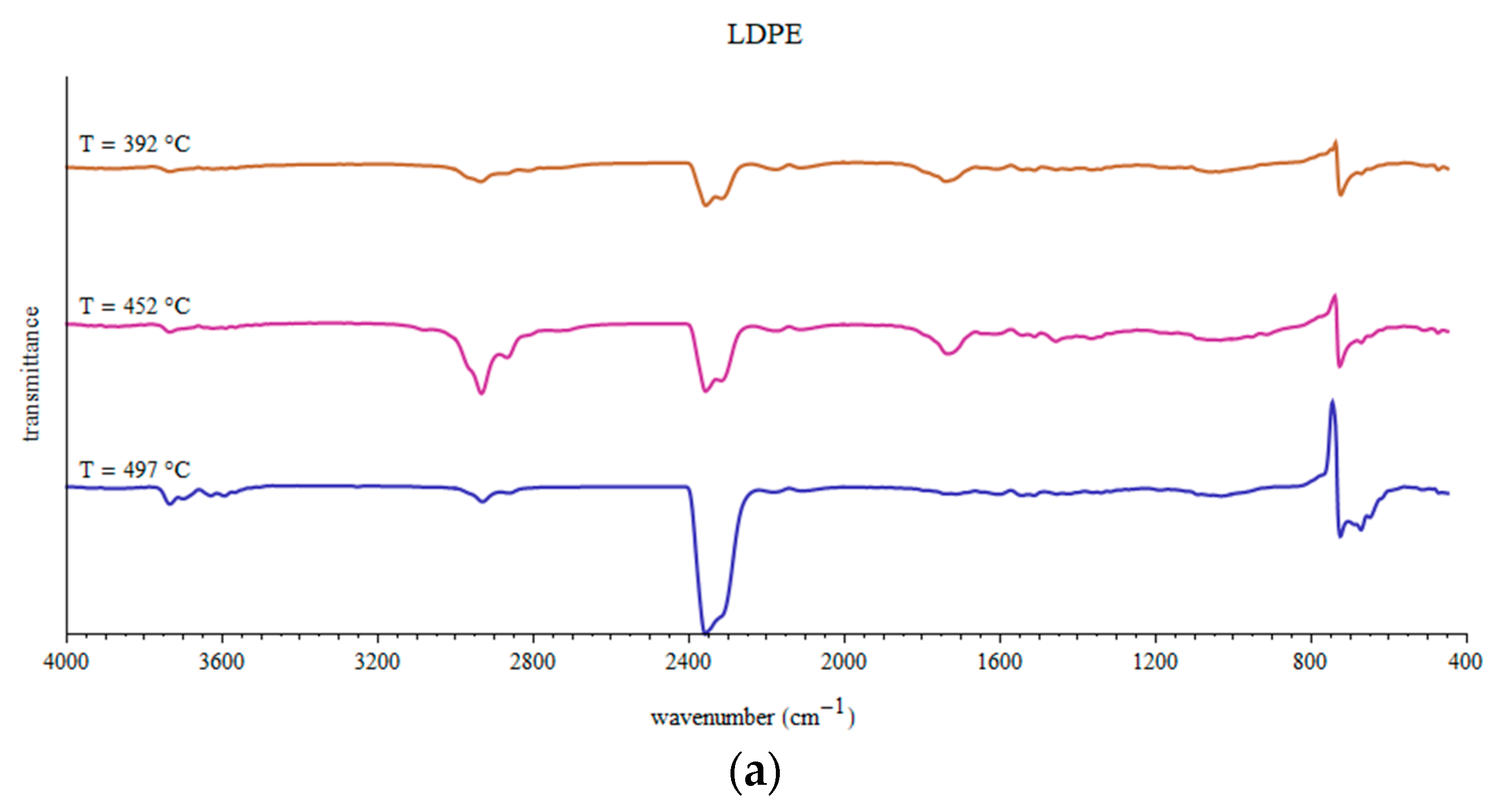
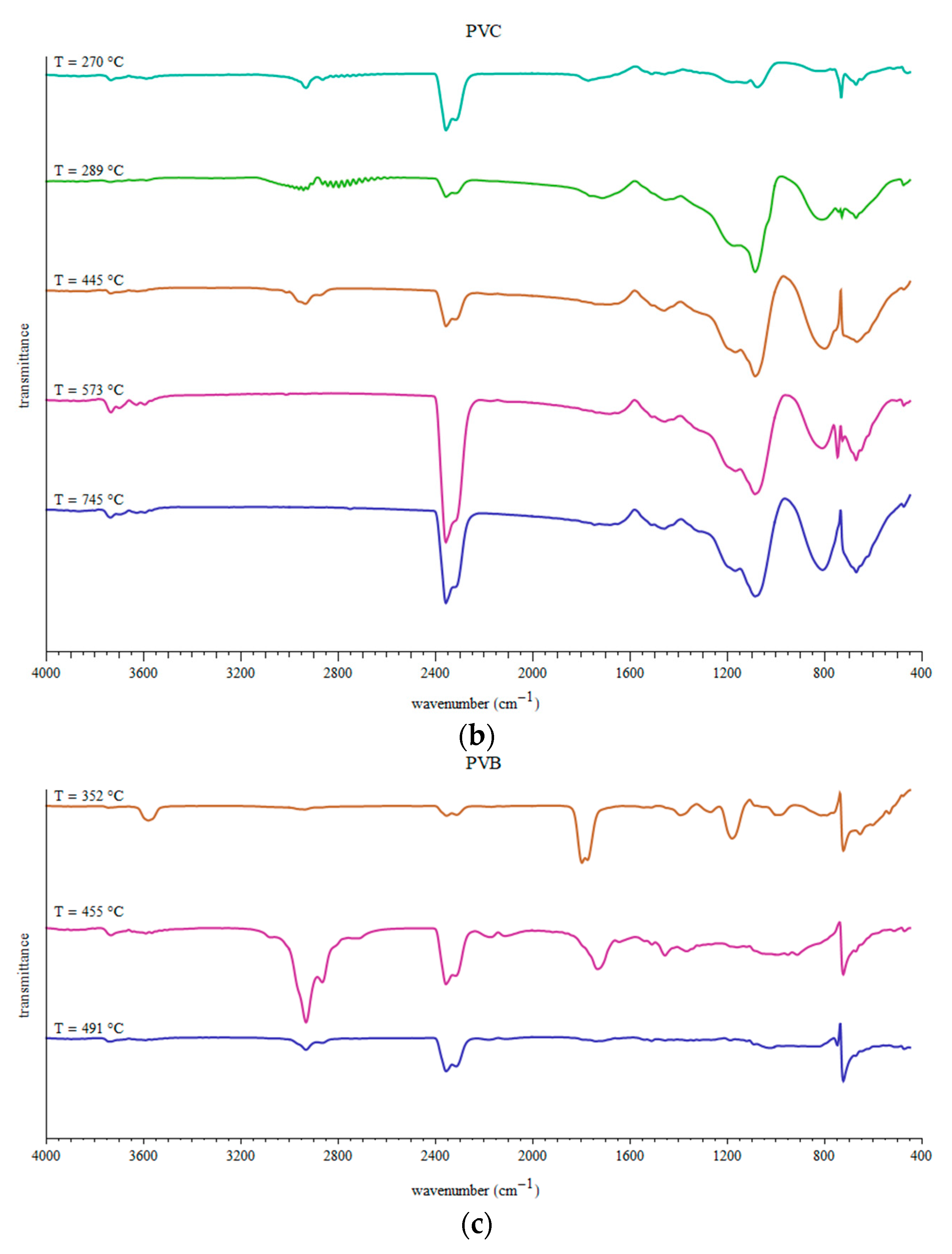
3.3. Fragments Arising during Pyrolysis of Considered WPs
- Several peaks around 2960, 2940, 2880 and 2845 cm−1 correspond to C-H stretching in alkanes (-CH3 and >CH2). Small peaks in region 3100–3000 cm−1 indicate C-H stretching in alkenes or aromatic compounds.
- Smaller series of peaks in region 3700–3500 cm−1 can be due to O-H stretching in free OH groups.
- Two usually strong double peaks around 2350 and 2315 cm−1 which should be attributed to CO2 presence in the gaseous products of pyrolysis.
- Weak peaks around 2170 and 2110 cm−1 can be assigned to C≡C in alkynes or C≡O in CO.
- Peaks around 1800–1540 cm−1 can be attributed to C=O stretching in carbonyl group.
- The peak around 1610 cm−1 can be related to C=C stretching.
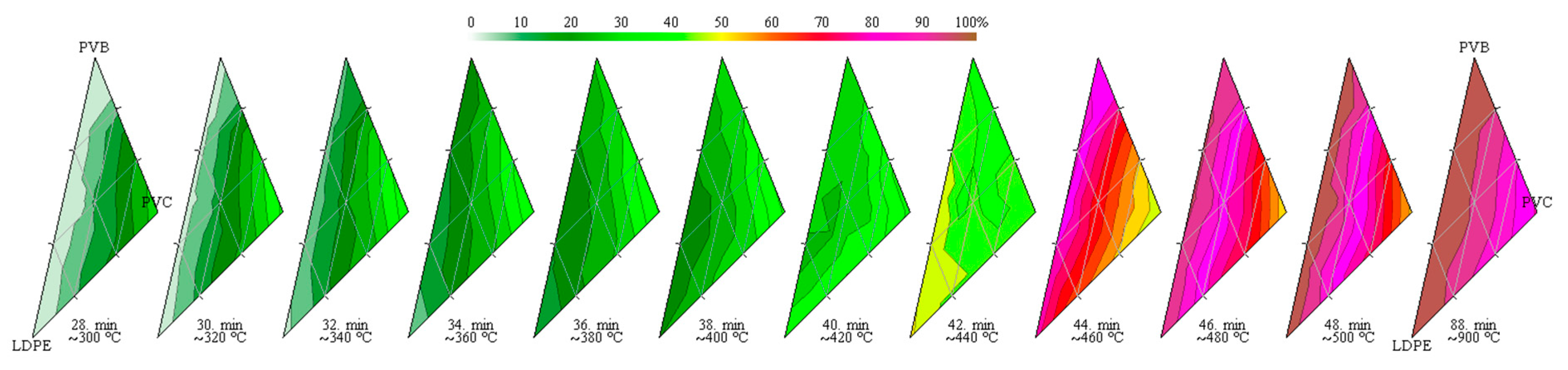
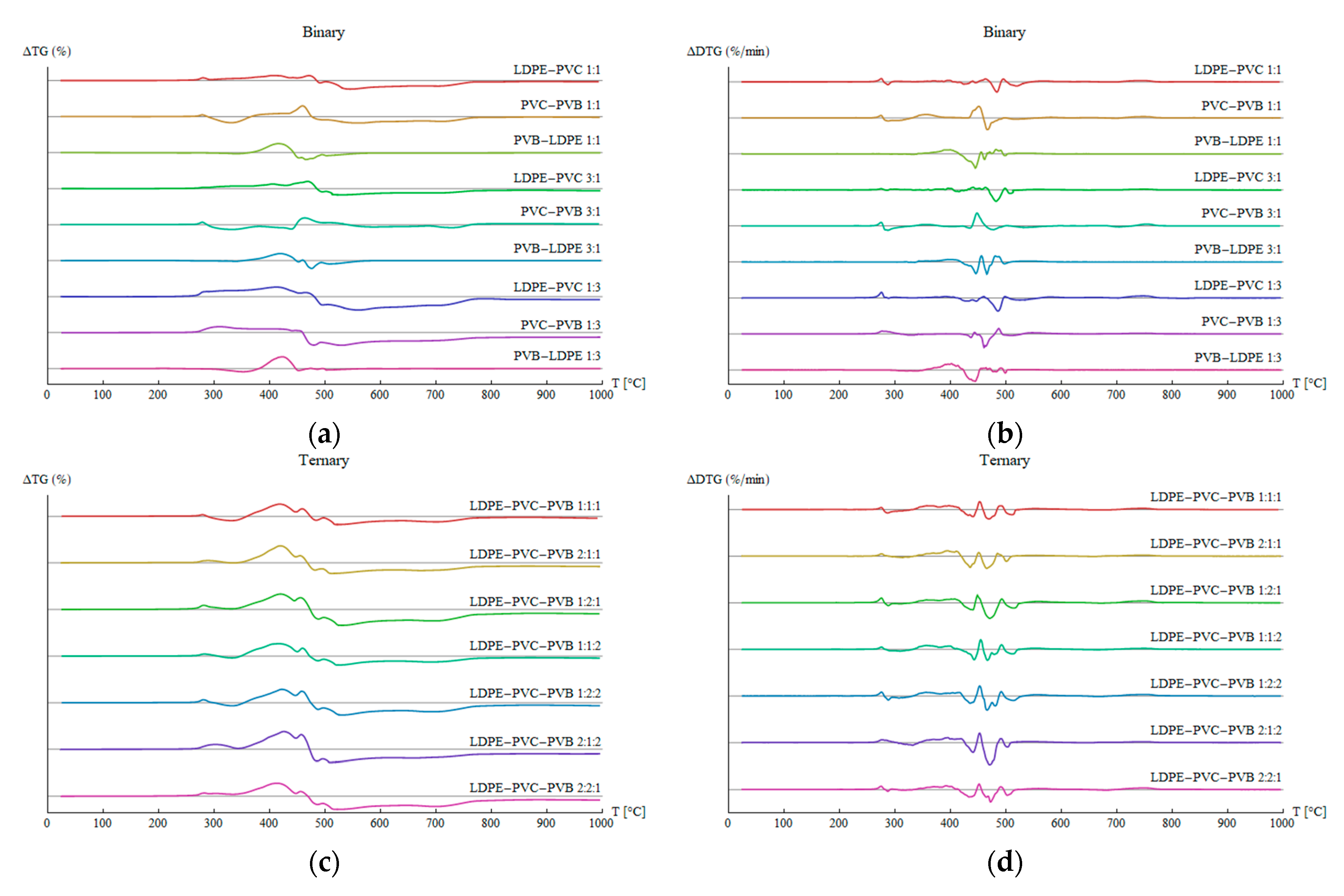
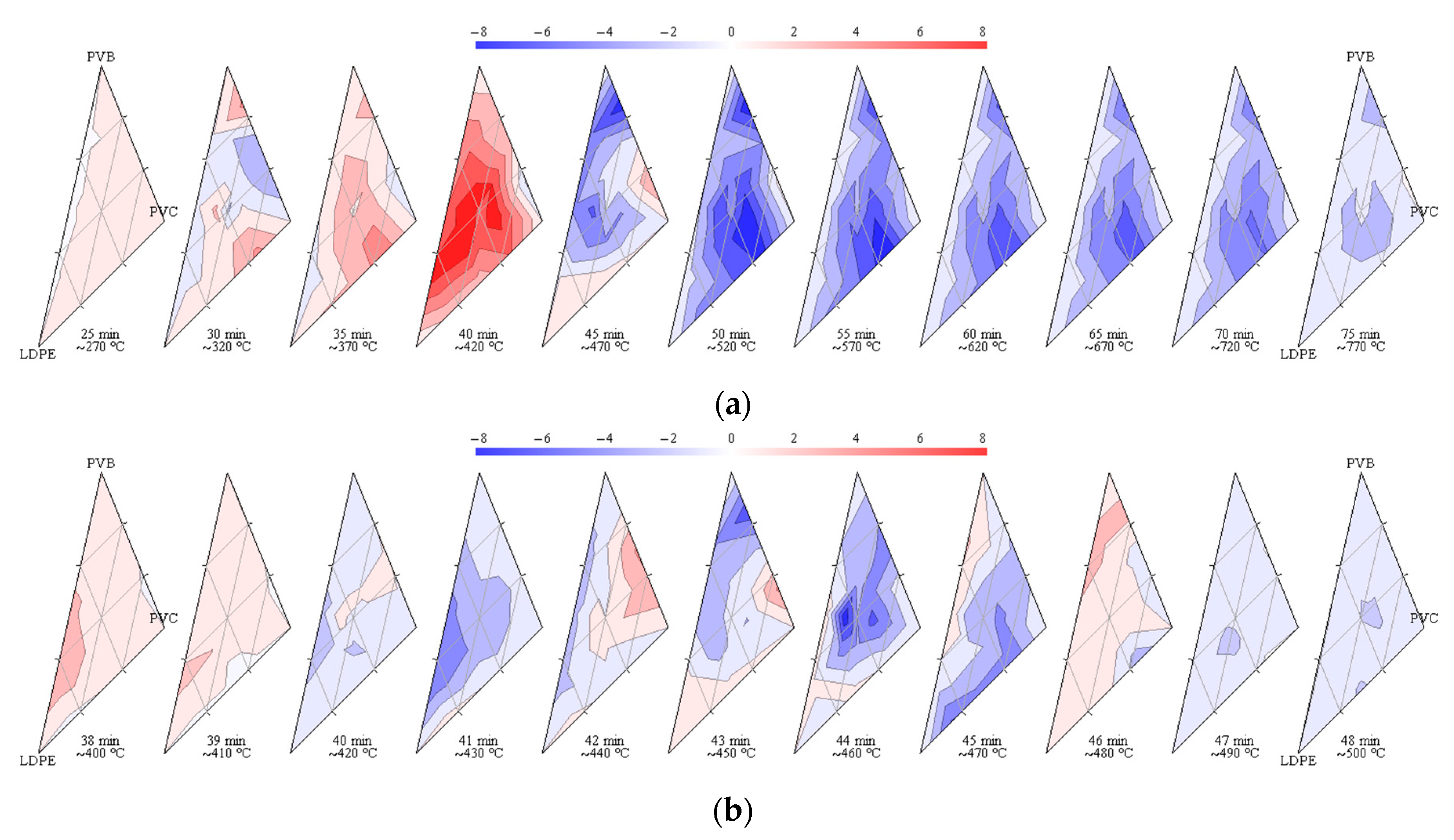
3.4. Synergy Effects
3.5. Deconvolution Results
3.6. Peak Parameters and CPI
3.7. Activation Energy
4. Conclusions
- Among the considered PWs, LDPE pyrolysis occurs in the narrowest range of temperatures, whereas PVC pyrolysis starts at the lowest temperature and ends at the highest one;
- The mass loss was around 99% for LDPE and PVB, whereas it was only around 80% for PVC;
- The highest mass loss rate was for PVB and the lowest was for PVC;
- The pyrolysis of the considered mixtures showed noticeable synergy effects—in the initial stage of pyrolysis up to a temperature around 450 °C, the mass loss is accelerated compared to the prediction based on a simple superposition, which means a higher conversion rate than expected;
- If the waste does not contain PVC, the pyrolysis is nearly complete at a temperature of around 500 °C for a heating rate of 10 °C/min, whereas PVC is not fully processed even at 995 °C;
- The stage related to the elimination of water from the vinyl alcohol copolymer during PVB pyrolysis seems considerably affected by the presence of PVC by lowering the temperature of that step, whereas other pyrolysis steps do not show significant shifts in temperatures;
- The activation energy varies significantly during the pyrolysis for all samples and all pyrolysis stages; it falls in the range around 70–380 kJ/mol; the activation energy related with particular pyrolysis steps was significantly lower in mixtures than in single-component samples;
- CPI values are highest for PVB, and lowest for PVC.
- If the waste contains only LDPE and PVB, and PVC is absent, the final pyrolysis temperature can be around 500 °C, i.e., there is no need to use higher temperatures. This may significantly accelerate the process and lower the costs.
- The synergistic effects influence significantly the pyrolysis process by accelerating some steps below around 450 °C and lowering the activation energy.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vijaykumar, S.; Patel, M.R.; Patel, J.V. Pet Waste Management by Chemical Recycling: A Review. J. Polym. Environ. 2010, 18, 8–25. [Google Scholar] [CrossRef]
- Plastics Europe, The Circular Economy for Plastics—A European Overview. Available online: https://plasticseurope.org/knowledge-hub/the-circular-economy-for-plastics-a-european-overview/ (accessed on 3 March 2024).
- Harussani, M.M.; Sapuan, S.M.; Rashid, U.; Khalina, A.; Ilyas, R.A. Pyrolysis of polypropylene plastic waste into carbonaceous char: Priority of plastic waste management amidst COVID-19 pandemic. Sci. Total Environ. 2022, 803, 149911. [Google Scholar] [CrossRef] [PubMed]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Lithner, D.; Larsson, A.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Nag, R.; Cummins, E. Human health concerns regarding microplastics in the aquatic environment—From marine to food systems. Sci. Total Environ. 2022, 823, 153730. [Google Scholar] [CrossRef] [PubMed]
- European Commission. A European Strategy for Plastics in a Circular Economy, Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. Strasburg, 2018. Document 52018DC0028. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2018%3A28%3AFIN (accessed on 3 March 2024).
- Environmental Investigation Agency. Available online: https://eia-international.org/about-us/reports/ (accessed on 3 March 2024).
- Jabłońska, B. Water consumption management in polyethylene terephthalate (PET) bottles washing process via wastewater pretreatment and reuse. J. Environ. Manag. 2018, 224, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, B.; Kiełbasa, P.; Korenko, M.; Dróżdż, T. Physical and Chemical Properties of Waste from PET Bottles Washing as A Component of Solid Fuels. Energies 2019, 12, 2197. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Supriyanto; Ylitervo, P.; Richards, T. Gaseous products from primary reactions of fast plastic pyrolysis. J. Anal. Appl. Pyrolysis 2021, 158, 105248. [Google Scholar] [CrossRef]
- Calero, M.; Solís, R.R.; Muñoz-Batista, M.J.; Pérez, A.; Blázquez, G.; Martín-Lara, M.A. Oil and gas production from the pyrolytic transformation of recycled plastic waste: An integral study by polymer families. Chem. Eng. Sci. 2023, 271, 118569. [Google Scholar] [CrossRef]
- Infiesta, L.R.; Ferreira, C.R.N.; Trovo, A.G.; Borges, V.L.; Carvalho, S.R. Design of an industrial solid waste processing line to produce refuse-derived fuel. J. Environ. Manag. 2019, 236, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.L.; Vaz de Miranda, D.M.; Pinto, J.C. Critical evaluation of life cycle assessment analyses of plastic waste pyrolysis. ACS Sustain. Chem. Eng. 2022, 10, 3799–3807. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Stasiulaitiene, I.; Zakarauskas, K.; Striūgas, N. Pyrolysis of all layers of surgical mask waste as a mixture and its life-cycle assessment. Sustain. Prod. Consum. 2022, 32, 519–531. [Google Scholar] [CrossRef]
- Punkkinen, H.; Oasmaa, A.; Laatikainen-Luntama, J.; Nieminen, M.; Laine-Ylijoki, J. Thermal Conversion of Plastic-Containing Waste: A Review; Research Report No D4.1-22; VTT Technical Research Centre of Finland: Helsinki, Finland, 2017. [Google Scholar]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Gabbar, H.A.; Aboughaly, M.; Stoute, C.A.B. DC thermal plasma design and utilization for the low density polyethylene to diesel oil pyrolysis reaction. Energies 2017, 10, 784. [Google Scholar] [CrossRef]
- Sharuddin, D.A.S.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Conesa, J.A.; Font, R.; Marcilla, A.; Garcia, A.N. Pyrolysis of polyethylene in a fluidized bed reactor. Energy Fuels 1994, 8, 1238–1246. [Google Scholar] [CrossRef]
- Poznańska, G.; Jabłońska, B. Europejskie trendy w recyklingu odpadów z tworzyw sztucznych. In Inżynieria Środowiska i Biotechnologia—Wyzwania i Nowe Technologie; Rosińska, A., Karwowska, B., Madeła, M., Eds.; Wyd. Politechniki Częstochowskiej: Częstochowa, Poland, 2022. [Google Scholar]
- Pielichowski, K.; Njuguna, J.; Majka, T.S. Thermal Degradation of Polymeric Materials, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Cho, M.H.; Song, Y.J.; Rhu, C.J.; Go, B.R. Pyrolysis Process of Mixed Microplastics Using TG-FTIR and TED-GC-MS. Polymers 2023, 15, 241. [Google Scholar] [CrossRef]
- Majewsky, M.; Bitter, H.; Eiche, E.; Horn, H. Determination of microplastic polyethylene (PE) and polypropylene (PP) in environmental samples using thermal analysis (TGA-DSC). Sci. Total Environ. 2016, 568, 507–511. [Google Scholar] [CrossRef]
- Genuino, H.C.; Ruiz, M.P.; Heeres, H.J.; Kersten, S.R.A. Pyrolysis of mixed plastic waste: Predicting the product yields. Waste Manag. 2023, 156, 208–215. [Google Scholar] [CrossRef]
- Hou, Q.; Zhen, M.; Qian, H.; Nie, Y.; Bai, X.; Xia, T.; Rehman, M.L.U.; Li, Q.; Ju, M. Upcycling and catalytic degradation of plastic wastes. Cell Rep. Phys. Sci. 2021, 2, 100514. [Google Scholar] [CrossRef]
- Dogu, O.; Pelucchi, M.; Van de Vijver, R.; Van Steenberge, P.H.M.; D’hooge, D.R.; Cuoci, A.; Mehl, M.; Frassoldati, A.; Faravelli, T.; Van Geem, K.M. The chemistry of chemical recycling of solid plastic waste via pyrolysis and gasification: State-of-the-art, challenges, and future directions. Prog. Energy Combust. Sci. 2021, 84, 100901. [Google Scholar] [CrossRef]
- Solis, M.; Silveira, S. Technologies for chemical recycling of household plastics—A technical review and TRL assessment. Waste Manag. 2020, 105, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.B.; Benedini, L.; Jensen, C.D.; Kamp, A.; Henriksen, U.B.; Thomsen, T.P. Climate footprint assessment of plastic waste pyrolysis and impacts on the Danish waste management system. J. Environ. Manag. 2024, 351, 119780. [Google Scholar] [CrossRef]
- Li, D.; Lei, S.; Wang, P.; Zhong, L.; Ma, W.; Chen, G. Study on the pyrolysis behaviors of mixed waste plastics. Renew. Energy 2021, 173, 662–674. [Google Scholar] [CrossRef]
- Mentes, D.; Nagy, G.; Szabó, T.J.; Hornyák-Mester, E.; Fiser, B.; Viskolcz, B.; Póliska, C. Combustion behaviour of plastic waste—A case study of PP, HDPE, PET, and mixed PES-EL. J. Clean. Prod. 2023, 402, 136850. [Google Scholar] [CrossRef]
- Alawa, B.; Choudhary, J.; Chakma, S. Discernment of synergism in co-pyrolysis of HDPE and PP waste plastics for production of pyro-oil: Mechanistic investigation with economic analysis and health risk assessment. Process Saf. Environ. Prot. 2023, 169, 107–131. [Google Scholar] [CrossRef]
- Martínez-Narro, G.; Royston, N.J.; Billsborough, K.L.; Phan, A.N. Kinetic modelling of mixed plastic waste pyrolysis. Chem. Thermodyn. Therm. Anal. 2023, 9, 100105. [Google Scholar] [CrossRef]
- Subhashini; Mondal, T. Experimental investigation on slow thermal pyrolysis of real-world plastic wastes in a fixed bed reactor to obtain aromatic rich fuel grade liquid oil. J. Environ. Manag. 2023, 344, 118680. [Google Scholar] [CrossRef]
- Jiang, G.; Fenwick, R.; Seville, J.; Mahood, H.B.; Thorpe, R.B.; Bhattacharya, S.; Sanchez Monsalve, D.A.; Leeke, G.A. Lumped kinetic modelling of polyolefin pyrolysis: A non-isothermal method to estimate rate constants. J. Anal. Appl. Pyrolysis 2022, 164, 105530. [Google Scholar] [CrossRef]
- European Standard CEN/TS 15414-1:2010; Solid Recovered Fuels—Determination of Moisture Content Using the Oven Dry Method—Part 1: Determination of Total Moisture by a Reference Method. European Committee for Standardization: Brussels, Belgium, 2010.
- Polish Standard PN-EN ISO 3451-1; Tworzywa Sztuczne. Oznaczanie Popiołu. Metody Ogólne. Polish Committee for Standardization: Warsaw, Poland, 2010.
- Skalmowski, K.; Wolska, K.; Pieniak, U.; Roszczyńska, I. Badania Właściwości Technologicznych Odpadów Komunalnych. Ćwiczenia Laboratoryjne; Oficyna Wydawnicza Politechniki Warszawskiej: Warszawa, Poland, 2004; pp. 94–97. [Google Scholar]
- Polish Standard PN-EN ISO 1183; Tworzywa Sztuczne—Metody Oznaczania Gęstości Tworzyw Sztucznych Nieporowatych—Część 1: Metoda Zanurzeniowa, Metoda Piknometru Cieczowego i Metoda Miareczkowa. Polish Committee for Standardization: Warsaw, Poland, 2019.
- Polish Standard PN-EN ISO 21663:2021-06; Solid Secondary Fuels—Instrumental Methods for the Determination of Carbon (C), Hydrogen (H), Nitrogen (N) and Sulphur (S). Polish Committee for Standardization: Warsaw, Poland, 2021.
- Polish Standard PN-EN ISO 21654:2021-12; Stałe Paliwa Wtórne—Oznaczanie Wartości Opałowej. Polish Committee for Standardization: Warsaw, Poland, 2021.
- Polish Standard PN-EN 15408:2011; Stałe Paliwa Wtórne—Metody Oznaczania Zawartości Siarki (S), Chloru (Cl), Fluoru (F) i Bromu (Br). Polish Committee for Standardization: Warsaw, Poland, 2011.
- da Silva, J.C.G.; de Albuquerque, J.G.; de Araujo Galdino, W.V.; de Sena, R.F.; Andersen, S.L.F. Single-step and multi-step thermokinetic study—Deconvolution method as a simple pathway for describe properly the biomass pyrolysis for energy conversion. Energy Convers. Manag. 2020, 209, 112653. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Starink, M. The determination of activation energy from linear heating rate experiments: A comparison of the accuracy of isoconversion methods. Thermochim. Acta 2003, 404, 163–176. [Google Scholar] [CrossRef]
- Brendgen, R.; Graßmann, C.; Grethe, T.; Mahltig, T.; Schwarz-Pfeiffer, A. Coatings with recycled polyvinyl butyral on polyester and polyamide mono- and multifilament yarns. J. Coat Technol Res. 2021, 18, 819–829. [Google Scholar] [CrossRef]
- Królikowski, M.; Żach, P.; Kalestyński, M. Selection of Conditions in PVB Polymer Dissolution Process for Laminated Glass Recycling Applications. Polymers 2022, 14, 5119. [Google Scholar] [CrossRef]
- Carrot, C.; Bendaoud, A.; Pillon, C. Polyvinyl Butyral. In Handbook of Thermoplastics, 2nd ed.; Olabisi, O., Adewale, K., Eds.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Singh, M.V.; Kumar, S.; Sarker, M. Waste HD-PE plastic, deformation into liquid hydrocarbon fuel using pyrolysis-catalytic cracking with a CuCO3 catalyst. Sust. Energy Fuels 2018, 2, 1057–1068. [Google Scholar] [CrossRef]
- Polish Standard PN-C-96024:2020-12; Petroleum Products—Fuel Oils. Polish Committee for Standardization: Warsaw, Poland, 2020. (In Polish)
- Onwudili, J.A.; Insura, N.; Williams, P.T. Composition of products from the pyrolysis of polyethylene and polystyrene in a closed batch reactor: Effects of temperature and residence time. J. Anal. Appl. Pyrolysis. 2009, 86, 293–303. [Google Scholar] [CrossRef]
- Lee, N.; Joo, J.; Lin, K.Y.A.; Lee, J. Waste-to-Fuels: Pyrolysis of Low-Density Polyethylene Waste in the Presence of H-ZSM-11. Polymers 2021, 13, 1198. [Google Scholar] [CrossRef]
- McNeil, I.C.; Memetea, L.; Cole, W.J. A study of the products of PVC thermal degradation. Polym. Degrad. Stab. 1995, 49, 181–191. [Google Scholar] [CrossRef]
- Liau, L.C.K.; Yang, T.C.K.; Viswanath, D.S. Reaction Pathways and Kinetic Analysis of PVB Thermal Degradation Using TG/FT-IR. Appl. Spectrosc. 1996, 50, 1058–1065. [Google Scholar] [CrossRef]
- Yan, H.; Cannon, W.R.; Shanefield, D.J. Poly(vinyl butyral) pyrolysis: Interactions with plasticizer and AIN ceramic powder. MRS Online Proc. Libr. 1992, 249, 377–382. [Google Scholar] [CrossRef]
- Iida, T.; Nakanishi, M.; Gotō, K. Investigations on poly(vinyl chloride). I. Evolution of aromatics on pyrolysis of poly(vinyl chloride) and its mechanism. J. Polym. Sci. Polym. Chem. Ed. 1974, 12, 737–749. [Google Scholar] [CrossRef]
- Mehl, M.; Marongiu, A.; Faravelli, T.; Bozzano, G.; Dente, M.; Ranzi, E. A kinetic modeling study of the thermal degradation of halogenated polymers. J. Anal. Appl. Pyrolysis. 2004, 72, 253–272. [Google Scholar] [CrossRef]
- Cepelioğullar, Ö.; Pütün, A. Utilization of two different types of plastic wastes from daily and industrial life. J. Selcuk Univ. Nat. Appl. Sci. 2013, 2, 694–706. [Google Scholar]
- Liau, L.C.K.; Viswanath, D.S. Thermal Degradation of Poly(vinylbutyral)/Ceramic Composites: A Kinetic Approach. Ind. Eng. Chem. Res. 1998, 37, 49–57. [Google Scholar] [CrossRef]
- McLafferty, F.W.; Tureček, F. Interpretation of Mass Spectra, 4th ed.; University Science Books: Sausalito, CA, USA, 1993. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]





| Group | No | Full Designation | Short Designation | Normalized Share | ||
|---|---|---|---|---|---|---|
| Components | 1 | LDPE | 1:0:0 | 1.00 | 0.00 | 0.00 |
| 2 | PVC | 0:1:0 | 0.00 | 1.00 | 0.00 | |
| 3 | PVB | 0:0:1 | 0.00 | 0.00 | 1.00 | |
| Binary | 4 | LDPE-PVC 1:1 | 1:1:0 | 0.50 | 0.50 | 0.00 |
| mixtures | 5 | PVC-PVB 1:1 | 0:1:1 | 0.00 | 0.50 | 0.50 |
| 6 | PVB-LDPE 1:1 | 1:0:1 | 0.50 | 0.00 | 0.50 | |
| 7 | LDPE-PVC 3:1 | 3:1:0 | 0.75 | 0.25 | 0.00 | |
| 8 | PVC-PVB 3:1 | 0:3:1 | 0.00 | 0.75 | 0.25 | |
| 9 | PVB-LDPE 3:1 | 1:0:3 | 0.25 | 0.00 | 0.75 | |
| 10 | LDPE-PVC 1:3 | 1:3:0 | 0.25 | 0.75 | 0.00 | |
| 11 | PVC-PVB 1:3 | 0:1:3 | 0.00 | 0.25 | 0.75 | |
| 12 | PVB-LDPE 1:3 | 3:0:1 | 0.75 | 0.00 | 0.25 | |
| Ternary | 13 | LDPE-PVC-PVB 1:1:1 | 1:1:1 | 0.33 | 0.33 | 0.33 |
| mixtures | 14 | LDPE-PVC-PVB 2:1:1 | 2:1:1 | 0.50 | 0.25 | 0.25 |
| 15 | LDPE-PVC-PVB 1:2:1 | 1:2:1 | 0.25 | 0.50 | 0.25 | |
| 16 | LDPE-PVC-PVB 1:1:2 | 1:1:2 | 0.25 | 0.25 | 0.50 | |
| 17 | LDPE-PVC-PVB 1:2:2 | 1:2:2 | 0.20 | 0.40 | 0.40 | |
| 18 | LDPE-PVC-PVB 2:1:2 | 2:1:2 | 0.40 | 0.20 | 0.40 | |
| 19 | LDPE-PVC-PVB 2:2:1 | 2:2:1 | 0.40 | 0.40 | 0.20 | |
| WP | Proximate Analysis | Ultimate Analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| W a | Ash d | VM daf | C | H | N | S | O | Cl | |||
| wt% | wt% | wt% | g/cm3 | kJ/g | wt% | wt% | wt% | wt% | wt% | wt% | |
| LDPE | 0.12 | 1.08 | 98.92 | 0.906 | 44.85 | 85.2 | 13.25 | 0.02 | 0.01 | 0.91 | 0.61 |
| PVC | 0.11 | 1.19 | 98.80 | 1.421 | 14.21 | 31.5 | 3.69 | 0.09 | 0.01 | 44.82 | 18.7 |
| PVB | 0.23 | 1.31 | 98.69 | 1.069 | 39.05 | 76.8 | 11.45 | 0.05 | 0.01 | 10.09 | 0.29 |
| Parameter | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | °C/min | °C | °C | °C | °C | % | %/min | %/min | – | * |
| LDPE | 10 | 334.0 | 492.3 | 470.0 | 36.8 | 92.6 | 16.9 | 6.4 | 0.96 | 1734 |
| Peak 1 | 20 | 394.5 | 509.3 | 485.7 | 39.3 | 93.4 | 41.6 | 16.5 | 0.99 | 8546 |
| 30 | 396.5 | 511.6 | 483.2 | 42.5 | 90.9 | 59.5 | 24.6 | 0.99 | 16,319 | |
| PVC | 10 | 254.8 | 340.6 | 283.1 | 27.3 | 37.9 | 11.2 | 4.6 | 0.48 | 983 |
| Peak 1 | 20 | 269.2 | 357.4 | 296.6 | 34.5 | 36.4 | 19.2 | 8.6 | 0.50 | 2159 |
| 30 | 272.9 | 360.5 | 300.1 | 37.6 | 35.6 | 28.1 | 12.9 | 0.50 | 4166 | |
| Peak 2 | 10 | 422.2 | 582.3 | 461.8 | 50.1 | 14.8 | 2.5 | 1.0 | 0.19 | 4 |
| 20 | 395.6 | 553.0 | 476.2 | 58.6 | 14.3 | 4.5 | 1.9 | 0.20 | 11 | |
| 30 | 391.5 | 551.4 | 473.2 | 60.0 | 14.6 | 6.9 | 2.8 | 0.20 | 26 | |
| Peak 5 | 10 | 701.6 | 778.7 | 748.9 | 47.1 | 5.8 | 1.2 | 0.7 | 0.07 | 0 |
| 20 | 708.1 | 836.5 | 794.2 | 66.2 | 7.3 | 2.2 | 1.1 | 0.10 | 0 | |
| 30 | 689.5 | 827.7 | 785.2 | 74.7 | 7.1 | 3.0 | 1.6 | 0.10 | 1 | |
| PVB | 10 | 276.1 | 401.7 | 352.1 | 45.1 | 26.4 | 5.2 | 2.2 | 0.27 | 68 |
| Peak 1 | 20 | 289.1 | 420.1 | 363.0 | 47.4 | 25.4 | 9.9 | 4.0 | 0.27 | 203 |
| 30 | 299.9 | 411.7 | 368.0 | 50.8 | 22.8 | 14.2 | 6.5 | 0.25 | 374 | |
| Peak 2 | 10 | 432.3 | 476.6 | 454.9 | 19.4 | 58.3 | 34.2 | 13.9 | 0.60 | 7285 |
| 20 | 442.5 | 506.2 | 471.7 | 35.4 | 59.8 | 37.8 | 19.3 | 0.63 | 5892 | |
| 30 | 438.3 | 508.1 | 471.0 | 35.9 | 62.4 | 54.9 | 27.5 | 0.68 | 12,724 | |
| LDPE-PVC | 10 | 266.8 | 313.8 | 286.0 | 15.2 | 13.0 | 6.6 | 2.8 | 0.15 | 207 |
| 1:1 | 20 | 272.3 | 359.9 | 298.5 | 29.4 | 17.0 | 10.0 | 4.0 | 0.20 | 284 |
| Peak 1 | 30 | 273.9 | 366.8 | 301.5 | 30.9 | 15.5 | 13.4 | 5.2 | 0.18 | 422 |
| Peak 2 | 10 | 355.0 | 496.5 | 477.9 | 42.3 | 48.1 | 10.0 | 3.7 | 0.54 | 251 |
| 20 | 401.9 | 518.7 | 491.4 | 42.2 | 58.7 | 25.4 | 10.4 | 0.68 | 1857 | |
| 30 | 398.5 | 520.5 | 491.1 | 46.1 | 59.3 | 36.3 | 15.1 | 0.70 | 3592 | |
| PVC-PVB | 10 | 267.8 | 330.8 | 284.8 | 19.6 | 16.5 | 6.4 | 2.6 | 0.19 | 186 |
| 1:1 | 20 | 273.3 | 358.9 | 297.5 | 28.2 | 17.1 | 10.5 | 4.1 | 0.20 | 322 |
| Peak 1 | 30 | 277.3 | 375.6 | 303.4 | 30.9 | 15.9 | 13.6 | 5.1 | 0.19 | 427 |
| Peak 2 | 10 | 226.6 | 377.5 | 316.0 | 59.5 | 12.9 | 2.0 | 0.9 | 0.15 | 5 |
| 20 | 234.2 | 409.0 | 353.6 | 65.9 | 12.8 | 3.6 | 1.5 | 0.15 | 13 | |
| 30 | 255.2 | 405.8 | 361.4 | 55.9 | 11.5 | 6.0 | 2.4 | 0.13 | 33 | |
| Peak 3 | 10 | 433.0 | 493.1 | 463.2 | 27.6 | 36.7 | 14.2 | 6.8 | 0.42 | 640 |
| 20 | 389.4 | 516.2 | 481.1 | 52.8 | 46.7 | 16.8 | 7.5 | 0.54 | 598 | |
| 30 | 389.8 | 515.5 | 482.0 | 47.4 | 50.4 | 29.8 | 12.5 | 0.59 | 2110 | |
| PVB-LDPE | 10 | 263.8 | 402.1 | 350.4 | 52.4 | 17.7 | 3.1 | 1.3 | 0.18 | 15 |
| 1:1 | 20 | 285.7 | 420.3 | 361.0 | 53.5 | 13.1 | 4.6 | 2.0 | 0.14 | 22 |
| Peak 1 | 30 | 284.6 | 413.7 | 364.5 | 49.1 | 12.2 | 7.2 | 3.0 | 0.13 | 52 |
| Peak 2 | 10 | 400.8 | 482.3 | 457.8 | 30.2 | 75.3 | 22.5 | 10.5 | 0.77 | 3196 |
| 20 | 421.3 | 513.6 | 485.1 | 37.1 | 81.4 | 40.7 | 18.1 | 0.87 | 7924 | |
| 30 | 416.8 | 511.1 | 486.1 | 37.7 | 80.7 | 60.5 | 26.3 | 0.88 | 16,824 | |
| LDPE-PVC- | 10 | 268.0 | 320.7 | 285.9 | 18.5 | 10.1 | 4.6 | 1.9 | 0.11 | 63 |
| PVB 1:1:1 | 20 | 274.2 | 349.4 | 298.6 | 28.5 | 5.7 | 3.7 | 1.6 | 0.06 | 14 |
| Peak 1 | 30 | 277.0 | 377.4 | 304.1 | 35.6 | 6.8 | 5.4 | 2.2 | 0.08 | 27 |
| Peak 2 | 10 | 223.3 | 392.5 | 320.7 | 67.4 | 12.3 | 1.7 | 0.7 | 0.13 | 3 |
| 20 | 248.7 | 411.6 | 353.6 | 62.6 | 6.9 | 2.0 | 0.9 | 0.08 | 2 | |
| 30 | 258.2 | 403.9 | 361.2 | 53.4 | 6.8 | 3.7 | 1.5 | 0.08 | 7 | |
| Peak 3 | 10 | 394.7 | 486.7 | 464.1 | 41.2 | 56.3 | 14.4 | 7.0 | 0.61 | 748 |
| 20 | 403.3 | 513.4 | 485.2 | 42.7 | 74.5 | 32.3 | 13.9 | 0.82 | 4012 | |
| 30 | 397.6 | 515.5 | 488.7 | 42.1 | 72.0 | 47.8 | 18.9 | 0.81 | 7967 |
| Friedman Method | Starink Method | |||||||
|---|---|---|---|---|---|---|---|---|
| Peak | Mean E | δE * | Mean R2 | δR2 * | Mean E | δE * | Mean R2 | δR2 * |
| kJ/mol | % | – | % | kJ/mol | % | – | % | |
| LDPE-1 | 183 | 96 | 0.89 | 11 | 126 | 116 | 0.82 | 6 |
| PVC-1 | 144 | 46 | 0.96 | 3 | 141 | 14 | 0.96 | 2 |
| PVC-2 ** | – | – | 0.70 | 115 | – | – | 0.76 | 106 |
| PVC-5 ** | 71 | 141 | 0.39 | 148 | 169 | 21 | 0.55 | 84 |
| PVB-1 | 212 | 10 | 0.88 | 45 | 191 | 29 | 0.94 | 22 |
| PVB-2 | 90 | 261 | 0.47 | 196 | 268 | 77 | 0.83 | 11 |
| LDPE-PVC-1 | 52 | 266 | 0.49 | 168 | 128 | 104 | 0.94 | 6 |
| LDPE-PVC-2 | 204 | 57 | 0.91 | 7 | 169 | 90 | 0.84 | 9 |
| PVC-PVB-1 | 89 | 88 | 0.99 | 1 | 128 | 73 | 1.00 | 0 |
| PVC-PVB-2 | 82 | 58 | 0.90 | 15 | 74 | 20 | 0.96 | 12 |
| PVC-PVB-3 ** | – | – | 0.55 | 161 | – | – | 0.83 | 67 |
| PVB-LDPE-1 | 197 | 28 | 0.82 | 41 | 177 | 38 | 0.84 | 20 |
| PVB-LDPE-2 | 143 | 88 | 0.83 | 25 | 163 | 8 | 0.84 | 13 |
| LDPE-PVC-PVB-1 | 60 | 156 | 0.96 | 11 | 122 | 95 | 1.00 | 1 |
| LDPE-PVC:PVB-2 | 109 | 92 | 0.90 | 18 | 85 | 48 | 0.92 | 14 |
| LDPE-PVC:PVB-3 | 146 | 51 | 0.82 | 33 | 199 | 49 | 0.87 | 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabłońska, B.; Poznańska, G.; Jabłoński, P.; Zwolińska, J. Thermochemical Valorization of Plastic Waste Containing Low Density Polyethylene, Polyvinyl Chloride and Polyvinyl Butyral into Thermal and Fuel Energy. Energies 2024, 17, 3458. https://doi.org/10.3390/en17143458
Jabłońska B, Poznańska G, Jabłoński P, Zwolińska J. Thermochemical Valorization of Plastic Waste Containing Low Density Polyethylene, Polyvinyl Chloride and Polyvinyl Butyral into Thermal and Fuel Energy. Energies. 2024; 17(14):3458. https://doi.org/10.3390/en17143458
Chicago/Turabian StyleJabłońska, Beata, Gabriela Poznańska, Paweł Jabłoński, and Joanna Zwolińska. 2024. "Thermochemical Valorization of Plastic Waste Containing Low Density Polyethylene, Polyvinyl Chloride and Polyvinyl Butyral into Thermal and Fuel Energy" Energies 17, no. 14: 3458. https://doi.org/10.3390/en17143458
APA StyleJabłońska, B., Poznańska, G., Jabłoński, P., & Zwolińska, J. (2024). Thermochemical Valorization of Plastic Waste Containing Low Density Polyethylene, Polyvinyl Chloride and Polyvinyl Butyral into Thermal and Fuel Energy. Energies, 17(14), 3458. https://doi.org/10.3390/en17143458






